High Performance Liquid Chromatography–Tandem Mass Spectrometry Method for Correlating the Metabolic Changes of Lactate, Pyruvate and L-Glutamine with Induced Tamoxifen Resistant MCF-7 Cell Line Potential Molecular Changes
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals and Materials
2.2. MCF-7 Cells Culturing Process
2.2.1. TMX Treatment
2.2.2. Media Collection, Cells Density, and Counting Process
2.3. HPLC-MS/MS Method Development
2.3.1. Instrumentation and Conditions
2.3.2. HPLC-MS/MS Method Validation
3. Results and Discussion
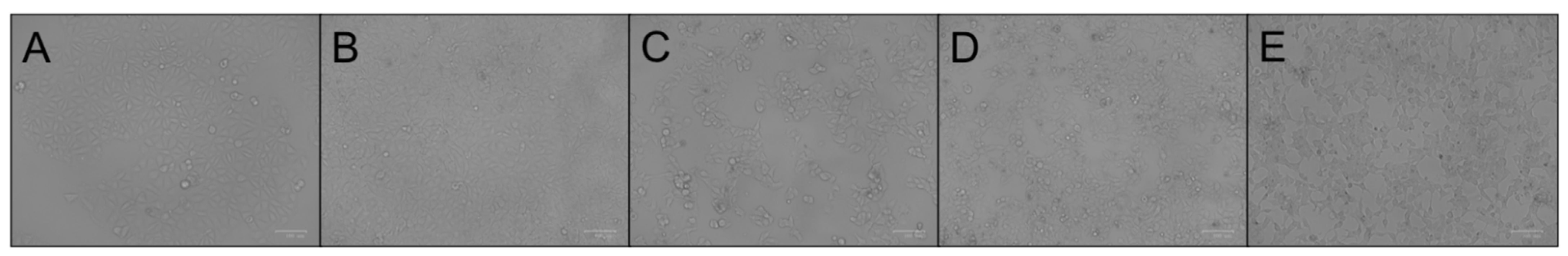
3.1. MCF-7 Cells Morphological Change
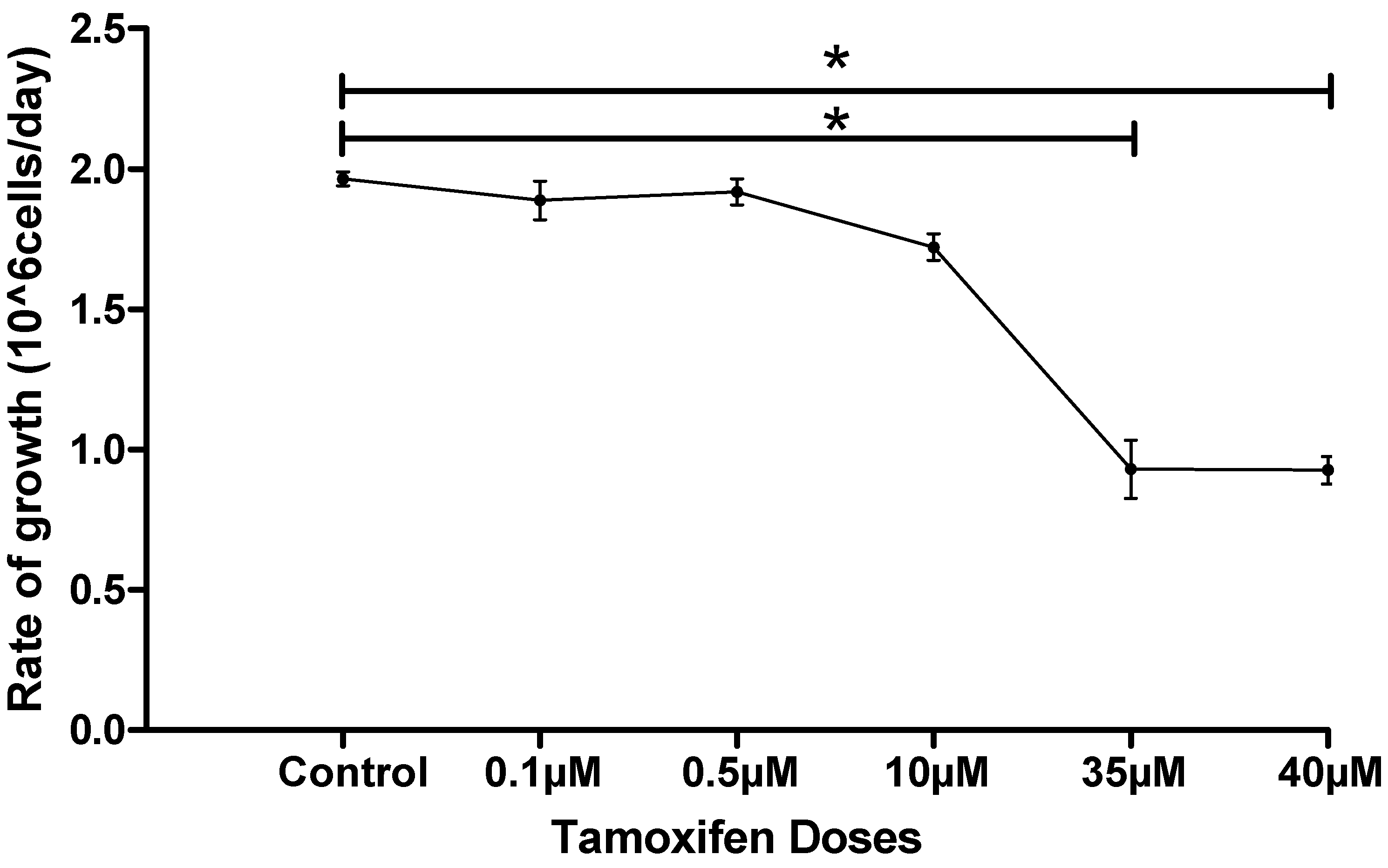
3.2. Cell Counting and Cell Density
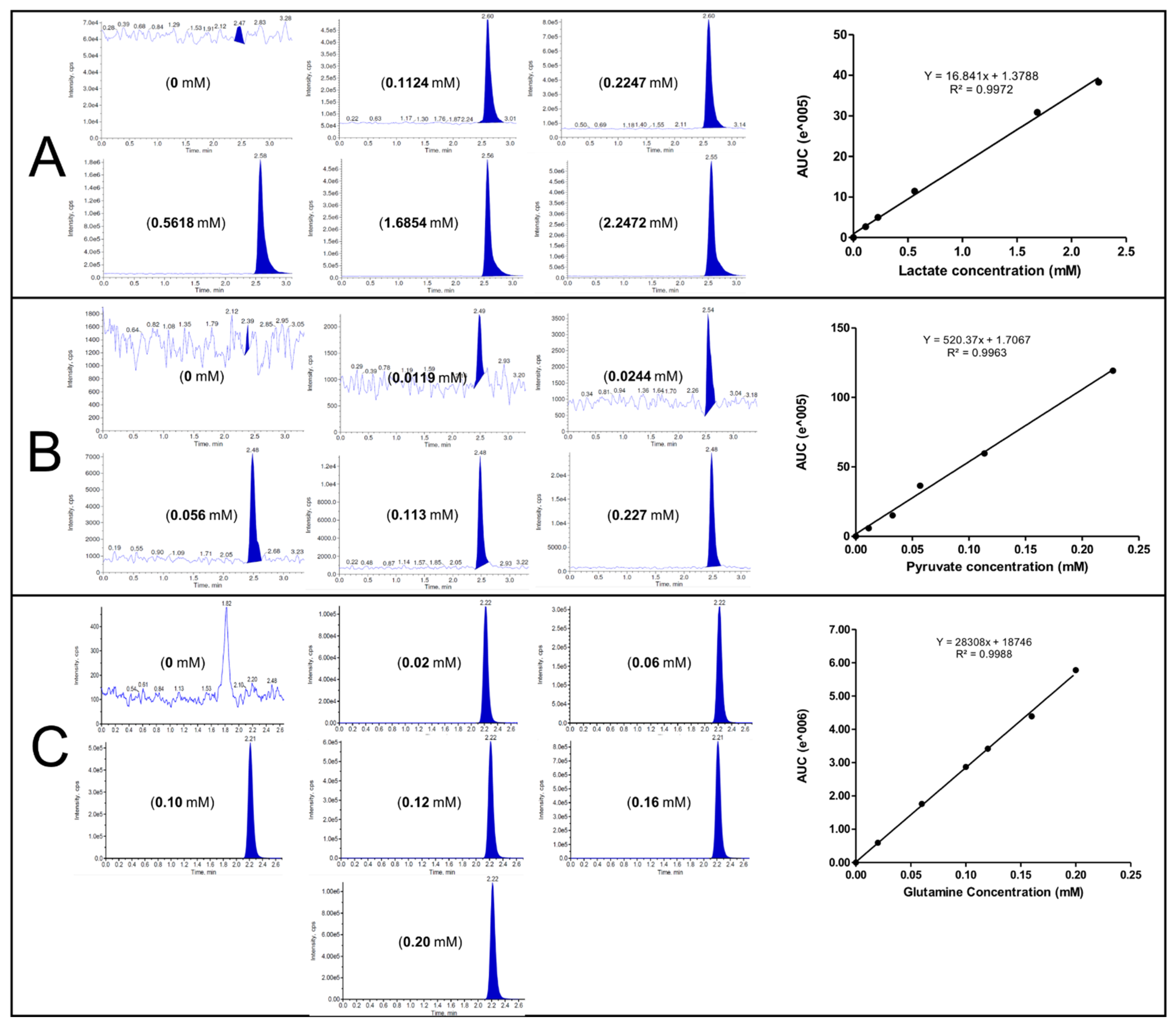
3.3. HPLC-MS/MS Method Validation
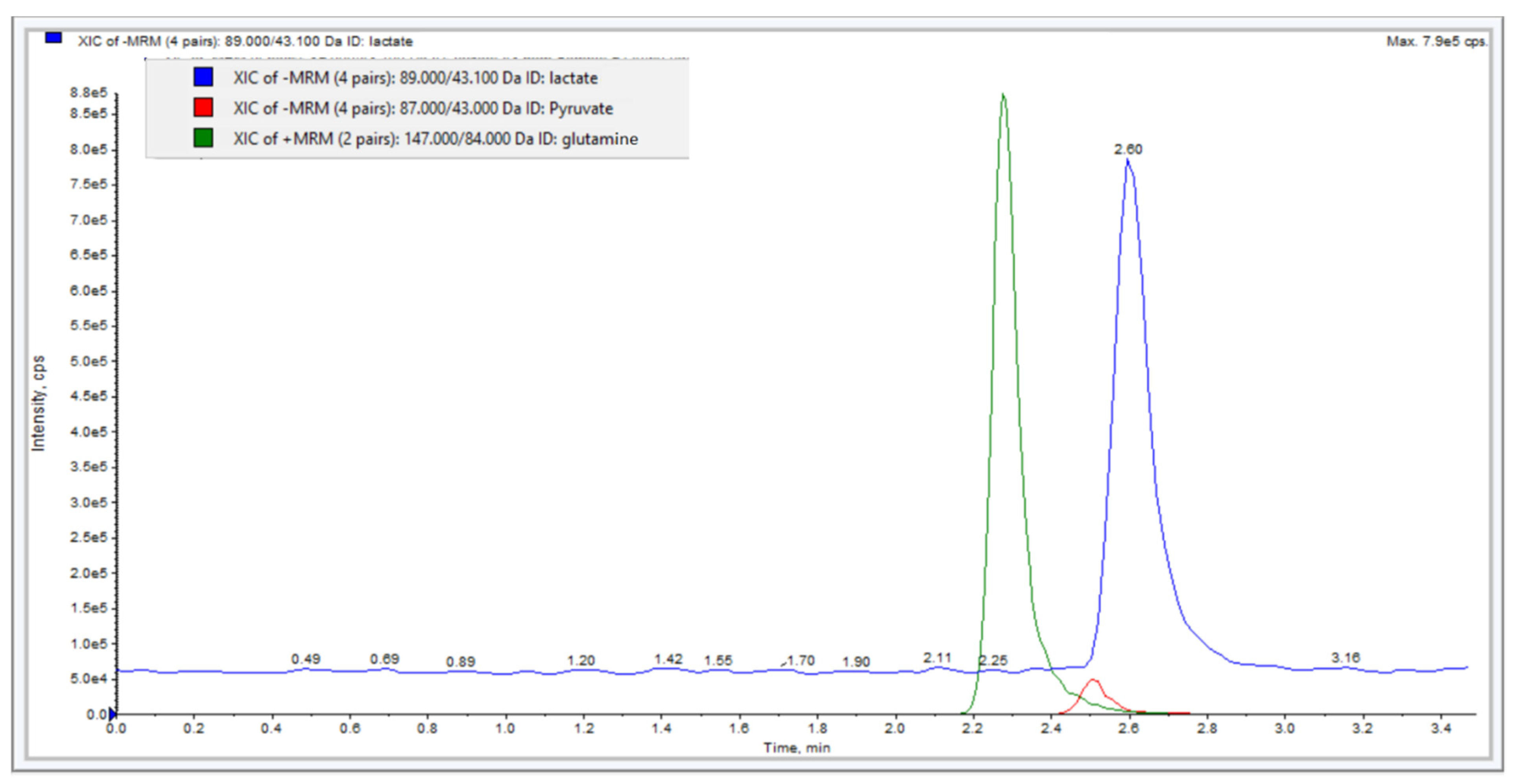
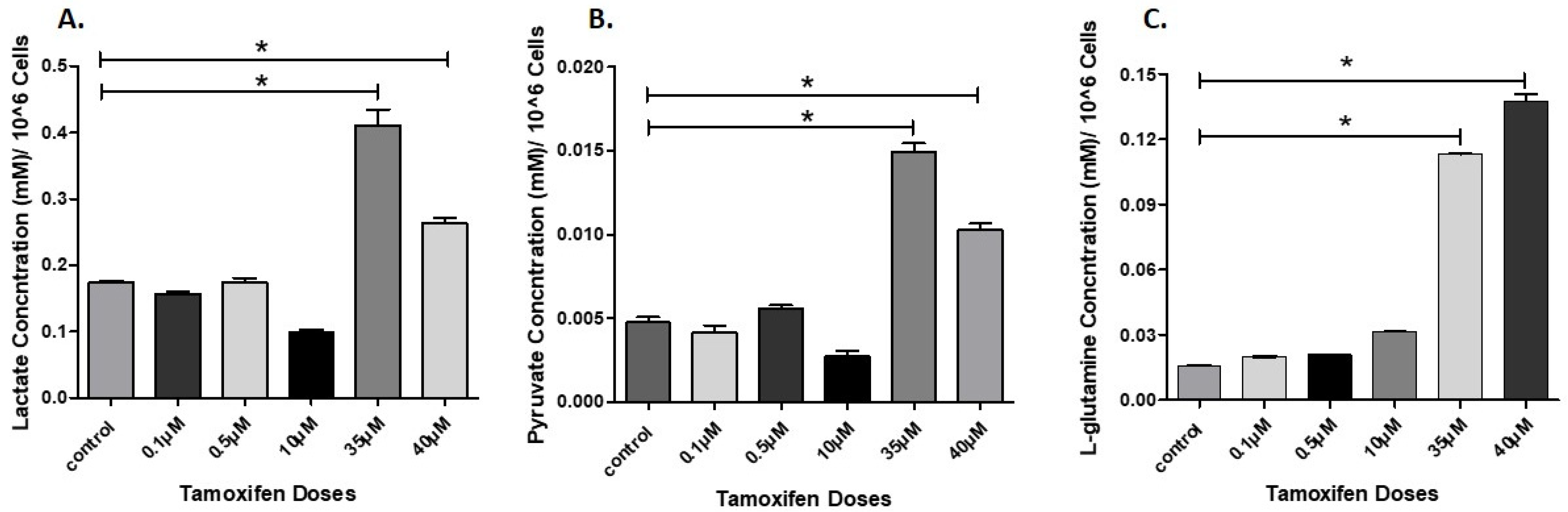
3.4. Determination of Lactate, Pyruvate, and Glutamine in Cell Culture Supernatant
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer, W.H.O. International Agency for Research on Cancer. Available online: https://gco.iarc.fr/today/data/factsheets/populations/400-jordan-fact-sheets.pdf (accessed on 26 February 2021).
- Day, C.M.; Hickey, S.M.; Song, Y.; Plush, S.E.; Garg, S. Novel tamoxifen nanoformulations for improving breast cancer treatment: Old wine in new bottles. Molecules 2020, 25, 1182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popova, M.; Koseva, N.; Trendafilova, I.; Lazarova, H.; Mitova, V.; Mihály, J.; Momekova, D.; Momekov, G.; Koleva, I.Z.; Aleksandrov, H.A. Tamoxifen Delivery System Based on PEGylated Magnetic MCM-41 Silica. Molecules 2020, 25, 5129. [Google Scholar] [CrossRef] [PubMed]
- Bitton, A.; Zheng, Y.; Houston, J.P.; Houston, K.D. Investigating differences between tamoxifen resistant and sensitive breast cancer cells with flow cytometry. Cytom. Part A 2021, 99, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.E.; El-Readi, M.Z.; Althubiti, M.A.; Almaimani, R.A.; Ismail, A.M.; Idris, S.; Refaat, B.; Almalki, W.H.; Babakr, A.T.; Mukhtar, M.H. Tamoxifen and the PI3K Inhibitor: LY294002 Synergistically Induce Apoptosis and Cell Cycle Arrest in Breast Cancer MCF-7 Cells. Molecules 2020, 25, 3355. [Google Scholar] [CrossRef] [PubMed]
- Mills, J.N.; Rutkovsky, A.C.; Giordano, A. Mechanisms of resistance in estrogen receptor positive breast cancer: Overcoming resistance to tamoxifen/aromatase inhibitors. Curr. Opin. Pharmacol. 2018, 41, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Ring, A.; Dowsett, M. Mechanisms of tamoxifen resistance. Endocr. Relat. Cancer 2004, 11, 643–658. [Google Scholar] [CrossRef]
- Clarke, R.; Tyson, J.J.; Dixon, J.M. Endocrine resistance in breast cancer–an overview and update. Mol. Cell. Endocrinol. 2015, 418, 220–234. [Google Scholar] [CrossRef] [Green Version]
- Massarweh, S.; Osborne, C.K.; Creighton, C.J.; Qin, L.; Tsimelzon, A.; Huang, S.; Weiss, H.; Rimawi, M.; Schiff, R. Tamoxifen resistance in breast tumors is driven by growth factor receptor signaling with repression of classic estrogen receptor genomic function. Cancer Res. 2008, 68, 826–833. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Li, X.; Zeng, C.; Liu, C.; Hao, Q.; Li, W.; Zhang, K.; Zhang, W.; Wang, S.; Zhao, H. CD63+ Cancer-Associated Fibroblasts Confer Tamoxifen Resistance to Breast Cancer Cells through Exosomal miR-22. Adv. Sci. 2020, 7, 2002518. [Google Scholar] [CrossRef]
- Xiao, Y.; Xie, Q.; Qin, Q.; Liang, Y.; Lin, H.; Zeng, D. Upregulation of SOX11 enhances tamoxifen resistance and promotes epithelial-to-mesenchymal transition via slug in MCF-7 breast cancer cells. J. Cell. Physiol. 2020, 235, 7295–7308. [Google Scholar] [CrossRef] [Green Version]
- Pagana, K.D.; Pagana, T.J.; Pike-MacDonald, S.A. Mosby’s Canadian Manual of Diagnostic and Laboratory Tests-E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Hamadneh, L.; Al-Lakkis, L.; Alhusban, A.A.; Tarawneh, S.; Abu-Irmaileh, B.; Albustanji, S.; Al-Bawab, A.Q. Changes in Lactate Production, Lactate Dehydrogenase Genes Expression and DNA Methylation in Response to Tamoxifen Resistance Development in MCF-7 Cell Line. Genes 2021, 12, 777. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Chen, Y.; Hu, M.; Lin, Y.; Zhang, S.; Kong, L.; Chen, Y. Diagnostic and prognostic value of the cancer-testis antigen lactate dehydrogenase C4 in breast cancer. Clin. Chim. Acta 2020, 503, 203–209. [Google Scholar] [CrossRef]
- Hamadneh, L.; Abuarqoub, R.; Alhusban, A.; Bahader, M. Upregulation of PI3K/AKT/PTEN pathway is correlated with glucose and glutamine metabolic dysfunction during tamoxifen resistance development in MCF-7 cells. Sci. Rep. 2020, 10, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.F.; Zhou, B.; Ressom, H.W. Metabolite identification and quantitation in LC-MS/MS-based metabolomics. TrAC Trends Anal. Chem. 2012, 32, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aslantürk, Ö.S. In Vitro cytotoxicity and cell viability assays: Principles, advantages, and disadvantages. In Genotoxicity-A Predictable Risk to Our Actual World; InTech: London, UK, 2018; Volume 2. [Google Scholar]
- Boer, D.P.; de Rijke, Y.B.; Hop, W.C.; Cransberg, K.; Dorresteijn, E.M. Reference values for serum creatinine in children younger than 1 year of age. Pediatr. Nephrol. 2010, 25, 2107–2113. [Google Scholar] [CrossRef] [Green Version]
- Hohenester, U.M.; Barbier Saint-Hilaire, P.; Fenaille, F.; Cole, R.B. Investigation of space charge effects and ion trapping capacity on direct introduction ultra-high-resolution mass spectrometry workflows for metabolomics. J. Mass Spectrom. 2020, 55, e4613. [Google Scholar] [CrossRef]
- Ranjan, R.; Sinha, N. Nuclear magnetic resonance (NMR)-based metabolomics for cancer research. NMR Biomed. 2019, 32, e3916. [Google Scholar] [CrossRef] [PubMed]
- Alhusban, A.A.; Breadmore, M.C.; Gueven, N.; Guijt, R.M. Capillary electrophoresis for automated on-line monitoring of suspension cultures: Correlating cell density, nutrients and metabolites in near real-time. Anal. Chim. Acta 2016, 920, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Alhusban, A.A.; Breadmore, M.C.; Gueven, N.; Guijt, R.M. Time-Resolved Pharmacological Studies using Automated, On-line Monitoring of Five Parallel Suspension Cultures. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Alhusban, A.A.; Breadmore, M.C.; Guijt, R.M. Capillary electrophoresis for monitoring bioprocesses. Electrophoresis 2013, 34, 1465–1482. [Google Scholar] [CrossRef] [PubMed]
- Alhusban, A.A.; Gaudry, A.J.; Breadmore, M.C.; Gueven, N.; Guijt, R.M. On-line sequential injection-capillary electrophoresis for near-real-time monitoring of extracellular lactate in cell culture flasks. J. Chromatogr. A 2014, 1323, 157–162. [Google Scholar] [CrossRef]
- Piestansky, J.; Olesova, D.; Galba, J.; Marakova, K.; Parrak, V.; Secnik, P.; Kovacech, B.; Kovac, A.; Zelinkova, Z.; Mikus, P. Profiling of amino acids in urine samples of patients suffering from inflammatory bowel disease by capillary electrophoresis-mass spectrometry. Molecules 2019, 24, 3345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barberini, L.; Noto, A.; Fattuoni, C.; Satta, G.; Zucca, M.; Cabras, M.G.; Mura, E.; Cocco, P. The metabolomic profile of lymphoma subtypes: A pilot study. Molecules 2019, 24, 2367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Łuczykowski, K.; Warmuzińska, N.; Operacz, S.; Stryjak, I.; Bogusiewicz, J.; Jacyna, J.; Wawrzyniak, R.; Struck-Lewicka, W.; Markuszewski, M.J.; Bojko, B. Metabolic Evaluation of Urine from Patients Diagnosed with High Grade (HG) Bladder Cancer by SPME-LC-MS Method. Molecules 2021, 26, 2194. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Jin, L.; Li, L.; Wang, W.; Zeng, S.; Jiang, H.; Zhou, H. Development and validation of a HPLC-ESI-MS/MS method for simultaneous quantification of fourteen alkaloids in mouse plasma after oral administration of the extract of Corydalis yanhusuo tuber: Application to pharmacokinetic study. Molecules 2018, 23, 714. [Google Scholar] [CrossRef] [Green Version]
- Alhusban, A.A.; Tarawneh, O.A.; Dawabsheh, S.O.; Alhusban, A.A.; Abumhareb, F.W. Liquid chromatography–tandem mass spectrometry for rapid and selective simultaneous determination of fluoroquinolones level in human aqueous humor. J. Pharmacol. Toxicol. Methods 2019, 97, 36–43. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency (EMA). Guidance on Bioanalytical Method Validation. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-bioanalytical-method-validation_en.pdf (accessed on 6 June 2021).
- Lee, A.V.; Oesterreich, S.; Davidson, N.E. MCF-7 cells—changing the course of breast cancer research and care for 45 years. JNCI J. Natl. Cancer Inst. 2015, 107. [Google Scholar] [CrossRef] [Green Version]
- Bui, Q.; Kang, K. Abstract P1-05-06: Essential role of notch-4/STAT3 signaling in epithelial-mesenchymal transition of tamoxifen-resistant human breast cancer. Mol. Cancer Res. 2016. [Google Scholar] [CrossRef]
- Liang, Y.-K.; Zeng, D.; Xiao, Y.-S.; Wu, Y.; Ouyang, Y.-X.; Chen, M.; Li, Y.-C.; Lin, H.-Y.; Wei, X.-L.; Zhang, Y.-Q. MCAM/CD146 promotes tamoxifen resistance in breast cancer cells through induction of epithelial–mesenchymal transition, decreased ERα expression and AKT activation. Cancer Lett. 2017, 386, 65–76. [Google Scholar] [CrossRef] [Green Version]
- Knowlden, J.M.; Hutcheson, I.R.; Jones, H.E.; Madden, T.; Gee, J.M.; Harper, M.E.; Barrow, D.; Wakeling, A.E.; Nicholson, R.I. Elevated levels of epidermal growth factor receptor/c-erbB2 heterodimers mediate an autocrine growth regulatory pathway in tamoxifen-resistant MCF-7 cells. Endocrinology 2003, 144, 1032–1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liberti, M.V.; Locasale, J.W. The Warburg effect: How does it benefit cancer cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef] [Green Version]
- Das, C.K.; Parekh, A.; Parida, P.K.; Bhutia, S.K.; Mandal, M. Lactate dehydrogenase A regulates autophagy and tamoxifen resistance in breast cancer. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2019, 1866, 1004–1018. [Google Scholar] [CrossRef] [PubMed]
- Kondaveeti, Y.; Reed, I.K.G.; White, B.A. Epithelial–mesenchymal transition induces similar metabolic alterations in two independent breast cancer cell lines. Cancer Lett. 2015, 364, 44–58. [Google Scholar] [CrossRef]
- Gatenby, R.A.; Smallbone, K.; Maini, P.K.; Rose, F.; Averill, J.; Nagle, R.B.; Worrall, L.; Gillies, R.J. Cellular adaptations to hypoxia and acidosis during somatic evolution of breast cancer. Br. J. Cancer 2007, 97, 646–653. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Choi, W.; Chen, Y.; Zhang, Q.; Deng, H.; He, W.; Shi, Y. A proposed role for glutamine in cancer cell growth through acid resistance. Cell Res. 2013, 23, 724–727. [Google Scholar] [CrossRef] [PubMed]
- Kung, H.-N.; Marks, J.R.; Chi, J.-T. Glutamine synthetase is a genetic determinant of cell type–specific glutamine independence in breast epithelia. PLoS Genet. 2011, 7, e1002229. [Google Scholar] [CrossRef] [Green Version]
| Tamoxifen Dose | Average of Started MCF-7 Cells Density (106 Cells/5 mL) | Average of Ended MCF-7 Cells Density (106 Cells/5 mL) |
|---|---|---|
| Control | 1.000 | 2.965 ± 0.95 |
| 0.1 µM | 1.000 | 2.888 ± 0.69 |
| 0.5 µM | 1.000 | 2.919 ± 0.47 |
| 10 µM | 1.000 | 2.721 ± 0.51 |
| 35 µM | 1.000 | 1.930 ± 0.93 |
| 40 µM | 1.000 | 1.936 ± 0.49 |
| Parameter | Lactate | Pyruvate | L-Glutamine |
|---|---|---|---|
| LOD (µM) | 0.16 | 0.28 | 4.6 |
| LOQ (µM) | 0.49 | 0.849 | 13.95 |
| R2 | 0.9972 | 0.9963 | 0.9988 |
| Linear Range (mM) | 0.11–2.25 | 0.012–0.227 | 0.02–0.20 |
| Within-run Accuracy % (n = 6) | 102.53 | 99.26 | 104.31 |
| Between-run Accuracy % (n = 6) | 104.11 | 98.94 | 105.50 |
| Within-run precision (RSD%, n = 6) | 0.39 | 0.32 | 0.48 |
| Between-run precision (RSD%, n = 6) | 0.61 | 0.77 | 0.86 |
| Recovery % | 99.85 | 100.34 | 100.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhusban, A.A.; Albustanji, S.; Hamadneh, L.A.; Shallan, A.I. High Performance Liquid Chromatography–Tandem Mass Spectrometry Method for Correlating the Metabolic Changes of Lactate, Pyruvate and L-Glutamine with Induced Tamoxifen Resistant MCF-7 Cell Line Potential Molecular Changes. Molecules 2021, 26, 4824. https://doi.org/10.3390/molecules26164824
Alhusban AA, Albustanji S, Hamadneh LA, Shallan AI. High Performance Liquid Chromatography–Tandem Mass Spectrometry Method for Correlating the Metabolic Changes of Lactate, Pyruvate and L-Glutamine with Induced Tamoxifen Resistant MCF-7 Cell Line Potential Molecular Changes. Molecules. 2021; 26(16):4824. https://doi.org/10.3390/molecules26164824
Chicago/Turabian StyleAlhusban, Ala A., Sokiyna Albustanji, Lama A. Hamadneh, and Aliaa I. Shallan. 2021. "High Performance Liquid Chromatography–Tandem Mass Spectrometry Method for Correlating the Metabolic Changes of Lactate, Pyruvate and L-Glutamine with Induced Tamoxifen Resistant MCF-7 Cell Line Potential Molecular Changes" Molecules 26, no. 16: 4824. https://doi.org/10.3390/molecules26164824
APA StyleAlhusban, A. A., Albustanji, S., Hamadneh, L. A., & Shallan, A. I. (2021). High Performance Liquid Chromatography–Tandem Mass Spectrometry Method for Correlating the Metabolic Changes of Lactate, Pyruvate and L-Glutamine with Induced Tamoxifen Resistant MCF-7 Cell Line Potential Molecular Changes. Molecules, 26(16), 4824. https://doi.org/10.3390/molecules26164824






