Theoretical Encapsulation of Fluorouracil (5-FU) Anti-Cancer Chemotherapy Drug into Carbon Nanotubes (CNT) and Boron Nitride Nanotubes (BNNT)
Abstract
:1. Introduction
2. Theoretical
- (1)
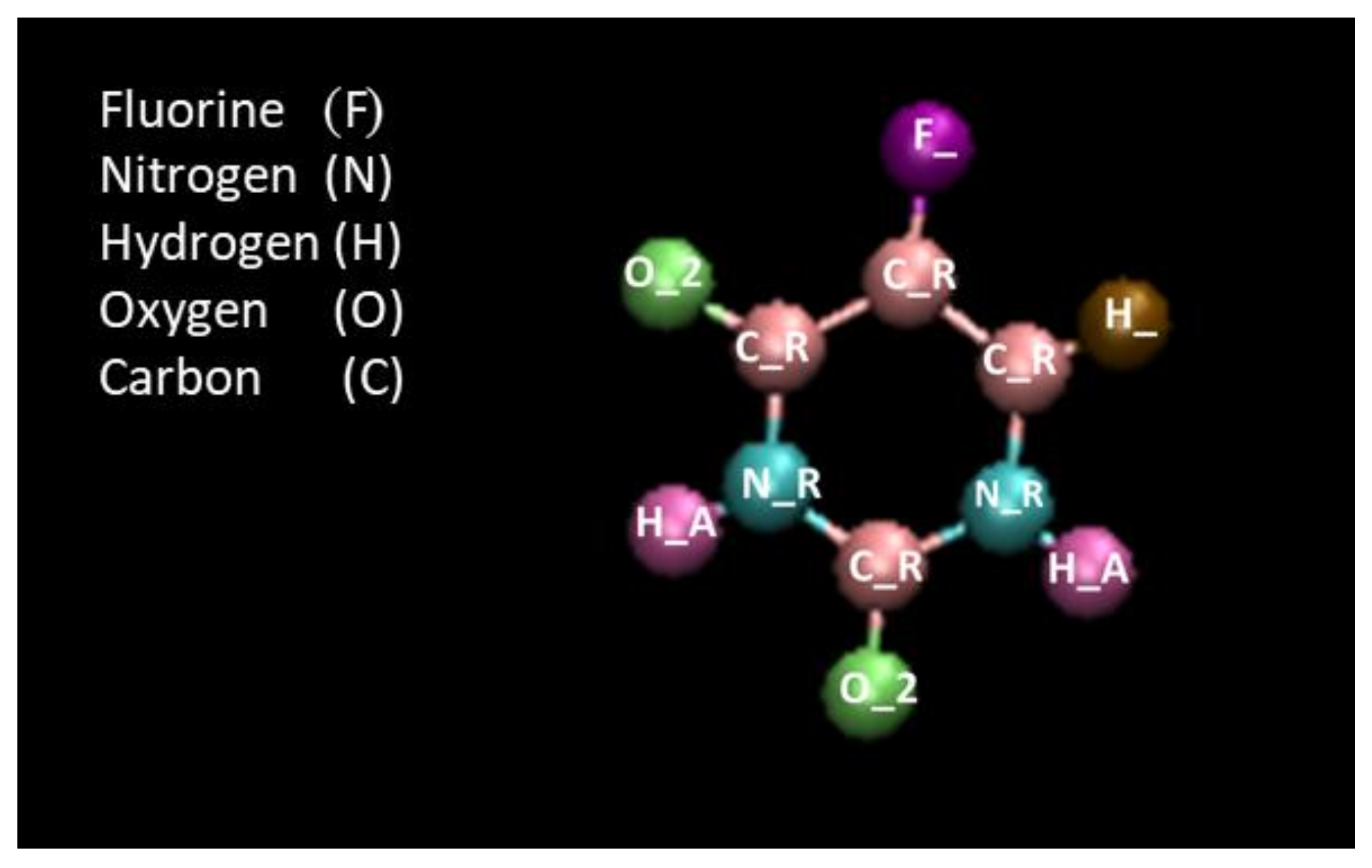
- In the first step, the insertion of 5-FU peptide into the CNT or BBNT and subsequently, the stability of the encapsulated drug inside the nanotubes was studied. Considering the size of the 5-FU guest molecule and in order to serve as a host drug nano-carrier, the nanotube was selected due to the chirality of an armchair (8,8), having the length and diameter of 20 and 6.26 Å, respectively. At time 0 nm, the 5-FU was situated at the initial distance of 2 Å from the nanotube in the MD simulation space. The axial direction of the nanotube was set parallel to the z-axis of the simulation box. The complex comprised of the nanotube- 5FU was immersed in the simulation box consisting of TIP3P3-point water molecules and counter-ions to neutralize the simulated solution with periodic boundary conditions. To assess the encapsulation process of the peptide, in the first stage, the minimization of the system was performed in the canonical NVT ensemble at 300 K, where moles (N), volume (V) and temperature (T) gradients were conserved, while the nanotube gradients were fixed. Next, the MD runs were performed in the NPT ensemble for 15 ns with the time step of 1 fs. The vdW interaction between the drug 5-FU and the nanotube was calculated according to the below equality as [45]:where refers to vdW Energy between 5-FU and the capped SWCNT, is vdW interaction of 5-FU combined with the capped SWCNT. and stand for vdW energies of 5-FU guest and the nanotube, respectively.
- (2)
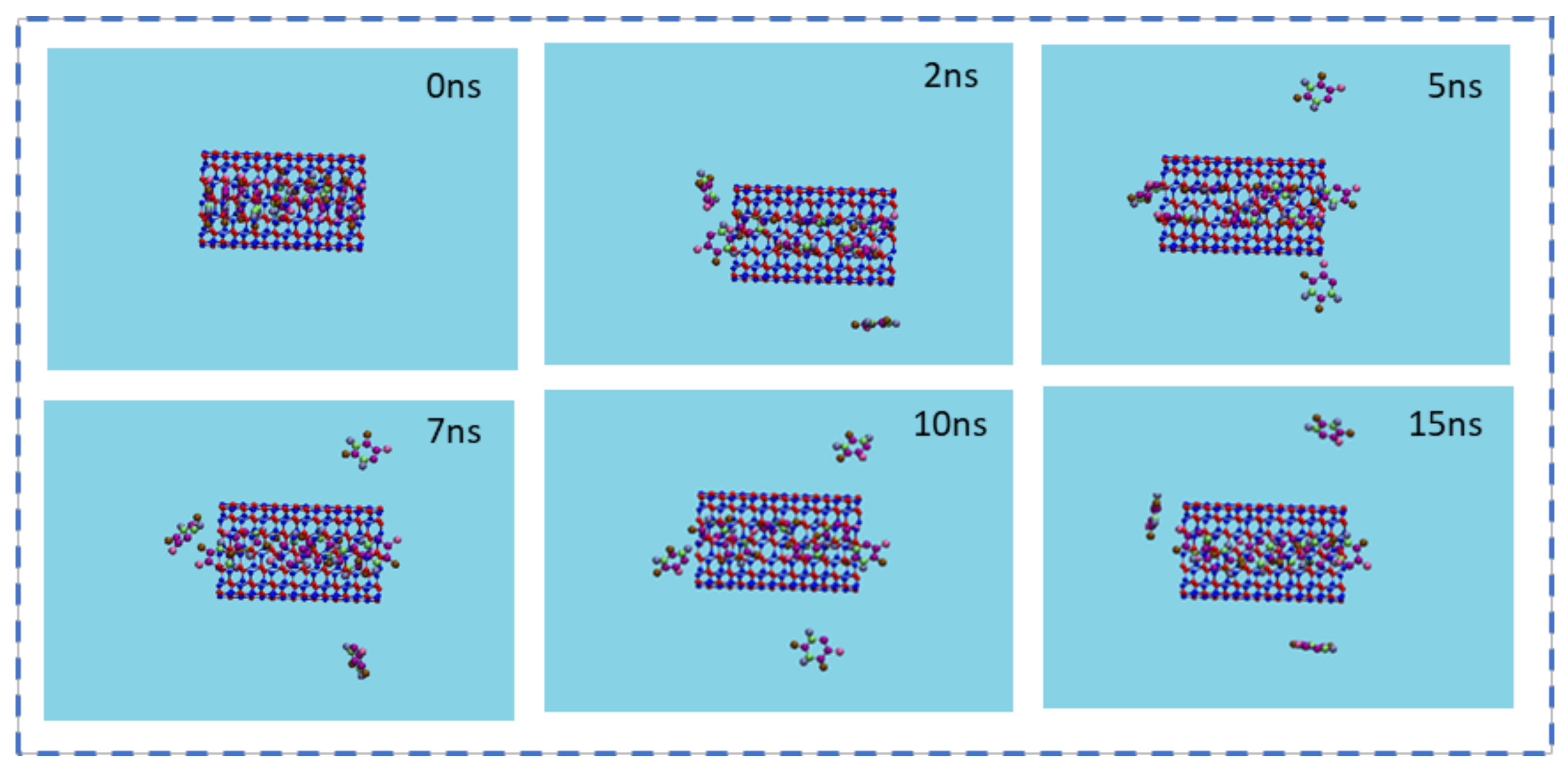
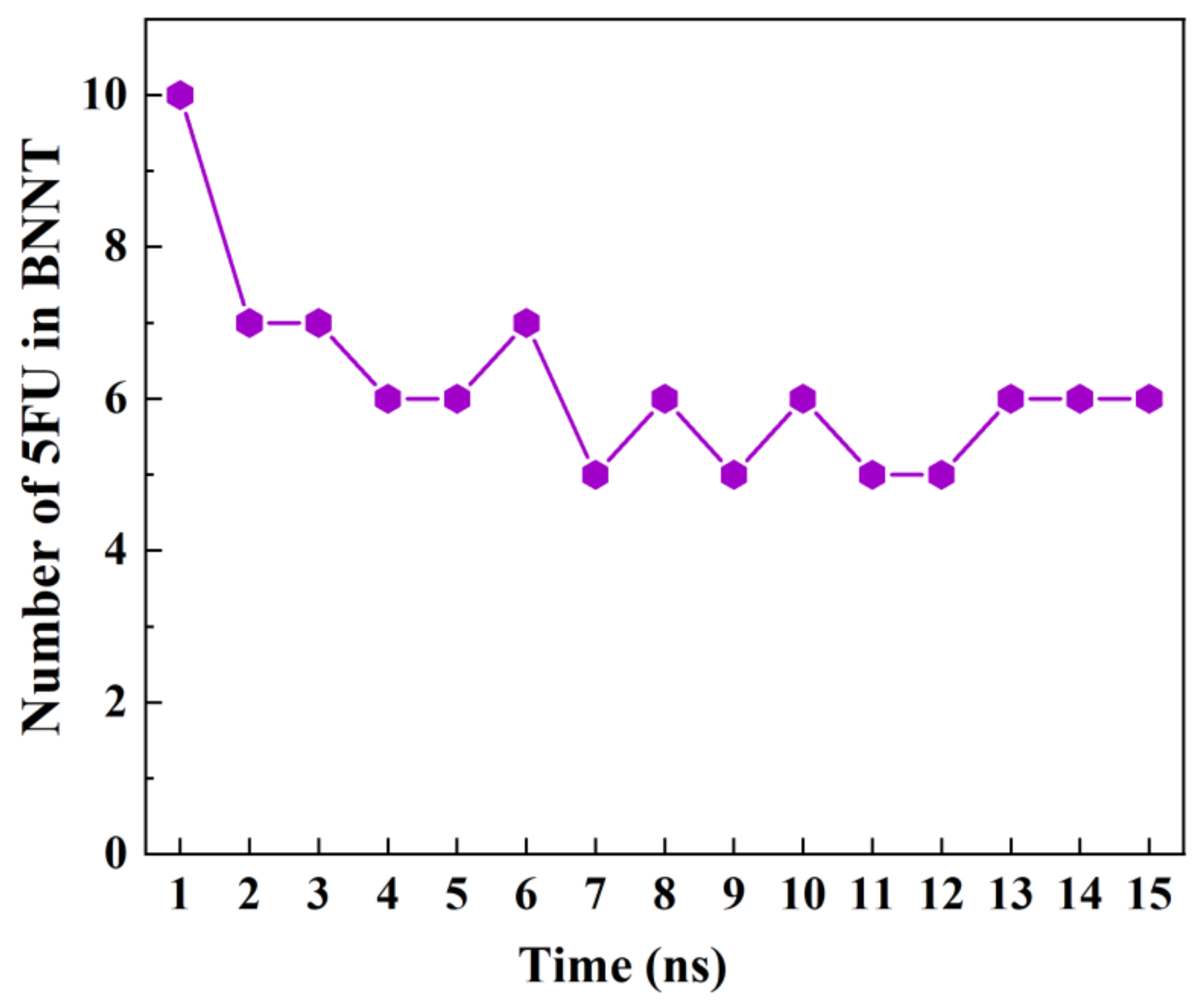
- At the second step, the storage capacity of the BNNT (8,8) was investigated. For this, 10 molecules of 5-FU were placed inside the nanotube. The axial directions of nanotubes were set to be parallel to the z-axis of the simulation box. The minimization of the system was done in the canonical NVT ensemble at 300 K while the SWCNT was fixed. Then, the MD run was performed in the NPT ensemble for 15 ns with the time step of 1 fs.
3. Results and Discussion
3.1. Localization of Drug 5-FU within the Nanotube-Drug Complex
3.2. Calculation of Free Energy from the MD Simulation
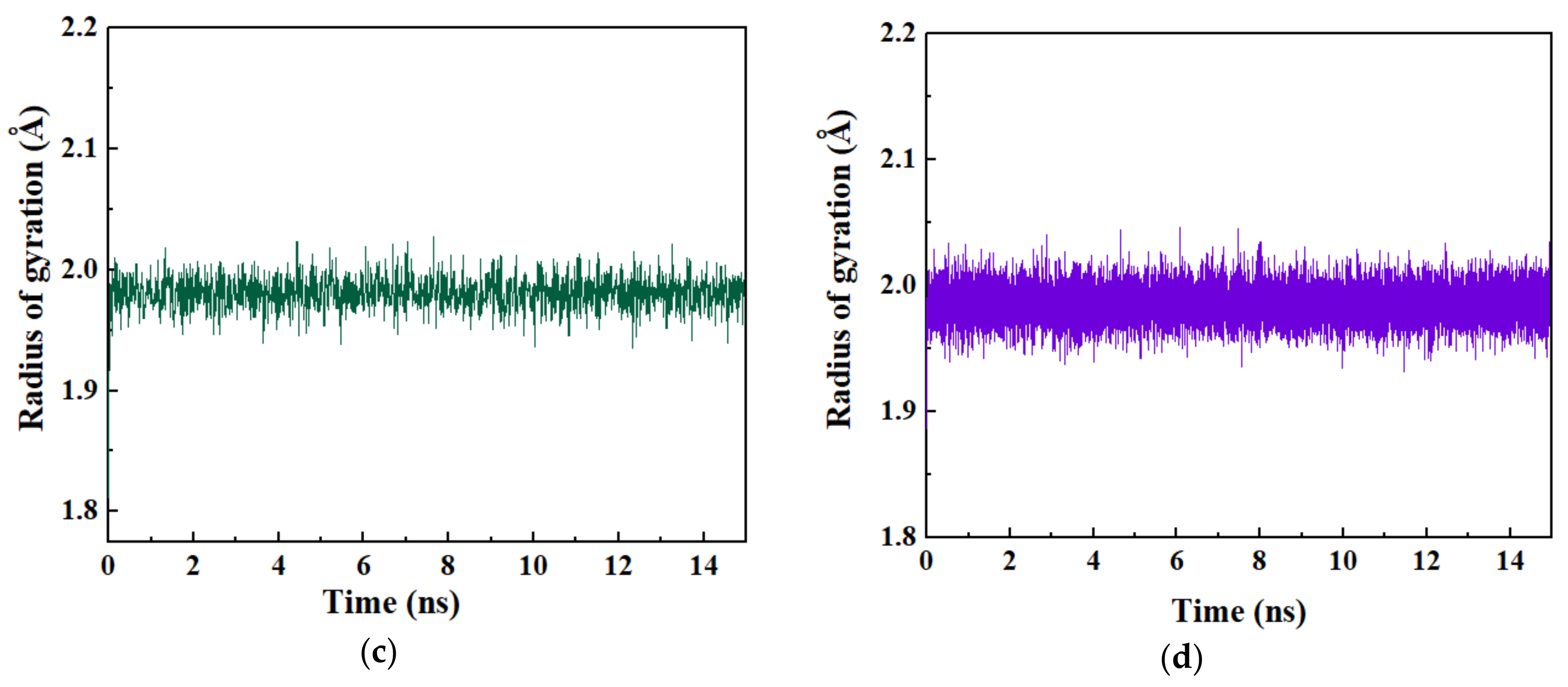
3.3. Analysis of Conformational Stability of Drug 5-FU
3.4. The Storage of 5-FU inside the BNNT
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Saberi, A.; Jabbari, F.; Zarrintaj, P.; Saeb, M.R.; Mozafari, M. Electrically Conductive Materials: Opportunities and Challenges in Tissue Engineering. Biomolecules 2019, 9, 488. [Google Scholar] [CrossRef] [Green Version]
- Zarrintaj, P.; Mostafapoor, F.; Milan, P.B.; Saeb, M.R. Theranostic platforms proposed for cancerous stem cells: A review. Curr. Stem Cell Res. Ther. 2019, 14, 137–145. [Google Scholar] [CrossRef]
- Zhao, L.; Xing, Y.; Wang, R.; Yu, F.; Yu, F. Self-Assembled Nanomaterials for Enhanced Phototherapy of Cancer. ACS Appl. Bio Mater. 2020, 3, 86–106. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, K.-T.; Lu, Z.-B.; Chen, J.-Y.; Liu, Y.-Y.; Lan, H.-R.; Dong, H.-Y.; Yang, F.; Zhao, Y.-Y.; Chen, X.-Y. Recent Trends in Nanocarrier-Based Targeted Chemotherapy: Selective Delivery of Anticancer Drugs for Effective Lung, Colon, Cervical, and Breast Cancer Treatment. J. Nanomater. 2020, 2020, 9184284. [Google Scholar] [CrossRef]
- Saeedi, M.; Vahidi, O.; Goodarzi, V.; Saeb, M.R.; Izadi, L.; Mozafari, M. A new prospect in magnetic nanoparticle-based cancer therapy: Taking credit from mathematical tissue-mimicking phantom brain models. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 2405–2414. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-Y.; Ju, D.-T.; Chang, C.-F.; Reddy, P.M.; Velmurugan, B.K. A review on the effects of current chemotherapy drugs and natural agents in treating non–small cell lung cancer. Biomedicine 2017, 7, 23. [Google Scholar] [CrossRef] [Green Version]
- Nussbaumer, S.; Bonnabry, P.; Veuthey, J.-L.; Fleury-Souverain, S. Analysis of anticancer drugs: A review. Talanta 2011, 85, 2265–2289. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, L.; Yang, T.; Wu, H. Stimuli-responsive polymeric micelles for drug delivery and cancer therapy. Int. J. Nanomed. 2018, 13, 2921. [Google Scholar] [CrossRef] [Green Version]
- Edis, Z.; Wang, J.; Waqas, M.K.; Ijaz, M.; Ijaz, M. Nanocarriers-Mediated Drug Delivery Systems for Anticancer Agents: An Overview and Perspectives. Int. J. Nanomed. 2021, 16, 1313–1330. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Ramsey, J.D.; Samadi, A.; Atoufi, Z.; Yazdi, M.K.; Ganjali, M.R.; Amirabad, L.M.; Zangene, E.; Farokhi, M.; Formela, K. Poloxamer: A versatile tri-block copolymer for biomedical applications. Acta Biomater. 2020, 110, 37–67. [Google Scholar] [CrossRef]
- Servatan, M.; Zarrintaj, P.; Mahmodi, G.; Kim, S.-J.; Ganjali, M.R.; Saeb, M.R.; Mozafari, M. Zeolites in drug delivery: Progress, challenges and opportunities. Drug Discov. Today 2020, 25, 642–656. [Google Scholar] [CrossRef]
- Skandani, A.A.; Al-Haik, M. Reciprocal effects of the chirality and the surface functionalization on the drug delivery permissibility of carbon nanotubes. Soft Matter. 2013, 9, 11645–11649. [Google Scholar] [CrossRef] [Green Version]
- Shaki, H.; Raissi, H.; Mollania, F.; Hashemzadeh, H. Modeling the interaction between anti-cancer drug penicillamine and pristine and functionalized carbon nanotubes for medical applications: Density functional theory investigation and a molecular dynamics simulation. J. Biomol. Struct. Dyn. 2019, 38, 1322–1334. [Google Scholar] [CrossRef] [PubMed]
- Ramos, M.A.D.S.; Da Silva, P.B.; Spósito, L.; De Toledo, L.G.; Bonifacio, B.V.; Rodero, C.F.; Dos Santos, K.C.; Chorilli, M.; Bauab, T.M. Nanotechnology-based drug delivery systems for control of microbial biofilms: A review. Int. J. Nanomed. 2018, 13, 1179. [Google Scholar] [CrossRef] [Green Version]
- Zarrintaj, P.; Khodadadi Yazdi, M.; Youssefi Azarfam, M.; Zare, M.; Ramsey, J.; Seidi, F.; Saeb, M.R.; Ramakrishna, S.; Mozafari, M. Injectable Cell-laden Hydrogels for Tissue Engineering: Recent Advances and Future Opportunities. Tissue Eng. 2021, 27, 821–843. [Google Scholar] [CrossRef]
- Bernkop-Schnürch, A.; Bratengeyer, I.; Valenta, C. Development and in vitro evaluation of a drug delivery system protecting from trypsinic degradation. Int. J. Pharm. 1997, 157, 17–25. [Google Scholar] [CrossRef]
- Dehaghani, M.Z.; Bagheri, B.; Nasiriasayesh, A.; Mashhadzadeh, A.H.; Zarrintaj, P.; Rabiee, N.; Bagherzadeh, M.; Habibzadeh, S.; Abida, O.; Saeb, M.R. Insight into the Self-Insertion of a Protein Inside the Boron Nitride Nanotube. ACS Omega 2020, 5, 32051. [Google Scholar] [CrossRef] [PubMed]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.K.; Kim, S.W. Recent advances in polymeric drug delivery systems. Biomater. Res. 2020, 24, 1–12. [Google Scholar] [CrossRef]
- Arsawang, U.; Saengsawang, O.; Rungrotmongkol, T.; Sornmee, P.; Wittayanarakul, K.; Remsungnen, T.; Hannongbua, S. How do carbon nanotubes serve as carriers for gemcitabine transport in a drug delivery system? J. Mol. Graph. Model. 2011, 29, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Yazdi, M.K.; Saeidi, H.; Zarrintaj, P.; Saeb, M.R.; Mozafari, M. PANI-CNT nanocomposites. In Fundamentals and Emerging Applications of Polyaniline; Elsevier: Amsterdam, The Netherlands, 2019; pp. 143–163. [Google Scholar]
- Jouyandeh, M.; Tikhani, F.; Hampp, N.; Yazdi, D.A.; Zarrintaj, P.; Ganjali, M.R.; Saeb, M.R. Highly curable self-healing vitrimer-like cellulose-modified halloysite nanotube/epoxy nanocomposite coatings. Chem. Eng. J. 2020, 396, 125196. [Google Scholar] [CrossRef]
- García-Merino, J.; Mercado-Zúñiga, C.; Martínez-González, C.; Torres-SanMiguel, C.; Vargas-García, J.; Torres-Torres, C. Magneto-conductive encryption assisted by third-order nonlinear optical effects in carbon/metal nanohybrids. Mater. Res. Express 2017, 4, 035601. [Google Scholar] [CrossRef]
- García-Beltrán, G.; Mercado-Zúñiga, C.; Torres-SanMiguel, C.R.; Trejo-Valdez, M.; Villalpando, I.; Torres-Torres, C. Navigation of Silver/Carbon Nanoantennas in Organic Fluids Explored by a Two-Wave Mixing. Nanomaterials 2020, 10, 1886. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ramírez-Hinestrosa, S.; Dobnikar, J.; Frenkel, D. The Lennard-Jones potential: When (not) to use it. Phys. Chem. Chem. Phys. 2020, 22, 10624–10633. [Google Scholar] [CrossRef] [Green Version]
- Maleki, R.; Afrouzi, H.H.; Hosseini, M.; Toghraie, D.; Rostami, S. Molecular dynamics simulation of Doxorubicin loading with N-isopropyl acrylamide carbon nanotube in a drug delivery system. Comput. Methods Programs Biomed. 2020, 184, 105303. [Google Scholar] [CrossRef]
- Moradnia, H.; Raissi, H.; Shahabi, M. The performance of the single-walled carbon nanotube covalently modified with polyethylene glycol to delivery of Gemcitabine anticancer drug in the aqueous environment. J. Biomol. Struct. Dyn. 2020, 39, 1–8. [Google Scholar] [CrossRef]
- Dehneshin, N.; Raissi, H.; Hasanzade, Z.; Farzad, F. Using molecular dynamics simulation to explore the binding of the three potent anticancer drugs sorafenib, streptozotocin, and sunitinib to functionalized carbon nanotubes. J. Mol. Model. 2019, 25, 159. [Google Scholar] [CrossRef]
- Khatti, Z.; Hashemianzadeh, S.M. Boron nitride nanotube as a delivery system for platinum drugs: Drug encapsulation and diffusion coefficient prediction. Eur. J. Pharm. Sci. 2016, 88, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Roosta, S.; Nikkhah, S.J.; Sabzali, M.; Hashemianzadeh, S.M. Molecular dynamics simulation study of boron-nitride nanotubes as a drug carrier: From encapsulation to releasing. RSC Adv. 2016, 6, 9344–9351. [Google Scholar] [CrossRef]
- Katiyar, R.S.; Jha, P.K. Molecular simulations in drug delivery: Opportunities and challenges. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2018, 8, e1358. [Google Scholar] [CrossRef]
- Singh, A.; Vanga, S.K.; Orsat, V.; Raghavan, V. Application of molecular dynamic simulation to study food proteins: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 2779–2789. [Google Scholar] [CrossRef]
- Fooladpanjeh, S.; Yousefi, F.; Molaei, F.; Zarghami Dehaghani, M.; Sajadi, S.M.; Abida, O.; Habibzadeh, S.; Hamed Mashhadzadeh, A.; Saeb, M.R. Thermal conductivity of random polycrystalline BC3 nanosheets: A step towards realistic simulation of 2D structures. J. Mol. Graph. Model. 2021, 107, 107977. [Google Scholar] [CrossRef]
- Zarghami Dehaghani, M.; Hamed Mashhadzadeh, A.; Salmankhani, A.; Karami, Z.; Habibzadeh, S.; Ganjali, M.R.; Saeb, M.R. Fracture toughness and crack propagation behavior of nanoscale beryllium oxide graphene-like structures: A molecular dynamics simulation analysis. Eng. Fract. Mech. 2020, 235, 107194. [Google Scholar] [CrossRef]
- Zarghami Dehaghani, M.; Salmankhani, A.; Hamed Mashhadzadeh, A.; Habibzadeh, S.; Abida, O.; Reza Saeb, M. Fracture mechanics of polycrystalline beryllium oxide nanosheets: A theoretical basis. Eng. Fract. Mech. 2021, 224, 107552. [Google Scholar] [CrossRef]
- Bagheri, B.; Dehaghani, M.Z.; Karami, Z.; Salmankhani, A.; Rostamiyan, Y.; Zarrintaj, P.; Mashhadzadeh, A.H.; Saeb, M.R. Correlation between surface topological defects and fracture mechanism of γ-graphyne-like boron nitride nanosheets. Comput. Mater. Sci. 2020, 188, 110152. [Google Scholar] [CrossRef]
- Albooyeh, A.; Dadrasi, A.; Mashhadzadeh, A.H. Effect of point defects and low-density carbon-doped on mechanical properties of BNNTs: A molecular dynamics study. Mater. Chem. Phys. 2020, 239, 122107. [Google Scholar] [CrossRef]
- Fatemi, S.M.; Foroutan, M. Review of recent studies on interactions between polymers and nanotubes using molecular dynamic simulation. J. Iran. Chem. Soc. 2017, 14, 269–283. [Google Scholar] [CrossRef]
- Sorrentino, M.F.; Kim, J.; Foderaro, A.E.; Truesdell, A.G. 5-fluorouracil induced cardiotoxicity: Review of the literature. Cardiol. J. 2012, 19, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Los, J.; Kroes, J.; Albe, K.; Gordillo, R.; Katsnelson, M.; Fasolino, A. Extended Tersoff potential for boron nitride: Energetics and elastic properties of pristine and defective h-BN. Phys. Rev. B 2017, 96, 184108. [Google Scholar] [CrossRef] [Green Version]
- Feller, S.E.; Zhang, Y.; Pastor, R.W.; Brooks, B.R. Constant pressure molecular dynamics simulation: The Langevin piston method. J. Chem. Phys. 1995, 103, 4613–4621. [Google Scholar] [CrossRef]
- Steinbach, P.J.; Brooks, B.R. New spherical-cutoff methods for long-range forces in macromolecular simulation. J. Comput. Chem. 1994, 15, 667–683. [Google Scholar] [CrossRef]
- Hirschfelder, J.O.; Curtiss, C.F.; Bird, R.B.; Mayer, M.G. Molecular Theory of Gases and Liquids; Wiley: New York, NY, USA, 1964; Volume 165. [Google Scholar]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Mayo, S.L.; Olafson, B.D.; Goddard, W.A. DREIDING: A generic force field for molecular simulations. J. Phys. Chem. 1990, 94, 8897–8909. [Google Scholar] [CrossRef]
- Kang, Y.; Liu, Y.-C.; Wang, Q.; Shen, J.-W.; Wu, T.; Guan, W.-J. On the spontaneous encapsulation of proteins in carbon nanotubes. Biomaterials 2009, 30, 2807–2815. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Schulten, K. Calculating potentials of mean force from steered molecular dynamics simulations. J. Chem. Phys. 2004, 120, 5946–5961. [Google Scholar] [CrossRef] [PubMed]
- Dehaghani, M.Z.; Bagheri, B.; Yousefi, F.; Nasiriasayesh, A.; Mashhadzadeh, A.H.; Zarrintaj, P.; Rabiee, N.; Bagherzadeh, M.; Fierro, V.; Celzard, A. Boron Nitride Nanotube as an Antimicrobial Peptide Carrier: A Theoretical Insight. Int. J. Nanomed. 2021, 16, 1837. [Google Scholar] [CrossRef]
- Mousavi, S.Z.; Amjad-Iranagh, S.; Nademi, Y.; Modarress, H. Carbon nanotube-encapsulated drug penetration through the cell membrane: An investigation based on steered molecular dynamics simulation. J. Membr. Biol. 2013, 246, 697–704. [Google Scholar] [CrossRef]
- Gao, H.; Kong, Y.; Cui, D.; Ozkan, C.S. Spontaneous insertion of DNA oligonucleotides into carbon nanotubes. Nano Lett. 2003, 3, 471–473. [Google Scholar] [CrossRef]
- Hasanzade, Z.; Raissi, H. Carbon and boron nanotubes as a template material for adsorption of 6-Thioguanine chemotherapeutic: A molecular dynamics and density functional approach. J. Biomol. Struct. Dyn. 2020, 38, 697–707. [Google Scholar] [CrossRef]
- Mortazavifar, A.; Raissi, H.; Shahabi, M. Comparative prediction of binding affinity of Hydroxyurea anti-cancer to boron nitride and carbon nanotubes as smart targeted drug delivery vehicles. J. Biomol. Struct. Dyn. 2019, 37, 4852–4862. [Google Scholar] [CrossRef]
- Veclani, D.; Melchior, A. Adsorption of ciprofloxacin on carbon nanotubes: Insights from molecular dynamics simulations. J. Mol. Liq. 2020, 298, 111977. [Google Scholar] [CrossRef]
- Dehaghani, M.Z.; Yousefi, F.; Bagheri, B.; Seidi, F.; Mashhadzadeh, A.H.; Rabiee, N.; Zarrintaj, P.; Mostafavi, E.; Saeb, M.R.; Kim, Y.-C. α-Helical Antimicrobial Peptide Encapsulation and Release from Boron Nitride Nanotubes: A Computational Study. Int. J. Nanomed. 2021, 16, 4277. [Google Scholar] [CrossRef] [PubMed]
- Khatti, Z.; Hashemianzadeh, S.M.; Shafiei, S.A. A molecular study on drug delivery system based on carbon nanotube compared to silicon carbide nanotube for encapsulation of platinum-based anticancer drug. Adv. Pharm. Bull. 2018, 8, 163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Wu, P.; Rousseas, M.; Okawa, D.; Gartner, Z.; Zettl, A.; Bertozzi, C.R. Boron Nitride Nanotubes Are Noncytotoxic and Can Be Functionalized for Interaction with Proteins and Cells. J. Am. Chem. Soc. 2009, 131, 890–891. [Google Scholar] [CrossRef] [Green Version]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zarghami Dehaghani, M.; Yousefi, F.; Sajadi, S.M.; Tajammal Munir, M.; Abida, O.; Habibzadeh, S.; Mashhadzadeh, A.H.; Rabiee, N.; Mostafavi, E.; Saeb, M.R. Theoretical Encapsulation of Fluorouracil (5-FU) Anti-Cancer Chemotherapy Drug into Carbon Nanotubes (CNT) and Boron Nitride Nanotubes (BNNT). Molecules 2021, 26, 4920. https://doi.org/10.3390/molecules26164920
Zarghami Dehaghani M, Yousefi F, Sajadi SM, Tajammal Munir M, Abida O, Habibzadeh S, Mashhadzadeh AH, Rabiee N, Mostafavi E, Saeb MR. Theoretical Encapsulation of Fluorouracil (5-FU) Anti-Cancer Chemotherapy Drug into Carbon Nanotubes (CNT) and Boron Nitride Nanotubes (BNNT). Molecules. 2021; 26(16):4920. https://doi.org/10.3390/molecules26164920
Chicago/Turabian StyleZarghami Dehaghani, Maryam, Farrokh Yousefi, S. Mohammad Sajadi, Muhammad Tajammal Munir, Otman Abida, Sajjad Habibzadeh, Amin Hamed Mashhadzadeh, Navid Rabiee, Ebrahim Mostafavi, and Mohammad Reza Saeb. 2021. "Theoretical Encapsulation of Fluorouracil (5-FU) Anti-Cancer Chemotherapy Drug into Carbon Nanotubes (CNT) and Boron Nitride Nanotubes (BNNT)" Molecules 26, no. 16: 4920. https://doi.org/10.3390/molecules26164920
APA StyleZarghami Dehaghani, M., Yousefi, F., Sajadi, S. M., Tajammal Munir, M., Abida, O., Habibzadeh, S., Mashhadzadeh, A. H., Rabiee, N., Mostafavi, E., & Saeb, M. R. (2021). Theoretical Encapsulation of Fluorouracil (5-FU) Anti-Cancer Chemotherapy Drug into Carbon Nanotubes (CNT) and Boron Nitride Nanotubes (BNNT). Molecules, 26(16), 4920. https://doi.org/10.3390/molecules26164920









