1. Introduction
Since their discovery, lanthanide-based single molecular magnets [
1,
2,
3] have been the subject of great interest to researchers exploring the field of molecular magnetism. From a theoretical perspective, the quest for adequate methods and models to predict the magnetic behavior of these compounds has generated lively debates and a fruitful exchange of ideas in recent years [
4,
5,
6,
7,
8,
9]. By virtue of their intrinsically large magnetic anisotropy, lanthanide series possess a great potential for application in magnetic memory storage nanounits [
10,
11,
12,
13] and stand as promising candidates for the realization of quantum logical devices. On the other hand, some lanthanide complexes, such as dysprosium and gadolinium based compounds, are ideal for implementation in magnetic resonance imaging [
14,
15,
16]. Dysprosium complexes play an essential role in gaining useful insights into the magnetic properties of lanthanide-based molecular magnets [
17,
18,
19]. The experimentally observed magnetic bistability at relatively high temperatures in Dy
complexes [
20,
21] and the Dy
cluster [
22], which displays a very high energy barrier of approximately
meV, are promising candidates for engineering future magnetic molecular devices. Other prominent lanthanide systems are the polyoxometalate-based single molecular magnets, [Ln(W
O
)
]
, Ln = (Tb, Dy, Ho and Er) [
4], with the erbium member (see e.g., Reference [
23]) demonstrating a relatively high energy barrier to magnetization reversal.
Recently [
9], a slow magnetic relaxation in the deuterated species, Na
[Ln(W
O
)
], with Ln = (Tb, Ho and Er) and a field-induced magnetic relaxation in the Ln = Nd member of this group, were reported. For this compound, the Er
ion is octacoordinated by four oxygen atoms from each W
O
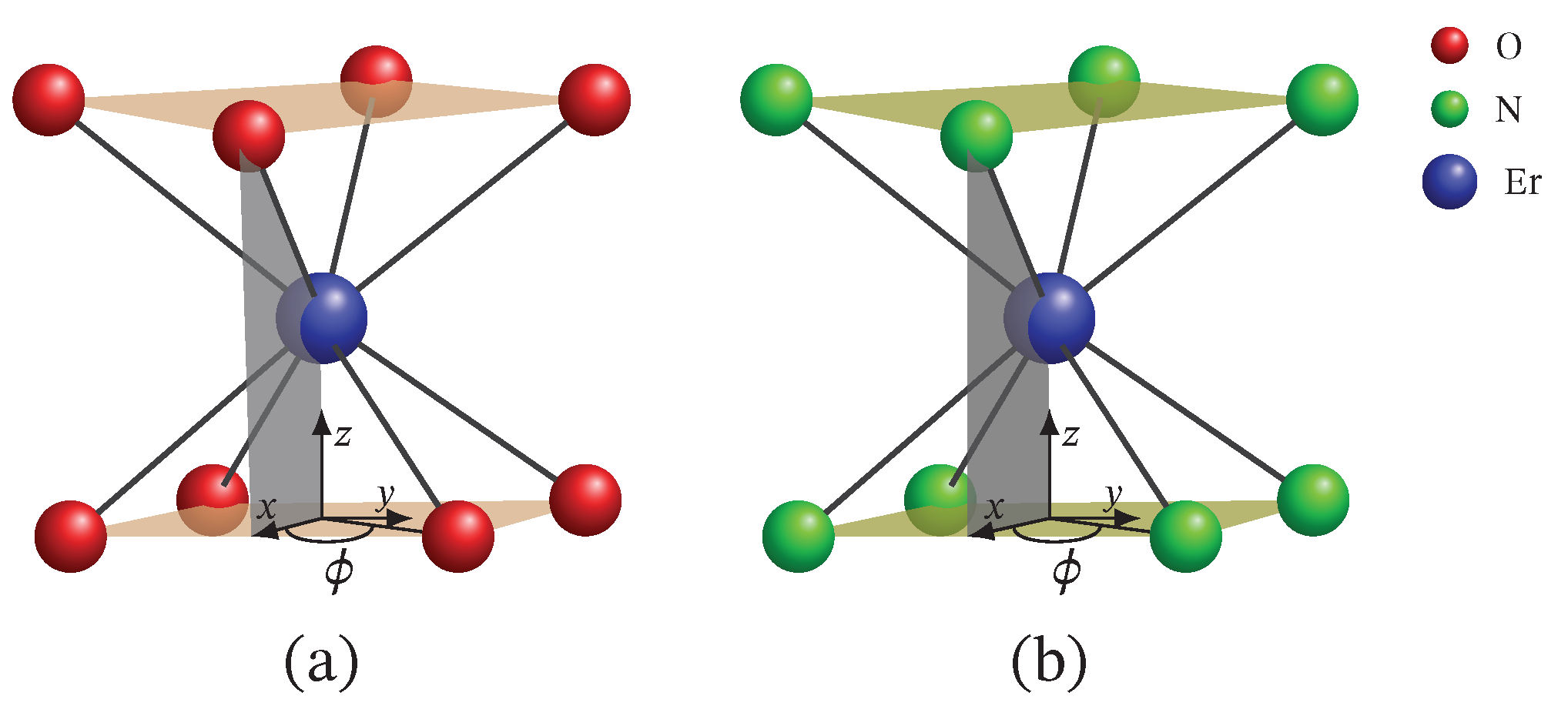
group, resulting in a slightly distorted square anti-prismatic geometry, as shown in
Figure 1a. The magnetic properties of this material clearly point to a profile characteristic of a single molecular magnet. According to inelastic neutron scattering (INS) measurements, the compound exhibits two ground states and one high-temperature magnetic excitations. The high-temperature transition is characterized by a peculiar high-scattering probability and energy loss of the neutrons. Apart from INS spectra, ac susceptibility measurements performed in the absence of an external magnetic field manifest the compound’s single molecular magnet behavior with an approximately
meV effective energy barrier. In addition to the slow magnetic relaxation, the dc magnetization and molar susceptibility data suggest a paramagnetic-like behavior. The static magnetic properties are well reproduced by particularly adapted crystal field calculations relying on the electronic structure in Reference [
4] and allowing some degree of mixing between some particular states.
Another class of attractive erbium–based single ion magnets includes [(Pc)Er{Pc{N(C
H
)
}
}]
[
24]. The Er
ion in these compounds is coordinated by eight nitrogen atoms in a square anti-prismatic structure with a different dihedral angle for each compound, see
Figure 1b. These compounds possess different field-induced dynamic magnetic properties. In contrast to the reduced compound, [(Pc)Er{Pc{N(C
H
)
}
}]
, which shows slow magnetic relaxation under the action of a dc external magnetic field with an energy barrier of approximately
meV, the unprotonated compound, [(Pc)Er{Pc{N(C
H
)
}
}]
, shows no trace of a relaxation pathway above 2 K and for a dc field
T. In the absence of a static magnetic field, both compounds are indistinguishable with respect to ac susceptibility data. On the other hand, they demonstrate similar dc magnetic behavior with a small difference with regard to the saturation of the magnetization. The reduced compound exhibits a lower saturation value of the magnetization and, in contrast to that of the neutral compound, it is almost temperature-independent within the range 2–6 K. It is worth noting that comparisons between theoretical results and experimental data for the overall magnetic properties are not reported. Furthermore, to our knowledge, experimental data for the magnetic spectrum of both compounds have not been reported so far.
In this paper, we study to what extent an effective spin exchange Hamiltonian may rationalize the magnetic properties of Er
single ion magnets. To this end, we adapt a recently proposed spin–sigma model [
25] and focus on the erbium-based molecular magnets, Na
[Er(W
O
)
] and [(Pc)Er{Pc{N(C
H
)
}
}]
. The proposed model is developed within the multi-configurational self-consistent field method, and was successfully used to characterize the magnetism of the molecular magnet, Ni
Mo
[
26,
27], and the magnetic spectrum of the trimeric compounds, A
Cu
(PO
)
A=(Ca, Sr, Pb) [
28]. This formalism describes all electrons as delocalized occupying molecular orbitals. Therefore, we show that, besides the use of localized electron states in the free-ion approximation and the crystal field formalism, there may exist an appropriate complete active space and spin coupling scheme, where the named model is able to reproduce the respective experimental findings reported in the literature [
9,
24]. In addition to the spin–sigma model, we apply the Heisenberg one and demonstrate that it fails at providing us with a complete picture of the corresponding magnetic properties.
For the complex Na
[Er(W
O
)
], we compute in detail the inelastic neutron scattering intensities and hence deduce the minimal effective energy levels sequence describing the experimentally observed magnetic excitations. Furthermore, we obtain theoretical results consistent with the magnetization and susceptibility measurements, providing us with the overall field-dependent energy spectrum in both the dc and ac regimes. We demonstrate that the effective energy barrier to magnetization reversal satisfies the inequality
meV, which agrees very well with the experimental value of
meV [
9], explaining the slow magnetic relaxation. The model further suggests an abrupt change in the ground state from a non-fully polarized spin ground state in the absence of an external magnetic field to a fully spin polarized one that does not result from the decreased spin rotational degeneracy caused by the relevant Zeeman interactions, but rather orbital ones that affect the system’s energy indirectly due to the used multi-configurational basis, see Reference [
25].
The magnetic properties of single ion magnets, [(Pc)Er{Pc{N(C
H
)
}
}]
, are also computed. Our results are quantitatively and qualitatively in good agreement with the existing magnetization, dc and ac susceptibility experimental data. The calculated value of the effective energy barrier to magnetization reversal for the reduced compound is consistent with the experimentally obtained one [
24]. With respect to the static magnetic properties, the spin–sigma model predicts a low-field induced change in the ground state from a non-fully to a fully polarized spin state, driven by the indirect external field effect rather than the direct spin Zeeman interactions. Further, calculating the temperature dependence of the magnetization, we found a small variation in the
g-factor value in the case of the reduced form [(Pc)Er{Pc{N(C
H
)
}
}]
. According to the applied method, such changes may be attributed to some nuclei spins that are uncoupled due to thermal effects or a change in the associated
g-factor anisotropy. Experimental data for the magnetic spectrum of both compounds are unavailable in the literature and all spectroscopic model parameters are fitted only to the available magnetization and susceptibility measurements.
The rest of the paper is organized as follows: In
Section 2, we present the physical models used to explore the magnetic properties of the considered compounds and introduce the relevant parameters, the used approximations and the pertinent physical relations. Our results for the magnetic spectrum, magnetization and susceptibility of the compound Na
[Er(W
O
)
], along with the corresponding analysis, are reported in
Section 3. In
Section 4, we compute and discuss the results for the magnetic properties of bis(phthalocyaninato) double-decker compounds [(Pc)Er{Pc{N(C
H
)
}
}]
.
Section 5 summarizes the results.
2. The Hamiltonian
To study the experimentally observed magnetic properties of the compounds Na
[Er(W
O
)
] [
9] and [(Pc)Er{Pc{N(C
H
)
}
}]
[
24], we rely on the method proposed in Reference [
25]. Here, we assume that a single molecule hosts no more than three unpaired valence electrons in its ground state with all non-bonding orbitals being fully occupied. The three active molecular orbitals are antibonding, such that the first one has lower energy than that of the 2-nd and 3-rd orbitals. Further, the quantization axis is oriented along the
z direction and the effective magnetic centers associated to the three electrons and active molecular orbitals are characterized by the same
g-factors value. Within the considered molecular orbital structure, we have three effective magnetic centers with a spin quantum number,
,
. Therefore, we have the spin coupling scheme
, with
and
, where
indicates the singlet
and triplet
spin–orbital configurations related to the 2-nd and the 3-rd molecular orbitals,
s is the effective total spin quantum number of the resulting Er
magnetic center.
Under the considered assumptions and the introduced effective spin coupling scheme, the spin–sigma Hamiltonian reads (see e.g., Reference [
25])
where
is the intramolecular exchange parameter between the spin centers, the effective three components’ spin operator
and the spin-like operator
are associated to the
i-th magnetic center. The operator
in the field dependent term satisfies the relation
where
, with
, is the
-th component of the
i-th spin operator and
Here,
B is the magnitude of the dc magnetic field and
b is that of the alternating field with frequency
. Under the action of an external magnetic field, the operators
obey the relations
Notice that, for all
,
s and
m,
is the
-th component of the effective
g-factor, say
,
and
are the corresponding spectroscopic and field parameters, respectively. Moreover, we would like to point out that the model parameters, related to Hamiltonian (
1), account for the contribution of all electrons occupying core molecular orbitals and that, due to the lack of exchange bridges, the value of the
g-factor does not depend directly on any of the three good spin quantum numbers as the general case described in Reference [
25] suggests. For more details about the physics behind all model parameters, the reader may consult Reference [
25].
The eigenvalues of (
1) are given by
where
n,
n,
n and
.
For a thorough study of the dc magnetic properties of the considered compounds and for the sake of comparison, in addition to the spin–sigma Hamiltonian given in (
1), we compute the same properties in the framework of the Heisenberg model. For
, the corresponding Hamiltonian reads
where
J and
are the corresponding exchange parameters,
is the
-th component of the corresponding
g-factor with
denoting the electron’s
g-factor and
being the
-th component of the unit vector
that defines the direction of the externally applied magnetic field.
The eigenvalues of (
3) are
where
are the respective eigenstates in the absence of the external magnetic field, that is,
.
The difference between
and
for all
components is that, in contrast to (
1), the Zeeman term in (
3) does not account for the magnetic field induced by all remaining electrons in the molecule. Thus, for a single electron system
, see Reference [
25]. It is worth mentioning that, since for rare earth elements the spin–orbital coupling is not a perturbation to the crystal field effect, the inclusion of the g-tensor is irrelevant.
We would like to emphasize that the energy spectra in (
2) and (
4) map only those energy levels from the initial variational spectrum that are relevant to the magnetic properties of the considered systems. Therefore, even the low-lying excited states related to transitions between molecular orbitals of different energies are ruled out.
5. Discussion
With the aim of exploring a possible role of the intramolecular exchange mechanism in governing the magnetic properties of Er
single ion magnets, we adapt a recently constructed effective spin-like model [
25] and study the magnetic properties of rare earth compounds Na
[Er(W
O
)
] and [(Pc)Er{Pc{N(C
H
)
}
}]
. To this end, in contrast to the methods working with free-ion basis states and the added perturbative crystal field effect, we considered the 4f unpaired electrons as delocalized occupying molecular orbitals, see
Section 2. The theoretical results obtained with the aid of the named model are in good agreement with the experimental findings [
9,
24].
In particular, calculating the magnetic properties of the single ion magnet Na
[Er(W
O
)
] we use the spin-sigma Hamiltonian (
1) and the Heisenberg (
3) models. Both models predict a Kramer’s doublet, with
,
,
as a ground state, and suggest the second doublet, characterized by the spin quantum numbers
,
,
, as a first excited state. Furthermore, in the energy spectra of both models, the quartet level appears as a second excited level. In this regard, both models describe reasonably well the two ground state transitions with intensities given by (
6), (
7) and shown in
Figure 3 and
Figure 4 with solid lines. Since for all
T, the difference in the values of
obtained via the spin–sigma and Heisenberg Hamiltonians is negligible, both models predict the same magnitudes for each integrated intensity, see (
6) and (
7). Nevertheless, only the energy spectrum (
2) accounts for the existence of a third excited level and therefore explains the appearance of the high temperature magnetic excitation. The high scattering probability related to the magnitude of the third peak is reproduced only by accounting for the probability of observing a transition from quartet non-magnetic states such as the spin–quadrupole ones [
31,
32,
33,
34,
35]. The respective intensity is given by (
8). On the other hand, the spectrum (
4) does not account for the contribution of electrons’ orbital moments to the system’s energy. Therefore, in the presence of an external magnetic field the Heisenberg model fails to explain the observed dc magnetization and susceptibility measurements. While the spin–sigma model provides a good qualitative and quantitative description of these properties, see
Figure 5 and
Figure 6. For
, it predicts an abrupt transition in the ground state from
and
to
and
that is stabilized by reaching the saturation of the magnetization above 2 T. Respectively, the total effective magnetic moment changes without exhibiting an intermediate magnetization step. According to the applied method, such shifting of the energy levels and hence variation in the total spin value is driven by unique interaction terms related to the electrons’ orbital moments that enter into the initial Hamiltonian for
, see Reference [
25]. The contribution of these interaction terms is effectively accounted for by the ‘
h’-field parameters with values given in
Table 1. The value of the total
g-factor points to the existence of a planar anisotropy, with
and
for
. The dynamic properties of Na
[Er(W
O
)
] are characterized only with the aid of the spin–sigma model. A comparison between the theoretical and experimental results is depicted in
Figure 8 and
Figure 9. We have a good agreement with the experimental data for both the in-phase and out-of-phase susceptibilities. The calculations yield an energy barrier to magnetization reversal in the interval
–
meV. This result is compatible with the experimentally observed value of
meV reported in Reference [
9].
To explore the magnetic properties of the single ion magnets, [(Pc)Er{Pc{N(C
H
)
}
}]
, we apply only the spin–sigma model (
1). As we have demonstrated for the other compound, see
Figure 5 and
Figure 6, Hamiltonian (
3) is not suitable for studying the magnetic properties of rare earth complexes. The results obtained with the aid of (
1) are in good agreement with the available experimental data provided in Reference [
24]. Reproducing the dc magnetization and susceptibility measurements, see
Figure 10 and
Figure 11, we detected a reversal of the quartet level from excited to ground state level. Accordingly, the total spin changes from
to
, as is the case with Na
[Er(W
O
)
]. As the theory suggests, the observed transition from partially to fully polarized spin state for low values of the applied field is a consequence of the interaction of the external magnetic field with that intrinsic to the molecule arising from the electrons’ orbitals. This effect is accounted for effectively by the ‘
h’ parameters with values given in
Table 3 and
Table 4. Moreover, according to the calculations for the saturation of the magnetization, the neutral compound [(Pc)Er{Pc{N(C
H
)
}
}]
is characterized by a larger
g-factor value than that of the reduced compound. On the other hand, the saturation of the magnetization for [(Pc)Er{Pc{N(C
H
)
}
}]
remains unchanged in the temperature domain 2–6 K. The stabilization of the saturation is related to a small variation of the corresponding
g-factor value, see
Table 4. According to the applied method and model, this effect is either due to a change in the planar anisotropy or from a contribution of uncoupled nuclear spins. The field-induced slow magnetic relaxation in the reduced member is qualitatively well reproduced by the energy spectrum of the spin–sigma model (
2). The calculated energy barrier is bounded in the domain
and it lies very close to the experimentally observed value of approximately
meV [
24]. The energy difference between the zero and ac field ground states and the second excited energy levels provides the amount of energy that has to be applied to observe a jump in the magnetization. It is accounted for by the ‘
h’-field parameters with values provided in
Table 5 and fitted to the in-phase and out-of-phase susceptibility data depicted in
Figure 14 and
Figure 15. The absence of a slow magnetic relaxation in the case
and
T, demonstrated by the neutral compound [(Pc)Er{Pc{N(C
H
)
}
}]
, leaves the zero field values of both ‘
h’-field parameters unchanged and suggests the absence of a preferential easy plain or axis, that is,
for all
. A slow magnetic relaxation may be observed for higher magnitudes of a dc or a ac generated field. Therefore, above 2 K the in-phase susceptibility describes the typical paramagnetic-like behavior, which is trivially reproduced. Accordingly, in the all-temperature domain, the out-of-phase susceptibility equals zero.