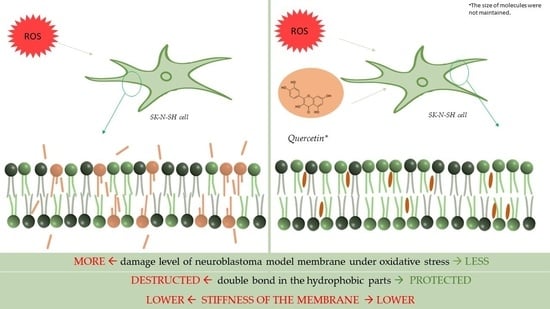
Advantageous/Unfavorable Effect of Quercetin on the Membranes of SK-N-SH Neuroblastoma Cells
Abstract
:1. Introduction
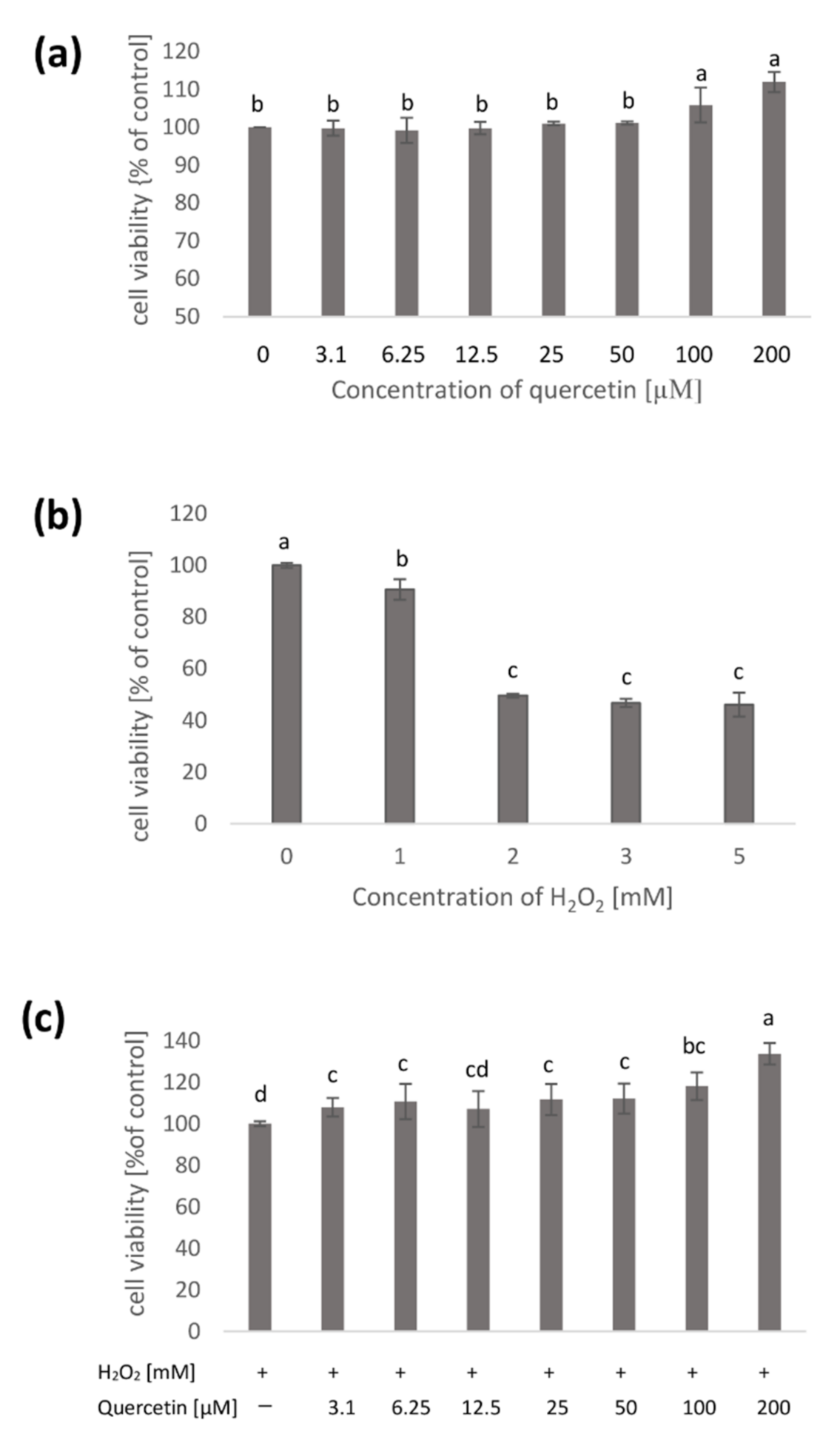
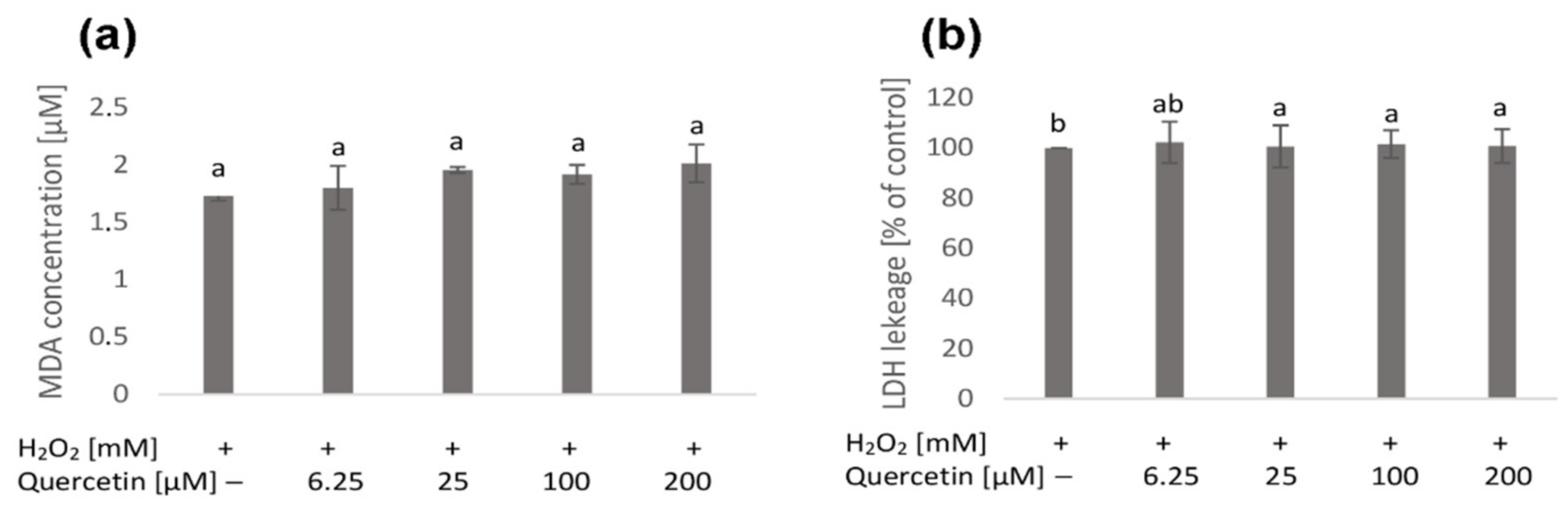
2. Results
2.1. Native Cell Membranes
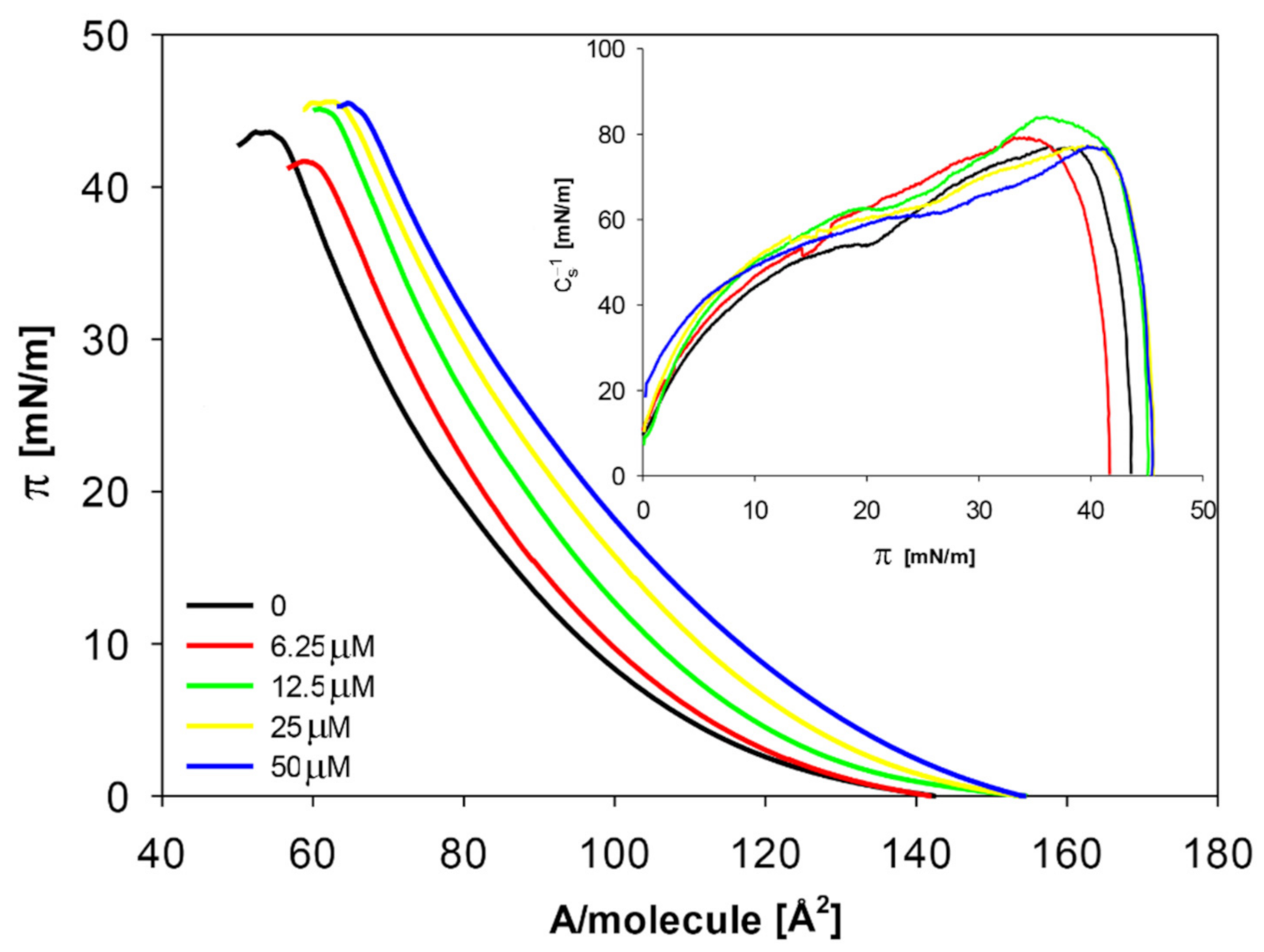
2.2. Model Membranes
2.2.1. The Influence of Quercetin on the Mechanical Properties of Membranes
2.2.2. Antioxidant Activity of Quercetin
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Measurement of Cell Viability (MTT Assay)
4.4. Membrane Damage Assay (LDH Assay)
4.5. Determination of Lipid Peroxidation (MDA Concentration)
4.6. Model Membranes
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hendrich, A.B. Flavonoid-membrane interactions: Possible consequences for biological effects of some polyphenolic compounds. Acta Pharmacol. Sin. 2006, 27, 27–40. [Google Scholar] [CrossRef] [Green Version]
- Boots, A.W.; Li, H.; Schins, R.P.; Duffin, R.; Heemskerk, J.W.; Bast, A.; Haenen, G.R. The quercetin paradox. Toxicol. Appl. Pharmacol. 2007, 222, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.F.; Borge, G.I.A.; Piskula, M.; Tudose, A.; Tudoreanu, L.; Valentová, K.; Santos, C.N. Bioavailability of quercetin in humans with a focus on interindividual variation. Compr. Rev. Food. Sci. Food Saf. 2018, 17, 714–731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen, B.; Egert, S.; Bosy-Westphal, A.; Müller, M.J.; Wolffram, S.; Hubbermann, E.M.; Rimbach, G.; Schwarz, K. Bioavailability of quercetin in humans and the influence of food matrix comparing quercetin capsules and different apple sources. Food. Res. Int. 2016, 88, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Rogerio, A.P.; Dora, C.L.; Andrade, E.L.; Chaves, J.S.; Silva, L.F.; Lemos-Senna, E.; Calixto, J.B. Anti-inflammatory effect of quercetin-loaded microemulsion in the airways allergic inflammatory model in mice. Pharmacol. Res. 2010, 61, 288–297. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, inflammation and immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Chien, S.Y.; Wu, Y.C.; Chung, J.G.; Yang, J.S.; Lu, H.F.; Tsou, M.F.; Chen, D.R. Quercetin-induced apoptosis acts through mitochondrial-and caspase-3-dependent pathways in human breast cancer MDA-MB-231 cells. Hum. Exp. Toxicol. 2009, 28, 493–503. [Google Scholar] [CrossRef]
- Iacopetta, D.; Grande, F.; Caruso, A.; Mordocco, R.A.; Plutino, M.R.; Scrivano, L.; Sinicropi, M.S. New insights for the use of quercetin analogs in cancer treatment. Future Med. Chem. 2017, 9, 2011–2028. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zhao, X.; Kariya, Y.; Fukala, H.; Teshigawara, K.; Uchida, A. Induction of apoptosis by quercetin: Involvement of heat shock protein. Cancer Res. 1994, 54, 4952–4957. [Google Scholar]
- Chou, C.C.; Yang, J.S.; Lu, H.F.; Ip, S.W.; Lo, C.; Wu, C.C.; Chen, D.R. Quercetin-mediated cell cycle arrest and apoptosis involving activation of a caspase cascade through the mitochondrial pathway in human breast cancer MCF-7 cells. Arch. Pharm. Res. 2010, 33, 1181–1191. [Google Scholar] [CrossRef]
- Heo, H.J.; Lee, C.Y. Protective effects of quercetin and vitamin C against oxidative stress-induced neurodegeneration. J. Agric. Food Chem. 2004, 52, 7514–7517. [Google Scholar] [CrossRef] [PubMed]
- Pavlica, S.; Gebhardt, R. Protective effects of flavonoids and two metabolites against oxidative stress in neuronal PC12 cells. Life Sci. 2010, 86, 79–86. [Google Scholar] [CrossRef]
- Suematsu, N.; Hosoda, M.; Fujimori, K. Protective effects of quercetin against hydrogen peroxide-induced apoptosis in human neuronal SH-SY5Y cells. Neurosci. Lett. 2011, 504, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Long, C.; Junming, T.; Qihuan, L.; Youshun, Z.; Chan, Z. Quercetin-induced apoptosis of HL-60 cells by reducing PI3K/Akt. Mol. Biol. Rep. 2012, 39, 7785–7793. [Google Scholar] [CrossRef] [PubMed]
- Rubio, V.; García-Pérez, A.I.; Herráez, A.; Diez, J.C. Different roles of Nrf2 and NFKB in the antioxidant imbalance produced by esculetin or quercetin on NB4 leukemia cells. Chem. Biol. Interact. 2018, 294, 158–166. [Google Scholar] [CrossRef]
- Brisdelli, F.; Coccia, C.; Cinque, B.; Cifone, M.G.; Bozzi, A. Induction of apoptosis by quercetin: Different response of human chronic myeloid (K562) and acute lymphoblastic (HSB-2) leukemia cells. Mol. Cell. Biochem. 2007, 296, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Kuo, P.C.; Liu, H.F.; Chao, J.I. Survivin and p53 modulate quercetin-induced cell growth inhibition and apoptosis in human lung carcinoma cells. J. Biol. Chem. 2004, 279, 55875–55885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.Y.; Kim, E.H.; Park, S.S.; Lim, J.H.; Kwon, T.K.; Choi, K.S. Quercetin sensitizes human hepatoma cells to TRAIL-induced apoptosis via Sp1-mediated DR5 up-regulation and proteasome-mediated c-FLIPS down-regulation. J. Cell. Biochem. 2008, 105, 1386–1398. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.F.; He, H.F.; Chen, Q. Quercetin inhibits proliferation and invasion acts by up-regulating miR-146a in human breast cancer cells. Mol. Cell. Biochem. 2015, 402, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Ranelletti, F.O.; Maggiano, N.; Serra, F.G.; Ricci, R.; Larocca, L.M.; Lanza, P.; Piantelli, M. Quercetin inhibits p21-RAS expression in human colon cancer cell lines and in primary colorectal tumors. Int. J. Cancer 2000, 85, 438–445. [Google Scholar] [CrossRef]
- Vijayababu, M.R.; Kanagaraj, P.; Arunkumar, A.; Ilangovan, R.; Aruldhas, M.M.; Arunakaran, J. Quercetin-induced growth inhibition and cell death in prostatic carcinoma cells (PC-3) are associated with increase in p21 and hypophosphorylated retinoblastoma proteins expression. J. Cancer Res. Clin. Oncol. 2005, 131, 765–771. [Google Scholar] [CrossRef]
- Senthilkumar, K.; Arunkumar, R.; Elumalai, P.; Sharmila, G.; Gunadharini, D.N.; Banudevi, S.; Arunakaran, J. Quercetin inhibits invasion, migration and signalling molecules involved in cell survival and proliferation of prostate cancer cell line (PC-3). Cell Biochem. Funct. 2011, 29, 87–95. [Google Scholar] [CrossRef]
- Spencer, J.P. The interactions of flavonoids within neuronal signalling pathways. Genes Nutr. 2007, 2, 257–273. [Google Scholar] [CrossRef] [Green Version]
- Jakubowicz-Gil, J.; Rzeski, W.; Zdzisinska, B.; Piersiak, T.; Weiksza, K.; Glowniak, K.; Gawron, A. Different sensitivity of neurons and neuroblastoma cells to quercetin treatment. Acta Neurobiol. Exp. 2008, 68, 463–476. [Google Scholar]
- Vauzour, D.; Vafeiadou, K.; Rodriguez-Mateos, A.; Rendeiro, C.; Spencer, J.P. The neuroprotective potential of flavonoids: A multiplicity of effects. Genes Nutr. 2008, 3, 115–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yen, G.C.; Duh, P.D.; Tsai, H.L.; Huang, S.L. Pro-oxidative properties of flavonoids in human lymphocytes. Biosci. Biotechnol. Biochem. 2003, 67, 1215–1222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendrich, A.B.; Malon, R.; Pola, A.; Shirataki, Y.; Motohashi, N.; Michalak, K. Differential interaction of Sophora isoflavonoids with lipidbilayers. Eur. J. Pharm. Sci. 2002, 16, 201–208. [Google Scholar] [CrossRef]
- Pawlikowska-Pawlęga, B.; Gruszecki, W.I.; Misiak, L.; Paduch, R.; Piersiak, T.; Zarzyka, B.; Gawron, A. Modification of membranes by quercetin, a naturally occurring flavonoid, via its incorporation in the polar head group. Biochim. Biophys. Acta Biomembr. 2007, 1768, 2195–2204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, W.M.; Kleinsmith, L.J.; Hardin, J.; Bertoni, G.P. The World of the Cell; Pearson/Benjamin Cummings: San Francisco, CA, USA, 2006. [Google Scholar]
- Morales, M.A.; Lozoya, X. Calcium-antagonist effect of quercetin onaortic smooth muscle. Planta Med. 1994, 60, 313–317. [Google Scholar] [CrossRef]
- Pawlikowska-Pawlęga, B.; Trębacz, K.; Król, E.; Gawron, A. Effects of quercetin and verapamil on membrane potential in the liver wort Conocephalum conicum. Acta Physiol. Plant 2000, 22, 61–68. [Google Scholar] [CrossRef]
- Bao, D.; Wang, J.; Pang, X.; Liu, H. Protective effect of quercetin against oxidative stress-induced cytotoxicity in rat pheochromocytoma (PC-12) cells. Molecules 2017, 22, 1122. [Google Scholar] [CrossRef]
- Ho, E.; Galougahi, K.K.; Liu, C.C.; Bhindi, R.; Figtree, G.A. Biological markers of oxidative stress: Applications to cardiovascular research and practice. Redox Biol. 2013, 1, 483–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metodiewa, D.; Jaiswal, A.K.; Cenas, N.; Dickancaité, E.; Segura-Aguilar, J. Quercetin may act as a cytotoxic prooxidant after its metabolic activation to semiquinone and quinoidal product. Free Radic. Biol. Med. 1999, 26, 107–116. [Google Scholar] [CrossRef]
- Bahnemann, D.; Hart, E.J. Rate Constants of the Reaction of the Hydrated Electron and Hydroxyl Radical with Ozone in Aqueous Solution. J. Phys. Chem. 1982, 86, 252–255. [Google Scholar] [CrossRef]
- Qiao, L.; Ge, A.; Osawa, M.; Ye, S. Structure and stability studies of mixed monolayers of saturated and unsaturated phospholipids under low-level ozone. Phys. Chem. Chem. Phys. 2013, 15, 17775–17785. [Google Scholar] [CrossRef] [PubMed]
- Rudolphi-Skórska, E.; Sieprawska, A. Adaptation of wheat cells to short-term ozone stress: The impact of a-tocopherol and gallic acid on natural and model membranes. Acta Physiol. Plant 2016, 38, 85. [Google Scholar] [CrossRef] [Green Version]
- Rudolphi-Skórska, E.; Dyba, B.; Kreczmer, B.; Filek, M. Impact of polyphenol-rich green tea extracts on the protection of DOPC monolayer against damage caused by ozone induced lipid oxidation. Acta Biochim. Pol. 2018, 65, 193–197. [Google Scholar] [CrossRef]
- Charalampous, F.C. Levels and Distributions of Phospholipids and Cholesterol in the Plasma Membrane of Neuroblastoma Cells. Biochim. Biophys. Acta Biomembr. 1979, 556, 38–51. [Google Scholar] [CrossRef]
- Yip, C.M.; Elton, E.A.; Darabie, A.A.; Morrison, M.R.; McLaurin, J. Cholesterol, a Modulator of Membrane-Associated Abeta-Fibrillogenesis and Neurotoxicity. J. Mol. Biol. 2001, 311, 723–734. [Google Scholar] [CrossRef]
- Jakubec, M.; Bariås, E.; Kryuchkov, F.; Hjørnevik, L.V.; Halskau, Ø. Fast and Quantitative Phospholipidomic Analysis of SH-SY5Y Neuroblastoma Cell Cultures Using Liquid Chromatography–Tandem Mass Spectrometry and 31P Nuclear Magnetic Resonance. ACS Omega 2019, 4, 21596–21603. [Google Scholar] [CrossRef] [Green Version]
- Bader, H.; Hoigné, J. Determination of ozone in water by the indigo method. Water Res. 1981, 15, 449–456. [Google Scholar] [CrossRef]
| Concentration of Qercetin [µM] | LDH Leakage [% of Control] |
|---|---|
| 0 | 100 a |
| 3.1 | 96.98 ± 0.86 c |
| 6.25 | 99.27 ± 1.02 a |
| 12.5 | 100.59 ± 1.53 a |
| 25 | 98.43 ± 0.50 b |
| 50 | 100.23 ± 2.38 a |
| 100 | 99.27 ± 0.67 a |
| 200 | 101.80 ± 0.50 a |
| Concentration of Quercetin [μM] | Alim [Å2] | πcoll [mN/m] | Cs−1max [mN/m] |
|---|---|---|---|
| 0 | 56.4 ± 0.1 a | 43.5 ± 0.2 b | 76.9 ± 0.2 d |
| 6.25 | 60.7 ± 0.2 b | 42.3 ± 0.3 a | 78.8 ± 0.2 b |
| 12.5 | 62.7 ± 0.1 c | 44.8 ± 0.3 c | 83.7 ± 0.2 a |
| 25 | 64.3 ± 0.3 d | 45.6 ± 0.2 d | 77.1 ± 0.2 c |
| 50 | 66.3 ± 0.2 e | 45.1 ± 0.2 e | 76.9 ± 0.2 d |
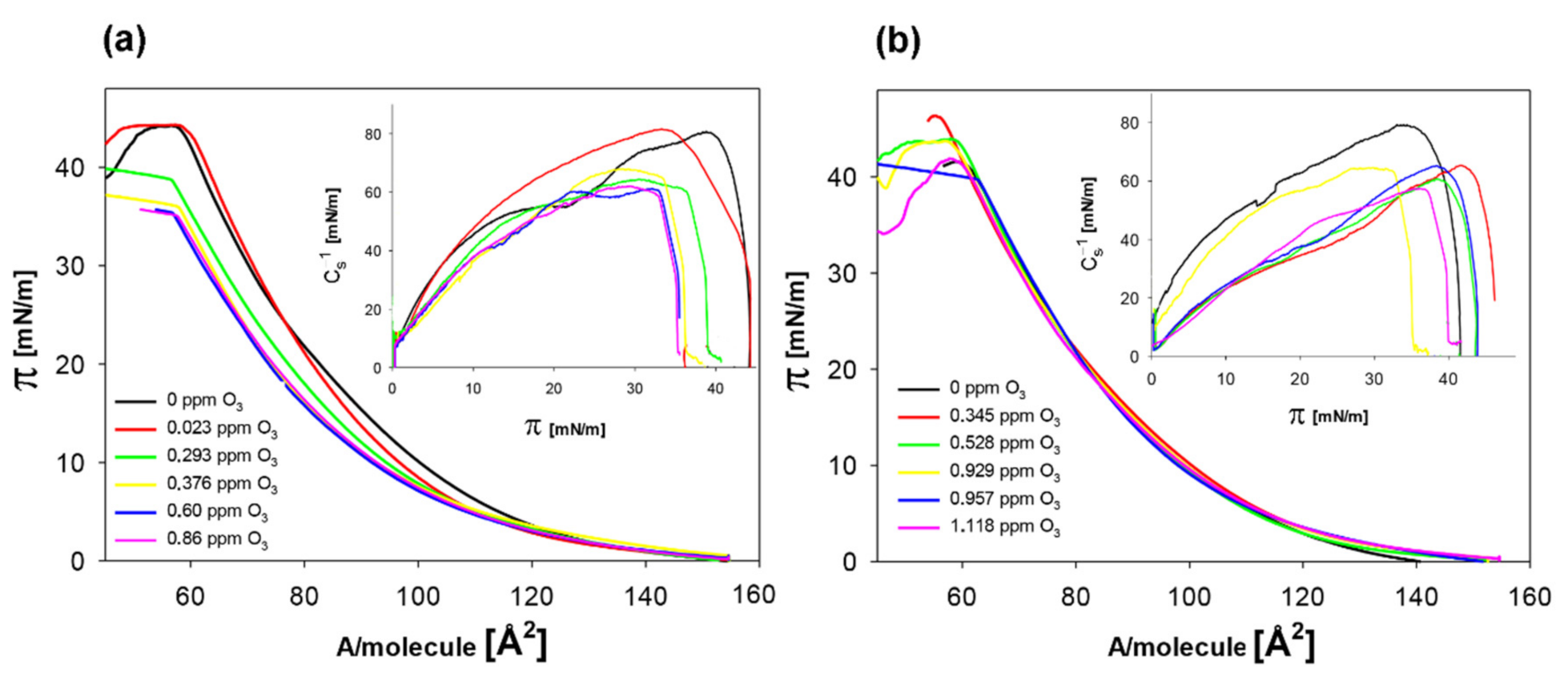
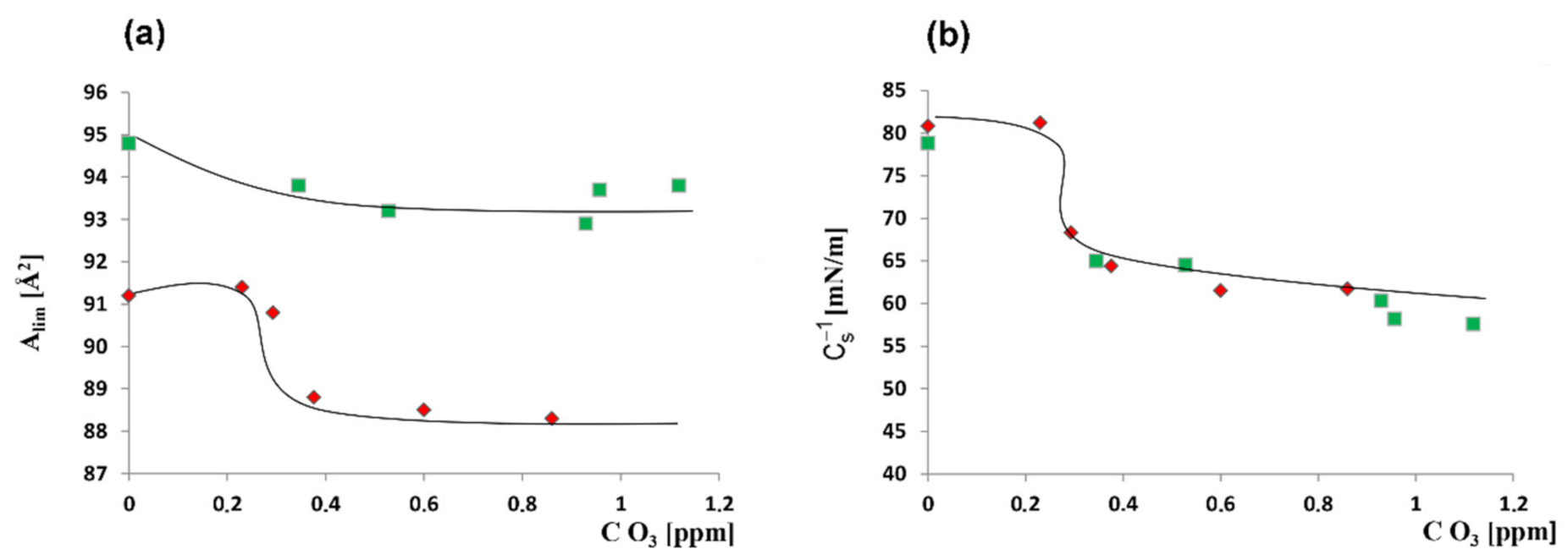
| O3 [ppm] | Alim [Å2] | Cs−1max [mN/m] |
|---|---|---|
| 0 | 91.2 ± 0.2 a | 80.8 ± 0.5 a |
| 0.023 | 91.4 ± 0.3 a | 81.2 ± 0.6 a |
| 0.293 | 90.8 ± 0.3 b | 68.3 ± 0.7 b |
| 0.376 | 88.8 ± 0.5 c | 64.4 ± 0.9 c |
| 0.60 | 88.5 ± 0.4 c | 61.5 ± 1.2 d |
| 0.86 | 88.3 ± 0.6 c | 61.7 ± 1.1 d |
| + 6.25 mM quercetin | ||
| 0 | 94.8 ± 0.4 a | 78.8 ± 1.2 a |
| 0.345 | 93.8 ± 0.5 b | 65.0 ± 1.4 b |
| 0.528 | 93.2 ± 0.4 b | 64.5 ± 1.3 b |
| 0.929 | 93.0 ± 0.6 b | 60.3 ± 1.1 c |
| 0.957 | 93.7 ± 0.5 b | 58.2 ± 0.9 d |
| 1.118 | 93.8 ± 0.4 b | 57.6 ± 0.8 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kreczmer, B.; Dyba, B.; Barbasz, A.; Rudolphi-Szydło, E. Advantageous/Unfavorable Effect of Quercetin on the Membranes of SK-N-SH Neuroblastoma Cells. Molecules 2021, 26, 4945. https://doi.org/10.3390/molecules26164945
Kreczmer B, Dyba B, Barbasz A, Rudolphi-Szydło E. Advantageous/Unfavorable Effect of Quercetin on the Membranes of SK-N-SH Neuroblastoma Cells. Molecules. 2021; 26(16):4945. https://doi.org/10.3390/molecules26164945
Chicago/Turabian StyleKreczmer, Barbara, Barbara Dyba, Anna Barbasz, and Elżbieta Rudolphi-Szydło. 2021. "Advantageous/Unfavorable Effect of Quercetin on the Membranes of SK-N-SH Neuroblastoma Cells" Molecules 26, no. 16: 4945. https://doi.org/10.3390/molecules26164945
APA StyleKreczmer, B., Dyba, B., Barbasz, A., & Rudolphi-Szydło, E. (2021). Advantageous/Unfavorable Effect of Quercetin on the Membranes of SK-N-SH Neuroblastoma Cells. Molecules, 26(16), 4945. https://doi.org/10.3390/molecules26164945