The Importance of Precursors and Modification Groups of Aerogels in CO2 Capture
Abstract
:1. Introduction
2. Inorganic Aerogels
3. Organic Aerogels
4. Hybrid Aerogels
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- García-González, C.A.; Camino-Rey, M.C.; Alnaief, M.; Zetzl, C.; Smirnova, I. Supercritical drying of aerogels using CO2: Effect of extraction time on the end material textural properties. J. Supercrit. Fluids 2012, 66, 297–306. [Google Scholar] [CrossRef]
- Keshavarz, L.; Ghaani, M.R.; MacElroy, J.M.D.; English, N.J. A comprehensive review on the application of aerogels in CO2-adsorption: Materials and characterisation. Chem. Eng. J. 2021, 412, 128604. [Google Scholar] [CrossRef]
- Cakmak, O.K.; Hassan, K.T.; Wang, J.; Han, X.; Šiller, L. Synthesis of sodium silicate-based silica aerogels with graphene oxide by ambient pressure drying. J. Porous Mater. 2021, 1–8. [Google Scholar] [CrossRef]
- Shen, J.; Zhang, Z.; Wu, G.; Zhou, B.; Ni, X.; Wang, J. Preparation and Characterization of Silica Aerogels Derived from Ambient Pressure. J. Mater. Sci. Technol. 2006, 22, 798–802. [Google Scholar]
- Muhammad, A.; Lee, D.; Shin, Y.; Park, J. Recent progress in polysaccharide aerogels: Their synthesis, application, and future outlook. Polymers 2021, 13, 1347. [Google Scholar] [CrossRef] [PubMed]
- Linneen, N.N.; Pfeffer, R.; Lin, Y.S. Amine distribution and carbon dioxide sorption performance of amine coated silica aerogel sorbents: Effect of synthesis methods. Ind. Eng. Chem. Res. 2013, 52, 14671–14679. [Google Scholar] [CrossRef]
- Begag, R.; Krutka, H.; Dong, W.; Mihalcik, D.; Rhine, W.; Gould, G.; Baldic, J.; Nahass, P. Superhydrophobic amine functionalized aerogels as sorbents for CO2 capture. Greenh. Gases Sci. Technol. 2013, 3, 30–39. [Google Scholar] [CrossRef]
- Marques, L.M.; Carrott, P.J.M.; Carrott, M.M.L.R. Amine-Modified Carbon Aerogels for CO2 Capture. Adsorpt. Sci. Technol. 2013, 31, 223–232. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Zhang, S.; Row, K.H.; Ahn, W.S. Amine—Silica composites for CO2 capture: A short review. J. Energy Chem. 2017, 26, 868–880. [Google Scholar] [CrossRef] [Green Version]
- Kamran, U.; Park, S.J. Chemically modified carbonaceous adsorbents for enhanced CO2 capture: A review. J. Clean. Prod. 2021, 290, 125776. [Google Scholar] [CrossRef]
- Fang, Q.; Huang, W.; Wang, H. Role of additives in silica-supported polyethylenimine adsorbents for CO2 adsorption. Mater. Res. Express 2020, 7, 035026. [Google Scholar] [CrossRef]
- Sun, T.; Fan, H.; Wang, Z. Hydrophobic monolithic silica with abundant pore as efficient adsorbent for organic contaminants removal. J. Mater. Sci. 2013, 48, 6713–6718. [Google Scholar] [CrossRef]
- Lin, Y.F.; Lin, Y.J.; Lee, C.C.; Lin, K.A.; Chung, T. Synthesis of mechanically robust epoxy cross-linked silica aerogel membranes for CO2 capture. J. Taiwan Inst. Chem. Eng. 2018, 87, 117–122. [Google Scholar] [CrossRef]
- Chen, Y.; Shao, G.; Kong, Y.; Shen, X.; Cui, S. Facile preparation of cross-linked polyimide aerogels with carboxylic functionalization for CO2 capture. Chem. Eng. J. 2017, 322, 1–9. [Google Scholar] [CrossRef]
- Kanamori, K. Handbook of Sol-Gel Science and Technology; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–22. [Google Scholar]
- Schwertfeger, F.; Frank, D.; Schmidt, M. Hydrophobic waterglass based aerogels without solvent exchange or supercritical drying. J. Non. Cryst. Solids 1998, 225, 24–29. [Google Scholar] [CrossRef]
- Nakanishi, K.; Minakuchi, H.; Soga, N.; Tanaka, N. Structure Design of Double-Pore Silica and Its Application to HPLC. J. Sol-Gel Sci. Technol. 1998, 13, 163–169. [Google Scholar] [CrossRef]
- Wagh, P.B.; Begag, R.; Pajonk, G.M.; Rao, A.V.; Haranath, D. Comparison of some physical properties of silica aerogel monoliths synthesized by different precursors. Mater. Chem. Phys. 1999, 57, 214–218. [Google Scholar] [CrossRef]
- Solimani, A.; Dorcheh, A.S.; Abbasi, M.H. Silica Aerogel; Synthesis, Properties and Characterization. J. Mater. Process. Technol. 2008, 199, 10–26. [Google Scholar] [CrossRef]
- Harreld, J.H.; Ebina, T.; Tsubo, N.; Stucky, G. Manipulation of pore size distributions in silica and ormosil gels dried under ambient pressure conditions. J. Non. Cryst. Solids 2002, 298, 241–251. [Google Scholar] [CrossRef]
- Nadargi, D.Y.; Latthe, S.S.; Hirashima, H.; Rao, A.V. Studies on rheological properties of methyltriethoxysilane (MTES) based flexible superhydrophobic silica aerogels. Microporous Mesoporous Mater. 2009, 117, 617–626. [Google Scholar] [CrossRef]
- Maleki, H.; Durães, L.; Portugal, A. An overview on silica aerogels synthesis and different mechanical reinforcing strategies. J. Non. Cryst. Solids 2014, 385, 55–74. [Google Scholar] [CrossRef] [Green Version]
- Yun, S.; Luo, H.; Gao, Y. Superhydrophobic silica aerogel microspheres from methyltrimethoxysilane: Rapid synthesis via ambient pressure drying and excellent absorption properties. RSC Adv. 2014, 4, 4535–4542. [Google Scholar] [CrossRef]
- Lin, Y.; Syu, C.; Huang, K.; Lin, K.A. Synthesis of silica aerogel membranes using low-cost silicate precursors for carbon dioxide capture. Chem. Phys. Lett. 2019, 726, 13–17. [Google Scholar] [CrossRef]
- Sarawade, P.B.; Kim, J.K.; Hilonga, A.; Kim, H.T. Production of low-density sodium silicate-based hydrophobic silica aerogel beads by a novel fast gelation process and ambient pressure drying process. Solid State Sci. 2010, 12, 911–918. [Google Scholar] [CrossRef]
- Pisal, A.A.; Rao, A.V. Comparative studies on the physical properties of TEOS, TMOS and Na2SiO3 based silica aerogels by ambient pressure drying method. J. Porous Mater. 2016, 23, 1547–1556. [Google Scholar] [CrossRef]
- Pignataro, B.; Licciardello, A.; Cataldo, S.; Marletta, G. SPM and TOF-SIMS investigation of the physical and chemical modification induced by tip writing of self-assembled monolayers. Mater. Sci. Eng. 2003, 23, 7–12. [Google Scholar] [CrossRef]
- English, N.J.; El-Hendawy, M.M.; Mooney, D.A.; MacElroy, J.M.D. Perspectives on atomospheric CO2 fixation in inorganic and biomimetic structures. Coord. Chem. Rev. 2014, 269, 85–95. [Google Scholar] [CrossRef]
- Yu, F.; Wu, Y.; Zhang, W.; Cai, T.; Xu, Y.; Chen, X. A novel aerogel sodium-based sorbent for low temperature CO2 capture. Greenh. Gases Sci. Technol. 2016, 6, 561–573. [Google Scholar] [CrossRef]
- Wang, W. Novel Freeze-dried Amine Functionalised 2D/3D Assemblies of Graphene and Mesoporous Silica for CO2 Capture. Ph.D. Thesis, The University of Queensland, Saint Lucia, Australia, 2019. [Google Scholar]
- Qi, G.; Fu, L.; Duan, X.; Choi, B.H.; Abraham, M.; Giannelis, E.P. Mesoporous amine-bridged polysilsesquioxane for CO2 capture. Greenh. Gases Sci. Technol. 2011, 1, 278–284. [Google Scholar] [CrossRef] [Green Version]
- Qi, G.; Wang, Y.; Estevez, L.; Duan, X.; Anako, N.; Park, A.A.; Li, W.; Jones, W.; Giannelis, E.P. High efficiency nanocomposite sorbents for CO2 capture based on amine-functionalized mesoporous capsules. R. Soc. Chem. 2011, 4, 444–452. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Dai, Z.; Wu, J.; Zhao, N.; Xu, J. Vacuum-dried robust bridged silsesquioxane aerogels. Adv. Mater. 2013, 25, 4494–4497. [Google Scholar] [CrossRef] [PubMed]
- Linneen, N.; Pfeffer, R.; Lin, Y.S. CO2 capture using particulate silica aerogel immobilized with tetraethylenepentamine. Microporous Mesoporous Mater. 2013, 176, 123–131. [Google Scholar] [CrossRef]
- Wang, L.; Yao, M.; Hu, X.; Hu, G.; Lu, J.; Luo, M.; Fan, M. Amine-modified ordered mesoporous silica: The effect of pore size on CO2 capture performance. Appl. Surf. Sci. 2015, 324, 286–292. [Google Scholar] [CrossRef]
- Linneen, N. Synthesis and Carbon Dioxide Adsorption Properties of Amine Modified Particulate Silica Aerogel Sorbents; Arizona State University: Tempe, AZ, USA, 2014. [Google Scholar]
- Fan, H.; Wu, Z.; Xu, Q.; Sun, T. Flexible, amine-modified silica aerogel with enhanced carbon dioxide capture performance. J. Porous Mater. 2016, 23, 131–137. [Google Scholar] [CrossRef]
- Cui, S.; Cheng, W.; Shen, X.; Fan, M.; Russell, A.T.; Wu, Z.; Yi, X. Mesoporous amine-modified SiO2 aerogel: A potential CO2 sorbent. Energy Environ. Sci. 2011, 4, 2070–2074. [Google Scholar] [CrossRef]
- Begag, R. Bench Scale Development and Testing of Aerogel Sorbents for CO2 Capture Final Technical Report; Pittsburgh, P.A., Morgantown, W.V., Eds.; Aspen Aerogels: Northborough, MA, USA, 2017. [Google Scholar]
- Amonette, J.E.; Matyáš, J. Functionalized silica aerogels for gas-phase purification, sensing, and catalysis: A review. Microporous Mesoporous Mater. 2017, 1–49. [Google Scholar] [CrossRef]
- Garip, M.; Gizli, N. Ionic liquid containing amine-based silica aerogels for CO2 capture by fixed bed adsorption. J. Mol. Liq. 2020, 310, 113227. [Google Scholar] [CrossRef]
- Minju, N.; Nair, B.N.; Mohamed, A.P.; Ananthakumar, S. Surface engineered silica mesospheres—A promising adsorbent for CO2 capture. Sep. Purif. Technol. 2017, 181, 192–200. [Google Scholar] [CrossRef]
- Santos, A.; Ajbary, M.; Kherbeche, A.; Piñero, M.; De La Rosa-Fox, N.; Esquivias, L. Fast CO2 sequestration by aerogel composites. J. Sol-Gel Sci. Technol. 2008, 45, 291–297. [Google Scholar] [CrossRef]
- Santos, A.; Ajbary, M.; Morales-Flórez, V.; Kherbeche, A.; Piñero, M.; Esquivias, L. Larnite powders and larnite/silica aerogel composites as effective agents for CO2 sequestration by carbonation. J. Hazard. Mater. 2009, 168, 1397–1403. [Google Scholar] [CrossRef] [Green Version]
- Wörmeyer, K.; Alnaief, M.; Smirnova, I. Amino functionalised Silica-Aerogels for CO2-adsorption at low partial pressure. Adsorption 2012, 18, 163–171. [Google Scholar] [CrossRef]
- Wörmeyer, K.; Smirnova, I. Adsorption of CO2, moisture and ethanol at low partial pressure using aminofunctionalised silica aerogels. Chem. Eng. J. 2013, 225, 350–357. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Y.; Chen, N.; Dai, S.; Jiang, H.; Wang, S. Effects of amine loading on the properties of cellulose nanofibrils aerogel and its CO2 capturing performance. Carbohydr. Polym. 2018, 194, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Minju, N.; Abhilash, P.; Nair, B.N.; Mohamed, A.P.; Ananthakumar, S. Amine impregnated porous silica gel sorbents synthesized from water-glass precursors for CO2 capturing. Chem. Eng. J. 2015, 269, 335–342. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Guo, Y.; Li, C.; Lu, S. Removal of low concentration CO2 at ambient temperature using several potassium-based sorbents. Appl. Energy 2014, 124, 241–247. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, C.; Li, C. Thermogravimetric analysis of carbonation behaviors of several potassium-based sorbents in low concentration CO2. J. Therm. Anal. Calorim. 2015, 119, 441–451. [Google Scholar] [CrossRef]
- Zhao, C.; Guo, Y.; Li, W.; Bu, C.; Wang, X.; Lu, P. Experimental and modeling investigation on CO2 sorption kinetics over K2CO3-modified silica aerogels. Chem. Eng. J. 2017, 312, 50–58. [Google Scholar] [CrossRef]
- Linneen, N.N.; Pfeffer, R.; Lin, Y.S. CO2 adsorption performance for amine grafted particulate silica aerogels. Chem. Eng. J. 2014, 254, 190–197. [Google Scholar] [CrossRef]
- Ello, A.S.; Yapo, J.A.; Trokourey, A. N-doped carbon aerogels for carbon dioxide (CO2) capture. Afr. J. Pure Appl. Chem. 2013, 7, 61–66. [Google Scholar]
- Al-Muhtaseb, B.S.A.; Ritter, J.A. Preparation and Properties of Resorcinol—Formaldehyde Organic and Carbon Gels. Adv. Mater. 2003, 15, 101–114. [Google Scholar] [CrossRef]
- Xie, W.; Yu, M.; Wang, R. CO2 capture behaviors of amine-modified resorcinol-based carbon aerogels adsorbents. Aerosol Air Qual. Res. 2017, 17, 2715–2725. [Google Scholar] [CrossRef] [Green Version]
- Guarín Romero, J.; Moreno-Piraján, J.; Giraldo Gutierrez, L. Kinetic and Equilibrium Study of the Adsorption of CO2 in Ultramicropores of Resorcinol-Formaldehyde Aerogels Obtained in Acidic and Basic Medium. C 2018, 4, 52. [Google Scholar] [CrossRef] [Green Version]
- Marques, L.; Carrott, P.; Ribeiro Carrott, M. Carbon aerogels used in carbon dioxide capture. Boletín Grup. Español Carbón 2016, 40, 9–12. [Google Scholar]
- Masika, E.; Mokaya, R. High surface area metal salt templated carbon aerogels via a simple subcritical drying route: Preparation and CO2 uptake properties. RSC Adv. 2013, 3, 17677–17681. [Google Scholar] [CrossRef]
- Biesmans, G.; Mertens, A.; Duffours, L.; Woignier, T.; Phalippou, J. Polyurethane based organic aerogels and their transformation into carbon aerogels. J. Non. Cryst. Solids 1998, 225, 64–68. [Google Scholar] [CrossRef]
- Li, W.; Guo, S. Preparation of low-density carbon aerogels from a cresol/formaldehyde mixture. Mater. Sci. Carbon 2000, 38, 1520–1523. [Google Scholar] [CrossRef]
- Majedi Far, H.; Rewatkar, P.M.; Donthula, S.; Taghvaee, T.; Saeed, A.M.; Sotiriou-Leventis, C.; Leventis, N. Exceptionally High CO2 Adsorption at 273 K by Microporous Carbons from Phenolic Aerogels: The Role of Heteroatoms in Comparison with Carbons from Polybenzoxazine and Other Organic Aerogels. Macromol. Chem. Phys. 2019, 220, 1–16. [Google Scholar] [CrossRef]
- Li, R.; Chen, C.; Li, J.; Xu, L.; Xiao, G.; Yan, D. A facile approach to superhydrophobic and superoleophilic graphene/polymer aerogels. J. Mater. Chem. A 2014, 2, 3057–3064. [Google Scholar] [CrossRef]
- Gromov, A.V.; Kulur, A.; Gibson, J.A.A.; Mangano, E.; Brandani, S.; Campbell, E.E.B. Carbon nanotube/PVA aerogels impregnated with PEI: Solid adsorbents for CO2 capture. Sustain. Energy Fuels 2018, 2, 1630–1640. [Google Scholar] [CrossRef] [Green Version]
- Guo, K.; Song, H.; Chen, X.; Du, X.; Zhong, L. Graphene oxide as an anti-shrinkage additive for resorcinol-formaldehyde composite aerogels. Phys. Chem. Chem. Phys. 2014, 16, 11603–11608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsan, N.; Dutta, P.K.; Kumar, S.; Bera, R.; Das, N. Chitosan grafted graphene oxide aerogel: Synthesis, characterization and carbon dioxide capture study. Int. J. Biol. Macromol. 2019, 125, 300–306. [Google Scholar] [CrossRef]
- Liu, Y.; Xiang, M.; Hong, L. Three-dimensional nitrogen and boron codoped graphene for carbon dioxide and oils adsorption. RSC Adv. 2017, 7, 6467–6473. [Google Scholar] [CrossRef] [Green Version]
- Khandaker, T.; Hossain, M.S.; Dhar, P.K.; Rahman, M.S.; Hossain, M.A.; Ahmed, M.B. Efficacies of Carbon-Based Adsorbents for Carbon Dioxide Capture. Processes 2020, 8, 654. [Google Scholar] [CrossRef]
- Pruna, A.; Cárcel, A.C.; Benedito, A.; Giménez, E. Effect of synthesis conditions on CO2 capture of ethylenediamine-modified graphene aerogels. Appl. Surf. Sci. 2019, 487, 228–235. [Google Scholar] [CrossRef]
- Bhanja, P.; Das, S.K.; Patra, A.K.; Bhaumik, A. Functionalized graphene oxide as an efficient adsorbent for CO2 capture and support for heterogeneous catalysis. RSC Adv. 2016, 6, 72055–72068. [Google Scholar] [CrossRef]
- Wang, W.; Motuzas, J.; Zhao, X.S.; Diniz Da Costa, J.C. 2D/3D amine functionalised sorbents containing graphene silica aerogel and mesoporous silica with improved CO2 sorption. Sep. Purif. Technol. 2019, 222, 381–389. [Google Scholar] [CrossRef]
- Reuß, M.; Ratke, L. RF-aerogels catalysed by ammonium carbonate. J. Sol-Gel Sci. Technol. 2010, 53, 85–92. [Google Scholar] [CrossRef]
- Zubair, N.A.; Abouzari-Lotf, E.; Mahmoud Nasef, M.; Abdullah, E.C. Aerogel-based materials for adsorbent applications in material domains. E3S Web Conf. 2019, 90, 1–12. [Google Scholar] [CrossRef]
- Yamamoto, T.; Nishimura, T.; Suzuki, T.; Tamon, H. Control of mesoporosity of carbon gels prepared by sol ± gel polycondensation and freeze drying. J. Non. Cryst. Solids 2001, 288, 46–55. [Google Scholar] [CrossRef]
- Montes, S.; Maleki, H. Aerogels and Their Applications; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 9780128133576. [Google Scholar]
- Liu, Q.; Han, Y.; Qian, X.; He, P.; Fei, Z.; Chen, X.; Zhang, Z.; Tang, J.; Cui, M.; Qiao, X. CO2 Adsorption over Carbon Aerogels: The Effect of Pore and Surface Properties. ChemistrySelect 2019, 4, 3161–3168. [Google Scholar] [CrossRef]
- Robertson, C.; Mokaya, R. Microporous activated carbon aerogels via a simple subcritical drying route for CO2 capture and hydrogen storage. Microporous Mesoporous Mater. 2013, 179, 151–156. [Google Scholar] [CrossRef]
- Zhuo, H.; Hu, Y.; Tong, X.; Zhong, L.; Peng, X.; Sun, R. Sustainable hierarchical porous carbon aerogel from cellulose for high-performance supercapacitor and CO2 capture. Ind. Crops Prod. 2016, 87, 229–235. [Google Scholar] [CrossRef]
- Kong, Y.; Shen, X.; Cui, S.; Fan, M. Development of monolithic adsorbent via polymeric sol-gel process for low-concentration CO2 capture. Appl. Energy 2015, 147, 308–317. [Google Scholar] [CrossRef]
- Chen, L.; Gong, M.; Cheng, Y.; Liu, Y.; Yin, S.; Luo, D. Effect of pore structure of supports on CO2 adsorption of tetraethylenepentamine/carbon aerogels prepared by incipient wetness impregnation method. Pol. J. Environ. Stud. 2019, 28, 4127–4137. [Google Scholar] [CrossRef]
- Yang, G.; Luo, H.; Ohba, T.; Kanoh, H. CO2 Capture by Carbon Aerogel-Potassium Carbonate Nanocomposites. Int. J. Chem. Eng. 2016, 2016, 1–9. [Google Scholar]
- Jeon, D.-H.; Min, B.-G.; Oh, J.G.; Nah, C.; Park, S.-J. Influence of Nitrogen moieties on CO2 capture of Carbon Aerogel. Carbon Lett. 2015, 16, 57–61. [Google Scholar] [CrossRef] [Green Version]
- Geng, S.; Wei, J.; Jonasson, S.; Hedlund, J.; Oksman, K. Multifunctional Carbon Aerogels with Hierarchical Anisotropic Structure Derived from Lignin and Cellulose Nanofibers for CO2 Capture and Energy Storage. ACS Appl. Mater. Interfaces 2020, 12, 7432–7441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, M.; Zhou, Q.; Du, A.; Van Kasteren, J.; Wang, Y. Preparation of nanoporous cellulose foams from cellulose-ionic liquid solutions. Mater. Lett. 2009, 63, 1851–1854. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Thomas, A.; Liao, Y. Ultra-High Surface Area Nitrogen-Doped Carbon Aerogels Derived from a Schiff-Base Porous Organic Polymer Aerogel for CO2 Storage and Supercapacitors. Adv. Funct. Mater. 2019, 29, 1904785. [Google Scholar] [CrossRef]
- Alhwaige, A.A.; Ishida, H.; Qutubuddin, S. Carbon Aerogels with Excellent CO2 Adsorption Capacity Synthesized from Clay-Reinforced Biobased Chitosan-Polybenzoxazine Nanocomposites. ACS Sustain. Chem. Eng. 2016, 4, 1286–1295. [Google Scholar] [CrossRef]
- Shi, F.; Wang, L.; Liu, J. Synthesis and characterization of silica aerogels by a novel fast ambient pressure drying process. Mater. Lett. 2006, 60, 3718–3722. [Google Scholar] [CrossRef]
- Hüsing, N.; Schubert, U.; Mezei, R.; Fratzl, P.; Riegel, B.; Kiefer, W.; Kohler, D.; Mader, W. Formation and Structure of Gel Networks from Si(OEt)4/(MeO)3Si(CH2)3NR’2 mixtures (NR’2 = NH2 or NHCH2CH2NH2). Chem. Mater. 1999, 11, 451–457. [Google Scholar] [CrossRef]
- Nguyen, B.N.; Meador, M.A.B.; Tousley, M.E.; Shonkwiler, B.; McCorkle, L.; Scheiman, D.A.; Palczer, A. Tailoring elastic properties of silica aerogels cross-linked with polystyrene. ACS Appl. Mater. Interfaces 2009, 1, 621–630. [Google Scholar] [CrossRef]
- Maleki, H. Recent advances in aerogels for environmental remediation applications: A review. Chem. Eng. J. 2016, 300, 98–118. [Google Scholar] [CrossRef]
- Agrawal, S.; Dev, P.; English, N.J.; Thampi, K.R.; MacElroy, J.M.D. A TD-DFT study of the effects of structural variations on the photochemistry of polyene dyes. Chem. Sci. 2012, 3, 416–424. [Google Scholar] [CrossRef]
- McDonnell, K.A.; Wadnerkar, N.; English, N.J.; Rahman, M.; Dowling, D.P. Photo-active and Optical Properties of Bismuth Ferrite (BiFeO3): An Experimental and Theoretical Study. Chem. Phys. Lett. 2013, 572, 78–84. [Google Scholar] [CrossRef]
- Kong, Y.; Shen, X.; Cui, S.; Fan, M. Facile synthesis of an amine hybrid aerogel with high adsorption efficiency and regenerability for air capture via a solvothermal-assisted sol-gel process and supercritical drying. Green Chem. 2012, 17, 3436–3445. [Google Scholar] [CrossRef]
- Kong, Y.; Shen, X.; Cui, S.; Fan, M. Use of monolithic silicon carbide aerogel as a reusable support for development of regenerable CO2 adsorbent. RSC Adv. 2014, 4, 64193–64199. [Google Scholar] [CrossRef]
- Kong, Y.; Shen, X.; Fan, M.; Yang, M.; Cui, S. Dynamic capture of low-concentration CO2 on amine hybrid silsesquioxane aerogel. Chem. Eng. J. 2016, 283, 1059–1068. [Google Scholar] [CrossRef]
- Kong, Y.; Zhang, J.; Shen, X. One-pot sol–gel synthesis of amine hybrid titania/silsesquioxane composite aerogel for CO2 capture. J. Sol-Gel Sci. Technol. 2017, 84, 422–431. [Google Scholar] [CrossRef]
- Kong, Y.; Shen, X.; Cui, S. Amine hybrid zirconia/silica composite aerogel for low-concentration CO2 capture. Microporous Mesoporous Mater. 2016, 236, 269–276. [Google Scholar] [CrossRef]
- Jiang, F.; Hu, S.; Hsieh, Y. Lo Aqueous Synthesis of Compressible and Thermally Stable Cellulose Nanofibril-Silica Aerogel for CO2 Adsorption. ACS Appl. Nano Mater. 2018, 1, 6701–6710. [Google Scholar] [CrossRef]
- Alhwaige, A.A.; Agag, T.; Ishida, H.; Qutubuddin, S. Biobased chitosan hybrid aerogels with superior adsorption: Role of graphene oxide in CO2 capture. RSC Adv. 2013, 3, 16011–16020. [Google Scholar] [CrossRef]
- Kong, Y.; Jiang, G.; Fan, M.; Shen, X.; Cui, S. Use of one-pot wet gel or precursor preparation and supercritical drying procedure for development of a high-performance CO2 sorbent. RSC Adv. 2014, 4, 43448–43453. [Google Scholar] [CrossRef]
- Kong, Y.; Jiang, G.; Fan, M.; Shen, X.; Cui, S.; Russell, A.G. A new aerogel based CO2 adsorbent developed using a simple sol-gel method along with supercritical drying. Chem. Commun. 2014, 50, 12158–12161. [Google Scholar] [CrossRef] [PubMed]

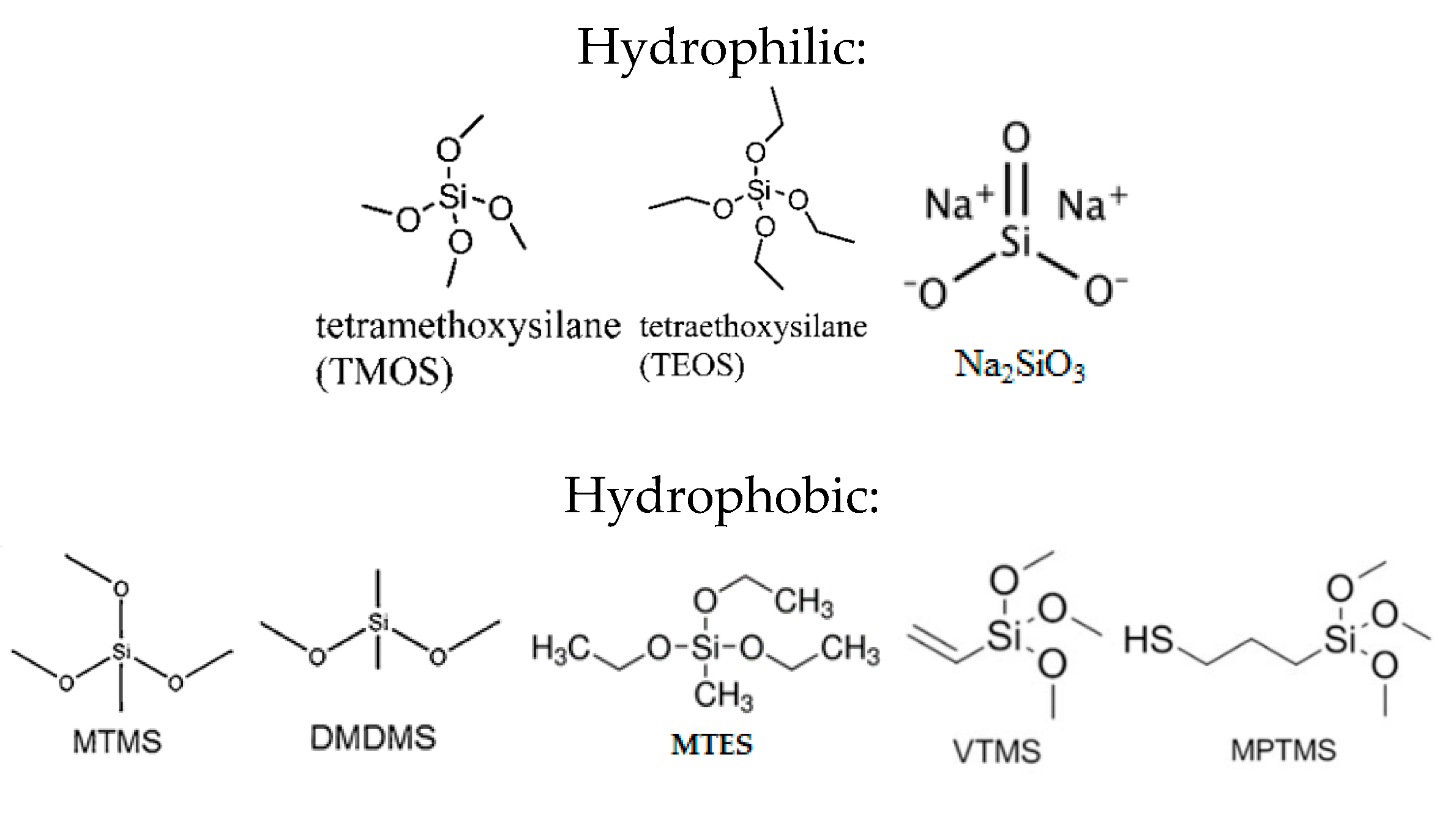


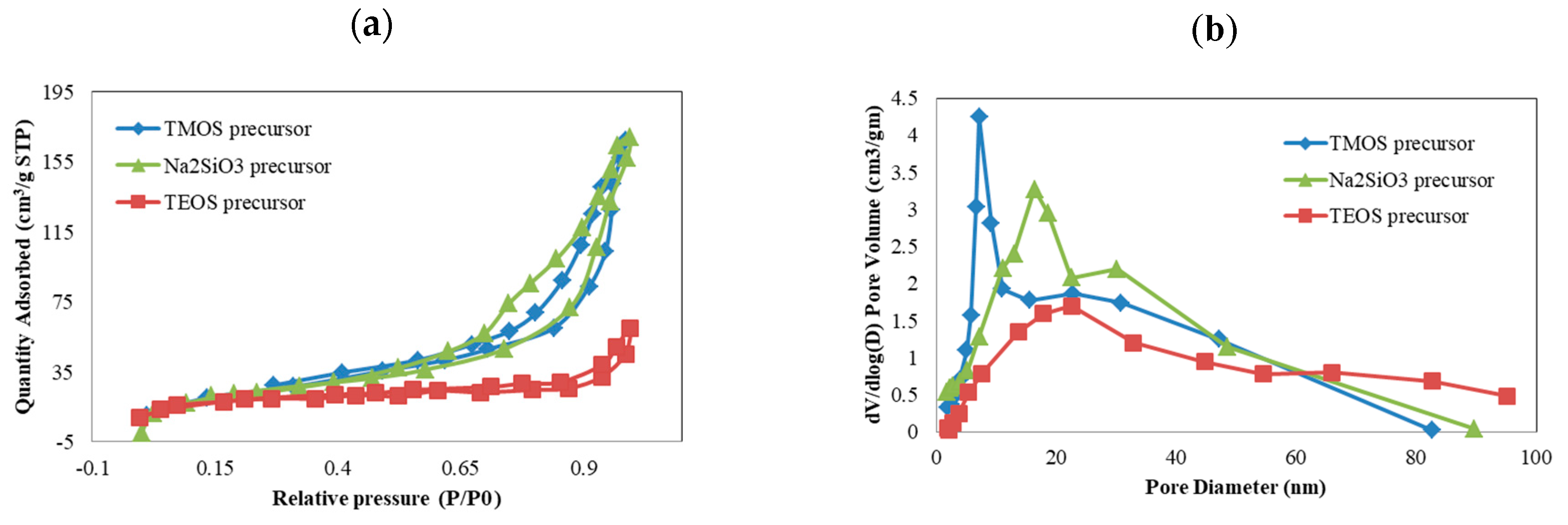
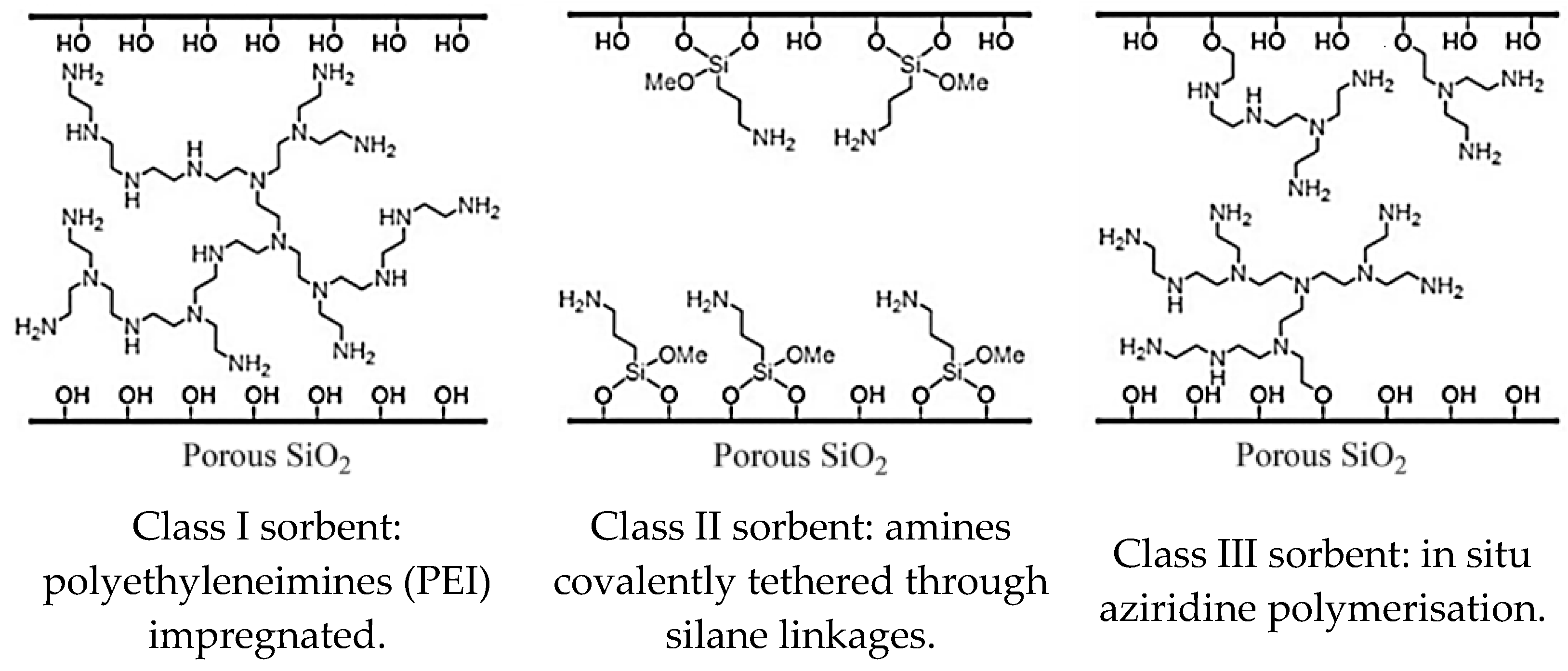

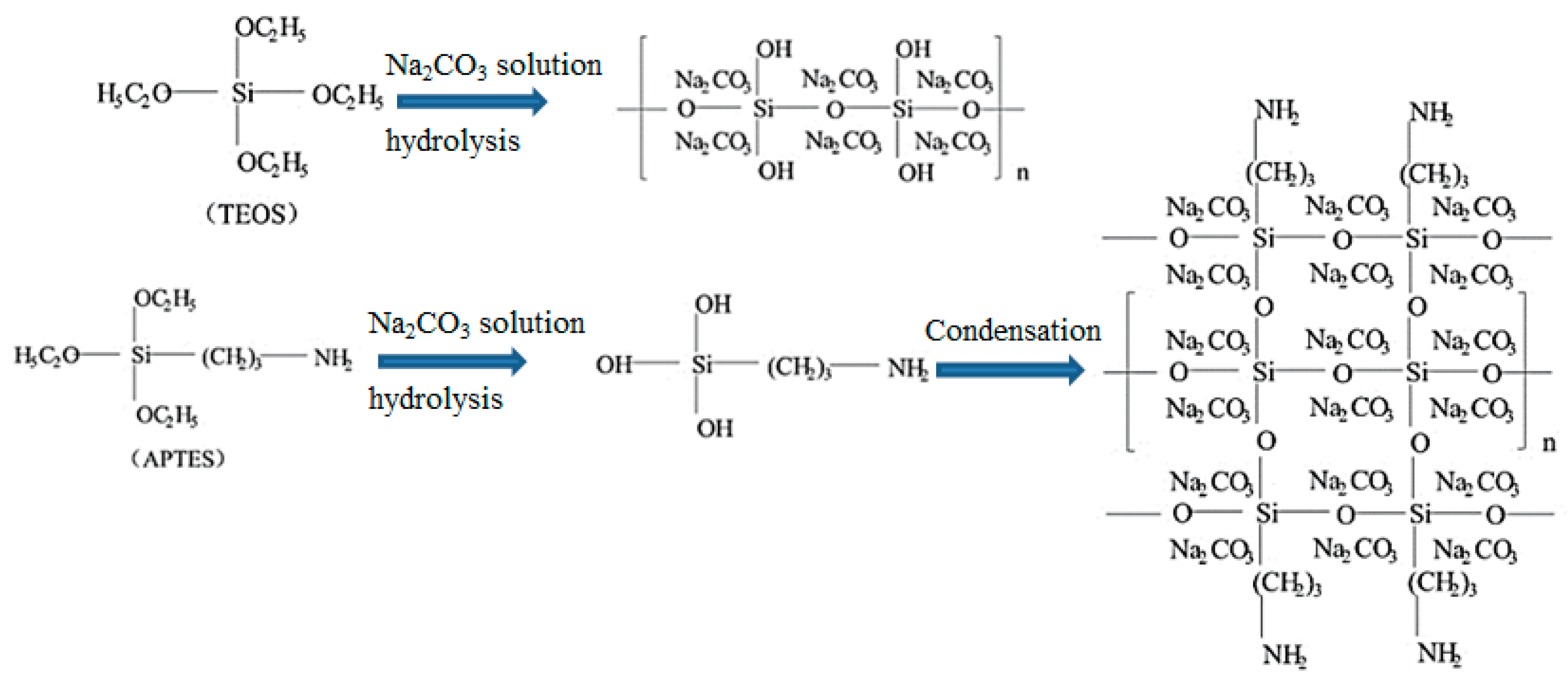

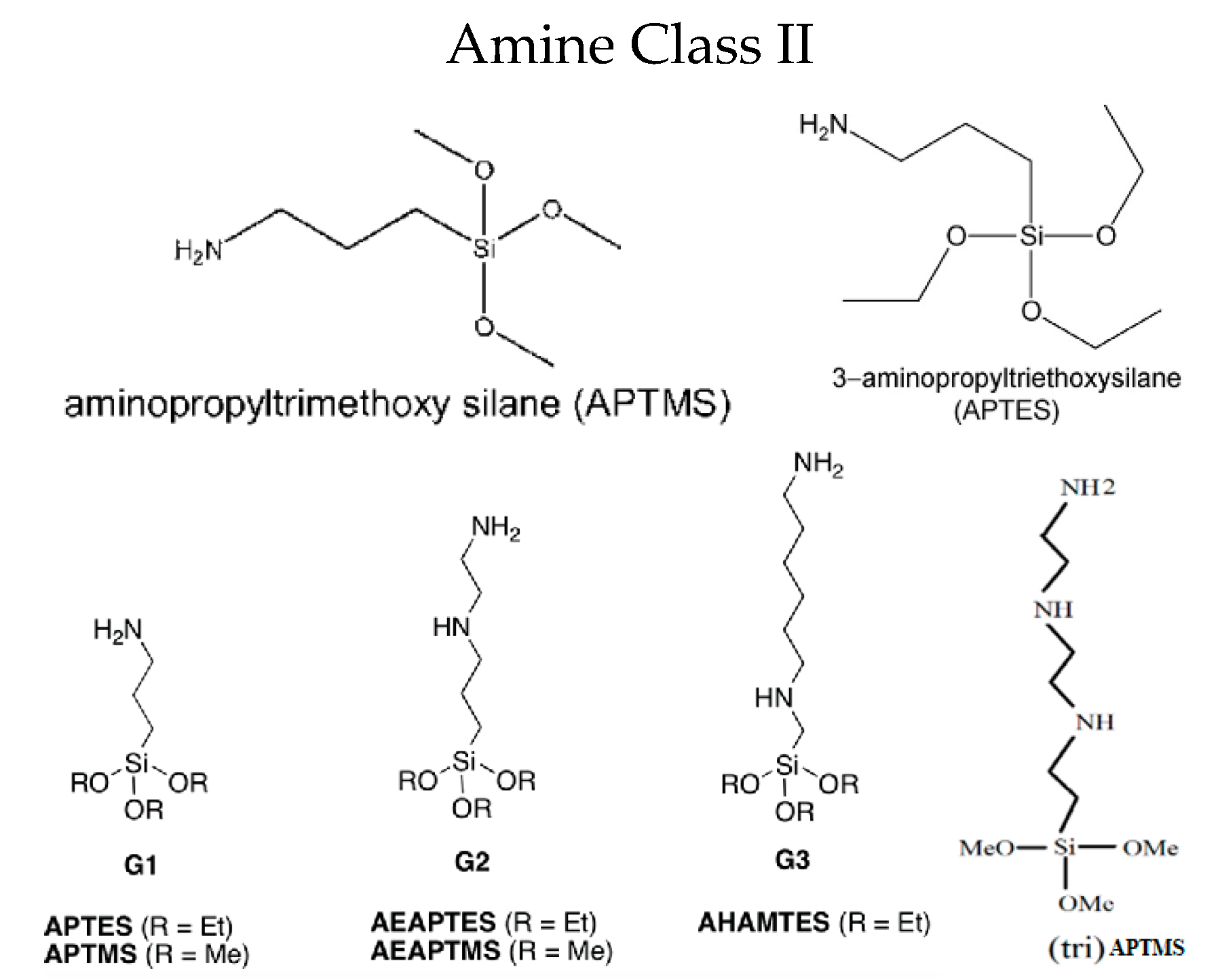

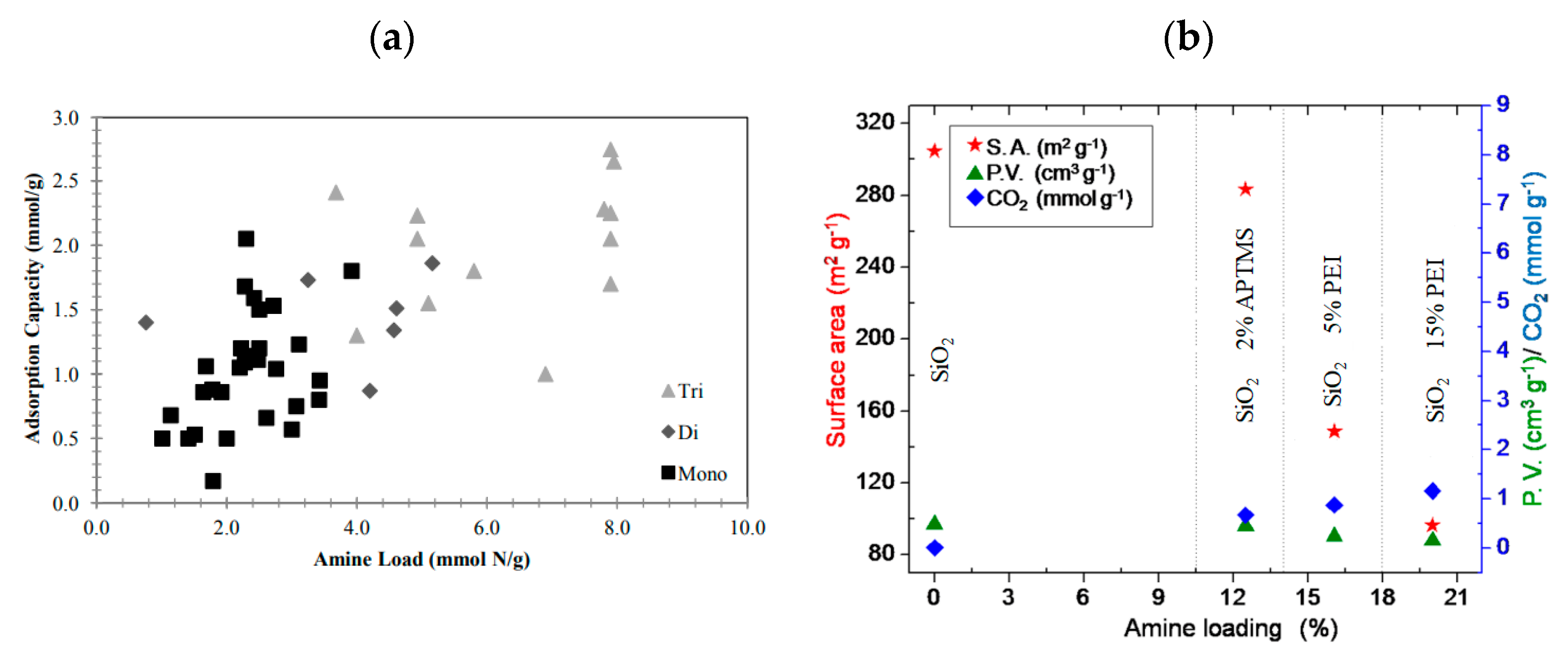
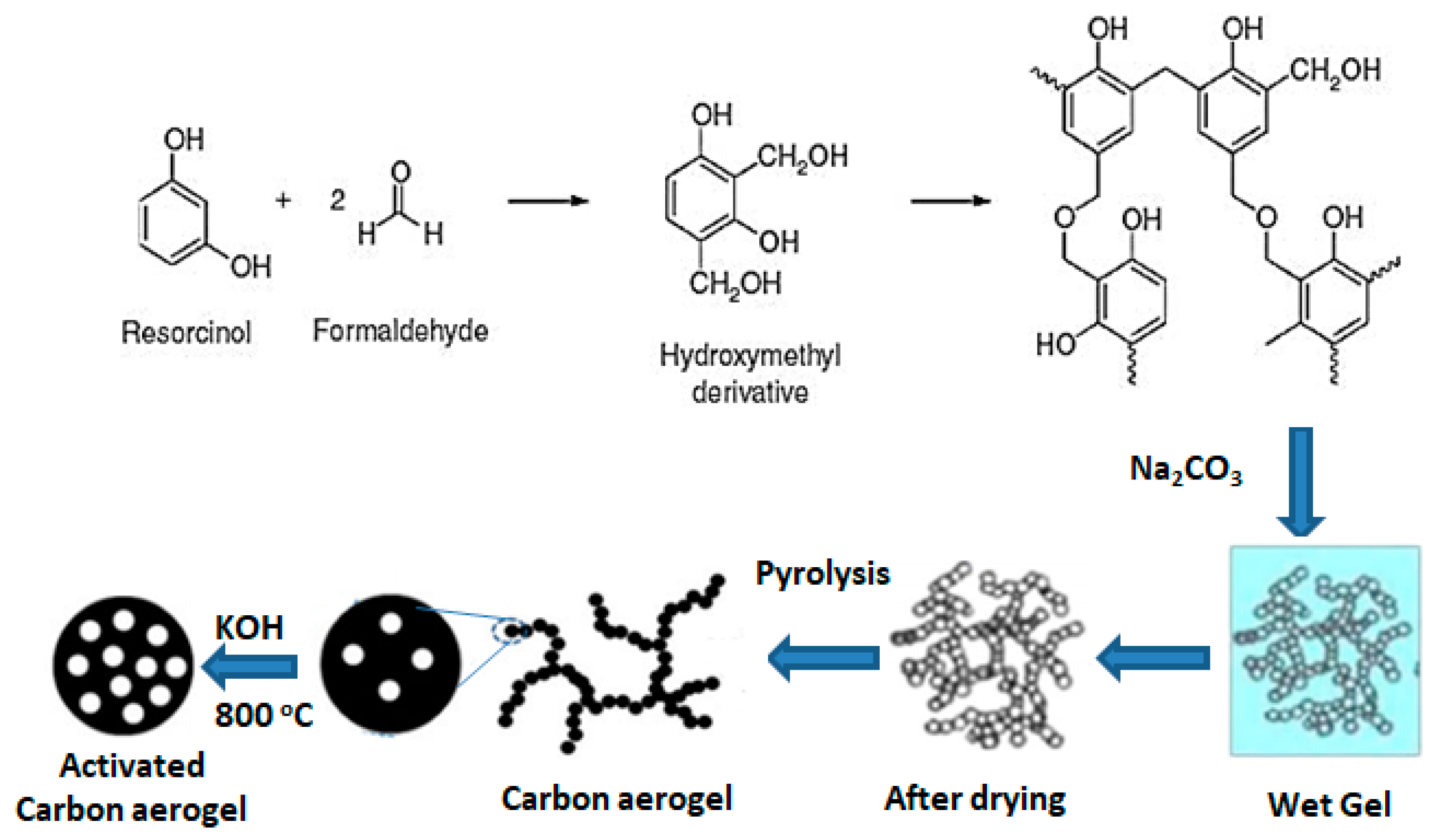
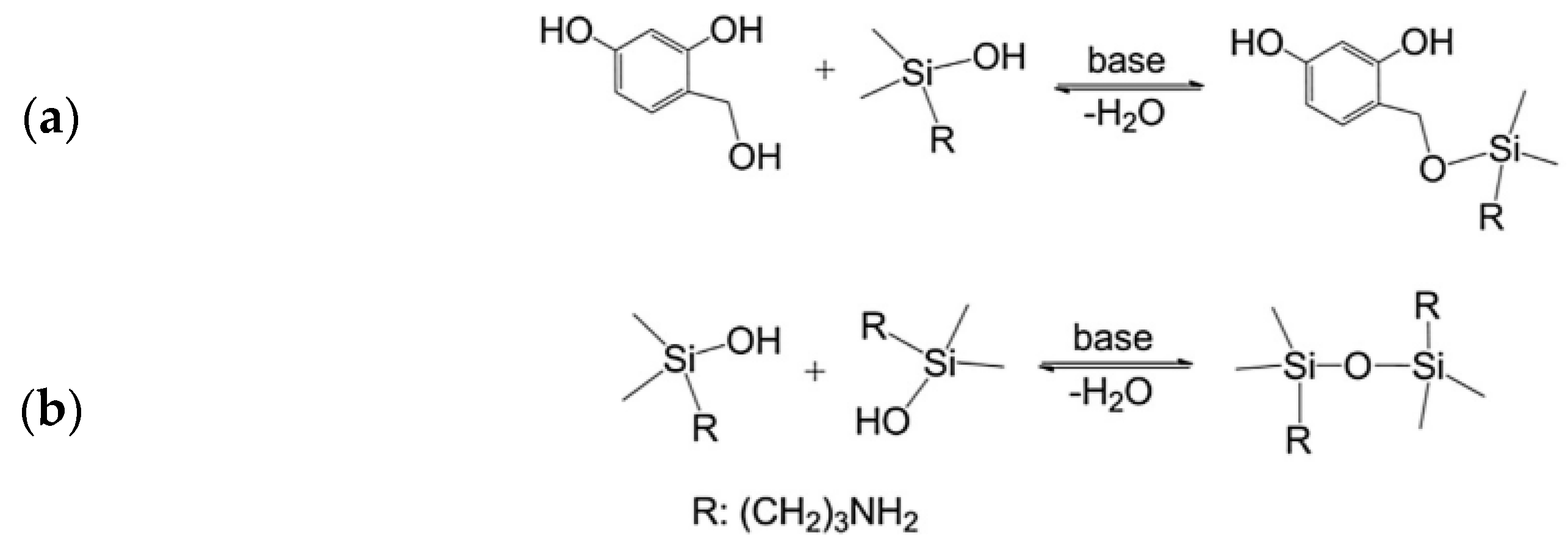
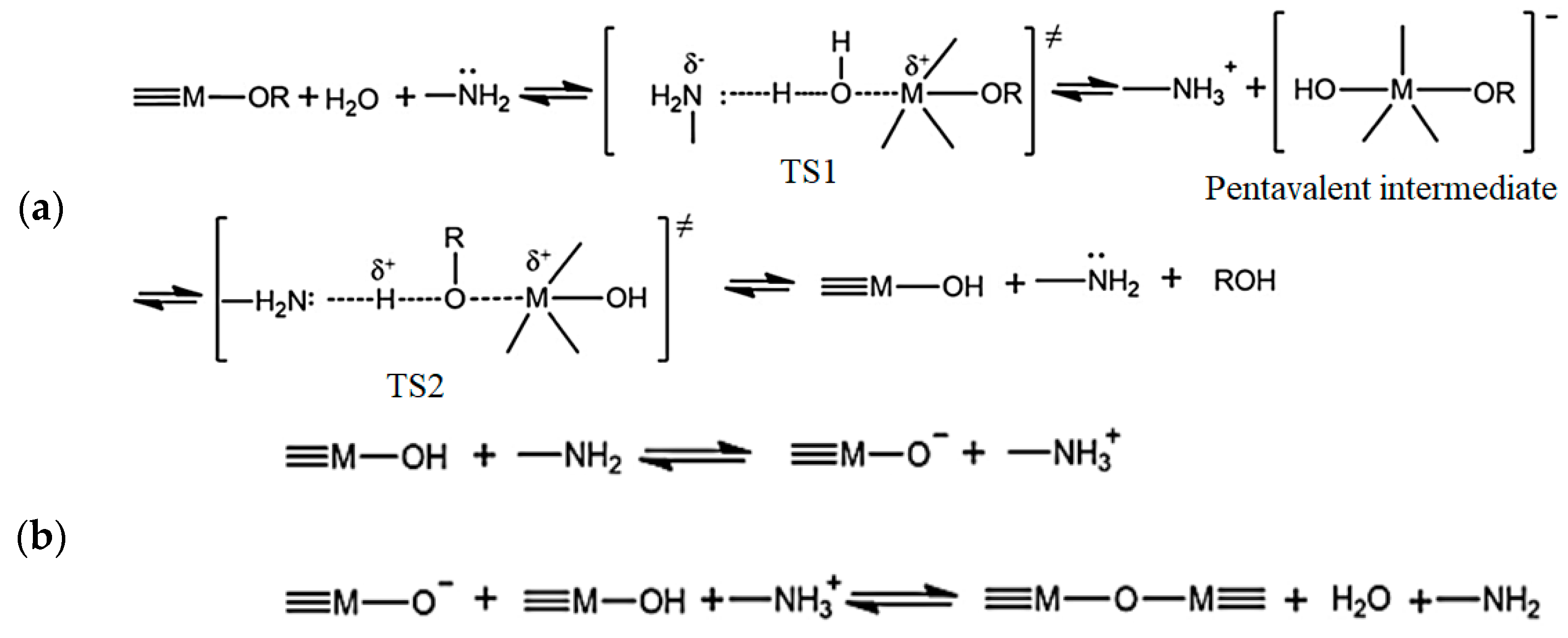
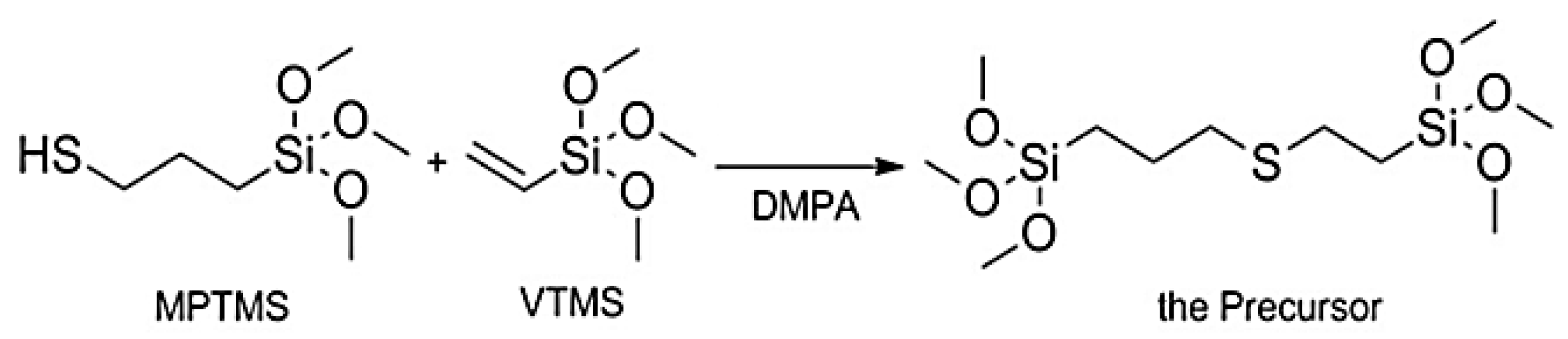
| Precursor | Amine Type, Amine Conc. [wt.%] | Modification Method | Adsorption Capacity [mmol∙g−1] & Adsorption Conditions | References |
|---|---|---|---|---|
| TEOS | TEPA, 80wt.% | Impregnation | 6.1 at 25 °C and 1 bar | [6] |
| TEOS | mono, di, and tri-amine trialkoxysilanes | Grafting | 0.67–1.64 at 25 °C, 100% CO2, 1 bar) | [52] |
| TEOS | TEPA, 80wt.% | Impregnation | 5.1 at 75 °C under a dry 10% CO2/Ar stream | [34] |
| TEOS (APTES and TEOS ratio:1:2) | APTES | Grafting | 1.95 at 25 °C and 1 bar | [38] |
| TMOS | APTMS 2 wt.%/ PEI 15 wt.% | Grafting/ Impregnation | 0.67 1.16 at 50 °C | [48] |
| TMOS | APTMS, 1.44 wt.% | co- condensation & post treatment | 0.523 at 250 Pa | [45] |
| TEOS | Mono-APTMS Tri-APTMS | Grafting | 0.52–1.07 at 250 Pa moist air | [46] |
| MTMS (MTMS and APTES:1:1), DMDMS 4 nmol | APTES: 1 mol ratio | One-pot | 6.45 at 1 bar of dry CO2 | [37] |
| TEOS | APTES, 24 wt.% + IL, 28 wt.% | One-pot | 5.53 at 25 °C and 1 bar | [41] |
| TEOS | PEI, 45wt.% | Grafting | 1.8 at 25 °C and 1 bar | [11] |
| TEOS, Na2CO3 (mole ratio:1:1) | APTES: 1 mol ratio | Direct | 2.51 at 50 °C (H2O/CO2 = 1:1) | [29] |
| Na2SiO3 coated on Al2O3 membrane | Fluoroalkylsilane (FAS) | Grafting | 1.5 at 25 °C and 1 bar | [24] |
| Precursor | Carbonization/Activation | Adsorption Capacity (mmol∙g−1) | References |
|---|---|---|---|
| RF | Carbonized at 1050 °C | 6.43 at 0 °C, 1 bar | [56] |
| RF/CTAB | Carbonized at 800 °C | 4.19 at 0 °C and atmospheric pressure | [75] |
| DHBA/F | AMPD | 1.59 at 35 °C and atmospheric pressure | [8] |
| PAB/TFB | N-doped Pyrolysis at 1000 °C | 6.1 at 0 °C, 1 bar, 33.1 at 50 °C, 30 bar | [84] |
| RF | Activation at 800 °C (KOH carbon ratio of 5) | 2.7–3.0 at 25 °C, 1 bar | [76] |
| MMT-CTS- PBZ | Pyrolysis at at 800 °C | 5.72 at 25 °C, 1 bar | [85] |
| RF | N-doped, Melamine | 2.68 at 25 °C, 1 bar | [81] |
| carbon nanotubes/PVA | 75–83% PEI | 3.3 ± 0.3 at 25 °C, 1 bar | [63] |
| PF, RF, FPOL and TPOL | Pyrolytic at 800 °C Etching at 1000 °C | 14.8 ± 3.9 at 25 °C, 1 bar | [61] |
| DHBA/F RF | Carbonisation at 800 °C | 1.2–2.14 at 25 °C, 1 bar | [57] |
| RF | N-doped, Urea Carbonisation at 800 °C | 3.6 at 25 °C and 4.5 at 25 °C, 1 bar | [53] |
| Melamine/F | Activation at 800 °C KOH/carbon ratio = 2 | 2.2 at 25 °C, 1 bar | [58] |
| Cellulose | N-doped, Urea Activation at 800 °C | 3.42 at 25 °C, 1 bar | [77] |
| RF | Impregnation of K2CO3 Carbonisation at 900 °C | 2.45 at 25 °C, 1 bar | [80] |
| RF/CTAB | Activation at 900 °C KOH (ratio to CA: 1), 55 wt.% PEI 60 wt.% TEPA | 2.06–2.84 at 75 °C, 1 bar | [55] |
| Precursor | Activating Group | Adsorption Capacity (mmol∙g−1) | References |
|---|---|---|---|
| CTS–GO (hybrid monolith aerogels of chitosan-Graphene oxide) aerogel | - | 1.92–4.15 | [98] |
| AHSA (Monolithic + SiC) | APTES | 2.69 at 30 °C | [94,99,100] |
| AHTSA titania + silsesquioxane | APTES | 4.19 at 30 °C | [95] |
| AHZSA Zirconia + Silica | APTES | 2.7 at 30 °C | [96] |
| AH-RFSA | APTES | 3.57 at 30 °C | [78,92] |
| AFMSiCA | APTES | 1.81 at 25 °C | [93] |
| CNT + PVA | APTES | 3.3 at 25 °C | [63] |
| MMPTMS, VTMS | PEI 0.35 mol∙L−1, Impregnation | 3.3 at 25 °C | [33] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keshavarz, L.; Ghaani, M.R.; English, N.J. The Importance of Precursors and Modification Groups of Aerogels in CO2 Capture. Molecules 2021, 26, 5023. https://doi.org/10.3390/molecules26165023
Keshavarz L, Ghaani MR, English NJ. The Importance of Precursors and Modification Groups of Aerogels in CO2 Capture. Molecules. 2021; 26(16):5023. https://doi.org/10.3390/molecules26165023
Chicago/Turabian StyleKeshavarz, Leila, Mohammad Reza Ghaani, and Niall J. English. 2021. "The Importance of Precursors and Modification Groups of Aerogels in CO2 Capture" Molecules 26, no. 16: 5023. https://doi.org/10.3390/molecules26165023
APA StyleKeshavarz, L., Ghaani, M. R., & English, N. J. (2021). The Importance of Precursors and Modification Groups of Aerogels in CO2 Capture. Molecules, 26(16), 5023. https://doi.org/10.3390/molecules26165023








