Characterization Techniques as Supporting Tools for the Interpretation of Biochar Adsorption Efficiency in Water Treatment: A Critical Review
Abstract
:1. Introduction
2. Activated Carbon and Biochar: The Different Approach to Characterization
2.1. Activated Carbon Characterization
2.1.1. Physical Tests
2.1.2. Adsorption Tests
2.1.3. Chemical and Physicochemical Tests
2.2. Biochar Characterization
2.2.1. Chemical and Physicochemical Tests
| Test | Ref Biochar |
|---|---|
| Adsorption Indices | |
| Iodine number | [56,76,86,87,88] |
| Methylene Blue number | [56,76,89] |
| Chemical Tests | |
| Total PAHs | [90] |
| pH, ash | [56,89] |
| Surface and Functional Groups Characterization | |
| Nitrogen-adsorption isotherm | [30,56,71,91,92] |
| FTIR | [56,89,92,93,94] |
| SEM | [92,94,95] |
| XRD | [56,92,93,95] |
| Zero-point charge | [56,93,94,96] |
| Boehm’s titration | [89] |
| Cation exchange capacity | [89] |
| Elemental Composition | |
| H/C, O/C, (O + N)/C (Van Krevelen plots) | [56,84,85,95] |
| Kinetic and isotherm studies | [56,97,98,99,100] |
2.2.2. Adsorption Tests
2.2.3. Adsorption Tests in Dynamic Bench Scale and Pilot Scale Conditions
2.2.4. Leaching Tests
3. Biochar as Adsorbent for Organic Micropollutants
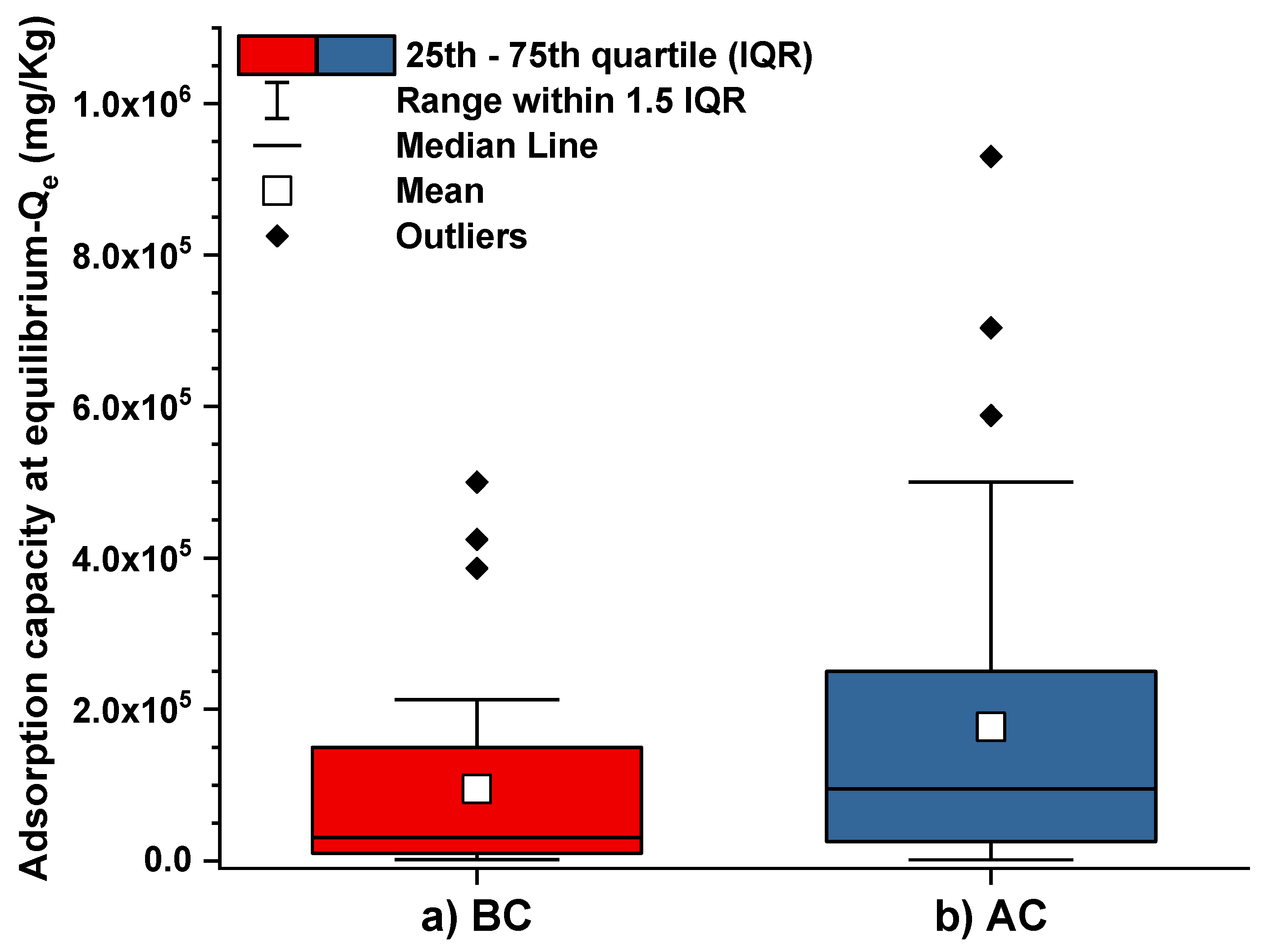
3.1. Typical Target Pollutants and Performance Capabilities
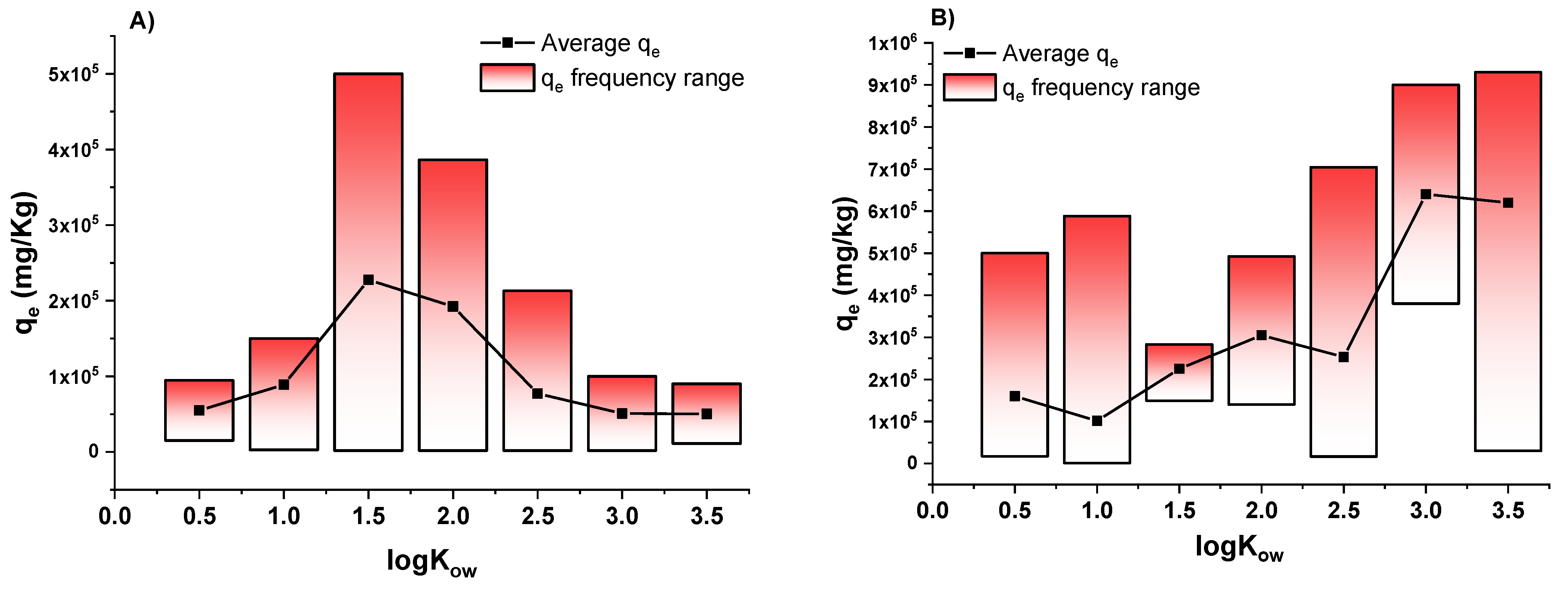
3.2. Effect of Hydrophobic Character of Analytes on Biochar Adsorption Efficiencies
4. Considerations on the Correlations of Physicochemical and Performance Indices with Biochar Adsorption Efficiencies through Chemometric Approaches
5. Economic Evaluation on Biochar Use over Activated Carbon
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Colantoni, A.; Evic, N.; Lord, R.; Retschitzegger, S.; Proto, A.R.; Gallucci, F.; Monarca, D. Characterization of biochars produced from pyrolysis of pelletized agricultural residues. Renew. Sustain. Energy Rev. 2016, 64, 187–194. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Hu, Y.; Zhao, X.; Wang, S.; Xing, G. Comparisons of Biochar Properties from Wood Material and Crop Residues at Different Temperatures and Residence Times. Energy Fuels 2013, 27, 5890–5899. [Google Scholar] [CrossRef]
- Cao, X.; Harris, W. Properties of dairy-manure-derived biochar pertinent to its potential use in remediation. Bioresour. Technol. 2010, 101, 5222–5228. [Google Scholar] [CrossRef]
- Méndez, A.; Cárdenas-Aguiar, E.; Paz-Ferreiro, J.; Plaza, C.; Gascó, G. The effect of sewage sludge biochar on peat-based growing media. Biol. Agric. Hortic. 2017, 33, 40–51. [Google Scholar] [CrossRef]
- DeSisto, W.J.; Hill, N.; Beis, S.H.; Mukkamala, S.; Joseph, J.; Baker, C.; Ong, T.-H.; Stemmler, E.A.; Wheeler, M.C.; Frederick, B.G. Fast pyrolysis of pine sawdust in a fluidized-bed reactor. Energy Fuels 2010, 24, 2642–2651. [Google Scholar] [CrossRef]
- Ahmad, M.; Lee, S.S.; Dou, X.; Mohan, D.; Sung, J.-K.; Yang, J.E.; Ok, Y.S. Effects of pyrolysis temperature on soybean stover-and peanut shell-derived biochar properties and TCE adsorption in water. Bioresour. Technol. 2012, 118, 536–544. [Google Scholar] [CrossRef]
- Brewer, C.E.; Schmidt-Rohr, K.; Satrio, J.A.; Brown, R.C. Characterization of biochar from fast pyrolysis and gasification systems. Environ. Prog. Sustain. Energy 2009, 28, 386–396. [Google Scholar] [CrossRef]
- Rillig, M.C.; Wagner, M.; Salem, M.; Antunes, P.M.; George, C.; Ramke, H.-G.; Titirici, M.-M.; Antonietti, M. Material derived from hydrothermal carbonization: Effects on plant growth and arbuscular mycorrhiza. Appl. Soil Ecol. 2010, 45, 238–242. [Google Scholar] [CrossRef]
- Antal, M.J.; Mochidzuki, K.; Paredes, L.S. Flash carbonization of biomass. Ind. Eng. Chem. Res. 2003, 42, 3690–3699. [Google Scholar] [CrossRef]
- Meyer, S.; Glaser, B.; Quicker, P. Technical, economical, and climate-related aspects of biochar production technologies: A literature review. Environ. Sci. Technol. 2011, 45, 9473–9483. [Google Scholar] [CrossRef]
- Mohan, D.; Sarswat, A.; Ok, Y.S.; Pittman, C.U., Jr. Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable adsorbent—A critical review. Bioresour. Technol. 2014, 160, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Woolf, D.; Amonette, J.E.; Street-Perrott, F.A.; Lehmann, J.; Joseph, S. Sustainable biochar to mitigate global climate change. Nat. Commun. 2010, 1, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Q.; Jung, J.; Chen, D.; Leong, K.; Song, S.; Li, F.; Mohan, B.C.; Yao, Z.; Prabhakar, A.K.; Lin, X.H. Biochar industry to circular economy. Sci. Total Environ. 2021, 757, 143820. [Google Scholar] [CrossRef] [PubMed]
- Khawkomol, S.; Neamchan, R.; Thongsamer, T.; Vinitnantharat, S.; Panpradit, B.; Sohsalam, P.; Werner, D.; Mrozik, W. Potential of Biochar Derived from Agricultural Residues for Sustainable Management. Sustainability 2021, 13, 8147. [Google Scholar] [CrossRef]
- Pan, S.-Y.; Dong, C.-D.; Su, J.-F.; Wang, P.-Y.; Chen, C.-W.; Chang, J.-S.; Kim, H.; Huang, C.-P.; Hung, C.-M. The Role of Biochar in Regulating the Carbon, Phosphorus, and Nitrogen Cycles Exemplified by Soil Systems. Sustainability 2021, 13, 5612. [Google Scholar] [CrossRef]
- Atkinson, C.J.; Fitzgerald, J.D.; Hipps, N.A. Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: A review. Plant Soil 2010, 337, 1–18. [Google Scholar] [CrossRef]
- Thompson, K.A.; Shimabuku, K.K.; Kearns, J.P.; Knappe, D.R.; Summers, R.S.; Cook, S.M. Environmental comparison of biochar and activated carbon for tertiary wastewater treatment. Environ. Sci. Technol. 2016, 50, 11253–11262. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef]
- Abbas, Z.; Ali, S.; Rizwan, M.; Zaheer, I.E.; Malik, A.; Riaz, M.A.; Shahid, M.R.; ur Rehman, M.Z.; Al-Wabel, M.I. A critical review of mechanisms involved in the adsorption of organic and inorganic contaminants through biochar. Arab. J. Geosci. 2018, 11, 448. [Google Scholar] [CrossRef]
- Dai, Y.; Zhang, N.; Xing, C.; Cui, Q.; Sun, Q. The adsorption, regeneration and engineering applications of biochar for removal organic pollutants: A review. Chemosphere 2019, 223, 12–27. [Google Scholar] [CrossRef]
- Gong, H.; Tan, Z.; Zhang, L.; Huang, Q. Preparation of biochar with high absorbability and its nutrient adsorption–desorption behaviour. Sci. Total Environ. 2019, 694, 133728. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhu, X.; He, H.; Fang, Y.; Dong, H.; Lü, J.; Li, J.; Li, Y. Adsorption of two antibiotics on biochar prepared in air-containing atmosphere: Influence of biochar porosity and molecular size of antibiotics. J. Mol. Liq. 2019, 274, 353–361. [Google Scholar] [CrossRef]
- Ni, B.-J.; Huang, Q.-S.; Wang, C.; Ni, T.-Y.; Sun, J.; Wei, W. Competitive adsorption of heavy metals in aqueous solution onto biochar derived from anaerobically digested sludge. Chemosphere 2019, 219, 351–357. [Google Scholar] [CrossRef]
- Cheng, N.; Wang, B.; Wu, P.; Lee, X.; Xing, Y.; Chen, M.; Gao, B. Adsorption of emerging contaminants from water and wastewater by modified biochar: A review. Environ. Pollut. 2021, 273, 116448. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.S.; Geza, M.; Vasquez, R.; Chilkoor, G.; Gadhamshetty, V. Enhanced heavy metal removal from synthetic stormwater using nanoscale zerovalent iron–modified biochar. Water Air Soil Pollut. 2020, 231, 1–15. [Google Scholar] [CrossRef]
- Yaashikaa, P.; Kumar, P.S.; Varjani, S.; Saravanan, A. A critical review on the biochar production techniques, characterization, stability and applications for circular bioeconomy. Biotechnol. Rep. 2020, 28, e00570. [Google Scholar] [CrossRef]
- Yargicoglu, E.N.; Sadasivam, B.Y.; Reddy, K.R.; Spokas, K. Physical and chemical characterization of waste wood derived biochars. Waste Manag. 2015, 36, 256–268. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Luo, L.; Deng, S.; Shi, G.; Zhang, S.; Zhang, Y.; Deng, O.; Wang, L.; Zhang, J.; Wei, L. Sorption of tetracycline on H3PO4 modified biochar derived from rice straw and swine manure. Bioresour. Technol. 2018, 267, 431–437. [Google Scholar] [CrossRef]
- Qian, W.C.; Luo, X.P.; Wang, X.; Guo, M.; Li, B. Removal of methylene blue from aqueous solution by modified bamboo hydrochar. Ecotoxicol. Environ. Saf. 2018, 157, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Meas, A.; Shan, S.; Yang, R.; Gai, X.; Wang, H.; Tsend, N. Hydrochars from bamboo sawdust through acid assisted and two-stage hydrothermal carbonization for removal of two organics from aqueous solution. Bioresour. Technol. 2018, 261, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Johir, M.A.H.; Sornalingam, K. Single and competitive sorption properties and mechanism of functionalized biochar for removing sulfonamide antibiotics from water. Chem. Eng. J. 2017, 311, 348–358. [Google Scholar] [CrossRef]
- Wei, Z.; Qi-Gang, C.; Wan-Dong, L.; Bing-Jing, L.; Wen-Xin, J.; Li-Jun, F.; Wei-Chi, Y. Selecting activated carbon for water and wastewater treatability studies. Environ. Prog. 2007, 26, 289–298. [Google Scholar]
- European Chemical Industry Council (CEFIC). Test Methods for Activated Carbon; European Council of Chemical Manufacturers’ Federation (CEFIC): Bruxelles, Belgium, 1986. [Google Scholar]
- ASTM D2854–09, Standard Test Method for Apparent Density of Activated Carbon; ASTM International: West Conshohocken, PA, USA, 2014; Available online: www.astm.org (accessed on 13 April 2021).
- ASTM D2862–16, Standard Test Method for Particle Size Distribution of Granular Activated Carbon; ASTM International: West Conshohocken, PA, USA, 2016; Available online: www.astm.org (accessed on 13 April 2021).
- ASTM D3802–16, Standard Test Method for Ball-Pan Hardness of Activated Carbon; ASTM International: West Conshohocken, PA, USA, 2016; Available online: www.astm.org (accessed on 13 April 2021).
- AWWA B604, Standard for Granular Activated Carbon; American Water Works Association: Denver, CO, USA, 2012; Available online: www.awwa.org (accessed on 13 April 2021).
- ASTM D3860-98, Standard Practice for Determination of Adsorptive Capacity of Activated Carbon by Aqueous Phase Isotherm Technique; ASTM International: West Conshohocken, PA, USA, 2014; Available online: www.astm.org (accessed on 13 April 2021).
- ASTM D 5919-96, Standard Practice for Determination of Adsorptive Capacity of Activated Carbon by a Micro-Isotherm Technique for Adsorbates at ppb Concentrations; ASTM International: West Conshohocken, PA, USA, 2017; Available online: www.astm.org (accessed on 13 April 2021).
- EN 12915-1, Products Used for the Treatment of Water Intended for Human Consumption-Granular Activated Carbon-Part 1: Virgin Granular Activated Carbon; European Committee for Standardization: Brussels, Belgium, 2009; Available online: www.cen.eu (accessed on 13 April 2021).
- AWWA B600-16, Powdered Activated Carbon; American Water Works Association: Denver, CO, USA, 2016; Available online: www.awwa.org (accessed on 13 April 2021).
- ASTM D4607-14, Standard Test Method for Determination of Iodine Number of Activated Carbon; ASTM International: West Conshohocken, PA, USA, 2014; Available online: www.astm.org (accessed on 13 April 2021).
- EPA 625171002A, Process Design Manual for Carbon Adsorption; U.S. Environmental Protection Agency: Washington, DC, USA, 1973. Available online: www.epa.gov (accessed on 13 April 2021).
- ASTM D3838-05, Standard Test Method for pH of Activated Carbon; ASTM International: West Conshohocken, PA, USA, 2017; Available online: www.astm.org (accessed on 13 April 2021).
- ASTM D6851-02, Standard Test Method for Determination of Contact pH with Activated Carbon; ASTM International: West Conshohocken, PA, USA, 2011; Available online: www.astm.org (accessed on 13 April 2021).
- Plantard, G.; Goetz, V.; Py, X. A direct method for porous particle density characterization applied to activated carbons. Adv. Powder Technol. 2010, 21, 592–598. [Google Scholar] [CrossRef]
- Ahmedna, M.; Johns, M.; Clarke, S.; Marshall, W.; Rao, R. Potential of agricultural by-product-based activated carbons for use in raw sugar decolourisation. J. Sci. Food Agric. 1997, 75, 117–124. [Google Scholar] [CrossRef]
- Caturla, F.; Molina-Sabio, M.; Rodriguez-Reinoso, F. Preparation of activated carbon by chemical activation with ZnCl2. Carbon 1991, 29, 999–1007. [Google Scholar] [CrossRef]
- Rodriguez-Reinoso, F.; Molina-Sabio, M. Activated carbons from lignocellulosic materials by chemical and/or physical activation: An overview. Carbon 1992, 30, 1111–1118. [Google Scholar] [CrossRef]
- Malusis, M.A.; Barben, E.J.; Evans, J.C. Hydraulic conductivity and compressibility of soil-bentonite backfill amended with activated carbon. J. Geotech. Geoenviron. Eng. 2009, 135, 664–672. [Google Scholar] [CrossRef]
- Achaw, O.-W. A study of the porosity of activated carbons using the scanning electron microscope. In Scanning Electron Microscopy; InTech: London, UK, 2012. [Google Scholar]
- EN 12902, Products Used for Treatment of Water Intended for Human Consumption. Inorganic Supporting and Filtering Materials; European Committee for Standardization: Brussels, Belgium, 2004.
- Nunes, C.A.; Guerreiro, M.C. Estimation of surface area and pore volume of activated carbons by methylene blue and iodine numbers. Química Nova 2011, 34, 472–476. [Google Scholar] [CrossRef] [Green Version]
- ASTM D 1783-70, Phenolic Compounds in Water; ASTM International: West Conshohocken, PA, USA, 2017; Available online: www.astm.org (accessed on 13 April 2021).
- Water Research Commission. WRC Report No. K5/1124; Water Research Commission: Pretoria, South Africa, 2003. [Google Scholar]
- Del Bubba, M.; Anichini, B.; Bakari, Z.; Bruzzoniti, M.C.; Camisa, R.; Caprini, C.; Checchini, L.; Fibbi, D.; El Ghadraoui, A.; Liguori, F. Physicochemical properties and sorption capacities of sawdust-based biochars and commercial activated carbons towards ethoxylated alkylphenols and their phenolic metabolites in effluent wastewater from a textile district. Sci. Total Environ. 2020, 708, 135217. [Google Scholar] [CrossRef]
- Wu, Y.; Xia, C.; Cai, L.; Shi, S.Q. Controlling pore size of activated carbon through self-activation process for removing contaminants of different molecular sizes. J. Colloid Interface Sci. 2018, 518, 41–47. [Google Scholar] [CrossRef]
- Wu, M.; Guo, Q.; Fu, G. Preparation and characteristics of medicinal activated carbon powders by CO2 activation of peanut shells. Powder Technol. 2013, 247, 188–196. [Google Scholar] [CrossRef]
- Ronowicz, J.; Kupcewicz, B.; Pałkowski, L.; Krysiński, J. Development and optimization of the activated charcoal suspension composition based on a mixture design approach. Acta Pharm. 2015, 65, 83–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flament, M.; M’bisi, R.; Leterme, P.; Gayot, A. Formulation of activated charcoal for per os administration to addicted subjects. Drug Dev. Ind. Pharm. 2000, 26, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Freddo, A.; Cai, C.; Reid, B.J. Environmental contextualisation of potential toxic elements and polycyclic aromatic hydrocarbons in biochar. Environ. Pollut. 2012, 171, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, M.; Bachmann, R.; Rafiq, M.T.; Shang, Z.; Joseph, S.; Long, R. Influence of Pyrolysis Temperature on Physico-Chemical Properties of Corn Stover (Zea mays L.) Biochar and Feasibility for Carbon Capture and Energy Balance. PLoS ONE 2016, 11, e0156894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- To, M.H.; Hadi, P.; Hui, C.W.; Lin, C.S.K.; McKay, G. Mechanistic study of atenolol, acebutolol and carbamazepine adsorption on waste biomass derived activated carbon. J. Mol. Liq. 2017, 241, 386–398. [Google Scholar] [CrossRef]
- Contescu, A.; Contescu, C.; Putyera, K.; Schwarz, J.A. Surface acidity of carbons characterized by their continuous pK distribution and Boehm titration. Carbon 1997, 35, 83–94. [Google Scholar] [CrossRef]
- Youssef, A.; El-Nabarawy, T.; Samra, S. Sorption properties of chemically-activated carbons: 1. Sorption of cadmium (II) ions. Colloids Surf. A Physicochem. Eng. Asp. 2004, 235, 153–163. [Google Scholar] [CrossRef]
- Munera-Echeverri, J.L.; Martinsen, V.; Strand, L.T.; Zivanovic, V.; Cornelissen, G.; Mulder, J. Cation exchange capacity of biochar: An urgent method modification. Sci. Total Environ. 2018, 642, 190–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Qiu, K. Preparation of activated carbons from walnut shells via vacuum chemical activation and their application for methylene blue removal. Chem. Eng. J. 2010, 165, 209–217. [Google Scholar] [CrossRef]
- Van Krevelen, D. Graphical-statistical method for the study of structure and reaction processes of coal. Fuel 1950, 29, 269–284. [Google Scholar]
- Benstoem, F.; Becker, G.; Firk, J.; Kaless, M.; Wuest, D.; Pinnekamp, J.; Kruse, A. Elimination of micropollutants by activated carbon produced from fibers taken from wastewater screenings using hydrothermal carbonization. J. Environ. Manag. 2018, 211, 278–286. [Google Scholar] [CrossRef]
- Tan, X.; Liu, Y.; Zeng, G.; Wang, X.; Hu, X.; Gu, Y.; Yang, Z. Application of biochar for the removal of pollutants from aqueous solutions. Chemosphere 2015, 125, 70–85. [Google Scholar] [CrossRef]
- Fan, S.; Wang, Y.; Wang, Z.; Tang, J.; Tang, J.; Li, X. Removal of methylene blue from aqueous solution by sewage sludge-derived biochar: Adsorption kinetics, equilibrium, thermodynamics and mechanism. J. Environ. Chem. Eng. 2017, 5, 601–611. [Google Scholar] [CrossRef]
- Li, R.; Wang, Z.; Guo, J.; Li, Y.; Zhang, H.; Zhu, J.; Xie, X. Enhanced adsorption of ciprofloxacin by KOH modified biochar derived from potato stems and leaves. Water Sci. Technol. 2018, 77, 1127–1136. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Tian, Y.; Yin, L.; Zhang, J.; Drewes, J.E. Insight into the effects of biochar as adsorbent and microwave receptor from one-step microwave pyrolysis of sewage sludge. Environ. Sci. Pollut. Res. 2018, 25, 18424–18433. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Bergman, R.; Anderson, N.; Alanya-Rosenbaum, S. Life cycle assessment of activated carbon from woody biomass. Wood Fiber Sci. 2018, 50, 229–243. [Google Scholar] [CrossRef] [Green Version]
- Gu, J.; Zhou, J.; Ma, H.; Ma, M.; Xing, M. Characteristics of camellia shell pyrolysis products and optimization of preparation parameters of activated carbon. Nongye Gongcheng Xuebao Trans. Chin. Soc. Agric. Eng. 2015, 31, 233–239. [Google Scholar]
- Strubinger, A.; Oliveros, A.R.; Araque, M.A.; Guerra, J. Assessment of the energy recovery of Aloe Vera solid residues by pyrolysis and hydrothermal conversion. Chem. Eng. Trans. 2017, 57, 19–24. [Google Scholar]
- Yin, Z.; Liu, Y.; Liu, S.; Jiang, L.; Tan, X.; Zeng, G.; Li, M.; Liu, S.; Tian, S.; Fang, Y. Activated magnetic biochar by one-step synthesis: Enhanced adsorption and coadsorption for 17β-estradiol and copper. Sci. Total Environ. 2018, 639, 1530–1542. [Google Scholar] [CrossRef]
- Igalavithana, A.D.; Mandal, S.; Niazi, N.K.; Vithanage, M.; Parikh, S.J.; Mukome, F.N.D.; Rizwan, M.; Oleszczuk, P.; Al-Wabel, M.; Bolan, N.; et al. Advances and future directions of biochar characterization methods and applications. Crit. Rev. Environ. Sci. Technol. 2017, 47, 2275–2330. [Google Scholar] [CrossRef]
- Maiti, S.; Purakayastha, S.; Ghosh, B. Production of Low-Cost Carbon Adsorbents from Agricultural Wastes and Their Impact on Dye Adsorption. Chem. Eng. Commun. 2007, 195, 386–403. [Google Scholar] [CrossRef]
- Long, L.; Xue, Y.; Zeng, Y.; Yang, K.; Lin, C. Synthesis, characterization and mechanism analysis of modified crayfish shell biochar possessed ZnO nanoparticles to remove trichloroacetic acid. J. Clean. Prod. 2017, 166, 1244–1252. [Google Scholar] [CrossRef]
- Lin, Y.; Munroe, P.; Joseph, S.; Ziolkowski, A.; van Zwieten, L.; Kimber, S.; Rust, J. Chemical and structural analysis of enhanced biochars: Thermally treated mixtures of biochar, chicken litter, clay and minerals. Chemosphere 2013, 91, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Gao, Y.; Sun, L.; Zhang, S.; Li, J.; Zhang, Y. Effective sorption of atrazine by biochar colloids and residues derived from different pyrolysis temperatures. Environ. Sci. Pollut. Res. 2018, 25, 18528–18539. [Google Scholar] [CrossRef]
- Komnitsas, K.A.; Zaharaki, D. Morphology of Modified Biochar and Its Potential for Phenol Removal from Aqueous Solutions. Front. Environ. Sci. 2016, 4, 26. [Google Scholar] [CrossRef] [Green Version]
- Vithanage, M.; Mayakaduwa, S.S.; Herath, I.; Ok, Y.S.; Mohan, D. Kinetics, thermodynamics and mechanistic studies of carbofuran removal using biochars from tea waste and rice husks. Chemosphere 2016, 150, 781–789. [Google Scholar] [CrossRef]
- Oliveira, F.R.; Patel, A.K.; Jaisi, D.P.; Adhikari, S.; Lu, H.; Khanal, S.K. Environmental application of biochar: Current status and perspectives. Bioresour. Technol. 2017, 246, 110–122. [Google Scholar] [CrossRef]
- Tan, Z.; Wang, Y.; Kasiulienė, A.; Huang, C.; Ai, P. Cadmium removal potential by rice straw-derived magnetic biochar. Clean Technol. Environ. Policy 2017, 19, 761–774. [Google Scholar] [CrossRef]
- Sun, J.; He, F.; Pan, Y.; Zhang, Z. Effects of pyrolysis temperature and residence time on physicochemical properties of different biochar types. Acta Agric. Scand. Sect. B-Soil Plant Sci. 2017, 67, 12–22. [Google Scholar] [CrossRef]
- Lawal, A.A.; Hassan, M.A.; Zakaria, M.R.; Yusoff, M.Z.M.; Norrrahim, M.N.F.; Mokhtar, M.N.; Shirai, Y. Effect of oil palm biomass cellulosic content on nanopore structure and adsorption capacity of biochar. Bioresour. Technol. 2021, 332, 125070. [Google Scholar] [CrossRef]
- Carrier, M.; Hardie, A.G.; Uras, Ü.; Görgens, J.; Knoetze, J. Production of char from vacuum pyrolysis of South-African sugar cane bagasse and its characterization as activated carbon and biochar. J. Anal. Appl. Pyrolysis 2012, 96, 24–32. [Google Scholar] [CrossRef]
- Hilber, I.; Blum, F.; Leifeld, J.; Schmidt, H.-P.; Bucheli, T.D. Quantitative Determination of PAHs in Biochar: A Prerequisite to Ensure Its Quality and Safe Application. J. Agric. Food Chem. 2012, 60, 3042–3050. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Gao, B.; Chen, H.; Jiang, L.; Inyang, M.; Zimmerman, A.R.; Cao, X.; Yang, L.; Xue, Y.; Li, H. Adsorption of sulfamethoxazole on biochar and its impact on reclaimed water irrigation. J. Hazard. Mater. 2012, 209–210, 408–413. [Google Scholar] [CrossRef]
- Shaaban, A.; Se, S.-M.; Mitan, N.M.M.; Dimin, M. Characterization of biochar derived from rubber wood sawdust through slow pyrolysis on surface porosities and functional groups. Procedia Eng. 2013, 68, 365–371. [Google Scholar] [CrossRef] [Green Version]
- Essandoh, M.; Kunwar, B.; Pittman, C.U., Jr.; Mohan, D.; Mlsna, T. Sorptive removal of salicylic acid and ibuprofen from aqueous solutions using pine wood fast pyrolysis biochar. Chem. Eng. J. 2015, 265, 219–227. [Google Scholar] [CrossRef]
- KS, A.S.; Shyang, O.; Iberahim, N.; Mohamed, A. Kinetic and Isotherm Studies Biochar on Ammonium Solution. Preprint 2020. [Google Scholar] [CrossRef]
- Suliman, W.; Harsh, J.B.; Abu-Lail, N.I.; Fortuna, A.-M.; Dallmeyer, I.; Garcia-Perez, M. Influence of feedstock source and pyrolysis temperature on biochar bulk and surface properties. Biomass Bioenergy 2016, 84, 37–48. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, W.; Wang, S.; Zhuang, L.; Yang, Y.; Qiu, R. Characterization of sewage sludge-derived biochars from different feedstocks and pyrolysis temperatures. J. Anal. Appl. Pyrolysis 2013, 102, 137–143. [Google Scholar] [CrossRef]
- Zhang, B.; Wu, Y.; Cha, L. Removal of methyl orange dye using activated biochar derived from pomelo peel wastes: Performance, isotherm, and kinetic studies. J. Dispers. Sci. Technol. 2020, 41, 125–136. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Adi, V.S.K.; Huang, H.-L.; Lin, H.-P.; Huang, Z.-H. Adsorption of metal ions with biochars derived from biomass wastes in a fixed column: Adsorption isotherm and process simulation. J. Ind. Eng. Chem. 2019, 76, 240–244. [Google Scholar] [CrossRef]
- Shin, J.; Lee, S.-H.; Kim, S.; Ochir, D.; Park, Y.; Kim, J.; Lee, Y.-G.; Chon, K. Effects of physicochemical properties of biochar derived from spent coffee grounds and commercial activated carbon on adsorption behavior and mechanisms of strontium ions (Sr2+). Environ. Sci. Pollut. Res. 2020, 28, 40623–40632. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, V.; Patel, M.; Pittman, C.U., Jr.; Mohan, D. Batch and Continuous Fixed-Bed Lead Removal Using Himalayan Pine Needle Biochar: Isotherm and Kinetic Studies. ACS Omega 2020, 5, 16366–16378. [Google Scholar] [CrossRef] [PubMed]
- Bruzzoniti, M.C.; de Carlo, R.M.; Sarzanini, C.; Caldarola, D.; Onida, B. Novel insights in Al-MCM-41 precursor as adsorbent for regulated haloacetic acids and nitrate from water. Environ. Sci. Pollut. Res. 2012, 19, 4176–4183. [Google Scholar] [CrossRef] [Green Version]
- Bruzzoniti, M.C.; Appendini, M.; Onida, B.; Castiglioni, M.; Del Bubba, M.; Vanzetti, L.; Jana, P.; Sorarù, G.D.; Rivoira, L. Regenerable, innovative porous silicon-based polymer-derived ceramics for removal of methylene blue and rhodamine B from textile and environmental waters. Environ. Sci. Pollut. Res. 2018, 25, 10619–10629. [Google Scholar] [CrossRef]
- Zand, A.D.; Abyaneh, M.R. Adsorption of Lead, manganese, and copper onto biochar in landfill leachate: Implication of non-linear regression analysis. Sustain. Environ. Res. 2020, 30, 1–16. [Google Scholar] [CrossRef]
- Zhao, S.; Ta, N.; Wang, X. Absorption of Cu (II) and Zn (II) from Aqueous Solutions onto Biochars Derived from Apple Tree Branches. Energies 2020, 13, 3498. [Google Scholar] [CrossRef]
- Choudhary, B.; Paul, D. Isotherms, kinetics and thermodynamics of hexavalent chromium removal using biochar. J. Environ. Chem. Eng. 2018, 6, 2335–2343. [Google Scholar] [CrossRef]
- Aygün, A.; Yenisoy-Karakaş, S.; Duman, I. Production of granular activated carbon from fruit stones and nutshells and evaluation of their physical, chemical and adsorption properties. Microporous Mesoporous Mater. 2003, 66, 189–195. [Google Scholar] [CrossRef]
- Yao, H.; Lu, J.; Wu, J.; Lu, Z.; Wilson, P.C.; Shen, Y. Adsorption of fluoroquinolone antibiotics by wastewater sludge biochar: Role of the sludge source. Water Air Soil Pollut. 2013, 224, 1370. [Google Scholar] [CrossRef]
- Cheng, G.; Sun, L.; Jiao, L.; Peng, L.-x.; Lei, Z.-h.; Wang, Y.-x.; Lin, J. Adsorption of methylene blue by residue biochar from copyrolysis of dewatered sewage sludge and pine sawdust. Desalin. Water Treat. 2013, 51, 7081–7087. [Google Scholar] [CrossRef]
- Park, J.-H.; Wang, J.J.; Kim, S.-H.; Kang, S.-W.; Cho, J.-S.; Delaune, R.D.; Ok, Y.S.; Seo, D.-C. Lead sorption characteristics of various chicken bone part-derived chars. Environ. Geochem. Health 2019, 41, 1675–1685. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, S.; Wang, J.; Ding, X. Phosphorus retention using iron (II/III) modified biochar in saline-alkaline soils: Adsorption, column and field tests. Environ. Pollut. 2020, 261, 114223. [Google Scholar] [CrossRef]
- Shao, F.; Zhang, X.; Sun, X.; Shang, J. Antibiotic removal by activated biochar: Performance, isotherm, and kinetic studies. J. Dispers. Sci. Technol. 2020, 42, 1–12. [Google Scholar] [CrossRef]
- Solanki, A.; Boyer, T.H. Physical-chemical interactions between pharmaceuticals and biochar in synthetic and real urine. Chemosphere 2019, 218, 818–826. [Google Scholar] [CrossRef] [PubMed]
- Lam, Y.Y.; Lau, S.S.; Wong, J.W. Removal of Cd (II) from aqueous solutions using plant-derived biochar: Kinetics, isotherm and characterization. Bioresour. Technol. Rep. 2019, 8, 100323. [Google Scholar] [CrossRef]
- Taghlidabad, R.H.; Sepehr, E.; Khodaverdiloo, H.; Samadi, A.; Rasouli-Sadaghiani, M.H. Characterization of cadmium adsorption on two cost-effective biochars for water treatment. Arab. J. Geosci. 2020, 13, 1–10. [Google Scholar]
- Jung, K.-W.; Jeong, T.-U.; Choi, J.-W.; Ahn, K.-H.; Lee, S.-H. Adsorption of phosphate from aqueous solution using electrochemically modified biochar calcium-alginate beads: Batch and fixed-bed column performance. Bioresour. Technol. 2017, 244, 23–32. [Google Scholar] [CrossRef]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and Interpretation of Adsorption Isotherms. J. Chem. 2017, 2017, 11. [Google Scholar] [CrossRef]
- Ahmad, M.; Lee, S.S.; Rajapaksha, A.U.; Vithanage, M.; Zhang, M.; Cho, J.S.; Lee, S.-E.; Ok, Y.S. Trichloroethylene adsorption by pine needle biochars produced at various pyrolysis temperatures. Bioresour. Technol. 2013, 143, 615–622. [Google Scholar] [CrossRef]
- Kajjumba, G.W.; Emik, S.; Öngen, A.; Özcan, H.K.; Aydın, S. Modelling of adsorption kinetic processes—Errors, theory and application. In Advanced Sorption Process Applications; IntechOpen: London, UK, 2018; pp. 187–206. [Google Scholar]
- Qiu, H.; Lv, L.; Pan, B.-C.; Zhang, Q.-J.; Zhang, W.-M.; Zhang, Q.-X. Critical review in adsorption kinetic models. J. Zhejiang Univ. Sci. A 2009, 10, 716–724. [Google Scholar] [CrossRef]
- Jung, C.; Park, J.; Lim, K.H.; Park, S.; Heo, J.; Her, N.; Oh, J.; Yun, S.; Yoon, Y. Adsorption of selected endocrine disrupting compounds and pharmaceuticals on activated biochars. J. Hazard. Mater. 2013, 263, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Ashoori, N.; Teixido, M.; Spahr, S.; LeFevre, G.H.; Sedlak, D.L.; Luthy, R.G. Evaluation of pilot-scale biochar-amended woodchip bioreactors to remove nitrate, metals, and trace organic contaminants from urban stormwater runoff. Water Res. 2019, 154, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, B.A.; Im, E.A.; Werner, D.; Higgins, C.P. Biochar and activated carbon for enhanced trace organic contaminant retention in stormwater infiltration systems. Environ. Sci. Technol. 2015, 49, 6222–6230. [Google Scholar] [CrossRef]
- Marsh, H.; Rodríguez-Reinoso, F. CHAPTER 4-Characterization of Activated Carbon. In Activated Carbon; Marsh, H., Rodríguez-Reinoso, F., Eds.; Elsevier Science Ltd.: Oxford, UK, 2006; pp. 143–242. [Google Scholar]
- Kearns, J.; Dickenson, E.; Knappe, D. Enabling organic micropollutant removal from water by full-scale biochar and activated carbon adsorbers using predictions from bench-scale column data. Environ. Eng. Sci. 2020, 37, 459–471. [Google Scholar] [CrossRef]
- Park, J.-H.; Cho, J.-S.; Ok, Y.S.; Kim, S.-H.; Kang, S.-W.; Choi, I.-W.; Heo, J.-S.; DeLaune, R.D.; Seo, D.-C. Competitive adsorption and selectivity sequence of heavy metals by chicken bone-derived biochar: Batch and column experiment. J. Environ. Sci. Health Part A 2015, 50, 1194–1204. [Google Scholar] [CrossRef]
- Mahdi, Z.; Qiming, J.Y.; El Hanandeh, A. Removal of lead (II) from aqueous solution using date seed-derived biochar: Batch and column studies. Appl. Water Sci. 2018, 8, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Mahdi, Z.; Yu, Q.J.; El Hanandeh, A. Investigation of the kinetics and mechanisms of nickel and copper ions adsorption from aqueous solutions by date seed derived biochar. J. Environ. Chem. Eng. 2018, 6, 1171–1181. [Google Scholar] [CrossRef] [Green Version]
- Boni, M.R.; Chiavola, A.; Marzeddu, S. Application of biochar to the remediation of Pb-contaminated solutions. Sustainability 2018, 10, 4440. [Google Scholar] [CrossRef] [Green Version]
- Hong, N.; Cheng, Q.; Goonetilleke, A.; Bandala, E.R.; Liu, A. Assessing the effect of surface hydrophobicity/hydrophilicity on pollutant leaching potential of biochar in water treatment. J. Ind. Eng. Chem. 2020, 89, 222–232. [Google Scholar] [CrossRef]
- Yuan, H.; Lu, T.; Wang, Y.; Chen, Y.; Lei, T. Sewage sludge biochar: Nutrient composition and its effect on the leaching of soil nutrients. Geoderma 2016, 267, 17–23. [Google Scholar] [CrossRef]
- Chen, X.; Yang, L.; Myneni, S.C.; Deng, Y. Leaching of polycyclic aromatic hydrocarbons (PAHs) from sewage sludge-derived biochar. Chem. Eng. J. 2019, 373, 840–845. [Google Scholar] [CrossRef]
- ChemAxon. Chemicalize. 2021. Available online: https://www.chemicalize.org (accessed on 15 July 2021).
- Inyang, M.; Gao, B.; Zimmerman, A.; Zhang, M.; Chen, H. Synthesis, characterization, and dye sorption ability of carbon nanotube–biochar nanocomposites. Chem. Eng. J. 2014, 236, 39–46. [Google Scholar] [CrossRef]
- Mahmoud, D.K.; Salleh, M.A.M.; Karim, W.A.W.A.; Idris, A.; Abidin, Z.Z. Batch adsorption of basic dye using acid treated kenaf fibre char: Equilibrium, kinetic and thermodynamic studies. Chem. Eng. J. 2012, 181, 449–457. [Google Scholar] [CrossRef]
- Park, J.-H.; Wang, J.J.; Meng, Y.; Wei, Z.; DeLaune, R.D.; Seo, D.-C. Adsorption/desorption behavior of cationic and anionic dyes by biochars prepared at normal and high pyrolysis temperatures. Colloids Surf. A Physicochem. Eng. Asp. 2019, 572, 274–282. [Google Scholar] [CrossRef]
- Jian, X.; Zhuang, X.; Li, B.; Xu, X.; Wei, Z.; Song, Y.; Jiang, E. Comparison of characterization and adsorption of biochars produced from hydrothermal carbonization and pyrolysis. Environ. Technol. Innov. 2018, 10, 27–35. [Google Scholar] [CrossRef]
- Parra-Marfíl, A.; Ocampo-Pérez, R.; Collins-Martínez, V.H.; Flores-Vélez, L.M.; Gonzalez-Garcia, R.; Medellín-Castillo, N.A.; Labrada-Delgado, G.J. Synthesis and characterization of hydrochar from industrial Capsicum annuum seeds and its application for the adsorptive removal of methylene blue from water. Environ. Res. 2020, 184, 109334. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Tran, H.N.; Chao, H.-P.; Lin, C.-C. Effect of nitric acid oxidation on the surface of hydrochars to sorb methylene blue: An adsorption mechanism comparison. Adsorpt. Sci. Technol. 2019, 37, 607–622. [Google Scholar] [CrossRef] [Green Version]
- Xu, R.-K.; Xiao, S.-C.; Yuan, J.-H.; Zhao, A.-Z. Adsorption of methyl violet from aqueous solutions by the biochars derived from crop residues. Bioresour. Technol. 2011, 102, 10293–10298. [Google Scholar] [CrossRef]
- Hameed, B.; Rahman, A. Removal of phenol from aqueous solutions by adsorption onto activated carbon prepared from biomass material. J. Hazard. Mater. 2008, 160, 576–581. [Google Scholar] [CrossRef]
- Liu, C.-H.; Chuang, Y.-H.; Li, H.; Teppen, B.J.; Boyd, S.A.; Gonzalez, J.M.; Johnston, C.T.; Lehmann, J.; Zhang, W. Sorption of lincomycin by manure-derived biochars from water. J. Environ. Qual. 2016, 45, 519. [Google Scholar] [CrossRef] [Green Version]
- Inyang, M.; Gao, B.; Zimmerman, A.; Zhou, Y.; Cao, X. Sorption and cosorption of lead and sulfapyridine on carbon nanotube-modified biochars. Environ. Sci. Pollut. Res. 2015, 22, 1868–1876. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Liu, W.-J.; Jiang, H.; Chen, J.-J.; Li, W.-W.; Yu, H.-Q. Modification of bio-char derived from fast pyrolysis of biomass and its application in removal of tetracycline from aqueous solution. Bioresour. Technol. 2012, 121, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Chen, W.; Xu, Z.; Zheng, S.; Zhu, D. Adsorption of sulfonamides to demineralized pine wood biochars prepared under different thermochemical conditions. Environ. Pollut. 2014, 186, 187–194. [Google Scholar] [CrossRef]
- Ni, J.; Pignatello, J.J.; Xing, B. Adsorption of aromatic carboxylate ions to black carbon (biochar) is accompanied by proton exchange with water. Environ. Sci. Technol. 2011, 45, 9240–9248. [Google Scholar] [CrossRef]
- Qiu, Y.; Zheng, Z.; Zhou, Z.; Sheng, G.D. Effectiveness and mechanisms of dye adsorption on a straw-based biochar. Bioresour. Technol. 2009, 100, 5348–5351. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Ho, S.-H.; Zhou, Y.; Ren, N.-Q. Highly efficient adsorption of dyes by biochar derived from pigments-extracted macroalgae pyrolyzed at different temperature. Bioresour. Technol. 2018, 259, 104–110. [Google Scholar]
- Li, Y.; Tsend, N.; Li, T.; Liu, H.; Yang, R.; Gai, X.; Wang, H.; Shan, S. Microwave assisted hydrothermal preparation of rice straw hydrochars for adsorption of organics and heavy metals. Bioresour. Technol. 2019, 273, 136–143. [Google Scholar] [CrossRef]
- Li, Y.; Meas, A.; Shan, S.; Yang, R.; Gai, X. Production and optimization of bamboo hydrochars for adsorption of Congo red and 2-naphthol. Bioresour. Technol. 2016, 207, 379–386. [Google Scholar] [CrossRef]
- Kearns, J.P.; Wellborn, L.S.; Summers, R.; Knappe, D. 2,4-D adsorption to biochars: Effect of preparation conditions on equilibrium adsorption capacity and comparison with commercial activated carbon literature data. Water Res. 2014, 62, 20–28. [Google Scholar] [CrossRef]
- Peng, B.; Chen, L.; Que, C.; Yang, K.; Deng, F.; Deng, X.; Shi, G.; Xu, G.; Wu, M. Adsorption of antibiotics on graphene and biochar in aqueous solutions induced by π-π interactions. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, H.M.; Kan, E. A novel hay-derived biochar for removal of tetracyclines in water. Bioresour. Technol. 2019, 274, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Li, J.; Shan, D. Adsorption of tetracycline in aqueous solution by biochar derived from waste Auricularia auricula dregs. Chemosphere 2020, 238, 124432. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Kong, X.; He, L.; Li, W.; Liao, Q. Low-cost biochar derived from herbal residue: Characterization and application for ciprofloxacin adsorption. Int. J. Environ. Sci. Technol. 2016, 13, 2449–2458. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Tong, L.; Zhu, P.; Huang, P.; Tan, Z.; Qin, F.; Shi, W.; Wang, M.; Nie, H.; Yan, G. Adsorption of chlortetracycline onto biochar derived from corn cob and sugarcane bagasse. Water Sci. Technol. 2018, 78, 1336–1347. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Chen, Z.; Lv, S. A novel magnetic biochar efficiently sorbs organic pollutants and phosphate. Bioresour. Technol. 2011, 102, 716–723. [Google Scholar] [CrossRef]
- Sun, K.; Ro, K.; Guo, M.; Novak, J.; Mashayekhi, H.; Xing, B. Sorption of bisphenol A, 17α-ethinyl estradiol and phenanthrene on thermally and hydrothermally produced biochars. Bioresour. Technol. 2011, 102, 5757–5763. [Google Scholar] [CrossRef]
- Cao, X.; Ma, L.; Gao, B.; Harris, W. Dairy-manure derived biochar effectively sorbs lead and atrazine. Environ. Sci. Technol. 2009, 43, 3285–3291. [Google Scholar] [CrossRef]
- Chen, B.; Chen, Z. Sorption of naphthalene and 1-naphthol by biochars of orange peels with different pyrolytic temperatures. Chemosphere 2009, 76, 127–133. [Google Scholar] [CrossRef]
- Kasozi, G.N.; Zimmerman, A.R.; Nkedi-Kizza, P.; Gao, B. Catechol and humic acid sorption onto a range of laboratory-produced black carbons (biochars). Environ. Sci. Technol. 2010, 44, 6189–6195. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, H.; Yu, L.; Sun, T. Adsorption and catalytic hydrolysis of carbaryl and atrazine on pig manure-derived biochars: Impact of structural properties of biochars. J. Hazard. Mater. 2013, 244, 217–224. [Google Scholar] [CrossRef]
- Karakoyun, N.; Kubilay, S.; Aktas, N.; Turhan, O.; Kasimoglu, M.; Yilmaz, S.; Sahiner, N. Hydrogel–Biochar composites for effective organic contaminant removal from aqueous media. Desalination 2011, 280, 319–325. [Google Scholar] [CrossRef]
- Reddy, K.R.; Xie, T.; Dastgheibi, S. Evaluation of biochar as a potential filter media for the removal of mixed contaminants from urban storm water runoff. J. Environ. Eng. 2014, 140, 04014043. [Google Scholar] [CrossRef] [Green Version]
- Inyang, M.; Dickenson, E. The potential role of biochar in the removal of organic and microbial contaminants from potable and reuse water: A review. Chemosphere 2015, 134, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Upton, G.; Cook, I. Understanding Statistics; Oxford University Press: Oxford, UK, 1996. [Google Scholar]
- Park, J.; Hung, I.; Gan, Z.; Rojas, O.J.; Lim, K.H.; Park, S. Activated carbon from biochar: Influence of its physicochemical properties on the sorption characteristics of phenanthrene. Bioresour. Technol. 2013, 149, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Ania, C.; Cabal, B.; Parra, J.; Pis, J. Importance of the hydrophobic character of activated carbons on the removal of naphthalene from the aqueous phase. Adsorpt. Sci. Technol. 2007, 25, 155–167. [Google Scholar] [CrossRef] [Green Version]
- Ding, Z.; Zhang, F.; Sun, N.; Chi, J. Sorption of Polycyclic Aromatic Hydrocarbons by Biochars of Wheat Straw with Different Pyrolysis Temperatures; IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2019; p. 052008. [Google Scholar]
- Ravichandran, P.; Sugumaran, P.; Seshadri, S.; Basta, A.H. Optimizing the route for production of activated carbon from Casuarina equisetifolia fruit waste. R. Soc. Open Sci. 2018, 5, 171578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gai, X.; Wang, H.; Liu, J.; Zhai, L.; Liu, S.; Ren, T.; Liu, H. Effects of feedstock and pyrolysis temperature on biochar adsorption of ammonium and nitrate. PLoS ONE 2014, 9, e113888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grung, B.; Manne, R. Missing values in principal component analysis. Chemom. Intell. Lab. Syst. 1998, 42, 125–139. [Google Scholar] [CrossRef]
- Varies Authors. Global and China Activated Carbon Industry Report, 2018–2023. 2018. Available online: https://www.researchandmarkets.com/reports/4478751/global-and-chinese-spherical-activated-carbon#rela0-4605170 (accessed on 13 April 2021).
- Gopinath, A.; Divyapriya, G.; Srivastava, V.; Laiju, A.; Nidheesh, P.; Kumar, M.S. Conversion of sewage sludge into biochar: A potential resource in water and wastewater treatment. Environ. Res. 2021, 194, 110656. [Google Scholar] [CrossRef]
- Karim, A.A.; Kumar, M.; Singh, E.; Kumar, A.; Kumar, S.; Ray, A.; Dhal, N.K. Enrichment of primary macronutrients in biochar for sustainable agriculture: A review. Crit. Rev. Environ. Sci. Technol. 2020, 1–42. [Google Scholar]
| Test | Method | Reference |
|---|---|---|
| Physical Tests | ||
| Bulk density | ASTM D2854, CEFIC | [33,34] |
| Absolute density | CEFIC | [33] |
| Particle density | CEFIC | [33] |
| Particle size | ASTM D2862, CEFIC | [33,35] |
| Pressure drop | CEFIC | [33] |
| Mechanical strength | ASTM D3802, AWWA B604, CEFIC | [33,36,37] |
| Adsorption Tests and Indices | ||
| Adsorption isotherm | ASTM: D3860-98, 5919-96, CEFIC | [33,38,39] |
| Iodine number | AWWA B600-16, EN 12915-1, ASTM D4607-14, CEFIC | [33,40,41,42] |
| Phenol number | CEFIC | [33] |
| Methylene Blue number | CEFIC | [33] |
| Molasses number | EPA625171002A | [43] |
| Tannin number | AWWA B600 | [41] |
| Chemical Tests | ||
| Ashes, water soluble material, and water-extractable substances (As, Cd, Cr, Hg, Ni, Pb, Sb, Se, CN-, fluoranthene, benzo[b]fluoranthene, benzo[k]fluoranthene, benzo[a]pyrene, benzo[g,h,i]perylene, indeno[1,2,3-cd]pyrene) | EN 12915-1 | [40] |
| pH | ASTM: D6851-02, D3838-05 | [44,45] |
| CATION | Class | logKow | pKa (Ionic Group) | Comparison with AC | Ref |
| Methylene blue | Dye | 0.75 | / | No | [133,134,135,136,137,138] |
| Methyl violet | Dye | 0.43 | 9.17 | No | [139] |
| Malachite green | Dye | 0.8 | / | No | [140] |
| Lincomycin | Antibiotic | −0.3 | 7.97 | No | [141] |
| ANION | Class | logKow | pKa (Ionic Group) | Comparison with AC | Ref |
| Sulphapyridine | Antibiotic | 0.35 | 6.24 | Yes | [142,143] |
| Sulfamethoxazole | Antibiotic | 0.79 | 6.16 | No | [144] |
| p-coumaric acid | Drug | 1.46 | 3.81 | No | [145] |
| Reactive brilliant blue | Dye | −1.33 | −2.69 | No | [146] |
| Congo Red | Dye | 2.63 | / | No | [135,147,148,149] |
| tris(2-carboxyethyl)phosphine | Flame retardant | 1.78 | 3.22; 4.38 | No | [121] |
| 2,4-dichlrophenenoxiacetic acid | Herbicide/Pesticide | 2.61 | 2.81 | Yes | [150] |
| t-Cinnamic acid | Precursor | 2.13 | 4.32 | No | [145] |
| NEUTRAL | Class | logKow | pKa (Ionic Group) | Comparison with AC | Ref |
| Amoxicillin | Antibiotic | −2.3 | 7.22 | No | [151] |
| Tetracycline | Antibiotic | −3.4 | 7.36 | No | [22,152,153] |
| Ciprofloxacin | Antibiotic | −0.8 | 5.56; 8.77 | No | [154] |
| Sulfadiazine | Antibiotic | 0.38 | 7 | No | [22] |
| Chlortetracycline | Antibiotic | −1.98 | 2.99 | No | [155] |
| 1H-benzotriazole | Corrosion Inhibitor | 1.44 | 9.04 | No | [121] |
| p-nitrotoluene | Dye | 2.37 | / | No | [156] |
| Bisphenol-a | Endocrine disruptors | 3.32 | 9.78; 10.39 | No | [157] |
| Atrazine | Herbicide | 2.61 | / | No/Yes | [121,158] |
| Diuron | Herbicide | 2.68 | 13.18 | No | [121] |
| 1-naphtol | Herbicide | 2.85 | 9.6 | Yes | [159] |
| Catechol | Herbicide | 0.9 | 9.34; 12.79 | No | [160] |
| Carbaryl | Herbicide | 0.9 | / | No | [161] |
| 17α-ethinyl estradiol | Estrogen | 3.67 | 10.33 | No | [157] |
| Phenol | Plastic production | 1.46 | 10.02 | No | [162] |
| Phenanthrene | Polycyclic Aromatic Hydrocarbon | 3.71 | / | No | [157,163] |
| Naphthalene | Polycyclic Aromatic Hydrocarbon | 2.96 | / | No/Yes/No | [156,159,163] |
| Trichloroethylene | Solvent | 2.42 | / | Yes | [6] |
| 4-tert-Octylphenol | Non-ionic Surfactant | 5.18 | 10.23 | Yes | [56] |
| TRITONTMX-45 (mixture of 4-t-octylphenol polyethoxylated) | Non-ionic Surfactant | 3.49–4.90 | 15.10 a | Yes | [56] |
| 4-(1-Ethyl-1,4-dimethylpentyl)-phenol | Surfactant | 5.79 | 10.22 | Yes | [56] |
| IGEPAL®CO-520 (mixture of branched 4-nonylphenol polyethoxylated oligomers) | Surfactant | 4.26–5.50 | / | Yes | [56] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castiglioni, M.; Rivoira, L.; Ingrando, I.; Del Bubba, M.; Bruzzoniti, M.C. Characterization Techniques as Supporting Tools for the Interpretation of Biochar Adsorption Efficiency in Water Treatment: A Critical Review. Molecules 2021, 26, 5063. https://doi.org/10.3390/molecules26165063
Castiglioni M, Rivoira L, Ingrando I, Del Bubba M, Bruzzoniti MC. Characterization Techniques as Supporting Tools for the Interpretation of Biochar Adsorption Efficiency in Water Treatment: A Critical Review. Molecules. 2021; 26(16):5063. https://doi.org/10.3390/molecules26165063
Chicago/Turabian StyleCastiglioni, Michele, Luca Rivoira, Irene Ingrando, Massimo Del Bubba, and Maria Concetta Bruzzoniti. 2021. "Characterization Techniques as Supporting Tools for the Interpretation of Biochar Adsorption Efficiency in Water Treatment: A Critical Review" Molecules 26, no. 16: 5063. https://doi.org/10.3390/molecules26165063






