Efficiency of Fe3O4 Nanoparticles with Different Pretreatments for Enhancing Biogas Yield of Macroalgae Ulva intestinalis Linnaeus
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Green Algae U. intestinalis
2.2. Chemical Analysis of Algae Powder
2.3. Ozonation Pretreatment of U. intestinalis
2.4. Sonication (US) Pretreatment of U. intestinalis
2.5. Microwave Pretreatment of U. intestinalis
2.6. Green Synthesis of Fe3O4 Nanoparticles
2.7. Fe3O4 NPs Pretreatment of U. intestinalis
2.8. Inoculum and Substrates Preparation
2.9. Biogas Tests
2.10. Characterization and Measurement
2.11. Kinetics Study and Statistical Analysis
3. Results
3.1. Characterization of Green Fe3O4 NPs
3.1.1. Fourier Transform Infrared Spectra (FTIR)
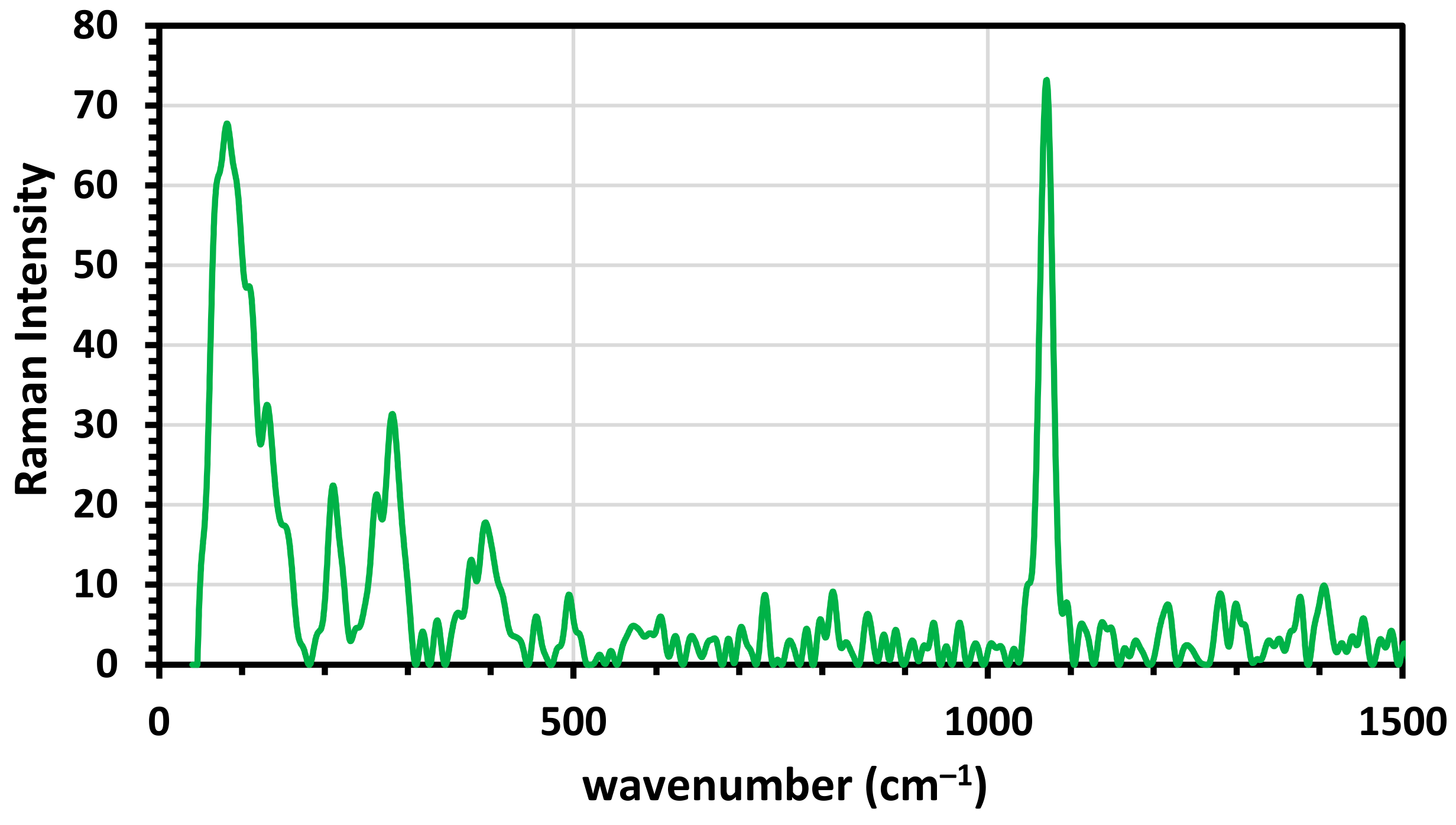
3.1.2. Raman Spectroscopy
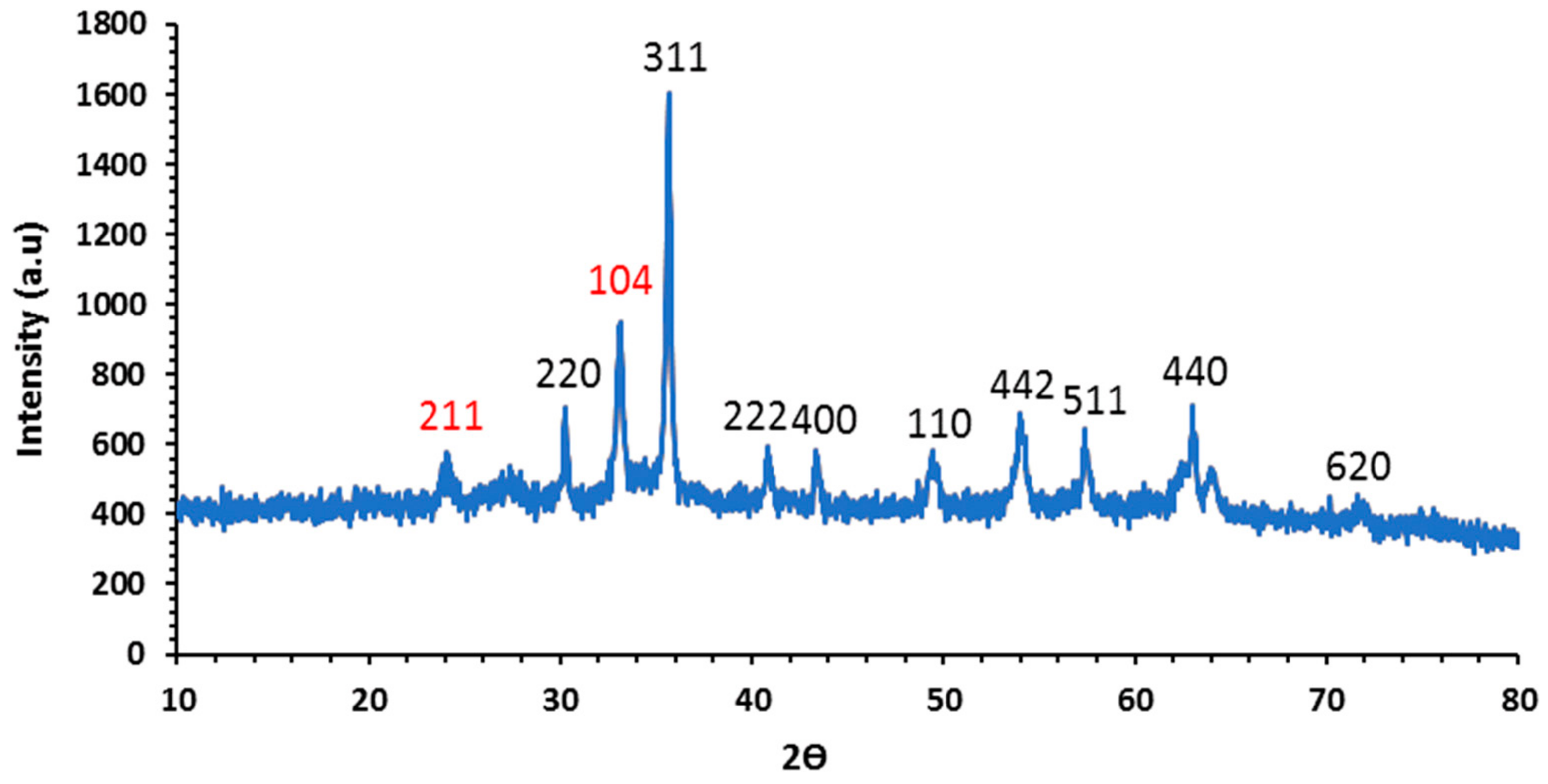
3.1.3. X-ray Diffraction (XRD)
3.1.4. Scanning Electron Microscopy (SEM)
3.1.5. Transmission Electron Microscopy (TEM)
3.1.6. Particle Size Analyzer (PSA) and BET Analysis of the Surface Area
3.2. Characterization of the Pretreatments Analysis of U. intestinalis
3.2.1. Fourier Transform Infrared Spectra (FTIR)
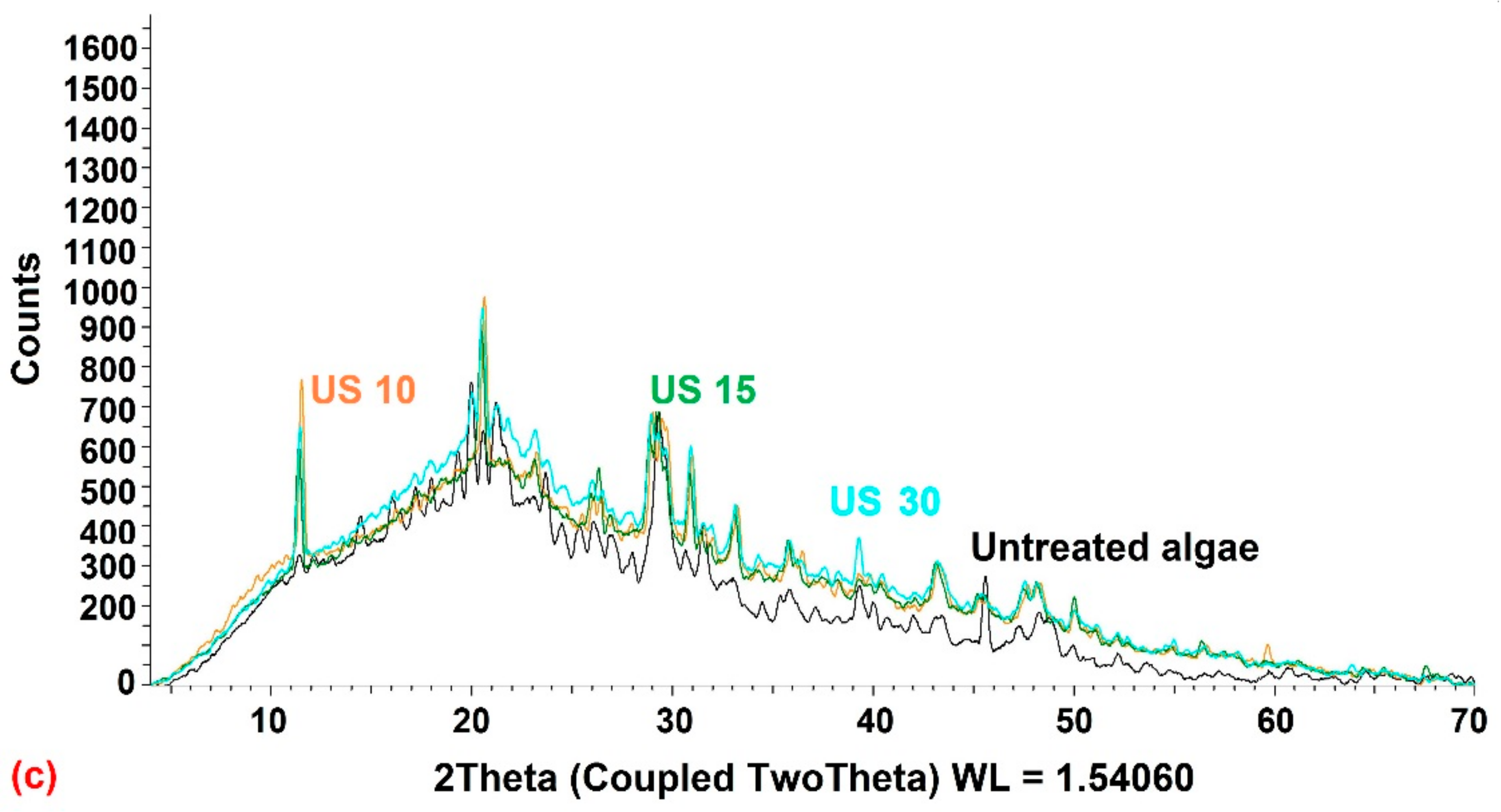
3.2.2. X-ray Diffraction (XRD)
3.2.3. Thermal Analysis (TGA)
3.2.4. Surface Morphology, Scanning Electron Microscopy (SEM)
3.3. Chemical Compositions of U. intestinalis
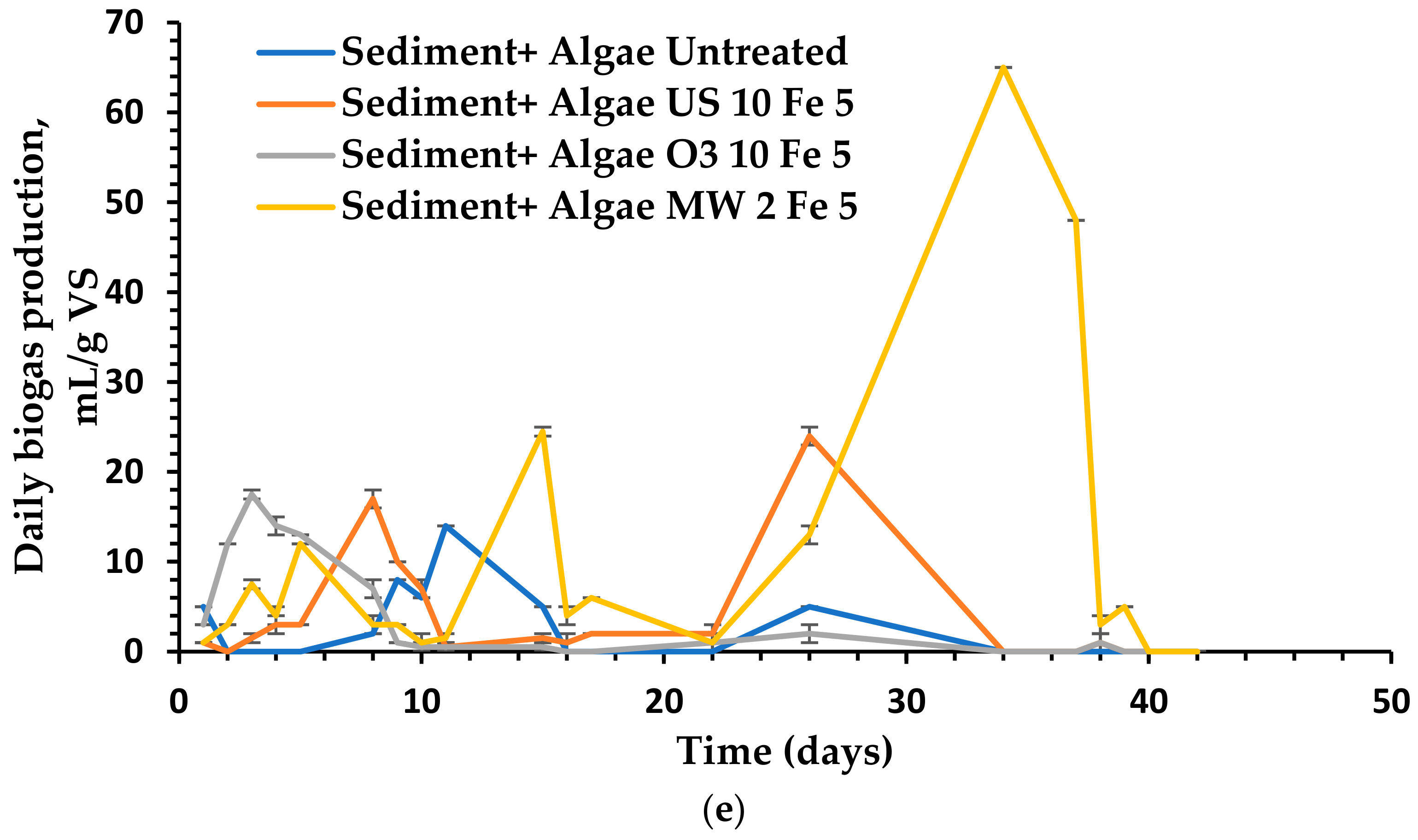
3.4. The Impact of Different Pretreatment Techniques on Anaerobic Digestion and Biogas Production
4. Discussion
5. Kinetic Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Abbreviations
| AD | Anaerobic digestion |
| NPs | Nanoparticles |
| Fe3O4 NPs | Magnetite nanoparticles |
| MW | Microwave |
| O3 | ozone |
| US | Ultrasonic |
| DDW | Double distilled water |
| FTIR | Fourier transform infrared |
| XRD | X-ray diffractograms |
| SEM | Scanning electron microscope |
| TEM | Transmission electron microscope |
| EDX | energy dispersive X-ray spectroscopy |
| BET | Brunauer–Emmett–Teller |
| TGA | Thermo gravimetric analysis |
| TS | Total solids |
| Rm | The maximum biogas production rate |
| VS | Volatile solids |
| λ | The lag phase time (days) |
References
- Montingelli, M.E.; Tedesco, S.; Olabi, A.G. Biogas production from algal biomass: A review. Renew. Sust. Energ. Rev. 2015, 43, 961–972. [Google Scholar] [CrossRef]
- Vivekanand, V.; Eijsink, V.G.H.; Horn, S.J. Biogas production from the brown seaweed Saccharina latissima: Thermal pretreatment and codigestion with wheat straw. J. Appl. Phycol. 2012, 24, 1295–1301. [Google Scholar] [CrossRef]
- Carlsson, A.S.; van Beilen, J.B.; Möller, R.; Clayton, D. Micro-and Macro-Algae: Utility for Industrial Applications: Outputs from the EPOBIO Project. 2007. Available online: http://www.etipbioenergy.eu/images/epobio_aquatic_report.pdf (accessed on 27 December 2018).
- Chen, P.; Min, M.; Chen, Y.; Wang, L.; Li, Y.; Chen, Q. Review of biological and engineering aspects of algae to fuels approach. Int. J. Agric. Biol. Eng. 2010, 2, 1–30. [Google Scholar] [CrossRef]
- Jung, K.A.; Lim, S.R.; Kim, Y.; Park, J.M. Potentials of macroalgae as feedstocks for biorefinery. Bioresour. Technol. 2013, 135, 182–190. [Google Scholar] [CrossRef]
- Murphy, J.D.; Drosg, B.; Allen, E.; Jerney, J.; Xia, A.; Herrmann, C. A Perspective on Algal Biogas; IEA Bioenergy: Paris, France, 2015; pp. 1–38. Available online: http://www.ieabioenergy.com/publications/a-perspective-on-algal-biogas/ (accessed on 3 August 2021).
- Passos, F.; Uggetti, E.; Carrère, H.; Ferrer, I. Pretreatment of microalgae to improvebiogas production: A review. Bioresour. Technol. 2014, 172, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.J.; Lewis, D.M.; Green, F.B. Anaerobic digestion of algae biomass: A review. Algal Res. 2014, 5, 204–214. [Google Scholar] [CrossRef]
- Alvira, P.; Tomás-Pejó, E.; Ballesteros, M.; Negro, M.J. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review. Bioresour. Technol. 2010, 101, 4851–4861. [Google Scholar] [CrossRef] [PubMed]
- Da Costa Sousa, L.; Chundawat, S.P.; Balan, V.; Dale, B.E. ‘Cradle-to-grave’ assessment of existing lignocelluloses pretreatment technologies. Curr. Opin. Biotechnol. 2009, 20, 339–347. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, J. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Lee, D.J.; Chang, J.S.; Liu, J.C. Effects of ozone and peroxone on algal separation via dispersed air flotation. Colloids Surf. B. Biointerfaces 2013, 105, 246–250. [Google Scholar] [CrossRef]
- Rodriguez, C.; Alaswad, A.; Mooney, J.; Prescott, T.; Olabi, A.G. Pretreatment techniques used for anaerobic digestion of algae. Fuel. Process Technol. 2015, 138, 765–779. [Google Scholar] [CrossRef]
- Kim, J.; Yoo, G.; Lee, H.; Lim, J.; Kim, K.; Kim, C.W. Methods of downstream processing for the production of biodiesel from microalgae. Biotechnol. Adv. 2013, 31, 862–876. [Google Scholar] [CrossRef] [PubMed]
- Rani, R.U.; Kumar, S.A.; Kaliappan, S.; Yeom, I.; Banu, J.R. Impacts of microwave pretreatments on the semi-continuous anaerobic digestion of dairy waste activated sludge. Waste Manag. 2013, 33, 1119–1127. [Google Scholar] [CrossRef]
- Wu, Y.N.; Mattsson, M.; Ding, M.W.; Wu, M.T.; Mei, J.; Shen, Y.L. Effects of dif-ferent pretreatments on improving biogas production of macroalgae Fucus vesiculosus and Fucus serratus in Baltic Sea. Energy Fuels 2019, 33, 2278–2284. [Google Scholar] [CrossRef]
- Garrote, G.; Dominguez, H.; Parajo, J.C. Hydrothermal processing of lignocellulosic materials. Holz als Roh. Werkstoff. 1999, 57, 191–202. [Google Scholar] [CrossRef]
- Onyeche, T.I.; Schläfer, O.; Bormann, H.; Schröder, C.; Sievers, M. Ultrasonic cell disruption of stabilised sludge with subsequent anaerobic digestion. Ultrasonics 2002, 40, 31–35. [Google Scholar] [CrossRef]
- Zhang, Q.; Benoit, M.; Vigier, K.D.O.; Barrault, J.; Jégou, G.; Philippe, M.; Jérôme, F. Pretreatment of microcrystalline cellulose by ultrasounds: Effect of particle size in the heterogeneously-catalyzed hydrolysis of cellulose to glucose. Green Chem. 2013, 15, 963–969. [Google Scholar] [CrossRef]
- Bharathiraja, S.; Suriya, J.; Krishnan, M.; Manivasagan, P.; Kim, S.K. Production of enzymes from agricultural wastes and their potential industrial applications. Adv. Food Nutr. Res. 2017, 80, 125–148. [Google Scholar]
- Montingelli, M.E.; Benyounis, K.Y.; Quilty, B.; Stokes, J.; Olabi, A.G. Influence of Mechanical Pretreatment and Organic Concentration of Irish Brown Seaweed for Methane Production. Energy 2017, 118, 1079–1089. [Google Scholar] [CrossRef]
- Hassaan, M.A.; El Nemr, A.; Elkatory, M.R.; Eleryan, A.; Ragab, S.; El Sikaily, A.; Pan-taleo, A. Enhancement of Biogas Production from Macroalgae Ulva latuca via Ozo-nation Pretreatment. Energies 2021, 14, 1703. [Google Scholar] [CrossRef]
- Cardeña, R.; Moreno, G.; Bakonyi, P.; Buitrón, G. Enhancement of methane production from various microalgae cultures via novel ozonation pretreatment. Chem. Eng. J. 2017, 307, 948–954. [Google Scholar] [CrossRef]
- Abdelsalam, E.; Samer, M.; Attia, Y.A.; Abdel-Hadi, M.A.; Hassan, H.E.; Badr, Y. Comparison of nanoparticles effects on biogas and methane production from anaerobic digestion of cattle dung slurry. Renew. Energy 2016, 87, 592–598. [Google Scholar] [CrossRef]
- Tsapekos, P.; Kougias, P.G.; Angelidaki, I. Biogas production from ensiled meadow grass; effect of mechanical pretreatments and rapid determination of substrate biodegradability via physicochemical methods. Bioresour. Technol. 2015, 182, 329–335. [Google Scholar] [CrossRef]
- Mu, H.; Chen, Y. Long-term effect of ZnO nanoparticles on waste activated sludge anaerobic digestion. Water Res. 2011, 45, 5612–5620. [Google Scholar] [CrossRef]
- Hassaan, M.A.; Pantaleo, A.; Tedone, L.; Elkatory, M.R.; Ali, R.M.; Nemr, A.E.; Mastro, G.D. Enhancement of biogas production via green ZnO nanoparticles: Experimental results of selected herbaceous crops. Chem. Eng. Commun. 2021, 208, 242–255. [Google Scholar] [CrossRef]
- Rafique, R.; Poulsen, T.G.; Nizami, A.-S.; Asam, Z.-Z.; Murphy, J.D.; Kiely, G. Effect of thermal, chemical and thermochemical pre-treatments to enhance methane production. Energy 2010, 35, 4556–4561. [Google Scholar] [CrossRef]
- Basavaiah, K.; Kahsay, M.H.; RamaDevi, D. Green synthesis of magnetite nanoparticles using aqueous pod extract of Dolichos lablab L for an efficient adsorption of crystal violet. Emergent Mater. 2018, 1, 121–132. [Google Scholar] [CrossRef]
- Santegoeds, C.M.; Damgaard, L.R.; Hesselink, G.; Zopfi, J.; Lens, P.; Muyzer, G.; de Beer, D. Distribution of sulfate-reducing and methanogenic bacteria in anaerobic aggregates determined by microsensor and molecular analyses. Appl. Environ. Microbiol. 1999, 65, 4618–4629. [Google Scholar] [CrossRef]
- Do Nascimento, M.; de los Angeles Dublan, M.; Ortiz-Marquez, J.C.F.; Curatti, L. High lipid productivity of an Ankistrodesmus–Rhizobium artificial consortium. Bioresour. Technol. 2013, 146, 400–407. [Google Scholar] [CrossRef]
- Amirante, R.; Demastro, G.; Distaso, E.; Hassaan, M.A.; Mormando, A.; Pantaleo, A.M.; Tamburrano, P.; Tedone, L.; Clodoveo, M.L. Effects of ultrasound and green synthesis ZnO nanoparticles on biogas production from Olive Pomace. Energy Proced. 2018, 148, 940–947. [Google Scholar]
- Hassaan, M.A.; Pantaleo, A.; Santoro, F.; Elkatory, M.R.; De Mastro, G.; El Sikaily, A.; Ragab, S.; El Nemr, A. Techno-Economic Analysis of ZnO Nanoparticles Pretreatments for Biogas Production from Barley Straw. Energies 2020, 13, 5001. [Google Scholar] [CrossRef]
- Remigi, E.U.; Buckley, C.A. Co-Digestion of High Strength/Toxic Organic Effluents in Anaerobic Digesters at Wastewater Treatment Works; Water Research Commission: Pretoria, South Africa, 2016. [Google Scholar]
- Ruíz-Baltazar, A.; Esparza, R.; Rosas, G.; Pérez, R. Effect of the surfactant on the growth and oxidation of iron nanoparticles. J. Nanomater. 2015, 16, 202. [Google Scholar] [CrossRef]
- El Nemr, A.; Eleryan, A.; Mashaly, M.; Khaled, A. Rapid synthesis of cellulose propionate and its conversion to cellulose nitrate propionate. Polym. Bull. 2021, 78, 4149–4182. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Jeon, B.-H.; Jeung, J.H.; Rene, E.R.; Banu, J.R.; Ravindran, B.; Vu, C.M.; Ngo, H.H.; Guo, W.; Chang, S.W. Thermophilic anaerobic digestion of model organic wastes: Evaluation of biomethane production and multiple kinetic models analysis. Bioresour. Technol. 2019, 280, 269–276. [Google Scholar] [CrossRef]
- Hassaan, M.A.; Nemr, A.E.; Elkatory, M.R.; Ragab, S.; El-Nemr, M.A.; Pantaleo, A. Synthesis, Characterization, and Synergistic Effects of Modified Biochar in Combination with -Fe2O3 NPs on Biogas Production from Red Algae Pterocladia capillacea. Sustainability 2021, 13, 9275. [Google Scholar] [CrossRef]
- Nnadozie, E.C.; Ajibade, P.A. Green synthesis and characterization of magnetite (Fe3O4) nanoparticles using Chromolaena odorata root extract for smart nanocomposite. Mater. Lett. 2020, 263, 127145. [Google Scholar] [CrossRef]
- Lu, W.; Shen, Y.; Xie, A.; Zhang, W. Green synthesis and characterization of superparamagnetic Fe3O4 nanoparticles. J. Magn. Magn. Mater. 2010, 322, 1828–1833. [Google Scholar] [CrossRef]
- Soliman, E.A.; Elkatory, M.R.; Hashem, A.I.; Ibrahim, H.S. Synthesis and performance of maleic anhydride copolymers with alkyl linoleate or tetra-esters as pour point depressants for waxy crude oil. Fuel 2018, 211, 535–547. [Google Scholar] [CrossRef]
- Salah, H.; Elkatory, M.R.; Fattah, M.A. Novel zinc-polymer complex with antioxidant activity for industrial lubricating oil. Fuel 2021, 305, 121536. [Google Scholar] [CrossRef]
- Slavov, L.; Abrashev, M.V.; Merodiiska, T.; Gelev, C.; Vandenberghe, R.E.; Markova-Deneva, I.; Nedkov, I. Raman spectroscopy investigation of magnetite nanoparticles in ferrofluids. J. Magn. Magn. Mater. 2010, 322, 1904–1911. [Google Scholar] [CrossRef]
- Panta, P.C.; Bergmann, C.P. Raman spectroscopy of iron oxide of nanoparticles (Fe3O4). J. Mater. Sci. Eng. 2015, 5, 1000217. [Google Scholar]
- Testa-Anta, M.; Ramos-Docampo, M.A.; Comesaña-Hermo, M.; Rivas-Murias, B.; Sal-gueiriño, V. Raman spectroscopy to unravel the magnetic properties of iron oxide nanocrystals for bio-related applications. Nanoscale Adv. 2019, 1, 2086–2103. [Google Scholar] [CrossRef]
- Lin, R.; Deng, C.; Ding, L.; Bose, A.; Murphy, J.D. Improving gaseous biofuel production from seaweed Saccharina latissima: The effect of hydrothermal pretreatment on energy efficiency. Energy Convers. Manag. 2019, 196, 1385–1394. [Google Scholar] [CrossRef]
- Ong, M.Y.; Syahira Abdul Latif, N.I.; Leong, H.Y.; Salman, B.; Show, P.L.; Nomanbhay, S. Characterization and analysis of Malaysian macroalgae biomass as potential feedstock for bio-oil production. Energies 2019, 12, 3509. [Google Scholar] [CrossRef]
- Ali, I.; Bahadar, A. Thermogravimetric characteristics and non-isothermal kinetics of macro-algae with an emphasis on the possible partial gasification at higher temperatures. Front. Energy Res. 2019, 7, 7. [Google Scholar] [CrossRef]
- Aketo, T.; Hoshikawa, Y.; Nojima, D.; Yabu, Y.; Maeda, Y.; Yoshino, T.; Takano, H.; Tanaka, T. Selection and characterization of microalgae with potential for nutrient removal from municipal wastewater and simultaneous lipid production. J. Biosci. Bioeng. 2020, 129, 565–572. [Google Scholar] [CrossRef]
- Friis, J.; Holm, C.; Halling-Sørensen, B. Evaluation of elemental composition of algal biomass as toxical endpoint. Chemosphere 1998, 37, 2665–2676. [Google Scholar] [CrossRef]
- Pang, Y.Z.; Liu, Y.P.; Li, X.J.; Wang, K.S.; Yuan, H.R. Improving Biodegradability and Biogas Production of Corn Stover through Sodium Hydroxide Solid State Pretreatment. Energy Fuels 2008, 22, 2761–2766. [Google Scholar] [CrossRef]
- Ragab, S.; El Nemr, A. Zirconyl chloride as a novel and efficient green Lewis acid catalyst for direct acetylation of cotton cellulose in the presence and absence of solvent. J. Polym. Res. 2019, 26, 156. [Google Scholar] [CrossRef]
- Nyns, E.J. Biomethanation Processes; Wiley-VCH: Berlin, Germany, 1986; pp. 207–267. [Google Scholar]
- Kivaisi, A.K.; Mtila, M. Production of biogas from water hyacinth (Eichhorniacrassipes) in a two stage bioreactor. World J. Microbiol. Biotechnol. 1997, 14, 125–131. [Google Scholar] [CrossRef]
- Mshandete, A.; Kivaisi, A.; Rubindamayugi, M.; Mattiasson, B. Anaerobic batch co-digestion of sisal pulp and fish wastes. Bioresour. Technol. 2004, 95, 19–24. [Google Scholar] [CrossRef]
- Ganzoury, M.A.; Allam, N.K. Impact of nanotechnology on biogas production: A mini-review. Renew. Sustain. Energy Rev. 2015, 50, 1392–1404. [Google Scholar] [CrossRef]
- Elijah, T.; Ibifuro, A.; Yahaya, S.M. The study of cow dung as co-substrate with rice husk in biogas production. Sci. Res. Essay 2009, 9, 861–866. [Google Scholar]
- Dehghani, H.M. Effectiveness of ultrasound on the destruction of Escherichia coli. Am. J. Environ. Sci. 2005, 1, 187–189. [Google Scholar] [CrossRef][Green Version]
- Mason, T.J.; Peters, D. Practical Sonochemistry: Power Ultrasound and Applications, 2nd ed.; Horwood Publishing Ltd.: Chichester, UK, 2004. [Google Scholar]
- Belgiorno, V.; Rizzo, L.; Fatta, D.; Della Rocca, C.; Lofrano, G.; Nikolaou, A.; Naddeo, V.; Meric, S. Review on endocrine disrupting-emerging compounds in urban wastewater: Occurrence and removal by photocatalysis and ultrasonic irradiation for wastewater reuse. Desalination 2007, 21, 166–176. [Google Scholar] [CrossRef]
- Naddeo, V.; Belgiorno, V.; Landi, M.; Zarra, T.; Napoli, R.M.A. Effect of sonolysis on waste activated sludge solubilisation and anaerobic biodegradability. Desalination 2009, 249, 762–767. [Google Scholar] [CrossRef]
- El-Hadj, T.B.; Dosta, J.; Márquez-Serrano, R.; Mata-Álvarez, J. Effect of ultrasound pretreatment in mesophilic and thermophilic anaerobic digestion with emphasis on naphthalene and pyrene removal. Water Res. 2007, 41, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Thiem, A.; Nickel, K.; Zellhorn, M.; Neis, U. Ultrasonic waste activated sludge disintegration for improving anaerobic stabilization. Water Sci. Technol. 2001, 35, 2003–2009. [Google Scholar] [CrossRef]
- Kim, J.; Park, C.; Kim, T.H.; Lee, M.; Kim, S.; Kim, S.W.; Lee, J. Effects of various pretreatments for enhanced anaerobic digestion with waste activated sludge. J. Biosci. Bioeng. 2003, 95, 271–275. [Google Scholar] [CrossRef]
- Kim, M.; Gomec, C.Y.; Ahn, Y.; Speece, R.E. Hydrolysis and acidogenesis of particulate organic material in mesophilic and thermophilic anaerobic digestion. Environ. Technol. 2003, 24, 1183–1190. [Google Scholar] [CrossRef]
- Bougrier, C.; Albasi, C.; Delgenés, J.P.; Carrère, H. Effect of ultrasonic, thermal and ozone pre-treatments on waste activated sludge solubilisation and anaerobic biodegradability. Chem. Eng. Process. 2006, 45, 711–718. [Google Scholar] [CrossRef]
- Erden, G.; Buyukkamaci, N.; Filibeli, A. Effect of low frequency ultrasound on anaerobic biodegradability of meat processing effluent. Desalination. 2010, 259, 223–227. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, D.; Dai, L.; Chen, Y.; Dai, X. Efects of metal nanoparticles on methane production from waste-activated sludge and microorganism community shift in anaerobic granular sludge. Sci. Rep. 2016, 6, 1–10. [Google Scholar]
- Zaidi, A.A.; Ruizhe, F.; Shi, Y.; Khan, S.Z. Nanoparticles augmentation on biogas yield from microalgal biomass anaerobic digestion. Int. J. Hydrogen Energy 2018, 43, 14202–14213. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, D.; Fang, H. Comparison of enhancement of anaerobic nano-zero valent iron and zero valent iron. RSC Adv. 2018, 8, 27181–27190. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, W.; Zhang, H.; Wang, Z.; Fan, C.; Zang, L. Recent achievements in enhancing anaerobic digestion with carbon-based functional materials. Bioresour. Technol. 2018, 266, 555–567. [Google Scholar] [CrossRef]
- Lovley, D.R. Syntrophy Goes Electric: Direct Interspecies Electron Transfer. Annu. Rev. Microbiol. 2017, 71, 643–664. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.M.E.; Eljamal, O.; Amen, T.W.M.; Sugihara, Y.; Matsunaga, N. Optimized nanoscale zero-valent iron supported on treated activated carbon for enhanced nitrate and phosphate removal from water. Chem. Eng. J. 2017, 309, 349–365. [Google Scholar] [CrossRef]
- Ali, R.M.; Elkatory, M.R.; Hamad, H.A. Highly active and stable magnetically recyclable CuFe2O4 as a heterogenous catalyst for efficient conversion of waste frying oil to biodiesel. Fuel 2020, 268, 117297. [Google Scholar] [CrossRef]
- Lei, Y.; Wei, L.; Liu, T.; Xiao, Y.; Dang, Y.; Sun, D.; Holmes, D.E. Magnetite enhances anaerobic digestion and methanogenesis of fresh leachate from a municipal solid waste incineration plant. Chem. Eng. J. 2018, 348, 992–999. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.; He, X.; Zheng, L.; Cheng, S.; Li, Z. Diminished inhibitory impact of ZnO nanoparticles on anaerobic fermentation by the presence of TiO2 nanoparticles: Phenomenon and mechanism. Sci. Total Environ. 2019, 647, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahab, T.A.M.; Mohanty, M.K.; Sahoo, P.K.; Behera, D. Impact of iron nanoparticles on biogas production and effluent chemical composition from anaerobic digestion of cattle manure. Biomass Convers. Biorefin. 2020, 1–13. [Google Scholar] [CrossRef]
















| Experiment | Pretreatment | Incubation Temp. (°C) | I/S Ratio |
|---|---|---|---|
| Batch 1 | Sediment + algae untreated | 37 ± 1 | 20:1.5 |
| Batch 2 | Sediment + Algae O3 (10 min) | 37 ± 1 | 20:1.5 |
| Batch 3 | Sediment + Algae O3 (15 min) | 37 ± 1 | 20:1.5 |
| Batch 4 | Sediment + Algae O3 (30 min) | 37 ± 1 | 20:1.5 |
| Batch 5 | Sediment + Algae US (10 min) | 37 ± 1 | 20:1.5 |
| Batch 6 | Sediment + Algae US (15 min) | 37 ± 1 | 20:1.5 |
| Batch 7 | Sediment + Algae US (30 min) | 37 ± 1 | 20:1.5 |
| Batch 8 | Sediment + Algae MW (2 min) | 37 ± 1 | 20:1.5 |
| Batch 9 | Sediment + Algae MW (4 min) | 37 ± 1 | 20:1.5 |
| Batch 10 | Sediment + Algae (Fe 5 mg/L) | 37 ± 1 | 20:1.5 |
| Batch 11 | Sediment + Algae (Fe 10 mg/L) | 37 ± 1 | 20:1.5 |
| Batch 12 | Sediment + Algae (Fe 20 mg/L) | 37 ± 1 | 20:1.5 |
| Batch 13 | Sediment + Algae 10 min O3 (Fe 5 mg/L) | 37 ± 1 | 20:1.5 |
| Batch 14 | Sediment + Algae 10 min US (Fe 5 mg/L) | 37 ± 1 | 20:1.5 |
| Batch 15 | Sediment + Algae 2 min MW (Fe 5 mg/L) | 37 ± 1 | 20:1.5 |
| Material | Elements Content | |
|---|---|---|
| O | Fe | |
| Green Fe3O4 | 18.87 ± 0.6 | 81.13 ± 1.2 |
| Sample | BET Surface Area (m2/g) | Mean Pore Diameter (nm) | Total Pore Volume (cm3/g) |
|---|---|---|---|
| Green Fe3O4 | 37.85 | 9.56 | 0.09 |
| Proximate Tests | U. intestinalis | Sediment |
|---|---|---|
| TS% | 85.11 | 57.19 |
| Ash% | 29.45 | 79.43 |
| VS% | 70.55 | 20.57 |
| C% | 23.05 | - |
| N% | 2.40 | - |
| H% | 4.6 | - |
| C/N | 9.60 | - |
| US | ||||||
|---|---|---|---|---|---|---|
| R2 | Predicted P (mL/g VS) | Differences (%) | Rmax mL/g VS.day | λ (Day) | RMSE | |
| untreated | 0.957 | 44.14 | 2.97 | 9.067 | 0.54 | 11.17 |
| 10 US | 0.987 | 182.87 | 2.13 | 6.18 | 0.0227 | 12.11 |
| 15 US | 0.968 | 24.83 | 4.480 | 3.087 | 0.298 | 1.188 |
| 30 US | 0.984 | 20.57 | 2.016 | 2.71 | 1.15 | 0.695 |
| O3 | ||||||
| untreated | 0.957 | 44.14 | 2.97 | 9.067 | 0.54 | 11.17 |
| 10 O3 | 0.996 | 187.6 | 3.42 | 17.51 | 0.0890 | 3.65 |
| 15 O3 | 0.985 | 91.12 | 7.48 | 9.47 | 0.163 | 3.86 |
| 30 O3 | 0.9 | 86.34 | 0.22 | 10.56 | 0.202 | 4.84 |
| MW | ||||||
| untreated | 0.957 | 44.14 | 2.97 | 9.067 | 0.54 | 11.17 |
| 2 MW | 0.992 | 83.63 | 0.55 | 8.27 | 0.198 | 2.66 |
| 4 MW | 0.926 | 49.53 | 8.27 | 2.38 | 0.88 | 3.57 |
| Fe3O4 | ||||||
| untreated | 0.957 | 44.14 | 2.97 | 9.067 | 0.54 | 11.17 |
| 5 Fe3O4 | 0.858 | 35.34 | 4.55 | 3.36 | 0.18 | 3.71 |
| 10 Fe3O4 | 0.975 | 51.63 | 2.58 | 3.09 | 0.35 | 2.21 |
| 20 Fe3O4 | 0.969 | 159.18 | 1.65 | 12.26 | 0.13 | 10.027 |
| Combined pretreatments—Fe3O4 | ||||||
| US-Fe3O4 | 0.951 | 74.96 | 1.14 | 8.85 | 0.13 | 5.70 |
| O3-Fe3O4 | 0.993 | 70.04 | 2.71 | 2.59 | 0.69 | 1.44 |
| MW-Fe3O4 | 0.979 | 776.43 | 7.49 | 49.89 | 0.0287 | 10.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Nemr, A.; Hassaan, M.A.; Elkatory, M.R.; Ragab, S.; Pantaleo, A. Efficiency of Fe3O4 Nanoparticles with Different Pretreatments for Enhancing Biogas Yield of Macroalgae Ulva intestinalis Linnaeus. Molecules 2021, 26, 5105. https://doi.org/10.3390/molecules26165105
El Nemr A, Hassaan MA, Elkatory MR, Ragab S, Pantaleo A. Efficiency of Fe3O4 Nanoparticles with Different Pretreatments for Enhancing Biogas Yield of Macroalgae Ulva intestinalis Linnaeus. Molecules. 2021; 26(16):5105. https://doi.org/10.3390/molecules26165105
Chicago/Turabian StyleEl Nemr, Ahmed, Mohamed A. Hassaan, Marwa R. Elkatory, Safaa Ragab, and Antonio Pantaleo. 2021. "Efficiency of Fe3O4 Nanoparticles with Different Pretreatments for Enhancing Biogas Yield of Macroalgae Ulva intestinalis Linnaeus" Molecules 26, no. 16: 5105. https://doi.org/10.3390/molecules26165105
APA StyleEl Nemr, A., Hassaan, M. A., Elkatory, M. R., Ragab, S., & Pantaleo, A. (2021). Efficiency of Fe3O4 Nanoparticles with Different Pretreatments for Enhancing Biogas Yield of Macroalgae Ulva intestinalis Linnaeus. Molecules, 26(16), 5105. https://doi.org/10.3390/molecules26165105







