Abstract
Gluconeogenesis is a key interface between organic acid/amino acid/lipid and sugar metabolism. The aims of this article are four-fold. First, to provide a concise overview of plant gluconeogenesis. Second, to emphasise the widespread occurrence of gluconeogenesis and its utilisation in diverse processes. Third, to stress the importance of the vacuolar storage and release of Krebs cycle acids/nitrogenous compounds, and of the role of gluconeogenesis and malic enzyme in this process. Fourth, to outline the contribution of fine control of enzyme activity to the coordinate-regulation of gluconeogenesis and malate metabolism, and the importance of cytosolic pH in this.
1. Introduction
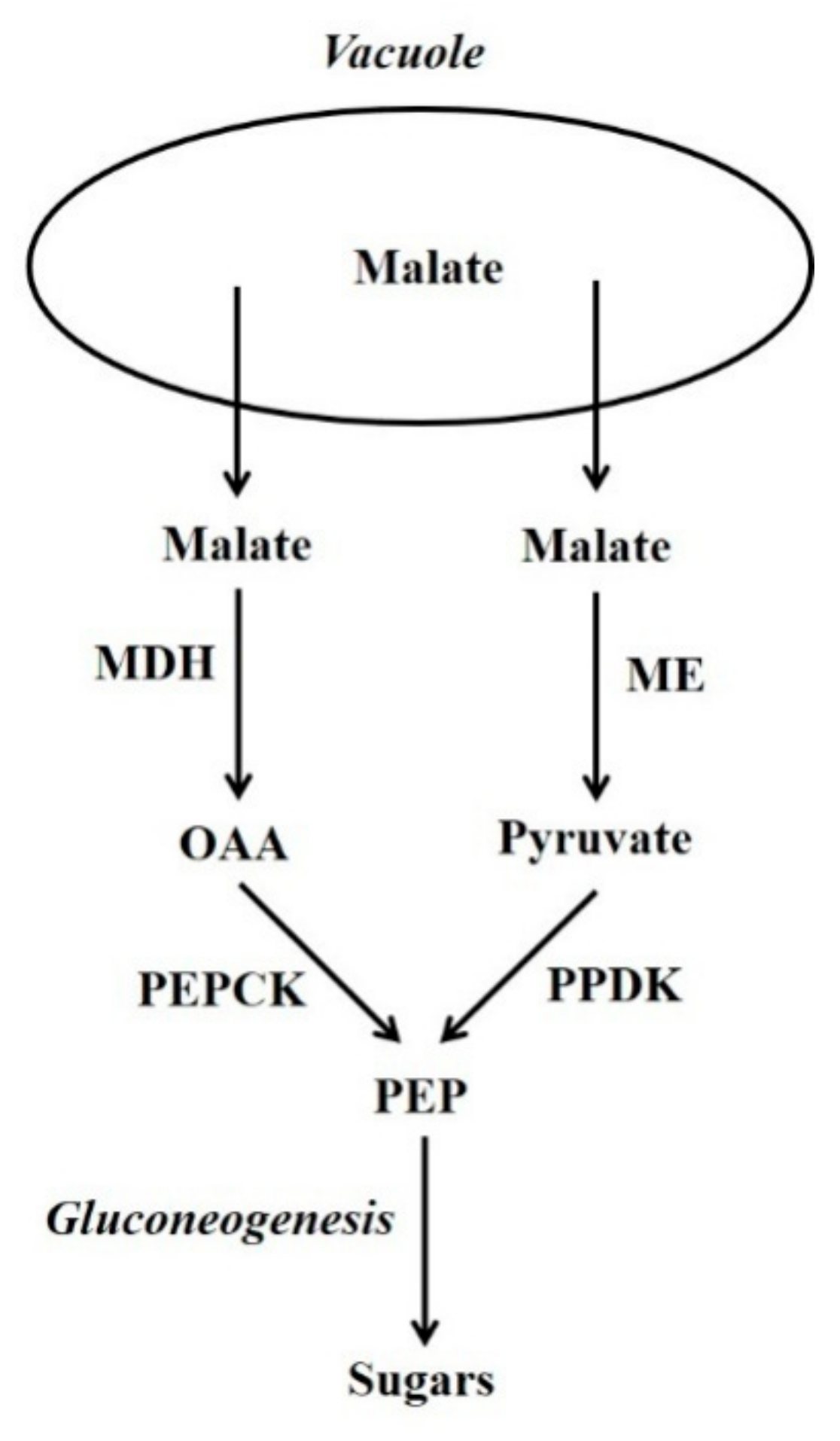
In plants, certain non-carbohydrate compounds such as lipids, amino acid carbon skeletons, and Krebs cycle organic acids (i.e., malic and other acids that are intermediates of the Krebs cycle) can be converted to sugars by a process called gluconeogenesis. Lipids and many amino acids are first converted to Krebs cycle organic acids, and the latter are then converted to sugars by gluconeogenesis. Thus, gluconeogenesis is a key interface between organic acid/amino acid/lipid metabolism and sugar metabolism. Two pathways can be used by plant gluconeogenesis [1,2,3]. One pathway employs phosphoenolpyruvate carboxykinase (PEPCK; oxalacetate (OAA) + ATP  phosphoenolpyruvate (PEP) + CO2 + ADP) in conjunction with malate dehydrogenase (MDH), whilst the alternate pathway employs pyruvate orthophosphate dikinase (PPDK; ATP + pyruvate + Pi
phosphoenolpyruvate (PEP) + CO2 + ADP) in conjunction with malate dehydrogenase (MDH), whilst the alternate pathway employs pyruvate orthophosphate dikinase (PPDK; ATP + pyruvate + Pi  AMP + PEP + PPi) in conjunction with malic enzyme (ME) (Figure 1) [1,2,4]. Although the presence of PEPCK protein is constitutive in some plant tissues (e.g. the flesh of fruits at certain stages of development and the leaves of many C4 and Crassulacean acid metabolism [CAM] plants) in others it is not; nevertheless, in many of the latter its presence can be induced by certain stimuli. The situation is similar for PPDK, and for both enzymes their presence confers on most tissues the capacity for gluconeogenesis [2,3]. It should be noted that, in the photosynthetic tissues of C4 leaves, both PEPCK and PPDK function in the C4 photosynthesis and not gluconeogenesis [2,3]. By contrast, MDH and ME are present in numerous tissues in which they do not function in gluconeogenesis [2,3]. Whether the PEPCK or PPDK pathway is utilised, is dependent on the species, tissue, developmental stage and potentially environmental factors such as N-supply.
AMP + PEP + PPi) in conjunction with malic enzyme (ME) (Figure 1) [1,2,4]. Although the presence of PEPCK protein is constitutive in some plant tissues (e.g. the flesh of fruits at certain stages of development and the leaves of many C4 and Crassulacean acid metabolism [CAM] plants) in others it is not; nevertheless, in many of the latter its presence can be induced by certain stimuli. The situation is similar for PPDK, and for both enzymes their presence confers on most tissues the capacity for gluconeogenesis [2,3]. It should be noted that, in the photosynthetic tissues of C4 leaves, both PEPCK and PPDK function in the C4 photosynthesis and not gluconeogenesis [2,3]. By contrast, MDH and ME are present in numerous tissues in which they do not function in gluconeogenesis [2,3]. Whether the PEPCK or PPDK pathway is utilised, is dependent on the species, tissue, developmental stage and potentially environmental factors such as N-supply.
 phosphoenolpyruvate (PEP) + CO2 + ADP) in conjunction with malate dehydrogenase (MDH), whilst the alternate pathway employs pyruvate orthophosphate dikinase (PPDK; ATP + pyruvate + Pi
phosphoenolpyruvate (PEP) + CO2 + ADP) in conjunction with malate dehydrogenase (MDH), whilst the alternate pathway employs pyruvate orthophosphate dikinase (PPDK; ATP + pyruvate + Pi  AMP + PEP + PPi) in conjunction with malic enzyme (ME) (Figure 1) [1,2,4]. Although the presence of PEPCK protein is constitutive in some plant tissues (e.g. the flesh of fruits at certain stages of development and the leaves of many C4 and Crassulacean acid metabolism [CAM] plants) in others it is not; nevertheless, in many of the latter its presence can be induced by certain stimuli. The situation is similar for PPDK, and for both enzymes their presence confers on most tissues the capacity for gluconeogenesis [2,3]. It should be noted that, in the photosynthetic tissues of C4 leaves, both PEPCK and PPDK function in the C4 photosynthesis and not gluconeogenesis [2,3]. By contrast, MDH and ME are present in numerous tissues in which they do not function in gluconeogenesis [2,3]. Whether the PEPCK or PPDK pathway is utilised, is dependent on the species, tissue, developmental stage and potentially environmental factors such as N-supply.
AMP + PEP + PPi) in conjunction with malic enzyme (ME) (Figure 1) [1,2,4]. Although the presence of PEPCK protein is constitutive in some plant tissues (e.g. the flesh of fruits at certain stages of development and the leaves of many C4 and Crassulacean acid metabolism [CAM] plants) in others it is not; nevertheless, in many of the latter its presence can be induced by certain stimuli. The situation is similar for PPDK, and for both enzymes their presence confers on most tissues the capacity for gluconeogenesis [2,3]. It should be noted that, in the photosynthetic tissues of C4 leaves, both PEPCK and PPDK function in the C4 photosynthesis and not gluconeogenesis [2,3]. By contrast, MDH and ME are present in numerous tissues in which they do not function in gluconeogenesis [2,3]. Whether the PEPCK or PPDK pathway is utilised, is dependent on the species, tissue, developmental stage and potentially environmental factors such as N-supply.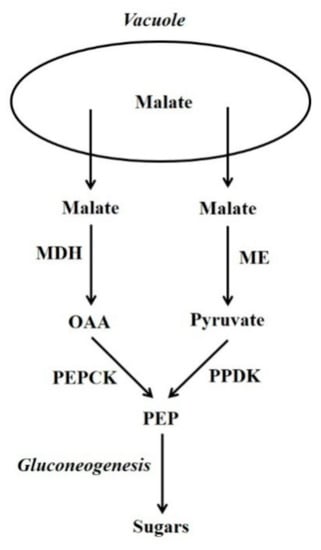
Figure 1.
The PEPCK and PPDK gluconeogenesis pathways. OAA = oxaloacetate; PEP = phosphoenolpyruvate; MDH = malate dehydrogenase; ME = malic enzyme; PEPCK = phosphoenolpyruvate carboxykinase; PPDK = pyruvate orthophosphate dikinase.
The vacuolar storage, subsequent release and metabolism of certain Krebs cycle acids (e.g., malic and citric) and nitrogenous compounds (e.g., ammonium and alanine) is of widespread occurrence in plants: and of fundamental importance when considering both gluconeogenesis [5,6,7], and certain other aspects of plant metabolism [8,9]. The vacuole occupies a considerable proportion of the volume of most plant cells, and this is one reason why it is well suited to the storage of these compounds. In addition, enzymes that metabolise malate and nitrogenous compounds are generally not present in the vacuole [7,10]. The storage-release of these metabolites can be associated with various processes such as osmoregulation, coordination of the import and utilisation of nitrogenous compounds, regulation of metabolite concentrations in different subcellular compartments and CAM. In association with gluconeogenesis this vacuolar storage-release is likely to occur in many plant organs/tissues (e.g., flowers, seeds, fruits, leaves, roots, vasculature, stomata and trichomes), and be involved in diverse processes such as vascular function, fruit and seed development, seed germination, osmoregulation, nitrogen metabolism, defence and responses to various stresses [7,11,12,13,14,15,16].
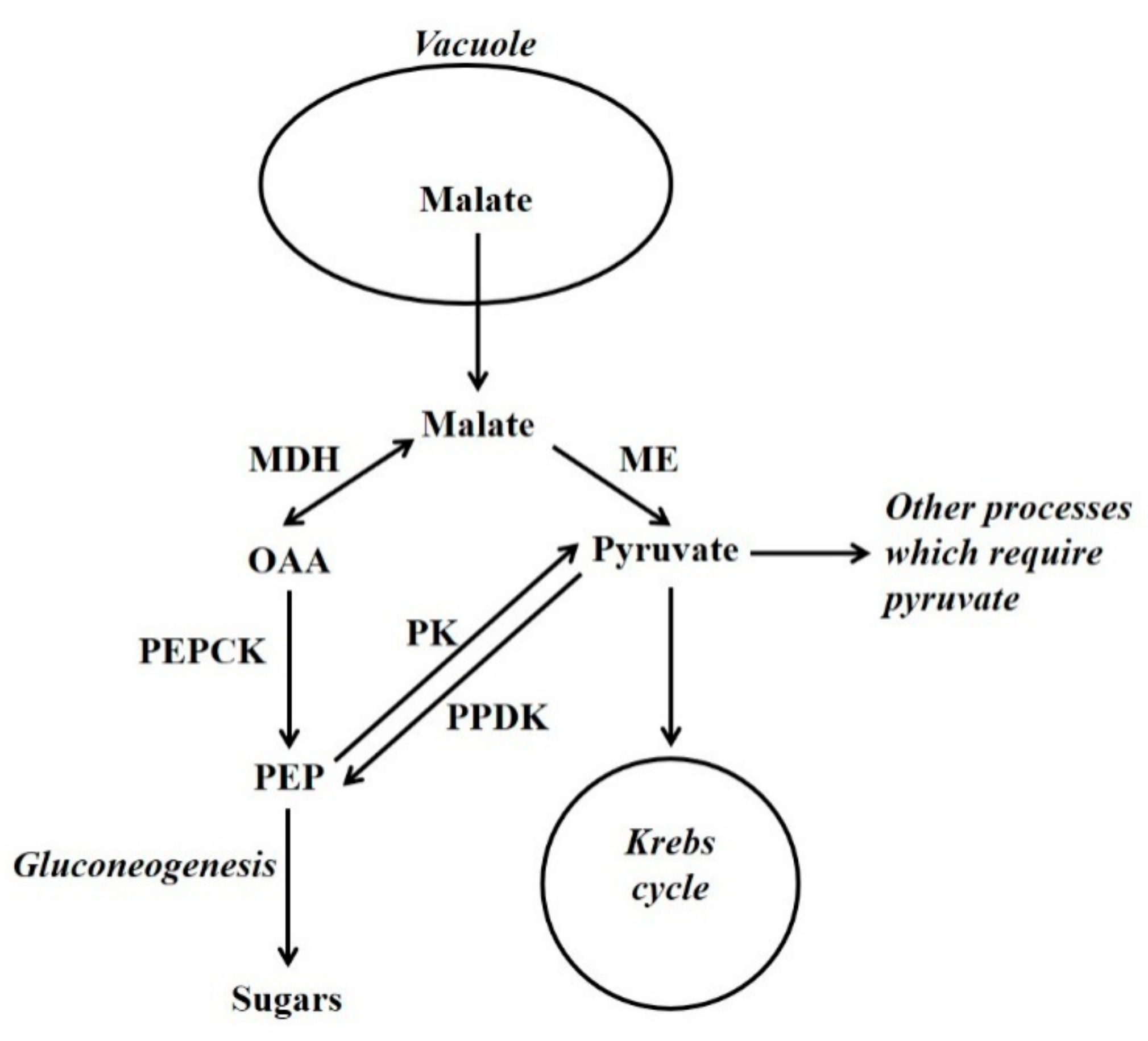
In many tissues gluconeogenesis is associated with the metabolism of Krebs cycle acids and/or nitrogenous compounds when they are released from the vacuole. These compounds, or their breakdown products, are used by processes such as the Krebs cycle. Gluconeogenesis occurs when the demand of other processes is exceeded (Figure 2) [7,16]. Thus, at these times the amount of these compounds released from the vacuole is such that glycolytic flux is not necessary to provide metabolic intermediates. The allocation of vacuolar-released Krebs cycle acids between gluconeogenesis and other processes must be regulated. There is evidence that in a range of tissues (e.g., leaves of both C3 and CAM plants, fruit flesh and stomata), there are times when Krebs cycle acids are synthesised and deposited in the vacuole, and times when they are released from the vacuole and metabolised [2,7,16,17,18]. In leaves of C3 plants, it appears that this release occurs largely during the nocturnal period [18,19]. Indeed, in these leaves modelling and NMR studies have shown that much of the carbon used in the synthesis of glutamate is derived from CO2 fixed in a previous nocturnal period, and stored in the vacuole as citrate [18,20,21]. Clearly, Krebs cycle acids are often synthesised from sugars, and a key enzyme utilised in this is phophoenolpyruvate carboxylase (PEPC; PEP + HCO3− → OAA + Pi) [2]. PEPC together with enzymes used in malate breakdown, such as malate dehydrogenase (MDH), PEPCK and cytosolic NADP-malic enzyme (NADP-ME), are present in the cytosol of the same cells [2,22]. Hence, there must be mechanisms that coordinate flux through malate synthetic and degradative enzymes.
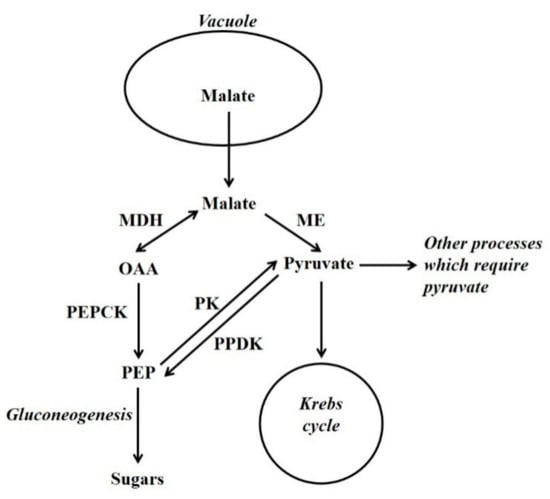
Figure 2.
The utilisation of vacuolar-released malate by gluconeogenesis and other processes such as the Krebs cycle. OAA = oxaloacetate; PEP = phosphoenolpyruvate; MDH = malate dehydrogenase; ME = malic enzyme; PEPCK = phosphoenolpyruvate carboxykinase; PK = pyruvate kinase; PPDK = pyruvate orthophosphate dikinase.
Malate serves an anaplerotic role in replenishing Krebs cycle intermediates. This is necessary because intermediates are withdrawn from the cycle, and used in biosynthesis (e.g., amino acids) or storage (e.g., citrate). When malate is released from the vacuole it can fulfil this anaplerotic requirement. However, for the Krebs cycle to function primarily in the generation of NADH/ATP (i.e., intermediates are not withdrawn from the cycle) pyruvate is required. This is because of the stoichiometry of the Krebs cycle: if malate/OAA (4C) enter the cycle they become citrate (6C) (in this situation pyruvate is also required and this forms acetyl CoA, that is utilised together with OAA, by citrate synthase, to form citrate). Citrate then flows through the cycle to form malate and 2C are lost as CO2. Thus, in this situation 6C have entered the cycle and only 2C have left it [2]. Thus, for malate to be fully oxidised it must first be converted to pyruvate (to prevent a build-up of intermediates of the cycle). This conversion of malate to pyruvate is an example of cataplerosis, and potentially both PEPCK (in conjunction with pyruvate kinase [PK]) and ME can function as cataplerotic enzymes [2,23] (Figure 2). The importance of a supply of pyruvate to the mitochondrion is illustrated by a recent study [24]. Gluconeogenesis can also occur from amino acid carbon skeletons [7,25,26,27,28]. For example, in maize endosperm there is evidence that vacuolar release of alanine results in gluconeogenesis via PPDK [7]. The process of storage, release and subsequent metabolism of Krebs cycle acids and the association with nitrogen metabolism has likely provided building blocks for the evolution of processes such as CAM, stomatal metabolism, C4 photosynthesis and the biochemical pH stat [7,18].
2. Potential Occurrence of Gluconeogenesis
2.1. Germinating Seeds
In germinating seeds, stored lipids and a proportion of stored proteins are converted to sugars by gluconeogenesis [25,29,30,31,32]. Malate is produced from lipids by the glyoxylate cycle [30,33,34]. It is possible, in order to assist in coordinating lipid/protein breakdown with the utilisation of these breakdown products, that a proportion of this malate is stored temporarily in the vacuole. Until recently it was thought that only the PEPCK gluconeogenic pathway was utilised in germinating seeds, however, recent work indicated that the PPDK pathway contributes in Arabidopsis [4]. Nevertheless, in cotyledons from germinating cucumber PEPCK was very abundant, whereas PPDK was not detected (our unpublished data), and this suggests that PPDK makes little or no contribution to gluconeogenesis in this organ. Clearly, in order to evaluate how widespread is the use of the PPDK pathway, the abundance of PEPCK and PPDK (protein/activity) in germinating seeds of a range of species needs to be determined.
2.2. Developing Seeds
Radiolabelling studies have shown that gluconeogenesis from amino acids occurs in developing seeds [7,26,27,28,35]. Although the glyoxylate cycle is active in many developing seeds, it is often not linked to gluconeogenesis [36]. PEPCK and PPDK are often present in developing seeds, however, their abundance depends on the species, stage of development and tissue [7,37,38,39]. In maize kernels PPDK is present in some tissues, such as the endosperm, and PEPCK in others, and both enzymes appear to have a role in gluconeogenesis associated with nitrogen metabolism [7]. In developing cherry and plum seeds PEPCK is abundant, whereas PPDK was not detected [40,41]. In the seeds of several species the abundance of PEPCK is correlated with the deposition of storage proteins, as is PPDK in maize endosperm [7,38,42]. The abundance of PEPCK in developing seeds can be increased greatly by either feeding seeds certain nitrogenous compounds in vitro, or by increasing the amount of nitrogenous compounds fed to the plant [38,42].
2.3. Senescing Tissues
The glyoxylate cycle occurs in both senescing and carbon-starved tissues, and it has been proposed to be linked to gluconeogenesis. However, this is unlikely, and one role may be in the anaplerotic replenishment of the Krebs cycle [43,44]. PPDK has been proposed to function in amino acid metabolism, associated with the export of nitrogenous compounds out of senescing leaves [45]. However, this role might not be associated with senescence per se, because PPDK protein is present in mature tomato leaves and its abundance g−1 FW does not increase during senescence [7].
2.4. Vasculature
PEPCK is present in the vasculature of a range of tissues, and this abundance is often increased by feeding the plant ammonium but not nitrate [46,47]. In the vasculature of rice leaves there is evidence that PEPCK plays a role in metabolism associated with xylem-phloem transfer of nitrogenous compounds [48]. In leaf vasculature PPDK functions in nitrogen metabolism [45], and gluconeogenesis from alanine occurs in cottonwood leaves [49]. Thus, it is possible that in leaves PPDK could also function in nitrogen metabolism that is associated with xylem-phloem transfer of nitrogenous compounds.
2.5. Roots
In roots PEPCK appears in response to feeding them ammonium, and is localised in the pericycle and vascular tissues. PPDK appears in roots subjected to anaerobic conditions [7,46,47,50]. Some studies have shown that PEPCK abundance in roots is not increased by anaerobic conditions, whilst others have, and reasons for these differences between studies need to be investigated [7,47,51].
2.6. Trichomes and Plant Defense
PEPCK is often present in defence tissues, such as some trichomes and the extrafascicular phloem of Curcurbits, in tissues undergoing lignification and in tissues challenged by pathogens. In many of these tissues large amounts of ammonium are likely to be produced by phenylalanine ammonia lyase (PAL) during the synthesis of phenolics [40,41,43,47,52,53,54,55]. Thus, gluconeogenesis could be used in the metabolism of malate released from the vacuole which is associated with vacuolar storage/release of ammonium. Further, it is possible that gluconeogenesis could be associated with osmoregulation. Thus, in trichomes and certain other tissues, both malate and sugars might be used to control processes such as expansion and import of materials [13,56,57].
2.7. CAM Plants
In photosynthetic tissues of CAM plants, malate/citrate is synthesised at night and stored in the vacuole. During the day, these acids are released from the vacuole, decarboxylated by PEPCK or ME and the released CO2 is used by photosynthesis. Pyruvate produced by ME is converted to PEP by PPDK. PEP is also produced from malate by malate dehydrogenase (MDH) in conjunction with PEPCK. PEP is then converted to sugars by gluconeogenesis [1,2].
2.8. Stomata
Malate/citrate and sugars are abundant osmotica in stomata, and both can be important in turgor regulation associated with stomatal opening/closing [58,59,60,61,62]. Both PEPCK and PPDK could potentially play a role in converting malate/citrate, which is released from the vacuole, to sugars [15,17,58,60]. However, there is evidence that at least in certain species PEPCK may be more important [58]. PEPCK has been localised in motor cells of rice leaves [63], and in these a similar role in osmoregulation is possible.
2.9. PPDK and Gluconeogenesis in Fruits
PPDK protein was not detected in the flesh of a range of soft fruits, plum, cherry or grape [22,41,64,65]. PPDK protein was detected in tomato flesh, and in this its abundance corresponded to an estimated activity of c0.003–0.014 µmol min−1 g−1 FW [12]. PEPCK activity in tomato, grape and cherry flesh is c0.1–0.3 µmol min−1 g−1 FW [16,64,66]. PPDK polypeptide has been detected in peach flesh [67,68]. However, throughout the ripening of peach flesh the amount of PPDK polypeptide is very low and its estimated activity is around 0.0003–0.0014 µmol min−1 g−1 FW [12]. By contrast PEPCK is quite abundant in peach flesh throughout ripening (c0.18 U g−1 FW), and this suggests that the bulk of any gluconeogenic flux utilises PEPCK [12]. Similarly, if PPDK is present in grape pericarp under normal conditions of growth it is likely to be at very low abundance compared to PEPCK [52]. Nevertheless, it is possible that the abundance of PPDK increases under certain conditions (e.g., low O2) [69]. Therefore, in the flesh of most fruits it appears that the PEPCK pathway makes the largest contribution to gluconeogenesis [12]. PPDK is abundant in the peel of cactus pear fruits, however, in these it is a component of CAM [70]. Substantial amounts of PPDK activity were detected in extracts of bean fruit (Phaseolus vulgaris pod) [71].
2.10. Glyoxylate Cycle and Gluconeogenesis in Fruits
Isocitrate lyase (ICL) and malate synthase (MS) are key enzymes of the glyoxylate cycle. In the flesh of ripening banana fruits both MS transcripts and ICL activity are present [72,73]. ICL activity has been measured in extracts of cucumber flesh [74], and there is a very low expression of a MS gene in this tissue [75]. In extracts of pumpkin flesh, from fruits at the time of commercial harvest, ICL polypeptide and activity were not detected, whereas, in slices of the fruits incubated under darkness they were [76]. Nevertheless, the activity of ICL is very low in the fruits in which it has been detected: in ripening banana flesh (c0.0001 µmol min−1 g−1 FW) [73] and in cucumber flesh (c0.008 µmol min−1 g−1 FW) [74]. ICL polypeptide was either absent or at very low abundance in the flesh of both grape and some soft fruits [22,65]. Therefore, in the flesh of most fruits under normal conditions of growth it appears that the glyoxylate cycle makes little or no contribution to providing substrate for gluconeogenesis [12].
2.11. PEPCK and Gluconeogenesis in Fruits
Radiolabelling studies in the 1960s provided evidence for the occurrence of gluconeogenesis from malate in the flesh of grape berries [77,78], and it was hypothesised that the PEPCK pathway was used [79]. A detailed study established the presence of PEPCK in grape berries [80]. Subsequently, radiolabelling studies demonstrated gluconeogenesis from malate in the flesh of both ripening tomato and cherry fruits [81,82,83]. Studies in transgenic tomato fruits containing altered amounts of PEPCK support the view that the enzyme can participate in gluconeogenesis [84,85,86]. The presence of PEPCK in the flesh of a range of other fruits suggests that gluconeogenesis can occur in these [11,22,41,64,87]. In the flesh of fruits, gluconeogenesis and certain other aspects of malate metabolism are not thought to be associated with photosynthesis [88]. Consistent with this there is no correlation between the abundance of Calvin cycle enzymes and PEPCK in either the flesh or endocarp of several fruits [22,41,87].
3. Gluconeogenesis and the Vacuolar Release of Malate/Citrate and Nitrogenous Compounds
3.1. VacuolarMalate/Citrate Storage and Release
The bulk of the malate content of fruit flesh is located in the vacuole [10]. In grape pericarp it was proposed that during ripening malate was released from the vacuole, and its metabolism by NADP-ME provided pyruvate for the Krebs cycle, whereas metabolism by PEPCK produced PEP that was used in gluconeogenesis [89]. The view developed that in ripening grape pericarp glycolysis was largely inhibited, and the predominant substrate used by metabolism was malate [89,90,91]. However, the latter was shown to be incorrect, and if the whole ripening period was considered sugars provided the bulk of metabolic substrate [5,92]. In the ripening flesh of other fruits, sugars are also likely to provide the bulk of the metabolic substrate [11,93]. In the fruits of many species, PEPCK is present when there is no net decrease in their Krebs cycle acid content [93]. Further, in grape pericarp radiolabelling studies have shown that malate can be converted to sugars before ripening, and at this time the malate content increases [94]. These studies raised the question as to why gluconeogenesis occurred. The most likely explanation is that there is a turnover of the vacuolar malate/citrate pool throughout development [11,16]. Thus, at certain times malate/citrate is released from the vacuole, and when their breakdown products exceed the demands of other processes, gluconeogenesis occurs. Clearly maintaining an appropriate concentration of malate in the extravacuolar compartment is crucial because perturbations in this affect various processes such as starch metabolism [95]. Then, sometime later malate efflux stops, and malate is resynthesised and transported into the vacuole [11,16]. These malate effluxes are likely to be associated with nitrogen metabolism (see below) and/or osmoregulation. Osmoregulation plays an important role in the pericarp of fruits; and is involved in fruit softening, cell expansion and the import of materials [14,57,96].
3.2. Ammonium Metabolism
In a number of tissues PEPCK is associated with ammonium metabolism [2,38,42,48,52,97,98,99,100,101]. For example, PEPCK protein and activity are increased greatly in a range of plant tissues such as: roots, vasculature and developing seeds when they are fed nitrogenous compounds such as ammonium or asparagine (ammonium is often released from asparagine when it is metabolised) [38,42,46,47,52]. Ammonium can accumulate in both the flesh of fruits and other tissues such as roots and developing seeds [11,40,42,46,47,64,102,103]. The bulk of the intracellular contents of both malate and ammonium are located in the vacuole [104]. In sinks, ammonium can arise from its import in the xylem, its synthesis from imported nitrate and from the metabolism of amino acids and amides by asparaginase or glutamate dehydrogenase. In addition, ammonium is produced during the synthesis of many phenolic compounds as a result of PAL [105].
In leaves the release of malate/citrate and ammonium from vacuoles, and their incorporation into amino acids, is coordinated and occurs at certain times during the diurnal cycle [9,106]. In other tissues, such as the flesh of fruits and developing seeds, vacuolar release of malate/citrate and ammonium and their assimilation into amino acids are also likely to be coordinated. In leaves malate/citrate metabolism is intimately linked with pH regulation that is associated with nitrogen metabolism [9,106]. In sink tissues, pH regulation is also involved in the utilisation of malate/citrate in ammonium assimilation [11,97]. For example, protons are consumed when ammonium, produced by the metabolism of glutamine or asparagine, is stored in the vacuole and the carbon skeletons of these amides are metabolised. If malic/citric acid is synthesised in the cytosol, and malate/citrate transported into the vacuole this will counteract an increase in cytosolic pH. Subsequently, when ammonium is released from the vacuole and assimilated into amino acids, protons will be produced if sugars provide the amino acid carbon skeletons. However, if malate/citrate is released from the vacuole and used together with sugars to produce these carbon skeletons no protons are produced [97].
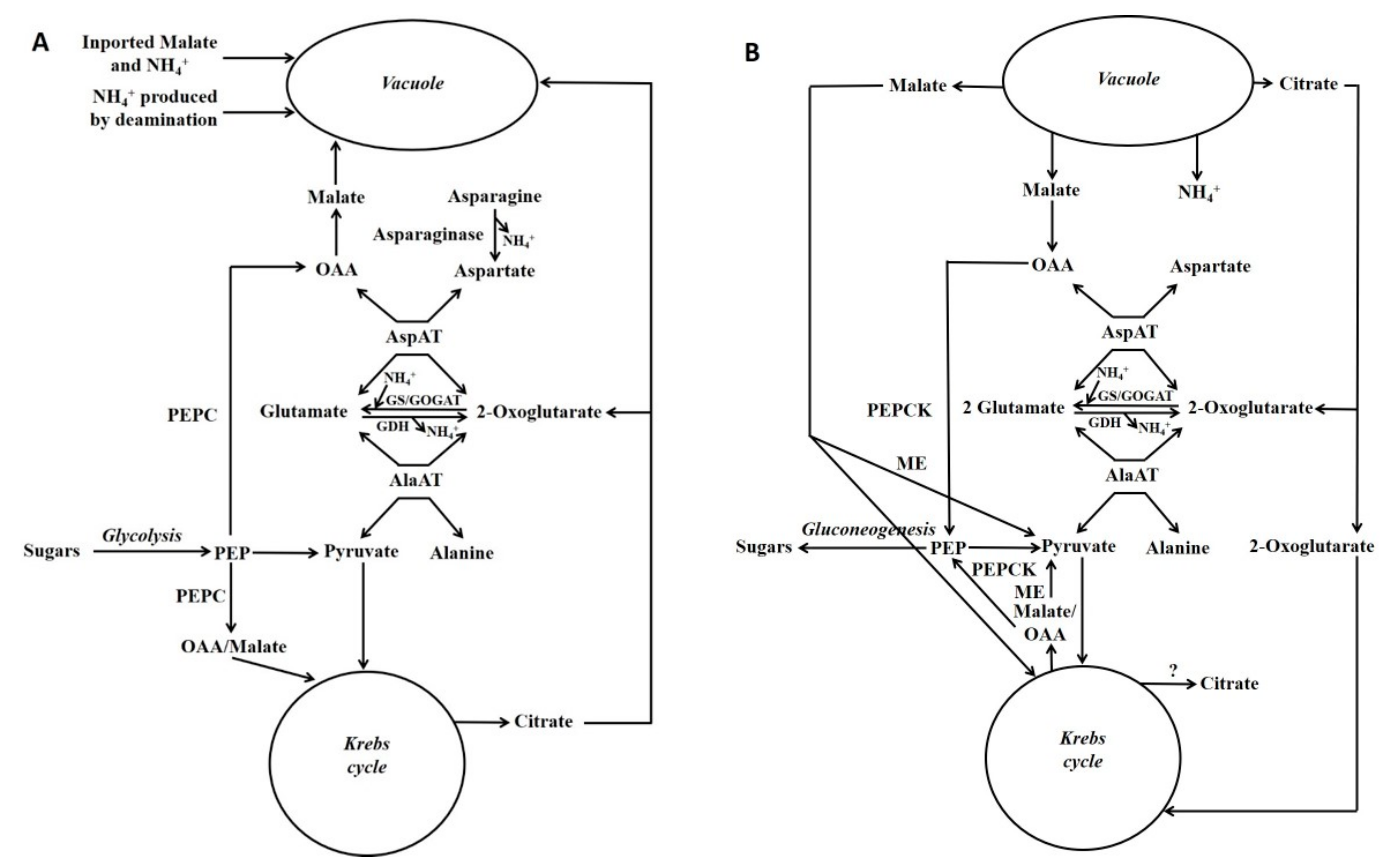
Schemes depicting events occurring during either the accumulation of malate/citrate in the vacuole or during their subsequent release are shown in Figure 3. Glutamate dehydrogenase (GDH) is considered to play an important role in regulating the cytoplasmic glutamate concentration [107]. Glutamate concentration is linked to the concentration of both aspartate and alanine because of the AspAT and AlaAT reactions. Thus, adjusting glutamate concentration by GDH can also potentially alter aspartate and alanine concentrations. For example, in the case of vacuolar efflux of alanine, GDH allows alanine to be metabolised without the requirement to synthesise large amounts of 2-OG (Figure 3 and Figure 4).
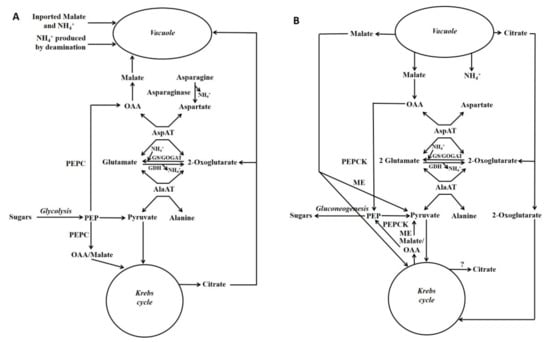
Figure 3.
Gluconeogenesis from malate and its association with nitrogen metabolism. Simplified scheme depicting gluconeogenesis associated with the metabolism of asparagine or ammonium: storage phase of these metabolites (A); utilisation phase (B) (Reproduced from Walker et al. [7]). Fluxes through different reactions in the schemes will differ according to factors such as which nitrogenous compound (e.g., asparagine [e.g., maize pedicel], glutamine or ammonium [e.g., maize root fed ammonium]) is the major input. Glutamate, aspartate, pyruvate and PEP are the precursors of most amino acids [105], and in both situations these metabolites could be produced. GDH = glutamate dehydrogenase; GOGAT = glutamine oxoglutarate aminotransferase; GS = glutamine synthase; ME = malic enzyme; OAA = oxalacetate; PEP = phosphoenolpyruvate; PEPC = phosphoenolpyruvate carboxylase.
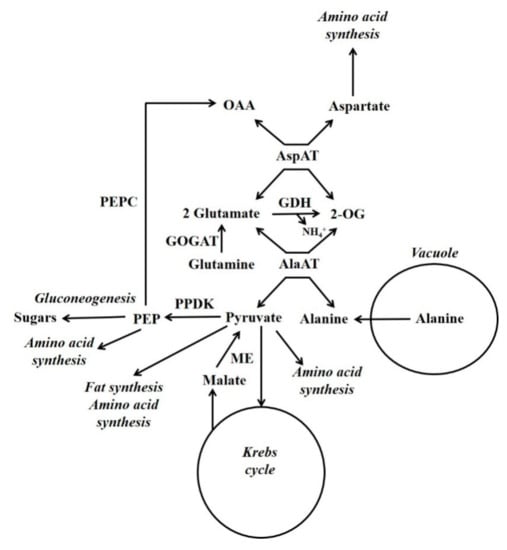
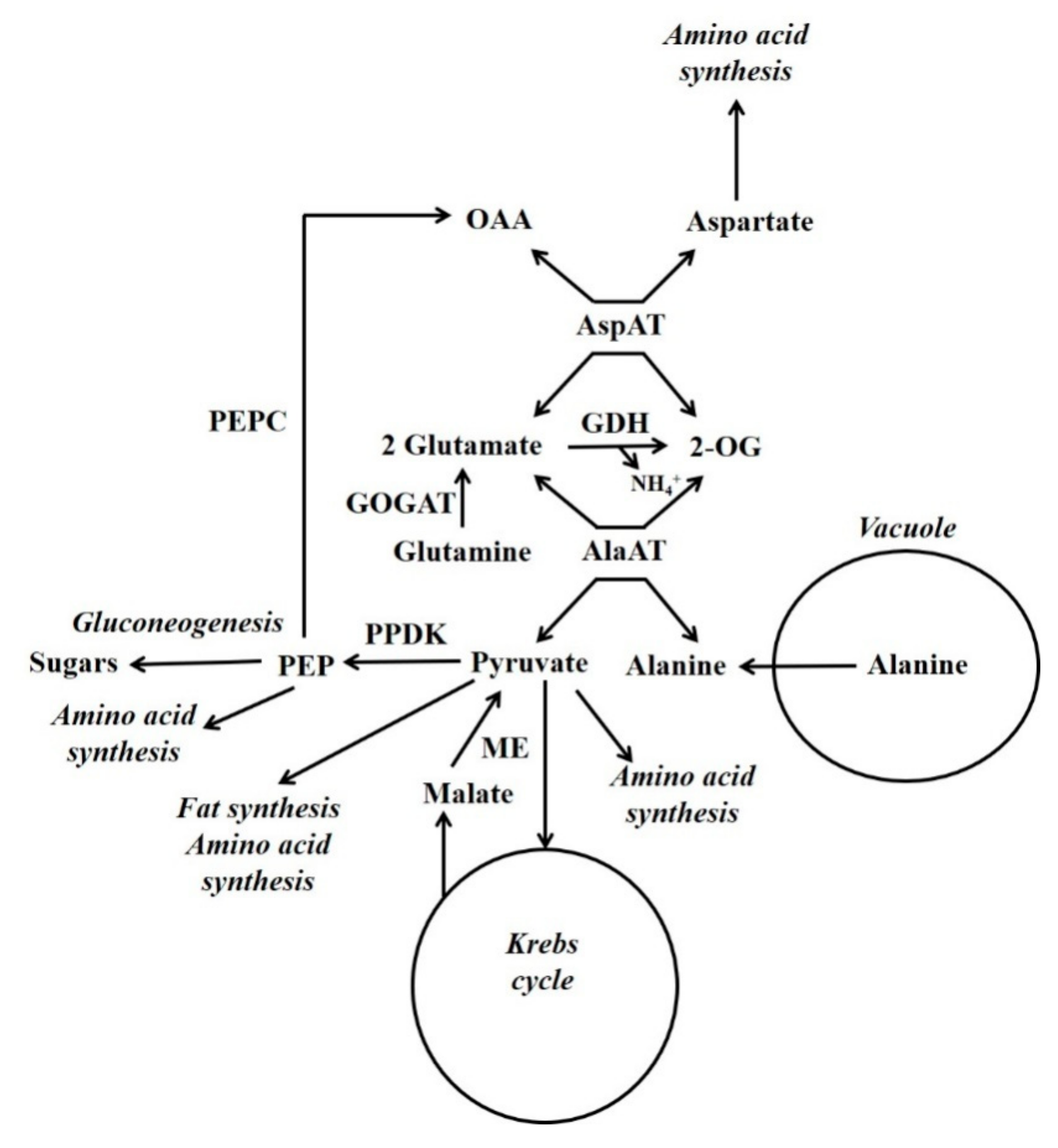
Figure 4.
Gluconeogenesis from vacuolar-released alanine. Simplified scheme outlining the functions of PPDK in gluconeogenesis and nitrogen metabolism (Reproduced from Walker et al. [7]). GDH = glutamate dehydrogenase; GOGAT = glutamine oxoglutarate aminotransferase; ME = malic enzyme; OAA = oxaloacetate; 2-OG = 2-oxoglutarate; PEP = phosphoenolpyruvate; PEPC = phosphoenolpyruvate carboxylase.
3.3. Alanine Metabolism
In developing maize endosperm radiolabelling has shown that there can be a massive gluconeogenic flux from alanine and glutamine [26,27] (Figure 4). In this tissue PPDK and not PEPCK is abundant, and alanine can account for a considerable proportion of the amino acid content [7]. In some plant tissues alanine is stored in the vacuole [108]. When alanine is released from the vacuole it is metabolised by cytosolic alanine aminotransferase (AlaAT) (alanine + 2-OG  glutamate + pyruvate). It is hypothesised that gluconeogenesis occurs when the amount of pyruvate produced by cytosolic AlaAT is in excess of the requirements of other processes [7]. Under hypoxia, roots accumulate alanine and PPDK abundance increases massively [7,50]. This accumulation of alanine allows the production of ATP under low O2 conditions, and involves a reconfiguration of central metabolism which is outlined by Rocha et al. and António et al. [109,110]. Alanine content decreases when O2 is supplied [111], and it is possible that one function of PPDK could be in the metabolism of this alanine in a similar way to that suggested for developing maize endosperm [7].
glutamate + pyruvate). It is hypothesised that gluconeogenesis occurs when the amount of pyruvate produced by cytosolic AlaAT is in excess of the requirements of other processes [7]. Under hypoxia, roots accumulate alanine and PPDK abundance increases massively [7,50]. This accumulation of alanine allows the production of ATP under low O2 conditions, and involves a reconfiguration of central metabolism which is outlined by Rocha et al. and António et al. [109,110]. Alanine content decreases when O2 is supplied [111], and it is possible that one function of PPDK could be in the metabolism of this alanine in a similar way to that suggested for developing maize endosperm [7].
 glutamate + pyruvate). It is hypothesised that gluconeogenesis occurs when the amount of pyruvate produced by cytosolic AlaAT is in excess of the requirements of other processes [7]. Under hypoxia, roots accumulate alanine and PPDK abundance increases massively [7,50]. This accumulation of alanine allows the production of ATP under low O2 conditions, and involves a reconfiguration of central metabolism which is outlined by Rocha et al. and António et al. [109,110]. Alanine content decreases when O2 is supplied [111], and it is possible that one function of PPDK could be in the metabolism of this alanine in a similar way to that suggested for developing maize endosperm [7].
glutamate + pyruvate). It is hypothesised that gluconeogenesis occurs when the amount of pyruvate produced by cytosolic AlaAT is in excess of the requirements of other processes [7]. Under hypoxia, roots accumulate alanine and PPDK abundance increases massively [7,50]. This accumulation of alanine allows the production of ATP under low O2 conditions, and involves a reconfiguration of central metabolism which is outlined by Rocha et al. and António et al. [109,110]. Alanine content decreases when O2 is supplied [111], and it is possible that one function of PPDK could be in the metabolism of this alanine in a similar way to that suggested for developing maize endosperm [7].4. Plant PEPCK Regulation and PEPCK Genes
There are three forms of PEPCK: PEPCK-ATP, PEPCK-GTP and PEPCK-PPi, however, plants only contain PEPCK-ATP [97,112]. These forms likely arose from a common ancestor, [97,112] (Figure 5). In plants there is often more than one form of a given enzyme. For example, there are different forms of malic enzyme, and each of these forms shows a higher sequence similarity to the same form from other plants species than to the other forms from the same species [113,114]. This is not the case for PEPCK (at least for the forms not involved in C4 photosynthesis), and if a plant contains more than one PEPCK gene, these genes are usually more closely related to each other than to PEPCK genes from other plants (Figure 5). A characteristic feature of PEPCK from both plants (angiosperms, gymnosperms and bryophytes) and green algae (from which plants evolved) is the possession of an N-terminal extension [31,115,116] (Figure 6). Although a number of amino acid residues that comprise plant PEPCK are phosphorylated [117], only one site (cAMP-dependent protein kinase site) is known to be subject to reversible phosphorylation [2,97,118,119]. In maize leaves, although this site is phosphorylated [117], it is not subject to large changes in phosphorylation status during the diurnal cycle [115,119]. This site is located within the N-terminal extension, and changes in its phosphorylation status alter the catalytic properties of the enzyme [119,120]. This phosphorylation site is present in the enzyme from mosses, gymnosperms and angiosperms but not green algae (Figure 6). Phosphorylation of PEPCK lowers its affinity for its substrate OAA, and this effect is dependent on both ATP/ADP ratio and concentrations of magnesium and manganese [97,119,121]. All three types of PEPCK are not generally thought to be modulated by most other metabolites if in vitro assay conditions approximate those within the plant cytosol [97,121]. However, more recent work reported that PEPCK activity is modulated by a plethora of metabolites [122,123]. Clearly this controversy needs to be resolved. The N-terminal extension of PEPCK is susceptible to proteolysis upon extraction of the tissue [31]. It has been suggested that this could be a mechanism used to modulate the activity of the enzyme in vivo [123]. However, such a mechanism is unlikely to contribute to the regulation of PEPCK in at least some plant tissues such as leaves of both CAM and C4 plants: because little or no cleavage of the enzyme was noted in leaves harvested from plants during either the night or day [118].
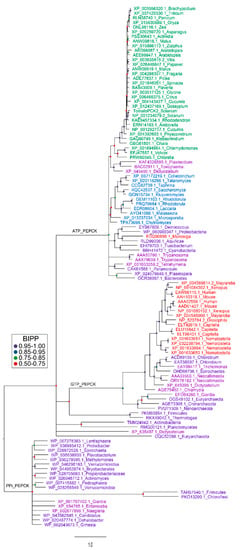
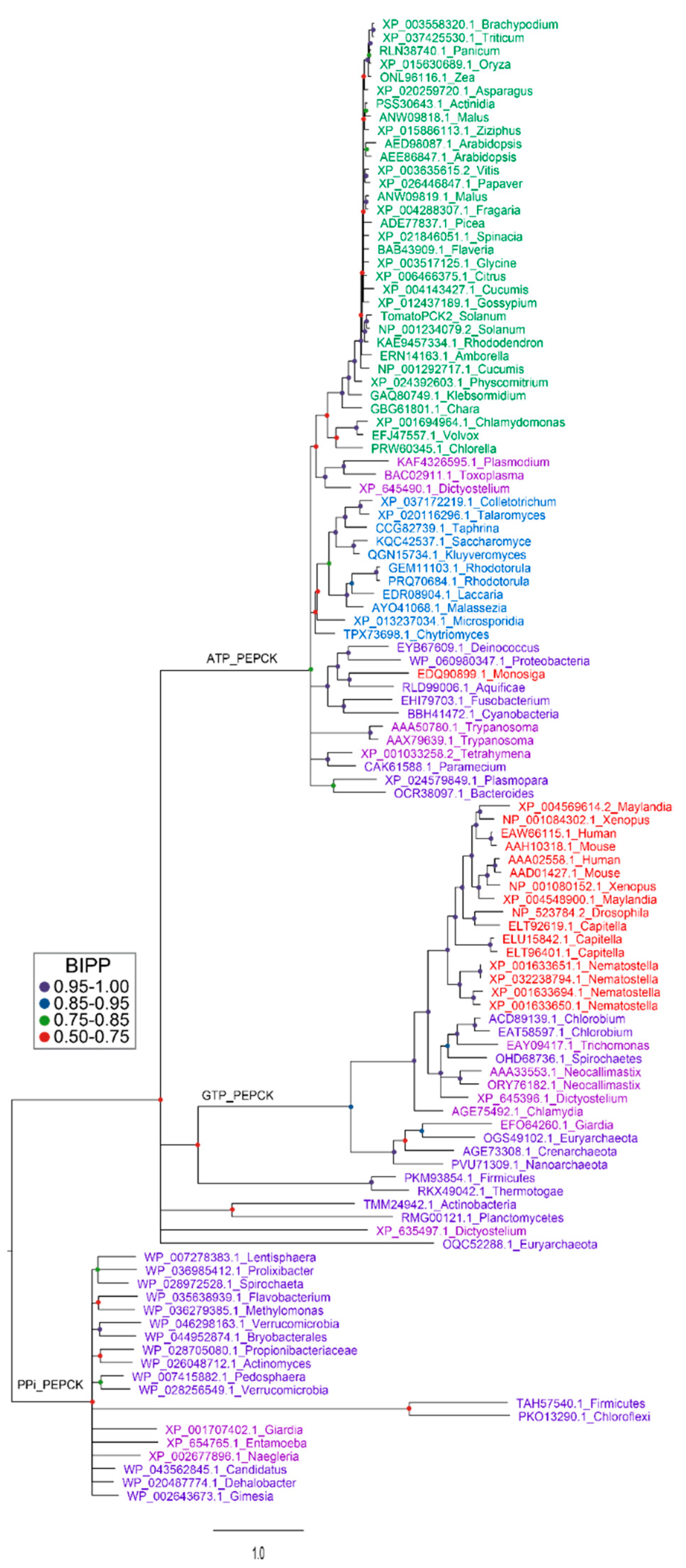
Figure 5.
Phylogenetic analysis of PEPCK proteins from three domains of life. Several representative PEPCK protein sequences were selected for each phylum of three domains of life [130] including bacteria [131,132], protozoans and fungi [133], plants [134] and metazoans in the NCBI database. Protein sequences were aligned using Clustal W [135]. After deletion of segments with poor consensus alignment, sequences were subjected to Bayesian inference for establishment of phylogenetic relationships between proteins [136]. Analysis were run for 5 million generations under a mixed amino-acid model with rate variation between sites estimated by a gamma distribution. Bayesian inference posterior probabilities (BIPPs) of tree nodes are indicated by coloured dots. Gene identifiers of the proteins are color-coded to represent the phyla from which they are derived. Green for plants, blue for fungi, pink for protozoans, purple for bacteria and red for metazoans. Corresponding species names are listed by the side of accession number on each branch of the tree. Note for the plant species there are very little differences in the amino acid sequence of the protein apart from in the c12 kD N-terminal extension. Thus, the reconstructed phylogeny of the plant enzyme is based largely on this part of the protein.
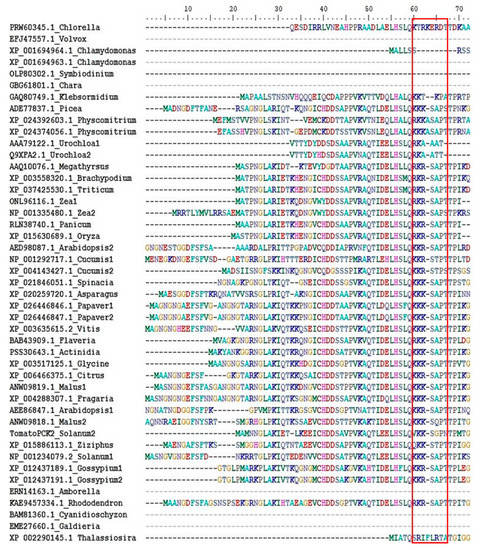
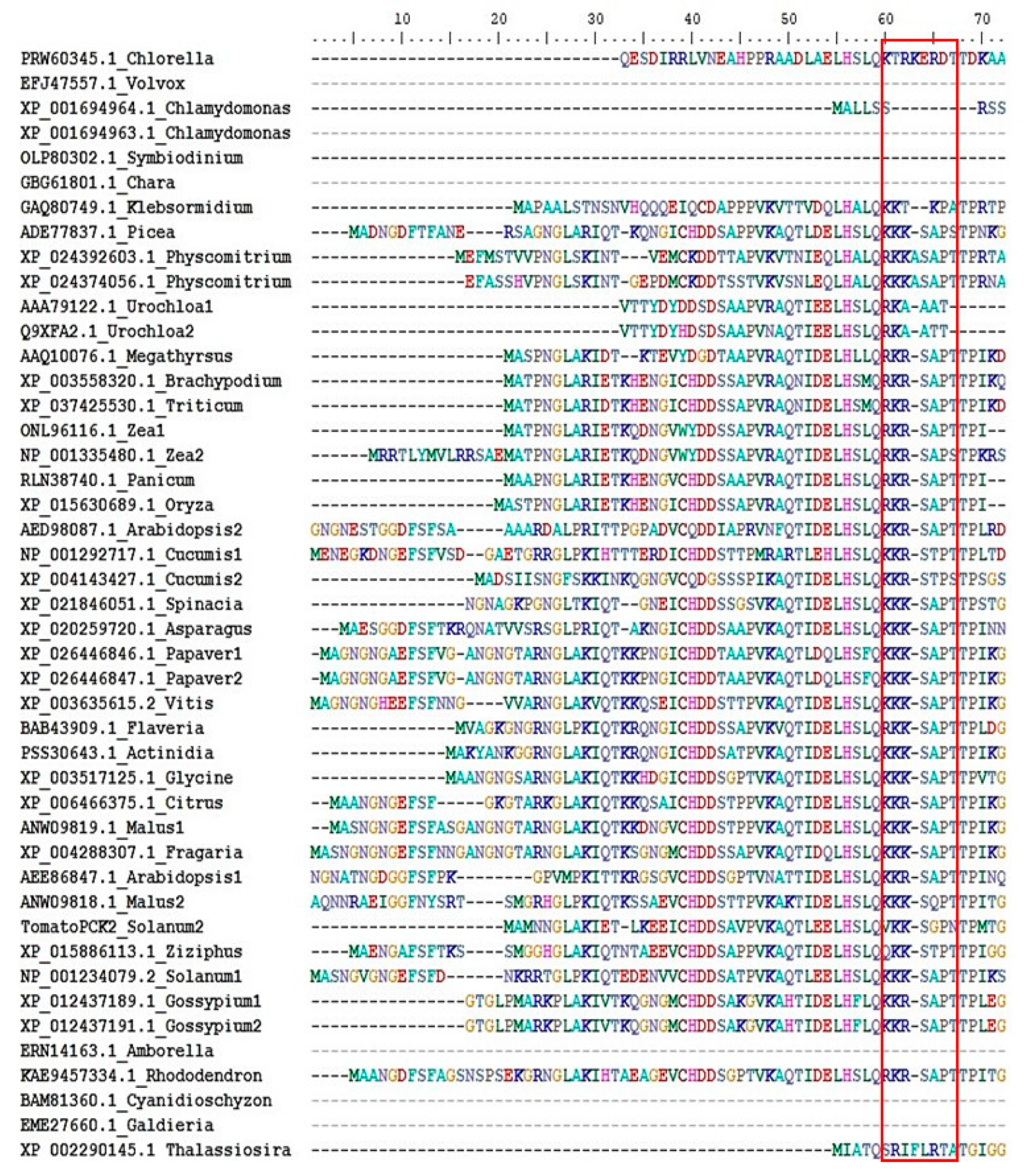
Figure 6.
Alignment of N-terminal extension sequences of plant PEPCKs. Several representative PEPCK protein sequences, from the phylum of plants and algae [134], were selected from the NCBI database. Protein sequences were aligned using Clustal W [135]. The alignment of N-terminal extension sequences was edited and extracted using the BioEdit programme. Species names are listed by the side of accession number. The phosphorylation motif (KK/RXSXPT) or its absence is shown in the red box.
5. Coordinate Regulation of Malate Metabolism and Gluconeogenesis
Enzymes involved in malate synthesis such as PEPC and MDH, and those involved in its degradation (e.g., MDH and PEPCK and cytosolic NADP-ME), are present in the cytosol. Thus, regulatory mechanisms are required to activate malate-synthetic enzymes and inactivate malate-degradation enzymes when malate is accumulated in the vacuole, and the reverse when malate is released from the vacuole [2]. The coordination of PEPCK and PEPC activities involves phosphorylation of both enzymes in conjunction with changes in metabolite concentration and pH [2,97,118,119,124,125]. Trehalose 6-P is a key regulatory metabolite that is involved in coordinating changes in flux between sucrose and organic acid/amino acid synthesis [19]. Clearly this metabolite is likely to play a role in the regulation of gluconeogenesis (e.g., by altering the phosphorylation status of PEPC and PEPCK).
The release of malate from the vacuole will have the following effects [2,97] (Figure 3B). Cytosolic oxaloacetate (OAA) concentration is largely determined by the following reactions (and will increase). Malate and OAA are rapidly interconverted by the reversible enzyme cytosolic MDH (OAA + NADH  malate + NAD). Further, OAA and 2-oxoglutarate (2-OG) are also interconverted by the reversible reaction catalysed by cytosolic aspartate aminotransferase (AspAT) (glutamate + OAA
malate + NAD). Further, OAA and 2-oxoglutarate (2-OG) are also interconverted by the reversible reaction catalysed by cytosolic aspartate aminotransferase (AspAT) (glutamate + OAA  aspartate + 2-OG). Hence an increase in malate concentration will increase OAA concentration (via MDH), and this will then increase aspartate concentration (via AspAT) [126,127,128]. These changes in metabolite concentrations will have a large effect on the in vivo activities of the cytosolic enzymes PEPC, NADP-ME, PEPCK and PK. These enzymes are key regulatory enzymes involved in controlling flux between PEP, pyruvate, malate and OAA [129].
aspartate + 2-OG). Hence an increase in malate concentration will increase OAA concentration (via MDH), and this will then increase aspartate concentration (via AspAT) [126,127,128]. These changes in metabolite concentrations will have a large effect on the in vivo activities of the cytosolic enzymes PEPC, NADP-ME, PEPCK and PK. These enzymes are key regulatory enzymes involved in controlling flux between PEP, pyruvate, malate and OAA [129].
 malate + NAD). Further, OAA and 2-oxoglutarate (2-OG) are also interconverted by the reversible reaction catalysed by cytosolic aspartate aminotransferase (AspAT) (glutamate + OAA
malate + NAD). Further, OAA and 2-oxoglutarate (2-OG) are also interconverted by the reversible reaction catalysed by cytosolic aspartate aminotransferase (AspAT) (glutamate + OAA  aspartate + 2-OG). Hence an increase in malate concentration will increase OAA concentration (via MDH), and this will then increase aspartate concentration (via AspAT) [126,127,128]. These changes in metabolite concentrations will have a large effect on the in vivo activities of the cytosolic enzymes PEPC, NADP-ME, PEPCK and PK. These enzymes are key regulatory enzymes involved in controlling flux between PEP, pyruvate, malate and OAA [129].
aspartate + 2-OG). Hence an increase in malate concentration will increase OAA concentration (via MDH), and this will then increase aspartate concentration (via AspAT) [126,127,128]. These changes in metabolite concentrations will have a large effect on the in vivo activities of the cytosolic enzymes PEPC, NADP-ME, PEPCK and PK. These enzymes are key regulatory enzymes involved in controlling flux between PEP, pyruvate, malate and OAA [129].In plants the affinity of PEPCK for OAA is such that physiological increases in OAA concentration will increase greatly flux through the enzyme [2,97,119]. PEPC activity is inhibited by malate, and further its activity is decreased by a fall in pH [137,138,139]. Hence enzyme activity is less at a lower pH, its sensitivity to inhibitory metabolites such as malate is greater, and its sensitivity to activator metabolites is lower [124,129,140]. In CAM plants, the pH of the cytosol decreases when malic acid is released from the vacuole, and this is thought to be important in decreasing flux through PEPC [141]. In addition, the above effects are modulated by the coordinated phosphorylation/dephosphorylation of PEPCK and PEPC. Dephosphorylation increases PEPCK activity, and this arises in part by increasing its affinity for OAA [119]. By contrast, dephosphorylation decreases PEPC activity, and this is brought about in part by increasing its sensitivity to inhibitory metabolites such as malate [129,140]. Thus, in leaves of CAM plants PEPCK and PEPC are dephosphorylated when malate is released from the vacuole, and the reverse occurs when malate is accumulated in the vacuole [2,118]. It is possible (but not certain) that the same protein kinase (PEPC-kinase) is used to phosphorylate PEPCK and PEPC [2,97]. Indeed, PEPC-kinase gene expression is enhanced by an increase in cytosolic pH and PEPCK gene expression is reduced [142]. Further, this change in PEPC-kinase gene expression can lead to rapid changes (less than an hour) in PEPC activity [142,143].
PK converts PEP to pyruvate (PEP + ADP → pyruvate + ATP). Cytosolic PK activity is increased by a decrease in pH, of the order that occurs in the cytosol of leaves of CAM plants when malic acid is released from the vacuole [138,141]. Aspartate concentration is a key factor in determining flux through cytosolic PK in vivo; because aspartate acts as an allosteric activator which over-rides inhibition of the enzyme by glutamate [138,139]. An increase in cytosolic malate concentration will increase cytosolic aspartate concentration (as a result of the reactions of cytosolic MDH and cytosolic AspAT), and hence increase the activity of cytosolic PK.
Cytosolic NADP-ME converts malate to pyruvate (malate + NADP → pyruvate + CO2 + NADPH). In the flesh of fruits such as apple, tomato and grape, cytosolic NADP-ME is important in supplying pyruvate to the Krebs cycle [81,89,144,145,146]. Mitochondrial NAD-malic enzyme (NAD-ME) converts malate to pyruvate (malate + NAD → pyruvate + CO2 + NADH). This enzyme is important in providing pyruvate for the Krebs cycle, and in Arabidopsis leaves this is associated with the release of malate from the vacuole at night [23]. All forms of ME require a divalent cation to be active [147,148,149,150]. In vitro enzyme assays of ME have employed as the cation either millimolar Mn2+ or Mg2+, and the catalytic properties depend on the metal ion that is used [148,149,150]. In the cytosol of plant cells, the concentration of Mn2+ is submicromolar and that of Mg2+ millimolar [121]. Unphysiological concentrations of Mn2+ and Mg2+ in in vitro assays alter the catalytic properties of many enzymes [121,151]. Studies of cytosolic NADP-ME from grape, and other fruits, have often used unphysiological concentrations of Mn2+ in vitro assays, and this has led to conflicting results [152,153]. However, some studies included assays that did not use millimolar concentrations of Mn2+ [154], and from these it is clear that the affinity of cytosolic-NADP-ME from grape pericarp for physiological concentrations of malate is sigmoidal at pH 7.3, and that this becomes hyperbolic as the pH drops to around 6.8. A fall in cytosolic pH from 7.3 to 6.8 massively increases the affinity of cytosolic NADP-ME for malate, and this will result in a large increase in the flux through the enzyme. A similar conclusion was reached for both NAD-ME and cytosolic NADP-ME from some other plant tissues [8,148]. Hence an increase in cytosolic malate concentration and a decrease in cytosolic pH will increase flux through PEPCK, cytosolic PK and cytosolic NADP-ME, and this will lead to an increase in pyruvate synthesis.
The Krebs cycle is a large consumer of pyruvate, and its supply must be coordinated with the demand for the products of the cycle (e.g., ATP, NADH and 2-oxoglutarate). In banana flesh an increase in ATP/ADP could be a factor that contributes to reducing flux through cytosolic PK in vivo [139]. An increase in cytosolic 2-oxoglutarate will decrease aspartate concentration (by the action of AspAT), and this will decrease cytosolic PK activity. A decrease in aspartate concentration arising from 2-oxoglutarate synthesis is thought to be important in coordinating cytosolic PK activity and flux through the Krebs cycle with amino acid metabolism [138,139]. Changes in the cytosolic ATP/ADP ratio in the range that can occur in plant cells [155] alter the activity of PEPCK in vivo. A higher ATP/ADP ratio increases the affinity of the enzyme for OAA and hence its activity [97,119]. Hence, when supply of pyruvate exceeds demand, the activity of PEPCK is likely to be maintained, whereas, the activity of cytosolic PK is likely to be reduced and this will increase cytosolic PEP concentration. The cytosolic concentration of PEP is a critical regulator of plant glycolysis, and a high concentration of PEP causes a switch to gluconeogenesis [129].
Subsequently when malic acid is no longer being released from the vacuole its metabolism in the cytoplasm will reduce its concentration. In addition, the metabolism of malate, as described above, consumes the protons arising from the release of malic acid from the vacuole [97]. This decrease in malate concentration and increase in cytosolic pH, will inhibit both PEPCK and cytosolic NADP-ME but activate PEPC. The decrease in flux through PEPCK, and an increase in flux through PEPC, will reduce PEP concentration and this will contribute to a switch from gluconeogenesis to glycolysis.
6. Conclusions
In plants, gluconeogenesis is of widespread occurrence and is associated with diverse processes. Often, it is associated with the release of Krebs cycle acids and/or certain nitrogenous compounds from the vacuole, and occurs when the breakdown products of these compounds exceed the demands of other processes. Fine control of enzyme activity is likely to make an important contribution to coordinating both malate synthesis-degradation and the utilisation of malate by gluconeogenesis and the Krebs cycle. The compartmentation of Krebs cycle acids/nitrogenous compounds in the vacuole almost certainly plays a key role in maintaining an appropriate cytoplasmic concentration of these compounds.
Funding
Authors received no external funding to write this review.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dittrich, P.; Campbell, W.H.; Black, C.C. Phosphoenolpyruvate carboxykinase in plants exhibiting crassulacean acid metabolism. Plant Physiol. 1973, 52, 357–361. [Google Scholar] [CrossRef]
- Leegood, R.C.; Walker, R.P. Regulation and roles of phosphoenolpyruvate carboxykinase in plants. Arch. Biochem. Biophys. 2003, 414, 204–210. [Google Scholar] [CrossRef]
- Chastain, C.J.; Failing, C.J.; Manandhar, L.; Zimmerman, M.A.; Lakner, M.M.; Nguyen, T.H. Functional evolution of C4 pyruvate, orthophosphate dikinase. J. Exp. Bot. 2011, 62, 3083–3091. [Google Scholar] [CrossRef] [PubMed]
- Eastmond, P.J.; Astley, H.M.; Parsley, K.; Aubry, S.; Williams, B.P.; Menard, G.N.; Craddock, C.P.; Nunes-Nesi, A.; Fernie, A.R.; Hibberd, J.M. Arabidopsis uses two gluconeogenic gateways for organic acids to fuel seedling establishment. Nat. Commun. 2015, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Famiani, F.; Farinelli, D.; Frioni, T.; Palliotti, A.; Battistelli, A.; Moscatello, S.; Walker, R.P. Malate as substrate for catabolism and gluconeogenesis during ripening in the pericarp of different grape cultivars. Biol. Plant. 2016, 60, 155–162. [Google Scholar] [CrossRef]
- Famiani, F.; Paoletti, A.; Proietti, P.; Battistelli, A.; Moscatello, S.; Cruz-Castillo, J.G.; Walker, R.P. The occurrence of phosphoenolpyruvate carboxykinase (PEPCK) in the pericarp of different grapevine genotypes and in grape leaves and developing seeds. J. Hortic. Sci. Biotechnol. 2018, 93, 456–465. [Google Scholar] [CrossRef]
- Walker, R.P.; Benincasa, P.; Battistelli, A.; Moscatello, S.; Técsi, l.; Leegood, R.C.; Famiani, F. Gluconeogenesis and nitrogen metabolism in maize. Plant Physiol. Biochem. 2018, 130, 324–333. [Google Scholar] [CrossRef]
- Davies, D.D. The fine control of cytosolic pH. Physiol. Plant. 1986, 67, 702–706. [Google Scholar] [CrossRef]
- Stitt, M.; Müller, C.; Matt, P.; Gibon, Y.; Carillo, P.; Morcuende, R.; Scheible, W.R.; Krapp, A. Steps towards an integrated view of nitrogen metabolism. J. Exp. Bot. 2002, 53, 959–970. [Google Scholar] [CrossRef]
- Walker, R.P.; Famiani, F. Organic acids in fruits: Metabolism, functions and contents. Hortic. Rev. 2018, 45, 371–430. [Google Scholar]
- Famiani, F.; Farinelli, D.; Moscatello, S.; Battistelli, A.; Leegood, R.C.; Walker, R.P. The contribution of stored malate and citrate to the substrate requirements of metabolism of ripening peach (Prunus persica L. Batsch) flesh is negligible. Implications for the occurrence of phosphoenolpyruvate carboxykinase and gluconeogenesis. Plant Physiol. Biochem. 2016, 101, 33–42. [Google Scholar] [CrossRef]
- Famiani, F.; Paoletti, A.; Battistelli, A.; Moscatello, S.; Chen, Z.H.; Leegood, R.C.; Walker, R.P. Phosphoenolpyruvate carboxykinase, pyruvate orthophosphate dikinase and isocitrate lyase in both tomato fruits and leaves, and in the flesh of peach and some other fruits. J. Plant Physiol. 2016, 202, 34–44. [Google Scholar] [CrossRef]
- Famiani, F.; Baldicchi, A.; Casulli, V.; Vaio, C.D.; Cruz-Castillo, J.G.; Walker, R.P. The occurrence of phosphoenolpyruvate carboxykinase (PEPCK) and enzymes related to photosynthesis and organic acid/nitrogen metabolism in apricot flowers (Prunus armeniaca L.). Acta Physiol. Plant. 2017, 39, 1–6. [Google Scholar] [CrossRef]
- Famiani, F.; Bonghi, C.; Chen, Z.H.; Drincovich, M.F.; Farinelli, D.; Lara, M.V.; Proietti, S.; Rosati, A.; Vizzotto, G.; Walker, R.P. Stone fruits: Growth and nitrogen and organic acid metabolism in the fruits and seeds—A review. Front. Plant Sci. 2020, 11, 1427. [Google Scholar] [CrossRef]
- Malone, S.; Chen, Z.H.; Bahrami, A.R.; Walker, R.P.; Gray, J.E.; Leegood, R.C. Phosphoenolpyruvate carboxykinase in arabidopsis: Changes in gene expression, protein and activity during vegetative and reproductive development. Plant Cell Physiol. 2007, 48, 441–450. [Google Scholar] [CrossRef]
- Walker, R.P.; Battistelli, A.; Moscatello, S.; Técsi, L.; Leegood, R.C.; Famiani, F. Phosphoenolpyruvate carboxykinase and gluconeogenesis in grape pericarp. Plant Physiol. Biochem. 2015, 97, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Penfield, S.; Clements, S.; Bailey, K.J.; Gilday, A.D.; Leegood, R.C.; Gray, J.E.; Graham, I.A. Expression and manipulation of phosphoenolpyruvate carboxykinase 1 identifies a role for malate metabolism in stomatal closure. Plant J. 2012, 69, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Bräutigam, A.; Schlüter, U.; Eisenhut, M.; Gowik, U. On the evolutionary origin of CAM134 photosynthesis. Plant Physiol. 2017, 174, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, C.M.; Feil, R.; Ishihara, H.; Watanabe, M.; Kölling, K.; Krause, U.; Höhne, M.; Encke, B.; Plaxton, W.C.; Zeeman, S.C.; et al. Trehalose 6-phosphate coordinates organic and amino acid metabolism with carbon availability. Plant J. 2016, 85, 410–423. [Google Scholar] [CrossRef]
- Cheung, C.Y.; Poolman, M.G.; Fell, D.A.; Ratcliffe, R.G.; Sweetlove, L.J. A Diel Flux Balance Model Captures Interactions between Light and Dark Metabolism during Day-Night Cycles in C3 and Crassulacean Acid Metabolism Leaves. Plant Physiol. 2014, 165, 917–929. [Google Scholar] [CrossRef]
- Abadie, C.; Lothier, J.; Boex-Fontvieille, E.; Carroll, A.; Tcherkez, G. Direct assessment of the metabolic origin of carbon atoms in glutamate from illuminated leaves using 13C-NMR. New Phytol. 2017, 216, 1079–1089. [Google Scholar] [CrossRef]
- Famiani, F.; Cultrera, N.G.M.; Battistelli, A.; Casulli, V.; Proietti, P.; Standardi, A.; Chen, Z.H.; Leegood, R.C.; Walker, R.P. Phosphoenolpyruvate carboxykinase and its potential role in the catabolism of organic acids in the flesh of soft fruit during ripening. J. Exp. Bot. 2005, 56, 2959–2969. [Google Scholar] [CrossRef]
- Tronconi, M.A.; Fahnenstich, H.; Weehler, M.C.G.; Andreo, C.S.; Flügge, U.I.; Drincovich, M.F.; Maurino, V.G. Arabidopsis NAD-malic enzyme functions as a homodimer and heterodimer and has a major impact on nocturnal metabolism. Plant Physiol. 2008, 146, 1540–1552. [Google Scholar] [CrossRef] [PubMed]
- Le, X.H.; Lee, C.P.; Millar, A.H. The mitochondrial pyruvate carrier (MPC) complex mediates one of three pyruvate-supplying pathways that sustain Arabidopsis respiratory metabolism. Plant Cell 2021, koab148. [Google Scholar] [CrossRef]
- Stewart, C.R.; Beevers, H. Gluconeogenesis from amino acids in germinating castor bean endosperm and its role in transport to the embryo. Plant Physiol. 1967, 42, 1587–1595. [Google Scholar] [CrossRef]
- Sodek, L. Biosynthesis of lysine and other amino acids in the developing maize endosperm. Phytochemistry 1976, 15, 1903–1906. [Google Scholar] [CrossRef]
- Misra, S.; Oaks, A. Glutamine metabolism in corn kernels cultured in vitro. Plant Physiol. 1985, 77, 520–523. [Google Scholar] [CrossRef] [PubMed]
- Murray, D.R. Nutritive role of seedcoats in developing legume seeds. Am. J. Bot. 1987, 74, 1122–1137. [Google Scholar] [CrossRef]
- Miller, E.C. A physiological study of the germination of Helianthus annuus. Ann. Bot. 1910, 24, 693–726. [Google Scholar] [CrossRef]
- Benedict, C.R.; Beevers, H. Formation of sucrose from malate in germinating castor beans. I. Conversion of malate to phosphoenol-pyruvate. Plant Physiol. 1961, 36, 540. [Google Scholar] [CrossRef]
- Walker, R.P.; Trevanion, S.J.; Leegood, R.C. Phosphoenolpyruvate carboxykinase from higher plants: Purification from cucumber and evidence of rapid proteolytic cleavage in extracts from a range of plant tissues. Planta 1995, 196, 58–63. [Google Scholar] [CrossRef]
- Rylott, E.L.; Gilday, A.D.; Graham, I.A. The gluconeogenic enzyme phosphoenolpyruvate carboxykinase in Arabidopsis is essential for seedling establishment. Plant Physiol. 2003, 131, 1834–1842. [Google Scholar] [CrossRef]
- Kornberg, H.L.; Beevers, H. The glyoxylate cycle as a stage in the conversion of fat to carbohydrate in castor beans. Biochim. Biophys. Acta 1957, 26, 531–537. [Google Scholar] [CrossRef]
- Graham, I.A. Seed storage oil mobilization. Annu. Rev. Plant Biol. 2008, 59, 115–142. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Kim, S.R.; Lee, S.K.; Choi, H.; Jeon, J.S.; An, G. Alanine aminotransferase 1 (OsAlaAT1) plays an essential role in the regulation of starch storage in rice endosperm. Plant Sci. 2015, 240, 79–89. [Google Scholar] [CrossRef]
- Chia, T.Y.; Pike, M.J.; Rawsthorne, S. Storage oil breakdown during embryo development of Brassica napus (L.). J. Exp. Bot. 2005, 56, 1285–1296. [Google Scholar] [CrossRef] [PubMed]
- Aoyagi, K.; Bassham, J.A.; Greene, F.C. Pyruvate orthophosphate dikinase gene expression in developing wheat seeds. Plant Physiol. 1984, 75, 393–396. [Google Scholar] [CrossRef]
- Walker, R.P.; Chen, Z.H.; Técsi, L.I.; Famiani, F.; Lea, P.J.; Leegood, R.C. Phosphoenolpyruvate carboxykinase plays a role in interactions of carbon and nitrogen metabolism during grape seed development. Planta 1999, 210, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Chastain, C.J.; Heck, J.W.; Colquhoun, T.A.; Voge, D.G.; Gu, X.Y. Posttranslational regulation of pyruvate, orthophosphate dikinase in developing rice (Oryza sativa) seeds. Planta 2006, 224, 924–934. [Google Scholar] [CrossRef]
- Walker, R.P.; Battistelli, A.; Moscatello, S.; Chen, Z.H.; Leegood, R.C.; Famiani, F. Metabolism of the seed and endocarp of cherry (Prunus avium L.) during development. Plant Physiol. Biochem. 2011, 49, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Famiani, F.; Casulli, V.; Baldicchi, A.; Battistelli, A.; Moscatello, S.; Walker, R.P. Development and metabolism of the fruit and seed of the Japanese plum Ozark Premier (Rosaceae). J. Plant Physiol. 2012, 169, 169,551–560. [Google Scholar] [CrossRef]
- Delgado-Alvarado, A.; Walker, R.P.; Leegood, R.C. Phosphoenolpyruvate carboxykinase in developing pea seeds is associated with tissues involved in solute transport and is nitrogen responsive. Plant Cell Environ. 2007, 30, 225–235. [Google Scholar] [CrossRef]
- Chen, Z.H.; Walker, R.P.; Acheson, R.M.; Técsi, L.I.; Wingler, A.; Lea, P.J.; Leegood, R.C. Are isocitrate lyase and phosphoenolpyruvate carboxykinase involved in gluconeogenesis during senescence of barley leaves and cucumber cotyledons? Plant Cell Physiol. 2000, 41, 960–967. [Google Scholar] [CrossRef]
- Eastmond, P.J.; Graham, I.A. Re-examining the role of the glyoxylate cycle in oilseeds. Trends Plant Sci. 2001, 6, 72–78. [Google Scholar] [CrossRef]
- Taylor, L.; Nunes-Nesi, A.; Parsley, K.; Leiss, A.; Leach, G.; Coates, S.; Wingler, A.; Fernie, A.R.; Hibberd, J.M. Cytosolic pyruvate, orthophosphate dikinase functions in nitrogen remobilization during leaf senescence and limits individual seed growth and nitrogen content. Plant J. 2010, 62, 641–652. [Google Scholar] [CrossRef]
- Walker, R.P.; Chen, Z.H.; Johnson, K.A.; Famiani, F.; Tecsi, L.; Leegood, R.C. Using immunohistochemistry to study plant metabolism: The examples of its use in the localization of amino acids in plant tissues, and of phosphoenolpyruvate carboxykinase and its possible role in pH regulation. J. Exp. Bot. 2001, 52, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.H.; Walker, R.P.; Técsi, L.I.; Lea, P.J.; Leegood, R.C. Phosphoenolpyruvate carboxykinase in cucumber plants is increased both by ammonium and by acidification, and is present in the phloem. Planta 2004, 219, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Bailey, K.J.; Leegood, R.C. Nitrogen recycling from the xylem in rice leaves: Dependence upon metabolism and associated changes in xylem hydraulics. J. Exp. Bot. 2016, 67, 2901–2911. [Google Scholar] [CrossRef] [PubMed]
- Vogelmann, T.C.; Dickson, R.E.; Larson, P.R. Comparative distribution and metabolism of xylem-borne amino compounds and sucrose in shoots of Populus deltoides. Plant Physiol. 1985, 77, 418–428. [Google Scholar] [CrossRef]
- Moons, A.; Valcke, R.; Van, M. Low-oxygen stress and water deficit induce cytosolic pyruvate orthophosphate dikinase (PPDK) expression in roots of rice, a C3 plant. Plant J. 1998, 15, 89–98. [Google Scholar] [CrossRef]
- Mustroph, A.; Hess, N. Hypoxic energy metabolism and PPi as an alternative energy currency. In Low-Oxygen Stress in Plants; Springer: Vienna, Austria, 2014; pp. 165–184. [Google Scholar]
- Lea, P.J.; Chen, Z.H.; Leegood, R.C.; Walker, R.P. Does phosphoenolpyruvate carboxykinase have a role in both amino acid and carbohydrate metabolism? Amino Acids 2001, 20, 225–241. [Google Scholar] [CrossRef] [PubMed]
- Zulak, K.G.; Aalim, M.W.; Vogel, H.J.; Facchini, P.J. Quantitative 1 H NMR metabolomics reveals extensive metabolic reprogramming of primary and secondary metabolism in elicitor-treated opium poppy cell cultures. BMC Plant Biol. 2008, 8, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Sallets, A.; Beyaert, M.; Boutry, M. Champagne, A. Comparative proteomics of short and tall glandular trichomes of Nicotiana tabacum reveals differential metabolic activities. J. Proteome Res. 2014, 13, 3386–3396. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.S.; Kim, N.H.; Hwang, B.K. The pepper phosphoenolpyruvate carboxykinase CaPEPCK1 is involved in plant immunity against bacterial and oomycete pathogens. Plant Mol. Biol. 2015, 89, 99–111. [Google Scholar] [CrossRef]
- Lu, W.; Ruan, Y.L. Unraveling mechanisms of cell expansion linking solute transport, metabolism, plasmodesmatal gating and cell wall dynamics. Plant Signal. Behav. 2010, 5, 1561–1564. [Google Scholar]
- Walker, R.P.; Bonghi, C.; Varotto, S.; Battistelli, A.; Burbidge, C.A.; Castellarin, S.D.; Chen, Z.-H.; Darriet, P.; Moscatello, S.; Rienth, M.; et al. Sucrose Metabolism and Transport in Grapevines, with Emphasis on Berries and Leaves, and Insights Gained from a Cross-Species Comparison. Int. J. Mol. Sci. 2021, 22, 7794. [Google Scholar] [CrossRef]
- Daloso, D.M.; dos Anjos, L.; Fernie, A.R. Roles of sucrose in guard cell regulation. New Phytol. 2016, 211, 809–818. [Google Scholar] [CrossRef]
- Antunes, W.C.; Daloso, D.M.; Pinheiro, D.P.; Rhys Williams, T.C.; Loureiro, M.E. Guard cell-specific down-regulation of the sucrose transporter SUT1 leads to improved water use efficiency and reveals the interplay between carbohydrate metabolism and K+ accumulation in the regulation of stomatal opening. Environ. Exp. Bot. 2017, 135, 73–85. [Google Scholar] [CrossRef]
- Daloso, D.M.; Medeiros, D.B.; dos Anjos, L.; Yoshida, T.; Araújo, W.L.; Fernie, A.R. Metabolism within the specialized guard cells of plants. New Phytol. 2017, 216, 1018–1033. [Google Scholar] [CrossRef]
- Flütsch, S.; Nigro, A.; Conci, F.; Fajkus, J.; Thalmann, M.; Trtílek, M.; Panzarová, K.; Santelia, D. Glucose uptake to guard cells via STP transporters provides carbon sources for stomatal opening and plant growth. EMBO Rep. 2020, 21, e49719. [Google Scholar] [CrossRef]
- Flütsch, S.; Wang, Y.; Takemiya, A.; Vialet-Chabrand, S.R.M.; Klejchová, M.; Nigro, A.; Hills, A.; Lawson, T.; Blatt, M.R.; Santelia, D. Guard cell starch degradation yields glucose for rapid stomatal opening in Arabidopsis. Pant Cell 2020, 32, 2325–2344. [Google Scholar] [CrossRef]
- Suzuki, S.; Burnell, J.N. The pck1 promoter from Urochloa panicoides (a C4 plant) directs expression differently in rice (a C3 plant) and maize (a C4 plant). Plant Sci. 2003, 165, 603–611. [Google Scholar] [CrossRef]
- Walker, R.P.; Battistelli, A.; Moscatello, S.; Chen, Z.H.; Leegood, R.C.; Famiani, F. Phosphoenolpyruvate carboxykinase in cherry (Prunus avium L.) fruit during development. J. Exp. Bot. 2011, 62, 5357–5365. [Google Scholar] [CrossRef]
- Famiani, F.; Moscatello, S.; Ferradini, N.; Gardi, T.; Battistelli, A.; Walker, R.P. Occurrence of a number of enzymes involved in either gluconeogenesis or other processes in the pericarp of three cultivars of grape (Vitis vinifera L.) during development. Plant Physiol. Biochem. 2014, 84, 261–270. [Google Scholar] [CrossRef]
- Bahrami, A.R.; Chen, Z.H.; Walker, R.P.; Leegood, R.C.; Gray, J.E. Ripening-related occurrence of phosphoenolpyruvate carboxykinase in tomato fruit. Plant Mol. Biol. 2001, 47, 499–506. [Google Scholar] [CrossRef]
- Borsani, J.; Budde, C.O.; Porrini, L.; Lauxmann, M.A.; Lombardo, V.A.; Murray, R.; Andreo, C.S.; Drincovich, M.F.; Lara, M.V. Carbon metabolism of peach fruit after harvest: Changes in enzymes involved in organic acid and sugar level modifications. J. Exp. Bot. 2009, 60, 1823–1837. [Google Scholar] [CrossRef]
- Lara, M.V.; Borsani, J.; Budde, C.O.; Lauxmann, M.A.; Lombardo, V.A.; Murray, R.; Andreo, S.C.; Drincovich, M.F. Biochemical and proteomic analysis of ‘Dixiland’peach fruit (Prunus persica) upon heat treatment. J. Exp. Bot. 2009, 60, 4315–4333. [Google Scholar] [CrossRef]
- Lara, M.V.; Budde, C.O.; Porrini, L.; Borsani, J.; Murray, R.; Andreo, C.S.; Drincovich, M.F. Peach (Prunus persica) fruit response to anoxia: Reversible ripening delay and biochemical changes. Plant Cell Physiol. 2011, 52, 392–403. [Google Scholar] [CrossRef]
- Walker, R.P.; Famiani, F.; Baldicchi, A.; Cruz-Castillo, J.G.; Inglese, P. Changes in enzymes involved in photosynthesis and other metabolic processes in the fruit of Opuntia ficus-indica during growth and ripening. Sci. Hortic. 2011, 128, 213–219. [Google Scholar] [CrossRef]
- Delgado-Alvarado, A.; Ortega-Delgado, M.L. Pyruvate orthophosphate dikinase from Phaseolus vulgaris L. pod. Annu. Rep. Bean Improv. Coop. 1996, 39, 251–252. [Google Scholar]
- Pua, E.C.; Chandramouli, S.; Han, P.; Liu, P. Malate synthase gene expression during fruit ripening of Cavendish banana (Musa acuminata cv. Williams). J. Exp. Bot. 2003, 54, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yang, Y.; Murayama, H.; Taira, S.; Fukushima, T. Effects of CO2 on respiratory metabolism in ripening banana fruit. Postharvest Biol. Technol. 2004, 33, 27–34. [Google Scholar] [CrossRef]
- Yang, Y.; Murayama, H.; Fukushima, T. Activation of glyoxylate cycle enzymes in cucumber fruits exposed to CO2. Plant Cell Physiol. 1998, 39, 533–539. [Google Scholar] [CrossRef]
- Graham, I.A.; Leaver, C.J.; Smith, S.M. Induction of malate synthase gene expression in senescent and detached organs of cucumber. Plant Cell 1992, 4, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Pistelli, L.; Nieri, B.; Smith, S.M.; Alpi, A.; De Bellis, L. Glycoxylate cycle enzyme activities are induced in senescent pumpkin fruits. Plant Sci. 1996, 119, 23–29. [Google Scholar] [CrossRef]
- Drawert, F.; Steffan, H. Biochemisch-physiologische Untersuchungen an Traubenbeeren. III. Stoffwechsel von zugeführten 14 C-Verbindungen und die Bedeutung des Säure-Zucker-Metabolismus für die Reifung von Traubenbeeren. Vitis 1966, 5, 377–384. [Google Scholar]
- Hardy, P.J. Metabolism of sugars and organic acids in immature grape berries. Plant Physiol. 1968, 43, 224–228. [Google Scholar] [CrossRef]
- Peynaud, E.; Ribereau-Gayon, P. The grape. In The Biochemistry of Fruits and Their Products; Academic Press: London, UK; New York, NY, USA, 1971; p. 787. [Google Scholar]
- Ruffner, H.P.; Kliewer, W.M. Phosphoenolpyruvate carboxykinase activity in grape berries. Plant Physiol. 1975, 56, 67–71. [Google Scholar] [CrossRef]
- Farineau, J.; Laval-Martin, D. Light versus Dark Carbon Metabolism in Cherry Tomato Fruits: II. Relationship Between Malate Metabolism and Photosynthetic Activity. Plant Physiol. 1977, 60, 877–880. [Google Scholar] [CrossRef]
- Halinska, A.; Frenkel, C. Acetaldehyde stimulation of net gluconeogenic carbon movement from applied malic acid in tomato fruit pericarp tissue. Plant Physiol. 1991, 95, 954–960. [Google Scholar] [CrossRef]
- Leegood, R.C.; Acheson, R.M.; Técsi, L.I.; Walker, R.P. The many-faceted function of phosphoenolpyruvate carboxykinase in plants. In Regulation of Primary Metabolic Pathways in Plants; Springer: Dordrecht, Germany, 1999; pp. 37–51. [Google Scholar]
- Osorio, S.; Vallarino, J.G.; Szecowka, M.; Ufaz, S.; Tzin, V.; Angelovici, R.; Galili, G.; Fernie, A.R. Alteration of the interconversion of pyruvate and malate in the plastid or cytosol of ripening tomato fruit invokes diverse consequences on sugar but similar effects on cellular organic acid, metabolism, and transitory starch accumulation. Plant Physiol. 2013, 161, 628–643. [Google Scholar] [CrossRef]
- Huang, Y.X.; Goto, Y.; Nonaka, S.; Fukuda, N.; Ezura, H.; Matsukura, C. Overexpression of the phosphoenolpyruvate carboxykinase gene (SlPEPCK) promotes soluble sugar accumulation in fruit and post-germination growth of tomato (Solanum lycopersicum L.). Plant Biotechnol. 2015, 32, 281–289. [Google Scholar] [CrossRef]
- Huang, Y.X.; Yin, Y.G.; Sanuki, A.; Fukuda, N.; Ezura, H.; Matsukura, C. Phosphoenolpyruvate carboxykinase (PEPCK) deficiency affects the germination, growth and fruit sugar content in tomato (Solanum lycopersicum L.). Plant Physiol. Biochem. 2015, 96, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Famiani, F.; Walker, R.P. Changes in abundance of enzymes involved in organic acid, amino acid and sugar metabolism, and photosynthesis during the ripening of blackberry fruit. J. Am. Soc. Hortic. Sci. 2009, 134, 167–175. [Google Scholar] [CrossRef]
- Blanke, M.M.; Lenz, F. Fruit photosynthesis. Plant Cell Environ. 1989, 12, 31–46. [Google Scholar] [CrossRef]
- Ruffner, H.P. Metabolism of tartaric and malic acid in Vitis: A review. Part A. Vitis 1982, 21, 247–259. [Google Scholar]
- Famiani, F.; Walker, R.P.; Tecsi, L.; Chen, Z.H.; Proietti, P.; Leegood, C.R. An immunohistochemical study of the compartmentation of metabolism during the development of grape (Vitis vinifera L.) berries. J. Exp. Bot. 2000, 51, 675–683. [Google Scholar]
- Sweetman, C.; Deluc, L.G.; Cramer, G.R.; Ford, C.M.; Soole, K.L. Regulation of malate metabolism in grape berry and other developing fruits. Phytochemistry 2009, 70, 1329–1344. [Google Scholar] [CrossRef]
- Famiani, F.; Farinelli, D.; Palliotti, A.; Moscatello, S.; Battistelli, A.; Walker, R.P. Is stored malate the quantitatively most important substrate utilised by respiration and ethanolic fermentation in grape berry pericarp during ripening? Plant Physiol. Biochem. 2014, 76, 52–57. [Google Scholar] [CrossRef]
- Famiani, F.; Battistelli, A.; Moscatello, S.; Cruz-Castillo, J.G.; Walker, R.P. The organic acids that are accumulated in the flesh of fruits: Occurrence, metabolism and factors affecting their contents-a review. Rev. Chapingo Ser. Hortic. 2015, 21, 97–128. [Google Scholar] [CrossRef]
- Terrier, N.; Romieu, C. Grape berry acidity. In Molecular Biology & Biotechnology of the Grapevine; Springer: Dordrecht, Germany, 2001; pp. 35–57. [Google Scholar]
- Centeno, D.C.; Osorio, S.; Nunes-Nesi, A.; Bertolo, A.L.F.; Carneiro, R.T.; Araújo, W.L.; Steinhauser, M.C.; Michalska, J.; Rohrmann, J.; Geigenberger, P.; et al. Malate Plays a Crucial Role in Starch Metabolism, Ripening, and Soluble Solid Content of Tomato Fruit and Affects Postharvest Softening. Plant Cell 2011, 23, 162–184. [Google Scholar] [CrossRef]
- Walker, R.P.; Battistelli, A.; Bonghi, C.; Drincovich, M.F.; Falchi, R.; Lara, M.V.; Moscatello, S.; Vizzotto, G.; Famiani, F. Non-structural Carbohydrate Metabolism in the Flesh of Stone Fruits of the Genus Prunus (Rosaceae)—A Review. Front. Plant Sci. 2020, 11, 549921. [Google Scholar] [CrossRef]
- Walker, R.P.; Chen, Z.H. Phosphoenolpyruvate carboxykinase: Structure, function and regulation. Adv. Bot. Res. 2002, 38, 93–189. [Google Scholar]
- Drincovich, M.F.; Lara, M.V.; Andreo, C.H.; Maurino, V.G. C 4 decarboxylases: Different solutions for the same biochemical problem, the provision of CO2 to RuBisCO in the bundle sheath cells. In C4 Photosynthesis and Related CO2 Concentrating Mechanisms; Springer: Dordrecht, Germany, 2010; pp. 277–300. [Google Scholar]
- Aubry, S.; Brown, N.J.; Hibberd, J.M. The role of proteins in C3 plants prior to their recruitment into the C4 pathway. J. Exp. Bot. 2011, 62, 3049–3059. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, S.; Rubio, M.B.; Cardoza, R.E.; Gutiérrez, S.; Nicolás, N.; Bettiol, W.; Hermosa, R.; Monte, E. Nitrogen metabolism and growth enhancement in tomato plants challenged with Trichoderma harzianum expressing the Aspergillus nidulans acetamidase amdS gene. Front. Microbiol. 2016, 7, 1182. [Google Scholar] [CrossRef]
- Ludwig, M. The roles of organic acids in C4 photosynthesis. Front. Plant Sci. 2016, 7, 647. [Google Scholar] [CrossRef]
- Roubelakis-Angelakis, K.A.; Kliewer, W.M. Nitrogen metabolism in grapevine. Hortic. Rev. 1992, 14, 407–452. [Google Scholar]
- Horchani, F.; Gallusci, P.; Baldet, P.; Cabasson, C.; Maucourt, M.; Rolin, D.; Aschi-Smiti, S.; Raymond, P. Prolonged root hypoxia induces ammonium accumulation and decreases the nutritional quality of tomato fruits. J. Plant Physiol. 2008, 165, 1352–1359. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.K.M.; Pang, M.K.L. Estimation of ammonium ion distribution between cytoplasm and vacuole using nuclear magnetic resonance spectroscopy. Plant Physiol. 1992, 100, 1571–1574. [Google Scholar] [CrossRef]
- Lea, P.J. Nitrogen metabolism. In Plant Biochemistry and Molecular Biology; Lea, P.J., Leegood, R.C., Eds.; John Wiley: New York, NY, USA, 1993; pp. 155–180. [Google Scholar]
- Scheible, W.R.; Krapp, A.; Stitt, M. Reciprocal diurnal changes of phosphoenolpyruvate carboxylase expression and cytosolic pyruvate kinase, citrate synthase and NADP-isocitrate dehydrogenase expression regulate organic acid metabolism during nitrate assimilation in tobacco leaves. Plant Cell Environ. 2000, 23, 1155–1167. [Google Scholar] [CrossRef]
- Forde, B.G.; Lea, P.J. Glutamate in plants: Metabolism, regulation, and signalling. J. Exp. Bot. 2007, 58, 2339–2358. [Google Scholar] [CrossRef]
- Tohge, T.; Ramos, M.S.; Nunes-Nesi, A.; Mutwil, M.; Giavalisco, P.; Steinhauser, D.; Schellenberg, M.; Willmitzer, L.; Persson, S.; Martinoia, E.; et al. Toward the storage metabolome: Profiling the barley vacuole. Plant physiol. 2011, 157, 1469–1482. [Google Scholar] [CrossRef]
- Rocha, M.; Licausi, F.; Araújo, W.L.; Nunes-Nesi, A.; Sodek, L.; Fernie, A.R.; van Dongen, J.T. Glycolysis and the Tricarboxylic Acid Cycle Are Linked by Alanine Aminotransferase during Hypoxia Induced by Waterlogging of Lotus japonicus. Plant Physiol. 2010, 152, 1501–1513. [Google Scholar] [CrossRef] [PubMed]
- António, C.; Päpke, C.; Rocha, M.; Diab, H.; Limami, A.M.; Obata, T.; Fernie, A.R.; van Dongen, J.T. Regulation of Primary Metabolism in Response to Low Oxygen Availability as Revealed by Carbon and Nitrogen Isotope Redistribution. Plant Physiol. 2016, 170, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, Y.; Dolferus, R.; Ismond, K.P.; Good, A.G. Alanine aminotransferase catalyses the breakdown of alanine after hypoxia in Arabidopsis thaliana. Plant J. 2007, 49, 1108–1121. [Google Scholar] [CrossRef]
- Chiba, Y.; Miyakawa, T.; Shimane, Y.; Takai, K.; Tanokura, M.; Nozaki, T. Structural comparisons of phosphoenolpyruvate carboxykinases reveal the evolutionary trajectories of these phosphodiester energy conversion enzymes. J. Biol. Chem. 2019, 294, 19269–19278. [Google Scholar] [CrossRef]
- Chi, S.; Wu, S.; Yu, J.; Wang, X.; Tang, X.; Liu, T. Phylogeny of C 4-photosynthesis enzymes based on algal transcriptomic and genomic data supports an archaeal/proteobacterial origin and multiple duplication for most C 4-related genes. PLoS ONE 2014, 9, e110154. [Google Scholar] [CrossRef] [PubMed]
- Tronconi, M.A.; Andreo, C.S.; Drincovich, M.F. Chimeric structure of plant malic enzyme family: Different evolutionary scenarios for NAD-and NADP-dependent isoforms. Front. Plant Sci. 2018, 9, 565. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.P.; Acheson, R.M.; Técsi, L.I.; Leegood, R.C. Phosphoenolpyruvate carboxykinase in C4 plants: Its role and regulation. Funct. Plant Biol. 1997, 24, 459–468. [Google Scholar] [CrossRef]
- Verma, N.K.; Bajpai, A.B.; Yadav, S.K. In-silico characterization and homology modeling of PEPCK enzyme of Medicago truncatula. J. Plant Dev. Sci. 2017, 9, 165–177. [Google Scholar]
- Shen, Z.; Dong, X.M.; Gao, Z.F.; Chao, Q.; Wang, B.C. Phylogenic and phosphorylation regulation difference of phosphoenolpyruvate carboxykinase of C3 and C4 plants. J. Plant Physiol. 2017, 213, 16–22. [Google Scholar] [CrossRef]
- Walker, R.P.; Leegood, R.C. Phosphorylation of phosphoenolpyruvate carboxykinase in plants: Studies in plants with C4 photosynthesis and Crassulacean acid metabolism and in germinating seeds. Biochem. J. 1996, 317, 653–658. [Google Scholar] [CrossRef]
- Walker, R.P.; Chen, Z.H.; Acheson, R.M.; Leegood, R.C. Effects of phosphorylation on phosphoenolpyruvate carboxykinase from the C4 plant, Guinea grass. Plant Physiol. 2002, 128, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.P.; Leegood, R.C. Purification, and phosphorylation in vivo and in vitro, of phosphoenolpyruvate carboxykinase from cucumber cotyledons. FEBS Lett. 1995, 362, 70–74. [Google Scholar] [CrossRef]
- Chen, Z.H.; Walker, R.P.; Acheson, R.M.; Leegood, R.C. Phosphoenolpyruvate carboxykinase assayed at physiological concentrations of metal ions has a high affinity for CO2. Plant Physiol. 2002, 128, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Rojas, B.E.; Hartman, M.D.; Figueroa, C.M.; Leaden, L.; Podestá, F.E.; Iglesias, A.A. Biochemical characterization of phosphoenolpyruvate carboxykinases from Arabidopsis thaliana. Biochem. J. 2019, 476, 2939–2952. [Google Scholar] [CrossRef]
- Rojas, B.E.; Hartman, M.D.; Figueroa, C.M.; Iglesias, A.A. Proteolytic cleavage of Arabidopsis thaliana phospho enol pyruvate carboxykinase-1 modifies its allosteric regulation. J. Exp. Bot. 2021, 72, 2514–2524. [Google Scholar] [CrossRef]
- Nimmo, H.G. Control of the phosphorylation of phosphoenolpyruvate carboxylase in higher plants. Arch. Biochem. Biophys. 2003, 414, 189–196. [Google Scholar] [CrossRef]
- Walker, R.P.; Paoletti, P.; Leegood, R.C.; Famiani, F. Phosphorylation of phosphoenolpyruvate carboxykinase (PEPCK) and phosphoenolpyruvate carboxylase (PEPC) in the flesh of fruits. Plant Physiol. Biochem. 2016, 108, 323–327. [Google Scholar] [CrossRef]
- Williamson, D.H.; Lund, P.; Krebs, H.A. The redox state of free nicotinamide-adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem. J. 1967, 103, 514–527. [Google Scholar] [CrossRef]
- Veech, R.L.; Eggleston, L.V.; Krebs, H.A. The redox state of free nicotinamide-adenine dinucleotide phosphate in the cytoplasm of rat liver. Biochem. J. 1996, 115, 609–619. [Google Scholar] [CrossRef]
- Heineke, D.; Riens, B.; Grosse, H.; Hoferichter, P.; Peter, U.; Flügge, U.I.; Heldt, H.W. Redox transfer across the inner chloroplast envelope membrane. Plant Physiol. 1991, 95, 1131–1137. [Google Scholar] [CrossRef]
- Plaxton, W.C. The organization of plant glycolysis. Annu. Rev. Plant Physiol. Mol. Biol. 1996, 47, 185–214. [Google Scholar] [CrossRef]
- Brown, J.R.; Douady, C.J.; Italia, M.J.; Marshall, W.E.; Stanhope, M.J. Universal trees based large combined protein sequence data sets. Nat. Genet. 2001, 28, 281–285. [Google Scholar] [CrossRef]
- Horiike, T.; Miyata, D.; Hamada, K.; Saruhashi, S.; Shinozawa, T.; Kumar, S.; Chakraborty, R.; Komiyama, T.; Tateno, Y. Phylogenetic construction of 17 bacterial phyla by new method and carefully selected orthologs. Gene 2009, 429, 59–64. [Google Scholar] [CrossRef]
- Chiba, Y.; Kamikawa, R.; Nakada-Tsukui, K.; Saito-Nakano, Y.; Nozaki, T. Discovery of PPi-type Phosphoenolpyruvate Carboxykinase Genes in Eukaryotes and Bacteria. J. Biol. Chem. 2015, 290, 23960–23970. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Kim, S.H. A genome Tree of Life for the Fungi kingdom. Proc. Natl. Acad. Sci. USA 2017, 114, 9391–9396. [Google Scholar] [CrossRef] [PubMed]
- Pryer, K.M.; Schneider, H.; Zimmer, E.A.; Banks, J.A. Deciding among green plants for whole genome studies. Trends Plant Sci. 2002, 7, 550–554. [Google Scholar] [CrossRef]
- Higgins, J.D.; Thompson, J.D.; Gibson, T.J.; Clustal, W. Improving the ensitivity ofprogressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar]
- Ronquist, F.; Huelsenbeck, J.P. Mr Bayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef]
- Law, R.D.; Plaxton, W.C. Purification and characterization of a novel phosphoenolpyruvate carboxylase from banana fruit. Biochem. J. 1995, 307, 807–816. [Google Scholar] [CrossRef]
- Smith, C.R.; Knowles, V.L.; Plaxton, W.C. Purification and characterization of cytosolic pyruvate kinase from Brassica napus (rapeseed) suspension cell cultures: Implications for the integration of glycolysis with nitrogen assimilation. Eur. J. Biochem. 2000, 267, 4477–4485. [Google Scholar] [CrossRef]
- Turner, W.L.; Plaxton, W.C. Purification and characterization of cytosolic pyruvate kinase from banana fruit. Biochem. J. 2000, 352, 875–882. [Google Scholar] [CrossRef]
- Chollet, R.; Vidal, J.; O’Leary, M.H. Phosphoenol pyruvate carboxylase: A ubiquitous, highly regulated enzyme in plants. Annu. Rev. Plant Biol. 1996, 47, 273–298. [Google Scholar] [CrossRef]
- Hafke, J.B.; Neff, R.; Hiitt, M.T.; Liittge, U.; Thiel, G. Day-to-night variations of cytoplasmic pH in a crassulacean acid metabolism plant. Protoplasma 2001, 216, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.H.; Jenkins, G.I.; Nimmo, H. pH and carbon supply control the expression of phosphoenolpyruvate carboxylase kinase genes in Arabidopsis thaliana. Plant Cell Environ. 2008, 31, 1844–1850. [Google Scholar] [CrossRef] [PubMed]
- Hartwell, J.; Gill, A.; Nimmo, G.A.; Wilkins, M.B.; Jenkins, G.I.; Nimmo, H.G. Phosphoenolpyruvate carboxylase kinase is a novel protein kinase regulated at the level of expression. Plant J. 1999, 20, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, P.W.; Prosser, I.M.; Young, K. NADP-linked malic enzyme and malate metabolism in ageing tomato fruit. Phytochemistry 1985, 24, 1157–1162. [Google Scholar] [CrossRef]
- Knee, M.; Finger, F.L. NADP+-malic enzyme and organic acid levels in developing tomato fruits. J. Am. Soc. Hortic. Sci. 1992, 117, 799–801. [Google Scholar] [CrossRef]
- Knee, M.; Finger, F.L.; Lagrimini, L.M. Evidence for a cytosolic NADP-malic enzyme in tomato. Phytochemistry 1996, 42, 11–16. [Google Scholar] [CrossRef]
- Davies, D.D.; Patil, K.D. Regulation of ‘malic’ enzyme of Solanum tuberosum by metabolites. Biochem. J. 1974, 137, 45–53. [Google Scholar] [CrossRef]
- Davies, D.D.; Patil, K.D. The control of NAD specific malic enzyme from cauliflower bud mitochondria by metabolites. Planta 1975, 126, 197–211. [Google Scholar] [CrossRef]
- Artus, N.N.; Edwards, G.E. NAD-malic enzyme from plants. FEBS Lett. 1985, 182, 225–233. [Google Scholar] [CrossRef]
- Wedding, R.T. Malic enzymes of higher plants: Characteristics, regulation, and physiological function. Plant Physiol. 1989, 90, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Bazaes, S.; Toncio, M.; Laivenieks, M.; Zeikus, J.G.; Cardemil, E. Comparative kinetic effects of Mn (II), Mg (II) and the ATP/ADP ratio on phosphoenolpyruvate carboxykinases from Anaerobiospirillum succiniciproducens and Saccharomyces cerevisiae. Protein J. 2007, 26, 265–269. [Google Scholar] [CrossRef]
- Drouet, A.G.; Hartmann, C.J.R. Activity of pear fruit malic enzyme; its regulation by metabolites. Phytochemistry 1977, 16, 505–508. [Google Scholar] [CrossRef]
- Ruffner, H.P.; Possner, D.; Brem, S.; Rast, D.M. The physiological role of malic enzyme in grape ripening. Planta 1984, 160, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Possner, D.; Ruffner, H.P.; Rast, D.M. Isolation and biochemical characterization of grape malic enzyme. Planta 1981, 151, 549–554. [Google Scholar] [CrossRef]
- Krömer, S.; Malmberg, G.; Gardestrom, P. Mitochondrial contribution to photosynthetic metabolism (A study with barley (Hordeum vulgare L.) leaf protoplasts at different light intensities and CO2 concentrations). Plant Physiol. 1993, 102, 947–955. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).