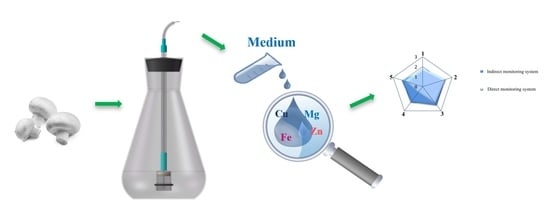
A New Biotechnology Method of Bioelements’ Accumulation Monitoring in In Vitro Culture of Agaricus bisporus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mushroom Materials
2.1.1. Initial Mycelial Cultures
2.1.2. Experimental Mycelial Cultures
2.2. Reagents
2.3. Direct Sampling Methods of Medium from In Vitro Cultures
2.4. Indirect Sampling Methods from Medium from In Vitro Cultures by Copyright Constructed Dipper
2.5. Determination of Elements with the Atomic Absorption Spectrometry (AAS)
3. Results and Discussion
3.1. Evaluation of the Reproducibility of Optimal A. bisporus In Vitro Cultures
3.2. Evaluation of Biomass Growth and Metals’ Accumulation Efficiency in Mycelium from In Vitro Cultures of A. bisporus
3.3. Evaluation of the Changes in Chemical Composition of Liquid Media during Biomass Growth Using Indirect and Direct Sampling Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Krakowska, A.; Reczyński, W.; Muszyńska, B. Optimization of the liquid culture medium composition to obtain the mycelium of Agaricus bisporus rich in essential minerals. Biol. Trace Elem. Res. 2016, 173, 231–240. [Google Scholar] [CrossRef]
- Krakowska, A.; Zięba, P.; Włodarczyk, A.; Kała, K.; Sułkowska-Ziaja, K.; Bernaś, E.; Muszyńska, B. Selected edible medicinal mushrooms from Pleurotus genus as an answer for human civilization diseases. Food Chem. 2020, 327, 127084. [Google Scholar] [CrossRef]
- Muszyńska, B.; Sułkowska-Ziaja, K.; Ekiert, H. An antioxidant in fruiting bodies and in mycelia from In Vitro cultures of Calocera viscosa (Basidiomycota)—Preliminary result. Acta Pol. Pharm. Drug Res. 2012, 69, 135–138. [Google Scholar]
- Muszyńska, B.; Krakowska, A.; Sułkowska-Ziaja, K.; Opoka, W.; Reczyński, W.; Baś, B. In Vitro cultures and fruiting bodies of culinary-medicinal Agaricus bisporus (white button mushroom) as a source of selected biologically-active elements. J. Food. Sci. Technol. 2015, 52, 7337–7344. [Google Scholar] [CrossRef]
- Ndungutse, V.; Mereddy, R.; Sultanbawa, Y. Bioactive properties of mushroom (Agaricus bisporus) stipe extracts. J. Food Process. Preserv. 2015, 39, 2225–2233. [Google Scholar] [CrossRef]
- Novaes, M.R.; Valadares, F.; Reis, M.C.; Gonçalves, D.R.; Menezes, M.C. The effects of dietary supplementation with Agaricales mushrooms and other medicinal fungi on breast cancer: Evidence-based medicine. Clinics 2011, 66, 2133–2139. [Google Scholar] [CrossRef] [Green Version]
- Raman, J.; Jang, K.Y.; Oh, Y.L.; Oh, M.; Im, J.H.; Lakshmanan, H.; Sabaratnam, V. Cultivation and nutritional value of prominent Pleurotus spp.: An Overview. Mycobiology 2021, 49, 1–14. [Google Scholar] [CrossRef]
- Sithole, S.C.; Mugivhisa, L.L.; Amoo, S.O.; Olowoyo, J.O. Pattern and concentrations of trace metals in mushrooms harvested from trace metal-polluted soils in Pretoria, South Africa. S. Afr. J. Bot. 2017, 108, 315–320. [Google Scholar] [CrossRef]
- Vamanu, E.; Nita, S. Biological activity of fluidized bed ethanol extracts from several edible mushrooms. Food Sci. Biotechnol. 2014, 23, 1483–1490. [Google Scholar] [CrossRef]
- Urbain, P.; Valverde, J.; Jakobsen, J. Impact on vitamin D2, vitamin D4 and agaritine in Agaricus bisporus mushrooms after artificial and natural solar uv light exposure. Plant Foods Hum. Nutr. 2016, 71, 314–321. [Google Scholar] [CrossRef]
- Kampmann, M.; Hoffrichter, A.C.; Stalinski, D.; Wichmann, R. Kinetic characterization of tyrosinase containing mushroom (Agaricus bisporus) cells immobilized in silica alginate. J. Mol. Catal. B Enzym. 2015, 116, 124–133. [Google Scholar] [CrossRef]
- Patel, S.; Goyal, A. Recent developments in mushrooms as anticancer therapeutics: A review. 3 Biotech. 2012, 2, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.C.; Ho, K.; Hsieh, Y.; Wang, T.; Mau, J. Contents of lovastatin, γ-aminobutyric acid and ergothioneine in mushroom fruiting bodies and mycelia. LWT-Food Sci. Technol. 2012, 47, 274–278. [Google Scholar] [CrossRef]
- Bosiacki, M.; Siwulski, M.; Sobieralski, K.; Krzebietke, S. The content of selected heavy metals in fruiting bodies of Agaricus bisporus (Lange) Imbach. wild growing in Poland. J. Elem. 2018, 23, 875–886. [Google Scholar] [CrossRef]
- Falandysz, J.; Borovička, J. Macro and trace mineral constituents and radionuclides in mushrooms: Health benefits and risk. Appl. Microbiol. Biotechnol. 2013, 97, 477–501. [Google Scholar] [CrossRef] [Green Version]
- Falandysz, J.; Drewnowska, M. Macro and trace elements in common chanterelle (Cantharellus cibarius) mushroom from the European background areas in Poland: Composition, accumulation, dietary exposure and data review for species. J. Environ. Sci. Health B 2015, 50, 374. [Google Scholar] [CrossRef]
- Glamočlija, J.; Stojković, D.; Nikolić, M.; Ćirić, A.; Reis, F.S.; Barros, L.; Ferreira, I.C.F.R. A comparative study on edible mushrooms as functional foods. Food Funct. 2015, 6, 1900–1910. [Google Scholar] [CrossRef] [Green Version]
- Haro, A.; Trescastro, A.; LaraIgnacio, L.; Fernández-Fígares, L.; Nieto, R.; Seiquer, I. Mineral elements content of wild growing edible mushrooms from the southeast of Spain. J. Food Compost. Anal. 2020, 91, 103504. [Google Scholar] [CrossRef]
- Siwulski, M.; Sobieralski, K.; Sas-Golak, I. Nutritive and health-promoting value of mushrooms. ŻNTJ 2014, 1, 16–28. [Google Scholar]
- Falandysz, J.; Szymczyk, K.; Ichihashi, H.; Bielawski, L.; Gucia, M.; Frankowska, A.; Yamasaki, S. ICP/MS and ICP/AES elemental anlysis (38 elemenets) of edible wild mushrooms growing in Poland. Food Addit. Contam. 2001, 18, 503–513. [Google Scholar] [CrossRef]
- Carvalho, M.; Pimentel, A.; Fernandes, B. Study of heavy metals in wild edible mushrooms under different pollutions conditions by X-ray fluorescence spectrometry. Anal. Sci. 2005, 21, 747–750. [Google Scholar] [CrossRef]
- Lalotra, P.; Gupta, D.; Yangdol, R.; Sharma, Y.; Gupta, S. Bioaccumulation of heavy metals in the sporocarps of some wild mushrooms. Curr. Res. Environ. Appl. Mycol. 2016, 6, 159–165. [Google Scholar] [CrossRef]
- Podkowa, A.; Kryczyk-Poprawa, A.; Opoka, W.; Muszyńska, B. Culinary–medicinal mushrooms: A review of organic compounds and bioelements with antioxidant activity. Eur. Food Res. Technol. 2021, 247, 513–533. [Google Scholar] [CrossRef]
- Reczyński, W.; Muszyńska, B.; Opoka, W.; Smalec, A.; Sułkowska-Ziaja, K. Comparative study of metals accumulation in cultured In Vitro mycelium and natural grown fruiting bodies of Boletus badius and Cantharellus cibarius. Biol. Trace Elem. Res. 2013, 153, 355–362. [Google Scholar] [CrossRef] [Green Version]
- Rashid, H.; Abed, I.; Owaid, M. Mycelial growth performance of Agaricus bisporus in culture medium of composts supplemented with Sesbania sesban straw and phosphate rock. Curr. Res. Environ. 2018, 8, 323–330. [Google Scholar]
- Tan, Y.; Moore, D. Convenient and effective methods for in vitro cultivation of mycelium and fruiting bodies of Lentinus edodes. Mycol. Res. 1992, 96, 1077–10984. [Google Scholar] [CrossRef]
- Hassan, F.; Ghada, M.; El-Kady, A. Mycelial biomass production of Enoke Mushroom (Flammulina velutipes) by submerged culture. Aust. J. Basic Appl. Sci. 2012, 6, 603–610. [Google Scholar]
- Muszyńska, B.; Kała, K.; Sułkowska-Ziaja, K. Mushrooms as a source of biological active indole compounds. In Indole Synthesis, Functions and Reactions; Chemistry Research and Applications; Nova Science Publishers: New York, NY, USA, 2019; Chapter 2; ISBN 1536147773. [Google Scholar]



| Metal | Amount of Additive [g] per 250 mL Oddoux Liquid Medium | |||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Mg | 0 | 0.10 | 0.50 | 1.00 |
| Zn | 0 | 0.01 | 0.05 | 0.10 |
| Cu | 0 | 0.01 | 0.05 | 0.10 |
| Fe | 0 | 0.10 | 0.50 | 1.00 |
| Symbol Identify In Vitro Culture | The Proportions of Added Metals [g] |
|---|---|
| A | Mg(2)Zn(1)Cu(2)Fe(1) |
| B | Mg(3)Zn(1)Cu(2)Fe(1) |
| C | Mg(1)Zn(1)Cu(3)Fe(1) |
| D | Mg(2)Zn(1)Cu(3)Fe(1) |
| E | Mg(3)Zn(1)Cu(3)Fe(1) |
| In Vitro Culture | Repetition | Weight [g] | Concentration [mg/g d. w.] | |||
|---|---|---|---|---|---|---|
| Mg | Zn | Cu | Fe | |||
| Control | 2.1263 | 0.91 ± 0.06 | 0.042 ± 0.004 | 0.011 ± 0.004 | 1.02 ± 0.08 | |
| A | A.1 | 3.3309 | 2.91 ± 0.01 * | 0.899 ± 0.001 * | 1.598 ± 0.008 * | 9.30 ± 0.01 * |
| A.2 | 3.3229 | 2.06 ± 0.01 * | 0.650 ± 0.006 * | 1.942 ± 0.003 * | 8.97 ± 0.01 * | |
| A.3 | 3.1996 | 2.90 ± 0.01 * | 0.705 ± 0.003 * | 2.175 ± 0.009 * | 8.43 ± 0.02 * | |
| A.4 | 3.2827 | 3.32 ± 0.03 * | 0.716 ± 0.011 * | 1.693 ± 0.007 * | 7.80 ± 0.01 * | |
| A.5 | 3.0178 | 2.78 ± 0.01 * | 0.576 ± 0.004 * | 2.234 ± 0.002 * | 7.42 ± 0.02 * | |
| B | B.1 | 3.1333 | 4.63 ± 0.02 * | 0.542 ± 0.006 * | 2.278 ± 0.001 * | 2.61 ± 0.02 * |
| B.2 | 3.2668 | 5.15 ± 0.01 * | 0.641 ± 0.009 * | 2.601 ± 0.011 * | 3.63 ± 0.01 * | |
| B.3 | 3.2095 | 4.10 ± 0.02 * | 0.677 ± 0.002 * | 3.141 ± 0.007 * | 2.16 ± 0.03 * | |
| B.4 | 3.1925 | 4.49 ± 0.02 * | 0.581 ± 0.005 * | 2.523 ± 0.008 * | 3.02 ± 0.01 * | |
| B.5 | 2.9832 | 4.42 ± 0.01 * | 0.488 ± 0.006 * | 2.994 ± 0.002 * | 2.12 ± 0.02 * | |
| C | C.1 | 3.3093 | 1.15 ± 0.02 * | 0.381 ± 0.009 * | 2.477 ± 0.012 * | 3.64 ± 0.03 * |
| C.2 | 3.0408 | 1.01 ± 0.01 | 0.168 ± 0.010 * | 2.377 ± 0.015 * | 1.37 ± 0.02 * | |
| C.3 | 3.4291 | 1.83 ± 0.01 * | 0.483 ± 0.007 * | 1.999 ± 0.028 * | 3.59 ± 0.03 * | |
| C.4 | 3.2193 | 0.90 ± 0.03 | 0.290 ± 0.001 * | 3.375 ± 0.001 * | 4.02 ± 0.02 * | |
| C.5 | 3.5032 | 1.33 ± 0.01 * | 0.462 ± 0.004 * | 3.455 ± 0.018 * | 4.80 ± 0.04 * | |
| D | D.1 | 3.2917 | 2.25 ± 0.03 * | 0.821 ± 0.009 * | 3.111 ± 0.022 * | 6.91 ± 0.03 * |
| D.2 | 3.3175 | 2.92 ± 0.01 * | 0.711 ± 0.002 * | 1.830 ± 0.010 * | 4.28 ± 0.03 * | |
| D.3 | 3.2144 | 2.70 ± 0.03 * | 0.655 ± 0.001 * | 2.107 ± 0.002 * | 5.44 ± 0.01 * | |
| D.4 | 3.8956 | 2.83 ± 0.01 * | 0.661 ± 0.008 * | 2.133 ± 0.021 * | 3.99 ± 0.03 * | |
| D.5 | 2.4965 | 2.17 ± 0.01 * | 0.581 ± 0.010 * | 1.597 ± 0.001 * | 5.59 ± 0.03 * | |
| E | E.1 | 2.6613 | 4.59 ± 0.04 * | 0.607 ± 0.009 * | 2.565 ± 0.008 * | 3.62 ± 0.02 * |
| E.2 | 2.9828 | 5.13 ± 0.03 * | 0.564 ± 0.002 * | 2.873 ± 0.009 * | 3.77 ± 0.02 * | |
| E.3 | 2.7405 | 5.00 ± 0.01 * | 0.713 ± 0.006 * | 3.462 ± 0.002 * | 2.75 ± 0.01 * | |
| E.4 | 2.7925 | 3.71 ± 0.03 * | 0.647 ± 0.005 * | 1.699 ± 0.012 * | 3.01 ± 0.03 * | |
| E.5 | 2.3925 | 3.77 ± 0.01 * | 0.665 ± 0.011 * | 1.974 ± 0.011 * | 3.96 ± 0.02 * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krakowska, A.; Reczyński, W.; Krakowski, T.; Szewczyk, K.; Opoka, W.; Muszyńska, B. A New Biotechnology Method of Bioelements’ Accumulation Monitoring in In Vitro Culture of Agaricus bisporus. Molecules 2021, 26, 5165. https://doi.org/10.3390/molecules26175165
Krakowska A, Reczyński W, Krakowski T, Szewczyk K, Opoka W, Muszyńska B. A New Biotechnology Method of Bioelements’ Accumulation Monitoring in In Vitro Culture of Agaricus bisporus. Molecules. 2021; 26(17):5165. https://doi.org/10.3390/molecules26175165
Chicago/Turabian StyleKrakowska, Agata, Witold Reczyński, Tomasz Krakowski, Karolina Szewczyk, Włodzimierz Opoka, and Bożena Muszyńska. 2021. "A New Biotechnology Method of Bioelements’ Accumulation Monitoring in In Vitro Culture of Agaricus bisporus" Molecules 26, no. 17: 5165. https://doi.org/10.3390/molecules26175165
APA StyleKrakowska, A., Reczyński, W., Krakowski, T., Szewczyk, K., Opoka, W., & Muszyńska, B. (2021). A New Biotechnology Method of Bioelements’ Accumulation Monitoring in In Vitro Culture of Agaricus bisporus. Molecules, 26(17), 5165. https://doi.org/10.3390/molecules26175165