Delta Sleep-Inducing Peptide Recovers Motor Function in SD Rats after Focal Stroke
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Drugs
2.3. Middle Cerebral Artery Occlusion Procedure (MCAO)
2.4. Groups and Dosing Regimen
2.5. Behavioral Testing
2.5.1. Rotarod Test
2.5.2. Drug-Induced Rotation Asymmetry
2.6. Brain Infarct Analysis
2.7. Statistical Analysis
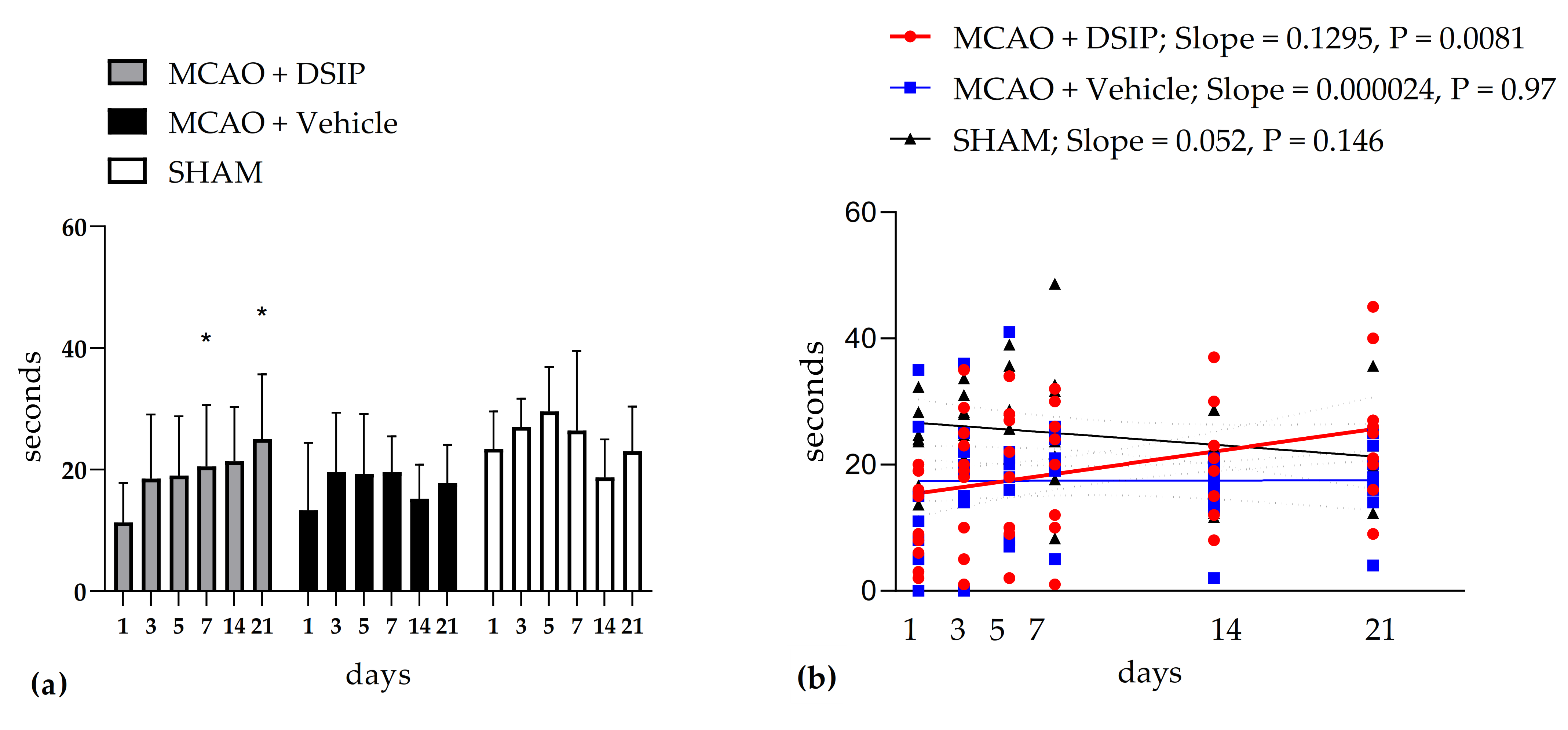
3. Results
3.1. Behavioral Testing
3.1.1. Motor Performance after MCAO in Rotarod Test
3.1.2. Ketamine-Induced Rotation
3.1.3. Brain Infarction Volume
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix A.1. Raw Data on Rotarod
| Animal Number | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Mean ± SD |
|---|---|---|---|---|---|---|---|---|
| Day 1 | 14 | 25 | 32 | 17 | 24 | 28 | 24 | 23 ± 6 |
| Day 3 | 28 | 21 | 31 | 25 | 22 | 28 | 34 | 27 ± 5 |
| Day 5 | 21 | 26 | 39 | 36 | 21 | 35 | 29 | 30 ± 7 |
| Day 7 | 21 | 8 | 49 | 18 | 32 | 33 | 24 | 26 ± 13 |
| Day 14 | 12 | 12 | 29 | 22 | 16 | 17 | 23 | 19 ± 6 |
| Day 21 | 23 | 25 | 12 | 19 | 20 | 26 | 36 | 23 ± 7 |
| Animal Number | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | Mean ± SD |
|---|---|---|---|---|---|---|---|---|---|---|
| Day 1 | 5 | 11 | 15 | 26 | 5 | 15 | 32 | 0 | 8 | 13 ± 11 |
| Day 3 | 20 | 15 | 19 | 36 | 14 | 25 | 25 | 0 | 22 | 20 ± 10 |
| Day 5 | 16 | 8 | 21 | 22 | 20 | 21 | 41 | 7 | 18 | 19 ± 10 |
| Day 7 | 20 | 19 | 19 | 26 | 21 | 24 | 21 | 5 | 21 | 20 ± 6 |
| Day 14 | 16 | 17 | 14 | 20 | 21 | 18 | 16 | 2 | 13 | 15 ± 5 |
| Day 21 | 23 | 14 | 17 | 18 | 23 | 16 | 25 | 4 | 20 | 18 ± 6 |
| Animal Number | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | Mean ± SD |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 1 | 15 | 8 | 9 | 2 | 16 | 6 | 3 | 19 | 20 | 15 | 11 ± 6 |
| Day 3 | 18 | 25 | 10 | 1 | 19 | 5 | 35 | 20 | 23 | 29 | 19 ± 11 |
| Day 5 | 18 | 27 | 10 | 2 | 18 | 9 | 22 | 28 | 22 | 34 | 19 ± 10 |
| Day 7 | 24 | 30 | 10 | 1 | 20 | 12 | 20 | 30 | 26 | 32 | 20 ± 10 |
| Day 14 | 30 | 37 | 12 | 8 | 23 | 19 | 21 | 19 | 15 | 30 | 21 ± 9 |
| Day 21 | 45 | 21 | 16 | 9 | 25 | 20 | 21 | 27 | 26 | 40 | 25 ± 11 |
Appendix A.2. Raw Data on Ketamine-Induced Rotation
| Animal Number | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Mean ± SD |
|---|---|---|---|---|---|---|---|---|
| Day 1 | 58 | 26 | 8 | 32 | 52 | 33 | 83 | 42 ± 25 |
| Day 3 | 72 | 64 | 18 | 25 | 55 | 100 | 100 | 62 ± 30 |
| Day 5 | 92 | 59 | 0 | 30 | 67 | 59 | 93 | 57 ± 32 |
| Day 7 | 22 | 61 | 16 | 92 | 52 | 65 | 71 | 54 ± 29 |
| Day 14 | 49 | 52 | 4 | 56 | 62 | 48 | 85 | 51 ± 25 |
| Day 21 | 64 | 41 | 19 | 58 | 37 | 64 | 92 | 54 ± 23 |
| Animal Number | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | Mean ± SD |
|---|---|---|---|---|---|---|---|---|---|---|
| Day 1 | 94 | 39 | 82 | 100 | 25 | 100 | 100 | 100 | 96 | 82 ± 31 |
| Day 3 | 90 | 93 | 92 | 90 | 75 | 100 | 100 | 100 | 85 | 92 ± 24 |
| Day 5 | 76 | 76 | 100 | 100 | 100 | 88 | 93 | 61 | 100 | 88 ± 13 |
| Day 7 | 96 | 96 | 85 | 93 | 77 | 73 | 65 | 89 | 95 | 85 ± 13 |
| Day 14 | 70 | 100 | 93 | 85 | 90 | 95 | 9 | 91 | 67 | 78 ± 22 |
| Day 21 | 72 | 85 | 83 | 76 | 83 | 86 | 100 | 98 | 92 | 86 ± 21 |
| Animal Number | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | Mean ± SD |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 1 | 71 | 59 | 0 | 69 | 67 | 71 | 100 | 75 | 100 | 87 | 70 ± 32 |
| Day 3 | 73 | 90 | 64 | 100 | 41 | 67 | 100 | 33 | 100 | 80 | 85 ± 29 |
| Day 5 | 100 | 100 | 81 | 100 | 0 | 100 | 100 | 79 | 95 | 91 | 85 ± 29 |
| Day 7 | 83 | 100 | 81 | 100 | 100 | 100 | 77 | 50 | 92 | 95 | 88 ± 24 |
| Day 14 | 83 | 100 | 38 | 99 | 91 | 95 | 100 | 67 | 64 | 98 | 84 ± 18 |
| Day 21 | 84 | 100 | 53 | 100 | 92 | 79 | 95 | 40 | 55 | 94 | 79 ± 22 |
Appendix A.3. Raw Data on Brain Damage
| Animal Number | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | Mean ± SD |
|---|---|---|---|---|---|---|---|---|---|---|
| 21.0 | 25.9 | 26.2 | 19.1 | 26.8 | 23.5 | 22.0 | 22.6 | 18.9 | 24.1 ± 4.6 |
| Animal Number | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | Mean ± SD |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 18.0 | 20.4 | 18.9 | 26.2 | 15.5 | 24.2 | 31.0 | 6.8 | 20.6 | 27.8 | 20.9 ± 6.9 |
References
- Schoenenberger, G.A.; Maier, P.F.; Tobler, H.J.; Monnier, M. A Naturally Occurring Delta-EEG Enhancing Nonapeptide in Rabbits X. Final Isolation, Characterization and Activity Test. Pflfigers Arch. 1977, 369, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Iyer, K.S.; Marks, G.A.; Kastin, A.J.; Mccann, S.M. Evidence for a role of delta sleep-inducing peptide in slow-wave sleep and sleep-related growth hormone release in the rat. Proc. NatI. Acad. Sci. USA 1988, 85, 3653–3656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karl, B.E.; Cespuglio, R.; Leger, L.; Marinesco, S.; Jouvet, M. Is the nucleus raphe dorsalis a target for the peptides possessing hypnogenic properties? Brain Res. 1994, 637, 211–221. [Google Scholar]
- Graf, M.V.; Kastin, A.J. Delta Sleep-Inducing Peptide (DSIP): A review. Neurosci. Biobehav. Rev. 1984, 8, 83–93. [Google Scholar] [CrossRef]
- Graf, M.V.; Kastin, A.J. Delta Sleep-Inducing Peptide (DSIP): Update. Peptides 1986, 7, 1165–1187. [Google Scholar] [CrossRef]
- Graf, M.V.; Kastin, A.J.; Coy, D.H.; Zadina, J.E. DSIP reduces amphetamine-induced hyperthermia in mice. Physiol. Behav. 1984, 33, 291–295. [Google Scholar] [CrossRef]
- Schoenenberger, G.A. Characterization, properties and multivariate functions of Delta-Sleep-Inducing Peptide (DSIP). Eur. Neurol. 1984, 23, 321–345. [Google Scholar] [CrossRef]
- Kovalzon, V.M. DSIP: A sleep peptide or unknown hypothalamic hormone? J. Evol. Biochem. Physiol. 1994, 30, 195–199. [Google Scholar]
- Yehuda, S.; Carasso, R. DSIP–a tool for investigating the sleep onset mechanism: A review. Int. J. Neurosci. 1988, 38, 345–353. [Google Scholar] [CrossRef]
- Chiodera, A.P.; Volpi, R.; Carpetti, L.; Giacalone, G.; Caffarri, G.; Davoli, C.; Nigro, E.; Coiro, V. Different effects of delta-sleep-inducing peptide on arginine-vasopressin and ACTH secretion in normal men. Horm. Res. 1994, 42, 267–272. [Google Scholar] [CrossRef]
- Prudchenko, I.A.; Mikhaleva, I.I. A problem of endogenity of Delta Sleep-Induced Peptide. Uspechi Sovremennoy Biol. 1994, 114, 727–740. [Google Scholar]
- Lysenko, A.V.; Mendzheritsky, A.M. Characteristics and mechanisms of realizing biological effects of the peptide inducing delta-sleep. Uspekhi Sovremennoy Biol. 1995, 115, 729–739. [Google Scholar]
- Strekalova, T.V. DSIP: Problems of its endogenous origin and biological activity. Neurokhimia 1998, 15, 227–239. [Google Scholar]
- Pollard, B.J.; Pomfrett, C.J.D. Delta sleep-inducing peptide. Eur. J. Anaesthesiol. 2001, 18, 419–422. [Google Scholar] [CrossRef]
- Kovalzon, V.M.; Strekalova, T.V. Delta sleep-inducing peptide (DSIP): A still unresolved riddle. J. Neurochem. 2006, 97, 303–309. [Google Scholar] [CrossRef] [Green Version]
- Mikhaleva, I.I.; Voitenkov, B.O. The delta-sleep inducing peptide and Deltaran: A way from biochemical studies to medicine. Novye Lekarstvennye Preparaty 2007, 3, 6–20. [Google Scholar]
- Shustanova, T.A.; Bondarenko, T.I.; Milyutina, N.P.; Mikhaleva, I.I. Regulation of freeradical processes in rat tissues by the delta-sleep inducing peptide during cold stress. Biokhimiya 2001, 66, 632–639. [Google Scholar] [CrossRef]
- Rikhireva, G.T.; Sokolova, I.S.; Rylova, A.V.; Kopylovskiĭ, S.A.; Mikhaleva, I.I.; Prudchenko, I.A. Changes in the rate of protein biosynthesis in the organs of mice under the action of the delta sleep-inducing peptide and psychoemotional stress. Izv. Akad. Nauk. Ser. Biol. 1995, 2, 142–148. [Google Scholar]
- Shmarin, I.P. Signaling molecules and social behavior. Neurochemistry 2001, 18, 243–250. [Google Scholar]
- Sudakov, K.V.; Ivanov, V.T.; Kolpik, E.V. Delta-sleep Indusing Peptide (DSIP) as factor facilitation animals resistance to acute emotional stress. Pavlov Biol. Sci. 1983, 18, 1–5. [Google Scholar] [CrossRef]
- Gershteĭn, L.M.; Dovedova, E.L.; Khrustalev, D.A. Morphochemical characteristic of rat brain structures after exposure to delta-sleep inducing peptide following long-term amphetamine administration. Morfologiia 2004, 125, 74–77. [Google Scholar]
- Konorova, I.L.; Gannushkina, I.V.; Koplik, E.V.; Antelava, A.L. Deltaran prevents an adverse effect of emotional stress on the course of cerebral ischemia in stress-low-resistant animals. Bull. Exp. Biol. Med. 2006, 141, 564–566. [Google Scholar] [CrossRef]
- Shandra, A.A.; Godlevskii, L.S.; Brusentsov, A.I.; Vast’yanov, R.S.; Karlyuga, V.A.; Dzygal, A.F.; Nikel, B. Effects of delta-sleep-inducing peptide in cerebral ischemia in rats. Neurosci. Behav. Physiol. 1998, 28, 443–446. [Google Scholar] [CrossRef]
- Kolpik, E.V. Delta-sleep indusing peptide and drug deltaran: Possible approaches to antistress protection. Zh. Nevrol. Psikhiatr. Im. SS Korsakova 2007, 107, 50–54. [Google Scholar]
- Kim, T.K.; Karantysh, G.V.; Mendzheritskiĭ, A.M.; Ryzhak, G.A. Effect of deltaran on the mediatory balance in the brain of young and old rats with left-side laterality profile in case of carotid arteries occlusion. Adv. Gerontol. 2007, 20, 138–142. [Google Scholar] [PubMed]
- Hrnčić, D.; Stanojlović, O.; Živanović, D.; Šušić, V. Delta-Sleep-Inducing Peptide Potentiates Anticonvulsive Activity of Valproate against Metaphit-Provoked Audiogenic Seizure in Rats. Pharmacology 2006, 77, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Belayev, L.; Alonso, O.F.; Busto, R.; Zhao, W.; Ginsberg, M.D. Middle cerebral artery occlusion in the rat by intraluminal suture. Neurological and pathological evaluation of an improved model. Stroke 1996, 27, 1616–1622. [Google Scholar] [CrossRef]
- Lebedev, S.V.; Petrov, S.V.; Blinov, D.V.; Lazarenko, I.P.; Chekhonin, V.P. Ketamine-induced rotational asymmetry in evaluation of motor disturbances in rats with middle cerebral artery occlusion. Bull. Exp. Biol. Med. 2003, 135, 424–427. [Google Scholar] [CrossRef]
- Tukhovskaya, E.A.; Yukin, A.Y.; Khokhlova, O.N.; Murashev, A.N. COG1410, a Novel Apolipoprotein-E Mimetic, Improves Functional and Morphological Recovery in a Rat Model of Focal Brain Ischemia. J. Neurosci. Res. 2009, 87, 677–682. [Google Scholar] [CrossRef] [Green Version]
- Gundersen, H.J.; Jensen, E.B. The efficiency of systematic sampling in stereology and its prediction. J. Microsc. 1987, 147, 229–263. [Google Scholar] [CrossRef]
- Swanson, R.A.; Morton, M.T.; Tsao-Wu, G.; Savalos, R.A.; Davidson, C.; Sharp, F.R. A semiautomated method for measuring brain infarct volume. J. Cereb. Blood Flow Metab. 1990, 10, 290–293. [Google Scholar] [CrossRef]
- Rathore, S.S.; Hinn, A.R.; Cooper, L.S.; Tyroler, H.A.; Rosamond, W.D. Characterization of incident stroke signs and symptoms: Findings from the atherosclerosis risk in communities study. Stroke 2002, 33, 2718–2721. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Xie, P.; Zhang, Y.; Chen, Y.; Cheng, S.; Zhang, L. Abnormal functional corticomuscular coupling after stroke. NeuroImage Clin. 2018, 19, 147–159. [Google Scholar] [CrossRef]
- Chen, X.L.; Xie, P.; Zhang, Y.Y.; Liu, L.X.; Du, Y.H.; Chen, X.L.; Xie, P.; Zhang, Y.Y.; Liu, L.X.; Du, Y.H. Distinction of functional corticomuscular coupling in synkinetic and separate movement following stroke. Brain Stimul. 2017, 10, 435–436. [Google Scholar] [CrossRef]
- Fang, Y.; Daly, J.J.; Sun, J.; Hvorat, K.; Fredrickson, E.; Pundik, S.; Sahgal, V.; Guang, H.Y. Functional Corticomuscular Connection During Reaching Is Weakened Following Stroke. Clin. Neurophysiol. 2009, 120, 994–1002. [Google Scholar] [CrossRef] [Green Version]
- Gardiner, R. The pathophysiological and clinical implications of neuro-muscular changes following cerebrovascular accident. Aust. J. Physiother. 1996, 42, 139–146. [Google Scholar] [CrossRef] [Green Version]
- Mima, T.; Hallett, M. Corticomuscular coherence: A review. Clin. Neurophysiol. 1999, 16, 501–511. [Google Scholar] [CrossRef]
- Langhorne, P.; Coupar, F.; Pollock, A. Motor recovery after stroke: A systematic review. Lancet Neurol. 2009, 8, 741–754. [Google Scholar] [CrossRef]
- Stinear, C.M. Prediction of motor recovery after stroke: Advances in biomarkers. Lancet Neurol. 2017, 16, 826–836. [Google Scholar] [CrossRef]
- Schwamm, L.H.; Pancioli, A.; Acker, J.E., 3rd; Goldstein, L.B.; Zorowitz, R.D.; Shephard, T.J.; Moyer, P.; Gorman, M.; Johnston, S.C.; Duncan, P.W.; et al. Recommendations for the Establishment of Stroke Systems of Care Recommendations From the American Stroke Association’s Task Force on the Development of Stroke Systems. Circulation 2005, 111, 1078–1091. [Google Scholar] [CrossRef]
- Desrosiers, J.; Rochette, A.; Corriveau, H. Validation of a New Lower-Extremity Motor Coordination Test. Arch. Phys. Med. Rehabil. 2005, 86, 993–998. [Google Scholar] [CrossRef] [PubMed]
- Winters, C.; van Wegen, E.E.; Daffertshofer, A.; Kwakkel, G. Generalizability of the proportional recovery model for the upper extremity after an ischemic stroke. Neurorehabil. Neural Repair. 2015, 29, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Buch, E.R.; Rizk, S.; Nicolo, P.; Cohen, L.G.; Schnider, A.; Guggisberg, A.G. Predicting motor improvement after stroke with clinical assessment and diffusion tensor imaging. Neurology 2016, 86, 1924–1925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byblow, W.D.; Stinear, C.M.; Barber, P.A.; Petoe, M.A.; Ackerley, S.J. Proportional recovery after stroke depends on corticomotor integrity. Ann. Neurol. 2015, 78, 848–859. [Google Scholar] [CrossRef]
- Feng, W.; Wang, J.; Chhatbar, P.Y.; Doughty, C.; Landsittel, D.; Lioutas, V.A.; Kautz, S.A.; Schlaug, G. Corticospinal tract lesion load: An imaging biomarker for stroke motor outcomes. Ann. Neurol. 2015, 78, 860–870. [Google Scholar] [CrossRef]
- Stinear, C.M.; Byblow, W.D.; Ackerley, S.J.; Smith, M.C.; Borges, V.M.; Barber, P.A. Proportional motor recovery after stroke: Implications for trial design. Stroke 2017, 48, 795–798. [Google Scholar] [CrossRef]
- Yeh, S.C.; Stewart, J.; McLaughlin, M.; Parsons, T.; Winstein, C.J.; Rizzo, A. Evaluation. Approach for Post-stroke Rehabilitation Via Virtual Reality Aided Motor Training. In International Conference on Ergonomics and Health Aspects of Work with Computers; Springer: Berlin/Heidelberg, Germany, 2007; LNCS 4566; pp. 378–387. [Google Scholar]
- Monnier, M.; Dudler, L.; Gächter, R.; Schoenenberger, G.A. Delta sleep-inducing peptide (DSIP): EEG and motor activity in rabbits following intravenous administration. Neurosci. Lett. 1977, 6, 9–13. [Google Scholar] [CrossRef]
- Makletsova, M.G.; Mikhaleva, I.I.; Prudchenko, I.A.; Rikhireva, G.T. Effect of delta sleep-inducing peptide on macromolecule biosynthesis in brain tissue of stressed rodents. Bull. Exp. Biol. Med. 2006, 141, 416–419. [Google Scholar] [CrossRef]
- Sudakov, K.V.; Umriukhin, P.E.; Rayevsky, K.S. Delta-sleep inducing peptide and neuronal activity after glutamate microiontophoresis: The role of NMDA-receptors. Pathophysiology 2004, 11, 81–86. [Google Scholar] [CrossRef]
- Grigor’ev, V.V.; Ivanova, T.A.; Kustova, E.A.; Petrova, L.N.; Serkova, T.P.; Bachurin, S.O. Effects of delta sleep-inducing peptide on pre- and postsynaptic glutamate and postsynaptic GABA receptors in neurons of the cortex, hippocampus, and cerebellum in rats. Bull. Exp. Biol. Med. 2006, 142, 186–188. [Google Scholar] [CrossRef]
- Khvatova, E.M.; Samartzev, V.N.; Zagoskin, P.P.; Prudchenko, I.A.; Mikhaleva, I.I. Delta sleep inducing peptide (DSIP): Effect on respiration activity in rat brain mitochondria and stress protective potency under experimental hypoxia. Peptides 2003, 24, 307–311. [Google Scholar] [CrossRef]
- Li, F.; Silva, M.D.; Liu, K.F.; Helmer, K.G.; Omae, T.; Fenstermacher, J.D.; Sotak, C.H.; Fisher, M. Secondary decline in apparent diffusion coefficient and neurological outcome after a short period of focal brain ischemia in rats. Ann. Neurol. 2000, 48, 236–244. [Google Scholar] [CrossRef]
- Kidwell, C.S.; Saver, J.L.; Mattiello, J.; Starkman, S.; Vinuela, F.; Duckwiler, G.; Gobin, Y.P.; Jahan, R.; Vespa, P.; Kalafut, M.; et al. Thrombolytic reversal of acute human cerebral ischemic injury shown by diffusion/perfusion magnetic resonance imaging. Ann. Neurol. 2000, 47, 462–469. [Google Scholar] [CrossRef]
- Lo, E.H.; Dalkara, T.; Moskowitz, M.A. Mechanisms, challenges and opportunities in stroke. Nat. Rev. Neurosci. 2003, 4, 399–415. [Google Scholar] [CrossRef]
- Warner, D.S.; Sheng, H.; Batini-Haberle, I. Oxidants, antioxidants and the ischemic brain. J. Exp. Biol. 2004, 207, 3221–3231. [Google Scholar] [CrossRef] [Green Version]
- Bondarenko, T.I.; Maiboroda, E.A.; Mikhaleva, I.I.; Prudchenko, I.A. Mechanism of geroprotective action of delta-sleep inducing peptide. Adv. Gerontol. 2011, 1, 328–339. [Google Scholar] [CrossRef]
- Kutilin, D.S.; Bondarenko, T.I.; Kornienko, I.V.; Mikhaleva, I.I. Effect of Delta Sleep-Inducing Peptide on the Expression of Antioxidant Enzyme Genes in the Brain and Blood of Rats during Physiological Aging. Bull. Exp. Biol. Med. 2014, 157, 616–619. [Google Scholar] [CrossRef]
- Tukhovskaya, E.A.; Shaykhutdinova, E.R.; Ismailova, A.M.; Slashcheva, G.A.; Prudchenko, I.A.; Mikhaleva, I.I.; Khokhlova, O.N.; Murashev, A.N.; Ivanov, V.T. DSIP-Like KND Peptide Reduces Brain Infarction in C57Bl/6 and Reduces Myocardial Infarction in SD Rats When Administered during Reperfusion. Biomedicines 2021, 9, 407. [Google Scholar] [CrossRef]
- Sudakov, K.V.; Umryukhin, P.E.; Koplik, E.V.; Anokhin, K.V. Expression of the c-fos gene during emotional stress in rats: The clocking effect of delta sleep-inducing peptide. Neurosci. Behav. Physiol. 2001, 31, 635–640. [Google Scholar] [CrossRef]
- Umriukhin, P.; Koplik, E.; Sudakov, K. Dizocilpine and cycloheximide prevent inhibition of c-Fos gene expression by delta sleep-inducing peptide in the paraventricular nucleus of the hypothalamus in rats with different resistance to emotional stress. Neurosci. Lett. 2012, 506, 184–187. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tukhovskaya, E.A.; Ismailova, A.M.; Shaykhutdinova, E.R.; Slashcheva, G.A.; Prudchenko, I.A.; Mikhaleva, I.I.; Khokhlova, O.N.; Murashev, A.N.; Ivanov, V.T. Delta Sleep-Inducing Peptide Recovers Motor Function in SD Rats after Focal Stroke. Molecules 2021, 26, 5173. https://doi.org/10.3390/molecules26175173
Tukhovskaya EA, Ismailova AM, Shaykhutdinova ER, Slashcheva GA, Prudchenko IA, Mikhaleva II, Khokhlova ON, Murashev AN, Ivanov VT. Delta Sleep-Inducing Peptide Recovers Motor Function in SD Rats after Focal Stroke. Molecules. 2021; 26(17):5173. https://doi.org/10.3390/molecules26175173
Chicago/Turabian StyleTukhovskaya, Elena A., Alina M. Ismailova, Elvira R. Shaykhutdinova, Gulsara A. Slashcheva, Igor A. Prudchenko, Inessa I. Mikhaleva, Oksana N. Khokhlova, Arkady N. Murashev, and Vadim T. Ivanov. 2021. "Delta Sleep-Inducing Peptide Recovers Motor Function in SD Rats after Focal Stroke" Molecules 26, no. 17: 5173. https://doi.org/10.3390/molecules26175173
APA StyleTukhovskaya, E. A., Ismailova, A. M., Shaykhutdinova, E. R., Slashcheva, G. A., Prudchenko, I. A., Mikhaleva, I. I., Khokhlova, O. N., Murashev, A. N., & Ivanov, V. T. (2021). Delta Sleep-Inducing Peptide Recovers Motor Function in SD Rats after Focal Stroke. Molecules, 26(17), 5173. https://doi.org/10.3390/molecules26175173





