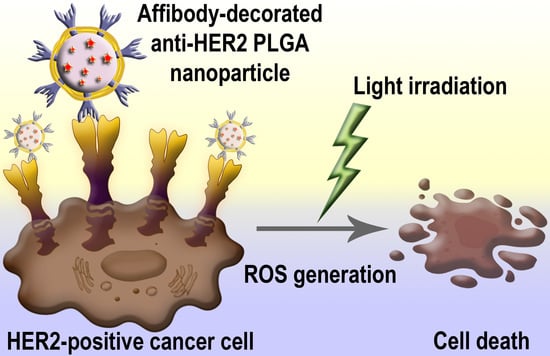
PLGA Nanoparticles Decorated with Anti-HER2 Affibody for Targeted Delivery and Photoinduced Cell Death
Abstract
:1. Introduction
2. Results
2.1. Synthesis and Modification of PLGA Nanoparticles
2.2. HER2-Overexpressing Cell Targeting by PLGANanoparticles Modified with Anti-HER2 Affibody ZHER2:342
2.3. Cellular Toxicity of Rose Bengal-Loaded PLGA Nanoparticles
3. Discussion
4. Materials and Methods
4.1. Electron Microscopy
4.2. Fluorescence Spectroscopy
4.3. Protein Quantification
4.4. Dynamic Light Scattering Measurements
4.5. Cell Culture
4.6. Protein Conjugation to FITC
4.7. Confocal Laser Scanning Microscopy
4.8. Cytotoxicity Assay
4.9. PLGA Conjugation with ZHER2:342
4.10. Flow Cytometry
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Sokolov, I.L.; Cherkasov, V.R.; Tregubov, A.A.; Buiucli, S.R.; Nikitin, M.P. Smart materials on the way to theranostic nanorobots: Molecular machines and nanomotors, advanced biosensors, and intelligent vehicles for drug delivery. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 1530–1544. [Google Scholar] [CrossRef]
- Raza, A.; Hayat, U.; Rasheed, T.; Bilal, M.; Iqbal, H.M.N. “Smart” materials-based near-infrared light-responsive drug delivery systems for cancer treatment: A review. J. Mater. Res. Tech-Nology 2019, 8, 1497–1509. [Google Scholar] [CrossRef]
- Belova, M.M.; Shipunova, V.O.; Kotelnikova, P.A.; Babenyshev, A.V.; Rogozhin, E.A.; Cherednichenko, M.Y.; Deyev, S.M. “Green” Synthesis of Cytotoxic Silver Nanoparticles Based on Secondary Metabolites of Lavandula Angustifolia Mill. Acta Nat. 2019, 11, 47–53. [Google Scholar] [CrossRef]
- Zelepukin, I.V.; Popov, A.A.; Shipunova, V.O.; Tikhonowski, G.V.; Mirkasymov, A.B.; Popova-Kuznetsova, E.A.; Klimentov, S.M.; Kabashin, A.V.; Deyev, S.M. Laser-synthesized TiN nanoparticles for biomedical applications: Evaluation of safety, biodistribution and pharmacokinetics. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 120, 111717. [Google Scholar] [CrossRef] [PubMed]
- Grebenik, E.A.; Kostyuk, A.B.; Deyev, S.M. Upconversion nanoparticles and their hybrid assemblies for bio-medical applications. Russ. Chem. Rev. 2016, 85, 1277–1296. [Google Scholar] [CrossRef]
- Kabashin, A.V.; Kravets, V.G.; Wu, F.; Imaizumi, S.; Shipunova, V.O.; Deyev, S.M.; Grigorenko, A.N. Phase-Responsive Fourier Nanotransducers for Probing 2D Materials and Functional Interfaces. Adv. Funct. Mater 2019, 29, 1902692. [Google Scholar] [CrossRef] [Green Version]
- Shipunova, V.O.; Nikitin, M.P.; Zelepukin, I.V.; Nikitin, P.I.; Deyev, S.M.; Petrov, R.V. A comprehensive study of interactions between lectins and glycoproteins for the development of effective theranostic nanoagents. Dokl. Biochem. Biophys. 2015, 464, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Ringaci, A.; Yaremenko, A.V.; Shevchenko, K.G.; Zvereva, S.D.; Nikitin, M.P. Metal-organic frameworks for simultaneous gene and small molecule delivery in vitro and in vivo. Chem. Eng. J. 2021, 418, 129386. [Google Scholar] [CrossRef]
- Shevchenko, K.G.; Cherkasov, V.R.; Nikitina, I.L.; Babenyshev, A.V.; Nikitin, M.P. Smart multifunctional nanoagents for in situ monitoring of small molecules with a switchable affinity towards biomedical targets. Appl Nanosci. 2018, 8, 195–203. [Google Scholar] [CrossRef] [Green Version]
- Slingerland, M.; Guchelaar, H.-J.; Gelderblom, H. Liposomal drug formulations in cancer therapy: 15 years along the road. Drug Discov. Today 2012, 17, 160–166. [Google Scholar] [CrossRef]
- Danhier, F. To exploit the tumor microenvironment: Since the EPR effect fails in the clinic, what is the future of nanomedicine? J. Control. Release 2016, 244, 108–121. [Google Scholar] [CrossRef]
- Nikitin, M.P.; Zelepukin, I.V.; Shipunova, V.O.; Sokolov, I.L.; Deyev, S.M.; Nikitin, P.I. Enhancement of the blood-circulation time and performance of nanomedicines via the forced clearance of erythrocytes. Nat. Bio-Med. Eng. 2020, 4, 717–731. [Google Scholar] [CrossRef]
- Zelepukin, I.V.; Yaremenko, A.V.; Shipunova, V.O.; Babenyshev, A.V.; Balalaeva, I.V.; Nikitin, P.I.; Deyev, S.M.; Nikitin, M.P. Nanoparticle-based drug delivery via RBC-hitchhiking for the inhibition of lung metastases growth. Nanoscale 2019, 11, 1636–1646. [Google Scholar] [CrossRef]
- Mirkasymov, A.B.; Zelepukin, I.V.; Nikitin, P.I.; Nikitin, M.P.; Deyev, S.M. In vivo blockade of mononuclear phagocyte system with solid nanoparticles: Efficiency and affecting factors. J. Control. Release 2021, 330, 111–118. [Google Scholar] [CrossRef]
- Zelepukin, I.V.; Yaremenko, A.V.; Yuryev, M.V.; Mirkasymov, A.B.; Sokolov, I.L.; Deyev, S.M.; Nikitin, P.I.; Ni-kitin, M.P. Fast processes of nanoparticle blood clearance: Comprehensive study. J. Control. Release 2020, 326, 181–191. [Google Scholar] [CrossRef]
- Shipunova, V.O.; Shramova, E.I.; Schulga, A.A.; Shilova, M.V.; Deyev, S.M.; Proshkina, G.M. Delivery of Bar-nase to Cells in Liposomes Functionalized by Her2-Specific DARPin Module. Russ. J. Bioorg. Chem. 2020, 46, 1156–1161. [Google Scholar] [CrossRef]
- Shipunova, V.O.; Komedchikova, E.N.; Kotelnikova, P.A.; Zelepukin, I.V.; Schulga, A.A.; Proshkina, G.M.; Shramova, E.I.; Kutscher, H.L.; Telegin, G.B.; Kabashin, A.V.; et al. Dual Regioselective Targeting the Same Re-ceptor in Nanoparticle-Mediated Combination Immuno/Chemotherapy for Enhanced Image-Guided Cancer Treatment. ACS Nano 2020, 14, 12781–12795. [Google Scholar] [CrossRef] [PubMed]
- Shipunova, V.O.; Kotelnikova, P.A.; Aghayeva, U.F.; Stremovskiy, O.A.; Novikov, I.A.; Schulga, A.A.; Nikitin, M.P.; Deyev, S.M. Self-assembling nanoparticles biofunctionalized with magnetite-binding protein for the tar-geted delivery to HER2/neu overexpressing cancer cells. J. Magn. Magn. Mater. 2019, 469, 450–455. [Google Scholar] [CrossRef]
- Shipunova, V.O.; Zelepukin, I.V.; Stremovskiy, O.A.; Nikitin, M.P.; Care, A.; Sunna, A.; Zvyagin, A.V.; Deyev, S.M. Versatile Platform for Nanoparticle Surface Bioengineering Based on SiO2-Binding Peptide and Proteina-ceous Barnase*Barstar Interface. ACS Appl. Mater. Interfaces 2018, 10, 17437–17447. [Google Scholar] [CrossRef]
- Shramova, E.; Proshkina, G.; Shipunova, V.; Ryabova, A.; Kamyshinsky, R.; Konevega, A.; Schulga, A.; Konovalova, E.; Telegin, G.; Deyev, S. Dual Targeting of Cancer Cells with DARPin-Based Toxins for Over-coming Tumor Escape. Cancers 2020, 12, 3014. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, E.; Proshkina, G.; Kutova, O.; Shilova, O.; Ryabova, A.; Schulga, A.; Stremovskiy, O.; Zdobnova, T.; Balalaeva, I.; Deyev, S. Recombinant targeted toxin based on HER2-specific DARPin possesses a strong selec-tive cytotoxic effect in vitro and a potent antitumor activity in vivo. J. Control. Release 2016, 233, 48–56. [Google Scholar] [CrossRef]
- Kotelnikova, P.A.; Shipunova, V.O.; Aghayeva, U.F.; Stremovskiy, O.A.; Nikitin, M.P.; Novikov, I.A.; Schulga, A.A.; Deyev, S.M.; Petrov, R.V. Synthesis of Magnetic Nanoparticles Stabilized by Magnetite-Binding Protein for Targeted Delivery to Cancer Cells. Dokl. Biochem. Biophys. 2018, 481, 198–200. [Google Scholar] [CrossRef] [PubMed]
- Shipunova, V.O.; Kolesnikova, O.A.; Kotelnikova, P.A.; Soloviev, V.D.; Popov, A.A.; Proshkina, G.M.; Nikitin, M.P.; Deyev, S.M. Comparative Evaluation of Engineered Polypeptide Scaffolds in HER2-Targeting Magnetic Nanocarrier Delivery. ACS Omega 2021. [Google Scholar] [CrossRef]
- Lee, S.B.; Hassan, M.; Fisher, R.; Chertov, O.; Chernomordik, V.; Kramer-Marek, G.; Gandjbakhche, A.; Capala, J. Affibody molecules for in vivo characterization of HER2-positive tumors by near-infrared imaging. Clin. Cancer Res. 2008, 14, 3840–3849. [Google Scholar] [CrossRef] [Green Version]
- Orlova, A.; Magnusson, M.; Eriksson, T.L.J.; Nilsson, M.; Larsson, B.; Höidén-Guthenberg, I.; Widström, C.; Carlsson, J.; Tolmachev, V.; Ståhl, S.; et al. Tumor imaging using a picomolar affinity HER2 binding affibody molecule. Cancer Res. 2006, 66, 4339–4348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Huang, Y.-Y.; Wang, Y.; Lyu, P.; Hamblin, M.R. Red (660 nm) or near-infrared (810 nm) photobio-modulation stimulates, while blue (415 nm), green (540 nm) light inhibits proliferation in human adi-pose-derived stem cells. Sci. Rep. 2017, 7, 7781. [Google Scholar] [CrossRef] [PubMed]
- Argyriou, A.A.; Bruna, J.; Marmiroli, P.; Cavaletti, G. Chemotherapy-induced peripheral neurotoxicity (CIPN): An update. Crit. Rev. Oncol. Hematol. 2012, 82, 51–77. [Google Scholar] [CrossRef]
- Love, R.R.; Leventhal, H.; Easterling, D.V.; Nerenz, D.R. Side effects and emotional distress during cancer chemotherapy. Cancer 1989, 63, 604–612. [Google Scholar] [CrossRef]
- Monsuez, J.-J.; Charniot, J.-C.; Vignat, N.; Artigou, J.-Y. Cardiac side-effects of cancer chemotherapy. Int. J. Cardiol. 2010, 144, 3–15. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic. Bioeng. Transl. Med. 2016, 1, 10–29. [Google Scholar] [CrossRef]
- Lagreca, E.; Onesto, V.; Di Natale, C.; La Manna, S.; Netti, P.A.; Vecchione, R. Recent advances in the formula-tion of PLGA microparticles for controlled drug delivery. Prog. Biomater. 2020, 9, 153–174. [Google Scholar] [CrossRef]
- Sadat Tabatabaei Mirakabad, F.; Nejati-Koshki, K.; Akbarzadeh, A.; Yamchi, M.R.; Milani, M.; Zarghami, N.; Zeighamian, V.; Rahimzadeh, A.; Alimohammadi, S.; Hanifehpour, Y.; et al. PLGA-based nanoparticles as cancer drug delivery systems. Asian Pac. J. Cancer Prev. 2014, 15, 517–535. [Google Scholar] [CrossRef] [Green Version]
- Soomherun, N. Enhancement Vehicles of Cardene Loading Poly(D,L-lactic-co-glycolic acid) Nanoparticles in Vitro Controlled Release for Biomedical Application. IJPMBS 2021, 10, 1–7. [Google Scholar] [CrossRef]
- Soonklang, C.; Tassanarangsan, C.; Soomherun, N.; Kreua-ongarjnukool, N.; Niyomthai, S.T. Study Kinetics Models of Clindamycin Hydrochloride from Poly(D,L-lactic-co-glycolic acid) Particles. IJPMBS 2021, 10, 68–74. [Google Scholar] [CrossRef]
- Valencia, A.; Morán, J. Reactive oxygen species induce different cell death mechanisms in cultured neurons. Free Radic. Biol. Med. 2004, 36, 1112–1125. [Google Scholar] [CrossRef]
- Foote, M.; Read, T.; Thomas, J.; Wagels, M.; Burmeister, B.; Smithers, B.M. Results of a phase II, open-label, non-comparative study of intralesional PV-10 followed by radiotherapy for the treatment of in-transit or met-astatic melanoma. J. Surg. Oncol. 2017, 115, 891–897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toshida, H.; Funaki, T.; Ono, K.; Tabuchi, N.; Watanabe, S.; Seki, T.; Otake, H.; Kato, T.; Ebihara, N.; Murakami, A. Efficacy and safety of retinol palmitate ophthalmic solution in the treatment of dry eye: A Japanese Phase II clinical trial. Drug Des. Devel. 2017, 11, 1871–1879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruangvaravate, N.; Prabhasawat, P.; Vachirasakchai, V.; Tantimala, R. High Prevalence of Ocular Surface Disease Among Glaucoma Patients in Thailand. J. Ocul. Pharm. 2018, 34, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Nord, K.; Gunneriusson, E.; Ringdahl, J.; Ståhl, S.; Uhlén, M.; Nygren, P.A. Binding proteins selected from com-binatorial libraries of an alpha-helical bacterial receptor domain. Nat. Biotechnol. 1997, 15, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Frejd, F.Y.; Kim, K.-T. Affibody molecules as engineered protein drugs. Exp. Mol. Med. 2017, 49, e306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sörensen, J.; Sandberg, D.; Sandström, M.; Wennborg, A.; Feldwisch, J.; Tolmachev, V.; Åström, G.; Lubberink, M.; Garske-Román, U.; Carlsson, J.; et al. First-in-human molecular imaging of HER2 expression in breast can-cer metastases using the 111In-ABY-025 affibody molecule. J. Nucl. Med. 2014, 55, 730–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shipunova, V.O.; Sogomonyan, A.S.; Zelepukin, I.V.; Nikitin, M.P.; Deyev, S.M. PLGA Nanoparticles Decorated with Anti-HER2 Affibody for Targeted Delivery and Photoinduced Cell Death. Molecules 2021, 26, 3955. https://doi.org/10.3390/molecules26133955
Shipunova VO, Sogomonyan AS, Zelepukin IV, Nikitin MP, Deyev SM. PLGA Nanoparticles Decorated with Anti-HER2 Affibody for Targeted Delivery and Photoinduced Cell Death. Molecules. 2021; 26(13):3955. https://doi.org/10.3390/molecules26133955
Chicago/Turabian StyleShipunova, Victoria Olegovna, Anna Samvelovna Sogomonyan, Ivan Vladimirovich Zelepukin, Maxim Petrovich Nikitin, and Sergey Mikhailovich Deyev. 2021. "PLGA Nanoparticles Decorated with Anti-HER2 Affibody for Targeted Delivery and Photoinduced Cell Death" Molecules 26, no. 13: 3955. https://doi.org/10.3390/molecules26133955
APA StyleShipunova, V. O., Sogomonyan, A. S., Zelepukin, I. V., Nikitin, M. P., & Deyev, S. M. (2021). PLGA Nanoparticles Decorated with Anti-HER2 Affibody for Targeted Delivery and Photoinduced Cell Death. Molecules, 26(13), 3955. https://doi.org/10.3390/molecules26133955