Toward a Greener World—Cyclodextrin Derivatization by Mechanochemistry
Abstract
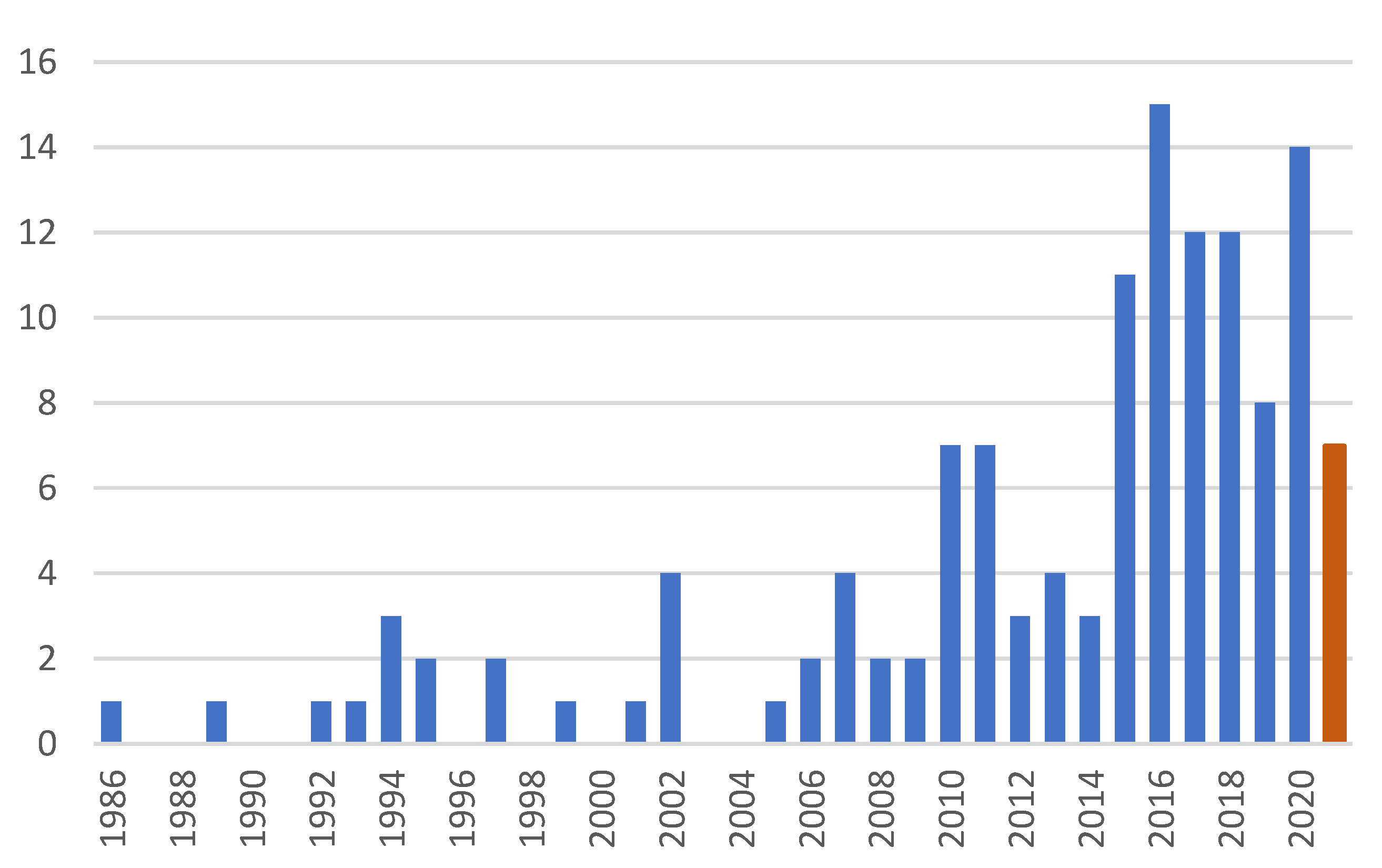
:1. Introduction
2. Discussion
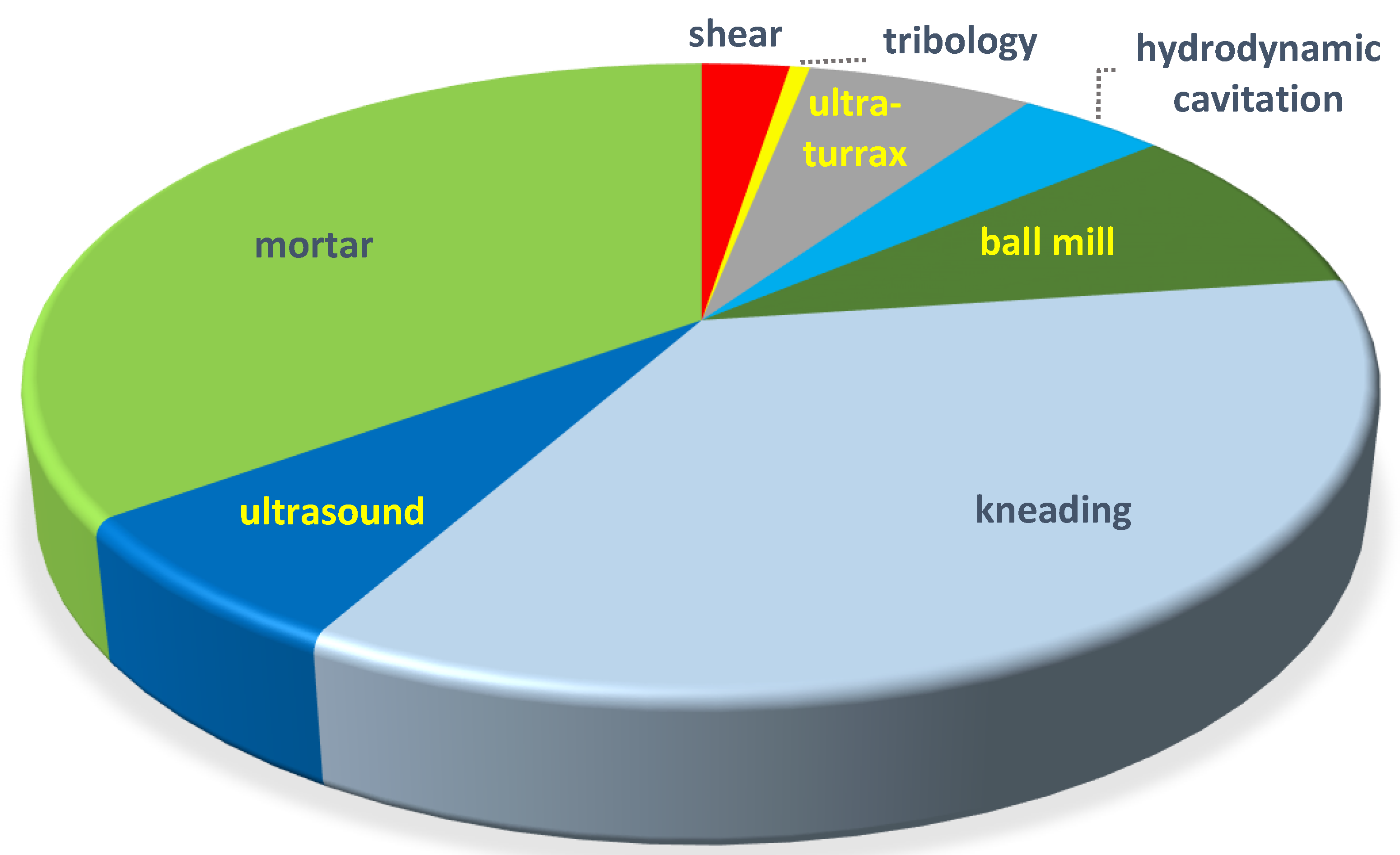
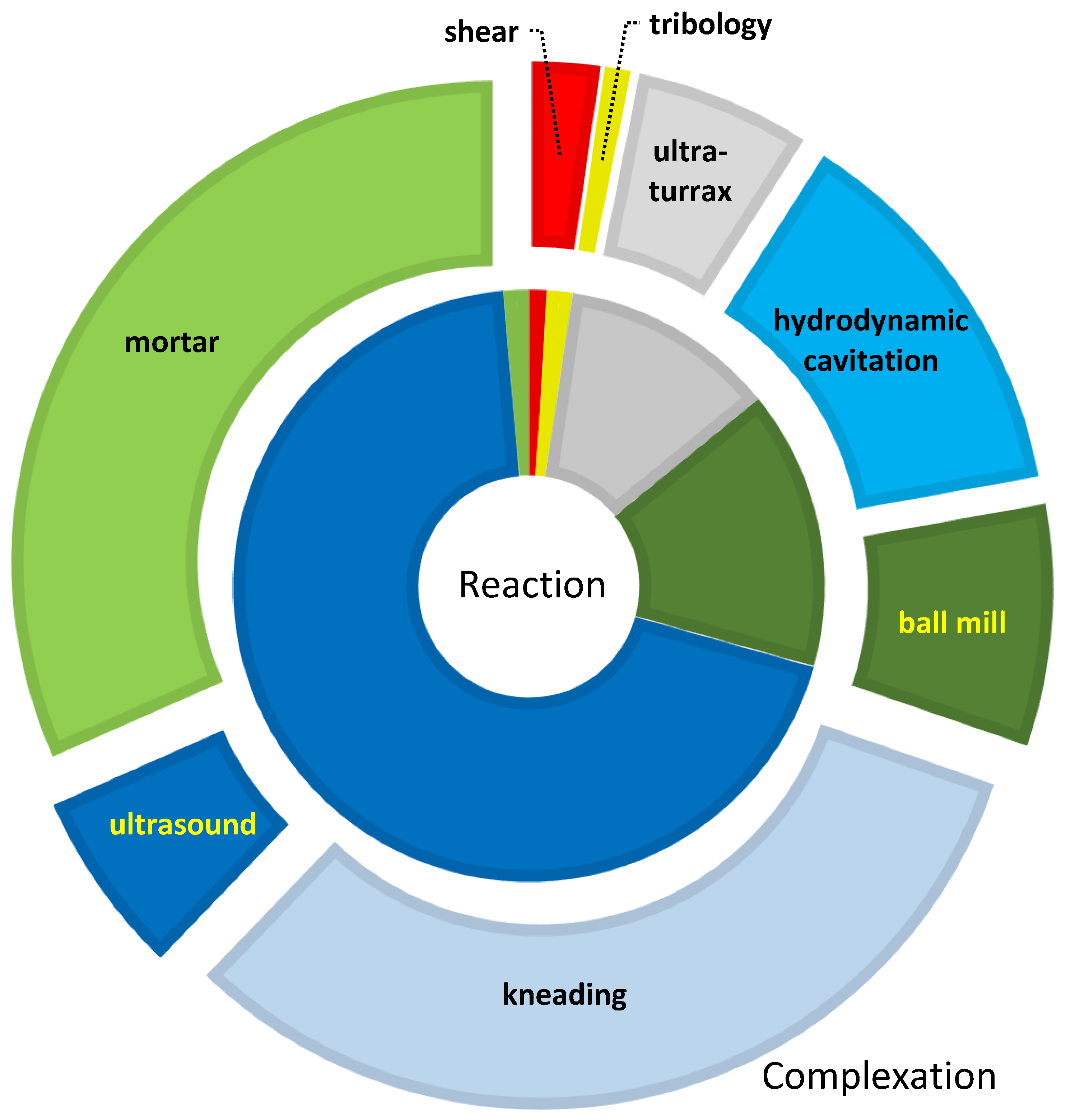
2.1. Complexes
2.1.1. Complexation in Mortar
2.1.2. Complexation in Mills
2.1.3. Complexation with Kneading
2.1.4. Complexation with Ultra-Turrax, a Transition to Hydrodynamic Cavitation
2.1.5. Spray-Drying, a Transition to Hydrodynamic Cavitation
2.1.6. Complexation with Hydrodynamic Cavitation
2.1.7. Complexation with Ultrasound
2.1.8. Complexes under Shearing
2.1.9. Tribology of Complexes
2.2. Chemical Transformations
2.2.1. Mechanochemical Transformation in Mortar
2.2.2. Mechanochemical Transformation in Mills
2.2.3. Mechanochemical Transformation Using Ultra-Turrax
2.2.4. Mechanochemical Transformation Using Ultrasound
2.2.5. Mechanochemical Transformation Using Hydrodynamic Cavitation
2.2.6. Mechanochemical Transformation Utilizes Shearing
2.2.7. Mechanochemical Transformation Using Tribology
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Ball mill | BM |
| Cyclodextrin | CD |
| Dialkyl pentasulfide | DPS |
| Dimethyl sulfoxide | DMSO |
| (2-Hydroxy)propyl-βCD | HPβCD |
| MW | Microwave |
| N,N-Dimethyl formamide | DMF |
| Nanoparticle | NP |
| Polyethylene glycol | PEG |
| 4-Sulfobutyl-βCD | SBβCD |
| Three-dimensional | 3D |
| Tris(hydroxymethyl)amine | TRIS |
| Ultrasound | US |
References
- Anastas, P.T.; Zimmerman, J.B. Peer Reviewed: Design through the 12 Principles of Green Engineering. Environ. Sci. Technol. 2003, 37, 94A–101A. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boldyreva, E. Mechanochemistry of inorganic and organic systems: What is similar, what is different? Chem. Soc. Rev. 2013, 42, 7719. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.L.; Cao, Q.; Browne, D.L. Mechanochemistry as an emerging tool for molecular synthesis: What can it offer? Chem. Sci. 2018, 9, 3080–3094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- James, S.L.; Adams, C.J.; Bolm, C.; Braga, D.; Collier, P.; Frišcic, T.; Grepioni, F.; Harris, K.D.M.; Hyett, G.; Jones, W.; et al. Mechanochemistry: Opportunities for new and cleaner synthesis. Chem. Soc. Rev. 2012, 41, 413–447. [Google Scholar] [CrossRef] [Green Version]
- Cravotto, G.; Tagliapietra, S.; Wu, Z. Chapter 7—Green Synthetic Procedures under Hydrodynamic and Acoustic Cavitation. In Green Synthetic Processes and Procedures; The Royal Society of Chemistry: London, UK, 2019; pp. 141–174. [Google Scholar] [CrossRef]
- James, S.L.; Friščić, T. Mechanochemistry. Chem. Soc. Rev. 2013, 42, 7494. [Google Scholar] [CrossRef] [PubMed]
- Suslick, K.S. Mechanochemistry and sonochemistry: Concluding remarks. Faraday Discuss. 2014, 170, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Cintas, P.; Cravotto, G.; Barge, A.; Martina, K. Interplay Between Mechanochemistry and Sonochemistry. In Polymer Mechanochemistry Topics in Current Chemistry; Springer International Publishing: Cham, Switzerland, 2014; pp. 239–284. [Google Scholar] [CrossRef]
- Yeon, J.; He, X.; Martini, A.; Kim, S.H. Mechanochemistry at Solid Surfaces: Polymerization of Adsorbed Molecules by Mechanical Shear at Tribological Interfaces. ACS Appl. Mater. Interfaces 2017, 9, 3142–3148. [Google Scholar] [CrossRef]
- Nakach, M.; Authelin, J.-R.; Chamayou, A.; Dodds, J. Comparison of various milling technologies for grinding pharmaceutical powders. Int. J. Miner. Process. 2004, 74, S173–S181. [Google Scholar] [CrossRef]
- Baláž, P.; Achimovičová, M.; Baláž, M.; Billik, P.; Cherkezova-Zheleva, Z.; Criado, J.M.; Delogu, F.; Dutková, E.; Gaffet, E.; Gotor, F.J.; et al. Hallmarks of mechanochemistry: From nanoparticles to technology. Chem. Soc. Rev. 2013, 42, 7571. [Google Scholar] [CrossRef] [Green Version]
- Capocelli, M.; Prisciandaro, M.; Lancia, A.; Musmarra, D. Comparison between hydrodynamic and acoustic cavitation in microbial cell disruption. Chem. Eng. Trans. 2014, 38, 13–18. [Google Scholar] [CrossRef]
- Stolle, A.; Szuppa, T.; Leonhardt, S.E.S.; Ondruschka, B. Ball Milling in Organic Synthesis: Solutions and Challenges. Chem. Soc. Rev. 2011, 40, 2317–2329. [Google Scholar] [CrossRef]
- Mukherjee, N.; Chatterjee, T.; Ranu, B.C. Reaction under Ball-Milling: Solvent-, Ligand-, and Metal-Free Synthesis of Unsymmetrical Diaryl Chalcogenides. J. Org. Chem. 2013, 78, 11110–11114. [Google Scholar] [CrossRef] [PubMed]
- Margetić, D.; Štrukil, V. Recent Advances in Mechanochemical Organic Synthesis. In Organic Synthesis—A Nascent Relook; Nandeshwarappa, B.P., Ed.; IntechOpen: London, UK, 2020; Available online: https://www.intechopen.com/chapters/71417 (accessed on 27 July 2021). [CrossRef] [Green Version]
- Jicsinszky, L.; Caporaso, M.; Tuza, K.; Martina, K.; Gaudino, E.C.; Cravotto, G. Nucleophilic Substitutions of 6I-O-Monotosyl-β-cyclodextrin in a Planetary Ball Mill. ACS Sustain. Chem. Eng. 2016, 4, 919–929. [Google Scholar] [CrossRef]
- Jicsinszky, L.; Caporaso, M.; Martina, K.; Gaudino, E.C.; Cravotto, G. Efficient Mechanochemical Synthesis of Regioselective Persubstituted Cyclodextrin. Beilstein J. Org. Chem. 2016, 12, 2364–2371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jicsinszky, L.; Caporaso, M.; Gaudino, E.C.; Giovannoli, C.; Cravotto, G. Synthesis of Randomly Substituted Anionic Cyclodextrins in Ball Milling. Molecules 2017, 22, 485. [Google Scholar] [CrossRef]
- El-Gendy, G.A.; Katsuhide, T.; Keiji, Y.; Yoshinobu, N. Molecular behavior, dissolution characteristics and chemical stability of aspirin in the ground mixture and in the inclusion complex with di-O-methyl-β-cyclodextrin. Int. J. Pharm. 1986, 31, 25–31. [Google Scholar] [CrossRef]
- Çelebi, N.; Erden, N. Interaction of naproxen with ß-cyclodextrin in ground mixture. Int. J. Pharm. 1992, 78, 183–187. [Google Scholar] [CrossRef]
- Uekama, K.; Horikawa, T.; Horiuchi, Y.; Hirayama, F. In vitro and in vivo evaluation of delayed-release behavior of diltiazem from its O-carboxymethyl-O-ethyl-β-cyclodextrin complex. J. Control. Release 1993, 25, 99–106. [Google Scholar] [CrossRef]
- Nozawa, Y.; Suzuki, K.; Sadzuka, Y.; Miyagishima, A.; Hirota, S. Effects of shear rate in roll mixing on mechanical complex formation of ibuprofen with β-cyclodextrin. Pharm. Acta. Helv. 1994, 69, 135–139. [Google Scholar] [CrossRef]
- Tsukushi, I.; Yamamuro, O.; Suga, H. Heat capacities and glass transitions of ground amorphous solid and liquid-quenched glass of tri-O-methyl-ß-cyclodextrin. J. Non-Cryst. Solids 1994, 175, 187–194. [Google Scholar] [CrossRef]
- Tabary, N.; Mahieu, A.; Willart, J.-F.; Dudognon, E.; Danède, F.; Descamps, M.; Bacquet, M.; Martel, B. Characterization of the hidden glass transition of amorphous cyclomaltoheptaose. Carbohydr. Res. 2011, 346, 2193–2199. [Google Scholar] [CrossRef]
- Rinaldi, L.; Binello, A.; Stolle, A.; Curini, M.; Cravotto, G. Efficient Mechanochemical Complexation of Various Steroid Compounds with α-, β- and γ-cyclodextrin. Steroids 2015, 98, 58–62. [Google Scholar] [CrossRef]
- Jug, M.; Mura, P. Grinding as Solvent-Free Green Chemistry Approach for Cyclodextrin Inclusion Complex Preparation in the Solid State. Pharmaceutics 2018, 10, 189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, J.; He, Y.; Zhan, X.; Sun, Z.; Shen, B.; Li, X. Study of kneading pressure and power consumption in a twin-blade planetary mixer for mixing highly viscous fluids. Chem. Eng. Sci. 2021, 241, 116723. [Google Scholar] [CrossRef]
- Ruiz, T.; Delalonde, M.; Bataille, B.; Baylac, G.; de Crescenzo, C.D. Texturing unsaturated granular media submitted to compaction and kneading processes. Powder Technol. 2005, 154, 43–53. [Google Scholar] [CrossRef]
- Furuta, T.; Yoshii, H.; Kobayashi, T.; Nishitarumi, T.; Yasunishi, A. Powdery encapsulation of d-limonene by kneading with mixed powders of.beta.-cyclodextrin and maltodextrin at low water content. Biosci. Biotechnol. Biochem. 1994, 58, 847–850. [Google Scholar] [CrossRef]
- Das, S.K.; Chakraborty, S.; Bose, A.; Rajabalaya, R.; Khanam, J. Effects of the preparation technique on the physicochemical characteristics and dissolution improvement of ketoprofen-SBE7-β-CD binary inclusion complexes. Colloids Surf. A 2021, 611, 125775. [Google Scholar] [CrossRef]
- Ozdemir, N.; Pola, C.C.; Teixeira, B.N.; Hill, L.E.; Bayrak, A.; Gomes, C.L. Preparation of black pepper oleoresin inclusion complexes based on beta-cyclodextrin for antioxidant and antimicrobial delivery applications using kneading and freeze drying methods: A comparative study. LWT 2018, 91, 439–445. [Google Scholar] [CrossRef]
- Bakshi, P.R.; Londhe, V.Y. Widespread applications of host-guest interactive cyclodextrin functionalized polymer nanocomposites: Its meta-analysis and review. Carbohydr. Polym. 2020, 242, 116430. [Google Scholar] [CrossRef]
- Mura, P.; Faucci, M.T.; Manderioli, A.; Bramanti, G. Influence of the preparation method on the physicochemical properties of binary systems of econazole with cyclodextrins. Int. J. Pharm. 1999, 193, 85–95. [Google Scholar] [CrossRef]
- Loftsson, T.; Brewster, M.E. Cyclodextrins as Functional Excipients: Methods to Enhance Complexation Efficiency. J. Pharm. Sci. 2012, 101, 3019–3032. [Google Scholar] [CrossRef]
- Kudryashova, O.; Muravlev, E.; Vorozhtsov, B.; Akhmadeev, I. The role of cavitation in submicron aerosol dispersion. MATEC Web Conf. 2018, 243, 00003. [Google Scholar] [CrossRef]
- Esfanjani, A.F.; Jafari, S.M. Biopolymer nano-particles and natural nano-carriers for nano-encapsulation of phenolic compounds. Colloids Surf. B 2016, 146, 532–543. [Google Scholar] [CrossRef]
- Patel, D.; Zode, S.S.; Bansal, A.K. Formulation aspects of intravenous nanosuspensions. Int. J. Pharm. 2020, 586, 119555. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, M.; Tang, S.Y.; Tan, K.W. Cavitation technology—A greener processing technique for the generation of pharmaceutical nanoemulsions. Ultrason. Sonochem. 2014, 21, 2069–2083. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, N.; Maddikeri, G.L.; Pandit, A.B. Sustained release formulations of citronella oil nanoemulsion using cavitational techniques. Ultrason. Sonochem. 2017, 36, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Tavares, M.; da Silva, M.R.M.; de Siqueira, L.B.O.; Rodrigues, R.A.S.; Bodjolle-d’Almeida, L.; dos Santos, E.P.; Ricci-Júnior, E. Trends in insect repellent formulations: A review. Int. J. Pharm. 2018, 539, 190–209. [Google Scholar] [CrossRef] [PubMed]
- Adeoye, O.; Costa, C.; Casimiro, T.; Aguiar-Ricardo, A.; Cabral-Marques, H. Preparation of ibuprofen/hydroxypropyl-γ-cyclodextrin inclusion complexes using supercritical CO2-assisted spray drying. J. Supercrit. Fluids 2018, 133, 479–485. [Google Scholar] [CrossRef]
- Hamdan, S.; Dumke, J.C.; El-Zahab, B.; Das, S.; Boldor, D.; Baker, G.A.; Warner, I.M. Strategies for controlled synthesis of nanoparticles derived from a group of uniform materials based on organic salts. J. Colloid Interface Sci. 2015, 446, 163–169. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.; Li, H. Recovery and purification of cholesterol from cholesterol-β-cyclodextrin inclusion complex using ultrasound-assisted extraction. Ultrason. Sonochem. 2017, 34, 281–288. [Google Scholar] [CrossRef]
- Li, H.; Han, C. Sonochemical synthesis of cyclodextrin-coated quantum dots for optical detection of pollutant phenols in water. Chem. Mater. 2008, 20, 6053–6059. [Google Scholar] [CrossRef]
- Le Provost, R.; Wille, T.; Louise, L.; Masurier, N.; Müller, S.; Reiter, G.; Renard, P.-Y.; Lafont, O.; Worek, F.; Estour, F. Optimized strategies to synthesize β-cyclodextrin-oxime conjugates as a new generation of organophosphate scavengers. Org. Biomol. Chem. 2011, 9, 3026–3032. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Ren, T.; Zhang, J.-R.; Chen, H.-Y.; Zhu, J.-J.; Burda, C. Spectroelectrochemistry of hollow spherical CdSe quantum dot assemblies in water. Electrochem. Commun. 2007, 9, 551–557. [Google Scholar] [CrossRef]
- Amanulla, B.; Subbu, H.K.R.; Ramaraj, S.K. A sonochemical synthesis of cyclodextrin functionalized Au-FeNPs for colorimetric detection of Cr6+ in different industrial waste water. Ultrason. Sonochem. 2018, 42, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Mohandoss, S.; Edison, T.N.J.I.; Atchudan, R.; Palanisamy, S.; Prabhu, N.M.; Napoleon, A.A.; You, S.; Lee, Y.R. Ultrasonic-assisted efficient synthesis of inclusion complexes of salsalate drug and β-cyclodextrin derivatives for potent biomedical applications. J. Mol. Liq. 2020, 319, 114358. [Google Scholar] [CrossRef]
- Cui, H.; Siva, S.; Lin, L. Ultrasound processed cuminaldehyde/2-hydroxypropyl-β-cyclodextrin inclusion complex: Preparation, characterization and antibacterial activity. Ultrason. Sonochem. 2019, 56, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Siva, S.; Li, C.; Cui, H.; Lin, L. Encompassment of isoeugenol in 2-hydroxypropyl-β-cyclodextrin using ultrasonication: Characterization, antioxidant and antibacterial activities. J. Mol. Liq. 2019, 296, 111777. [Google Scholar] [CrossRef]
- Kumar, R.; Kaur, K.; Uppal, S.; Mehta, S.K. Ultrasound processed nanoemulsion: A comparative approach between resveratrol and resveratrol cyclodextrin inclusion complex to study its binding interactions, antioxidant activity and UV light stability. Ultrason. Sonochem. 2017, 37, 478–489. [Google Scholar] [CrossRef]
- Maraulo, G.E.; dos Santos Ferreira, C.; Mazzobre, M.F. β-Cyclodextrin Enhanced Ultrasound-assisted Extraction As a Green Method to Recover Olive Pomace Bioactive Compounds. J. Food Process. Preserv. 2021, 45. [Google Scholar] [CrossRef]
- Kaur, P.; Kaur, P. β-Cyclodextrin-chitosan biocomposites for synergistic removal of imazethapyr and imazamox from soils: Fabrication, performance and mechanisms. Sci. Total Environ. 2020, 710, 135659. [Google Scholar] [CrossRef]
- Schwartz, D.P.; Brewington, C.R.; Burgwald, L.H. Rapid quantitative procedure for removing cholesterol from butter fat. J. Lipid Res. 1967, 8, 54–55. [Google Scholar] [CrossRef]
- Courregelongue, J.; Maffrand, J.-P. Procédé d’Élimination du Cholestérol Contenu Dans une Matière Grasse d’Origine Animale et Matière Grasse Appauvrie en Cholestérol Obtenue. European Patent Application EP0256911, 24 February 1988. [Google Scholar]
- Boudreau, A.; Arul, J. Cholesterol Reduction and Fat Fractionation Technologies for Milk Fat: An Overview. J. Dairy Sci. 1993, 76, 1772–1781. [Google Scholar] [CrossRef]
- Rani, D.; Sethi, A.; Kaur, K.; Agarwal, J. Ultrasonication-Assisted Synthesis of a dGlucosamine-Based CD Inclusion Complex and Its Application as an Aqueous Heterogeneous Organocatalytic System. J. Org. Chem. 2020, 85, 9548–9557. [Google Scholar] [CrossRef] [PubMed]
- Bhasarkar, J.B.; Dikshit, P.K.; Moholkar, V.S. Ultrasound assisted biodesulfurization of liquid fuel using free and immobilized cells of Rhodococcus rhodochrous MTCC 3552: A mechanistic investigation. Bioresour. Technol. 2015, 187, 369–378. [Google Scholar] [CrossRef]
- George, H.F.; Qureshi, F. Newton’s Law of Viscosity, Newtonian and Non-Newtonian Fluids. In Encyclopedia of Tribology; Wang, Q.J., Chung, Y.-W., Eds.; Springer: Boston, MA, USA, 2013; pp. 2416–2420. [Google Scholar] [CrossRef]
- Phunpee, S.; Suktham, K.; Surassmo, S.; Jarussophon, S.; Rungnim, C.; Soottitantawat, A.; Puttipipatkhachorn, S.; Ruktanonchai, U.R. Controllable encapsulation of α-mangostin with quaternized β-cyclodextrin grafted chitosan using high shear mixing. Int. J. Pharm. 2018, 538, 21–29. [Google Scholar] [CrossRef]
- Zhao, N.; Yan, L.; Xue, J.; Zhang, K.; Xu, F.-J. Degradable one-dimensional dextran-iron oxide nanohybrids for MRI-guided synergistic gene/photothermal/magnetolytic therapy. Nano Today 2021, 38, 101118. [Google Scholar] [CrossRef]
- Hu, X.; Ke, Y.; Zhao, Y.; Lu, S.; Yu, C.; Peng, F. Synthesis and characterization of a β-cyclodextrin modified polyacrylamide and its rheological properties by hybriding with silica nanoparticles. Colloids Surf. A 2018, 548, 10–18. [Google Scholar] [CrossRef]
- Hu, X.; Ke, Y.; Zhao, Y.; Lu, S.; Deng, Q.; Yu, C.; Peng, F. Synthesis, characterization and solution properties of β-cyclodextrin-functionalized polyacrylamide/montmorillonite nanocomposites. Colloids Surf. A 2019, 560, 336–343. [Google Scholar] [CrossRef]
- Kang, W.; Zhang, H.; Lu, Y.; Yang, H.; Zhu, T.; Zhang, X.; Chen, C.; Sarsenbekuly, B.; Besembaevna, O.Z. Study on the enhanced viscosity mechanism of the cyclodextrin polymer and betaine-type amphiphilic polymer inclusion complex. J. Mol. Liq. 2019, 296, 111792. [Google Scholar] [CrossRef]
- Hu, X.; Zhao, X.; Ke, Y. Effects of silica nanoparticle on the solution properties of hydrophobically associating polymer based on acrylamide and β-cyclodextrin. J. Mol. Liq. 2019, 296, 111885. [Google Scholar] [CrossRef]
- Jalalvandi, E.; Shavandi, A. Shear thinning/self-healing hydrogel based on natural polymers with secondary photocrosslinking for biomedical applications. J. Mech. Behav. Biomed. Mater. 2019, 90, 191–201. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Taguchi, T. Bonding a titanium plate and soft tissue interface by using an adhesive bone paste composed of α-tricalcium phosphate and α-cyclodextrin/nonanyl group-modified poly(vinyl alcohol) inclusion complex. Colloids Surf. B 2021, 203, 111757. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Williamson, G.S.; Yang, H. Branched polyrotaxane hydrogels consisting of alpha-cyclodextrin and low-molecular-weight four-arm polyethylene glycol and the utility of their thixotropic property for controlled drug release. Colloids Surf. B 2018, 165, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Yang, Y.; Hu, Q.; Guo, Z.; Liu, T.; Xu, J.; Wu, J.; Kirk, T.B.; Ma, D.; Xue, W. Injectable supramolecular hydrogel formed from α-cyclodextrin and PEGylated arginine-functionalized poly(l-lysine) dendron for sustained MMP-9 shRNA plasmid delivery. Acta Biomater. 2017, 49, 456–471. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, G.; Wang, C.; Li, C.; Yao, P. Shear-responsive injectable supramolecular hydrogel releasing doxorubicin loaded micelles with pH-sensitivity for local tumor chemotherapy. Int. J. Pharm. 2017, 530, 53–62. [Google Scholar] [CrossRef]
- McNeel, K.E.; Siraj, N.; Negulescu, I.; Warner, I.M. Sodium deoxycholate/TRIS-based hydrogels for multipurpose solute delivery vehicles: Ambient release, drug release, and enantiopreferential release. Talanta 2018, 177, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Araki, J.; Ito, K. Strongly thixotropic viscosity behavior of dimethylsulfoxide solution of polyrotaxane comprising α-cyclodextrin and low molecular weight poly(ethylene glycol). Polymer 2007, 48, 7139–7144. [Google Scholar] [CrossRef] [Green Version]
- Hu, T.; Cui, X.; Zhu, M.; Wu, M.; Tian, Y.; Yao, B.; Song, W.; Niu, Z.; Huang, S.; Fu, X. 3D-printable supramolecular hydrogels with shear-thinning property: Fabricating strength tunable bioink via dual crosslinking. Bioact. Mater. 2020, 5, 808–818. [Google Scholar] [CrossRef]
- Liao, D.; Dai, S.; Tam, K.C. Rheological properties of hydrophobic ethoxylated urethane (HEUR) in the presence of methylated β-cyclodextrin. Polymer 2004, 45, 8339–8348. [Google Scholar] [CrossRef]
- Busche, B.J.; Tonelli, A.E.; Balik, C.M. Morphology of polystyrene/poly(dimethyl siloxane) blends compatibilized with star polymers containing a γ–cyclodextrin core and polystyrene arms. Polymer 2010, 51, 1465–1471. [Google Scholar] [CrossRef]
- Busche, B.J.; Tonelli, A.E.; Balik, C.M. Properties of polystyrene/poly(dimethyl siloxane) blends partially compatibilized with star polymers containing a γ-cyclodextrin core and polystyrene arms. Polymer 2010, 51, 6013–6020. [Google Scholar] [CrossRef]
- Busche, B.J.; Tonelli, A.E.; Balik, C.M. Compatibilization of polystyrene/poly(dimethyl siloxane) solutions with star polymers containing a -cyclodextrin core and polystyrene arms. Polymer 2010, 51, 454–462. [Google Scholar] [CrossRef]
- Margetić, D.; Štrukil, V. Practical Considerations in Mechanochemical Organic Synthesis. Mechanochem. Org. Synth. 2016, 1–54. [Google Scholar] [CrossRef]
- Ling, A.R.; Baker, J.L. XCVI.—Halogen derivatives of quinone. Part III. Derivatives of quinhydrone. J. Chem. Soc. Trans. 1893, 63, 1314–1327. [Google Scholar] [CrossRef]
- Patil, A.O.; Curtin, D.Y.; Paul, I.C. Solid-state formation of quinhydrones from their components. Use of solid-solid reactions to prepare compounds not accessible from solution. J. Am. Chem. Soc. 1984, 106, 348–353. [Google Scholar] [CrossRef]
- Aakeröy, C.B.; Chopade, P.D. Oxime Decorated Cavitands Functionalized through Solvent-Assisted Grinding. Org. Lett. 2011, 13, 1–3. [Google Scholar] [CrossRef]
- Margetić, D.; Štrukil, V. Experiments for Introduction of Mechanochemistry in the Undergraduate Curriculum. Mechanochem. Org. Synth. 2016, 351–360. [Google Scholar] [CrossRef]
- Adeoye, O.; Conceição, J.; Serra, P.A.; da Silva, A.B.; Duarte, N.; Guedes, R.C.; Corvo, M.C.; Ricardo, A.-A.; Jicsinszky, L.; Casimiro, T.; et al. Cyclodextrin solubilization and complexation of antiretroviral drug lopinavir: In silico prediction; effects of derivatization, molar ratio and preparation method. Carbohydr. Polym. 2020, 227, 115287. [Google Scholar] [CrossRef]
- Oliva, E.; Mathiron, D.; Rigaud, S.; Monflier, E.; Sevin, E.; Bricout, H.; Tilloy, S.; Gosselet, F.; Fenart, L.; Bonnet, V.; et al. New Lipidyl-Cyclodextrins Obtained by Ring Opening of Methyl Oleate Epoxide Using Ball Milling. Biomolecules 2020, 10, 339. [Google Scholar] [CrossRef] [Green Version]
- Jicsinszky, L.; Calsolaro, F.; Martina, K.; Bucciol, F.; Manzoli, M.; Cravotto, G. Reaction of oxiranes with cyclodextrins under high-energy ball-milling conditions. Beilstein J. Org. Chem. 2019, 15, 1448–1459. [Google Scholar] [CrossRef]
- Menuel, S.; Doumert, B.; Saitzek, S.; Ponchel, A.; Delevoye, L.; Monflier, E.; Hapiot, F. Selective Secondary Face Modification of Cyclodextrins by Mechanosynthesis. J. Org. Chem. 2015, 80, 6259–6266. [Google Scholar] [CrossRef]
- Menuel, S.; Saitzek, S.; Monflier, E.; Hapiot, F. Particle size effect in the mechanically assisted synthesis of β-cyclodextrin mesitylene sulfonate. Beilstein J. Org. Chem. 2020, 16, 2598–2606. [Google Scholar] [CrossRef]
- Giancarlo, C.; László, J.; Foglia, E.; European Patent Application. Method for the Production of Metal Oxide Pigment Composite of Controlled Agglomerating Properties and Respective Product. EP3798272, 1 April 2021. [Google Scholar]
- Tabary, N.; Garcia-Fernández, M.J.; Danède, F.; Descamps, M.; Martel, B.; Willart, J.-F. Determination of the glass transition temperature of cyclodextrin polymers. Carbohydr. Polym. 2016, 148, 172–180. [Google Scholar] [CrossRef]
- Fuerstenau, D.W. Grinding Aids. KONA Powder Part. J. 1995, 13, 5–18. [Google Scholar] [CrossRef] [Green Version]
- Martina, K.; Baricco, F.; Berlier, G.; Caporaso, M.; Cravotto, G. Efficient green protocols for preparation of highly functionalized β-cyclodextrin-grafted silica. ACS Sustain. Chem. Eng. 2014, 2, 2595–2603. [Google Scholar] [CrossRef]
- Jicsinszky, L.; Bucciol, F.; Manzoli, M.; Cravotto, G. Comparative Studies of Mechanochemically Synthesized Insoluble Beta-Cyclodextrin Polymers. Curr. Org. Chem. 2021, 25. in press. [Google Scholar] [CrossRef]
- Argenziano, M.; Haimhoffer, A.; Bastiancich, C.; Jicsinszky, L.; Caldera, F.; Trotta, F.; Scutera, S.; Alotto, D.; Fumagalli, M.; Musso, T.; et al. In Vitro Enhanced Skin Permeation and Retention of Imiquimod Loaded in β-Cyclodextrin Nanosponge Hydrogel. Pharmaceutics 2019, 11, 138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trotta, F.; Zanetti, M.; Cavalli, R. Cyclodextrin-based nanosponges as drug carriers. Beilstein J. Org. Chem. 2012, 8, 2091–2099. [Google Scholar] [CrossRef]
- Pedrazzo, A.R.; Caldera, F.; Zanetti, M.; Appleton, S.L.; Dhakar, N.K.; Trotta, F. Mechanochemical green synthesis of hyper-crosslinked cyclodextrin polymers. Beilstein J. Org. Chem. 2020, 16, 1554–1563. [Google Scholar] [CrossRef] [PubMed]
- Concheiro, A.; Alvarez-Lorenzo, C. Chemically cross-linked and grafted cyclodextrin hydrogels: From nanostructures to drug-eluting medical devices. Adv. Drug Deliv. Rev. 2013, 65, 1188–1203. [Google Scholar] [CrossRef] [PubMed]
- Bashari, A.; Hemmatinejad, N.; Pourjavadi, A. Smart and fragrant garment via surface modification of cotton fabric with cinnamon oil/stimuli responsive PNIPAAm/chitosan nano hydrogels. IEEE Trans. Nanobiosci. 2017, 16, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Wang, Z.; Xiao, Y.; Zhang, S.; Wang, J. Advances in crosslinking strategies of biomedical hydrogels. Biomater. Sci. 2019, 7, 843–855. [Google Scholar] [CrossRef] [PubMed]
- Bhangu, S.K.; Ashokkumar, M. Theory of Sonochemistry. Top. Curr. Chem. 2016, 374, 56. [Google Scholar] [CrossRef]
- Zhao, J.; Shao, H.; Wu, X.; Shi, S. A rapid synthesis of pyranoid glycals promoted by β-cyclodextrin and ultrasound. Chin. J. Chem. 2011, 29, 1434–1440. [Google Scholar] [CrossRef]
- Karthika, A.; Selvarajan, S.; Karuppasamy, P.; Suganthi, A.; Rajarajan, M. A novel highly efficient and accurate electrochemical detection of poisonous inorganic Arsenic (III) ions in water and human blood serum samples based on SrTiO3/β-cyclodextrin composite. J. Phys. Chem. Solids 2019, 127, 11–18. [Google Scholar] [CrossRef]
- Xu, J.-Z.; Xu, S.; Geng, J.; Li, G.-X.; Zhu, J.-J. The fabrication of hollow spherical copper sulfide nanoparticle assemblies with 2-hydroxypropyl-β-cyclodextrin as a template under sonication. Ultrason. Sonochem. 2006, 13, 451–454. [Google Scholar] [CrossRef]
- Xu, L.; Zhu, J.-J. Green synthesis of Cu/Cu2O composite hollow nanospheres via a facile sonochemical route. Sci. Adv. Mater. 2009, 1, 121–124. [Google Scholar] [CrossRef]
- Suárez-Cerda, J.; Espinoza-Gómez, H.; Alonso-Núñez, G.; Rivero, I.A.; Gochi-Ponce, Y.; Flores-López, L.Z. A green synthesis of copper nanoparticles using native cyclodextrins as stabilizing agents. J. Saudi Chem. Soc. 2017, 21, 341–348. [Google Scholar] [CrossRef]
- Law, H.; Benito, J.M.; García Fernández, J.M.; Jicsinszky, L.; Crouzy, S.; Defaye, J. Copper(II)-complex Directed Regioselective Mono-p-toluenesulfonylation of Cyclomaltoheptaose at a Primary Hydroxyl Group Position: An NMR and Molecular Dynamics-aided Design. J. Phys. Chem. B 2011, 115, 7524–7532. [Google Scholar] [CrossRef] [PubMed]
- Martina, K.; Calsolaro, F.; Zuliani, A.; Berlier, G.; Chávez-Rivas, F.; Moran, M.J.; Luque, R.; Cravotto, G. Sonochemically-promoted preparation of silica-anchored cyclodextrin derivatives for efficient copper catalysis. Molecules 2019, 24, 2490. [Google Scholar] [CrossRef] [Green Version]
- Pirouzmand, M.; Sani, P.S.; Ghasemi, Z.; Azizi, S. Citric acid-crosslinked β-cyclodextrin supported zinc peroxide as a biocompatible H2O2 scavenger. J. Biol. Inorg. Chem. 2020, 25, 411–417. [Google Scholar] [CrossRef]
- Xu, F.; Wang, Y.; Liu, Z.; Wei, X.; Chen, J.; He, X.; Li, H.; Zhou, Y. A deep eutectic solvent modified magnetic β-cyclodextrin particle for solid-phase extraction of trypsin. Anal. Chim. Acta 2020, 1137, 125–135. [Google Scholar] [CrossRef]
- Zhao, M.-X.; Su, H.; Mao, Z.-W.; Ji, L.-N. Synthesis, biocompatibility and luminescence properties of quantum dots conjugated with amino acid-functionalized β-cyclodextrin. J. Lumin. 2012, 132, 16–22. [Google Scholar] [CrossRef]
- Rajkumar, T.; Kukkar, D.; Kim, K.-H.; Sohn, J.R.; Deep, A. Cyclodextrin-metal–organic framework (CD-MOF): From synthesis to applications. J. Ind. Eng. Chem. 2019, 72, 50–66. [Google Scholar] [CrossRef]
- Arai, T.; Hayashi, M.; Takagi, N.; Takata, T. One-pot synthesis of native and permethylated α-cyclodextrin- containing polyrotaxanes in water. Macromolecules 2009, 42, 1881–1887. [Google Scholar] [CrossRef]
- Cravotto, G.; Bicchi, C.; Tagliapietra, S.; Costa, L.; Di Carlo, S.; Nervi, C. New chiral selectors: Design and synthesis of 6-TBDMS-2,3-methyl β-cyclodextrin 2-2′ thioureido dimer and 6-TBDMS-2,3-methyl (or 2-methyl-3-acetyl) β-cyclodextrin bearing an (R) mosher acid moiety. Chirality 2004, 16, 526–533. [Google Scholar] [CrossRef]
- Bicchi, C.; Cagliero, C.; Liberto, E.; Sgorbini, B.; Martina, K.; Cravotto, G.; Rubiolo, P. New asymmetrical per-substituted cyclodextrins (2-O-methyl-3-O-ethyl- and 2-O-ethyl-3-O-methyl-6-O-t-butyldimethylsilyl-β-derivatives) as chiral selectors for enantioselective gas chromatography in the flavour and fragrance field. J. Chromatogr. A 2010, 1217, 1106–1113. [Google Scholar] [CrossRef]
- Kalakuntla, R.K.; Wille, T.; Provost, R.L.; Letort, S.; Reiter, G.; Müller, S.; Thiermann, H.; Worek, F.; Gouhier, G.; Lafont, O.; et al. New modified β-cyclodextrin derivatives as detoxifying agents of chemical warfare agents (I). Synthesis and preliminary screening: Evaluation of the detoxification using a half-quantitative enzymatic assay. Toxicol. Lett. 2013, 216, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Martina, K.; Trotta, F.; Robaldo, B.; Belliardi, N.; Jicsinszky, L.; Cravotto, G. Efficient Regioselective Functionalizations of Cyclodextrins Carried Out under Microwaves or Power Ultrasound. Tetrahedron Lett. 2007, 48, 9185–9189. [Google Scholar] [CrossRef]
- Carmona, T.; Marcelo, G.; Rinaldi, L.; Martina, K.; Cravotto, G.; Mendicuti, F. Soluble cyanine dye/β-cyclodextrin derivatives: Potential carriers for drug delivery and optical imaging. Dyes Pigm. 2015, 114, 204–214. [Google Scholar] [CrossRef]
- Trotta, F.; Cavalli, R. Characterization and Applications of New Hyper-Cross-Linked Cyclodextrins. Compos. Interfaces 2009, 16, 39–48. [Google Scholar] [CrossRef]
- Alongi, J.; Poskovic, M.; Frache, A.; Trotta, F. Role of β-cyclodextrin nanosponges in polypropylene photooxidation. Carbohydr. Polym. 2011, 86, 127–135. [Google Scholar] [CrossRef]
- Trotta, F.; Martina, K.; Robaldo, B.; Barge, A.; Cravotto, G. Recent advances in the synthesis of cyclodextrin derivatives under microwaves and power ultrasound. J. Inclusion Phenom. Macrocycl. Chem. 2007, 57, 3–7. [Google Scholar] [CrossRef]
- Jicsinszky, L.; Ivanyi, R. Catalytic Transfer Hydrogenation of Sugar Derivatives. Carbohydr. Polym. 2001, 45, 139–145. [Google Scholar] [CrossRef]
- Noro, J.; Reis, R.L.; Cavaco-Paulo, A.; Silva, C. Ultrasound-assisted biosynthesis of novel methotrexate-conjugates. Ultrason. Sonochem. 2018, 48, 51–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, G.B.; Tang, K.W. Preparation of gas chromatographic capillary columns with beta-cyclodextrin polymer stationary phase modified with methyl phenyl silicone(OV-17). Chin. J. Chrom./Zhongguo Hua Xue Hui 2000, 18, 343–345. [Google Scholar]
- Guan, J.; Xu, X.; Li, G.; Peng, W. Preparation and tribological properties of inclusion complex of β-cyclodextrin/dialkyl pentasulfide as additive in PEG-600 aqueous solution. Appl. Surf. Sci. 2014, 289, 400–406. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jicsinszky, L.; Cravotto, G. Toward a Greener World—Cyclodextrin Derivatization by Mechanochemistry. Molecules 2021, 26, 5193. https://doi.org/10.3390/molecules26175193
Jicsinszky L, Cravotto G. Toward a Greener World—Cyclodextrin Derivatization by Mechanochemistry. Molecules. 2021; 26(17):5193. https://doi.org/10.3390/molecules26175193
Chicago/Turabian StyleJicsinszky, László, and Giancarlo Cravotto. 2021. "Toward a Greener World—Cyclodextrin Derivatization by Mechanochemistry" Molecules 26, no. 17: 5193. https://doi.org/10.3390/molecules26175193






