Recent Progress on Biological Activity of Amaryllidaceae and Further Isoquinoline Alkaloids in Connection with Alzheimer’s Disease
Abstract
:1. Introduction
2. Isolation of Alkaloids from Plant Material for Biological Evaluation
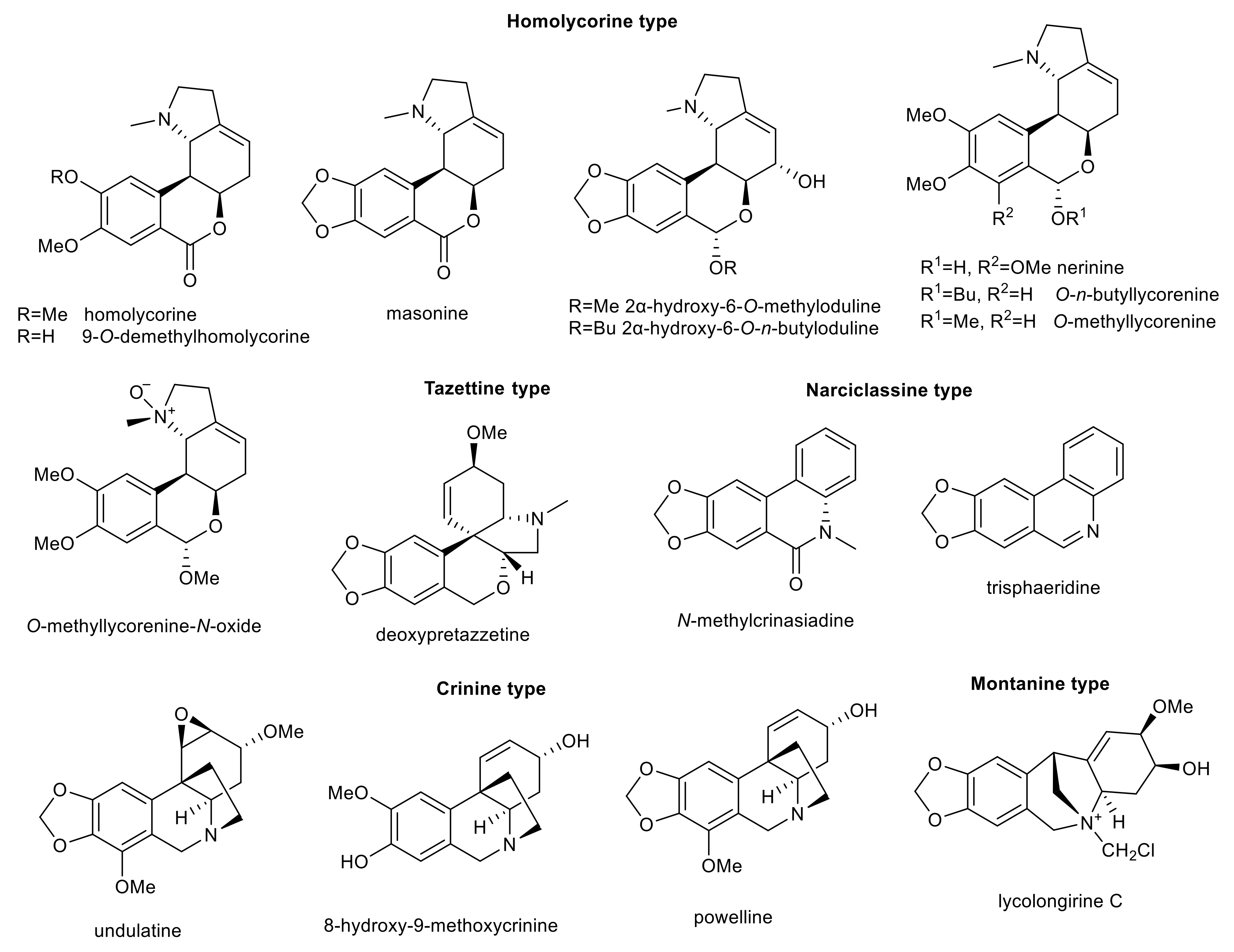
3. Amaryllidaceae Alkaloids
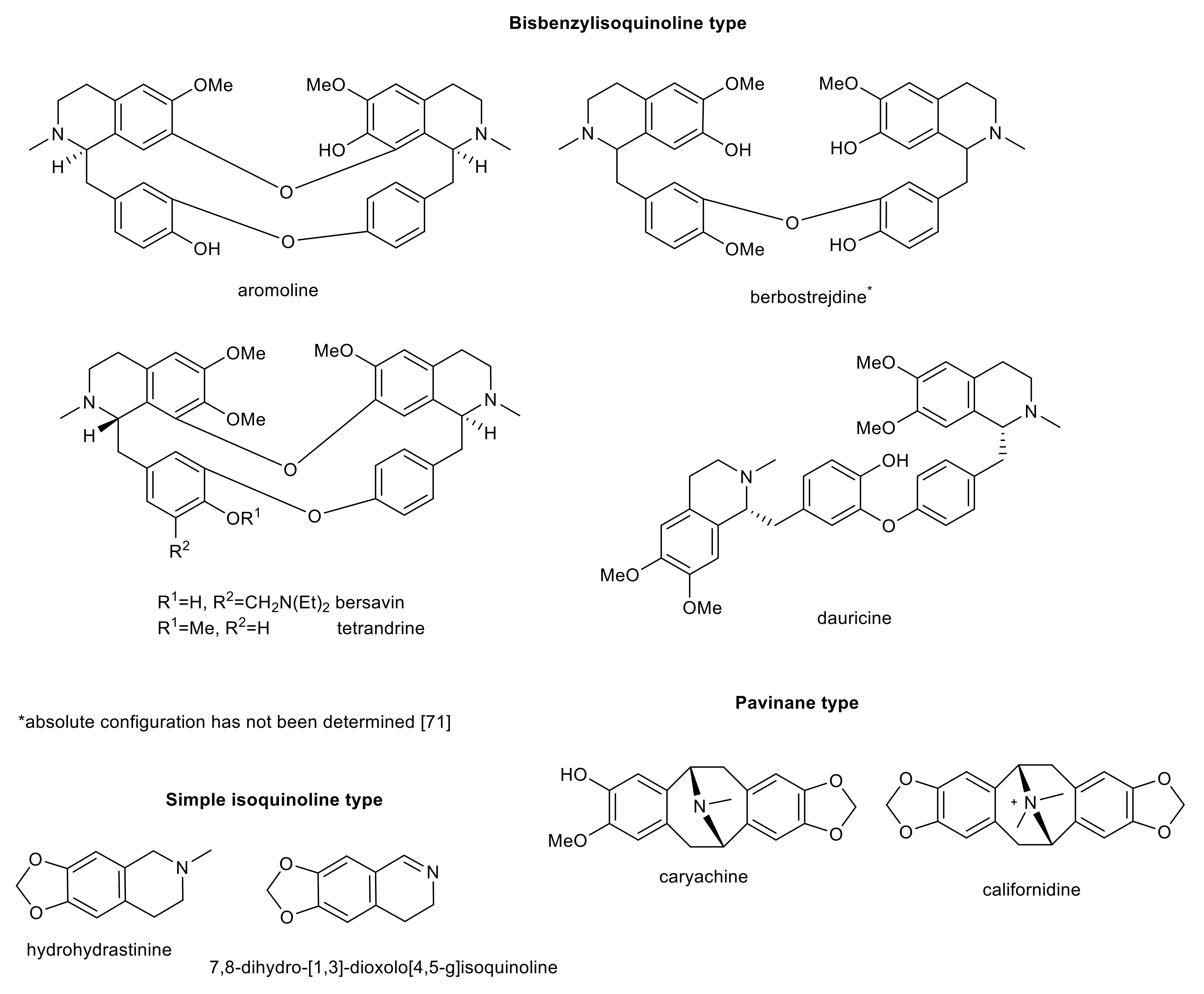
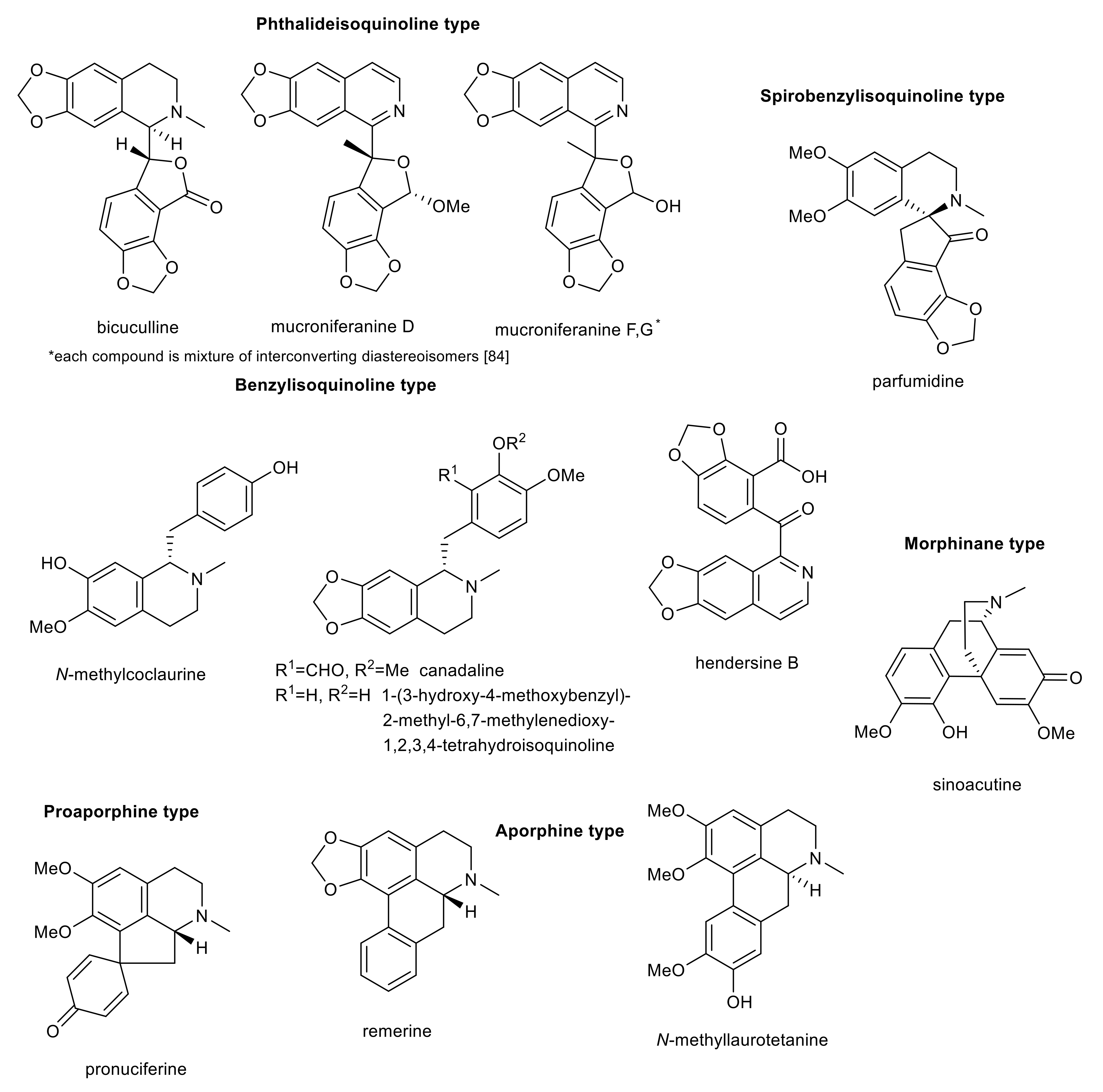
4. Further Isoquinoline Alkaloids
| Isoquinoline Alkaloid | Structural-Type | Plant Source | IC50 AChE (μM) | IC50 BuChE (μM) | IC50 POP (μM) | IC50 MAO-A (μM) | IC50 Aβ (μM) | Ref. |
|---|---|---|---|---|---|---|---|---|
| avicine | benzophenanthridine | Zanthoxylum rigidum | 0.15 ± 0.01 a 0.52 ± 0.05 b | 0.88 ± 0.08 c | n.s | 0.41 ± 0.02 | 5.56 ± 0.94 | [111] |
| nitidine | benzophenanthridine | Zanthoxylum rigidum | 0.65 ± 0.09 a 1.25 ± 0.09 b | 5.73 ± 0.60 c | n.s | 1.89 ± 0.17 | 1.89 ± 0.40 | [111] |
| 6-ethoxydihydrochelerythrine | benzophenanthridine | Chelidonium majus | 0.83 ± 0.04 b | 4.20 ± 0.19 d | n.s. | n.s. | n.s. | [112] |
| chelerythrine | benzophenanthridine | Chelidonium majus | 1.54 ± 0.07 b 3.78 ± 0.15 a | 6.33 ± 0.93 c 10.34 ± 0.24 d | n.s. | 0.55 ± 0.04 | 4.20 ± 0.43 | [113,114] |
| sanguinarine | benzophenanthridine | Corydalis saxicola | 1.93 ± 0.01 a | >200 c | n.s. | n.s. | n.s. | [96] |
| mucroniferanine H (racemic mixture) | protoberberine | Corydalis mucronifera | 2.31 ± 0.20 a | 36.71 ± 1.12 c | n.s. | n.s. | n.s. | [109] |
| 6-ethoxydihydrosanguinarine | benzophenanthridine | Chelidonium majus | 3.25 ± 0.24 b | 4.51 ± 0.31 d | n.s. | n.s. | n.s. | [112] |
| hydrohydrastinine | simple isoquinoline | Corydalis mucronifera | 9.13 ± 0.15 a | >100 c | n.s. | n.s. | n.s. | [109] |
| dehydrocavidine | protoberberine | Corydalis saxicola | 9.92 ± 0.24 * | >200 * | n.s. | n.s. | n.s. | [96] |
| mucroniferanine G (diastereoisomeric mixture 1:1) | phthalideisoquinoline | Corydalis mucronifera | 11.3 ± 0.8 a | n.s. | n.s. | n.s. | n.s. | [110] |
| mucroniferanine F (diastereosomeric mixture 1:1) | phthalideisoquinoline | Corydalis mucronifera | 12.2 ± 0.2 a | n.s. | n.s. | n.s. | n.s. | [110] |
| (+)-canadine | protoberberine | Corydalis cava | 12.4 ± 0.9 b | >100 d | 152.0 ± 12.5 | n.s. | n.s. | [115,116,117] |
| hendersine B | benzylisoquinoline | Corydalis mucronifera | 14.22 ± 0.34 a | >100 c | n.s. | n.s. | n.s. | [109] |
| 7,8-dihydro-[1,3]-dioxolo[4,5-g]isoquinoline | simple isoquinoline | Stephania rotunda | 18.4 ± 1.4 e | n.s. | n.s. | n.s. | n.s. | [95] |
| caryachine | pavinane | Eschscholzia californica | 19.6 ± 0.4 b | >100 d | n.s. | n.s. | n.s. | [118] |
| (+)-canadaline | benzylisoquinoline | Corydalis cava | 20.1 ± 1.1 b | 85.2 ± 2.2 d | n.s. | n.s. | n.s. | [115] |
| remerine | aporphine | Stephania rotunda | 20.7 ± 1.3 e | n.s. | n.s. | n.s. | n.s. | [95] |
| chelidonine | benzophenanthridine | Chelidonium majus | 26.8 ± 1.2 b | 31.9 ± 1.4 d | n.s. | n.s. | n.s. | [112] |
| (−)-mucroniferanine D | phthalideisoquinoline | Corydalis mucronifera | 28.3 ± 0.4 a | n.s. | n.s. | n.s. | n.s. | [110] |
| aromoline | bisbenzylisoquinoline | Berberis vulgaris | >100 b | 0.82 ± 0.10 d | 189 ± 32 | n.s. | n.s. | [97] |
| berbostrejdine | bisbenzylisoquinoline | Berberis vulgaris | 66.0 ± 8.0 b | 6.9 ± 1.0 d | n.s. | n.s. | n.s. | [97] |
| N-methylcoclaurine | benzylisoquinoline | Peumus boldus | >100 b | 15.0 ± 1.4 d | n.s. | n.s. | n.s. | [119] |
| 1-(3-hydroxy-4-methoxybenzyl)-2-methyl-6,7-methylenedioxy-1,2,3,4-tetrahydroisoquinoline | benzylisoquinoline | Eschscholzia californica | >100 b | 27.8 ± 0.4 d | n.s. | n.s. | n.s. | [118] |
| sinactine | protoberberine | Fumaria offcinalis | >100 b | >100 d | 53 ± 2 | n.s. | n.s. | [120] |
| californidine iodide | pavinane | Eschscholzia californica | 36.7 ± 0.9 b | >100 d | 55.6 ± 3.5 | n.s. | n.s. | [118,121] |
| bersavine | bisbenzylisoquinoline | Berberis vulgaris | 68.0 ± 11.0 b | >100 d | 67 ± 6 | n.s. | n.s. | [97] |
| parfumidine | spirobenzylisoquinoline | Fumaria offcinalis | >100 b | >100 d | 99 ± 5 | n.s. | n.s. | [120] |
| dihydrosanguinarine | benzophenanthridine | Macleaya cordata, Corydalis mucronifera | >100 b | n.s. | 99.1 ± 7.6 | n.s. | n.s. | [110,121] |
| corypalmine | protoberberine | Corydalis cava | >100 b | >100 d | 128.0 ± 10.5 | n.s. | n.s. | [117,121] |
| N-methyllaurotetanine | aporphine | Peumus boldus | >100 b | >100 d | 135.0 ± 11.7 | n.s. | n.s. | [119,121] |
| sinoacutine | morphinane | Peumus boldus | >100 b | >100 d | 143.1 ± 25.4 | n.s. | n.s. | [119] |
| bicuculline | phthalideisoquinoline | Fumaria offcinalis | >100 b | >100 d | 190 ± 50 | n.s. | n.s. | [120] |
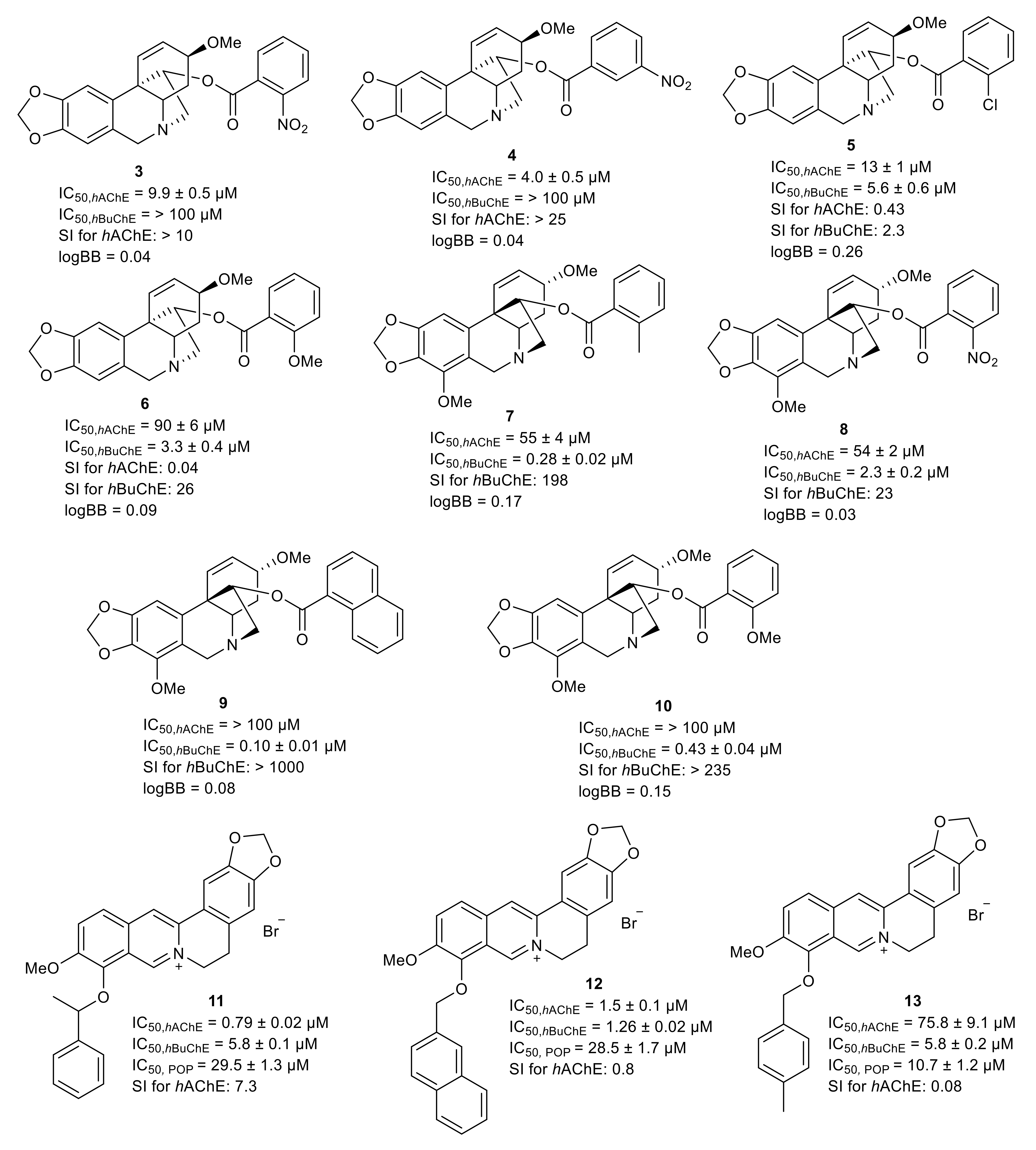
5. Selected Semisynthetic Derivatives of Amaryllidaceae and Isoquinoline Alkaloids as Inspiration for Drug Development for Neurodegenerative Diseases
6. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marešová, P.; Klímová, B.; Novotný, M.; Kuča, K. Alzheimer’s and Parkinson’s diseases: Expected economic impact on Europe—A call for a Uniform European Strategy. J. Alzheimers Dis. 2016, 54, 1123–1133. [Google Scholar] [CrossRef]
- Silva, T.; Reis, J.; Teixeira, J.; Borges, F. Alzheimer’s disease, enzyme targets and drug discovery struggles: From natural products to drug prototypes. Ageing Res. Rev. 2014, 15, 116–145. [Google Scholar] [CrossRef]
- Ballard, C.; Gauthier, S.; Corbett, A.; Brayne, C.; Aarsland, D.; Jones, E. Alzheimer’s disease. Lancet 2011, 377, 1019–1031. [Google Scholar] [CrossRef]
- Nichols, E.; Szoeke, C.; Vollset, S.; Abbasi, N.; Abd-Allah, F.; Ebro, J.; Aichour, M.; Akinyemi, R.; Alahdab, F.; Asgedom, S.; et al. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 88–106. [Google Scholar] [CrossRef] [Green Version]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef] [Green Version]
- Mehta, D.C.; Short, J.L.; Hilmer, S.N.; Nicolazzo, J.A. Drug access to the central nervous system in Alzheimer’s disease: Preclinical and clinical insights. Pharm. Res. 2015, 32, 819–839. [Google Scholar] [CrossRef] [PubMed]
- Anand, R.; Gill, K.D.; Mahdi, A.A. Therapeutics of Alzheimer’s disease: Past, present and future. Neuropharmacology 2014, 76, 27–50. [Google Scholar] [CrossRef] [PubMed]
- Dubey, H.; Gulati, K.; Ray, A. Recent studies on cellular and molecular mechanisms in Alzheimer’s disease: Focus on epigenetic factors and histone deacetylase. Rev. Neurosci. 2018, 29, 241–260. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Schneider, L.S.; Giacobini, E.; Kivipelto, M.; Sindi, S.; Dubois, B.; Broich, K.; Nisticò, R.; Aisen, P.S.; Lista, S. Advances in the therapy of Alzheimer’s disease: Targeting amyloid beta and tau and perspectives for the future. Expert Rev. Neurother. 2015, 15, 83–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, M.L.; Cleveland, D.W. Going new places using an old MAP: Tau, microtubules and human neurodegenerative disease. Curr. Opin. Cell Biol. 2001, 13, 41–48. [Google Scholar] [CrossRef]
- Hampel, H.; Mesulam, M.M.; Cuello, A.C.; Farlow, M.R.; Giacobini, E.; Grossberg, G.T.; Khachaturian, A.S.; Vergallo, A.; Cavedo, E.; Snyder, P.J.; et al. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain 2018, 141, 1917–1933. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, J.P.; Saldanha, C. Nonneuronal cholinergic system in human erythrocytes: Biological role and clinical relevance. J. Membr. Biol. 2010, 234, 227–234. [Google Scholar] [CrossRef] [Green Version]
- Cahlíková, L.; Macáková, K.; Benešová, N.; Chlebek, J.; Hošťálková, A.; Opletal, L. Chapter 6—Natural compounds (small molecules) as potential and real drugs of Alzheimer’s disease: A critical review. In Studies in Natural Products Chemistry, 1st ed.; Rahman, A., Ed.; Elsevier: Oxford, UK, 2014; Volume 42, pp. 153–194. ISBN 978-0-444-63281-4. [Google Scholar]
- Ciro, A.; Park, J.; Burkhard, G.; Yan, N.; Geula, C. Biochemical differentiation of cholinesterases from normal and Alzheimer’s disease cortex. Curr. Alzheimer Res. 2012, 9, 138–143. [Google Scholar] [CrossRef]
- García-Ayllón, M.S.; Silveyra, M.X.; Sáez-Valero, J. Association between acetylcholinesterase and beta-amyloid peptide in Alzheimer’s cerebrospinal fluid. Chem. Biol. Interact. 2008, 175, 209–215. [Google Scholar] [CrossRef]
- Sridhar, G.R.; Nirmala, G.; Apparao, A.; Madhavi, A.S.; Sreelatha, S.; Rani, J.S.; Vijayalakshmi, P. Serum butyrylcholinesterase in type 2 diabetes mellitus: A biochemical and bioinformatics approach. Lipids Health Dis. 2005, 4, 18. [Google Scholar] [CrossRef] [Green Version]
- Paz, M.L.; Barrantes, F.J. Autoimmune attack of the neuromuscular junction in myasthenia gravis: Nicotinic acetylcholine receptors and other targets. ACS Chem. Neurosci. 2019, 10, 2186–2194. [Google Scholar] [CrossRef]
- Bajgar, J. Organophosphates/nerve agent poisoning: Mechanism of action, diagnosis, prophylaxis, and treatment. Adv. Clin. Chem. 2004, 38, 151–216. [Google Scholar] [CrossRef]
- Wang, R.; Reddy, P.H. Role of glutamate and NMDA receptors in Alzheimer’s disease. J. Alzheimers Dis. 2017, 57, 1041–1048. [Google Scholar] [CrossRef] [Green Version]
- Kornhuber, J.; Wiltfang, J. The role of glutamate in dementia. J. Neural. Transm. Suppl. 1998, 53, 277–287. [Google Scholar] [CrossRef]
- Hernández, F.; Avila, J. The role of glycogen synthase kinase 3 in the early stages of Alzheimer’s disease. FEBS Lett. 2008, 582, 3848–3854. [Google Scholar] [CrossRef] [Green Version]
- Hooper, C.; Killick, R.; Lovestone, S. The GSK3 hypothesis of Alzheimer’s disease. J. Neurochem. 2008, 104, 1433–1439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plattner, F.; Angelo, M.; Giese, K.P. The roles of cyclin-dependent kinase 5 and glycogen synthase kinase 3 in tau hyperphosphorylation. J. Biol. Chem. 2006, 281, 25457–25465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez, A.; Perez, D.I. GSK-3 inhibitors: A ray of hope for the treatment of Alzheimer’s disease? J. Alzheimers Dis. 2008, 15, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z. Monoamine oxidase inhibitors: Promising therapeutic agents for Alzheimer’s disease (Review). Mol. Med. Rep. 2014, 9, 1533–1541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramsay, R.R.; Popovic-Nikolic, M.R.; Nikolic, K.; Uliassi, E.; Bolognesi, M.L. A perspective on multi-target drug discovery and design for complex diseases. Clin. Transl. Med. 2018, 7, 3. [Google Scholar] [CrossRef] [Green Version]
- Schedin-Weiss, S.; Inoue, M.; Hromádková, L.; Teranishi, Y.; Yamamoto, N.G.; Wiehager, B.; Bogdanovic, N.; Winblad, B.; Sandebring-Matton, A.; Frykman, S.; et al. Monoamine oxidase B is elevated in Alzheimer disease neurons, is associated with γ-secretase and regulates neuronal amyloid β-peptide levels. Alzheimers Res. Ther. 2017, 9, 57. [Google Scholar] [CrossRef]
- Svarcbahs, R.; Jäntti, M.; Kilpeläinen, T.; Julku, U.H.; Urvas, L.; Kivioja, S.; Norrbacka, S.; Myöhänen, T.T. Prolyl oligopeptidase inhibition activates autophagy via protein phosphatase 2A. Pharmacol. Res. 2020, 151, 104558. [Google Scholar] [CrossRef]
- Szeltner, Z.; Polgár, L. Structure, function and biological relevance of prolyl oligopeptidase. Curr. Protein Pept. Sci. 2008, 9, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Laitinen, K.S.; van Groen, T.; Tanila, H.; Venäläinen, J.; Männistö, P.T.; Alafuzoff, I. Brain prolyl oligopeptidase activity is associated with neuronal damage rather than beta-amyloid accumulation. Neuroreport 2001, 12, 3309–3312. [Google Scholar] [CrossRef]
- García-Horsman, J.A.; Männistö, P.T.; Venäläinen, J.I. On the role of prolyl oligopeptidase in health and disease. Neuropeptides 2007, 41, 1–24. [Google Scholar] [CrossRef]
- Zemek, F.; Drtinová, L.; Nepovimová, E.; Šepsová, V.; Korábečný, J.; Klimeš, J.; Kuča, K. Outcomes of Alzheimer’s disease therapy with acetylcholinesterase inhibitors and memantine. Expert Opin. Drug Saf. 2014, 13, 759–774. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, S.; Kishi, T.; Iwata, N. Combination therapy with cholinesterase inhibitors and memantine for Alzheimer’s disease: A systematic review and meta-analysis. Int. J. Neuropsychopharmacol. 2014, 18, pyu115. [Google Scholar] [CrossRef]
- Lalli, G.; Schott, J.M.; Hardy, J.; De Strooper, B. Aducanumab: A new phase in therapeutic development for Alzheimer’s disease? EMBO Mol. Med. 2021, 13, e14781. [Google Scholar] [CrossRef]
- Mahase, E. Three FDA advisory panel members resign over approval of Alzheimer’s drug. BMJ 2021, 373, n1503. [Google Scholar] [CrossRef]
- Servick, K. Alzheimer’s drug approval spotlights blood tests. Science 2021, 373, 373–374. [Google Scholar] [CrossRef]
- Wang, S.; Dong, G.; Sheng, C. Structural simplification of natural products. Chem. Rev. 2019, 119, 4180–4220. [Google Scholar] [CrossRef]
- Konrath, E.L.; Passos Cdos, S.; Klein, L.C., Jr.; Henriques, A.T. Alkaloids as a source of potential anticholinesterase inhibitors for the treatment of Alzheimer’s disease. J. Pharm. Pharmacol. 2013, 65, 1701–1725. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.; Faraoni, M.; Castro, M.; Alza, N.; Cavallaro, V. Natural AChE inhibitors from plants and their contribution to Alzheimer’s disease therapy. Curr. Neuropharmacol. 2013, 11, 388–413. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos, T.C.; Gomes, T.M.; Pinto, B.A.S.; Camara, A.L.; Paes, A.M.D.A. Naturally occurring acetylcholinesterase inhibitors and their potential use for Alzheimer’s disease therapy. Front. Pharmacol. 2018, 9, 1192. [Google Scholar] [CrossRef] [Green Version]
- Moodie, L.W.K.; Sepčić, K.; Turk, T.; Frangež, R.; Svenson, J. Natural cholinesterase inhibitors from marine organisms. Nat. Prod. Rep. 2019, 36, 1053–1092. [Google Scholar] [CrossRef]
- Panda, S.S.; Jhanji, N. Natural products as potential anti-Alzheimer agents. Curr. Med. Chem. 2020, 27, 5887–5917. [Google Scholar] [CrossRef]
- Svendsen, A.B.; Verpoorte, R. (Eds.) Chapter 4—Isolation of alkaloids. In Chromatography of Alkaloids; Elsevier: Amsterdam, The Netherlands, 1983; Volume 23A, pp. 51–58. ISBN 978-0-444-42145-6. [Google Scholar]
- Lee, K.T. Quantitative isolation of alkaloids from plant materials. Nature 1960, 188, 65–66. [Google Scholar] [CrossRef]
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K.M.; Latha, L.Y. Extraction, isolation and characterization of bioactive compounds from plants’ extracts. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Maldoni, B. Alkaloids: Isolation and purification. J. Chem. Educ. 1991, 68, 700–703. [Google Scholar] [CrossRef]
- Kornienko, A.; Evidente, A. Chemistry, biology, and medicinal potential of narciclasine and its congeners. Chem. Rev. 2008, 108, 1982–2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cahlíková, L.; Breiterová, K.; Opletal, L. Chemistry and biological activity of alkaloids from the genus Lycoris (Amaryllidaceae). Molecules 2020, 25, 4797. [Google Scholar] [CrossRef]
- Berkov, S.; Osorio, E.; Viladomat, F.; Bastida, J. Chapter Two—Chemodiversity, chemotaxonomy and chemoecology of Amaryllidaceae alkaloids. In The Alkaloids: Chemistry and Biology; Knölker, H.J., Ed.; Academic Press: Cambridge, MA, USA, 2020; Volume 83, pp. 113–185. ISBN 978-0-12-820981-3. [Google Scholar]
- Xu, T.; Chen, W.; Zhou, J.; Dai, J.; Li, Y.; Zhao, Y. Virtual screening for reactive natural products and their probable artifacts of solvolysis and oxidation. Biomolecules 2020, 10, 1486. [Google Scholar] [CrossRef]
- Jin, A.; Li, X.; Zhu, Y.Y.; Yu, H.Y.; Pi, H.F.; Zhang, P.; Ruan, H.L. Four new compounds from the bulbs of Lycoris aurea with neuroprotective effects against CoCl₂ and H₂O₂-induced SH-SY5Y cell injuries. Arch. Pharm. Res. 2014, 37, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Maltese, F.; van der Kooy, F.; Verpoorte, R. Solvent derived artifacts in natural products chemistry. Nat. Prod. Commun. 2009, 4, 447–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miana, G.A. Tertiary dihydroprotoberberine alkaloids of Berberis lycium. Phytochemistry 1973, 12, 1822–1823. [Google Scholar] [CrossRef]
- Kilgore, M.B.; Kutchan, T.M. The Amaryllidaceae alkaloids: Biosynthesis and methods for enzyme discovery. Phytochem. Rev. 2016, 15, 317–337. [Google Scholar] [CrossRef] [Green Version]
- Nair, J.J.; Wilhelm, A.; Bonnet, S.L.; van Staden, J. Antibacterial constituents of the plant family Amaryllidaceae. Bioorg. Med. Chem. Lett. 2017, 27, 4943–4951. [Google Scholar] [CrossRef]
- Al Mamun, A.; Maříková, J.; Hulcová, D.; Janoušek, J.; Šafratová, M.; Nováková, L.; Kučera, T.; Hrabinová, M.; Kuneš, J.; Korábečný, J.; et al. Amaryllidaceae alkaloids of belladine-type from Narcissus pseudonarcissus cv. carlton as new selective inhibitors of butyrylcholinesterase. Biomolecules 2020, 10, 800. [Google Scholar] [CrossRef] [PubMed]
- Fulton, B.; Benfield, P. Galanthamine. Drugs Aging 1996, 9, 60–65. [Google Scholar] [CrossRef]
- Raskind, M.A.; Peskind, E.R.; Wessel, T.; Yuan, W. Galantamine in AD: A 6-month randomized, placebo-controlled trial with a 6-month extension. The Galantamine USA-1 Study Group. Neurology 2000, 54, 2261–2268. [Google Scholar] [CrossRef]
- Kotra, L.P.; Park, J. 5.14—Therapeutic approaches to MS and other neurodegenerative diseases. In Comprehensive Medicinal Chemistry III; Chackalamannil, S., Rotella, D., Ward, S.E., Eds.; Elsevier: Oxford, UK, 2017; pp. 439–473. ISBN 978-0-12-803201-5. [Google Scholar]
- López, S.; Bastida, J.; Viladomat, F.; Codina, C. Acetylcholinesterase inhibitory activity of some Amaryllidaceae alkaloids and Narcissus extracts. Life Sci. 2002, 71, 2521–2529. [Google Scholar] [CrossRef]
- Šafratová, M.; Hošťálková, A.; Hulcová, D.; Breiterová, K.; Hrabcová, V.; Machado, M.; Fontinha, D.; Prudêncio, M.; Kuneš, J.; Chlebek, J.; et al. Alkaloids from Narcissus poeticus cv. Pink Parasol of various structural types and their biological activity. Arch. Pharm. Res. 2018, 41, 208–218. [Google Scholar] [CrossRef]
- Hulcová, D.; Maříková, J.; Korábečný, J.; Hošťálková, A.; Jun, D.; Kuneš, J.; Chlebek, J.; Opletal, L.; De Simone, A.; Nováková, L.; et al. Amaryllidaceae alkaloids from Narcissus pseudonarcissus L. cv. Dutch Master as potential drugs in treatment of Alzheimer’s disease. Phytochemistry 2019, 165, 112055. [Google Scholar] [CrossRef]
- Kohelová, E.; Maříková, J.; Korábečný, J.; Hulcová, D.; Kučera, T.; Jun, D.; Chlebek, J.; Jenčo, J.; Šafratová, M.; Hrabinová, M.; et al. Alkaloids of Zephyranthes citrina (Amaryllidaceae) and their implication to Alzheimer’s disease: Isolation, structural elucidation and biological activity. Bioorg. Chem. 2021, 107, 104567. [Google Scholar] [CrossRef]
- Maříková, J.; Mamun, A.A.; Shammari, L.A.; Korábečný, J.; Kučera, T.; Hulcová, D.; Kuneš, J.; Malaník, M.; Vašková, M.; Kohelová, E.; et al. Structure elucidation and cholinesterase inhibition activity of two new minor Amaryllidaceae alkaloids. Molecules 2021, 26, 1279. [Google Scholar] [CrossRef]
- Sibanyoni, M.N.; Chaudhary, S.K.; Chen, W.; Adhami, H.-R.; Combrinck, S.; Maharaj, V.; Schuster, D.; Viljoen, A. Isolation, in vitro evaluation and molecular docking of acetylcholinesterase inhibitors from South African Amaryllidaceae. Fitoterapia 2020, 146, 104650. [Google Scholar] [CrossRef] [PubMed]
- Vaněčková, N.; Hošťálková, A.; Šafratová, M.; Kuneš, J.; Hulcová, D.; Hrabinová, M.; Doskočil, I.; Štěpánková, Š.; Opletal, L.; Nováková, L.; et al. Isolation of Amaryllidaceae alkaloids from Nerine bowdenii W. Watson and their biological activities. RSC Adv. 2016, 6, 80114–80120. [Google Scholar] [CrossRef]
- Elgorashi, E.E.; Stafford, G.I.; van Staden, J. Acetylcholinesterase enzyme inhibitory effects of Amaryllidaceae alkaloids. Planta Med. 2004, 70, 260–262. [Google Scholar] [CrossRef]
- Nair, J.J.; Aremu, A.O.; van Staden, J. Isolation of narciprimine from Cyrtanthus contractus (Amaryllidaceae) and evaluation of its acetylcholinesterase inhibitory activity. J. Ethnopharmacol. 2011, 137, 1102–1106. [Google Scholar] [CrossRef] [PubMed]
- Orhan, I.E.; Senol Deniz, F.S.; Eren, G.; Sener, B. Molecular approach to promising cholinesterase inhibitory effect of several Amaryllidaceae alkaloids: Further re-investigation. S. Afr. J. Bot. 2021, 136, 175–181. [Google Scholar] [CrossRef]
- Zhu, Y.Y.; Li, X.; Yu, H.Y.; Xiong, Y.F.; Zhang, P.; Pi, H.F.; Ruan, H.L. Alkaloids from the bulbs of Lycoris longituba and their neuroprotective and acetylcholinesterase inhibitory activities. Arch. Pharm. Res. 2015, 38, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Cahlíková, L.; Hrabinová, M.; Kulhánková, A.; Benešová, N.; Chlebek, J.; Jun, D.; Novák, Z.; Macáková, K.; Kuneš, J.; Kuča, K.; et al. Alkaloids from Chlidanthus fragrans and their acetylcholinesterase, butyrylcholinesterase and prolyl oligopeptidase activities. Nat. Prod. Commun. 2013, 8, 1541–1544. [Google Scholar] [CrossRef] [Green Version]
- Hulcová, D.; Breiterová, K.; Siatka, T.; Klímová, K.; Davani, L.; Šafratová, M.; Hošťálková, A.; De Simone, A.; Andrisano, V.; Cahlíková, L. Amaryllidaceae alkaloids as potential glycogen synthase kinase-3β inhibitors. Molecules 2018, 23, 719. [Google Scholar] [CrossRef] [Green Version]
- Al Shammari, L.; Hulcová, D.; Maříková, J.; Kučera, T.; Šafratová, M.; Nováková, L.; Schmidt, M.; Pulkrábková, L.; Janoušek, J.; Soukup, O.; et al. Amaryllidaceae alkaloids from Hippeastrum X hybridum cv. Ferrari, and preparation of vittatine derivatives as potential ligands for Alzheimer’s disease. S. Afr. J. Bot. 2020, 137–146. [Google Scholar] [CrossRef]
- Šafratová, M.; Novák, Z.; Kulhánková, A.; Kuneš, J.; Hrabinová, M.; Jun, D.; Macákova, K.; Opletal, L.; Cahlíková, L. Revised NMR data for 9-O-demethylgalanthine: An alkaloid from Zephyranthes robusta (Amaryllidaceae) and its biological activity. Nat. Prod. Commun. 2014, 9, 787–788. [Google Scholar] [CrossRef] [Green Version]
- Tarrago, T.; Kichik, N.; Seguí, J.; Giralt, E. The natural product berberine is a human prolyl oligopeptidase inhibitor. ChemMedChem 2007, 2, 354–359. [Google Scholar] [CrossRef]
- Muehlbacher, M.; Spitzer, G.M.; Liedl, K.R.; Kornhuber, J. Qualitative prediction of blood-brain barrier permeability on a large and refined dataset. J. Comput. Aided Mol. Des. 2011, 25, 1095–1106. [Google Scholar] [CrossRef] [Green Version]
- Di, L.; Kerns, E.H.; Fan, K.; McConnell, O.J.; Carter, G.T. High throughput artificial membrane permeability assay for blood–brain barrier. Eur. J. Med. Chem. 2003, 38, 223–232. [Google Scholar] [CrossRef]
- Zenaro, E.; Piacentino, G.; Constantin, G. The blood-brain barrier in Alzheimer’s disease. Neurobiol. Dis. 2017, 107, 41–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carmona-Viglianco, F.; Zaragoza-Puchol, D.; Parravicini, O.; Garro, A.; Enriz, R.D.; Feresin, G.E.; Kurina-Sanz, M.; Orden, A.A. Synthesis, biological evaluation and molecular modeling studies of substituted N-benzyl-2-phenylethanamines as cholinesterase inhibitors. N. J. Chem. 2020, 44, 9466–9476. [Google Scholar] [CrossRef]
- Mamun, A.A.; Pidaný, F.; Hulcová, D.; Maříková, J.; Kučera, T.; Schmidt, M.; Catapano, M.C.; Hrabinová, M.; Jun, D.; Múčková, L.; et al. Amaryllidaceae alkaloids of norbelladine-type as inspiration for development of highly selective butyrylcholinesterase inhibitors: Synthesis, biological activity evaluation, and docking studies. Int. J. Mol. Sci. 2021, 22, 8308. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.M.; Zhu, Y.Y.; Li, H.R.; Yu, H.Y.; Zhang, P.; Pi, H.F.; Ruan, H.L. Two new alkaloids from the bulbs of Lycoris sprengeri. J. Asian Nat. Prod. Res. 2014, 16, 192–199. [Google Scholar] [CrossRef]
- Trujillo-Chacón, L.M.; Alarcón-Enos, J.E.; Céspedes-Acuña, C.L.; Bustamante, L.; Baeza, M.; López, M.G.; Fernández-Mendívil, C.; Cabezas, F.; Pastene-Navarrete, E.R. Neuroprotective activity of isoquinoline alkaloids from of Chilean Amaryllidaceae plants against oxidative stress-induced cytotoxicity on human neuroblastoma SH-SY5Y cells and mouse hippocampal slice culture. Food Chem. Toxicol. 2019, 132, 110665. [Google Scholar] [CrossRef]
- Cortes, N.; Posada-Duque, R.A.; Alvarez, R.; Alzate, F.; Berkov, S.; Cardona-Gómez, G.P.; Osorio, E. Neuroprotective activity and acetylcholinesterase inhibition of five Amaryllidaceae species: A comparative study. Life Sci. 2015, 122, 42–50. [Google Scholar] [CrossRef]
- Ibrakaw, A.S.; Omoruyi, S.I.; Ekpo, O.E.; Hussein, A.A. Neuroprotective activities of Boophone haemanthoides (Amaryllidaceae) extract and its chemical constituents. Molecules 2020, 25, 5376. [Google Scholar] [CrossRef]
- Yang, E.J.; Park, G.H.; Song, K.S. Neuroprotective effects of liquiritigenin isolated from licorice roots on glutamate-induced apoptosis in hippocampal neuronal cells. Neurotoxicology 2013, 39, 114–123. [Google Scholar] [CrossRef]
- Niedzielska, E.; Smaga, I.; Gawlik, M.; Moniczewski, A.; Stankowicz, P.; Pera, J.; Filip, M. Oxidative stress in neurodegenerative diseases. Mol. Neurobiol. 2016, 53, 4094–4125. [Google Scholar] [CrossRef] [Green Version]
- Traykova, M.; Traykov, T.; Hadjimitova, V.; Krikorian, K.; Bojadgieva, N. Antioxidant properties of galantamine hydrobromide. Z. Naturforsch. C J. Biosci. 2003, 58, 361–365. [Google Scholar] [CrossRef]
- Bakhtiari, M.; Panahi, Y.; Ameli, J.; Darvishi, B. Protective effects of flavonoids against Alzheimer’s disease-related neural dysfunctions. Biomed. Pharmacother. 2017, 93, 218–229. [Google Scholar] [CrossRef]
- Cortes, N.; Castañeda, C.; Osorio, E.H.; Cardona-Gomez, G.P.; Osorio, E. Amaryllidaceae alkaloids as agents with protective effects against oxidative neural cell injury. Life Sci. 2018, 203, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Hoang, T.H.X.; Ho, D.V.; Van Phan, K.; Le, Q.V.; Raal, A.; Nguyen, H.T. Effects of Hippeastrum reticulatum on memory, spatial learning and object recognition in a scopolamine-induced animal model of Alzheimer’s disease. Pharm. Biol. 2020, 58, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Cortes, N.; Sabogal-Guaqueta, A.M.; Cardona-Gomez, G.P.; Osorio, E. Neuroprotection and improvement of the histopathological and behavioral impairments in a murine Alzheimer’s model treated with Zephyranthes carinata alkaloids. Biomed. Pharmacother. 2019, 110, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Bisset, N.G. Plants as a source of isoquinoline alkaloids. In The Chemistry and Biology of Isoquinoline Alkaloids. Proceedings in Life Sciences; Phillipson, J.D., Roberts, M.F., Zenk, M.H., Eds.; Springer: Berlin/Heidelberg, Germany, 1985; pp. 1–22. ISBN 978-3-642-70130-6. [Google Scholar]
- Kukula-Koch, W.A.; Widelski, J. Chapter 9—Alkaloids. In Pharmacognosy: Fundamentals, Applications and Strategies; Badal, S., Delgoda, R., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 163–198. ISBN 978-0-12-802104-0. [Google Scholar]
- Ingkaninan, K.; Phengpa, P.; Yuenyongsawad, S.; Khorana, N. Acetylcholinesterase inhibitors from Stephania venosa tuber. J. Pharm. Pharmacol. 2006, 58, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Hung, T.M.; Nguyen, H.D.; Kim, J.C.; Jang, H.S.; Ryoo, S.W.; Lee, J.H.; Choi, J.S.; Bae, K.H.; Min, B.S. Alkaloids from roots of Stephania rotunda and their cholinesterase inhibitory activity. Planta Med. 2010, 76, 1762–1764. [Google Scholar] [CrossRef] [Green Version]
- Huang, Q.Q.; Bi, J.L.; Sun, Q.Y.; Yang, F.M.; Wang, Y.H.; Tang, G.H.; Zhao, F.W.; Wang, H.; Xu, J.J.; Kennelly, E.J.; et al. Bioactive isoquinoline alkaloids from Corydalis saxicola. Planta Med. 2012, 78, 65–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hošťálková, A.; Maříková, J.; Opletal, L.; Korábečná, J.; Hulcová, D.; Kuneš, J.; Nováková, L.; Perez, D.I.; Jun, D.; Kučera, T.; et al. Isoquinoline alkaloids from Berberis vulgaris as potential lead compounds for the treatment of Alzheimer’s disease. J. Nat. Prod. 2019, 82, 239–248. [Google Scholar] [CrossRef]
- Nachon, F.; Carletti, E.; Ronco, C.; Trovaslet, M.; Nicolet, Y.; Jean, L.; Renard, P.Y. Crystal structures of human cholinesterases in complex with huprine W and tacrine: Elements of specificity for anti-Alzheimer’s drugs targeting acetyl- and butyryl-cholinesterase. Biochem. J. 2013, 453, 393–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, C.; Su, P.; Lv, C.; Guo, L.; Cao, G.; Qin, C.; Zhang, W. Berberine alleviates amyloid β-induced mitochondrial dysfunction and synaptic loss. Oxid. Med. Cell. Longev. 2019, 2019, 7593608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wai, A.; Yeung, A.W.K.; Erdogan Orhan, I.; Aggarwal, B.; Battino, M.; Belwal, T.; Bishayee, A.; Daglia, M.; Devkota, H.; El-Demerdash, A.; et al. Berberine, a popular dietary supplement for human and animal health: Quantitative research literature analysis—A review. Anim. Sci. Pap. Rep. 2020, 38, 5–19. [Google Scholar]
- Chen, Y.; Chen, Y.; Liang, Y.; Chen, H.; Ji, X.; Huang, M. Berberine mitigates cognitive decline in an Alzheimer’s Disease Mouse Model by targeting both tau hyperphosphorylation and autophagic clearance. Biomed. Pharmacother. 2020, 121, 109670. [Google Scholar] [CrossRef]
- Dahua, F.; Liping, L.; Zhengzhi, W.; Meiqun, C. Combating neurodegenerative diseases with the plant alkaloid berberine: Molecular mechanisms and therapeutic potential. Curr. Neuropharmacol. 2019, 17, 563–579. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, C.; Yang, W. Role of berberine in Alzheimer’s disease. Neuropsychiatr. Dis. Treat. 2016, 12, 2509–2520. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Huang, M.; Jiang, X.; Liu, Q.; Chang, X.; Guo, Y. The neuroprotective effects of Berberine against amyloid β-protein-induced apoptosis in primary cultured hippocampal neurons via mitochondria-related caspase pathway. Neurosci. Lett. 2017, 655, 46–53. [Google Scholar] [CrossRef]
- Huang, M.; Jiang, X.; Liang, Y.; Liu, Q.; Chen, S.; Guo, Y. Berberine improves cognitive impairment by promoting autophagic clearance and inhibiting production of β-amyloid in APP/tau/PS1 mouse model of Alzheimer’s disease. Exp. Gerontol. 2017, 91, 25–33. [Google Scholar] [CrossRef]
- Ahmed, T.; Gilani, A.H.; Abdollahi, M.; Daglia, M.; Nabavi, S.F.; Nabavi, S.M. Berberine and neurodegeneration: A review of literature. Pharmacol. Rep. 2015, 67, 970–979. [Google Scholar] [CrossRef]
- Ji, H.F.; Shen, L. Berberine: A potential multipotent natural product to combat Alzheimer’s disease. Molecules 2011, 16, 6732–6740. [Google Scholar] [CrossRef]
- Long, J.; Song, J.; Zhong, L.; Liao, Y.; Liu, L.; Li, X. Palmatine: A review of its pharmacology, toxicity and pharmacokinetics. Biochimie 2019, 162, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, C.; Xu, F.C.; Quesheng; Zhang, Q.Y.; Tu, P.F.; Liang, H. Cholinesterase inhibitory isoquinoline alkaloids from Corydalis mucronifera. Phytochemistry 2019, 159, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, Q.Y.; Tu, P.F.; Xu, F.C.; Liang, H. Mucroniferanines A–G, isoquinoline alkaloids from Corydalis mucronifera. J. Nat. Prod. 2018, 81, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Plazas, E.; Hagenow, S.; Avila Murillo, M.; Stark, H.; Cuca, L.E. Isoquinoline alkaloids from the roots of Zanthoxylum rigidum as multi-target inhibitors of cholinesterase, monoamine oxidase A and Aβ1-42 aggregation. Bioorg. Chem. 2020, 98, 103722. [Google Scholar] [CrossRef]
- Cahlíková, L.; Opletal, L.; Kurfürst, M.; Macáková, K.; Kulhánková, A.; Hošťálková, A. Acetylcholinesterase and butyrylcholinesterase inhibitory compounds from Chelidonium majus (Papaveraceae). Nat. Prod. Commun. 2010, 5, 1751–1754. [Google Scholar] [CrossRef] [Green Version]
- Baek, S.C.; Ryu, H.W.; Kang, M.G.; Lee, H.; Park, D.; Cho, M.L.; Oh, S.R.; Kim, H. Selective inhibition of monoamine oxidase A by chelerythrine, an isoquinoline alkaloid. Bioorg. Med. Chem. Lett. 2018, 28, 2403–2407. [Google Scholar] [CrossRef]
- Brunhofer, G.; Fallarero, A.; Karlsson, D.; Batista-Gonzalez, A.; Shinde, P.; Gopi Mohan, C.; Vuorela, P. Exploration of natural compounds as sources of new bifunctional scaffolds targeting cholinesterases and beta amyloid aggregation: The case of chelerythrine. Bioorg. Med. Chem. 2012, 20, 6669–6679. [Google Scholar] [CrossRef]
- Chlebek, J.; Simone, A.; Hošťálková, A.; Opletal, L.; Perez, C.; Perez, D.; Havlíková, L.; Cahlíková, L.; Andrisano, V. Application of BACE1 immobilized enzyme reactor for the characterization of multifunctional alkaloids from Corydalis cava (Fumariaceae) as Alzheimer’s disease targets. Fitoterapia 2016, 109, 241–247. [Google Scholar] [CrossRef]
- Chlebek, J.; Macáková, K.; Cahlíková, L.; Kurfürst, M.; Kuneš, J.; Opletal, L. Acetylcholinesterase and butyrylcholinesterase inhibitory compounds from Corydalis cava (Fumariaceae). Nat. Prod. Commun. 2011, 6, 607–610. [Google Scholar] [CrossRef] [Green Version]
- Siatka, T.; Adamcová, M.; Opletal, L.; Cahlíková, L.; Jun, D.; Hrabinová, M.; Kuneš, J.; Chlebek, J. Cholinesterase and prolyl oligopeptidase inhibitory activities of alkaloids from Argemone platyceras (Papaveraceae). Molecules 2017, 22, 1181. [Google Scholar] [CrossRef] [PubMed]
- Cahlíková, L.; Macáková, K.; Kuneš, J.; Kurfürst, M.; Opletal, L.; Cvačka, J.; Chlebek, J.; Blunden, G. Acetylcholinesterase and butyrylcholinesterase inhibitory compounds from Eschscholzia californica (Papaveraceae). Nat. Prod. Commun. 2010, 5, 1035–1038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hošťálková, A.; Opletal, L.; Kuneš, J.; Novák, Z.; Hrabinová, M.; Chlebek, J.; Čegan, L.; Cahlíková, L. Alkaloids from Peumus boldus and their acetylcholinesterase, butyrylcholinesterase and prolyl oligopeptidase inhibition activity. Nat. Prod. Commun. 2015, 10, 577–580. [Google Scholar] [CrossRef] [Green Version]
- Chlebek, J.; Novák, Z.; Kassemová, D.; Šafratová, M.; Kostelník, J.; Malý, L.; Ločárek, M.; Opletal, L.; Hošťálková, A.; Hrabinová, M.; et al. Isoquinoline alkaloids from Fumaria officinalis L. and their biological activities related to Alzheimer’s disease. Chem. Biodivers. 2016, 13, 91–99. [Google Scholar] [CrossRef]
- Cahlíková, L.; Hulová, L.; Hrabinová, M.; Chlebek, J.; Hošťálková, A.; Adamcová, M.; Šafratová, M.; Jun, D.; Opletal, L.; Ločárek, M.; et al. Isoquinoline alkaloids as prolyl oligopeptidase inhibitors. Fitoterapia 2015, 103, 192–196. [Google Scholar] [CrossRef]
- Toušek, J.; Dommisse, R.; Dostál, J.; Žák, Z.; Pieters, L.; Marek, R. Configurations and conformations of sanguinarine and chelerythrine free bases stereoisomers. J. Mol. Struct. 2002, 613, 103–113. [Google Scholar] [CrossRef]
- Marasco, D.; Vicidomini, C.; Krupa, P.; Cioffi, F.; Huy, P.D.Q.; Li, M.S.; Florio, D.; Broersen, K.; de Pandis, M.F.; Roviello, G.N. Plant isoquinoline alkaloids as potential neurodrugs: A comparative study of the effects of benzo[c]phenanthridine and berberine-based compounds on β-amyloid aggregation. Chem. Biol. Interact. 2021, 334, 109300. [Google Scholar] [CrossRef]
- Sugimoto, Y.; Yoshida, A.; Uchida, S.; Inanaga, S.; Yamada, Y. Dauricine production in cultured roots of Menispermum dauricum. Phytochemistry 1994, 36, 679–683. [Google Scholar] [CrossRef]
- Liu, P.; Chen, X.; Zhou, H.; Wang, L.; Zhang, Z.; Ren, X.; Zhu, F.; Guo, Y.; Huang, X.; Liu, J.; et al. The isoquinoline alkaloid dauricine targets multiple molecular pathways to ameliorate Alzheimer-like pathological changes in vitro. Oxid. Med. Cell. Longev. 2018, 2018, 2025914. [Google Scholar] [CrossRef] [Green Version]
- Thinakaran, G.; Teplow, D.B.; Siman, R.; Greenberg, B.; Sisodia, S.S. Metabolism of the “Swedish” amyloid precursor protein variant in neuro2a (N2a) cells. Evidence that cleavage at the “beta-secretase” site occurs in the golgi apparatus. J. Biol. Chem. 1996, 271, 9390–9397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Liu, J.; Wang, T.; Xiao, H.; Yin, C. Effects of tetrandrine on cAMP and cGMP levels in rabbit corpus cavernosum in vitro. Nat. Prod. Res. 2010, 24, 1095–1103. [Google Scholar] [CrossRef] [PubMed]
- He, F.Q.; Qiu, B.Y.; Zhang, X.H.; Li, T.K.; Xie, Q.; Cui, D.J.; Huang, X.L.; Gan, H.T. Tetrandrine attenuates spatial memory impairment and hippocampal neuroinflammation via inhibiting NF-κB activation in a rat model of Alzheimer’s disease induced by amyloid-β(1-42). Brain Res. 2011, 1384, 89–96. [Google Scholar] [CrossRef]
- Fajardo, V.; Araya, M.; Cuadra, P.; Oyarzun, A.; Gallardo, A.; Cueto, M.; Diaz-Marrero, A.R.; Darias, J.; Villarroel, L.; Álvarez, C.; et al. Pronuciferine N-oxide, a proaporphine N-oxide alkaloid from Berberis coletioides. J. Nat. Prod. 2009, 72, 1355–1356. [Google Scholar] [CrossRef] [PubMed]
- Bayazeid, O.; Nemutlu, E.; Eylem, C.C.; Yalçın, F.N. Neuroactivity of naturally occurring proaporphine alkaloid, pronuciferine. J. Biochem. Mol. Toxicol. 2020, 34, e22601. [Google Scholar] [CrossRef] [PubMed]
- Kohelová, E.; Peřinová, R.; Maafi, N.; Korábečný, J.; Hulcová, D.; Maříková, J.; Kučera, T.; Martínez González, L.; Hrabinová, M.; Vorčáková, K.; et al. Derivatives of the β-crinane Amaryllidaceae alkaloid haemanthamine as multi-target directed ligands for Alzheimer’s disease. Molecules 2019, 24, 1307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peřinová, R.; Maafi, N.; Korábečný, J.; Kohelová, E.; De Simone, A.; Al Mamun, A.; Hulcová, D.; Marková, J.; Kučera, T.; Jun, D.; et al. Functionalized aromatic esters of the Amaryllidaceae alkaloid haemanthamine and their in vitro and in silico biological activity connected to Alzheimer’s disease. Bioorg. Chem. 2020, 100, 103928. [Google Scholar] [CrossRef]
- Maříková, J.; Ritomská, A.; Korábečný, J.; Peřinová, R.; Al Mamun, A.; Kučera, T.; Kohelová, E.; Hulcová, D.; Kobrlová, T.; Kuneš, J.; et al. Aromatic esters of the crinane Amaryllidaceae alkaloid ambelline as selective inhibitors of butyrylcholinesterase. J. Nat. Prod. 2020, 83, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Sobolová, K.; Hrabinová, M.; Hepnarová, V.; Kučera, T.; Kobrlová, T.; Benková, M.; Janočková, J.; Doležal, R.; Prchal, L.; Benek, O.; et al. Discovery of novel berberine derivatives with balanced cholinesterase and prolyl oligopeptidase inhibition profile. Eur. J. Med. Chem. 2020, 203, 112593. [Google Scholar] [CrossRef]
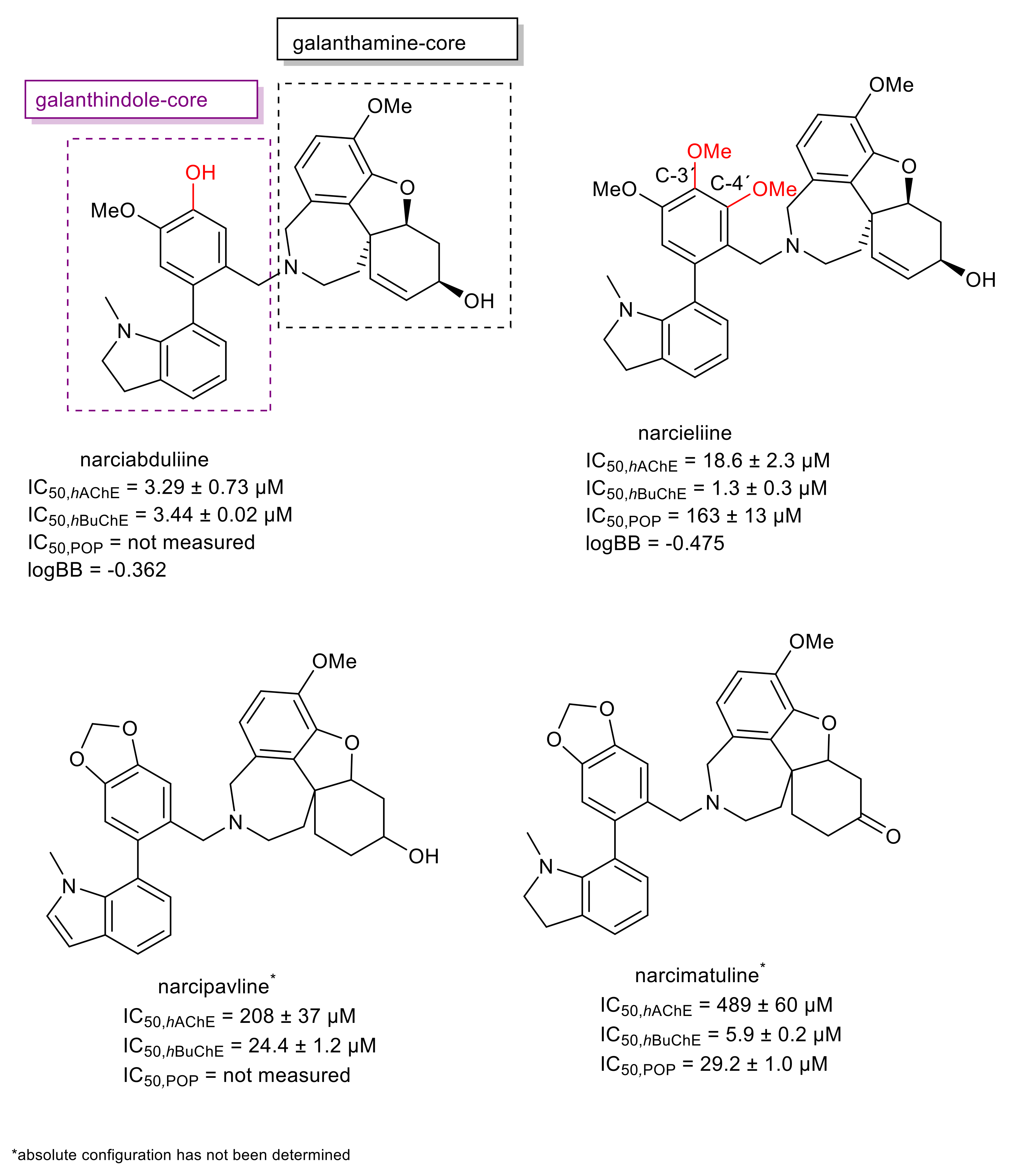
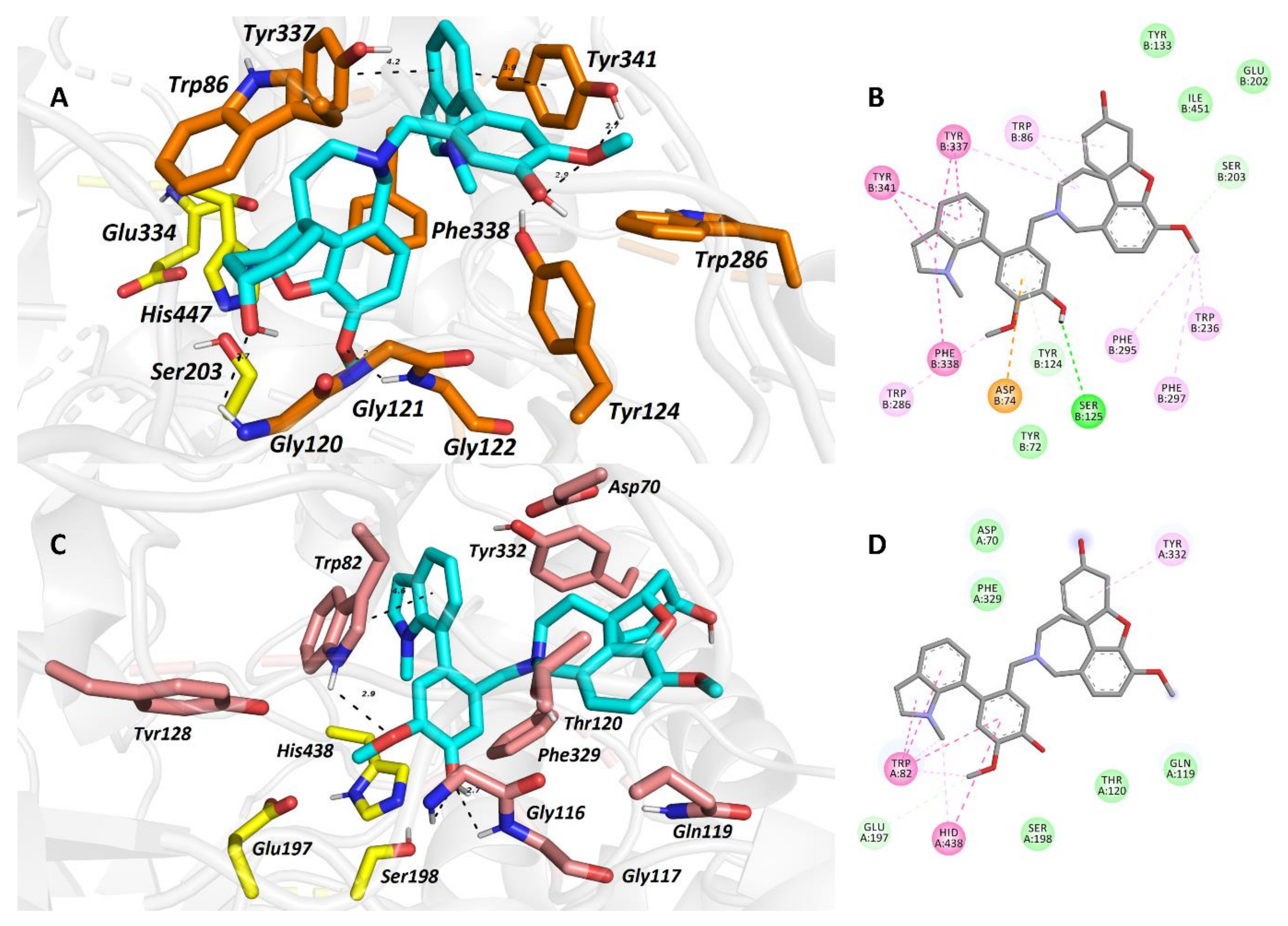
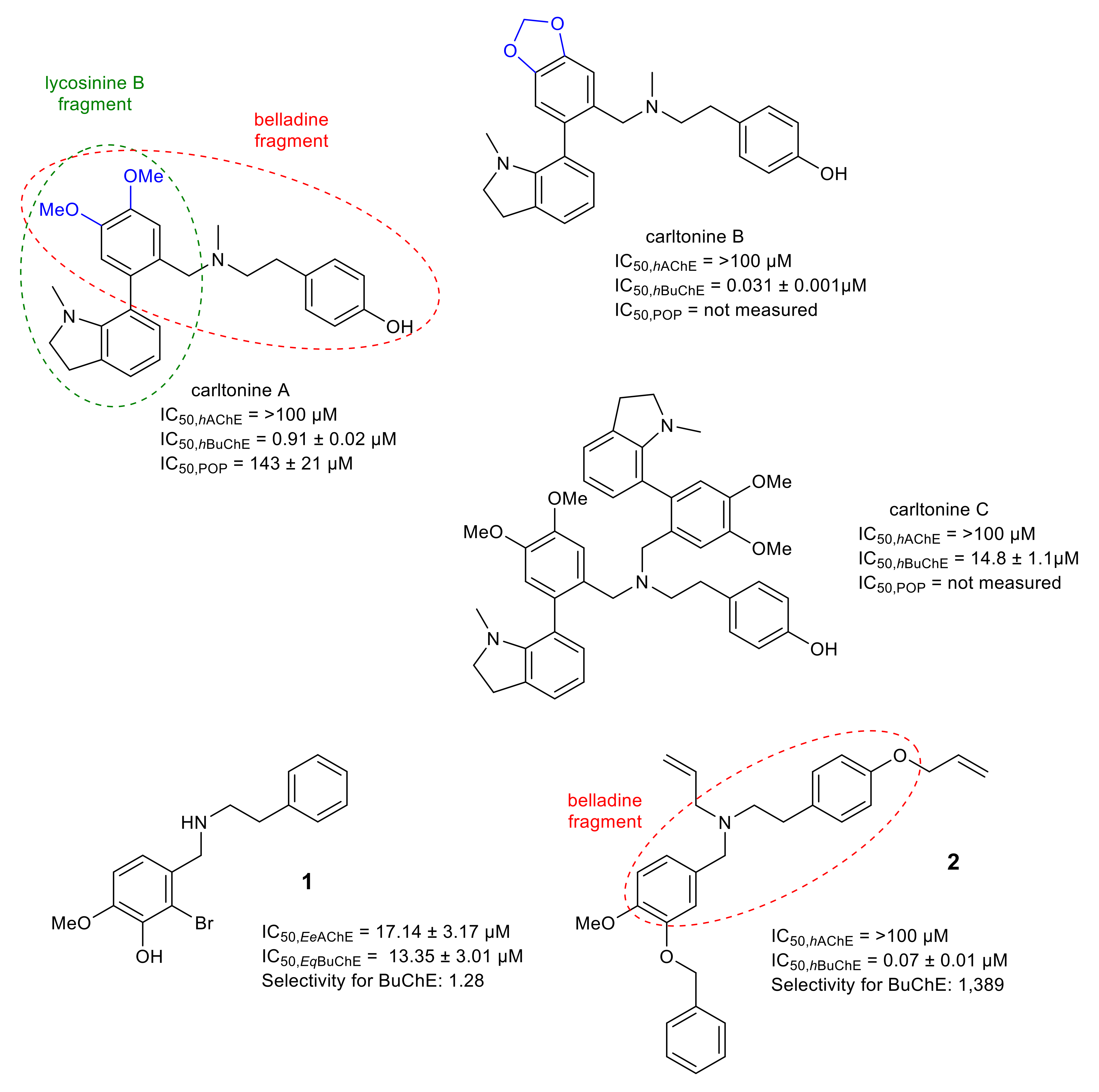
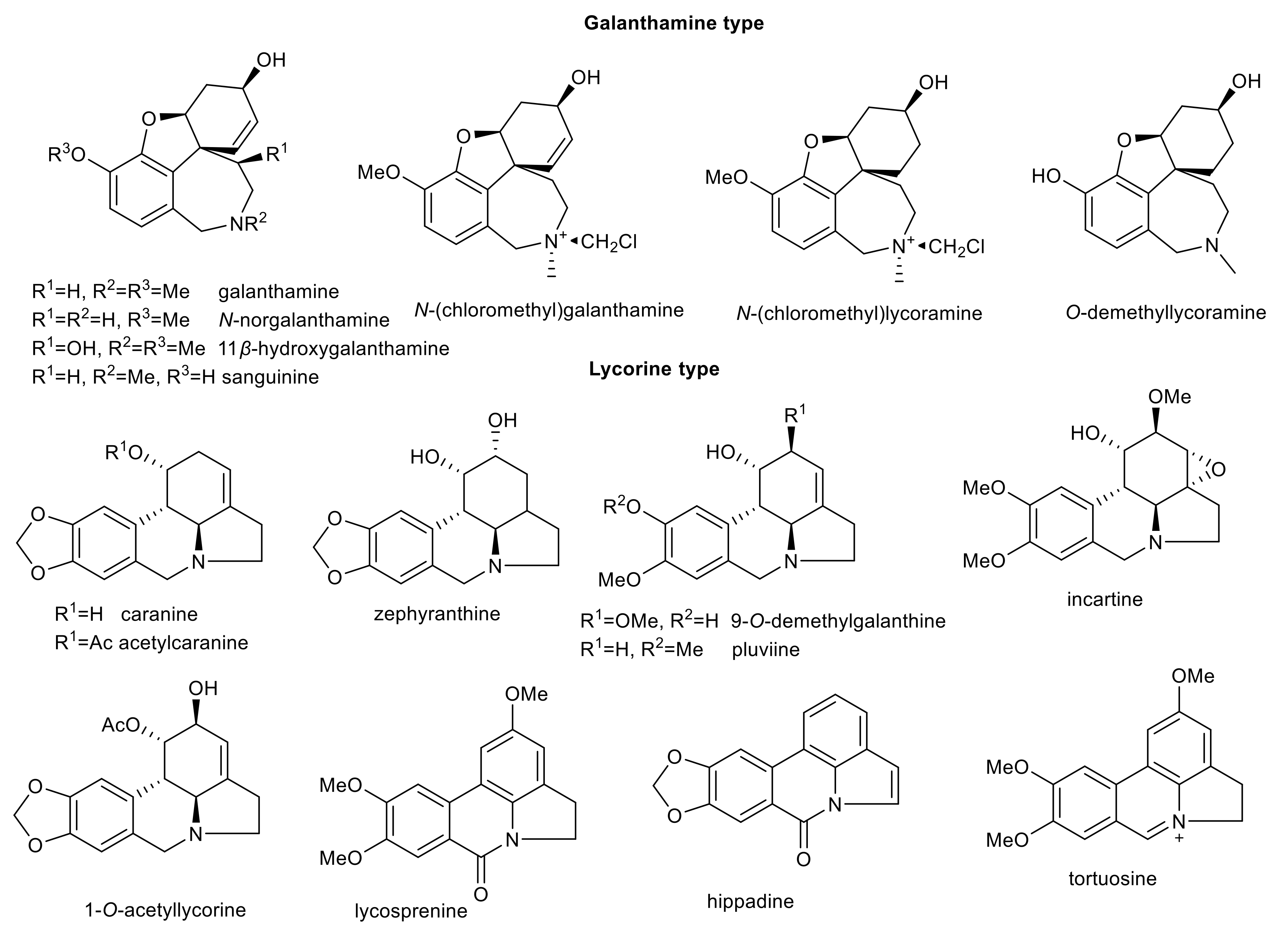

| Alkaloid | Structural Type | Source | IC50 AChE (μM) | IC50 BuChE (μM) | IC50 POP (μM) | GSK-3β (μM) | Ref. |
|---|---|---|---|---|---|---|---|
| N-norgalanthamine | galanthamine | Pancratium maritimum Lycoris longituba | 2.76 ± 0.65 a 8.85 ± 0.58 b | 76.4 ± 3.7 c | n.s. | n.s. | [69,70] |
| 11β-hydroxygalanthamine | galanthamine | Lycoris longituba | 3.04 ± 0.61 a | n.s. | n.s. | n.s. | [70] |
| narciabduliine | narcikachnine | Narcissus pseudonarcissus cv. Carlton | 3.29 ± 0.73 b | 3.44 ± 0.02 c | n.s. | n.s. | [64] |
| N-methylcrinasiadine | narciclassine | Lycoris longituba | 4.23 ± 1.13 a | n.s. | n.s. | n.s. | [70] |
| N-(chloromethyl)galanthamine | galanthamine | Lycoris longituba | 5.55 ± 0.6 a | n.s. | n.s. | n.s. | [70] |
| O-demethyllycoramine | galanthamine | Lycoris longituba | 8.13 ± 1.49 a | n.s. | n.s. | n.s. | [70] |
| deoxypretazzetine | tazettine | Lycoris longituba | 8.44 ± 0.83 a | n.s. | n.s. | n.s. | [70] |
| acetylcaranine | lycorine | Amaryllis belladona | 11.7 ± 0.7 a >30 b | n.s. | >200 | n.s. | [65,66] |
| narcieliine | narcikachnine | Zephyranthes citrina | 18.7 ± 2.3 b | 1.34 ± 0.31 c | 163 ± 13 | n.s. | [63] |
| undulatine | crinine | Chlidanthus fragrans Nerine bowdenii | 23.5 ± 1.2 b | >100 c | >200 | 43.3 ± 4.0 e | [66,71,72] |
| 8-hydroxy-9-methoxycrinine | crinine | Pancratium maritimum | 25.3 ± 1.8 a | >365 d | n.s. | n.s. | [69] |
| N-(chloromethyl)lycoramine | galanthamine | Lycoris longituba | 25.76 ± 1.09 a | n.s. | n.s. | n.s. | [70] |
| powelline | crinine | Nerine bowdenii | 29.1 ± 1.6 b | >30 c | >200 | n.s. | [66] |
| carltonine B | belladine | Narcissus pseudonarcissus cv. Carlton | >30 b | 0.031 ± 0.001 c | n.s. | n.s. | [56] |
| carltonine A | belladine | Narcissus pseudonarcissus cv. Carlton | >30 b | 0.913 ± 0.020 c | 143 ± 12 | n.s. | [56] |
| narcimatuline | narcikachnine | Narcissus pseudonarcissus cv. Dutch Master | >30 b | 5.9 ± 0.2 c | 29.2 ± 1.0 | 20.7 ± 2.4 | [62] |
| carltonine C | belladine | Narcissus pseudonarcissus cv. Carlton | >30 b | 14.8 ± 1.1 c | n.s. | n.s. | [56] |
| narcipavline | narcikachnine | Narcissus poeticus cv. Pink Parasol | >30 b | 24.4 ± 1.2 c | n.s. | n.s. | [61] |
| zephyranthine | lycorine | Hippeastrum hybridum cv. Ferrari | >30 b | >30 c | 142 ± 10 | n.s. | [73] |
| 9-O-demethylgalanthine | lycorine | Zephyranthes robusta | >30 b | >30 c | 150 ± 20 | 50.9 ± 8.9 e | [74] |
| homolycorine | homolycorine | Narcissus poeticus cv. Pink Parasol | >30 b | >30 c | 173 ± 41 | 54.4 ± 8.9 e | [61] |
| nerinine | homolycorine | Zephyranthes citrina | >30 b | >30 c | 190 ± 10 | n.s. | [63] |
| masonine | homolycorine | Narcissus poeticus cv. Pink Parasol | >30 b | >30 c | >200 | 27.81 ± 0.05 | [61,72] |
| caranine | lycorine | Nerine bowdenii | >30 b | >30 c | >200 | 30.75 ± 0.04 | [66,72] |
| 9-O-demethylhomolycorine | homolycorine | Narcissus poeticus cv. Pink Parasol | n.s. | n.s. | n.s. | 30.01 ± 0.04 | [72] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cahlíková, L.; Vrabec, R.; Pidaný, F.; Peřinová, R.; Maafi, N.; Mamun, A.A.; Ritomská, A.; Wijaya, V.; Blunden, G. Recent Progress on Biological Activity of Amaryllidaceae and Further Isoquinoline Alkaloids in Connection with Alzheimer’s Disease. Molecules 2021, 26, 5240. https://doi.org/10.3390/molecules26175240
Cahlíková L, Vrabec R, Pidaný F, Peřinová R, Maafi N, Mamun AA, Ritomská A, Wijaya V, Blunden G. Recent Progress on Biological Activity of Amaryllidaceae and Further Isoquinoline Alkaloids in Connection with Alzheimer’s Disease. Molecules. 2021; 26(17):5240. https://doi.org/10.3390/molecules26175240
Chicago/Turabian StyleCahlíková, Lucie, Rudolf Vrabec, Filip Pidaný, Rozálie Peřinová, Negar Maafi, Abdullah Al Mamun, Aneta Ritomská, Viriyanata Wijaya, and Gerald Blunden. 2021. "Recent Progress on Biological Activity of Amaryllidaceae and Further Isoquinoline Alkaloids in Connection with Alzheimer’s Disease" Molecules 26, no. 17: 5240. https://doi.org/10.3390/molecules26175240
APA StyleCahlíková, L., Vrabec, R., Pidaný, F., Peřinová, R., Maafi, N., Mamun, A. A., Ritomská, A., Wijaya, V., & Blunden, G. (2021). Recent Progress on Biological Activity of Amaryllidaceae and Further Isoquinoline Alkaloids in Connection with Alzheimer’s Disease. Molecules, 26(17), 5240. https://doi.org/10.3390/molecules26175240









