Nickel Complexes in C‒P Bond Formation
Abstract
1. Introduction
2. Synthesis of Tricoordinated Organophosphorus Compounds
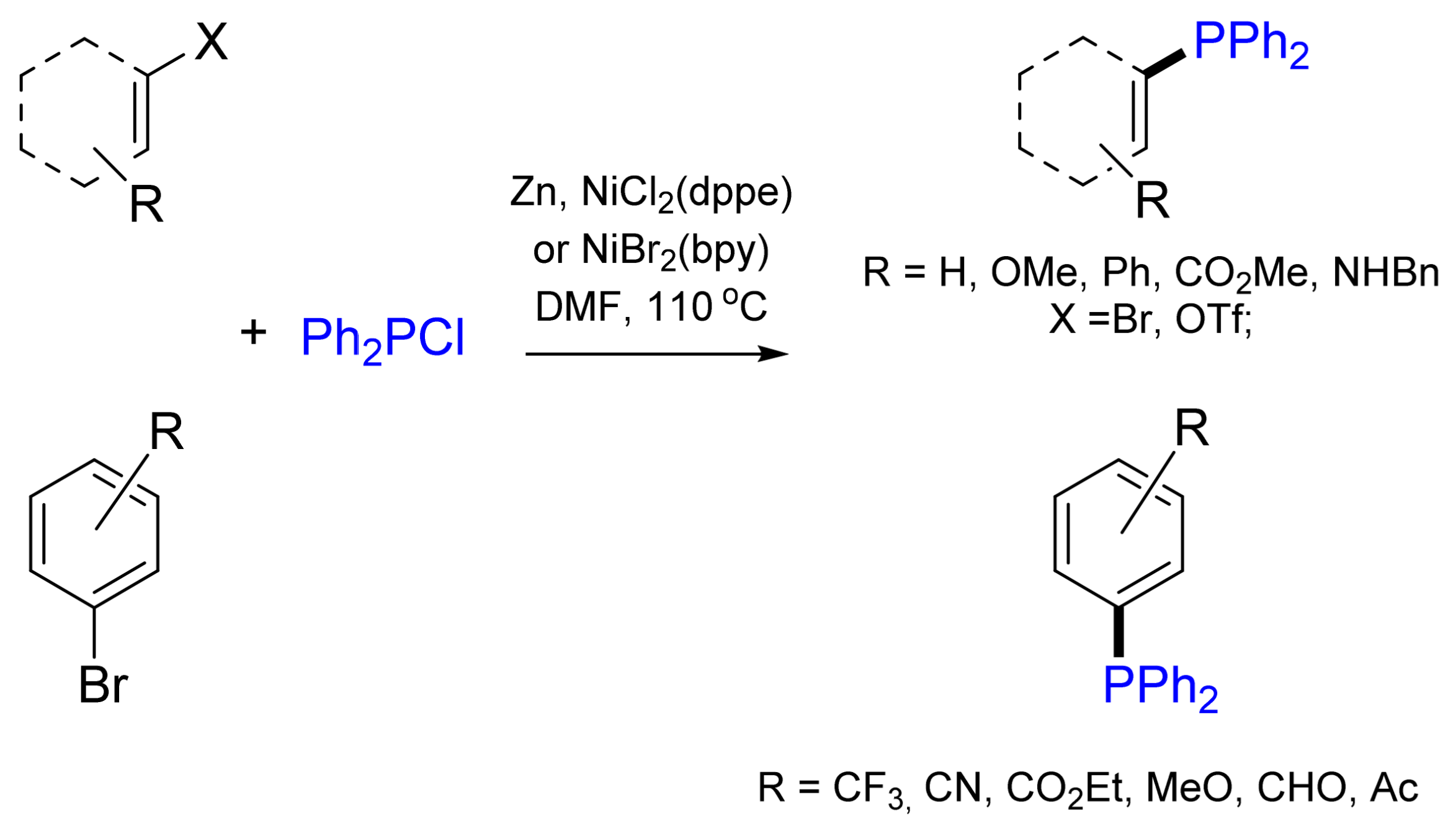
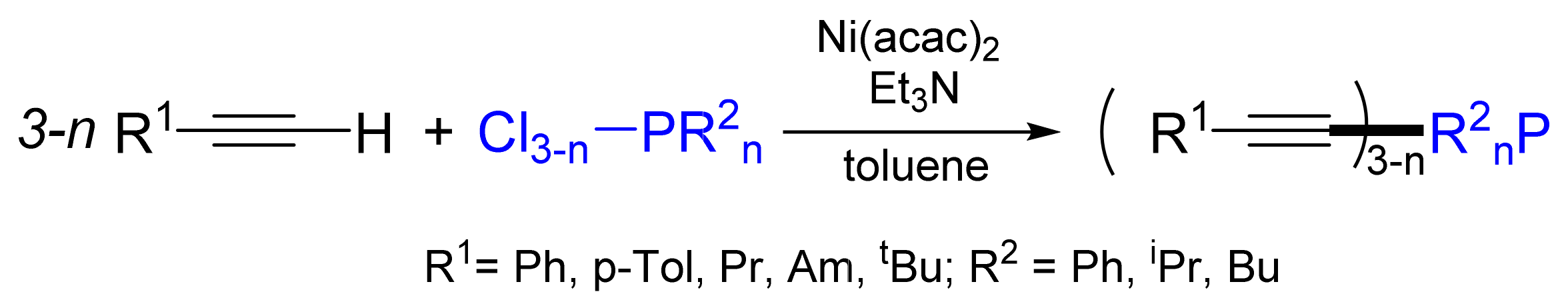
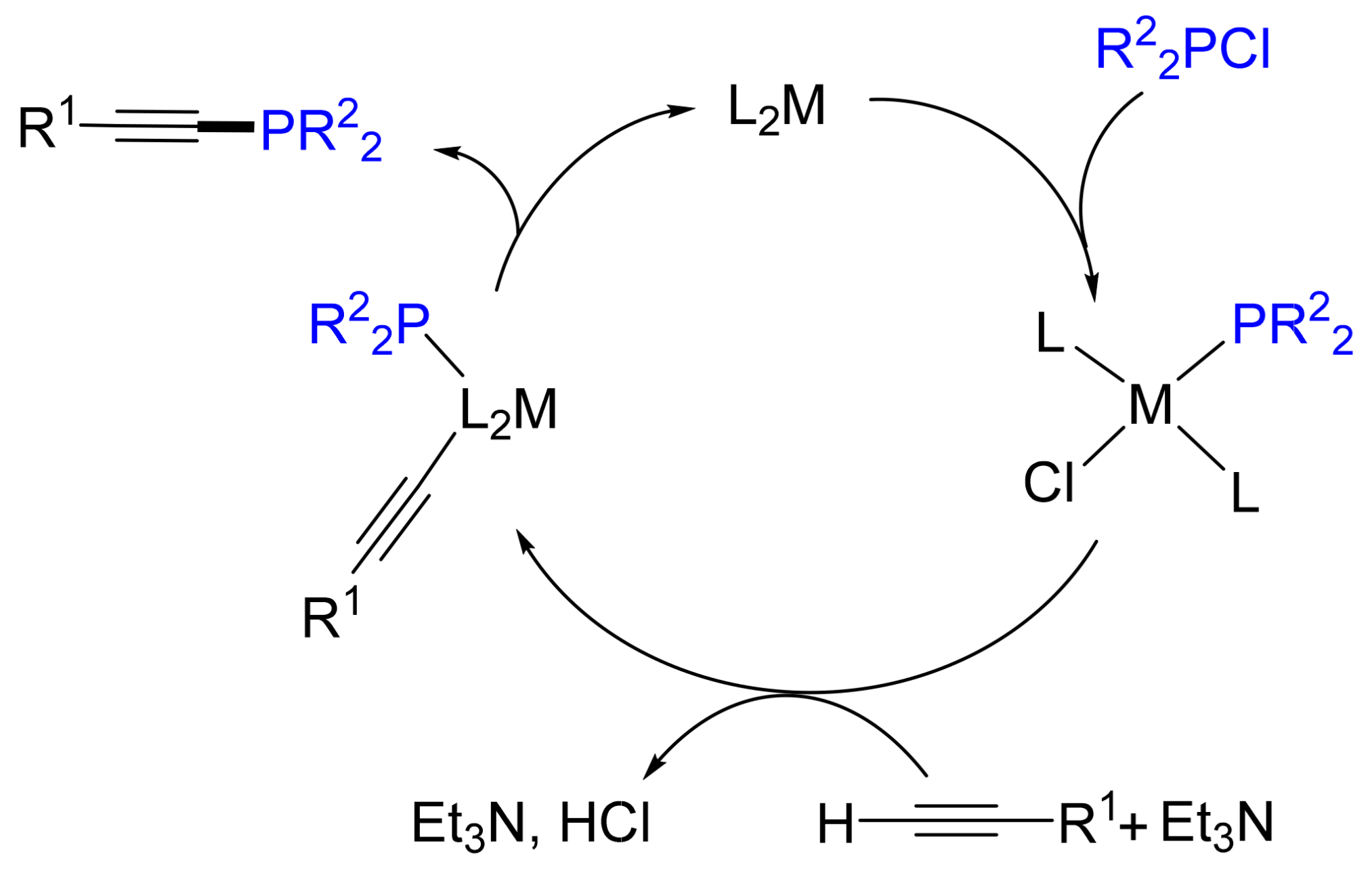
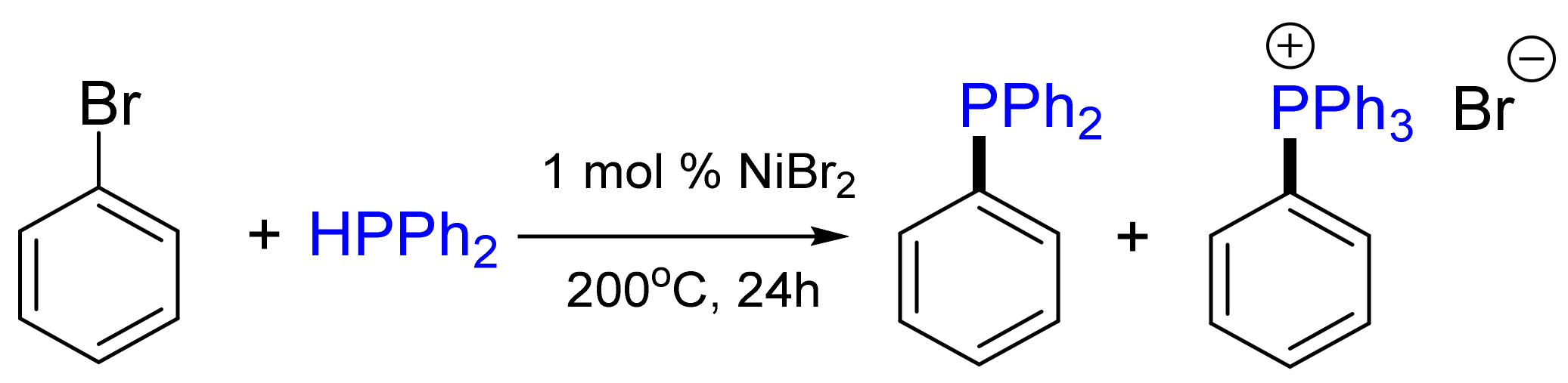
2.1. Synthesis of Phosphanes Using Phosphane Chlorides
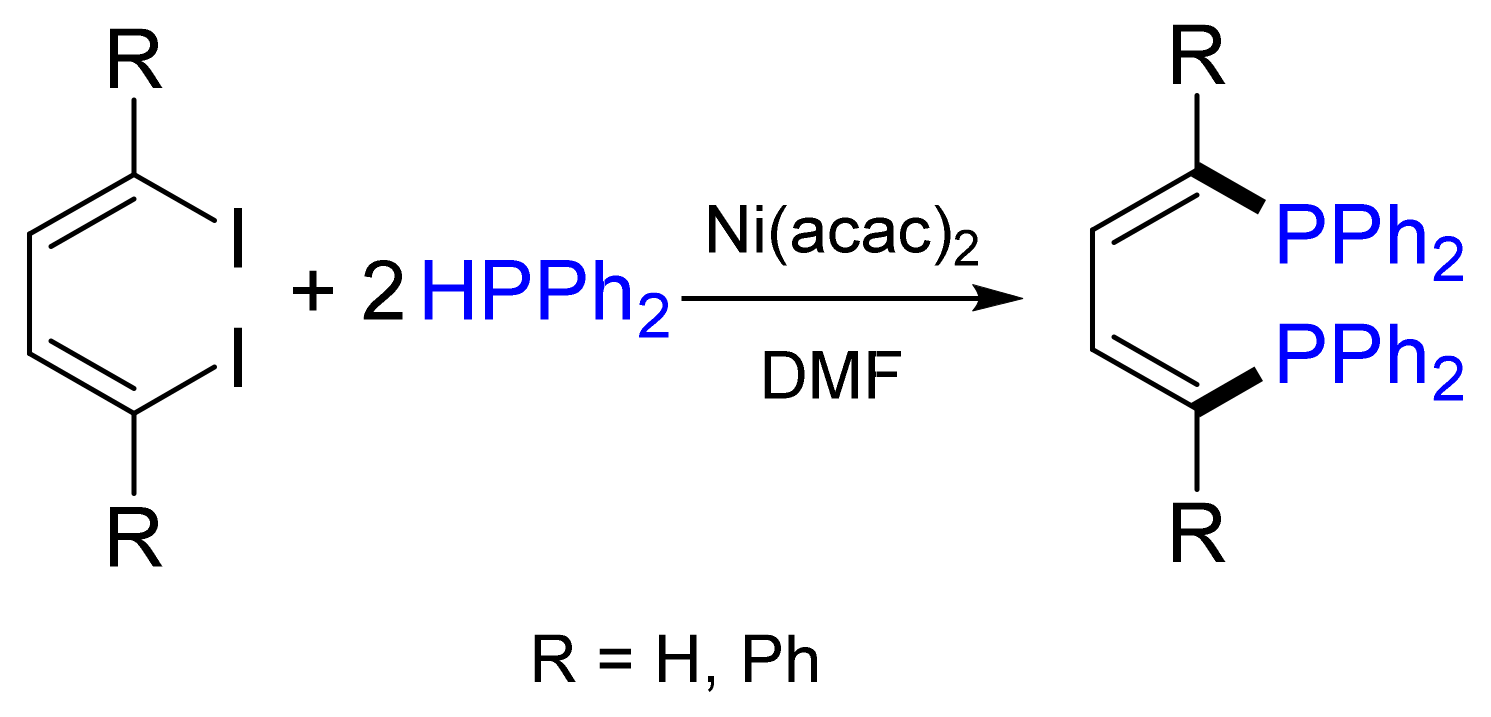
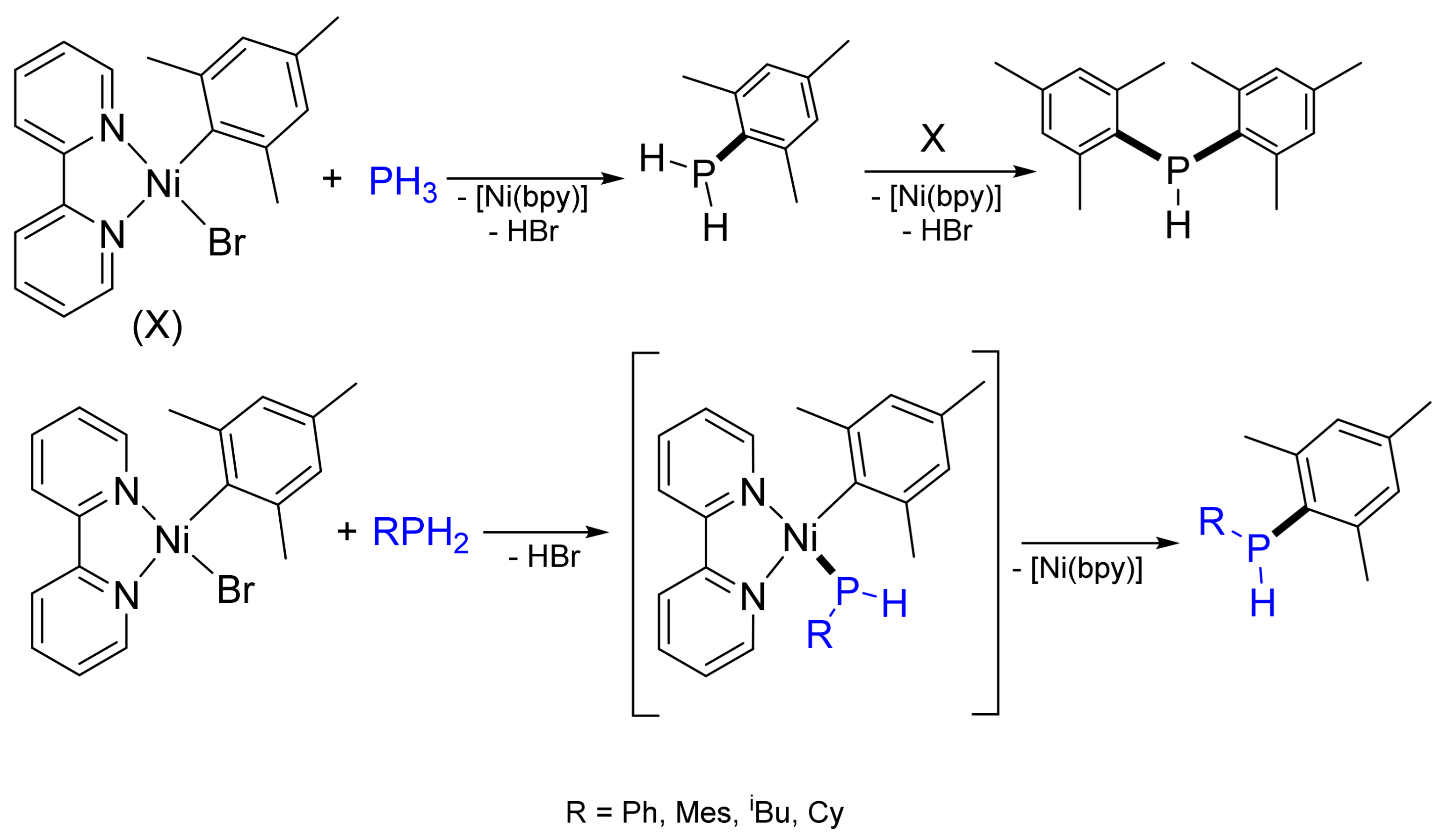
2.2. Arylation and Vinylation of Secondary and Primary Phosphanes
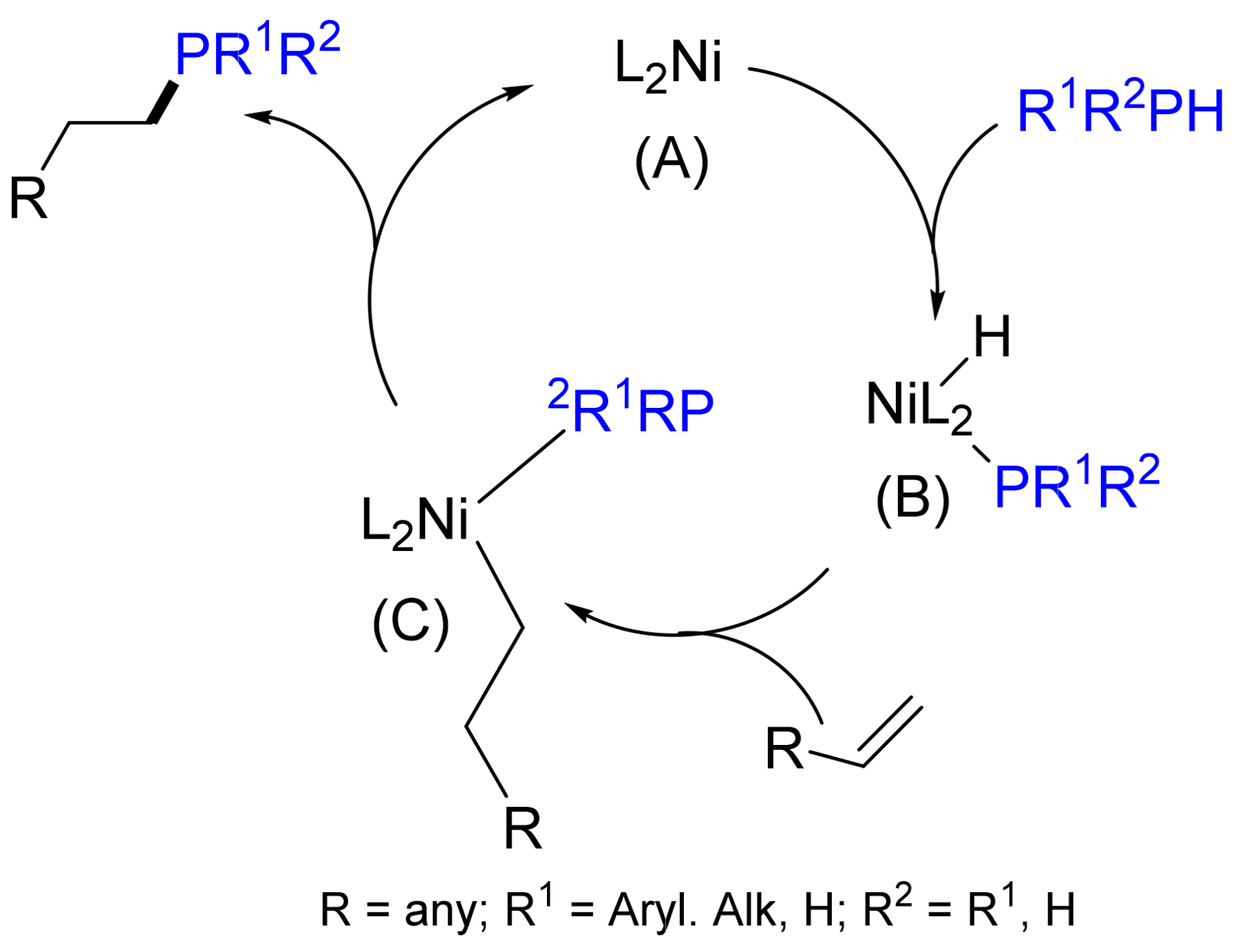
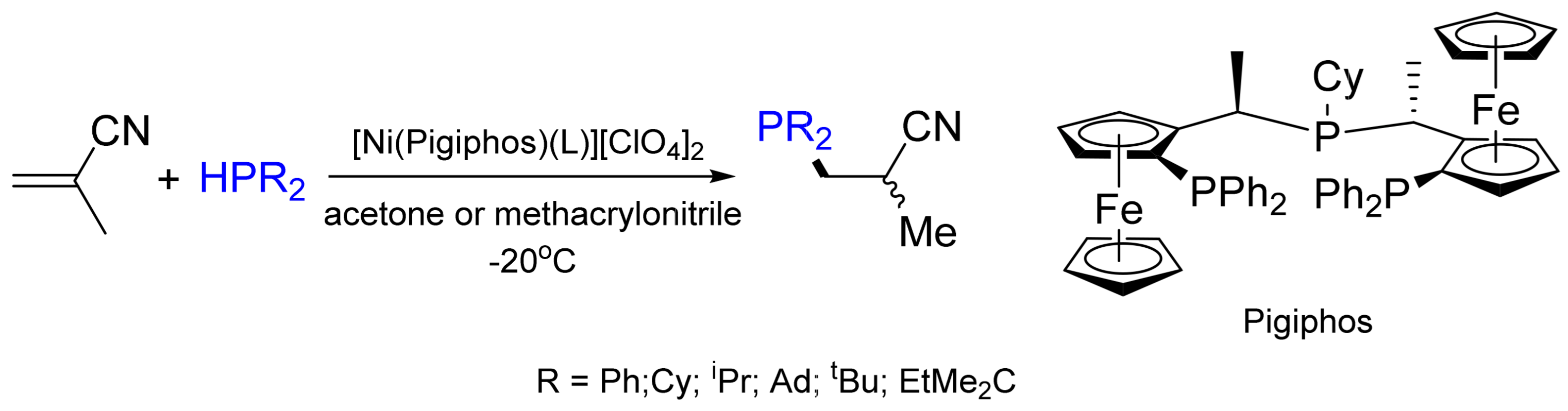
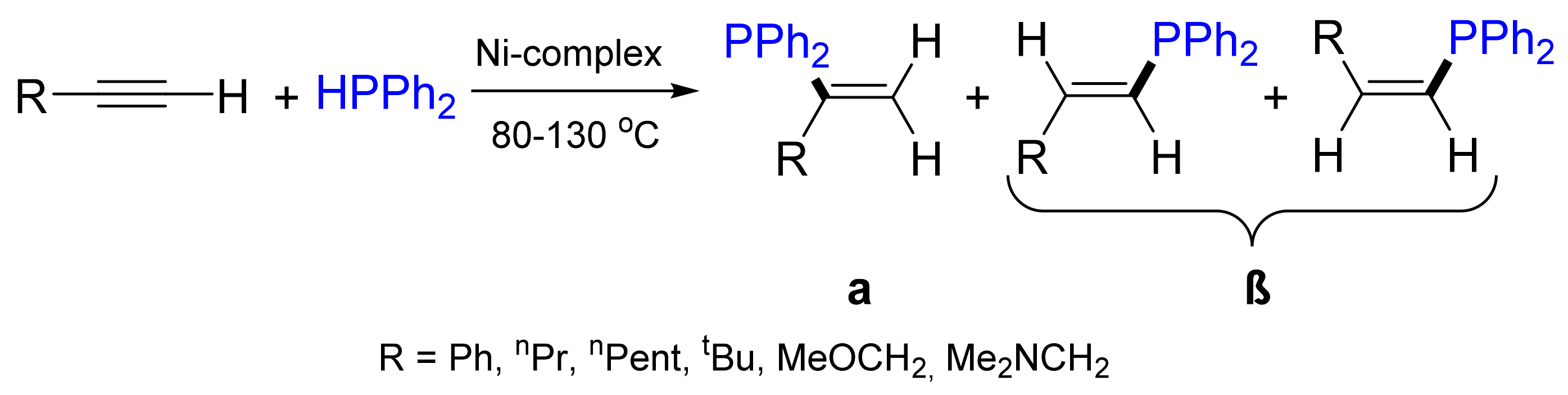
2.3. Hydrophosphination Reactions of Alkenes and Alkynes
3. Synthesis of Tetracoordinated Organophosphorus Compounds
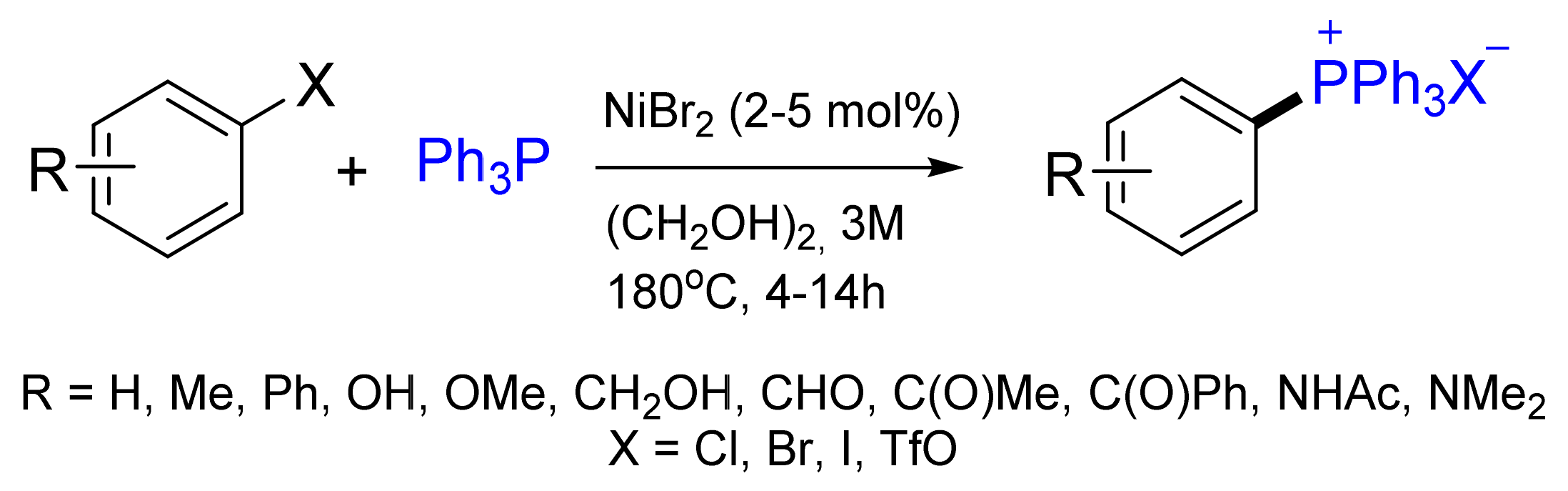
3.1. Synthesis of Phosphonium Salts in the Presence of Ni Salts
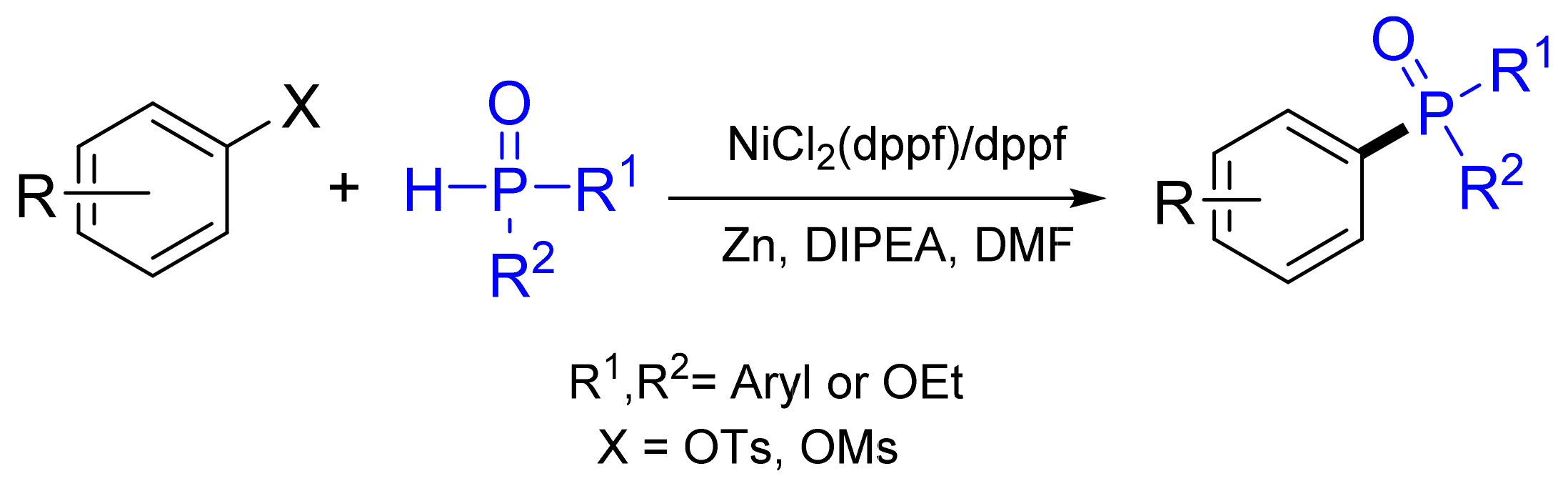
3.2. Synthesis of Phosphane Oxides by C‒P Cross-Coupling
3.3. Synthetic Routes of Phosphinates
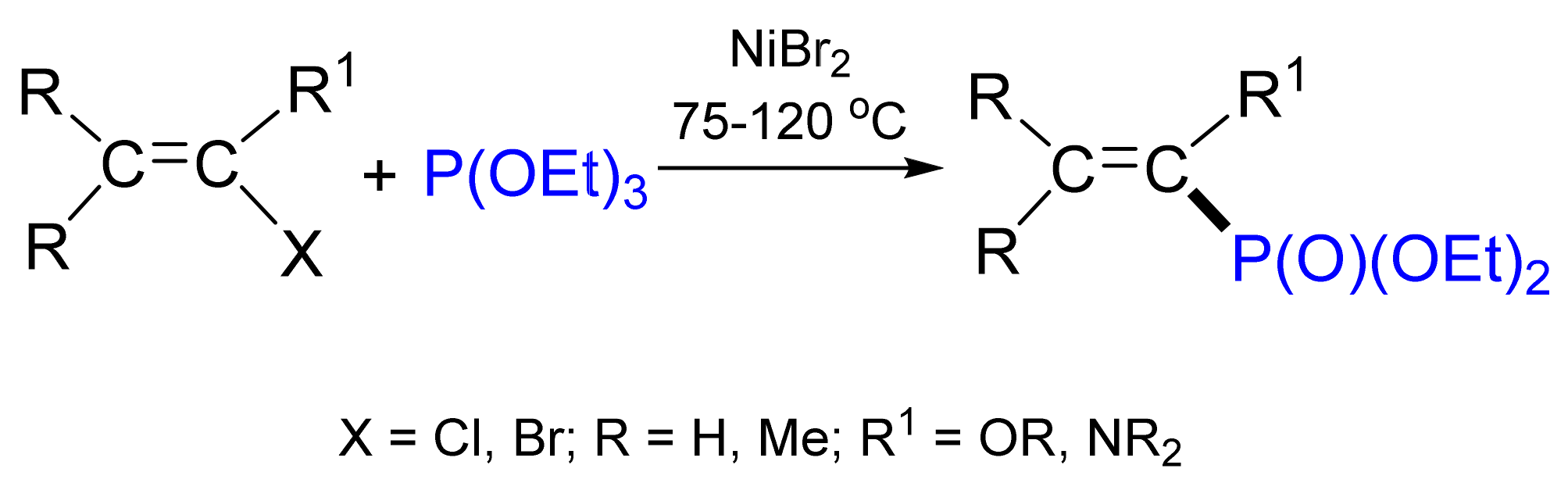
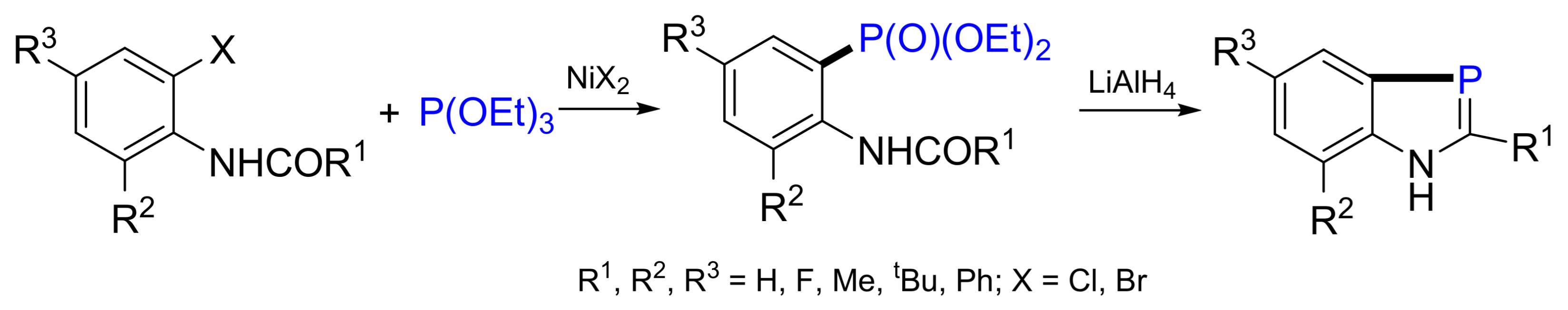
3.4. Synthesis of Phosphonates by C‒P Cross-Coupling
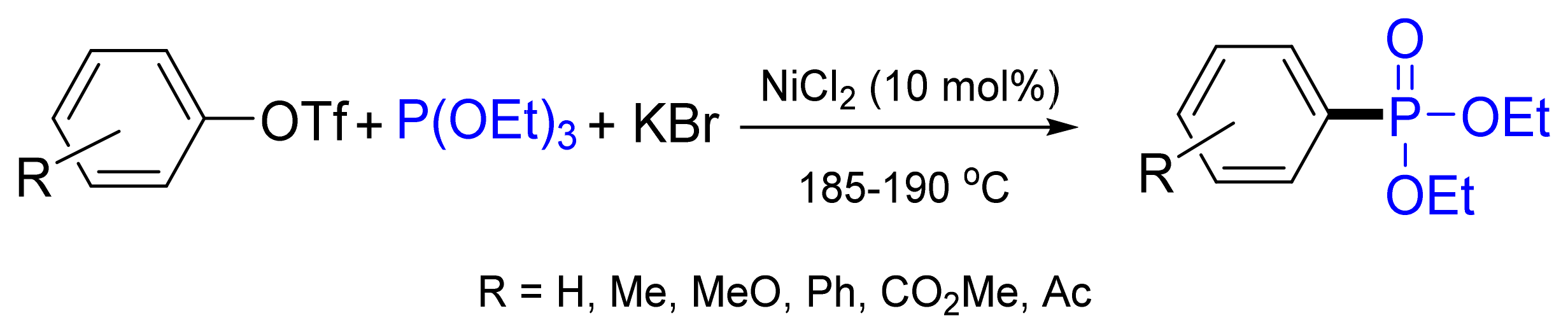
3.4.1. Ni-Catalyzed Phosphonylation with Phosphites
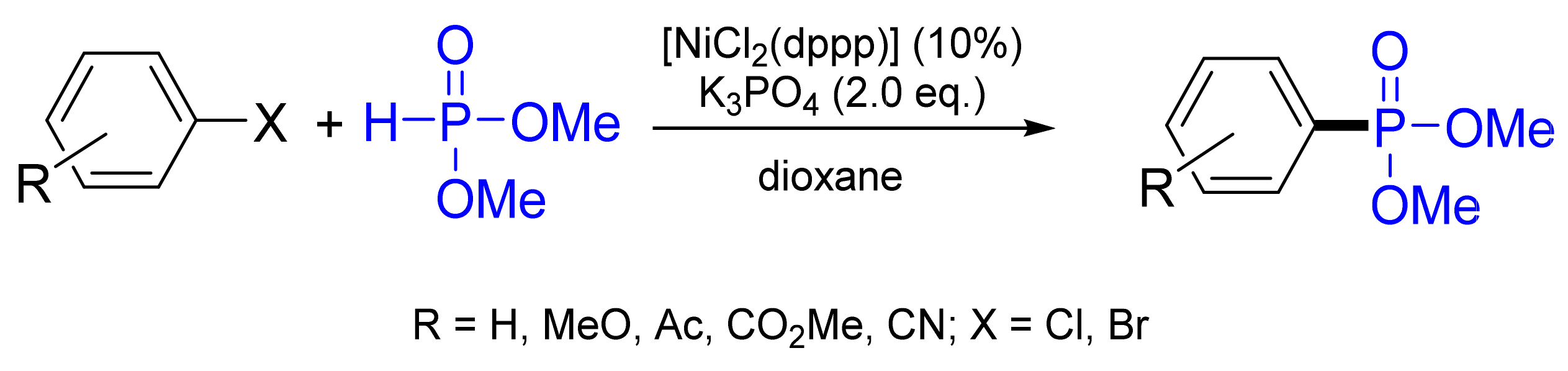
3.4.2. Phosphonylation by Hirao Reaction
4. Summary and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Ac | acetyl radical |
| acac | acetylacetone |
| BDPP | (2S,4S)-2,4-bis(diphenylphosphino)pentane |
| BINAP | 2,2′-bis(diphenylphosphino)-1,1′-binaphthyl |
| bpy | 2,2′-bipyridine |
| BSA | bis(trimethylsilyl)acetamide |
| DABCO | 1,4-diazabicyclo [2.2.2]octane |
| DCE | 1,2-dichloroethane |
| dcypt | bis(dicyclohexylphosphano)thiophene |
| DIPEA | diethylisopropylamine |
| DME | 1,2-dimethoxyethane |
| DMF | N,N-dimethylformamide |
| Dmp | 2,6-dimesitylphenyl radical |
| dppe | 1,2-bis(diphenylphosphano)ethane |
| dppf | 1,1′-bis(diphenylphosphino)ferrocene |
| dppp | 1,3-bis(diphenylphosphano)propane). |
| dtbpe | 1,2-bis(di-tert-butylphosphino)ethane |
| MW irradiation | microwave irradiation |
| naphthyl | naphthyl radical |
| NMP | N-methyl-2-pyrrolidone |
| Pigiphos | bis{(R)-1-[(S)-2-(diphenylphosphino)ferrocenyl]ethyl}cyclohexylphosphane) |
| PINAP | (R)-(+)-4-[2-(diphenylphosphino)-1-naphthalenyl]-N-[-1-phenylethyl]-1-phthalazinamine |
| Py | pyridine |
| PyBroP | bromotripyrrolidinophosphonium hexafluorophosphate |
References
- Allen, D.W. Phosphines and related C–P bonded compounds. Organophosphorus Chem. 2016, 45, 1–50. [Google Scholar] [CrossRef]
- Peruzzini, M.; Gonsalvi, L. Phosphorus Compounds: Advanced Tools in Catalysis and Material Sciences; Springer: Dordrecht, The Netherlands, 2011. [Google Scholar]
- Borner, A. Phosphorus Ligands in Asymmetric Catalysis; Wiley: Weinheim, Germany, 2008. [Google Scholar]
- Rodriguez, J.B.; Gallo-Rodriguez, C. The role of the phosphorus atom in drug design. ChemMedChem 2018, 14, 190–216. [Google Scholar] [CrossRef]
- Zagidullin, A.A.; Bezkishko, I.A.; Miluykov, V.A.; Sinyashin, O.G. Phospholes–development and recent advances. Mendeleev Commun. 2013, 23, 117–130. [Google Scholar] [CrossRef]
- Schwan, A. Palladium catalyzed cross-coupling reactions for phosphorus–carbon bond formation. Chem. Soc. Rev. 2004, 33, 218–224. [Google Scholar] [CrossRef]
- Oestreich, M.; Tappe, F.; Trepohl, V. Transition-Metal-Catalyzed C-P Cross-Coupling Reactions. Synthesis 2010, 2010, 3037–3062. [Google Scholar] [CrossRef]
- Wauters, I.; Debrouwer, W.; Stevens, C.V. Preparation of phosphines through C–P bond formation. Beilstein J. Org. Chem. 2014, 10, 1064–1096. [Google Scholar] [CrossRef]
- Koshti, V.; Gaikwad, S.; Chikkali, S.H. Contemporary avenues in catalytic PH bond addition reaction: A case study of hydrophosphination. Coord. Chem. Rev. 2014, 265, 52–73. [Google Scholar] [CrossRef]
- Tasker, S.Z.; Standley, E.A.; Jamison, T.F. Recent advances in homogeneous nickel catalysis. Nat. Cell Biol. 2014, 509, 299–309. [Google Scholar] [CrossRef]
- Henrion, M.; Ritleng, V.; Chetcuti, M.J. Nickel N-Heterocyclic Carbene-Catalyzed C–C Bond Formation: Reactions and Mechanistic Aspects. ACS Catal. 2015, 5, 1283–1302. [Google Scholar] [CrossRef]
- Trotuş, I.-T.; Zimmermann, T.; Schüth, F. Catalytic reactions of acetylene: A feedstock for the chemical industry revisited. Chem. Rev. 2013, 114, 1761–1782. [Google Scholar] [CrossRef]
- Ananikov, V. Nickel: The “Spirited Horse” of Transition Metal Catalysis. ACS Catal. 2015, 5, 1964–1971. [Google Scholar] [CrossRef]
- Cristau, H.-J.; Chêne, A.; Christol, H. Arylation catalytique d’organophosphorés. Produits de l’arylation, catalysée par les sels de nickel (II), de composés du phosphore tricoordiné. J. Organomet. Chem. 1980, 185, 283–295. [Google Scholar] [CrossRef]
- Ager, D.J.; Laneman, S.A. Convenient and direct preparation of tertiary phosphines via nickel-catalysed cross-coupling. Chem. Commun. 1997, 2359–2360. [Google Scholar] [CrossRef]
- Le Gall, E.; Troupel, M.; Nédélec, J.-Y. Nickel-catalyzed reductive coupling of chlorodiphenylphosphine with aryl bromides into functionalized triarylphosphines. Tetrahedron 2003, 59, 7497–7500. [Google Scholar] [CrossRef]
- Sun, M.; Zang, Y.-S.; Hou, L.-K.; Chen, X.-X.; Sun, W.; Yang, S.-D. Convenient Formation of Triarylphosphines by Nickel-Catalyzed C-P Cross-Coupling with Aryl Chlorides. Eur. J. Org. Chem. 2014, 2014, 6796–6801. [Google Scholar] [CrossRef]
- Beletskaya, I.P.; Afanasiev, V.V.; Kazankova, M.A.; Efimova, I.V. New Approach to Phosphinoalkynes Based on Pd- and Ni-Catalyzed Cross-Coupling of Terminal Alkynes with Chlorophosphanes. Org. Lett. 2003, 5, 4309–4311. [Google Scholar] [CrossRef]
- Di Credico, B.; De Biani, F.F.; Gonsalvi, L.; Guerri, A.; Ienco, A.; Laschi, F.; Peruzzini, M.; Reginato, G.; Rossin, A.; Zanello, P. Cyclopentadienyl ruthenium(II) complexes with bridging alkynylphosphine ligands: Synthesis and electrochemical studies. Chem. Eur. J. 2009, 15, 11985–11998. [Google Scholar] [CrossRef] [PubMed]
- Beletskaya, I.P.; Afanasiev, V.V.; Kazankova, M.A.; Efimova, I.V.; Antipin, M.U. A convenient and direct route to phosphinoalkynes via copper-catalyzed cross-coupling of terminal alkynes with chlorophosphanes. Synthesis 2003, 2003, 2835–2838. [Google Scholar] [CrossRef]
- Kazankova, M.A.; Shulyupin, M.O.; Chirkov, E.A.; Beletskaya, I.P. Nickel-catalyzed cross-coupling of diphenylphosphine with vinyl bromides and chlorides as a route to diphenylvinylphosphines. Synlett 2005, 2005, 658–660. [Google Scholar] [CrossRef]
- Beletskaya, I.; Kazankova, M.A. Catalytic methods for building up phosphorus-carbon bond. Russ. J. Org. Chem. 2002, 38, 1391–1430. [Google Scholar] [CrossRef]
- Trostyanskaya, I.G.; Titskiy, D.Y.; Anufrieva, E.A.; Borisenko, A.A.; Kazankova, M.A.; Beletskaya, I.P. Cross-coupling of E,E-1,4-diiodobuta-1,3-diene with nucleophiles catalyzed by Pd or Ni complexes: A new route to functionalized dienes. Russ. Chem. Bull. 2001, 50, 2095–2100. [Google Scholar] [CrossRef]
- Berthod, M.; Mignani, G.; Woodward, G.; Lemaire, M. Modified BINAP: The how and the why. Chem. Rev. 2005, 105, 1801–1836. [Google Scholar] [CrossRef]
- Cai, D.; Payack, J.F.; Bender, D.R.; Hughes, D.L.; Verhoeven, T.R.; Reider, P.J. Synthesis of Chiral 2,2’-Bis(diphenylphosphino)-1,1’-binaphthyl (BINAP) via a Novel Nickel-Catalyzed Phosphine Insertion. J. Org. Chem. 1994, 59, 7180–7181. [Google Scholar] [CrossRef]
- Kerrigan, N.J.; Dunne, E.C.; Cunningham, D.; McArdle, P.; Gilligan, K.; Gilheany, D.G. Studies in the preparation of novel P-chirogenic binaphthyl monophosphanes (MOPs). Tetrahedron Lett. 2003, 44, 8461–8465. [Google Scholar] [CrossRef]
- Morris, D.J.; Docherty, G.; Woodward, G.; Wills, M. Modification of ligand properties of phosphine ligands for C–C and C–N bond-forming reactions. Tetrahedron Lett. 2007, 48, 949–953. [Google Scholar] [CrossRef]
- Fleming, W.J.; Müller-Bunz, H.; Lillo, V.; Fernandez, E.; Guiry, P.J. Axially chiral P-N ligands for the copper catalyzed β-borylation of α,β-unsaturated esters. Org. Biomol. Chem. 2009, 7, 2520–2524. [Google Scholar] [CrossRef]
- Fleming, W.J.; Müller-Bunz, H.; Guiry, P.J. Synthesis and post-resolution modification of new axially chiral ligands for asymmetric catalysis. Eur. J. Org. Chem. 2010, 2010, 5996–6004. [Google Scholar] [CrossRef]
- Rafter, E.; Muldoon, J.; Bunz, H.M.; Gilheany, D.G. Synthesis of P-stereogenic BINAP bissulfide analogues. Tetrahedron Asymmetry 2011, 22, 1680–1686. [Google Scholar] [CrossRef]
- Pereira, M.M.; Calvete, M.J.F.; Carrilho, R.M.B.; Abreu, A.R. Synthesis of binaphthyl based phosphine and phosphite ligands. Chem. Soc. Rev. 2013, 42, 6990–7027. [Google Scholar] [CrossRef]
- Enev, V.; Ewers, C.L.J.; Harre, M.; Nickisch, K.; Mohr, J.T. A Bis-steroidal phosphine as new chiral hydrogenation ligand. J. Org. Chem. 1997, 62, 7092–7093. [Google Scholar] [CrossRef]
- Lacey, P.M.; McDonnell, C.M.; Guiry, P.J. Synthesis and resolution of 2-methyl-Quinazolinap, a new atropisomeric phosphinamine ligand for asymmetric catalysis. Tetrahedron Lett. 2000, 41, 2475–2478. [Google Scholar] [CrossRef]
- Knöpfel, T.F.; Aschwanden, P.; Ichikawa, T.; Watanabe, T.; Carreira, E.M. Readily Available Biaryl P,N Ligands for Asymmetric Catalysis. Angew. Chem. Int. Ed. 2004, 43, 5971–5973. [Google Scholar] [CrossRef] [PubMed]
- Knöpfel, T.F.; Zarotti, P.; Ichikawa, T.; Carreira, E. Catalytic, enantioselective, conjugate alkyne addition. J. Am. Chem. Soc. 2005, 127, 9682–9683. [Google Scholar] [CrossRef]
- Birdsall, D.J.; Hope, E.G.; Stuart, A.M.; Chen, W.; Hu, Y.; Xiao, J. Synthesis of fluoroalkyl-derivatised BINAP ligands. Tetrahedron Lett. 2001, 42, 8551–8553. [Google Scholar] [CrossRef]
- Nakamura, Y.; Takeuchi, S.; Zhang, S.; Okumura, K.; Ohgo, Y. Preparation of a fluorous chiral BINAP and application to an asymmetric Heck reaction. Tetrahedron Lett. 2002, 43, 3053–3056. [Google Scholar] [CrossRef]
- Zhao, Y.-L.; Wu, G.-J.; Li, Y.; Gao, L.-X.; Han, F.-S. [NiCl2(dppp)]-catalyzed cross-coupling of aryl halides with dialkyl phosphite, diphenylphosphine oxide, and diphenylphosphine. Chem. Eur. J. 2012, 18, 9622–9627. [Google Scholar] [CrossRef]
- Klein, A.; Budnikova, Y.H.; Sinyashin, O. Electron transfer in organonickel complexes of α-diimines: Versatile redox catalysts for C–C or C–P coupling reactions—A review. J. Organomet. Chem. 2007, 692, 3156–3166. [Google Scholar] [CrossRef]
- Budnikova, Y.H.; Perichon, J.; Yakhvarov, D.G.; Kargin, Y.M.; Sinyashin, O. Highly reactive σ-organonickel complexes in electrocatalytic processes. J. Organomet. Chem. 2001, 630, 185–192. [Google Scholar] [CrossRef]
- Yakhvarov, D.G.; Khusnuriyalova, A.F.; Sinyashin, O. Electrochemical synthesis and properties of organonickel σ-complexes. Organometallics 2014, 33, 4574–4589. [Google Scholar] [CrossRef]
- Yakhvarov, D.G.; Kvashennikova, S.V.; Sinyashin, O. Reactions of activated organonickel σ-complexes with elemental (white) phosphorus. Russ. Chem. Bull. 2013, 62, 2472–2476. [Google Scholar] [CrossRef]
- Gafurov, Z.; Musin, L.; Sakhapov, I.F.; Babaev, V.; Musina, E.I.; Karasik, A.A.; Sinyashin, O.; Yakhvarov, D.G. The formation of secondary arylphosphines in the reaction of organonickel sigma-complex [NiBr(Mes)(bpy)], where Mes = 2,4,6-trimethylphenyl, bpy = 2,2′-bipyridine, with phenylphosphine. Phosphorus Sulfur Silicon Relat. Elements 2016, 191, 1475–1477. [Google Scholar] [CrossRef]
- Gafurov, Z.; Sakhapov, I.F.; Kagilev, A.A.; Kantyukov, A.O.; Khayarov, K.R.; Sinyashin, O.; Yakhvarov, D.G. The formation of mesitylphosphine and dimesitylphosphine in the reaction of organonickel σ-complex [NiBr(Mes)(bpy)] (Mes = 2,4,6-trimethylphenyl, bpy = 2,2′-bipyridine) with phosphine PH3. Phosphorus Sulfur Silicon Relat. Elements 2020, 195, 726–729. [Google Scholar] [CrossRef]
- Bange, C.A.; Waterman, R. Challenges in Catalytic Hydrophosphination. Chem. Eur. J. 2016, 22, 12598–12605. [Google Scholar] [CrossRef]
- Kazankova, M.A.; Shulyupin, M.O.; Borisenko, A.A.; Beletskaya, I. Synthesis of alkyl(diphenyl)phosphines by hydrophosphination of vinylarenes catalyzed by transition metal complexes. Russ. J. Org. Chem. 2002, 38, 1479–1484. [Google Scholar] [CrossRef]
- Beletskaya, I.P.; Kazankova, M.A.; Shulyupin, M.O. Catalytic Hydrophosphination of Alkenylalkyl Ethers. Synlett 2003, 2003, 2155–2158. [Google Scholar] [CrossRef]
- Ananikov, V.; Gayduk, K.A.; Starikova, Z.A.; Beletskaya, I. Ni(acac)2/phosphine as an excellent precursor of Nickel(0) for catalytic systems. Organometallics 2010, 29, 5098–5102. [Google Scholar] [CrossRef]
- Webster, R.L. Room Temperature Ni(II) Catalyzed Hydrophosphination and Cyclotrimerization of Alkynes. Inorganics 2018, 6, 120. [Google Scholar] [CrossRef]
- Shulyupin, M.O.; Kazankova, M.A.; Beletskaya, I. Catalytic Hydrophosphination of Styrenes. Org. Lett. 2002, 4, 761–763. [Google Scholar] [CrossRef]
- Ganushevich, Y.S.; Miluykov, V.A.; Polyancev, F.M.; Latypov, S.; Lönnecke, P.; Hey-Hawkins, E.; Yakhvarov, D.G.; Sinyashin, O. Nickel Phosphanido Hydride Complex: An Intermediate in the Hydrophosphination of Unactivated Alkenes by Primary Phosphine. Organometallics 2013, 32, 3914–3919. [Google Scholar] [CrossRef]
- Latypov, S.K.; Kondrashova, S.A.; Polyancev, F.M.; Sinyashin, O.G. Quantum Chemical Calculations of 31P NMR Chemical Shifts in Nickel Complexes: Scope and Limitations. Organometallics 2020, 39, 1413–1422. [Google Scholar] [CrossRef]
- Latypov, S.K.; Polyancev, F.M.; Ganushevich, Y.S.; Miluykov, V.A.; Sinyashin, O.G. Mechanism of intramolecular transformations of nickel phosphanido hydride complexes. Dalton Trans. 2015, 45, 2053–2059. [Google Scholar] [CrossRef]
- Wang, C.; Huang, K.; Ye, J.; Duan, W.-L. Asymmetric Synthesis of P-Stereogenic Secondary Phosphine-Boranes by an Unsymmetric Bisphosphine Pincer-Nickel Complex. J. Am. Chem. Soc. 2021, 143, 5685–5690. [Google Scholar] [CrossRef]
- Sadow, A.D.; Haller, I.; Fadini, A.L.; Togni, A. Nickel(II)-Catalyzed Highly Enantioselective Hydrophosphination of Methacrylonitrile. J. Am. Chem. Soc. 2004, 126, 14704–14705. [Google Scholar] [CrossRef] [PubMed]
- Sadow, A.D.; Togni, A. Enantioselective addition of secondary phosphines to methacrylonitrile: Catalysis and mechanism. J. Am. Chem. Soc. 2005, 127, 17012–17024. [Google Scholar] [CrossRef]
- Kazankova, M.A.; Efimova, I.V.; Kochetkov, A.N.; Afanas’Ev, V.V.; Beletskaya, I.; Dixneuf, P.H. New Approach to Vinylphosphines Based on Pd- and Ni-Catalyzed Diphenylphosphine Addition to Alkynes. Synlett 2001, 2001, 0497–0500. [Google Scholar] [CrossRef]
- Kazankova, M.A.; Efimova, I.V.; Kochetkov, A.; Afanas’Ev, V.V.; Beletskaya, I.P. Synthesis of Vinylphosphines by Hydrophosphination of Alkynes in the Presence of Transition Metal Complexes. Russ. J. Org. Chem. 2002, 38, 1465–1474. [Google Scholar] [CrossRef]
- Ananikov, V.P.; Beletskaya, I. Alkyne insertion into the M-P and M-H bonds (M=Pd, Ni, Pt, and Rh): A theoretical mechanistic study of the C-P and C-H bond-formation steps. Chem. Asian J. 2011, 6, 1423–1430. [Google Scholar] [CrossRef]
- Yan, J.; Wang, Y.; Hou, S.; Shi, L.; Zhu, X.; Hao, X.; Song, M. NCC Pincer Ni (II) complexes Catalyzed hydrophosphination of Nitroalkenes with diphenylphosphine. Appl. Organomet. Chem. 2020, 34, 5954. [Google Scholar] [CrossRef]
- Ribiere, P.; Bravo-Altamirano, K.; Antczak, M.I.; Hawkins, J.D.; Montchamp, J.-L. NiCl2-Catalyzed Hydrophosphinylation. J. Org. Chem. 2005, 70, 4064–4072. [Google Scholar] [CrossRef]
- Ananikov, V.; Khemchyan, L.L.; Beletskaya, I.; Starikova, Z.A. Acid-Free Nickel Catalyst for Stereo- and Regioselective Hydrophosphorylation of Alkynes: Synthetic Procedure and Combined Experimental and Theoretical Mechanistic Study. Adv. Synth. Catal. 2010, 352, 2979–2992. [Google Scholar] [CrossRef]
- Han, L.-B.; Ono, Y.; Yazawa, H. Nickel-Catalyzed Addition of P(O)−H Bonds to Propargyl Alcohols: One-Pot Generation of Phosphinoyl 1,3-Butadienes. Org. Lett. 2005, 7, 2909–2911. [Google Scholar] [CrossRef]
- Han, L.-B.; Zhang, C.; Yazawa, A.H.; Shimada, S. Efficient and Selective Nickel-Catalyzed Addition of H−P(O) and H−S Bonds to Alkynes. J. Am. Chem. Soc. 2004, 126, 5080–5081. [Google Scholar] [CrossRef]
- Cassar, L.; Foà, M. Nickel-catalyzed synthesis of phosphonium salts. J. Organomet. Chem. 1974, 74, 75. [Google Scholar] [CrossRef]
- Allen, D.W.; Coles, S.J.; Light, M.E.; Hursthouse, M.B. Synthesis and X-ray crystal structures of organotri(2-furyl)phosphonium salts: Effects of 2-furyl substituents at phosphorus on intramolecular nitrogen to phosphorus hypervalent coordinative interactions. Inorganica Chim. Acta 2004, 357, 1558–1564. [Google Scholar] [CrossRef]
- Monkowius, U.V.; Nogai, S.; Schmidbaur, H. Unsuccessful/successful attempts to produce penta(heteroaryl)-phosphoranes/-arsoranes R5E (E = P, As; R = 2-furyl, 2-thienyl). Dalton Trans. 2004, 10, 1610–1617. [Google Scholar] [CrossRef] [PubMed]
- Asay, M.; Donnadieu, B.; Baceiredo, A.; Soleilhavoup, M.; Bertrand, G. Cyclic (Amino)[bis(ylide)]carbene as an Anionic Bidentate Ligand for Transition-Metal Complexes. Inorg. Chem. 2008, 47, 3949–3951. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Manabe, K. Synthesis of novel chiral quaternary phosphonium salts with a multiple hydrogen-bonding site, and their application to asymmetric phase-transfer alkylation. Tetrahedron 1998, 54, 14465–14476. [Google Scholar] [CrossRef]
- Marcoux, D.; Charette, A.B. Nickel-Catalyzed Synthesis of Phosphonium Salts from Aryl Halides and Triphenylphosphine. Adv. Synth. Catal. 2008, 350, 2967–2974. [Google Scholar] [CrossRef]
- Wan, W.; Yang, X.; Smith, R.C. Convenient route to tetraarylphosphonium polyelectrolytes via metal-catalysed P–C coupling polymerisation of aryl dihalides and diphenylphosphine. Chem. Commun. 2016, 53, 252–254. [Google Scholar] [CrossRef]
- Budnikova, Y.H.; Sinyashin, O.G.; Budnikova, Y.H. Phosphorylation of C–H bonds of aromatic compounds using metals and metal complexes. Russ. Chem. Rev. 2015, 84, 917–951. [Google Scholar] [CrossRef]
- Zhang, H.-Y.; Sun, M.; Ma, Y.-N.; Tian, Q.-P.; Yang, S.-D. Nickel-catalyzed C–P cross-coupling of diphenylphosphine oxide with aryl chlorides. Org. Biomol. Chem. 2012, 10, 9627–9633. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, H.; Hu, X.; Tang, G.; Zhu, J.; Zhao, Y. Ni(II)/Zn Catalyzed Reductive Coupling of Aryl Halides with Diphenylphosphine Oxide in Water. Org. Lett. 2011, 13, 3478–3481. [Google Scholar] [CrossRef]
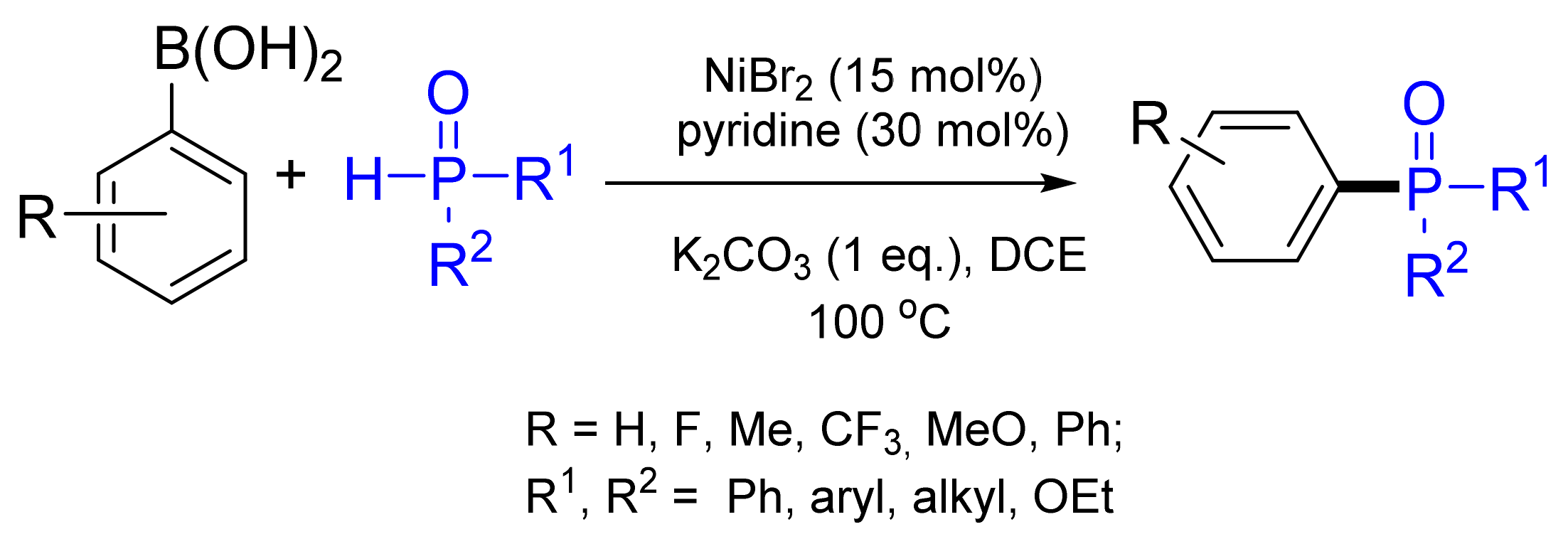
- Hu, G.; Chen, W.; Fu, T.; Peng, Z.; Qiao, H.; Gao, Y.; Zhao, Y. Nickel-Catalyzed C–P Cross-Coupling of Arylboronic Acids with P(O)H Compounds. Org. Lett. 2013, 15, 5362–5365. [Google Scholar] [CrossRef]
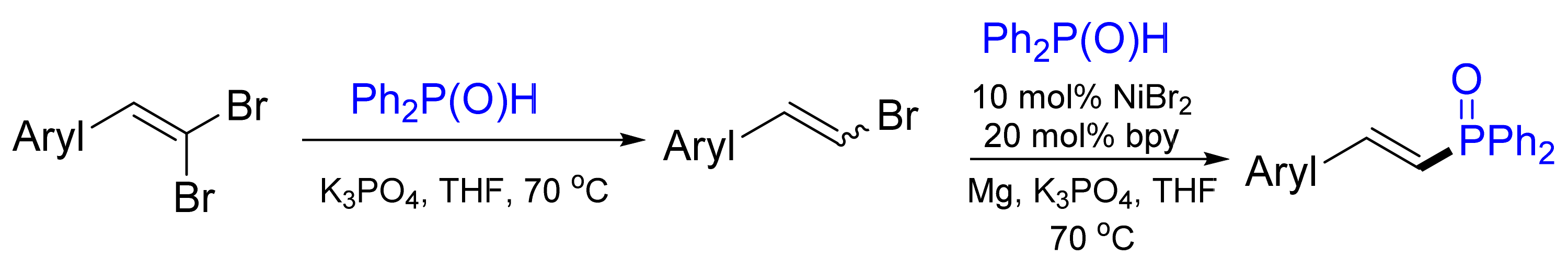
- Liu, L.L.; Wang, Y.; Zeng, Z.; Xu, P.; Gao, Y.; Yin, Y.; Zhao, Y. Nickel(II)-Magnesium-Catalyzed Cross-Coupling of 1,1-Dibromo-1-alkenes with Diphenylphosphine Oxide: One-Pot Synthesis of (E)-1-Alkenylphosphine Oxides or Bisphosphine Oxides. Adv. Synth. Catal. 2013, 355, 659–666. [Google Scholar] [CrossRef]
- Xuan, J.; Zeng, T.-T.; Chen, J.-R.; Lu, L.-Q.; Xiao, W.-J. Room Temperature C-P Bond Formation Enabled by Merging Nickel Catalysis and Visible-Light-Induced Photoredox Catalysis. Chem. Eur. J. 2015, 21, 4962–4965. [Google Scholar] [CrossRef]
- Isshiki, R.; Muto, K.; Yamaguchi, J. Decarbonylative C–P Bond Formation Using Aromatic Esters and Organophosphorus Compounds. Org. Lett. 2018, 20, 1150–1153. [Google Scholar] [CrossRef]
- Liu, X.-T.; Zhang, Y.-Q.; Han, X.-Y.; Sun, S.-P.; Zhang, Q.-W. Ni-Catalyzed Asymmetric Allylation of Secondary Phosphine Oxides. J. Am. Chem. Soc. 2019, 141, 16584–16589. [Google Scholar] [CrossRef]
- Hou, H.; Zhou, B.; Wang, J.; Sun, D.; Yu, H.; Chen, X.; Han, Y.; Shi, Y.; Yan, C.; Zhu, S. Visible-light-induced ligand to metal charge transfer excitation enabled phosphorylation of aryl halides. Chem. Commun. 2021, 57, 5702–5705. [Google Scholar] [CrossRef]
- Łastawiecka, E.; Flis, A.; Stankevič, M.; Greluk, M.; Słowik, G.; Gac, W. P-Arylation of secondary phosphine oxides catalyzed by nickel-supported nanoparticles. Org. Chem. Front. 2018, 5, 2079–2085. [Google Scholar] [CrossRef]
- Balthazor, T.M. Phosphindolin-3-one. A useful intermediate for phosphindole synthesis. J. Org. Chem. 1980, 45, 2519–2522. [Google Scholar] [CrossRef]
- Hirao, T.; Masunaga, T.; Ohshiro, Y.; Agawa, T. Stereoselective synthesis of vinylphosphonate. Tetrahedron Lett. 1980, 21, 3595–3598. [Google Scholar] [CrossRef]
- Shen, C.; Yang, G.; Zhang, W. Nickel-catalyzed C–P coupling of aryl mesylates and tosylates with H(O)PR1R2. Org. Biomol. Chem. 2012, 10, 3500–3505. [Google Scholar] [CrossRef]
- Tavs, P. Reaktion von Arylhalogeniden mit Trialkylphosphiten und Benzolphosphonigsäure-Dialkylestern zu Aromatischen Phosphonsäureestern und Phosphinsäureestern unter Nickelsalzkatalyse. Eur. J. Inorg. Chem. 1970, 103, 2428–2436. [Google Scholar] [CrossRef]
- Tavs, P.; Weitkamp, H. Herstellung und KMR-spektren einiger α,β-ungesättigter phosphonsäureester: Nickelsalzkatalysierte reaktion von vinylhalogeniden mit trialkylphosphiten. Tetrahedron 1970, 26, 5529–5534. [Google Scholar] [CrossRef]
- Comins, D.L.; Ollinger, C.G. Inter- and intramolecular Horner–Wadsworth–Emmons reactions of 5-(diethoxyphosphoryl)-1-acyl-2-alkyl(aryl)-2,3-dihydro-4-pyridones. Tetrahedron Lett. 2001, 42, 4115–4118. [Google Scholar] [CrossRef]
- DeMik, N.N.; Kabachnik, M.M.; Novikova, Z.S.; Beletskaya, I.P. Preparation of arylphosphonates by the reaction of aryl halides with tris(trimethylsilyl) phosphite under homogeneous catalysis conditions. Russ. Chem. Bull. 1991, 40, 1300–1301. [Google Scholar] [CrossRef]
- Kazankova, M.A.; Trostyanskaya, I.G.; Lutsenko, S.V.; Efimova, I.V.; Beletskaya, I.P. Synthesis of 1- and 2-alkoxy- and dialkylaminoalkenyl-phosphonates, catalyzed by transition metal complexes. Russ. J. Org. Chem. 1999, 35, 452–458. [Google Scholar]
- Kazankova, M.A.; Trostyanskaya, I.G.; Lutsenko, S.V.; Beletskaya, I. Nickel- and palladium-catalyzed cross-coupling as a route to 1- and 2-alkoxy- or dialkylaminovinylphosphonates. Tetrahedron Lett. 1999, 40, 569–572. [Google Scholar] [CrossRef]
- Balthazor, T.M.; Grabiak, R.C. Nickel-catalyzed Arbuzov reaction: Mechanistic observations. J. Org. Chem. 1980, 45, 5425–5426. [Google Scholar] [CrossRef]
- Heinicke, J.; Gupta, N.; Surana, A.; Peulecke, N.; Witt, B.; Steinhauser, K.; Bansal, R.K.; Jones, P.G. Synthesis of 1H-1,3-benzazaphospholes: Substituent influence and mechanistical aspects. Tetrahedron 2001, 57, 9963–9972. [Google Scholar] [CrossRef]
- Yang, G.; Shen, C.; Zhang, L.; Zhang, W. Nickel-catalyzed Arbuzov reactions of aryl triflates with triethyl phosphite. Tetrahedron Lett. 2011, 52, 5032–5035. [Google Scholar] [CrossRef]
- Hirao, T.; Masunaga, T.; Yamada, N.; Ohshiro, Y.; Agawa, T. Palladium-catalyzed New Carbon-Phosphorus Bond Formation. Bull. Chem. Soc. Jpn. 1982, 55, 909–913. [Google Scholar] [CrossRef]
- Lu, X.; Zhu, J. Nickel(0)-catalyzed Reaction of O,O-Dialkyl Phosphonates with Allyl Acetates or Carbonates. A Novel Method of Preparing Allyl Phosphonates. Synthesis 1986, 1986, 563–564. [Google Scholar] [CrossRef]
- Lu, X.; Tao, X.; Zhu, J.; Sun, X.; Xu, J. Regio- and Stereoselective Synthesis of Symmetrical and Unsymmetrical 1,3-Diphosphoryl(Phosphonyl, Phosphinyl) Substituted (E)-Propenes. Synthesis 1989, 1989, 848–850. [Google Scholar] [CrossRef]
- Yang, J.; Xiao, J.; Chen, T.; Han, L.-B. Nickel-catalyzed phosphorylation of aryl triflates with P(O)H compounds. J. Organomet. Chem. 2016, 820, 120–124. [Google Scholar] [CrossRef]
- Yang, J.; Chen, T.; Han, L.-B. C–P Bond-Forming Reactions via C–O/P–H Cross-Coupling Catalyzed by Nickel. J. Am. Chem. Soc. 2015, 137, 1782–1785. [Google Scholar] [CrossRef]
- Yang, J.; Xiao, J.; Chen, T.; Han, L.-B. Nickel-Catalyzed Phosphorylation of Phenol Derivatives via C–O/P–H Cross-Coupling. J. Org. Chem. 2016, 81, 3911–3916. [Google Scholar] [CrossRef]
- Liao, L.-L.; Gui, Y.-Y.; Zhang, X.-B.; Shen, G.; Liu, H.-D.; Zhou, W.-J.; Li-Li, L.; Yong-Yuan, G. Phosphorylation of Alkenyl and Aryl C–O Bonds via Photoredox/Nickel Dual Catalysis. Org. Lett. 2017, 19, 3735–3738. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Wang, Z.-X. Ni-Catalyzed C–P Coupling of Aryl, Benzyl, or Allyl Ammonium Salts with P(O)H Compounds. J. Org. Chem. 2019, 84, 1500–1509. [Google Scholar] [CrossRef]
- Yang, J.; Xiao, J.; Chen, T.; Yin, S.-F.; Han, L.-B. Efficient nickel-catalyzed phosphinylation of C–S bonds forming C–P bonds. Chem. Commun. 2016, 52, 12233–12236. [Google Scholar] [CrossRef]
- Zhang, J.-S.; Chen, T.; Yang, J.; Han, L.-B. Nickel-catalysed P–C bond formation via P–H/C–CN cross coupling reactions. Chem. Commun. 2015, 51, 7540–7542. [Google Scholar] [CrossRef]
- Sun, M.; Zhang, H.-Y.; Han, Q.; Yang, K.; Yang, S.-D. Nickel-Catalyzed C–P Cross-Coupling by C–CN Bond Cleavage. Chem. A Eur. J. 2011, 17, 9566–9570. [Google Scholar] [CrossRef]
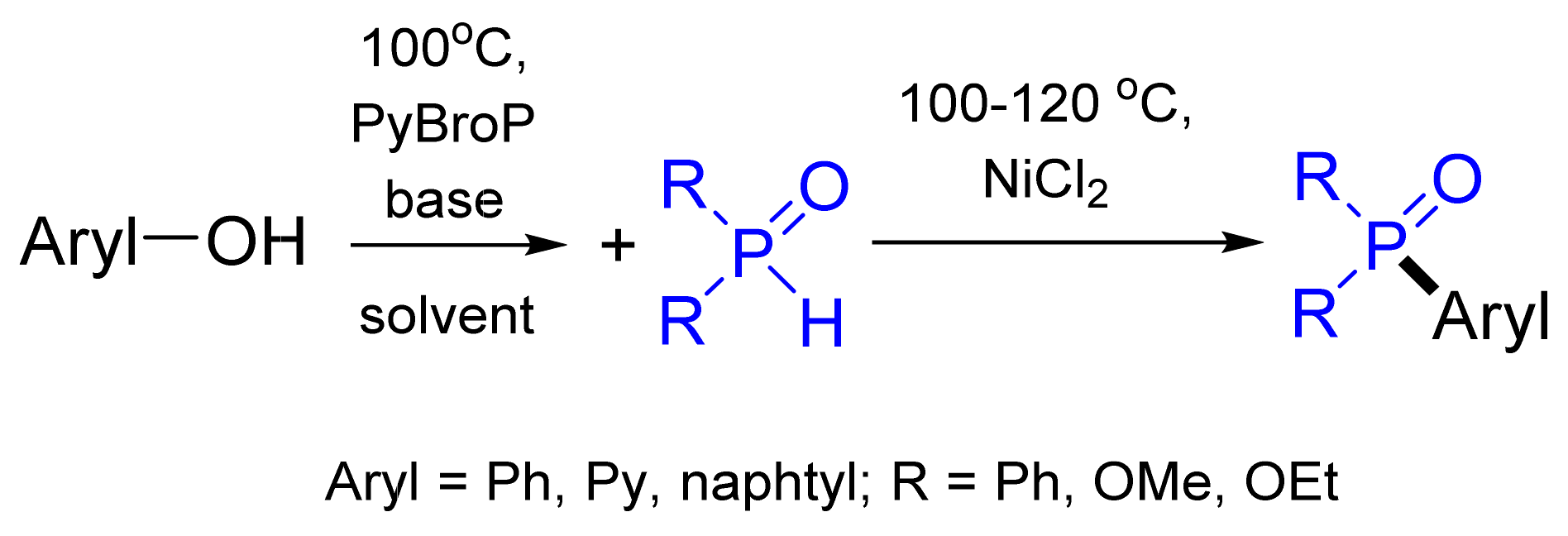
- Zhao, Y.-L.; Wu, G.-J.; Han, F.-S. Ni-catalyzed construction of C–P bonds from electron-deficient phenols via the in situ aryl C–O activation by PyBroP. Chem. Commun. 2012, 48, 5868–5870. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liu, L.L.; Yan, K.; Xu, P.; Gao, Y.; Zhao, Y. Nickel-Catalyzed Decarboxylative C–P cross-coupling of alkenyl acids with P(O)H compounds. J. Org. Chem. 2014, 79, 8118–8127. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Szostak, M. Decarbonylative phosphorylation of amides by palladium and nickel catalysis: The Hirao cross-coupling of amide derivatives. Angew. Chem. Int. Ed. 2017, 56, 12718–12722. [Google Scholar] [CrossRef]
- Jablonkai, E.; Balazs, L.; Keglevich, G. A P-ligand-free nickel-catalyzed variation of the hirao reaction under microwave conditions. Curr. Org. Chem. 2015, 19, 197–202. [Google Scholar] [CrossRef]
- Keglevich, G.; Henyecz, R.; Mucsi, Z. Focusing on the Catal. of the Pd- and Ni-Catalyzed Hirao Reactions. Molecules 2020, 25, 3897. [Google Scholar] [CrossRef] [PubMed]
- Henyecz, R.; Mucsi, Z.; Keglevich, G. A surprising mechanism lacking the Ni(0) state during the Ni(II)-catalyzed P–C cross-coupling reaction performed in the absence of a reducing agent–An experimental and a theoretical study. Pure Appl. Chem. 2020, 92, 493–503. [Google Scholar] [CrossRef]
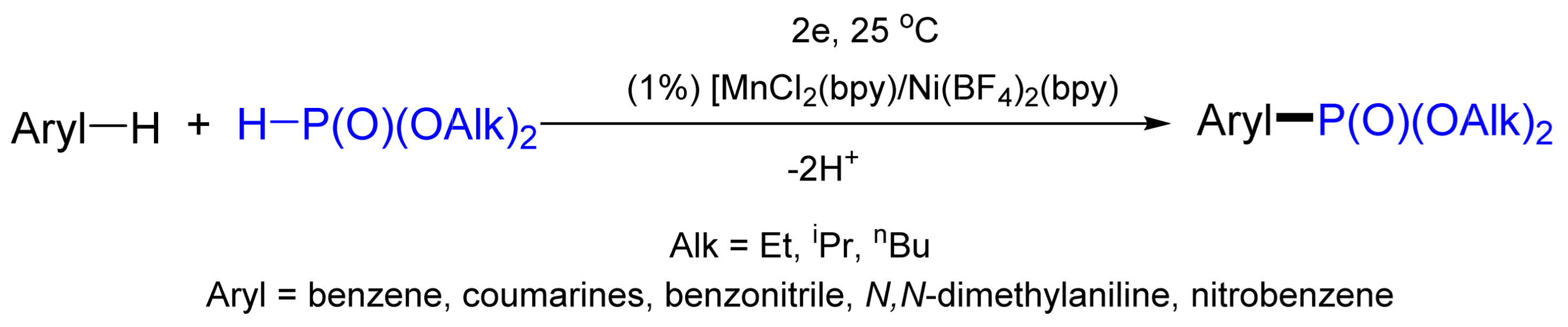
- Khrizanforov, M.; Strekalova, S.; Kholin, K.; Khrizanforova, V.; Kadirov, M.; Gryaznova, T.; Budnikova, Y. Novel approach to metal-induced oxidative phosphorylation of aromatic compounds. Catal. Today 2017, 279, 133–141. [Google Scholar] [CrossRef]
- Strekalova, S.; Khrizanforov, M.; Sinyashin, O.; Budnikova, Y. Catalytic Phosphorylation of Aromatic C-H Bonds: From Traditional Approaches to Electrochemistry. Curr. Org. Chem. 2019, 23, 1756–1770. [Google Scholar] [CrossRef]
- Gryaznova, T.V.; Khrizanforov, M.; Strekalova, S.O.; Budnikova, Y.H.; Sinyshin, O.G. Electrochemical oxidative phosphonation of azoles. Phosphorus Sulfur Silicon Relat. Elem. 2016, 191, 1658–1659. [Google Scholar] [CrossRef]
- Budnikova, Y.H.; Gryaznova, T.V.; Grinenko, V.; Dudkina, Y.; Khrizanforov, M. Eco-efficient electrocatalytic C–P bond formation. Pure Appl. Chem. 2017, 89, 311–330. [Google Scholar] [CrossRef]





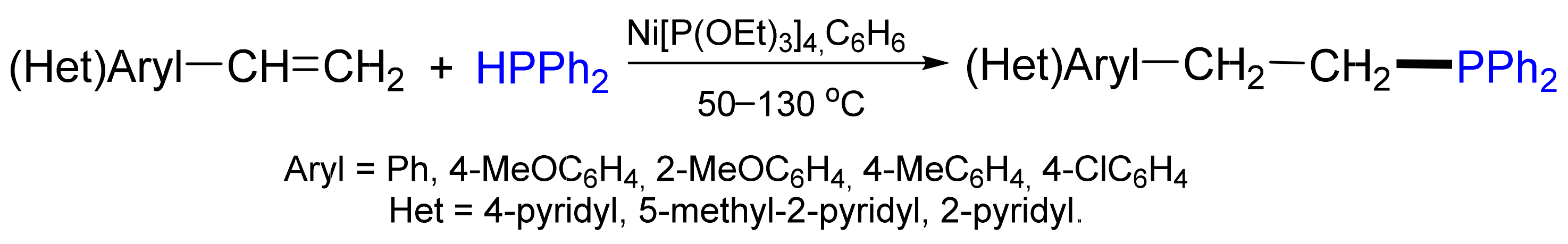

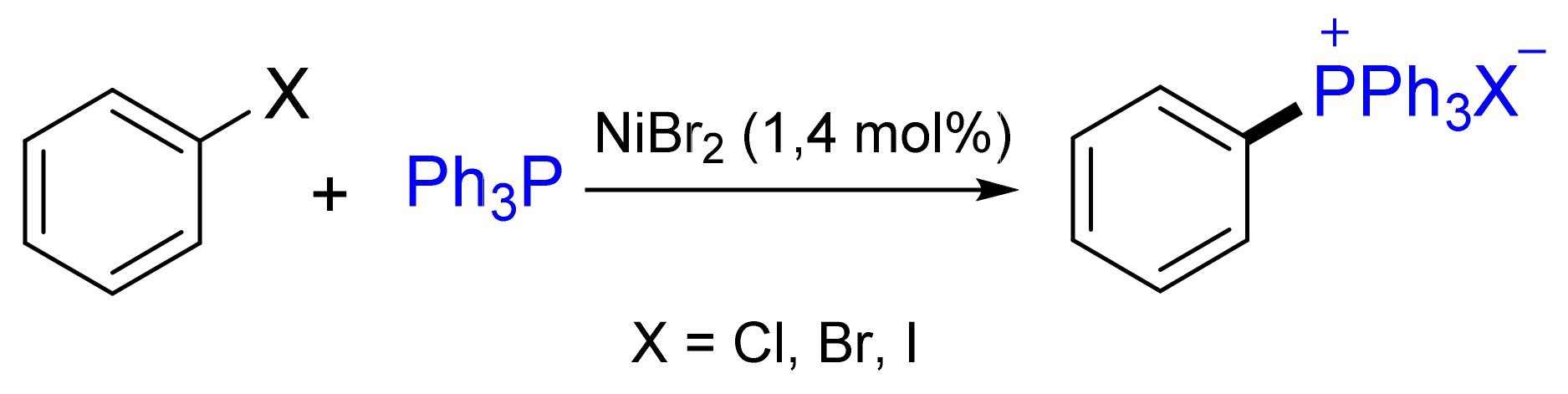

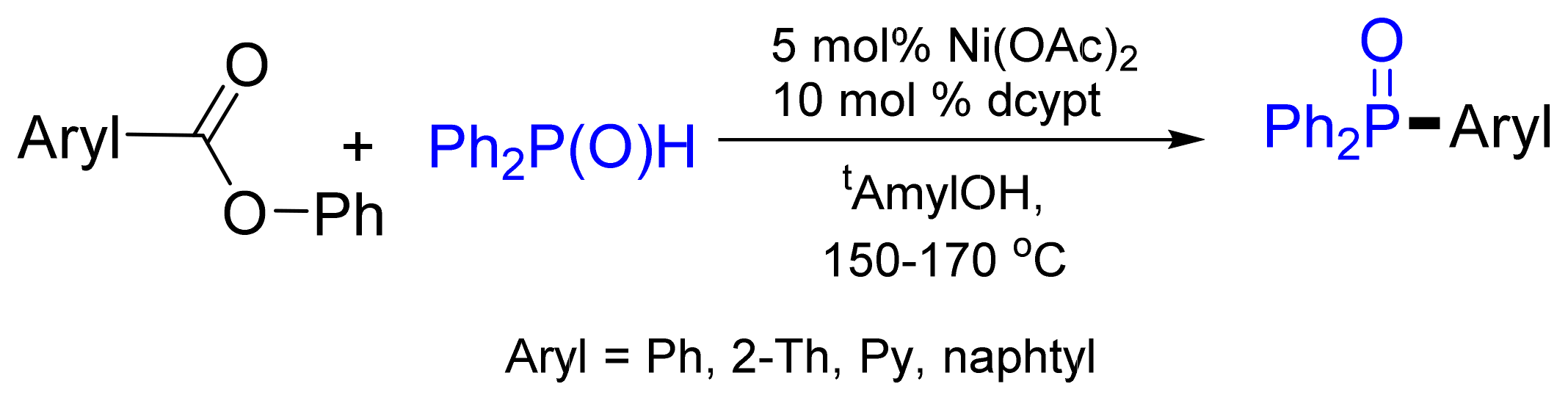

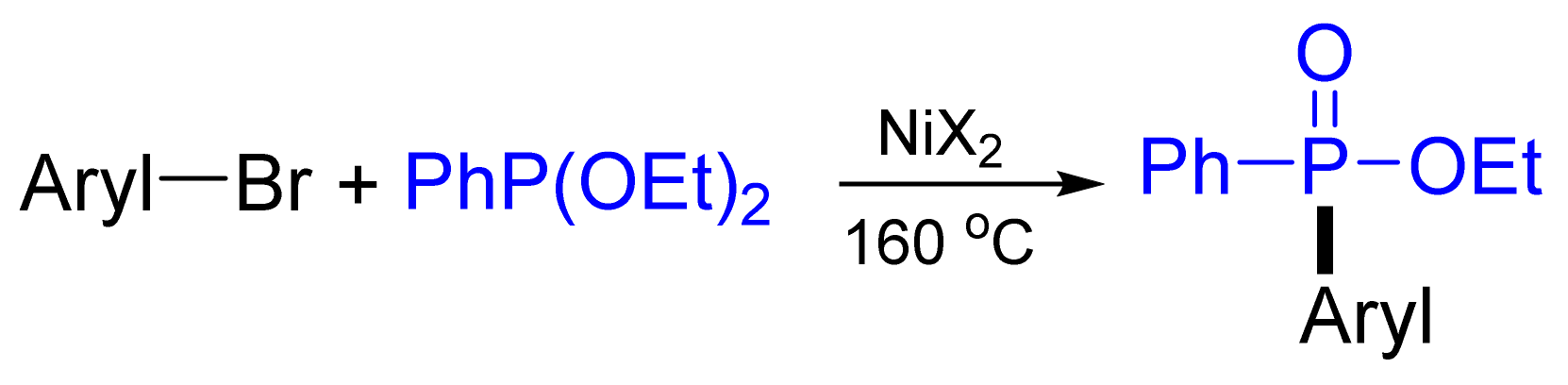
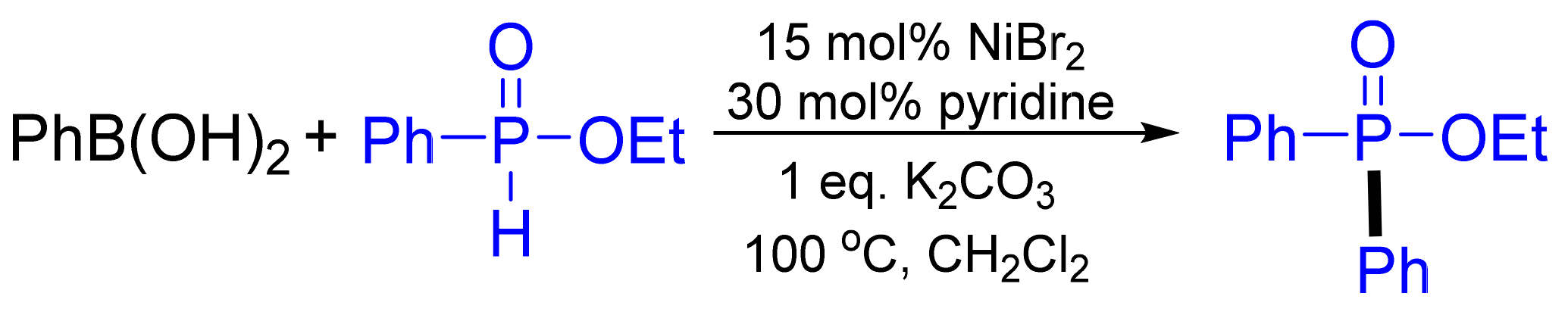
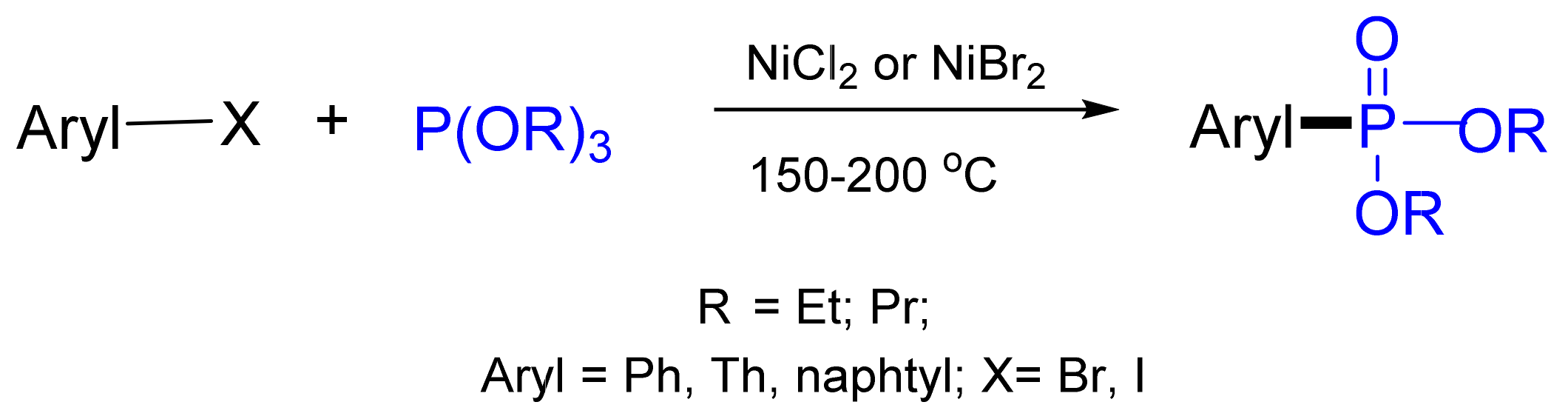
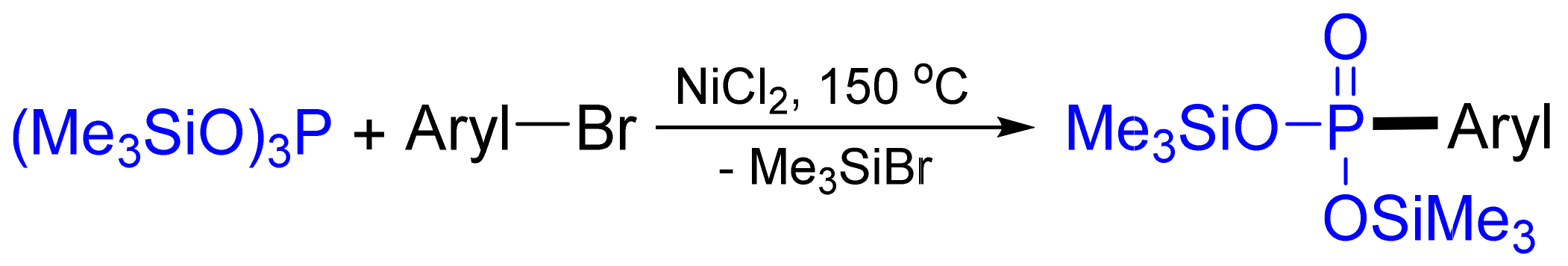




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zagidullin, A.A.; Sakhapov, I.F.; Miluykov, V.A.; Yakhvarov, D.G. Nickel Complexes in C‒P Bond Formation. Molecules 2021, 26, 5283. https://doi.org/10.3390/molecules26175283
Zagidullin AA, Sakhapov IF, Miluykov VA, Yakhvarov DG. Nickel Complexes in C‒P Bond Formation. Molecules. 2021; 26(17):5283. https://doi.org/10.3390/molecules26175283
Chicago/Turabian StyleZagidullin, Almaz A., Il’yas F. Sakhapov, Vasili A. Miluykov, and Dmitry G. Yakhvarov. 2021. "Nickel Complexes in C‒P Bond Formation" Molecules 26, no. 17: 5283. https://doi.org/10.3390/molecules26175283
APA StyleZagidullin, A. A., Sakhapov, I. F., Miluykov, V. A., & Yakhvarov, D. G. (2021). Nickel Complexes in C‒P Bond Formation. Molecules, 26(17), 5283. https://doi.org/10.3390/molecules26175283





