Organic Functionalized Carbon Nanostructures for Solar Energy Conversion
Abstract
:1. Introduction
2. Chemistry of Carbon Nanostructures
3. Dye-Sensitized Solar Cells
4. Perovskite and Other Hybrid and Organic Photovoltaics
5. Photocatalytic Fuel Production
6. Conclusions and/or Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations and Acronyms
| AFM | atomic force microscopy |
| BNAH | 1-benzyl-1,4-dihydronicotinamide |
| CDT1 | carbonaceous material binding peptide-Dps-titanium binding peptide |
| CHP | 1-cyclohexyl-2-pyrrolidone |
| CNS | carbon nanostructure |
| CNT | carbon nanotube |
| DFT | density functional theory |
| DLS | dynamic light scattering |
| DMF | dimethylformamide |
| Dps | DNA-binding protein from starved cells |
| DSSC | dye-sensitized solar cell |
| D–π–A | donor–π–acceptor system |
| EPR | electron paramagnetic resonance spectroscopy |
| EQE | external quantum efficiency |
| ETM | electron-transporting materials |
| FD | functionalization degree |
| FGO | polymerizable styryl-functionalized GO |
| f-MWCNTs-PIN | carboxylic acid-functionalized multi-walled carbon nanotubes-polyindole |
| FT-IR | Fourier-transform infrared spectroscopy |
| FTO | glass |
| GBM | graphene-based materials |
| GDQ | graphene quantum dot |
| GO | graphene oxide |
| GQD | graphene quantum dots |
| H2SO4 | sulfuric acid |
| HEL | hole-extraction layer |
| HiPco | high-pressure carbon monoxide |
| HNO3 | nitric acid |
| HOMO | highest occupied molecular orbital |
| HTM | hole-transporting materials |
| IHM | [FeFe]-hydrogenase model complex |
| ITO | indium tin oxide electrode |
| Jsc | short current density |
| LUMO | lowest unoccupied molecular orbital |
| MnPcG | manganese phthalocyanine GO hybrid |
| MnTPP | tetraphenylporphyrin |
| MOCPc | metallo-octacarboxyphthalocyanines |
| MOF | metal–organic framework |
| MPC | metallophthalocyanines |
| MtrC/OmcA | membrane-bound redox proteins |
| MV | methyl viologen |
| MWCNT | multi-walled carbon nanotube |
| N719 | Di-tetrabutylammonium cis-bis(isothiocyanato)bis(2,2′-bipyridyl-4,4′-dicarboxylato)ruthenium(II) |
| NE | norepinephrine |
| NH2-TPA-Th-H | triphenylamine-thiophene-cyan acrylic acid |
| NMR | nuclear magnetic resonance |
| OPVs | organic photovoltaics |
| P3HT | poly-3-hexyl-thiophene |
| PBC | porous biomass carbon |
| PCE | power conversion efficiency |
| PEDOT:PSS | poly(3,4-ethylenedioxythiophene)polystyrene sulfonate |
| PhBiTh | 4-[(2-20-bithiophene)-5-yl]phenyl |
| PhMeTh | 4-(5-methylthien-2-yl)phenyl |
| PhOEtHex | 4-[(2-ethyl)hexyloxy]phenyl |
| PhOHex | 4-(hexyloxy)phenyl |
| PhOMe | anisole |
| PhTh | 4-(thien-2-yl)phenyl |
| PIN | polyindole |
| polyNE | poly-norepinephrine |
| PRGO | polyacrylonitrile-grafted reduced graphene oxide |
| PSC | perovskite solar cell |
| RGO | reduced graphene oxide |
| rGO | reduced graphene oxide |
| SWCNT | single-walled carbon nanotube |
| TCE | transparent conducting electrode |
| TEA | triethylamine |
| TEM | transmission electron microscopy |
| TGA | thermogravimetric analysis |
| Ti[BALDH] | titanium(IV)bis(ammonium lactato)dihydroxide) |
| TiO2 | titanium dioxide |
| TPA-Et | methyl-2-cyano-3-(4-(diphenylamino)phenyl)acrylate |
| TPP | tetraphenylporphyrin |
| TPP-NH2 | tetraphenylporphyrin |
| UV–vis | ultraviolet–visible spectroscopy |
| UV–vis–NIR | ultraviolet–visible–near infrared |
| VOC | open circuit voltage |
| XPS | X-ray photoelectron spectroscopy |
| XRD | X-ray diffraction spectroscopy |
| ZnTNP–OH | 5-(4-hydroxyphenyl)-10,15,20-trinaphthylporphyrin zinc |
| ZnTNP–PAES | copolymer of phenyl sulfone, (p-amino)-phenylhydroquinone, and zinc dinaphthylporphyrin |
References
- Ganesamoorthy, R.; Sathiyan, G.; Sakthivel, P. Review: Fullerene based acceptors for efficient bulk heterojunction organic solar cell applications. Sol. Energy Mater. Sol. Cells 2017, 161, 102–148. [Google Scholar] [CrossRef]
- Po, R.; Maggini, M.; Camaioni, N. Polymer Solar Cells: Recent Approaches and Achievements. J. Phys. Chem. C 2010, 114, 695–706. [Google Scholar] [CrossRef]
- Thompson, B.C.; Frechet, J.M.J. Organic photovoltaics—Polymer-fullerene composite solar cells. Angew. Chem.-Int. Ed. 2008, 47, 58–77. [Google Scholar] [CrossRef]
- Gatti, T.; Menna, E.; Meneghetti, M.; Maggini, M.; Petrozza, A.; Lamberti, F. The Renaissance of fullerenes with perovskite solar cells. Nano Energy 2017, 41, 84–100. [Google Scholar] [CrossRef]
- Gatti, T.; Menna, E. Use of Carbon Nanostructures in Hybrid Photovoltaic Devices. In Photoenergy and Thin Film Materials; Yang, X.-Y., Ed.; Scrivener Publishing: Beverly, MA, USA, 2019; pp. 1–47. [Google Scholar]
- Cataldo, S.; Salice, P.; Menna, E.; Pignataro, B. Carbon nanotubes and organic solar cells. Energy Environ. Sci. 2012, 5, 5919–5940. [Google Scholar] [CrossRef]
- Petridis, C.; Kakavelakis, G.; Kymakis, E. Renaissance of graphene-related materials in photovoltaics due to the emergence of metal halide perovskite solar cells. Energy Environ. Sci. 2018, 11, 1030–1061. [Google Scholar] [CrossRef]
- Albero, J.; Mateo, D.; Garcia, H. Graphene-Based Materials as Efficient Photocatalysts for Water Splitting. Molecules 2019, 24, 906. [Google Scholar] [CrossRef] [Green Version]
- Amollo, T.A.; Mola, G.T.; Nyamori, V.O. Organic solar cells: Materials and prospects of graphene for active and interfacial layers. Crit. Rev. Solid State Mater. Sci. 2020, 45, 261–288. [Google Scholar] [CrossRef]
- Das, S.; Pandey, D.; Thomas, J.; Roy, T. The Role of Graphene and Other 2D Materials in Solar Photovoltaics. Adv. Mater. 2019, 31, 1802722. [Google Scholar] [CrossRef] [Green Version]
- Jeon, I.; Matsuo, Y.; Maruyama, S. Single-Walled Carbon Nanotubes in Solar Cells. Top. Curr. Chem. 2018, 376, 4. [Google Scholar] [CrossRef]
- Paulo, S.; Palomares, E.; Martinez-Ferrero, E. Graphene and Carbon Quantum Dot-Based Materials in Photovoltaic Devices: From Synthesis to Applications. Nanomaterials 2016, 6, 157. [Google Scholar] [CrossRef] [PubMed]
- Loh, K.P.; Tong, S.W.; Wu, J.S. Graphene and Graphene-like Molecules: Prospects in Solar Cells. J. Am. Chem. Soc. 2016, 138, 1095–1102. [Google Scholar] [CrossRef]
- Gatti, T.; Vicentini, N.; Mba, M.; Menna, E. Organic Functionalized Carbon Nanostructures for Functional Polymer-Based Nanocomposites. Eur. J. Org. Chem. 2016, 2016, 1071–1090. [Google Scholar] [CrossRef]
- Wick, P.; Louw-Gaume, A.E.; Kucki, M.; Krug, H.F.; Kostarelos, K.; Fadeel, B.; Dawson, K.A.; Salvati, A.; Vazquez, E.; Ballerini, L.; et al. Classification Framework for Graphene-Based Materials. Angew. Chem.-Int. Ed. 2014, 53, 7714–7718. [Google Scholar] [CrossRef] [Green Version]
- Gavrel, G.; Jousselme, B.; Filoramo, A.; Campidelli, S.; Marcaccio, M.; Paolucci, F. Supramolecular Chemistry of Carbon Nanotubes. Mak. Exploit. Fuller. Graphene Carbon Nanotub. 2014, 348, 95–126. [Google Scholar] [CrossRef]
- Maggini, M.; Scorrano, G.; Prato, M. Addition of azomethine ylides to C-60—Synthesis, characterization, and functionalization of fullerene pyrrolidines. J. Am. Chem. Soc. 1993, 115, 9798–9799. [Google Scholar] [CrossRef]
- Georgakilas, V.; Kordatos, K.; Prato, M.; Guldi, D.M.; Holzinger, M.; Hirsch, A. Organic functionalization of carbon nanotubes. J. Am. Chem. Soc. 2002, 124, 760–761. [Google Scholar] [CrossRef]
- Quintana, M.; Spyrou, K.; Grzelczak, M.; Browne, W.R.; Rudolf, P.; Prato, M. Functionalization of Graphene via 1,3-Dipolar Cycloaddition. ACS Nano 2010, 4, 3527–3533. [Google Scholar] [CrossRef] [Green Version]
- Koutsioukis, A.; Belessi, V.; Georgakilas, V. Solid phase functionalization of MWNTs: An eco-friendly approach for carbon-based conductive inks. Green Chem. 2021, 23, 5442–5448. [Google Scholar] [CrossRef]
- Garrido, M.; Gualandi, L.; Di Noja, S.; Filippini, G.; Bosi, S.; Prato, M. Synthesis and applications of amino-functionalized carbon nanomaterials. Chem. Commun. 2020, 56, 12698–12716. [Google Scholar] [CrossRef]
- Bahr, J.L.; Tour, J.M. Highly functionalized carbon nanotubes using in situ generated diazonium compounds. Chem. Mater. 2001, 13, 3823–3824. [Google Scholar] [CrossRef]
- Salice, P.; Fabris, E.; Sartorio, C.; Fenaroli, D.; Figà, V.; Casaletto, M.P.; Cataldo, S.; Pignataro, B.; Menna, E. An Insight into the Functionalisation of Carbon Nanotubes by Diazonium Chemistry: Towards a Controlled Decoration. Carbon 2014, 74, 73–82. [Google Scholar] [CrossRef]
- Mo, F.Y.; Qiu, D.; Zhang, L.; Wang, J.B. Recent Development of Aryl Diazonium Chemistry for the Derivatization of Aromatic Compounds. Chem. Rev. 2021, 121, 5741–5829. [Google Scholar] [CrossRef] [PubMed]
- Sinitskii, A.; Dimiev, A.; Corley, D.A.; Fursina, A.A.; Kosynkin, D.V.; Tour, J.M. Kinetics of Diazonium Functionalization of Chemically Converted Graphene Nanoribbons. ACS Nano 2010, 4, 1949–1954. [Google Scholar] [CrossRef]
- Liu, J.; Rinzler, A.G.; Dai, H.J.; Hafner, J.H.; Bradley, R.K.; Boul, P.J.; Lu, A.; Iverson, T.; Shelimov, K.; Huffman, C.B.; et al. Fullerene pipes. Science 1998, 280, 1253–1256. [Google Scholar] [CrossRef]
- Jonoush, Z.A.; Farahani, M.; Bohlouli, M.; Niknam, Z.; Golchin, A.; Hatamie, S.; Rezaei-Tavirani, M.; Omidi, M.; Zali, H. Surface Modification of Graphene and its Derivatives for Drug Delivery Systems. Mini-Rev. Org. Chem. 2021, 18, 78–92. [Google Scholar] [CrossRef]
- Sun, Y.P.; Fu, K.F.; Lin, Y.; Huang, W.J. Functionalized carbon nanotubes: Properties and applications. Acc. Chem. Res. 2002, 35, 1096–1104. [Google Scholar] [CrossRef]
- Perez, E.; Martin, N. pi-pi interactions in carbon nanostructures. Chem. Soc. Rev. 2015, 44, 6425–6433. [Google Scholar] [CrossRef]
- Salice, P.; Gambarin, A.; Daldosso, N.; Mancin, F.; Menna, E. Noncovalent Interaction between Single-Walled Carbon Nanotubes and Pyrene-Functionalized Gold Nanoparticles in Water-Soluble Nanohybrids. J. Phys. Chem. C 2014, 118, 27028–27038. [Google Scholar] [CrossRef]
- Wang, H.; Bi, S.G.; Ye, Y.S.; Xue, Y.; Xie, X.L.; Mai, Y.W. An effective non-covalent grafting approach to functionalize individually dispersed reduced graphene oxide sheets with high grafting density, solubility and electrical conductivity. Nanoscale 2015, 7, 3548–3557. [Google Scholar] [CrossRef]
- Ehli, C.; Guldi, D.M.; Herranz, M.A.; Martin, N.; Campidelli, S.; Prato, M. Pyrene-tetrathiafulvalene supramolecular assembly with different types of carbon nanotubes. J. Mater. Chem. 2008, 18, 1498–1503. [Google Scholar] [CrossRef]
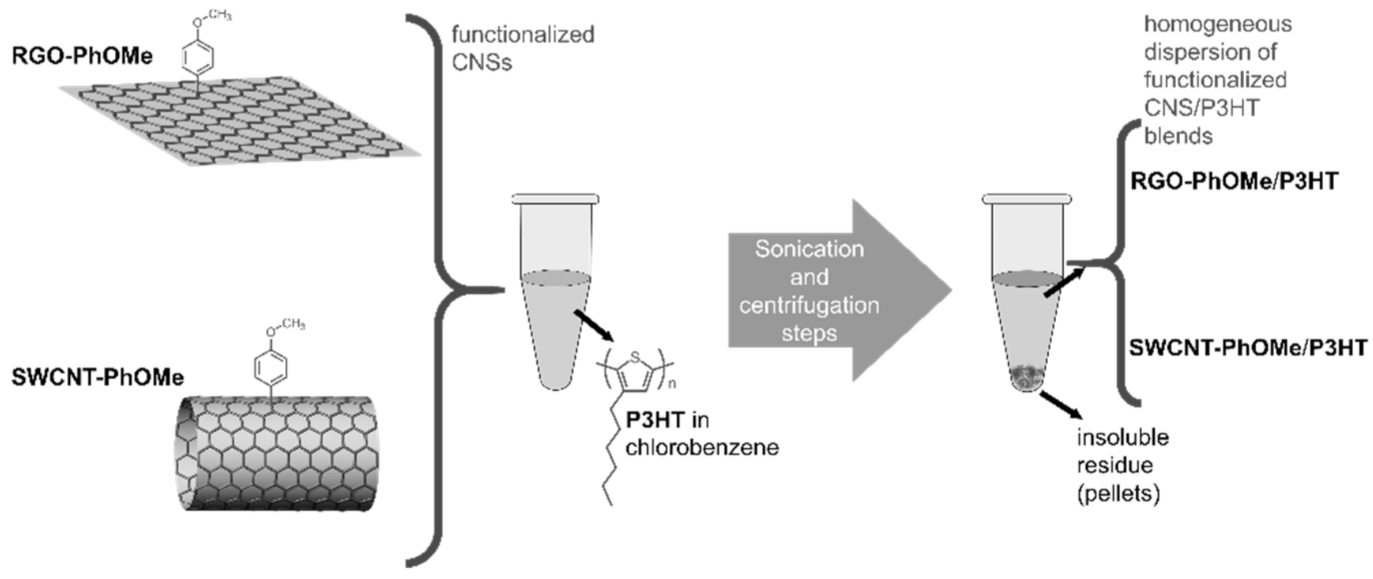
- Samanta, S.K.; Fritsch, M.; Scherf, U.; Gomulya, W.; Bisri, S.Z.; Loi, M.A. Conjugated Polymer-Assisted Dispersion of Single-Wall Carbon Nanotubes: The Power of Polymer Wrapping. Acc. Chem. Res. 2014, 47, 2446–2456. [Google Scholar] [CrossRef]
- Gomulya, W.; Gao, J.; Loi, M. Conjugated polymer-wrapped carbon nanotubes: Physical properties and device applications. Eur. Phys. J. B 2013, 86, 404. [Google Scholar] [CrossRef]
- Monteiro, A.R.; Neves, M.; Trindade, T. Functionalization of Graphene Oxide with Porphyrins: Synthetic Routes and Biological Applications. Chempluschem 2020, 85, 1857–1880. [Google Scholar] [CrossRef] [PubMed]
- Umadevi, D.; Panigrahi, S.; Sastry, G.N. Noncovalent Interaction of Carbon Nanostructures. Acc. Chem. Res. 2014, 47, 2574–2581. [Google Scholar] [CrossRef]
- Martín, N.; Nierengarten, J.-F. (Eds.) Supramolecular Chemistry of Fullerenes and Carbon Nanotubes; Wiley: Weinheim, Germany, 2012. [Google Scholar]
- Yuan, K.; Zhao, R.-S.; Zheng, J.-J.; Zheng, H.; Nagase, S.; Zhao, S.-D.; Liu, Y.-Z.; Zhao, X. Van Der Waals heterogeneous layer-layer carbon nanostructures involving π···H-C-C-H···π···H-C-C-H stacking based on graphene and graphane sheets. J. Comput. Chem. 2017, 38, 730–739. [Google Scholar] [CrossRef]
- Smith, B.W.; Monthioux, M.; Luzzi, D.E. Encapsulated C-60 in carbon nanotubes. Nature 1998, 396, 323–324. [Google Scholar] [CrossRef]
- Campestrini, S.; Corvaja, C.; De Nardi, M.; Ducati, C.; Franco, L.; Maggini, M.; Meneghetti, M.; Menna, E.; Ruaro, G. Investigation of the Inner Environment of Carbon Nanotubes with a Fullerene-Nitroxide Probe. Small 2008, 4, 350–356. [Google Scholar] [CrossRef]
- Loi, M.A.; Gao, J.; Cordella, F.; Blondeau, P.; Menna, E.; Bartova, B.; Hebert, C.; Lazar, S.; Botton, G.A.; Milko, M.; et al. Encapsulation of Conjugated Oligomers in Single-Wall Carbon Nanotubes: Towards Nanohybrids for Photonic Devices. Adv. Mater. 2010, 22, 1635–1639. [Google Scholar] [CrossRef]
- D’Este, M.; De Nardi, M.; Menna, E. A co-functionalization approach to soluble and functional Single-Walled Carbon Nanotubes. Eur. J. Org. Chem. 2006, 2006, 2517–2522. [Google Scholar] [CrossRef]
- Ahmad, A.; Kurkina, T.; Kern, K.; Balasubramanian, K. Applications of the Static Quenching of Rhodamine B by Carbon Nanotubes. Chemphyschem 2009, 10, 2251–2255. [Google Scholar] [CrossRef]
- Liu, L.; Wang, T.X.; Li, J.X.; Guo, Z.X.; Dai, L.M.; Zhang, D.Q.; Zhu, D.B. Self-assembly of gold nanoparticles to carbon nanotubes using a thiol-terminated pyrene as interlinker. Chem. Phys. Lett. 2003, 367, 747–752. [Google Scholar] [CrossRef]
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Boschloo, G.; Sun, L.C.; Kloo, L.; Pettersson, H. Dye-Sensitized Solar Cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef] [PubMed]
- Park, N.G.; Kim, K. Transparent solar cells based on dye-sensitized nanocrystalline semiconductors. Phys. Status Solidi A Appl. Mater. Sci. 2008, 205, 1895–1904. [Google Scholar] [CrossRef]
- Mishra, A.; Fischer, M.K.R.; Bäuerle, P. Metal-Free Organic Dyes for Dye-Sensitized Solar Cells: From Structure: Property Relationships to Design Rules. Angew. Chem. Int. Ed. 2009, 48, 2474–2499. [Google Scholar] [CrossRef]
- Kim, B.-G.; Chung, K.; Kim, J. Molecular Design Principle of All-organic Dyes for Dye-Sensitized Solar Cells. Chem.–A Eur. J. 2013, 19, 5220–5230. [Google Scholar] [CrossRef] [Green Version]
- Roy-Mayhew, J.D.; Aksay, I.A. Graphene Materials and Their Use in Dye-Sensitized Solar Cells. Chem. Rev. 2014, 114, 6323–6348. [Google Scholar] [CrossRef]
- Batmunkh, M.; Biggs, M.J.; Shapter, J.G. Carbon Nanotubes for Dye-Sensitized Solar Cells. Small 2015, 11, 2963–2989. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Cui, X.; Li, B.; Li, L.-S. Large, Solution-Processable Graphene Quantum Dots as Light Absorbers for Photovoltaics. Nano Lett. 2010, 10, 1869–1873. [Google Scholar] [CrossRef]
- Mak, K.F.; Sfeir, M.Y.; Wu, Y.; Lui, C.H.; Misewich, J.A.; Heinz, T.F. Measurement of the Optical Conductivity of Graphene. Phys. Rev. Lett. 2008, 101, 196405. [Google Scholar] [CrossRef]
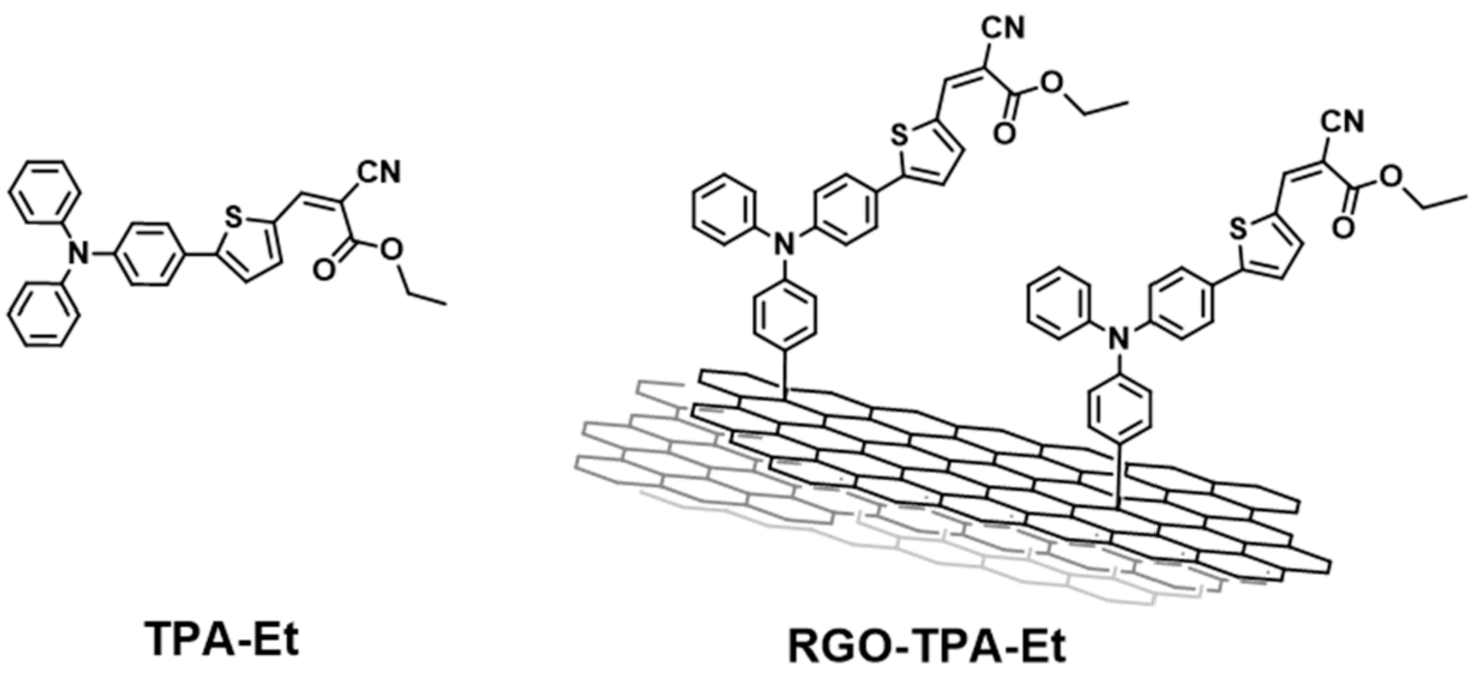
- Gatti, T.; Manfredi, N.; Boldrini, C.; Lamberti, F.; Abbotto, A.; Menna, E. A D-π-A organic dye—Reduced graphene oxide covalent dyad as a new concept photosensitizer for light harvesting applications. Carbon 2017, 115, 746–753. [Google Scholar] [CrossRef]
- Setaro, A.; Bluemmel, P.; Maity, C.; Hecht, S.; Reich, S. Non-Covalent Functionalization of Individual Nanotubes with Spiropyran-Based Molecular Switches. Adv. Funct. Mater. 2012, 22, 2425–2431. [Google Scholar] [CrossRef]
- Guarracino, P.; Gatti, T.; Canever, N.; Abdu-Aguye, M.; Loi, M.A.; Menna, E.; Franco, L. Probing photoinduced electron-transfer in graphene–dye hybrid materials for DSSC. Phys. Chem. Chem. Phys. 2017, 19, 27716–27724. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Roy, M.S.; Thomas, K.R.J.; Balaiah, S.; Bhanuprakash, K.; Sharma, G.D. New Triphenylamine-Based Organic Dyes with Different Numbers of Anchoring Groups for Dye-Sensitized Solar Cells. J. Phys. Chem. C 2012, 116, 5941–5950. [Google Scholar] [CrossRef]
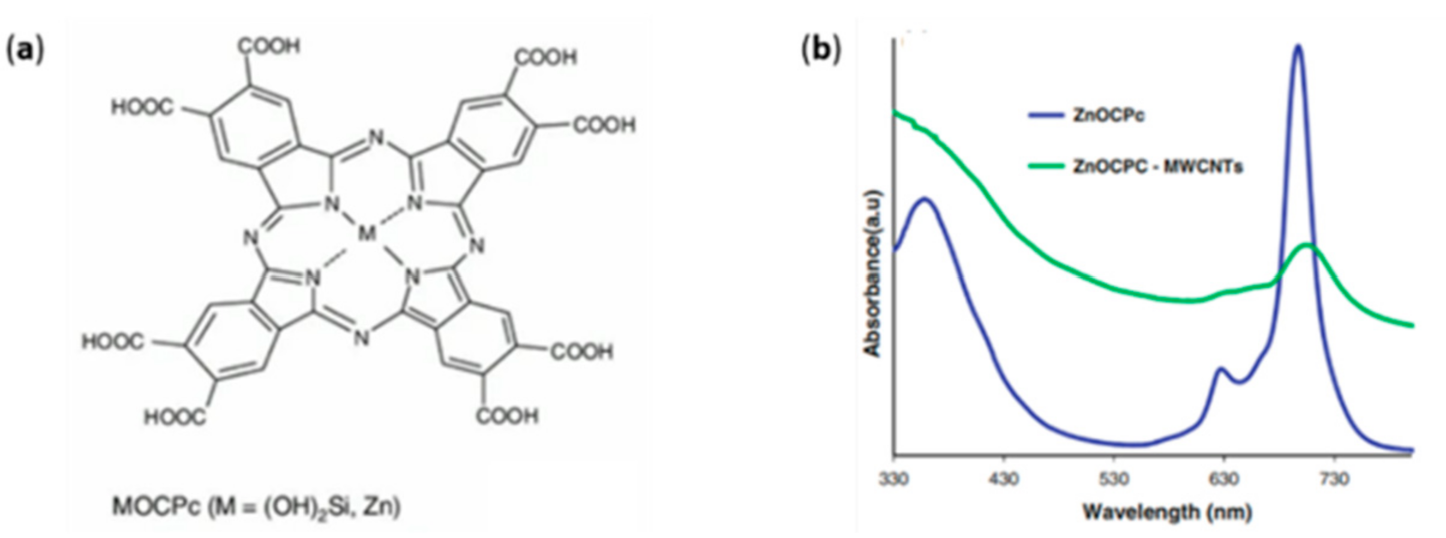
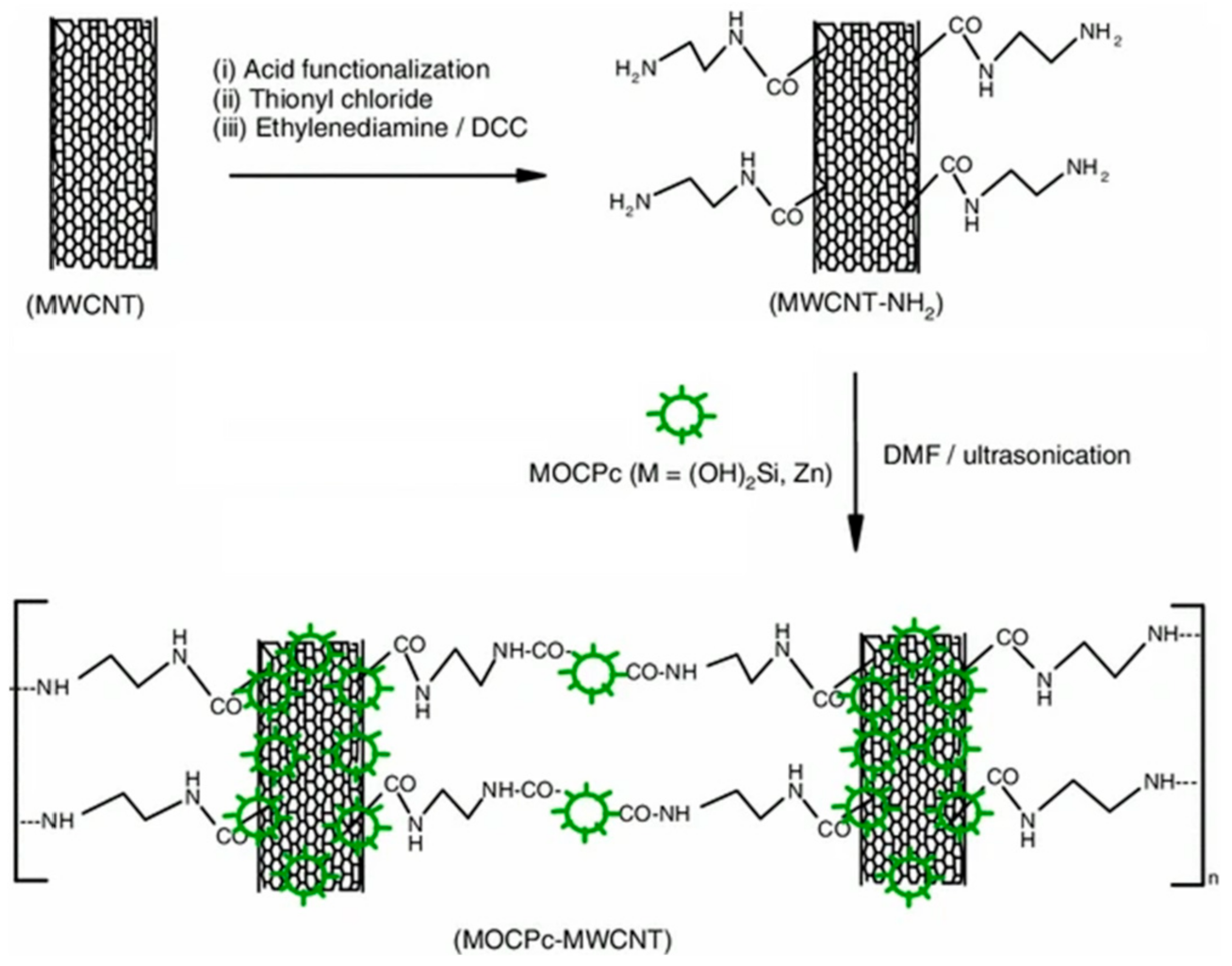
- Mphahlele, N.E.; Le Roux, L.; Jafta, C.J.; Cele, L.; Mathe, M.K.; Nyokong, T.; Kobayashi, N.; Ozoemena, K.I. Carbon nanotube-enhanced photoelectrochemical properties of metallo-octacarboxyphthalocyanines. J. Mater. Sci. 2014, 49, 340–346. [Google Scholar] [CrossRef]
- Jang, S.-R.; Vittal, R.; Kim, K.-J. Incorporation of Functionalized Single-Wall Carbon Nanotubes in Dye-Sensitized TiO2 Solar Cells. Langmuir 2004, 20, 9807–9810. [Google Scholar] [CrossRef]
- Volland, M.; Lennert, A.; Roth, A.; Ince, M.; Torres, T.; Guldi, D.M. Azulenocyanines immobilized on graphene; on the way to panchromatic absorption and efficient DSSC blocking layers. Nanoscale 2019, 11, 10709–10715. [Google Scholar] [CrossRef]
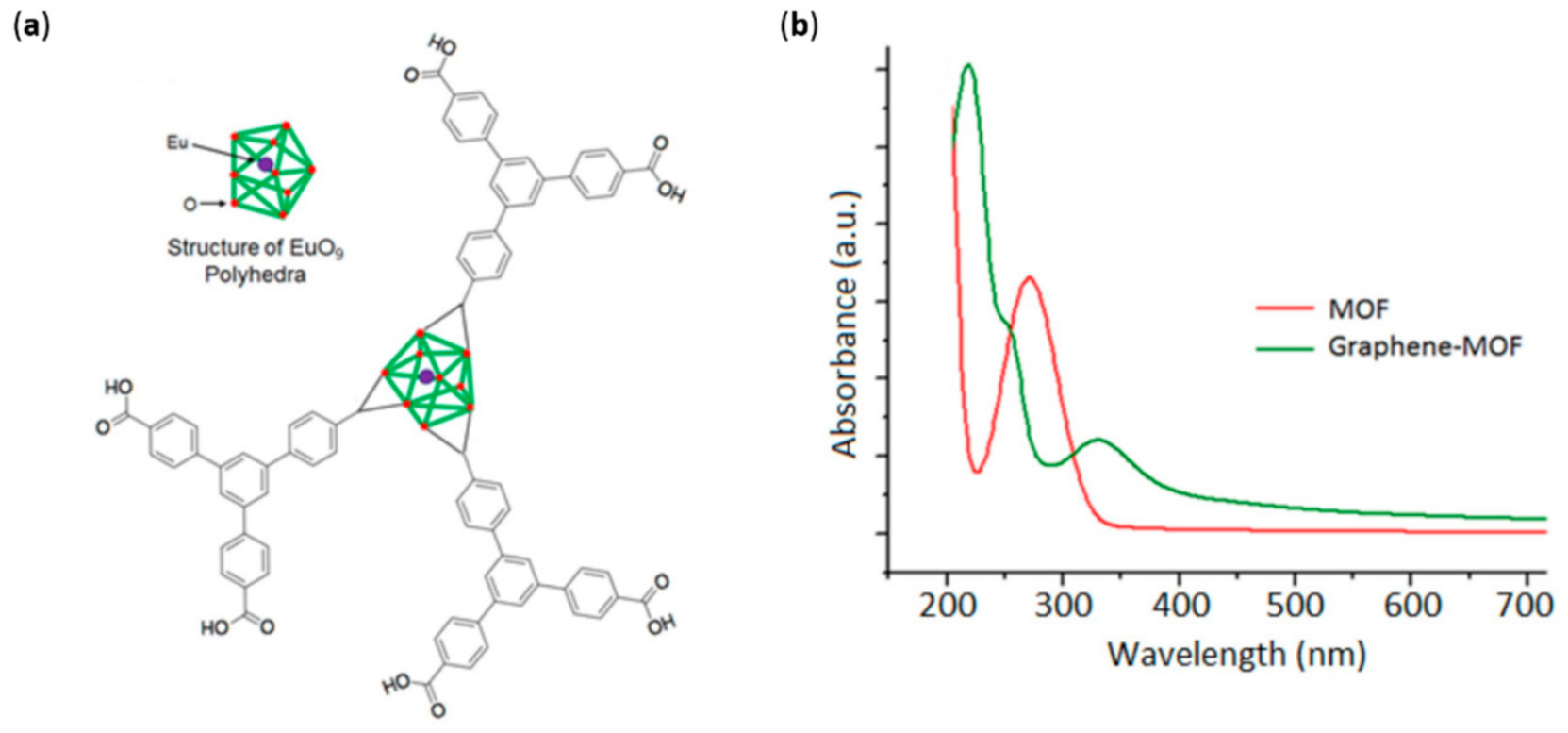
- Kaur, R.; Kim, K.-H.; Deep, A. A convenient electrolytic assembly of graphene-MOF composite thin film and its photoanodic application. Appl. Surf. Sci. 2017, 396, 1303–1309. [Google Scholar] [CrossRef]
- So, M.C.; Wiederrecht, G.P.; Mondloch, J.E.; Hupp, J.T.; Farha, O.K. Metal–organic framework materials for light-harvesting and energy transfer. Chem. Commun. 2015, 51, 3501–3510. [Google Scholar] [CrossRef]
- Lee, C.Y.; Farha, O.K.; Hong, B.J.; Sarjeant, A.A.; Nguyen, S.T.; Hupp, J.T. Light-Harvesting Metal–Organic Frameworks (MOFs): Efficient Strut-to-Strut Energy Transfer in Bodipy and Porphyrin-Based MOFs. J. Am. Chem. Soc. 2011, 133, 15858–15861. [Google Scholar] [CrossRef]
- Li, Y.; Tang, J.; He, L.; Liu, Y.; Liu, Y.; Chen, C.; Tang, Z. Core-Shell Upconversion Nanoparticle@Metal-Organic Framework Nanoprobes for Luminescent/Magnetic Dual-Mode Targeted Imaging. Adv. Mater. 2015, 27, 4075–4080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.Y.; Shinde, D.V.; Yoon, S.J.; Cho, K.N.; Lee, W.; Shrestha, N.K.; Han, S.-H. Cu-Based Metal–Organic Frameworks for Photovoltaic Application. J. Phys. Chem. C 2014, 118, 16328–16334. [Google Scholar] [CrossRef]
- Wang, W.; Cui, Q.Y.; Sun, D.; Yang, Q.; Xu, J.; Liao, W.; Zuo, X.Q.; Tang, H.B.; Li, G.; Jin, S.W. Enhanced electrocatalytic performance in dye-sensitized solar cell via coupling CoSe2@N-doped carbon and carbon nanotubes. J. Mater. Chem. C 2021, 9, 7046–7056. [Google Scholar] [CrossRef]
- Rafique, S.; Rashid, I.; Sharif, R. Cost effective dye sensitized solar cell based on novel Cu polypyrrole multiwall carbon nanotubes nanocomposites counter electrode. Sci. Rep. 2021, 11, 14830. [Google Scholar] [CrossRef] [PubMed]
- Ludin, N.A.; Al-Alwani Mahmoud, A.M.; Bakar Mohamad, A.; Kadhum, A.A.H.; Sopian, K.; Abdul Karim, N.S. Review on the development of natural dye photosensitizer for dye-sensitized solar cells. Renew. Sustain. Energy Rev. 2014, 31, 386–396. [Google Scholar] [CrossRef]
- Calogero, G.; Citro, I.; Di Marco, G.; Armeli Minicante, S.; Morabito, M.; Genovese, G. Brown seaweed pigment as a dye source for photoelectrochemical solar cells. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 117, 702–706. [Google Scholar] [CrossRef]
- Enciso, P.; Cerdá, M.F. Solar cells based on the use of photosensitizers obtained from Antarctic red algae. Cold Reg. Sci. Technol. 2016, 126, 51–54. [Google Scholar] [CrossRef]
- Armeli Minicante, S.; Ambrosi, E.; Back, M.; Barichello, J.; Cattaruzza, E.; Gonella, F.; Scantamburlo, E.; Trave, E. Development of an eco-protocol for seaweed chlorophylls extraction and possible applications in dye sensitized solar cells. J. Phys. D Appl. Phys. 2016, 49, 295601. [Google Scholar] [CrossRef]
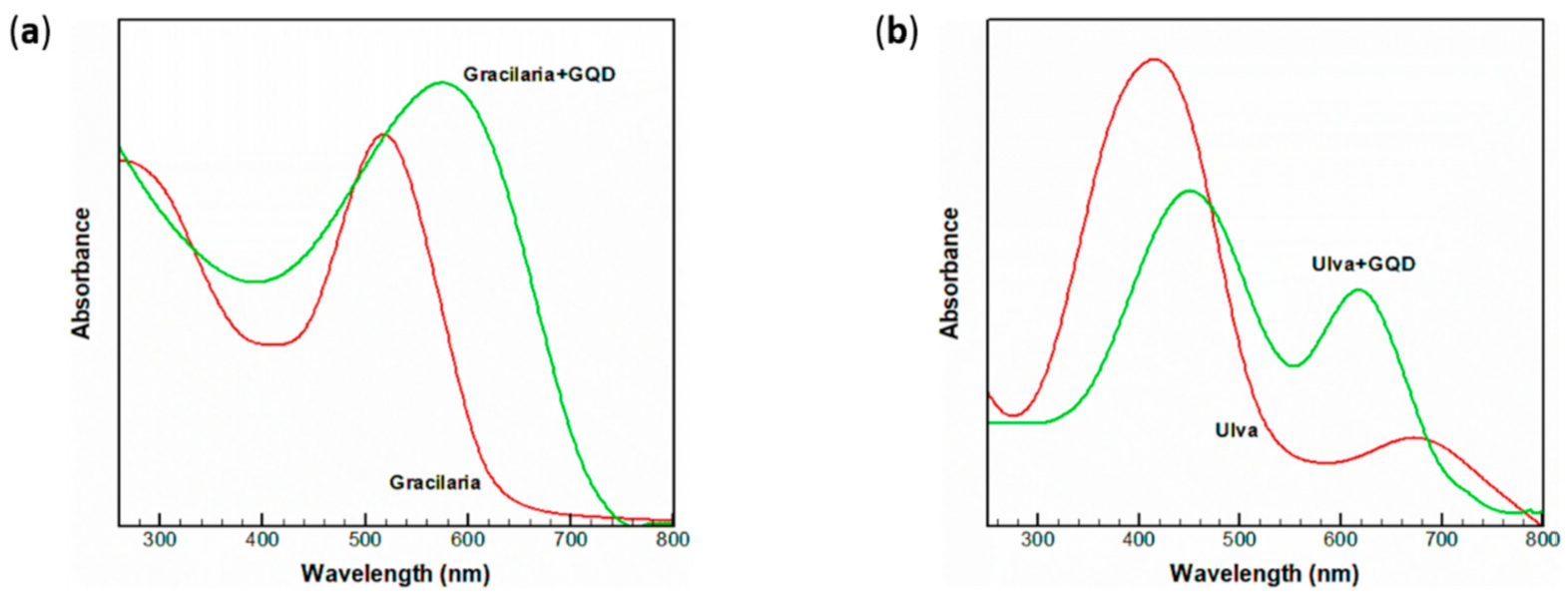
- Saedi, A.; Moradi, A.M.; Kimiagar, S.; Panahi, H.A. Efficiency Enhancement of Dye-Sensitized Solar Cells Based on Gracilaria/Ulva Using Graphene Quantum Dot. Int. J. Environ. Res. 2020, 14, 393–402. [Google Scholar] [CrossRef]
- Nguyen-Phan, T.-D.; Pham, V.H.; Shin, E.W.; Pham, H.-D.; Kim, S.; Chung, J.S.; Kim, E.J.; Hur, S.H. The role of graphene oxide content on the adsorption-enhanced photocatalysis of titanium dioxide/graphene oxide composites. Chem. Eng. J. 2011, 170, 226–232. [Google Scholar] [CrossRef]
- Zhu, Z.; Ma, J.; Wang, Z.; Mu, C.; Fan, Z.; Du, L.; Bai, Y.; Fan, L.; Yan, H.; Phillips, D.L.; et al. Efficiency Enhancement of Perovskite Solar Cells through Fast Electron Extraction: The Role of Graphene Quantum Dots. J. Am. Chem. Soc. 2014, 136, 3760–3763. [Google Scholar] [CrossRef]
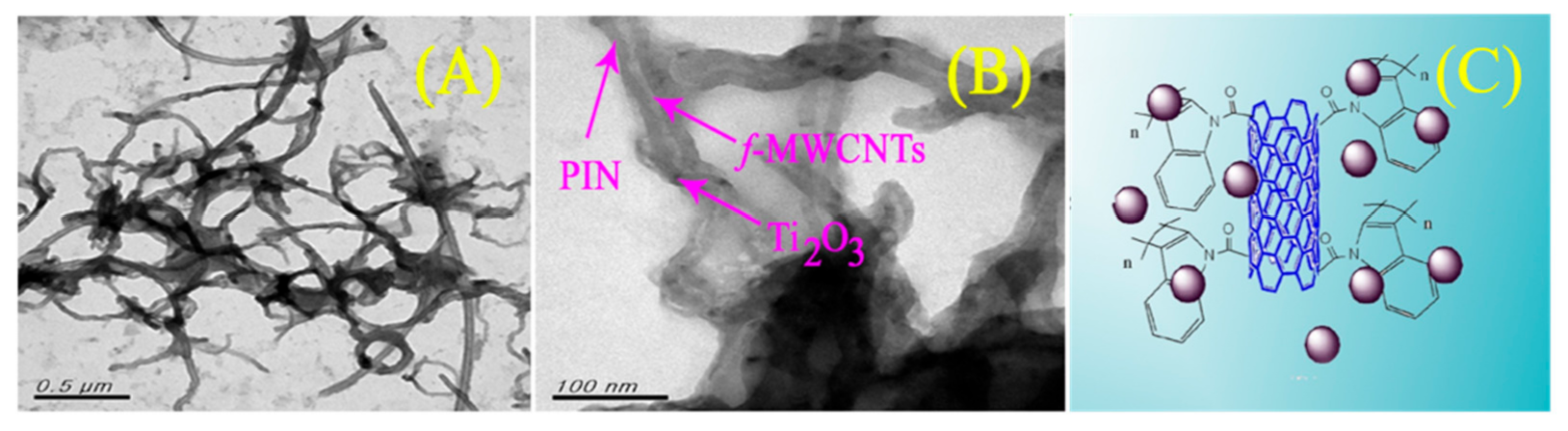
- Sireesha, P.; Sasikumar, R.; Chen, S.-M.; Su, C.; Ranganathan, P.; Rwei, S.-P. Carboxylic acid-functionalized multi-walled carbon nanotubes-polyindole/Ti2O3: A novel hybrid nanocomposite as highly efficient photo-anode for dye-sensitized solar cells (DSSCs). Appl. Surf. Sci. 2017, 423, 147–153. [Google Scholar] [CrossRef]
- Kokkonen, M.; Talebi, P.; Zhou, J.; Asgari, S.; Soomro, S.A.; Elsehrawy, F.; Halme, J.; Ahmad, S.; Hagfeldt, A.; Hashmi, S.G. Advanced research trends in dye-sensitized solar cells. J. Mater. Chem. A 2021, 9, 10527–10545. [Google Scholar] [CrossRef]
- Mahalingam, S.; Manap, A.; Omar, A.; Low, F.W.; Afandi, N.F.; Chia, C.H.; Rahim, N.A. Functionalized graphene quantum dots for dye-sensitized solar cell: Key challenges, recent developments and future prospects. Renew. Sustain. Energy Rev. 2021, 144, 110999. [Google Scholar] [CrossRef]
- Muchuweni, E.; Martincigh, B.S.; Nyamori, V.O. Recent advances in graphene-based materials for dye-sensitized solar cell fabrication. RSC Adv. 2020, 10, 44453–44469. [Google Scholar] [CrossRef]
- Samantaray, M.R.; Mondal, A.K.; Murugadoss, G.; Pitchaimuthu, S.; Das, S.; Bahru, R.; Mohamed, M.A. Synergetic Effects of Hybrid Carbon Nanostructured Counter Electrodes for Dye-Sensitized Solar Cells: A Review. Materials 2020, 13, 2779. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Song, W.; Ge, J.; Tang, B.; Zhang, X.; Wu, T.; Ge, Z. Recent progress of organic photovoltaics for indoor energy harvesting. Nano Energy 2021, 82, 105770. [Google Scholar] [CrossRef]
- Wu, X.; Liu, Y.; Qi, F.; Lin, F.; Fu, H.; Jiang, K.; Wu, S.; Bi, L.; Wang, D.; Xu, F.; et al. Improved stability and efficiency of perovskite/organic tandem solar cells with an all-inorganic perovskite layer. J. Mater. Chem. A 2021. [Google Scholar] [CrossRef]
- Dou, L.; You, J.; Hong, Z.; Xu, Z.; Li, G.; Street, R.; Yang, Y. 25th Anniversary Article: A Decade of Organic/Polymeric Photovoltaic Research. Adv. Mater. 2013, 25, 6642–6671. [Google Scholar] [CrossRef] [PubMed]
- Rafique, S.; Abdullah, S.; Sulaiman, K.; Iwamoto, M. Fundamentals of bulk heterojunction organic solar cells: An overview of stability/degradation issues and strategies for improvement. Renew. Sustain. Energy Rev. 2018, 84, 43–53. [Google Scholar] [CrossRef]
- Duan, L.; Elumalai, N.; Zhang, Y.; Uddin, A. Progress in non-fullerene acceptor based organic solar cells. Sol. Energy Mat. Sol. C 2019, 193, 22–65. [Google Scholar] [CrossRef]
- Tonui, P.; Oseni, S.; Sharma, G.; Yan, Q.; Mola, G. Perovskites photovoltaic solar cells: An overview of current status. Renew. Sustain. Energy Rev. 2018, 91, 1025–1044. [Google Scholar] [CrossRef]
- Pham, H.; Li, X.; Li, W.; Manzhos, S.; Kyaw, A.; Sonar, P. Organic interfacial materials for perovskite-based optoelectronic devices. Energy Environ. Sci. 2019, 12, 1177–1209. [Google Scholar] [CrossRef]
- Carulli, F.; Scavia, G.; Lassi, E.; Pasini, M.; Galeotti, F.; Brovelli, S.; Giovanella, U.; Luzzati, S. A bifunctional conjugated polyelectrolyte for the interfacial engineering of polymer solar cells. J. Colloid Interface Sci. 2019, 538, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Lassi, E.; Squeo, B.; Sorrentino, R.; Scavia, G.; Mrakic-Sposta, S.; Gussoni, M.; Vercelli, B.; Galeotti, F.; Pasini, M.; Luzzati, S. Sulfonate-Conjugated Polyelectrolytes as Anode Interfacial Layers in Inverted Organic Solar Cells. Molecules 2021, 26, 763. [Google Scholar] [CrossRef]
- Cho, H.; Liao, W.; Lin, W.; Yoshimura, M.; Wu, J. Pristine reduced graphene oxide as an energy-matched auxiliary electron acceptor in nanoarchitectural metal oxide/poly(3-hexylthiophene) hybrid solar cell. J. Power Source 2015, 293, 246–252. [Google Scholar] [CrossRef]
- Jiao, Y.; Ma, F.; Gao, G.; Wang, H.; Bell, J.; Frauenheim, T.; Du, A. Graphene-covered perovskites: An effective strategy to enhance light absorption and resist moisture degradation. RSC Adv. 2015, 5, 82346–82350. [Google Scholar] [CrossRef] [Green Version]
- Hu, R.; Chu, L.; Zhang, J.; Li, X.; Huang, W. Carbon materials for enhancing charge transport in the advancements of perovskite solar cells. J. Power Source 2017, 361, 259–275. [Google Scholar] [CrossRef]
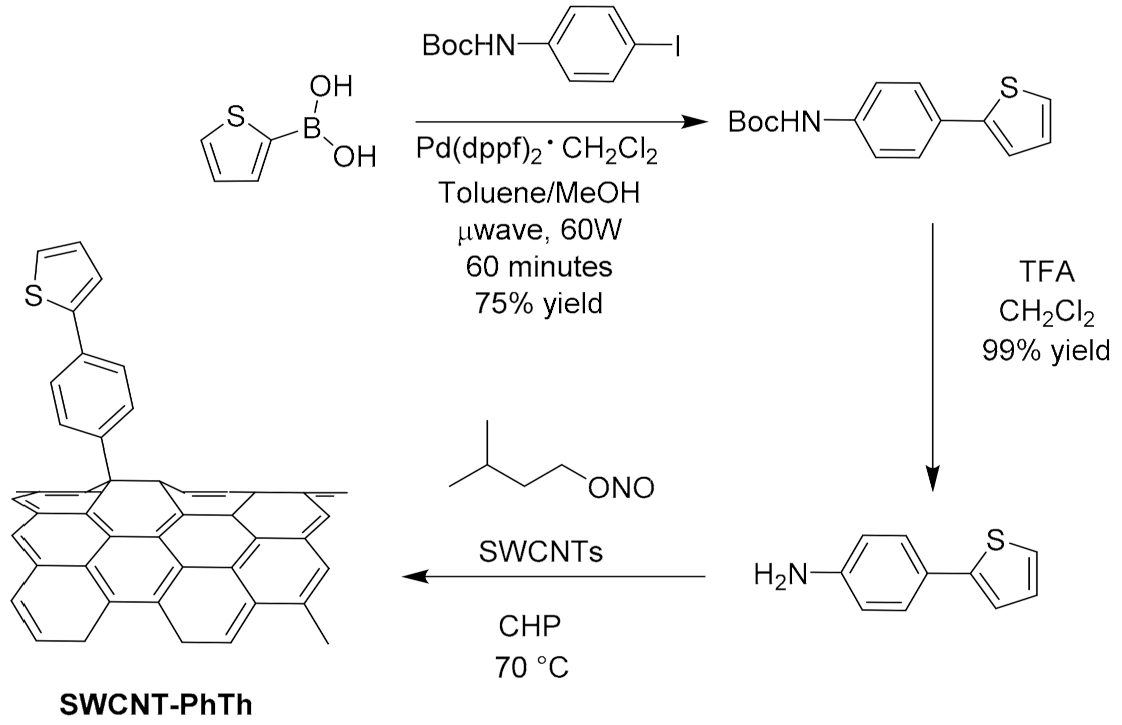
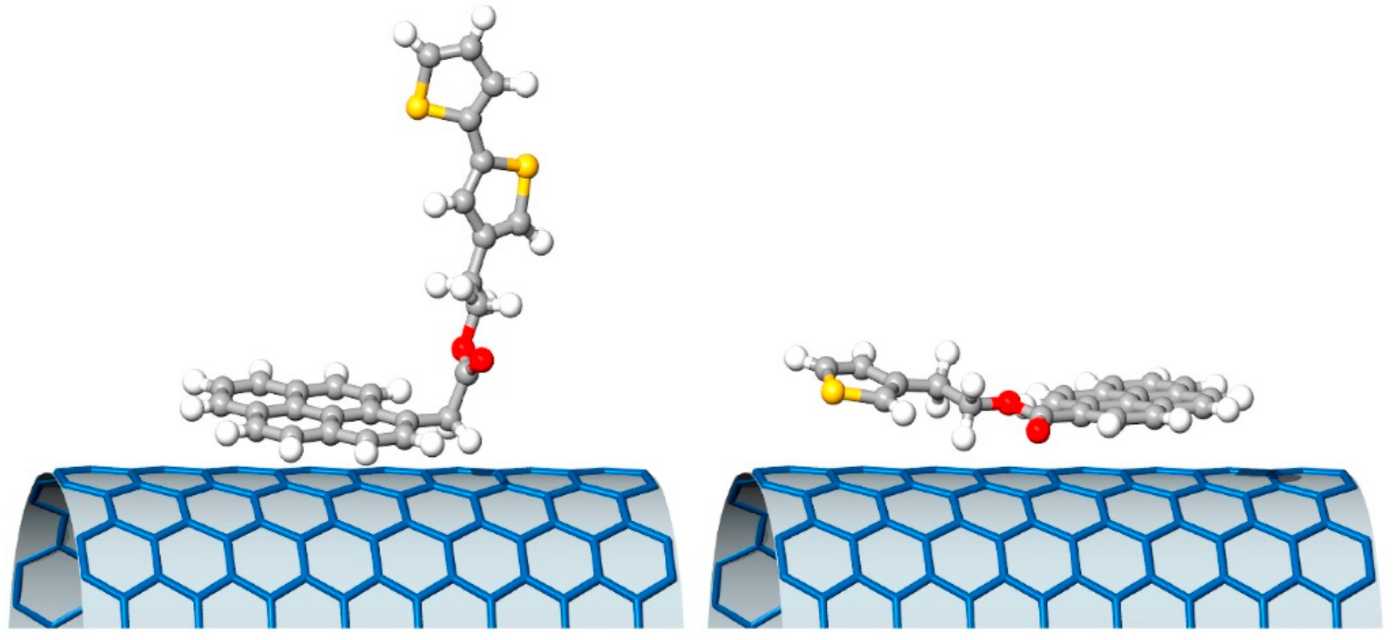
- Sartorio, C.; Figa, V.; Salice, P.; Gragnato, D.; Cataldo, S.; Scopelliti, M.; Improta, R.; Menna, E.; Pignataro, B. Thiophene pyrenyl derivatives for the supramolecular processability of single-walled carbon nanotubes in thin film heterojunction. Synth. Met. 2017, 229, 7–15. [Google Scholar] [CrossRef]
- Bachilo, S.; Strano, M.; Kittrell, C.; Hauge, R.; Smalley, R.; Weisman, R. Structure-assigned optical spectra of single-walled carbon nanotubes. Science 2002, 298, 2361–2366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Connell, M.; Bachilo, S.; Huffman, C.; Moore, V.; Strano, M.; Haroz, E.; Rialon, K.; Boul, P.; Noon, W.; Kittrell, C.; et al. Band gap fluorescence from individual single-walled carbon nanotubes. Science 2002, 297, 593–596. [Google Scholar] [CrossRef] [Green Version]
- Salice, P.; Maity, P.; Rossi, E.; Carofiglio, T.; Menna, E.; Maggini, M. The continuous-flow cycloaddition of azomethine ylides to carbon nanotubes. Chem. Commun. 2011, 47, 9092–9094. [Google Scholar] [CrossRef] [PubMed]
- Salice, P.; Sartorio, C.P.; Burlini, A.; Improta, R.; Pignataro, B.; Menna, E. On the trade-off between processability and opto-electronic properties of single wall carbon nanotube derivatives in thin film heterojunctions. J. Mater. Chem. C 2015, 3, 303–312. [Google Scholar] [CrossRef]
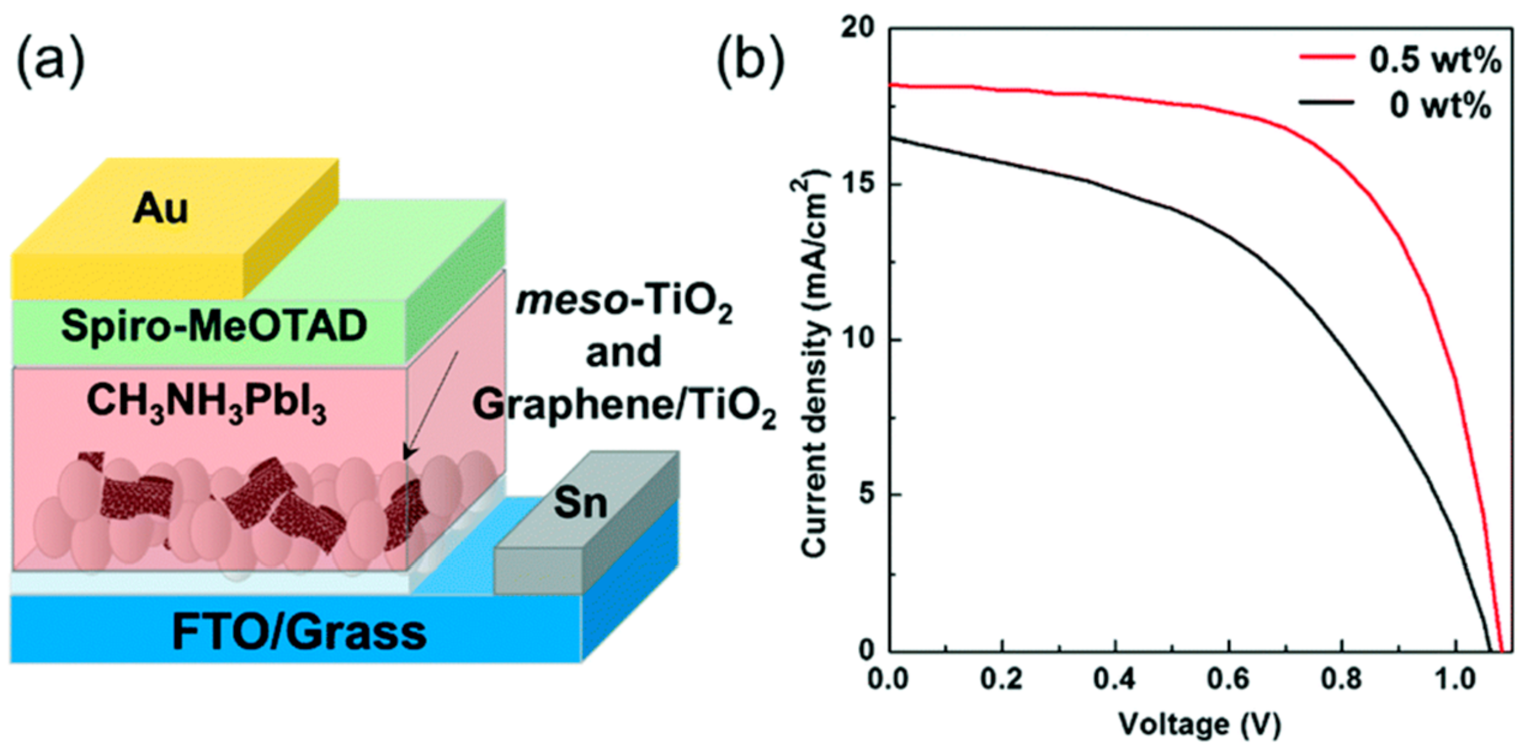
- Gatti, T.; Casaluci, S.; Prato, M.; Salerno, M.; Di Stasio, F.; Ansaldo, A.; Menna, E.; Di Carlo, A.; Bonaccorso, F. Boosting Perovskite Solar Cells Performance and Stability through Doping a Poly-3(hexylthiophene) Hole Transporting Material with Organic Functionalized Carbon Nanostructures. Adv. Funct. Mater. 2016, 26, 7443–7453. [Google Scholar] [CrossRef]
- Gatti, T.; Lamberti, F.; Topolovsek, P.; Abdu-Aguye, M.; Sorrentino, R.; Perino, L.; Salerno, M.; Girardi, L.; Marega, C.; Rizzi, G.; et al. Interfacial Morphology Addresses Performance of Perovskite Solar Cells Based on Composite Hole Transporting Materials of Functionalized Reduced Graphene Oxide and P3HT. Sol. RRL 2018, 2, 1800013. [Google Scholar] [CrossRef]
- Habisreutinger, S.N.; Leijtens, T.; Eperon, G.E.; Stranks, S.D.; Nicholas, R.J.; Snaith, H.J. Carbon Nanotube/Polymer Composites as a Highly Stable Hole Collection Layer in Perovskite Solar Cells. Nano Lett. 2014, 14, 5561–5568. [Google Scholar] [CrossRef]
- Habisreutinger, S.; Leijtens, T.; Eperon, G.; Stranks, S.; Nicholas, R.; Snaith, H. Enhanced Hole Extraction in Perovskite Solar Cells Through Carbon Nanotubes. J. Phys. Chem. Lett. 2014, 5, 4207–4212. [Google Scholar] [CrossRef]
- Ihly, R.; Dowgiallo, A.-M.; Yang, M.; Schulz, P.; Stanton, N.J.; Reid, O.G.; Ferguson, A.J.; Zhu, K.; Berry, J.J.; Blackburn, J.L. Efficient charge extraction and slow recombination in organic–inorganic perovskites capped with semiconducting single-walled carbon nanotubes. Energy Environ. Sci. 2016, 9, 1439–1449. [Google Scholar] [CrossRef]
- Jeon, N.J.; Lee, H.G.; Kim, Y.C.; Seo, J.; Noh, J.H.; Lee, J.; Seok, S.I. o-Methoxy Substituents in Spiro-OMeTAD for Efficient Inorganic–Organic Hybrid Perovskite Solar Cells. J. Am. Chem. Soc. 2014, 136, 7837–7840. [Google Scholar] [CrossRef]
- Jung, C.; Noh, Y.; Bae, J.; Yu, J.; Hwang, I.; Shin, J.; Shin, K.; Lee, J.; Choi, J.; Na, S. Polyacrylonitrile-grafted reduced graphene oxide hybrid: An all-round and efficient hole-extraction material for organic and inorganic-organic hybrid photovoltaics. Nano Energy 2017, 31, 19–27. [Google Scholar] [CrossRef]
- Chen, J.; Yao, B.; Li, C.; Shi, G. An improved Hummers method for eco-friendly synthesis of graphene oxide. Carbon 2013, 64, 225–229. [Google Scholar] [CrossRef]
- Tung, V.; Allen, M.; Yang, Y.; Kaner, R. High-throughput solution processing of large-scale graphene. Nat. Nanotechnol. 2009, 4, 25–29. [Google Scholar] [CrossRef]
- Dreyer, D.; Todd, A.; Bielawski, C. Harnessing the chemistry of graphene oxide. Chem. Soc. Rev. 2014, 43, 5288–5301. [Google Scholar] [CrossRef]
- Jung, C.; Park, Y.; Hwang, I.; Go, Y.; Na, S.; Shin, K.; Lee, J.; Choi, J. Eco-friendly and simple radiation-based preparation of graphene and its application to organic solar cells. J. Phys. D Appl. Phys. 2014, 47, 015105. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Peng, C.; Yu, M.; Li, L.; Deng, B.; Hu, P.; Fan, C.; Li, J.; Huang, Q. Preparation of polymer decorated graphene oxide by gamma-ray induced graft polymerization. Nanoscale 2012, 4, 1742–1748. [Google Scholar] [CrossRef]
- Gupta, B.; Kumar, N.; Panda, K.; Melvin, A.; Joshi, S.; Dash, S.; Tyagi, A. Effective Noncovalent Functionalization of Poly(ethylene glycol) to Reduced Graphene Oxide Nanosheets through gamma-Radiolysis for Enhanced Lubrication. J. Phys. Chem. C 2016, 120, 2139–2148. [Google Scholar] [CrossRef]
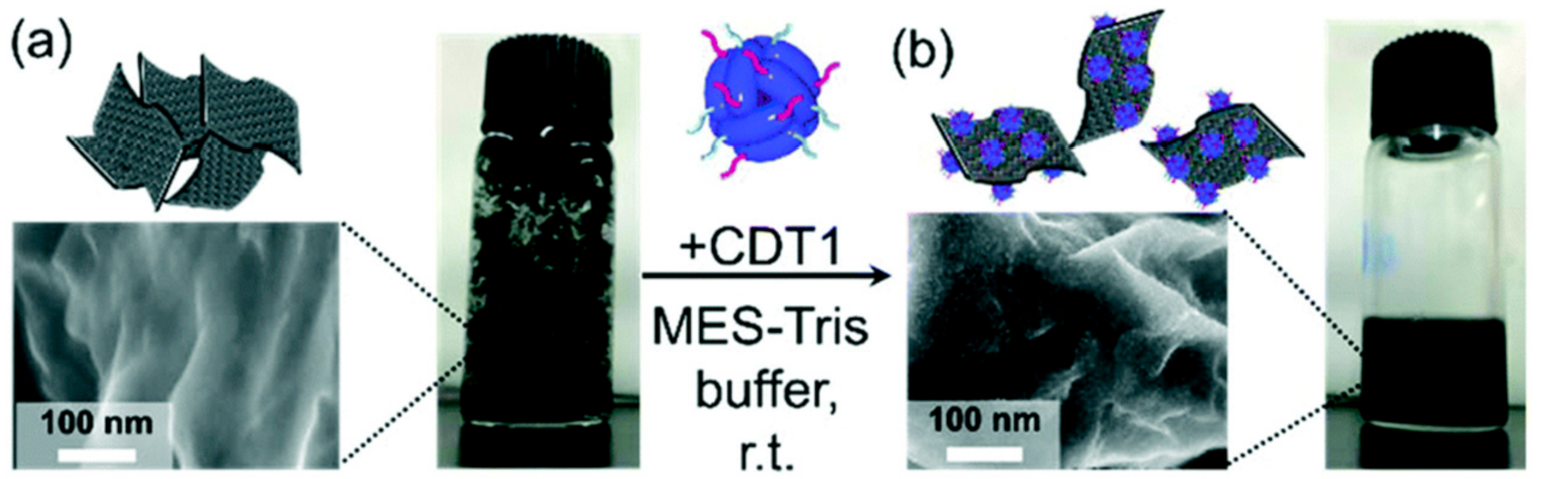
- Hashima, Y.; Ishikawa, Y.; Raifuku, I.; Inoue, I.; Okamoto, N.; Yamashita, I.; Minami, T.; Uraoka, Y. Easy and green preparation of a graphene–TiO2 nanohybrid using a supramolecular biomaterial consisting of artificially bifunctionalized proteins and its application for a perovskite solar cell. Nanoscale 2018, 10, 19249–19253. [Google Scholar] [CrossRef]
- Inou, I.; Zheng, B.; Watanabe, K.; Ishikawa, Y.; Shiba, K.; Yasueda, H.; Uraoka, Y.; Yamashita, I. A novel bifunctional protein supramolecule for construction of carbon nanotube-titanium hybrid material. Chem. Commun. 2011, 47, 12649–12651. [Google Scholar] [CrossRef] [PubMed]
- Inoue, I.; Yamauchi, H.; Okamoto, N.; Toyoda, K.; Horita, M.; Ishikawa, Y.; Yasueda, H.; Uraoka, Y.; Yamashita, I. Thermo-stable carbon nanotube-TiO2 nanocompsite as electron highways in dye-sensitized solar cell produced by bio-nano-process. Nanotechnology 2015, 26, 285601. [Google Scholar] [CrossRef]
- Yamashita, I. Biosupramolecules for nano-devices: Biomineralization of nanoparticles and their applications. J. Mater. Chem. 2008, 18, 3813–3820. [Google Scholar] [CrossRef]
- Uenuma, M.; Ban, T.; Okamoto, N.; Zheng, B.; Kakihara, Y.; Horita, M.; Ishikawa, Y.; Yamashita, I.; Uraoka, Y. Memristive nanoparticles formed using a biotemplate. RSC Adv. 2013, 3, 18044–18048. [Google Scholar] [CrossRef]
- Haikarainen, T.; Paturi, P.; Linden, J.; Haataja, S.; Meyer-Klaucke, W.; Finne, J.; Papageorgiou, A. Magnetic properties and structural characterization of iron oxide nanoparticles formed by Streptococcus suis Dpr and four mutants. J. Biol. Inorg. Chem. 2011, 16, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Kumagai, S.; Zheng, B.; Uraoka, Y.; Douglas, T.; Yamashita, I. A water-soluble carbon nanotube network conjugated by nanoparticles with defined nanometre gaps. Chem. Commun. 2011, 47, 3475–3477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haikarainen, T.; Papageorgiou, A. Dps-like proteins: Structural and functional insights into a versatile protein family. Cell. Mol. Life Sci. 2010, 67, 341–351. [Google Scholar] [CrossRef]
- Okuda, M.; Suzumoto, Y.; Iwahori, K.; Kang, S.; Uchida, M.; Douglas, T.; Yamashita, I. Bio-templated CdSe nanoparticle synthesis in a cage shaped protein, Listeria-Dps, and their two dimensional ordered array self-assembly. Chem. Commun. 2010, 46, 8797–8799. [Google Scholar] [CrossRef]
- Suppavorasatit, I.; Cadwallader, K. Effect of Enzymatic Deamidation of Soy Protein by Protein-Glutaminase on the Flavor-Binding Properties of the Protein under Aqueous Conditions. J. Agric. Food Chem. 2012, 60, 7817–7823. [Google Scholar] [CrossRef]
- Husain, A.; Hasan, W.; Shafie, S.; Hamidon, M.; Pandey, S. A review of transparent solar photovoltaic technologies. Renew. Sustain. Energy Rev. 2018, 94, 779–791. [Google Scholar] [CrossRef]
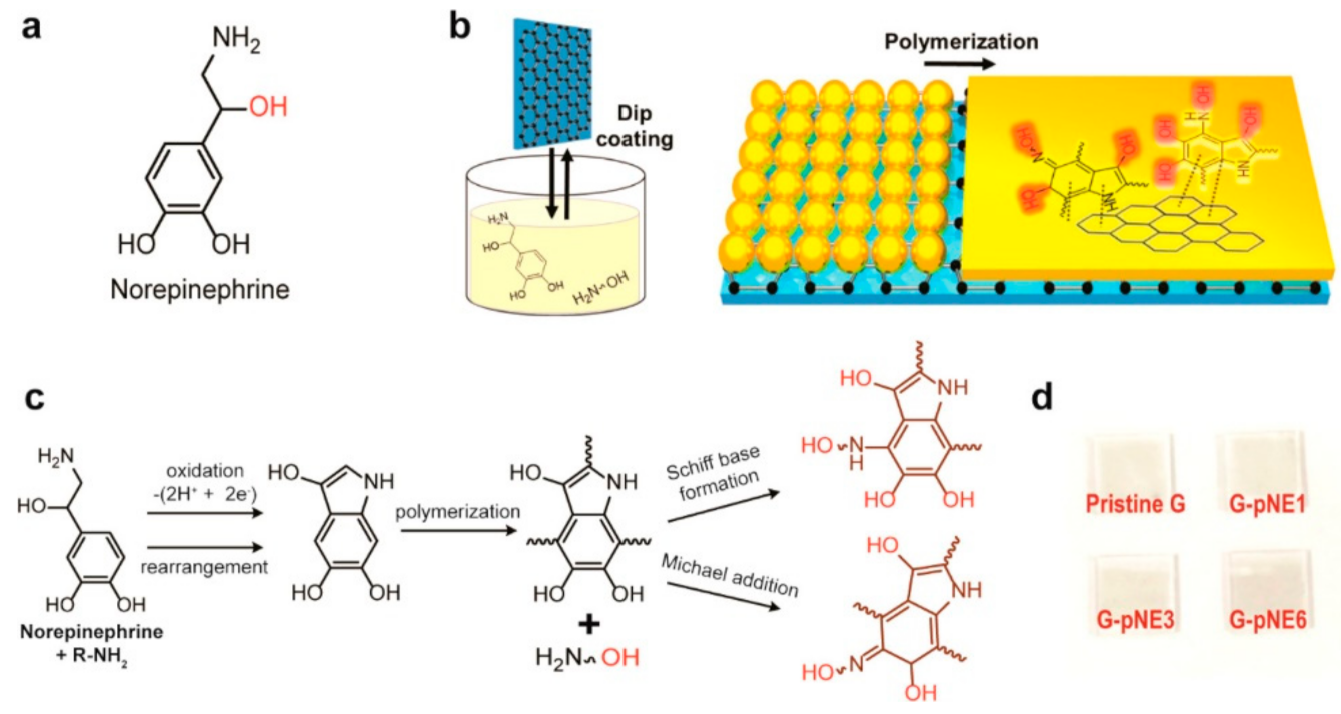
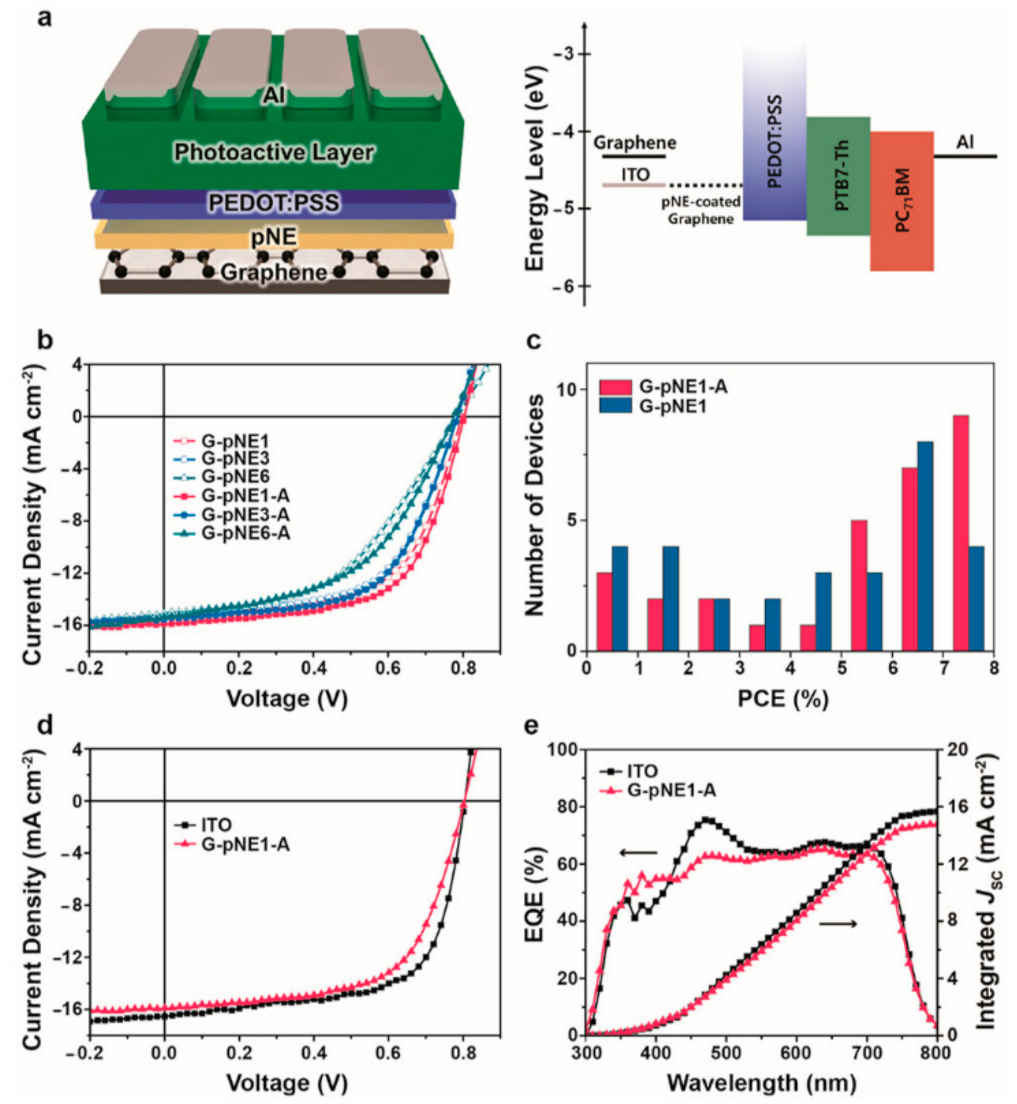
- Jung, S.; Kim, H.; Lee, J.; Jeong, G.; Park, J.; Park, H. Bio-Inspired Catecholamine-Derived Surface Modifier for Graphene-Based Organic Solar Cells. ACS Appl. Energy Mater. 2018, 1, 6463–6468. [Google Scholar] [CrossRef]
- Hong, S.; Kim, J.; Na, Y.; Park, J.; Kim, S.; Singha, K.; Im, G.; Han, D.; Kim, W.; Lee, H. Poly(norepinephrine): Ultrasmooth Material-Independent Surface Chemistry and Nanodepot for Nitric Oxide. Angew. Chem.-Int. Ed. 2013, 52, 9187–9191. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.; Miller, W.; Messersmith, P. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.; Yeom, J.; Song, I.; Kang, S.; Lee, H. Pyrogallol 2-Aminoethane: A Plant Flavonoid-Inspired Molecule for Material-Independent Surface Chemistry. Adv. Mater. Interfaces 2014, 1, 1400113. [Google Scholar] [CrossRef]
- Yao, T.T.; An, X.R.; Han, H.X.; Chen, J.Q.; Li, C. Photoelectrocatalytic Materials for Solar Water Splitting. Adv. Energy Mater. 2018, 8, 1800210. [Google Scholar] [CrossRef]
- Ragoussi, M.E.; Malig, J.; Katsukis, G.; Butz, B.; Spiecker, E.; de la Torre, G.; Torres, T.; Guldi, D.M. Linking Photo- and Redoxactive Phthalocyanines Covalently to Graphene. Angew. Chem.-Int. Ed. 2012, 51, 6421–6425. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, K.Z.; Wang, D.D.; Huang, J.; Zhang, C.Y.; Du, Y.K.; Yang, P. One-pot synthesis of manganese porphyrin covalently functionalized graphene oxide for enhanced photocatalytic hydrogen evolution. J. Porphyr. Phthalocyanines 2017, 21, 179–188. [Google Scholar] [CrossRef]
- Xu, Y.F.; Liu, Z.B.; Zhang, X.L.; Wang, Y.; Tian, J.G.; Huang, Y.; Ma, Y.F.; Zhang, X.Y.; Chen, Y.S. A Graphene Hybrid Material Covalently Functionalized with Porphyrin: Synthesis and Optical Limiting Property. Adv. Mater. 2009, 21, 1275–1279. [Google Scholar] [CrossRef]
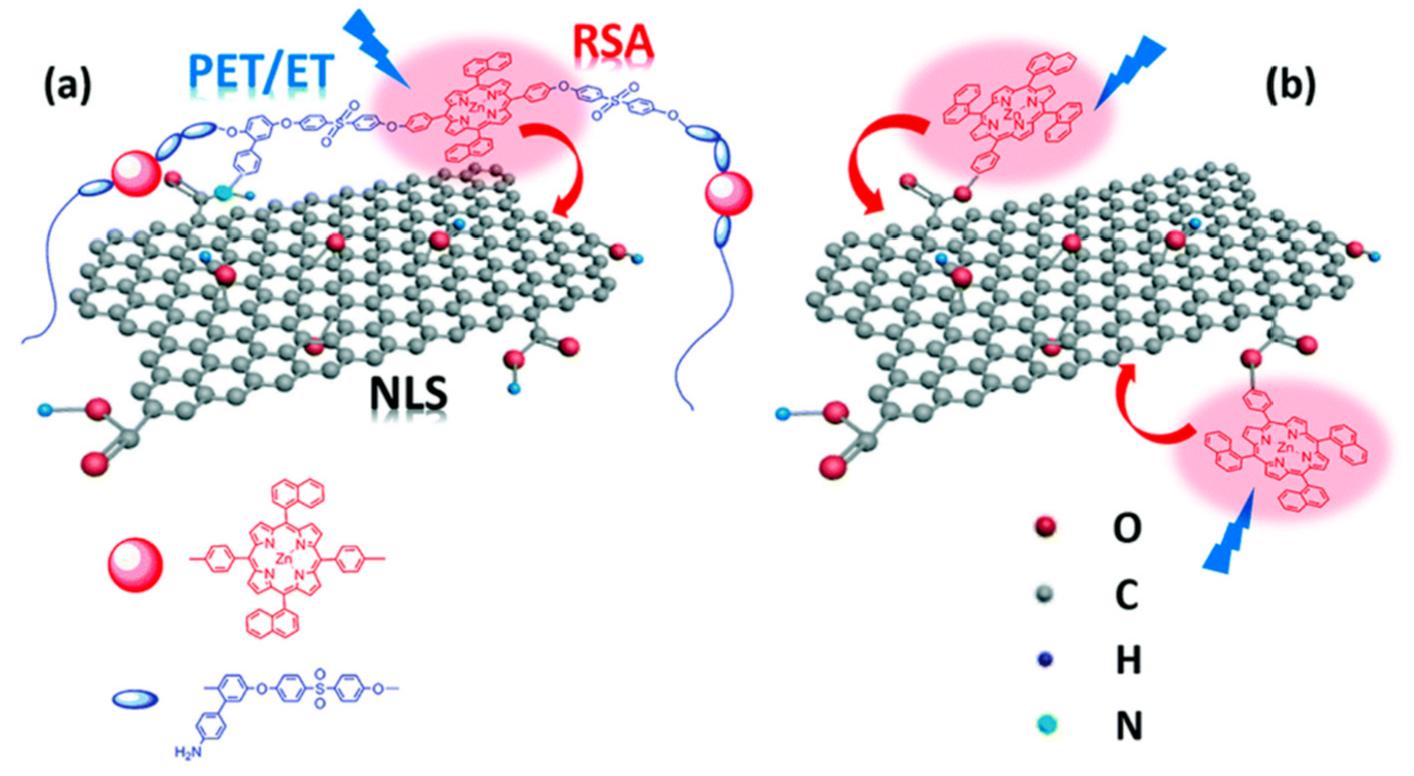
- Li, R.X.; Liu, X.F.; Liu, T.; Yin, Y.B.; Zhou, Y.; Mei, S.K.; Yan, J. Electrocatalytic properties of FeFe-hydrogenases models and visible-light-driven hydrogen evolution efficiency promotion with porphyrin functionalized graphene nanocomposite. Electrochim. Acta 2017, 237, 207–216. [Google Scholar] [CrossRef]
- Kowalska, A.M.; Trzaskowski, B.; Osella, S. Assessing the Charge Transfer at the Cytochrome c(553)/Graphene Interface: A Multiscale Investigation. J. Phys. Chem. C 2018, 122, 29405–29413. [Google Scholar] [CrossRef] [Green Version]
- Du, Y.L.; Dong, N.N.; Zhang, M.H.; Zhu, K.; Na, R.Q.; Zhang, S.L.; Sun, N.W.; Wang, G.B.; Wang, J. Covalent functionalization of graphene oxide with porphyrin and porphyrin incorporated polymers for optical limiting. Phys. Chem. Chem. Phys. 2017, 19, 2252–2260. [Google Scholar] [CrossRef]
- Guldi, D.M.; Taieb, H.; Rahman, G.M.A.; Tagmatarchis, N.; Prato, M. Novel photoactive single-walled carbon nanotube-porphyrin polymer wraps: Efficient and long-lived intracomplex charge separation. Adv. Mater. 2005, 17, 871–875. [Google Scholar] [CrossRef]
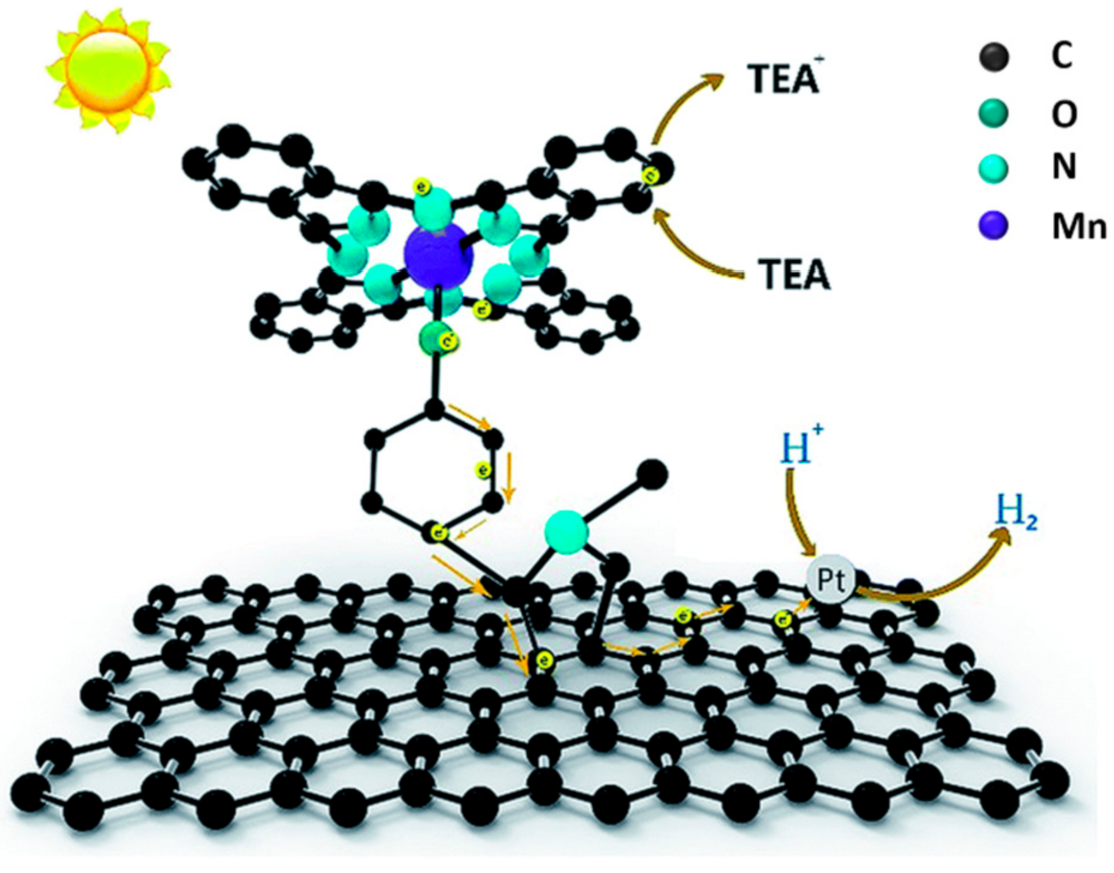
- Wang, D.D.; Huang, J.; Li, X.; Yang, P.; Du, Y.K.; Goh, C.M.; Lu, C. Photocatalytic H-2 production under visible-light irradiation based on covalent attachment of manganese phthalocyanine to graphene. J. Mater. Chem. A 2015, 3, 4195–4202. [Google Scholar] [CrossRef]
- Lewandowska, K.; Rosiak, N.; Bogucki, A.; Cielecka-Piontek, J.; Mizera, M.; Bednarski, W.; Suchecki, M.; Szacilowski, K. Supramolecular Complexes of Graphene Oxide with Porphyrins: An Interplay between Electronic and Magnetic Properties. Molecules 2019, 24, 688. [Google Scholar] [CrossRef] [Green Version]
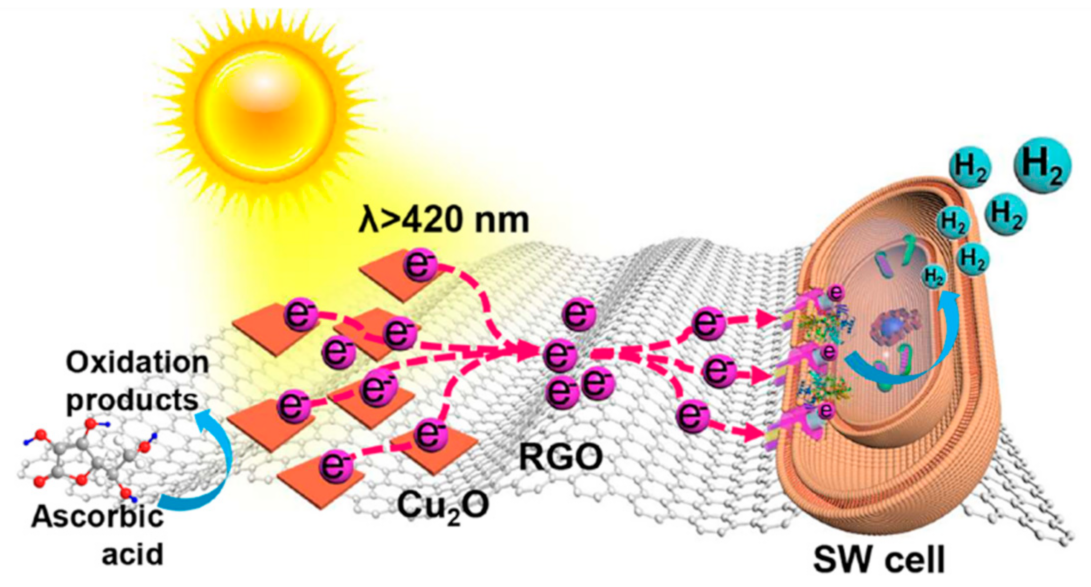
- Shen, H.; Wang, Y.-Z.; Liu, G.; Li, L.; Xia, R.; Luo, B.; Wang, J.; Suo, D.; Shi, W.; Yong, Y.-C. A Whole-Cell Inorganic-Biohybrid System Integrated by Reduced Graphene Oxide for Boosting Solar Hydrogen Production. ACS Catal. 2020, 10, 13290–13295. [Google Scholar] [CrossRef]
- Takaguchi, Y.; Sako, Y.; Yanagimoto, Y.; Tsuboi, S.; Motoyoshiya, J.; Aoyama, H.; Wakahara, T.; Akasaka, T. Facile and reversible synthesis of an acidic water-soluble poly(amidoamine) fullerodendrimer. Tetrahedron Lett. 2003, 44, 5777–5780. [Google Scholar] [CrossRef]
- Murakami, N.; Miyake, H.; Tajima, T.; Nishikawa, K.; Hirayama, R.; Takaguchi, Y. Enhanced Photosensitized Hydrogen Production by Encapsulation of Ferrocenyl Dyes into Single-Walled Carbon Nanotubes. J. Am. Chem. Soc. 2018, 140, 3821–3824. [Google Scholar] [CrossRef]
- Tajima, T.; Yamagami, M.; Sagawa, R.; Miyake, H.; Takaguchi, Y. Dye-sensitized H-2 evolution from water facilitated by photoinduced electron transfer between molecules on the inside and the outside of a carbon nanotube. J. Appl. Phys. 2021, 129, 014303. [Google Scholar] [CrossRef]
- Zeng, L.; Li, X.; Fan, S.; Yin, Z.; Mu, J.; Qin, M.; Chen, A. Solar-driven bio-electro-chemical system for synergistic hydrogen evolution and pollutant elimination simultaneously over defect-rich CoN–MoS2/biomass nanosheets. J. Power Source 2020, 478, 228755. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazzarin, L.; Pasini, M.; Menna, E. Organic Functionalized Carbon Nanostructures for Solar Energy Conversion. Molecules 2021, 26, 5286. https://doi.org/10.3390/molecules26175286
Lazzarin L, Pasini M, Menna E. Organic Functionalized Carbon Nanostructures for Solar Energy Conversion. Molecules. 2021; 26(17):5286. https://doi.org/10.3390/molecules26175286
Chicago/Turabian StyleLazzarin, Luca, Mariacecilia Pasini, and Enzo Menna. 2021. "Organic Functionalized Carbon Nanostructures for Solar Energy Conversion" Molecules 26, no. 17: 5286. https://doi.org/10.3390/molecules26175286
APA StyleLazzarin, L., Pasini, M., & Menna, E. (2021). Organic Functionalized Carbon Nanostructures for Solar Energy Conversion. Molecules, 26(17), 5286. https://doi.org/10.3390/molecules26175286







