A Reusable Efficient Green Catalyst of 2D Cu-MOF for the Click and Knoevenagel Reaction
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis of (4-Chloro-phenyl)-pyridin-4-ylmethylene-amine (CPA)
2.2. Synthesis of Cu-MOF, [Cu(CPA)(BDC)]n
2.3. Crystal Structure Determination of Cu-MOF
2.4. Characterization of MOF Catalyst
2.5. Gas Adsorption Measurements
2.6. Catalytic Response
3. Results and Discussion
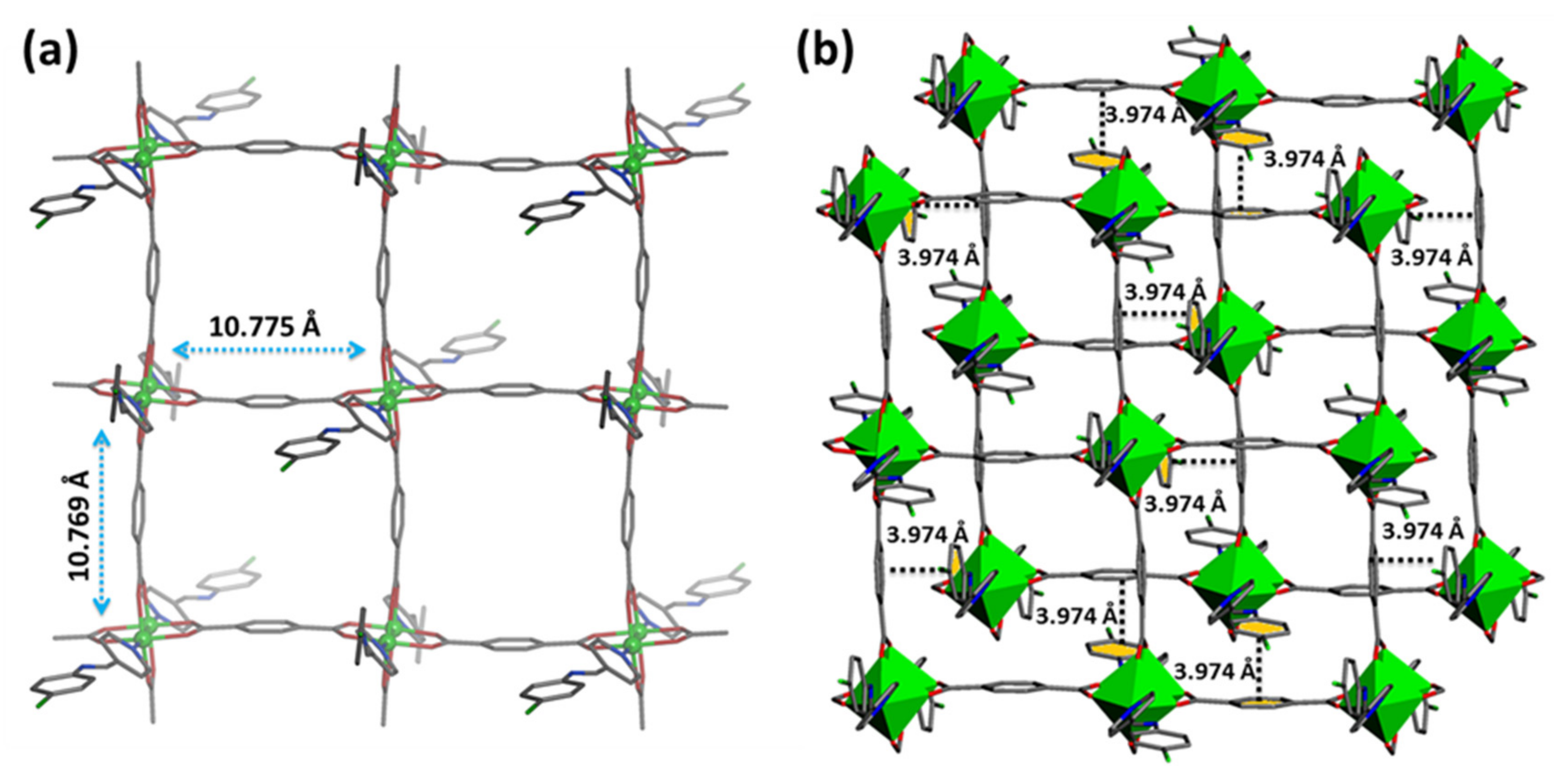
3.1. Structural Descriptions of [Cu(CPA)(BDC)]n,
3.2. Catalytic Activity of Cu-MOF
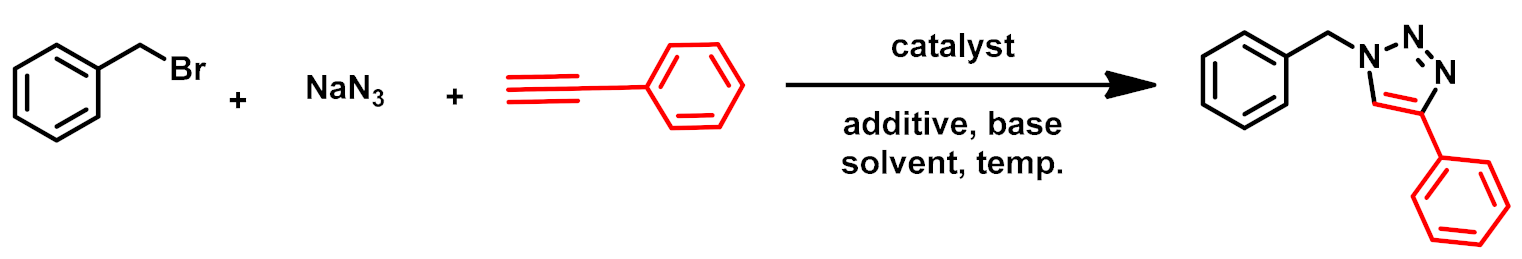
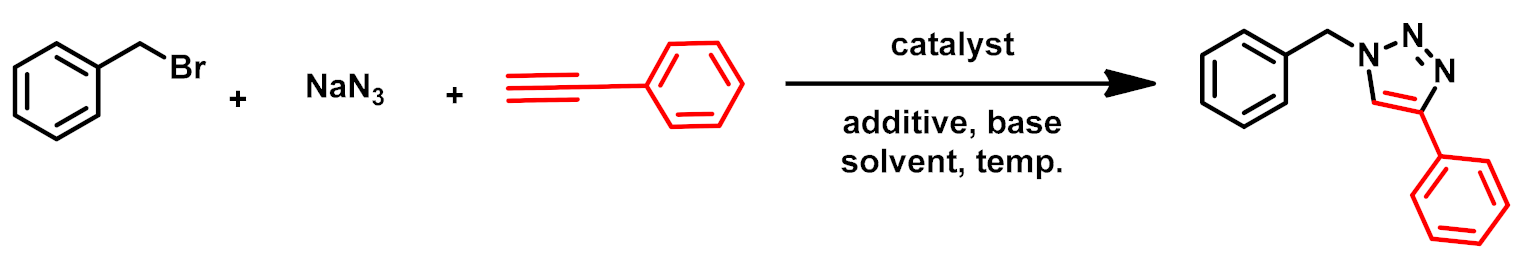
3.2.1. Optimization of Click Reaction
3.2.2. Substrate Scope
3.3. Knoevenagel Condensation Catalytic Experiments
3.4. Recyclability Cu-MOF Catalyst
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Katz, M.J.; Howarth, A.J.; Moghadam, P.Z.; DeCoste, J.B.; Snurr, R.Q.; Huppa, J.T.; Farha, O.K. High volumetric uptake of ammonia using Cu-MOF-74/Cu-CPO-27. Dalton Trans. 2016, 45, 4150–4153. [Google Scholar] [CrossRef]
- Li, H.; Wang, K.; Sun, Y.; Lollar, C.T.; Li, J.; Zhou, H.-C. Recent advances in gas storage and separation using metal–organic frameworks. Mater. Today 2018, 21, 108–121. [Google Scholar] [CrossRef]
- Forgan, R.S.; Smaldone, R.A.; Gassensmith, J.J.; Furukawa, H.; Cordes, D.B.; Li, Q.; Wilmer, C.E.; Botros, Y.Y.; Snurr, R.Q.; Slawin, A.M.Z.; et al. Nanoporous Carbohydrate Metal–Organic Frameworks. J. Am. Chem. Soc. 2012, 134, 406–417. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, Q.-L.; Zou, R.; Xu, Q. Metal-Organic Frameworks for Energy Applications. Chem 2017, 2, 52–80. [Google Scholar] [CrossRef] [Green Version]
- Rice, A.M.; Leith, G.A.; Ejegbavwo, O.A.; Dolgopolova, E.A.; Shustova, N.B. Heterometallic Metal–Organic Frameworks (MOFs): The Advent of Improving the Energy Landscape. ACS Energy Lett. 2019, 4, 1938–1946. [Google Scholar] [CrossRef]
- Naskar, K.; Dey, A.; Maity, S.; Ray, P.P.; Ghosh, P.; Sinha, C. Biporous Cd(II) Coordination Polymer via in Situ Disulfide Bond Formation: Self-Healing and Application to Photosensitive Optoelectronic Device. Inorg. Chem. 2020, 59, 5518–5528. [Google Scholar] [CrossRef]
- Naskar, K.; Dey, A.; Dutta, B.; Ahmed, F.; Sen, C.; Mir, M.H.; Roy, P.P.; Sinha, C. Intercatenated Coordination Polymers (ICPs) of Carboxylato Bridged Zn(II)-Isoniazid and Their Electrical Conductivity. Cryst. Growth Des. 2017, 17, 3267–3276. [Google Scholar] [CrossRef]
- Naskar, K.; Dey, A.; Maity, S.; Bhunia, M.K.; Ray, P.P.; Sinha, C. Novel porous polycatenated Iodo–cadmium coordination polymer for iodine sorption and electrical conductivity measurement. Cryst. Growth Des. 2019, 19, 2206–2218. [Google Scholar] [CrossRef]
- Naskar, K.; Sil, S.; Sahu, N.; Dutta, B.; Slawin, A.M.Z.; Ray, P.P.; Sinha, C. Enhancement of Electrical Conductivity due to Structural Distortion from Linear to Nonlinear Dicarboxylato-Bridged Zn(II) 1D-Coordination Polymers. Cryst. Growth Des. 2019, 19, 2632–2641. [Google Scholar] [CrossRef]
- Maity, S.; Naskar, K.; Bhowmik, T.; Bera, A.; Weyhermüller, T.; Sinha, C.; Ghosh, P. Coordination polymers of Ag(I) and Hg(I) ions with 2,2′-azobispyridine: Synthesis, characterization and enhancement of conductivity in the presence of Cu(II) ions. Dalton Trans. 2020, 49, 8438–8442. [Google Scholar] [CrossRef] [PubMed]
- Maity, K.; Mukherjee, D.; Sen, M.; Biradha, K. Fluorescent Dye-Based Metal–Organic Framework Piezochromic and Multicolor-Emitting Two-Dimensional Materials for Light-Emitting Devices. ACS Appl. Nano Mater. 2019, 2, 1614–1620. [Google Scholar] [CrossRef]
- Khatua, S.; Goswami, S.; Biswas, S.; Tomar, K.; Jena, H.S.; Konar, S. Stable Multiresponsive Luminescent MOF for Colorimetric Detection of Small Molecules in Selective and Reversible Manner. Chem. Mater. 2015, 27, 5349–5360. [Google Scholar] [CrossRef]
- Naskar, K.; Bhanja, A.K.; Paul, S.; Pal, K.; Sinha, C. Trace Quantity Detection of H2PO4− by Fluorescent Metal–Organic Framework (FMOF) and Bioimaging Study. Cryst. Growth Des. 2020, 20, 6453–6460. [Google Scholar] [CrossRef]
- Robison, L.; Zhang, L.; Drout, R.J.; Li, P.; Haney, C.R.; Brikha, A.; Noh, H.; Mehdi, B.L.; Browning, N.D.; Dravid, V.P.; et al. A Bismuth Metal–Organic Framework as a Contrast Agent for X-ray Computed Tomography. ACS Appl. Bio Mater. 2019, 2, 1197–1203. [Google Scholar] [CrossRef]
- Zhao, H.; Hou, S.; Zhao, X.; Liu, D. Adsorption and pH-Responsive Release of Tinidazole on Metal–Organic Framework CAU-1. J. Chem. Eng. Data 2019, 64, 1851–1858. [Google Scholar] [CrossRef]
- Lin, S.X.; Pan, W.L.; Niu, R.J.; Liu, Y.; Chen, J.X.; Zhang, W.H.; Lang, J.P.; Young, D.J. Effective loading of cisplatin into a nanoscale UiO-66 metal-organic framework with preformed defects. Dalton Trans. 2019, 48, 5308–5314. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Liang, Z.B.; Zou, R.Q.; Zhao, Y.L. Heterogeneous Catalysis in Zeolites, Mesoporous Silica, and Metal-Organic Frameworks. Adv. Mater. 2017, 29, 1701139. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Garcia, H. Metal–organic frameworks as solid catalysts for the synthesis of nitrogen-containing heterocycles. Chem. Soc. Rev. 2014, 43, 5750–5765. [Google Scholar] [CrossRef]
- Abdelbaky, M.S.M.; Amghouz, Z.; Blanco, D.M.; García-Granda, S.; García, J.R. Crystal structure and characterization of a novel layered copper-lithium phosphonate with antiferromagnetic intrachain Cu(II)•••Cu(II) interactions. J. Solid State Chem. 2017, 248, 61–67. [Google Scholar] [CrossRef]
- Jana, S.; Ray, A.; Chandra, A.; El Fallah, M.S.; Das, S.; Sinha, C. Studies on Magnetic and Dielectric Properties of Antiferromagnetically Coupled Dinuclear Cu(II) in a One-Dimensional Cu(II) Coordination Polymer. ACS Omega 2020, 5, 274–280. [Google Scholar] [CrossRef]
- Park, K.S.; Ni, Z.; Côté, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qutaish, H.; Lee, J.; Hyeon, Y.; Han, S.A.; Lee, I.-H.; Heo, Y.-U.; Whang, D.; Moon, J.; Park, M.-S.; Kim, J.H. Design of cobalt catalysed carbon nanotubes in bimetallic zeolitic imidazolate frameworks. Appl. Surf. Sci. 2021, 547, 149134. [Google Scholar] [CrossRef]
- Hayashi, H.; Côté, A.P.; Furukawa, H.; O’Keeffe, M.; Yaghi, O.M. Zeolite A imidazolate frameworks. Nat. Mater. 2007, 6, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Zanon, A.; Chaemchuen, S.; Verpoort, F. Zn@ZIF-67 as Catalysts for the Knoevenagel Condensation of Aldehyde Derivatives with Malononitrile. Catal. Lett. 2017, 147, 2410–2420. [Google Scholar] [CrossRef]
- Horiuchi, Y.; Toyao, T.; Fujiwaki, M.; Dohshi, S.; Kim, T.-H.; Matsuoka, M. Zeolitic imidazolate frameworks as heterogeneous catalysts for a one-pot P–C bond formation reaction via Knoevenagel condensation and phospha-Michael addition. RSC Adv. 2015, 5, 24687–24690. [Google Scholar] [CrossRef]
- Fan, H.; Yang, Y.; Song, J.; Ding, G.; Wu, C.; Yang, G.; Han, B. One-pot sequential oxidation and aldol-condensation reactions of veratryl alcohol catalyzed by the Ru@ZIF-8 + CuO/basic ionic liquid system. Green Chem. 2014, 16, 600–604. [Google Scholar] [CrossRef]
- Vermoortele, F.; Ameloot, R.; Vimont, A.; Serre, C.; De Vos, D. An amino-modified Zr-terephthalate metal–organic framework as an acid–base catalyst for cross-aldol condensation. Chem. Commun. 2011, 47, 1521–1523. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Jee, S.; Choi, K.M.; Shin, D.-S. Single-atom Pd catalyst anchored on Zr-based metal-organic polyhedra for Suzuki-Miyaura cross coupling reactions in aqueous media. Nano Res. 2021, 14, 486–492. [Google Scholar] [CrossRef]
- Gong, X.-F.; Zhang, L.-Y.; Zhang, H.-X.; Cui, Y.-M.; Jin, F.-C.; Liu, Y.; Zhai, Y.-F.; Li, J.-H.; Liu, G.-Y.; Zeng, Y.-F. Highly Active Heterogeneous PdCl2/MOF Catalyst for Suzuki–Miyaura Cross-Coupling Reactions of Aryl Chloride. Z. Anorg. Allg. Chem. 2020, 646, 1336–1341. [Google Scholar] [CrossRef]
- Nguyen, L.T.L.; Le, K.K.A.; Phan, N.T.S. A Zeolite Imidazolate Framework ZIF-8 Catalyst for Friedel-Crafts Acylation. Chin. J. Catal. 2012, 33, 688–696. [Google Scholar] [CrossRef]
- Calleja, G.; Sanz, R.; Orcajo, G.; Briones, D.; Leo, P.; Martínez, F. Copper-based MOF-74 material as effective acid catalyst in Friedel–Crafts acylation of anisole. Catal. Today 2014, 227, 130–137. [Google Scholar] [CrossRef]
- Nagarjun, N.; Concepcion, P.; Dhakshinamoorthy, A. MIL-101(Fe) as an active heterogeneous solid acid catalyst for the regioselective ring opening of epoxides by indoles. Mol. Catal. 2020, 482, 110628. [Google Scholar] [CrossRef]
- Srivastava, D.; Rani, P.; Srivastava, R. ZIF-8-Nanocrystalline Zirconosilicate Integrated Porous Material for the Activation and Utilization of CO2 in Insertion Reactions. Chem. Asian J. 2020, 15, 1132–1139. [Google Scholar] [CrossRef]
- Lee, J.Y.; Farha, O.K.; Roberts, J.; Scheidt, K.A.; Nguyen, S.B.T.; Hupp, J.T. Metal–organic framework materials as catalysts. Chem. Soc. Rev. 2009, 38, 1450. [Google Scholar] [CrossRef]
- Liu, J.; Chen, L.; Cui, H.; Zhang, J.; Zhang, L.; Su, C.-Y. Applications of metal–organic frameworks in heterogeneous supramolecular catalysis. Chem. Soc. Rev. 2014, 43, 6011–6061. [Google Scholar] [CrossRef] [Green Version]
- Gawande, M.B.; Goswami, A.; Felpin, F.-X.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and Cu-Based Nanoparticles: Synthesis and Applications in Catalysis. Chem. Rev. 2016, 116, 3722–3811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berg, R.; Straub, B.F. Advancements in the mechanistic understanding of the copper-catalyzed Azide—Alkyne cycloaddition. Beilstein J. Org. Chem. 2013, 9, 2715–2750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, J.; Rinaldi, A.; Plodinec, M.; Huang, X.; Willinger, E.; Hammud, A.; Hieke, S.; Beeg, S.; Gregoratti, L.; Colbea, C.; et al. In situ observation of oscillatory redox dynamics of copper. Nat. Commun. 2020, 11, 3554. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Martinez, A.; Wang, C.; Ciganda, R.; Yate, L.; Escobar, A.; Moya, S.; Fouquet, E.; Ruiz, J.; Astruc, D. Exposure to air boosts CuAAC reactions catalyzed by PEG-stabilized Cu nanoparticles. Chem. Commun. 2017, 53, 5384–5387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szanyi, J.; Daturi, M.; Clet, G.; Baerc, D.R.; Peden, C.H.F. Well-studied Cu–BTC still serves surprises: Evidence for facile Cu2+/Cu+ interchange. Phys. Chem. Chem. Phys. 2012, 14, 4383–4390. [Google Scholar] [CrossRef]
- Ahmed, A.; Robertson, C.M.; Steiner, A.; Whittles, T.; Ho, A.; Dhanak, V.; Zhang, H. Cu(i)Cu(ii)BTC, a microporous mixed-valence MOF via reduction of HKUST-1. RSC Adv. 2016, 6, 8902–8905. [Google Scholar] [CrossRef]
- Pasini, D. The Click Reaction as an Efficient Tool for the Construction of Macrocyclic Structures. Molecules 2013, 18, 9512–9530. [Google Scholar] [CrossRef]
- Schneider, E.M.; Zeltner, M.; Kränzlin, N.; Grass, R.N.; Stark, W.J. Base-free Knoevenagel condensation catalyzed by copper metal surfaces. Chem. Commun. 2015, 51, 10695–10698. [Google Scholar] [CrossRef] [Green Version]
- Pandey, R.; Singh, D.; Thakur, N.; Raj, K.K. Catalytic C–H Bond Activation and Knoevenagel Condensation Using Pyridine-2,3 Dicarboxylate-Based Metal–Organic Frameworks. ACS Omega 2021, 6, 13240–13259. [Google Scholar] [CrossRef]
- Tehrania, A.A.; Morsalia, A.; Kubicki, M. The role of weak hydrogen and halogen bonding interactions in the assembly of a series of Hg(II) coordination polymers. Dalton Trans. 2015, 44, 5703–5712. [Google Scholar] [CrossRef] [PubMed]
- Kouznetsov, V.V.; Robles-Castellanos, M.L.; Sojo, F.; Rojas-Ruiz, F.A.; Arvelo, F. Diverse C-6 substituted 4-methyl-2-(2-, 3- and 4-pyridinyl)quinolines: Synthesis, in vitro anticancer evaluation and in silico studies. Med. Chem. Res. 2017, 26, 551–561. [Google Scholar] [CrossRef]
- SMART and SAINT; Bruker AXS Inc.: Madison, WI, USA, 1998.
- Bruker; SADABS; Bruker AXS Inc.: Madison, WI, USA, 2001.
- Sheldrick, G.M. SHELXL 2014, SHELXL-2016/6 and SHELXL-2017/1; Program for Crystal Structure Solution, University of Göttingen: Göttingen, Germany, 2017. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Spek, A.L. Structure validation in chemical crystallography. Acta Cryst. 2009, D65, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-S.; Jin, F.-Z.; Ma, H.-C.; Li, X.-B.; Liu, M.-Y.; Kan, J.-L.; Chen, G.-J.; Dong, Y.-B. Au@Cu(II)-MOF: Highly Efficient Bifunctional Heterogeneous Catalyst for Successive Oxidation–Condensation Reactions. Inorg. Chem. 2016, 55, 6685–6691. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-A.; Yang, S.; Liu, Q.-K.; Chen, G.-J.; Ma, J.-P.; Dong, Y.-B. Pd(0)@UiO-68-AP: Chelation-directed bifunctional heterogeneous catalyst for stepwise organic transformations. Chem. Commun. 2016, 52, 6517–6520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srivastava, S.; Aggarwal, H.; Gupta, R. Three-Dimensional Heterometallic Coordination Networks: Syntheses, Crystal Structures, Topologies, and Heterogeneous Catalysis. Cryst. Growth Des. 2015, 15, 4110–4122. [Google Scholar] [CrossRef]





| CCDC No. | 2094389 |
|---|---|
| formula | C20H12.4 ClCuN2O4 |
| formula weight | 443.74 |
| crystal system | monoclinic |
| space group | P21/n |
| a (Å) | 10.2699 (4) |
| b (Å) | 15.3188 (6) |
| c (Å) | 14.1931 (6) |
| β (°) | 105.404 (2) |
| V (Å3) | 2152.68 (15) |
| T (K) | 293 (2) |
| Z | 4 |
| Dcalcd (g/cm3) | 1.369 |
| µ (mm−1) | 1.164 |
| λ(Å) | 0.71073 |
| θ range (°) | 2.98–25.01 |
| total reflections | 3795 |
| unique reflections | 2336 |
| refined parameters | 296 |
| R1 a [I > 2σ(I)] | 0.0533 |
| wR2b | 0.1569 |
| Goodness-of-fit | 1.008 |
| difference between peak and hole (e·Å−3) | 0.595−0.443 |
| Entry | Catalyst | Amount of Catalyst | Solvent | Time (h) | T (°C) | Yield (%) b |
|---|---|---|---|---|---|---|
| 1 | Cu-MOF | 5 mol% | Neat | 7 | 70 | trace |
| 2 | Cu-MOF | 5 mol% | DMF | 7 | 70 | 42 |
| 3 | Cu-MOF | 5 mol% | THF | 7 | 70 | 43 |
| 4 | Cu-MOF | 5 mol% | CH3CN | 7 | 70 | 48 |
| 5 | Cu-MOF | 5 mol% | H2O | 7 | 70 | 68 |
| 6 | Cu-MOF | 5 mol% | Toluene | 7 | 70 | 29 |
| 7 | Cu-MOF | 5 mol% | Ethanol | 7 | 70 | 64 |
| 8 | Cu-MOF | 5 mol% | Methanol | 7 | 70 | 59 |
| 9 | Cu-MOF | 5 mol% | H2O-MeOH | 7 | 70 | 83 |
| 10 | Cu-MOF | 5 mol% | H2O-THF | 7 | 70 | 49 |
| 11 | Cu-MOF | 5 mol% | H2O-DMF | 7 | 70 | 55 |
| 12 | Cu-MOF | 5 mol% | H2O-MeOH | 9 | 70 | 39 |
| 13 | --- | - | H2O-MeOH | 12 | 80 | -- |
| 14 | Cu-MOF | 3 mol% | H2O-MeOH | 7 | 70 | 54 |
| 15 | Cu-MOF | 5 mol% | H2O-MeOH | 7 | 70 c | 83 |
| 16 | Cu-MOF | 5 mol% | H2O-MeOH | 7 | 70 d | 22 |
| 17 | Cu-MOF | 10 mol% | H2O-MeOH | 7 | 70 | 93 |
| 18 | Cu-MOF | 15 mol% | H2O-MeOH | 7 | 70 | 93 |
| 19 | Cu-MOF | 10 mol% | H2O-MeOH | 7 | rt | 11 |
| 20 | Cu-MOF | 10 mol% | H2O-MeOH | 7 | 90 | 93 |
| 21 | Cu-MOF | 10 mol% | H2O-MeOH | 7 | 70 e | 93 |
| 22 | Cu-MOF | 10 mol% | H2O-MeOH | 7 | 70 f | 57 |

| Entry | Aliphatic Halide | Alkyne | Triazole | Yield (%) a |
|---|---|---|---|---|
| 1 | 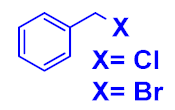 | 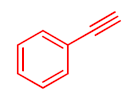 | 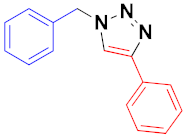 | 84 93 |
| 2 | 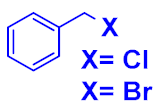 | 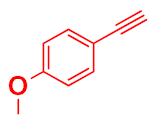 | 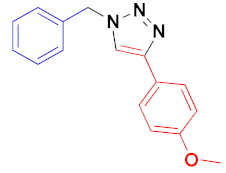 | 81 89 |
| 3 |  | 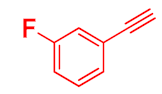 | 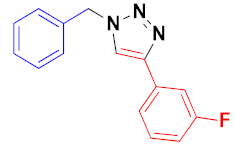 | 78 |
| 4 |  | 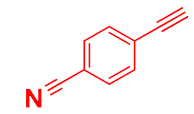 |  | 80 |
| 5 | 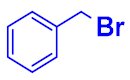 |  | 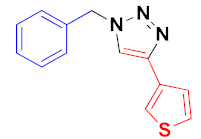 | 82 |
| Entry | R1 | Time | Product | Conversion (%) |
|---|---|---|---|---|
| 1. | -OH | 15 min |  | >99 |
| 2. | -OH | 15 min |  | >98 |
| 3. | -OMe | 15 min | 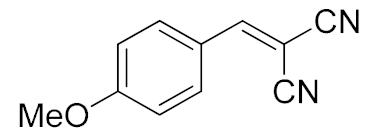 | >99 |
| 4. | -OMe | 15 min | 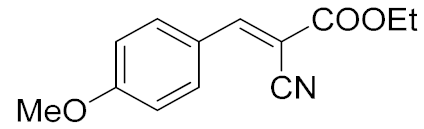 | >97 |
| 5. | -OMe, -OH | 15 min | 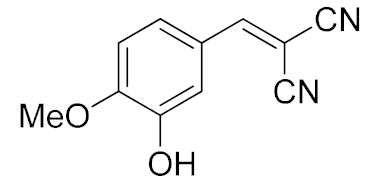 | >99 |
| 6. | -OMe, -OH | 15 min | 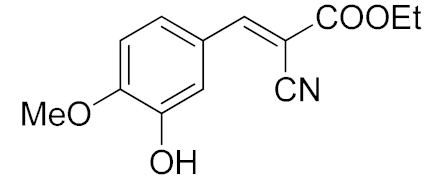 | >99 |
| 7. | -OMe, -OH | 15 min |  | >99 |
| 8. | -OMe, -OH | 15 min | 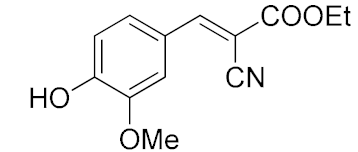 | >98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naskar, K.; Maity, S.; Maity, H.S.; Sinha, C. A Reusable Efficient Green Catalyst of 2D Cu-MOF for the Click and Knoevenagel Reaction. Molecules 2021, 26, 5296. https://doi.org/10.3390/molecules26175296
Naskar K, Maity S, Maity HS, Sinha C. A Reusable Efficient Green Catalyst of 2D Cu-MOF for the Click and Knoevenagel Reaction. Molecules. 2021; 26(17):5296. https://doi.org/10.3390/molecules26175296
Chicago/Turabian StyleNaskar, Kaushik, Suvendu Maity, Himadri Sekhar Maity, and Chittaranjan Sinha. 2021. "A Reusable Efficient Green Catalyst of 2D Cu-MOF for the Click and Knoevenagel Reaction" Molecules 26, no. 17: 5296. https://doi.org/10.3390/molecules26175296







