Synthesis, Structural and Physicochemical Characterization of a Titanium(IV) Compound with the Hydroxamate Ligand N,2-Dihydroxybenzamide
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of 1 and Comparison with the Reported Higher Nuclearity TOCs/H2dihybe
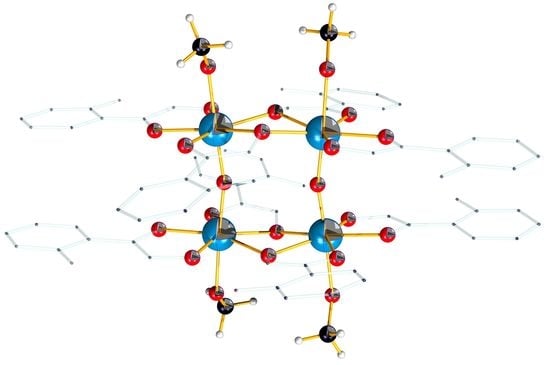
2.2. Description of the Structure
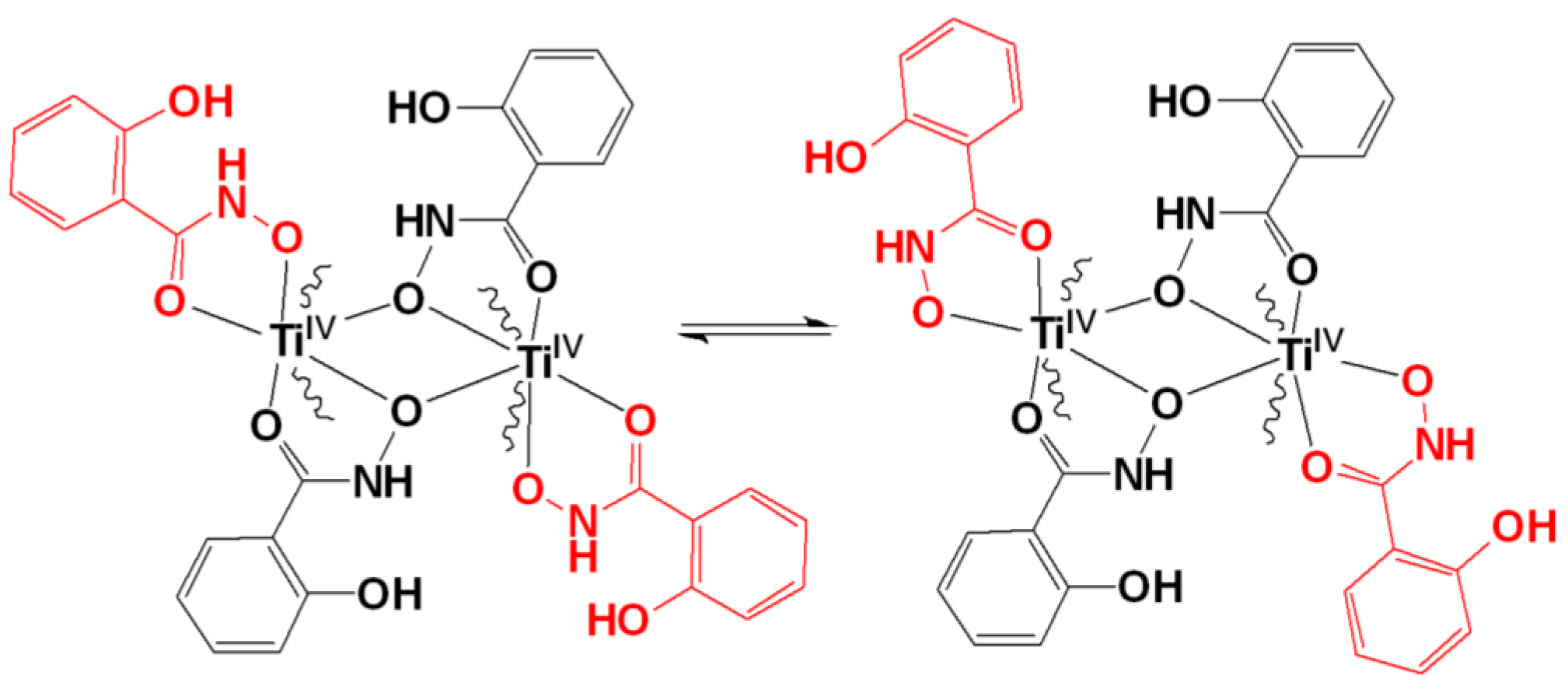
2.3. ESI-MS Spectrometry
2.4. IR Spectroscopy
2.5. NMR Spectroscopy
2.6. Solution UV–Vis and Luminescence Spectroscopies
2.7. Solid-State UV−Vis Spectroscopy
3. Materials and Methods
3.1. Experimental Details
3.2. Synthesis of [TiIV4(μ-O)2(HOCH3)4(μ-η1,η2-Hdihybe-O,O′)4(η1,η1-hdihybe-O,O′)4]Cl4.10H2O.12CH3OH (1)
3.3. X-ray Crystallographic Details
3.4. ESI MS Experimental Details
3.5. FT-IR Spectroscopy
3.6. Ab Initio Modeling of Ligand and Ti-Complex
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Liu, P.Y.; Cui, L.N.; Yang, L.; Shu, X.P.; Zhu, Q.Y.; Dai, J. Bio-compatible fluorescent nano TiO materials prepared from titanium-oxo-cluster precursors. Chem. Commun. 2019, 55, 12360–12363. [Google Scholar] [CrossRef]
- Chen, X.; Selloni, I.A. Introduction: Titanium dioxide (TiO2) nanomaterials. Chem. Rev. 2014, 114, 9281–9282. [Google Scholar] [CrossRef]
- Rajh, T.; Dimitrijevic, N.M.; Bissonnette, M.; Koritarov, T.; Konda, V. Titanium Dioxide in the Service of the Biomedical Revolution. Chem. Rev. 2014, 114, 10177–10216. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Zhou, B. Titanium Dioxide Nanomaterials for Sensor Applications. Chem. Rev. 2014, 114, 10131–10176. [Google Scholar] [CrossRef]
- Bai, Y.; Mora-Sero’, I.; Angelis, F.D.; Bisquert, J.; Wang, P. Titanium dioxide nanomaterials for photovoltaic applications. Chem. Rev. 2014, 114, 10095–10130. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, X.; Jia, Y.; Chen, X.; Han, H.; Li, C. Titanium Dioxide-Based Nanomaterials for Photocatalytic Fuel Generations. Chem. Rev. 2014, 114, 9987–10043. [Google Scholar] [CrossRef]
- Fan, X.; Wang, J.H.; Wu, K.F.; Zhang, L.; Zhang, J. Isomerism in Titanium-oxo Clusters: Molecular Anatase Model with Atomic Structure and Improved Photocatalytic Activity. Angew. Chem. Int. Ed. 2019, 58, 1320–1323. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, C.; Li, L.J.; Sun, Z.M. Synthesis, Crystal Structures, and Photochemical Properties of a Family of Heterometallic Titanium Oxo Clusters. Inorg. Chem. 2019, 58, 6312–6319. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, Y.; Wang, Z.; Zheng, J.; Bi, Y.; Zheng, Z. Thiacalix[4]arene-Protected Titanium−Oxo Clusters: Influence of Ligand Conformation and Ti−S Coordination on the Visible-Light Photocatalytic Hydrogen Production. Inorg. Chem. 2020, 59, 7150–7157. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.B.; Wang, X.C. Multifunctional Metal-Organic Frameworks for Photocatalysis. Small 2015, 11, 3097–3112. [Google Scholar] [CrossRef]
- Janek, M.; Radtke, A.; Muzioł, T.M.; Jerzykiewicz, M.; Piszczek, P. Tetranuclear Oxo-Titanium Clusters with Different Carboxylate Aromatic Ligands: Optical Properties, DFT Calculations, and Photoactivity. Materials 2018, 11, 1661. [Google Scholar] [CrossRef] [Green Version]
- Pearce, A.J.; Harkins, R.P.; Reiner, B.R.; Wotal, A.C.; Dunscomb, R.J.; Tonks, I.A. Multicomponent Pyrazole Synthesis from Alkynes, Nitriles, and Titanium Imido Complexes via Oxidatively Induced N−N Bond Coupling. J. Am. Chem. Soc. 2020, 142, 4390–4399. [Google Scholar] [CrossRef]
- Tuskaev, V.A.; Gagieva, S.C.; Kurmaev, D.A.; Melnikova, E.K.; Zubkevich, S.V.; Buzin, M.I.; Nikiforova, G.G.; Vasil’ev, V.G.; Saracheno, D.; Bogdanov, V.S.; et al. Olefin polymerization behavior of titanium(IV) alkoxo complexes with fluorinated diolate ligands: The impact of the chelate ring size and the nature of organoaluminum compounds. Appl. Organomet. Chem. 2020, 34, e5953. [Google Scholar] [CrossRef]
- Robinson, S.G.; Wu, X.; Jiang, B.; Sigman, M.S.; Lin, S. Mechanistic Studies Inform Design of Improved Ti(salen) Catalysts for Enantioselective [3 + 2] Cycloaddition. J. Am. Chem. Soc. 2020, 142, 18471–18482. [Google Scholar] [CrossRef] [PubMed]
- Ayla, E.Z.; Potts, D.S.; Bregante, D.T.; Flaherty, D.W. Alkene Epoxidations with H2O2 over Groups 4−6 Metal-Substituted BEA Zeolites: Reactive Intermediates, Reaction Pathways, and Linear Free-Energy Relationships. ACS Catal. 2021, 11, 139–154. [Google Scholar] [CrossRef]
- Engler, H.; Lansing, M.; Gordon, C.P.; Neudörfl, J.M.; Schafer, M.; Schlörer, N.E.; Copéret, C.; Berkessel, A. Olefin Epoxidation Catalyzed by Titanium–Salalen Complexes: Synergistic H2O2 Activation by Dinuclear Ti Sites, Ligand H-Bonding, and π-Acidity. ACS Catal. 2021, 11, 3206–3217. [Google Scholar] [CrossRef]
- Zhang, T.; Solé-Daura, A.; Fouilloux, H.; Poblet, J.M.; Proust, A.; Carbó, J.J.; Guillemot, G. Reaction Pathway Discrimination in Alkene Oxidation Reactions by Designed Ti-Siloxy-Polyoxometalates. ChemCatChem 2021, 13, 1220–1229. [Google Scholar] [CrossRef]
- Hou, J.L.; Huo, P.; Tang, Z.Z.; Cui, L.N.; Zhu, Q.Y.; Dai, J. A Titanium Oxo Cluster Model Study of Synergistic Effect of Cocoordinated Dye Ligands on Photocurrent Responses. Inorg. Chem. 2018, 57, 7420–7427. [Google Scholar] [CrossRef]
- Zou, D.H.; Cui, L.N.; Liu, P.Y.; Yang, S.; Zhu, Q.Y.; Dai, J. Molecular Model of Dye Sensitized Titanium Oxides Based on ArylAmine Dye Anchored Titanium Oxo Clusters. Inorg. Chem. 2019, 58, 9246–9252. [Google Scholar] [CrossRef]
- Lakshmi Suresh, L.; Ralte Lalrempuia, R.; Ekeli, J.B.; Gillis-D’Hamers, F.; Törnroos, K.W.; Jensen, V.R.; Le Roux, E. Unsaturated and Benzannulated N-Heterocyclic Carbene Complexes of Titanium and Hafnium: Impact on Catalysts Structure and Performance in Copolymerization of Cyclohexene Oxide with CO2. Molecules 2020, 25, 4364. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Azambuja, F.; Parac-Vogt, T.N. The forgotten chemistry of group(IV) metals: A survey on the synthesis, structure, and properties of discrete Zr(IV), Hf(IV), and Ti(IV) oxo clusters. Coord. Chem. Rev. 2021, 438, 213886. [Google Scholar] [CrossRef]
- Wang, J.F.; Fang, W.H.; Li, D.S.; Zhang, L.; Zhang, J. Cocrystal of {Ti4} and {Ti6} Clusters with Enhanced Photochemical Properties. Inorg. Chem. 2017, 56, 2367–2370. [Google Scholar] [CrossRef]
- Wang, C.; Liu, C.; Tian, H.R.; Li, L.J.; Sun, Z.M. Designed Cluster Assembly of Multidimensional Titanium Coordination Polymers: Syntheses, Crystal Structure and Properties. Chem.-Eur. J. 2018, 24, 2952–2961. [Google Scholar] [CrossRef] [PubMed]
- Passadis, S.S.; Papanikolaou, M.G.; Elliott, A.; Tsiafoulis, C.G.; Tsipis, A.C.; Keramidas, A.D.; Miras, H.N.; Kabanos, T.A. Synthesis, Structural, and Physicochemical Characterization of a Ti6 and a Unique Type of Zr6 Oxo Clusters Bearing an Electron-Rich Unsymmetrical {OON} Catecholate/Oxime Ligand and Exhibiting Metalloaromaticity. Inorg. Chem. 2020, 59, 18345–18357. [Google Scholar] [CrossRef] [PubMed]
- Schwyn, B.; Neilands, J.B. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef]
- Ahmed, E.; Holmström, S.J.M. Siderophores in environmental research: Roles and applications. Microb. Biotechnol. 2014, 7, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Boukhalfa, H.; Lack, J.; Reilly, S.D.; Hersman, L.; Neu, M.P. Siderophore production and facilitated uptake of iron and plutonium in P. putida. Proc. AIP Conf. Proc. 2003, 673, 343. [Google Scholar]
- Brennan, B.J.; Chen, J.; Rudshteyn, B.; Chaudhuri, S.; Mercado, B.Q.; Batista, V.S.; Crabtree, R.H.; Brudvig, G.W. Molecular titanium–hydroxamate complexes as models for TiO2 surface binding. Chem. Commun. 2016, 52, 2972–2975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brewster, T.P.; Konezny, S.J.; Sheehan, S.W.; Martini, L.A.; Schmuttenmaer, C.A.; Batista, V.S.; Crabtree, R.H. Hydroxamate Anchors for Improved Photoconversion in Dye-Sensitized Solar Cells. Inorg. Chem. 2013, 52, 6752–6764. [Google Scholar] [CrossRef]
- McNamara, W.R.; Snoeberger, R.C.; Li, G.; Richter, C.; Allen, L.J.; Milot, R.L.; Schmuttenmaer, C.A.; Crabtree, R.H.; Brudvig, G.W.; Batista, V.S. Hydroxamate anchors for water-stable attachment to TiO2 nanoparticles. Energy Environ. Sci. 2009, 2, 1173–1175. [Google Scholar] [CrossRef]
- Zaleski, C.M.; Kampf, J.W.; Mallah, T.; Kirk, M.L.; Pecoraro, V.L. Assessing the Slow Magnetic Relaxation Behavior of LnIII4MnIII6 Metallacrowns. Inorg. Chem. 2007, 46, 1954–1956. [Google Scholar] [CrossRef] [PubMed]
- Boron, T.T., III; Kampf, J.W.; Pecoraro, V.L. A Mixed 3d-4f 14-Metallacrown-5 Complex That Displays Slow Magnetic Relaxation through Geometric Control of Magnetoanisotropy. Inorg. Chem. 2010, 49, 9104–9106. [Google Scholar] [CrossRef]
- Zaleski, C.M.; Depperman, E.C.; Kampf, J.W.; Kirk, M.L.; Pecoraro, V.L. Synthesis, Structure, and Magnetic Properties of a Large Lanthanide− Transition-Metal Single-Molecule Magnet. Angew. Chem. Int. Ed. 2004, 43, 3912–3914. [Google Scholar] [CrossRef] [PubMed]
- Deb, A.; Boron, T.T., III; Itou, M.; Sakurai, Y.; Mallah, T.; Pecoraro, V.L.; Penner-Hahn, J.E. Understanding Spin Structure in Metallacrown Single-Molecule Magnets using Magnetic Compton Scattering. J. Am. Chem. Soc. 2014, 136, 4889–4892. [Google Scholar] [CrossRef] [PubMed]
- Chow, C.Y.; Trivedi, E.R.; Pecoraro, V.; Zaleski, C.M. Heterometallic Mixed 3d-4f Metallacrowns: Structural Versatility, Luminescence, and Molecular Magnetism. Comments Inorg. Chem. 2015, 35, 214–253. [Google Scholar] [CrossRef]
- Ostrowska, M.; Toporivska, Y.; Golenya, I.A.; Shova, S.; Fritsky, I.O.; Pecoraro, V.L.; Gumienna-Kontecka, E. Explaining How α-Hydroxamate Ligands Control the Formation of Cu(II)-, Ni(II)-, and Zn(II)-Containing Metallacrowns. Inorg. Chem. 2019, 58, 16642–16659. [Google Scholar] [CrossRef]
- Eliseeva, S.V.; Salerno, E.V.; Bermudez, B.A.L.; Petoud, S.; Pecoraro, V.L. Dy3+ White Light Emission Can Be Finely Controlled by Tuning the First Coordination Sphere of Ga3+/Dy3+ Metallacrown Complexes. J. Am. Chem. Soc. 2020, 142, 16173–16176. [Google Scholar] [CrossRef]
- Salerno, E.V.; Eliseeva, S.V.; Schneider, B.L.; Kampf, J.W.; Petoud, S.; Pecoraro, V.L. Visible, Near-Infrared, and Dual-Range Luminescence Spanning the 4f Series Sensitized by a Gallium(III)/Lanthanide(III) Metallacrown Structure. J. Phys. Chem. A 2020, 124, 10550–10564. [Google Scholar] [CrossRef]
- Alaimo, A.A.; Koumousi, E.S.; Cunha-Silva, L.; McCormick, L.J.; Teat, S.J.; Psycharis, V.; Raptopoulou, C.P.; Mukherjee, S.; Li, C.; Gupta, S.D.; et al. Structural Diversities in Heterometallic Mn−Ca Cluster Chemistry from the Use of Salicylhydroxamic Acid: {MnIII 4Ca2}, {MnII/III 6Ca2}, {MnIII/IV8Ca}, and {MnIII8Ca2} Complexes with Relevance to Both Highand Low-Valent States of the Oxygen-Evolving Complex. Inorg. Chem. 2017, 56, 10760–10774. [Google Scholar] [CrossRef] [Green Version]
- Sun, O.; Chen, P.; Li, H.F.; Gao, T.; Yan, P.F. Wheel-like {Ln6} luminescent lanthanide complexes covering the visible and near-infrared domains. CrystEngComm 2020, 22, 5200–5206. [Google Scholar] [CrossRef]
- Pathak, A.; Blair, V.L.; Ferrero, R.L.; Junk, P.C.; Tabora, R.F.; Andrews, P.C. Synthesis and structural characterisation of bismuth(III) hydroxamates and their activity against Helicobacter pylori. Dalton Trans. 2015, 44, 16903–16913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doua, M.; Yanga, H.; Zhaoa, X.; Zhangb, Z.; Lia, D.; Dou, J. A novel “sawtooth-like” heterometallic Sr-Mo 18-metallacrown-6 complex: Synthesis, structure and anticancer activity. Inorg. Chem. Commun. 2020, 119, 108127–108130. [Google Scholar] [CrossRef]
- Wang, B.; Luo, X. A first-principles study on potential chelation agents and indicators of Alzheimer’s disease. RSC Adv. 2020, 10, 35574–35581. [Google Scholar] [CrossRef]
- Gao, D.D.; Gao, Q.; Chen, Y.M.; Li, Y.H.; Li, W. Syntheses, Structures, and Luminescent Properties of the Zn-II and Cd-II 1-D Chain Polymers Assembled by Salicylhydroxamic Acid. Chin. J. Struct. Chem. 2015, 34, 1371–1378. [Google Scholar]
- Sun, O.; Chen, P.; Li, H.F.; Gao, T.; Yan, P.F. Structural, photophysical and magnetic studies of {Ln2} assembled about oxime. Inorg. Chem. Commun. 2020, 114, 107841–197844. [Google Scholar] [CrossRef]
- Chen, S.; Fang, W.H.; Zhang, L.; Zhang, J. Synthesis, Structures, and Photocurrent Responses of PolyoxoTitanium Clusters with Oxime Ligands: From Ti4 to Ti18. Inorg. Chem. 2018, 57, 8850–8856. [Google Scholar] [CrossRef] [PubMed]
- Ito, W.; Hattori, S.; Kondo, M.; Sakagami, H.; Kobayashi, O.; Ishimoto, T.; Shinozaki, K. Dual emission from an iridium(iii) complex/counter anion ion pair. Dalton Trans. 2021, 50, 1887–1894. [Google Scholar] [CrossRef]
- Hong, Q.M.; Wang, S.Y.; An, D.L.; Li, H.Y.; Zhou, J.M.; Deng, Y.F.; Zhou, Z.H. Transformations of dimeric and tetrameric glycolato peroxotitanates and their thermal decompositions for the preparations of anatase and rutile oxides. J. Solid State Chem. 2019, 277, 169–174. [Google Scholar] [CrossRef]
- Tomita, K.; Petrykin, V.; Kobayashi, M.; Shiro, M.; Yoshimura, M.; Kakihana, M. A Water-Soluble Titanium Complex for the Selective Synthesis of Nanocrystalline Brookite, Rutile, and Anatase by a Hydrothermal Method. Angew. Chem. Int. Ed. 2006, 45, 2378–2381. [Google Scholar] [CrossRef]
- Janas, Z.; Jerzykiewicz, L.; Przybylak, K.; Sobota, P.; Szczegot, K. Titanium Complexes Stabilized by a Sulfur-Bridged Chelating Bis(aryloxo) Ligand as Active Catalysts for Olefin Polymerization. Eur. J. Inorg. Chem. 2004, 1639–1645. [Google Scholar] [CrossRef]
- Miras, H.N.; Stone, D.; Long, L.; McInnes, E.J.L.; Kögerler, P.; Cronin, L. Exploring the Structure and Properties of Transition Metal Templated {VM17(VO4)2} Dawson-Like Capsules. Inorg. Chem. 2011, 50, 8384–8391. [Google Scholar] [CrossRef]
- Xu, F.; Scullion, R.A.; Yan, J.; Miras, H.N.; Busche, C.; Scandurra, A.; Pignataro, B.; Long, D.L.; Cronin, L. A Supramolecular Heteropolyoxopalladate {Pd15} Cluster Host Encapsulating a {Pd2} Dinuclear Guest: [PdII2⊂{H7PdII15O10(PO4)10}]9-. J. Am. Chem. Soc. 2011, 133, 4684–4686. [Google Scholar] [CrossRef]
- Miras, H.N.; Sorus, M.; Hawkett, J.; Sells, D.O.; McInnes, E.J.L.; Cronin, L. Oscillatory Template Exchange in Polyoxometalate Capsules: A Ligand-Triggered, Redox-Powered, Chemically Damped Oscillation. J. Am. Chem. Soc. 2012, 134, 6980–6983. [Google Scholar] [CrossRef]
- Miras, H.N.; Wilson, E.F.; Cronin, L. Unravelling the complexities of inorganic and supramolecular self-assembly in solution with electrospray and cryospray mass spectrometry. Chem. Commun. 2009, 11, 1297–1311. [Google Scholar] [CrossRef] [PubMed]
- Zang, H.; Surman, A.; Long, D.; Cronin, L.; Miras, H.N. Exploiting the equilibrium dynamics in the self-assembly of inorganic macrocycles based upon polyoxothiometalate building blocks. Chem. Commun. 2016, 52, 9109–9112. [Google Scholar] [CrossRef] [Green Version]
- Zang, H.Y.; Chen, J.J.; Long, D.L.; Cronin, L.; Miras, H.N. Assembly of Thiometalate-Based {Mo16} and {Mo36} Composite Clusters Combining [Mo2 O2S2],2+ Cations and Selenite Anions. Adv. Mater. 2013, 25, 6245–6249. [Google Scholar] [CrossRef]
- Miras, H.N.; Zang, H.Y.; Long, D.L.; Cronin, L. Direct Synthesis and Mass Spectroscopic Observation of the {M40} Polyoxothiometalate Wheel. Eur. J. Inorg. Chem. 2011, 33, 5105–5111. [Google Scholar] [CrossRef]
- Drouza, C.; Hadjithoma, S.; Nicolaou, M.; Keramidas, A.D. Structural characterization, hydrolytic stability, and dynamics of cis-MoVIO22+ hydroquinonate/phenolate complexes. Polyhedron 2018, 152, 22–30. [Google Scholar] [CrossRef]
- Drouza, C.; Stylianou, M.; Keramidas, A.D. NMR characterization and dynamics of vanadium(V) complexes with tripod (hydroquinonate/phenolate) iminodiacetate ligands in aqueous solution. Pure Appl. Chem. 2009, 81, 1313–1321. [Google Scholar] [CrossRef]
- Drouza, C.; Stylianou, M.; Papaphilippou, P.; Keramidas, A.D. Structural and electron paramagnetic resonance (EPR) characterization of novel vanadium(V/IV) complexes with hydroquinonate-iminodiacetate ligands exhibiting “noninnocent” activity. Pure Appl. Chem. 2013, 85, 329–342. [Google Scholar] [CrossRef]
- Passadis, S.S.; Hadjithoma, S.; Kalampounias, A.G.; Tsipis, A.C.; Sproules, S.; Miras, H.N.; Keramidas, A.D.; Kabanos, T.A. Synthesis, structural and physicochemical characterization of a new type Ti6-oxo cluster protected by a cyclic imide dioxime ligand. Dalton Trans. 2019, 48, 5551–5559. [Google Scholar] [CrossRef] [Green Version]
- Shi, C.R.; Zhang, M.; Hang, X.; Bi, Y.; Huang, L.; Zhou, K.; Xu, Z.; Zheng, Z. Assembly of thiacalix[4]arene-supported high-nuclearity Cd24 cluster with enhanced photocatalytic activity. Nanoscale 2018, 10, 14448–14454. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Phase Annealing in SHELX-90: Direct Methods for Larger Structures. Acta Crystallogr. Sect. A 1990, 46, 467. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112. [Google Scholar] [CrossRef] [Green Version]
- Farrugia, L.J. WinGX Program Features. J. Appl. Cryst. 1999, 32, 837. [Google Scholar] [CrossRef]
- Clark, R.C.; Reid, J.S. The Analytical Calculation of Absorption in Multifaceted Crystals. Acta Crystallogr. Sect. A 1995, 51, 887. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA. Available online: https://gaussian.com/g09citation/ (accessed on 15 July 2021).
- Available online: https://pubchem.ncbi.nlm.nih.gov (accessed on 15 July 2021).
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colic-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785. [Google Scholar] [CrossRef] [Green Version]















| Bond Distances | |||
| Ti(1)-O(1) | 2.112(3) | Ti(1)-O(4) | 1.978(3) |
| Ti(1)-O(1)’ | 2.087(3) | Ti(1)-O(9) | 2.114(3) |
| Ti(1)-O(2) | 2.043(3) | Ti(1)-O(15) | 1.7945(10) |
| Ti(1)-O(3) | 2.027(3) | ||
| Bond Angles | |||
| Ti(1)’-O(1)-Ti(1) | 113.65(13) | O(15)-Ti(1)-O(1) | 91.07(13) |
| Ti(1)’’-O(15)-Ti(1) | 165.8(2) | O(4)-Ti(1)-O(1) | 138.26(12) |
| O(15)-Ti(1)-O(4) | 97.77(10) | O(3)-Ti(1)-O(1) | 144.82(12) |
| O(15)-Ti(1)-O(3) | 94.60(15) | O(2)-Ti(1)-O(1) | 72.89(11) |
| O(4)-Ti(1)-O(3) | 75.20(12) | O(1)’-Ti(1)-O(1) | 63.64(13) |
| O(15)-Ti(1)-O(2) | 90.87(10) | O(15)-Ti(1)-O(9) | 176.42(13) |
| O(4)-Ti(1)-O(2) | 146.94(12) | O(4)-Ti(1)-O(9) | 85.45(13) |
| O(3)-Ti(1)-O(2) | 72.33(12) | O(3)-Ti(1)-O(9) | 87.73(2) |
| O(15)-Ti(1)-O(1)’ | 93.56(14) | O(2)-Ti(1)-O(9) | 87.23(12) |
| O(4)-Ti(1)-O(1)’ | 75.11(12) | O(1)’-Ti(1)-O(9) | 85.73(12) |
| O(3)-Ti(1)-O(1)’ | 150.00(12) | O(1)-Ti(1)-O(9) | 85.47(12) |
| O(2)-Ti(1)-O(1)’ | 136.35(12) | ||
| Experimental | Theoretical | Charge | Formula |
|---|---|---|---|
| 698.98 | 698.09 | −1 | {TiIII2O2(OCH3)3(C7H5NO3)3(OH2)H6}– |
| 736.93 | 737.03 | −1 | {TiIII2O2(C7H5NO3)4H5}– |
| 768.95 | 769.06 | −1 | {TiIII2O2(OCH3)(C7H5NO3)4H6}– |
| 895.91 | 896.04 | −1 | {TiIII3O2(OCH3)(C7H5NO3)4(HOCH3)2(OH2)H3}– |
| 931.90 | 932.10 | −1 | {TiIII3O2(OCH3)(C7H5NO3)4(HOCH3)2(OH2)3H3}– |
| 1048.95 | 1049.05 | −1 | {TiIITiIII3O2(OCH3)(C7H5NO3)5(H2O)2H3}– |
| 1086.90 | 1086.97 | −1 | {TiII3TiIIIO2(OCH3)(C7H5NO3)5(H2O)4H5}– |
| 1124.90 | 1125.03 | −1 | {TiIVTiIII3O2(OCH3)4(C7H5NO3)5(OH2)H4}– |
| 1245.90 | 1246.05 | −1 | {TiIII2TiIV2O2(OCH3)3(C7H5NO3)6(OH2)H5}– |
| 1281.90 | 1281.06 | −1 | {TiIIITiIV3O2(OCH3)3(C7H5NO3)6(OH2)3H3}– |
| 1315.90 | 1316.05 | −1 | {TiIIITiIV3O2(OCH3)3(C7H5NO3)6(OH2)5H3}– |
| 1 | H2dihybe | |||||
|---|---|---|---|---|---|---|
| 13C | 1H | 13C | 1H | 13C (Δδ, ppm) β | 1H (Δδ, ppm) β | |
| C(d)γ | 129.9 | 7.837 | 128.2 | 7.669 | 1.7 | 0.168 |
| C(c)γ | 119.9 | 7.004 | 119.4 | 6.897 | 0.5 | 0.107 |
| C(b)γ | 134.3 | 7.436 | 133.6 | 7.338 | 0.7 | 0.098 |
| C(a)γ | 115.5 | 7.017 | 117.2 | 6.928 | −1.7 | 0.089 |
| C(g)γ | 163.3 | 167.2 | −3.9 | |||
| C(f)γ | 156.6 | 159.2 | −2.6 | |||
| C(e)γ | 111.1 | 114.1 | −3.0 | |||
| Formula | C72H132N8O52Cl4Ti4 | |
| Formula weight | 2275.112 g mol−1 | |
| Temperature | 150(2) K | |
| Wavelength | 0.71073 Å | |
| Crystal system | Orthorhombic | |
| Space group | F d d d | |
| Unit cell dimensions | a = 20.040(4) Å b = 26.118(5) Å c = 36.745(11) Å | a = 90° b = 90° g = 90° |
| Volume | 19232(8) Å3 | |
| Z | 16 | |
| Density (calculated) | 1.453 Mg/m3 | |
| Absorption coefficient | 0.587 mm−1 | |
| F(000) | 8624 | |
| Crystal size | 0.200 × 0.170 × 0.100 mm3 | |
| Theta range for data collection | 1.396 to 26.515°. | |
| Index ranges | −24 ≤ h ≤ 25, −32 ≤ k ≤ 32, −45 ≤ l ≤ 45 | |
| Reflections collected | 45,786 | |
| Independent reflections | 4976 [R(int) = 0.1227] | |
| Completeness to theta = 25.242° | 100.0% | |
| Absorption correction | Empirical | |
| Max. and min. transmission | 0.7454 and 0.6576 | |
| Refinement method | Full-matrix least-squares on F2 | |
| Data/restraints/parameters | 4976/5/304 | |
| Goodness-of-fit on F2 | 0.967 | |
| Final R indices [I>2sigma(I)] | R1 = 0.0657, wR2 = 0.1703 | |
| R indices (all data) | R1 = 0.1101, wR2 = 0.2058 | |
| Extinction coefficient | n/a | |
| Largest diff. peak and hole | 1.117 and −0.473 e.Ε−3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Passadis, S.S.; Hadjithoma, S.; Siafarika, P.; Kalampounias, A.G.; Keramidas, A.D.; Miras, H.N.; Kabanos, T.A. Synthesis, Structural and Physicochemical Characterization of a Titanium(IV) Compound with the Hydroxamate Ligand N,2-Dihydroxybenzamide. Molecules 2021, 26, 5588. https://doi.org/10.3390/molecules26185588
Passadis SS, Hadjithoma S, Siafarika P, Kalampounias AG, Keramidas AD, Miras HN, Kabanos TA. Synthesis, Structural and Physicochemical Characterization of a Titanium(IV) Compound with the Hydroxamate Ligand N,2-Dihydroxybenzamide. Molecules. 2021; 26(18):5588. https://doi.org/10.3390/molecules26185588
Chicago/Turabian StylePassadis, Stamatis S., Sofia Hadjithoma, Panagiota Siafarika, Angelos G. Kalampounias, Anastasios D. Keramidas, Haralampos N. Miras, and Themistoklis A. Kabanos. 2021. "Synthesis, Structural and Physicochemical Characterization of a Titanium(IV) Compound with the Hydroxamate Ligand N,2-Dihydroxybenzamide" Molecules 26, no. 18: 5588. https://doi.org/10.3390/molecules26185588