Efficient Direct Nitrosylation of α-Diimine Rhenium Tricarbonyl Complexes to Structurally Nearly Identical Higher Charge Congeners Activable towards Photo-CO Release
Abstract
:1. Introduction
2. Results
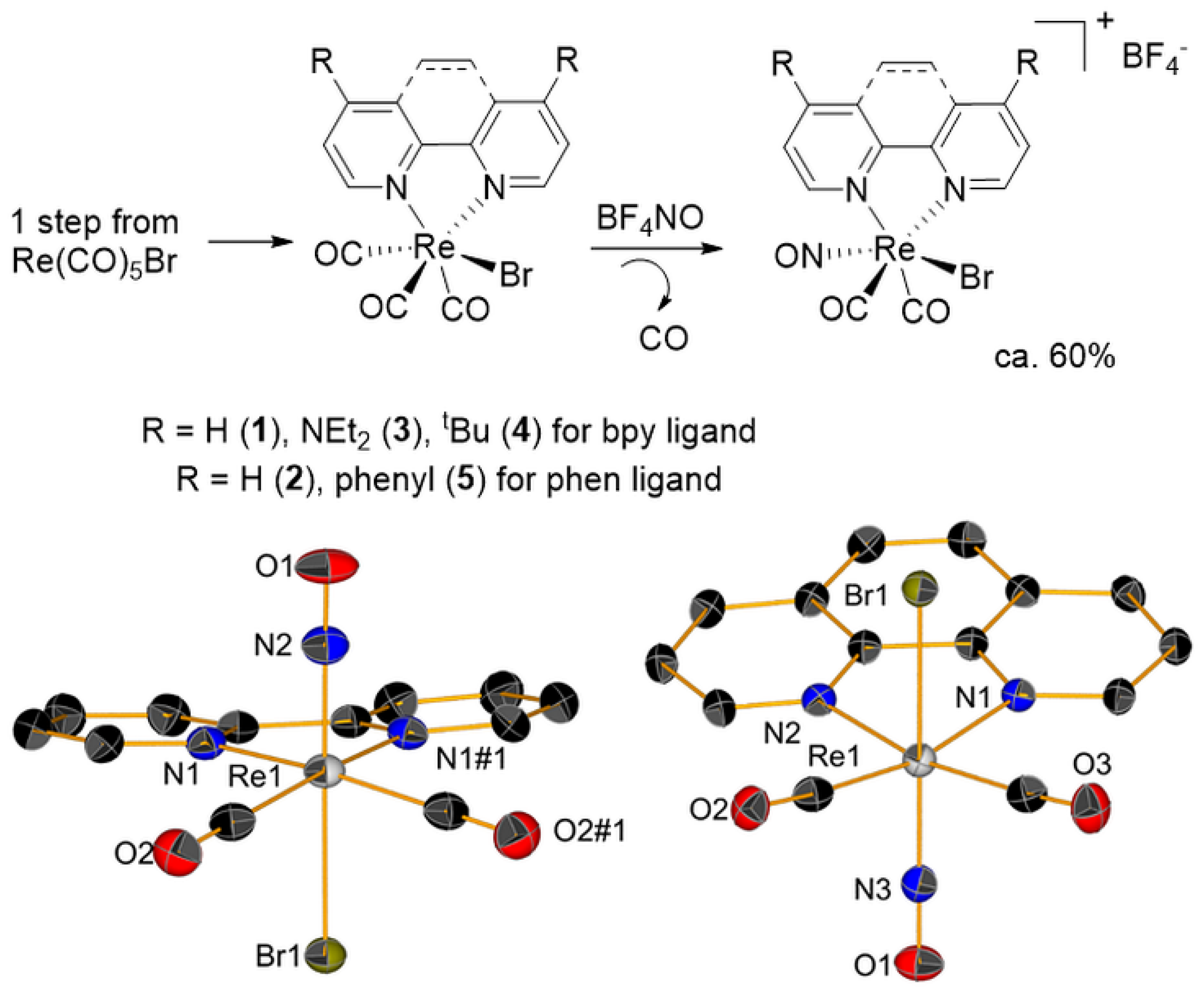
2.1. Synthesis of fac-[Re(CO)2(NO)(N-N)X]+ Species
2.2. Spectroscopic Properties of fac-[Re(CO)2(NO)(N-N)X]+ Species
2.3. Attempted Synthesis of fac-[Re(CO)2(NO)(N-N)L]2+ Species
2.4. Reactivity of fac-[ReI(CO)3(N-N)OR] Species (OR = π-Base) with NO+
2.5. X-ray Crystallography
2.6. CO Releasing Properties
2.7. Antimicrobial Properties of Selected Complexes
3. Materials and Methods
3.1. Reagents and Chemicals
3.2. Instruments and Analysis
3.3. Synthetic Procedures
3.4. Detection of CO Release Using the Myoglobin Assay
3.5. Strains and Culture Conditions
3.6. In Vitro Antimicrobial Activity Determination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Interagency Coordination Group on Antimicrobial Resistance. Meeting the Challenge of Antimicrobial Resistance: From Communication to Collective Action. 2018. Available online: https://www.who.int/antimicrobial-resistance/interagency-coordination-group/IACG_Meeting_challenge_AMR_communication_to_collective_action_270718.pdf?ua=270711 (accessed on 31 January 2021).
- Dadgostar, P. Antimicrobial Resistance: Implications and Costs. Infect. Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef] [Green Version]
- Frei, A. Metal Complexes, an Untapped Source of Antibiotic Potential? Antibiotics 2020, 9, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasiri Sovari, S.; Zobi, F. Recent Studies on the Antimicrobial Activity of Transition Metal Complexes of Groups 6–12. Chemistry 2020, 2, 418–452. [Google Scholar] [CrossRef]
- Frei, A.; Zuegg, J.; Elliott, A.G.; Baker, M.; Braese, S.; Brown, C.; Chen, F.; Dowson, C.G.; Dujardin, G.; Jung, N.; et al. Metal complexes as a promising source for new antibiotics. Chem. Sci. 2020, 11, 2627–2639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, F.; Moat, J.; McFeely, D.; Clarkson, G.; Hands-Portman, I.J.; Furner-Pardoe, J.P.; Harrison, F.; Dowson, C.G.; Sadler, P.J. Biguanide Iridium(III) Complexes with Potent Antimicrobial Activity. J. Med. Chem. 2018, 61, 7330–7344. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, M.; Patra, M.; Senges, C.H.; Ott, I.; Stepanek, J.J.; Pinto, A.; Prochnow, P.; Vuong, C.; Langklotz, S.; Metzler-Nolte, N.; et al. Analysis of the mechanism of action of potent antibacterial hetero-tri-organometallic compounds: A structurally new class of antibiotics. ACS Chem. Biol. 2013, 8, 1442–1450. [Google Scholar] [CrossRef]
- Patra, M.; Wenzel, M.; Prochnow, P.; Pierroz, V.; Gasser, G.; Bandow, J.E.; Metzler-Nolte, N. An organometallic structure-activity relationship study reveals the essential role of a Re(CO)3 moiety in the activity against gram-positive pathogens including MRSA. Chem. Sci. 2015, 6, 214–224. [Google Scholar] [CrossRef]
- Siegmund, D.; Lorenz, N.; Gothe, Y.; Spies, C.; Geissler, B.; Prochnow, P.; Nuernberger, P.; Bandow, J.E.; Metzler-Nolte, N. Benzannulated Re(I)-NHC complexes: Synthesis, photophysical properties and antimicrobial activity. Dalton Trans. 2017, 46, 15269–15279. [Google Scholar] [CrossRef]
- Frei, A.; Amado, M.; Cooper, M.A.; Blaskovich, M.A.T. Light-activated Rhenium Complexes with Dual Mode of Action against Bacteria. Chem. Eur. J. 2019, 26, 2852–2858. [Google Scholar] [CrossRef] [PubMed]
- Delasoie, J.; Radakovic, N.; Pavic, A.; Zobi, F. Neovascularization Effects of Carbon Monoxide Releasing Drugs Chemisorbed on Coscinodiscus Diatoms Carriers Characterized by Spectromicroscopy Imaging. Appl. Sci. 2020, 10, 7380. [Google Scholar] [CrossRef]
- Santoro, G.; Beltrami, R.; Kottelat, E.; Blacque, O.; Bogdanova, A.Y.; Zobi, F. N-Nitrosamine-{cis-Re[CO]2}2+ cobalamin conjugates as mixed CO/NO-releasing molecules. Dalton Trans. 2016, 45, 1504–1513. [Google Scholar] [CrossRef] [PubMed]
- Prieto, L.; Rossier, J.; Derszniak, K.; Dybas, J.; Oetterli, R.M.; Kottelat, E.; Chlopicki, S.; Zelder, F.; Zobi, F. Modified biovectors for the tuneable activation of anti-platelet carbon monoxide release. Chem. Commun. 2017, 53, 6840–6843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suliman, H.B.; Zobi, F.; Piantadosi, C.A. Heme Oxygenase-1/Carbon Monoxide System and Embryonic Stem Cell Differentiation and Maturation into Cardiomyocytes. Antioxid. Redox Signal. 2016, 24, 345–360. [Google Scholar] [CrossRef] [Green Version]
- Delasoie, J.; Pavic, A.; Voutier, N.; Vojnovic, S.; Crochet, A.; Nikodinovic-Runic, J.; Zobi, F. Identification of novel potent and non-toxic anticancer, anti-angiogenic and antimetastatic rhenium complexes against colorectal carcinoma. Eur. J. Med. Chem. 2020, 204, 112583. [Google Scholar] [CrossRef] [PubMed]
- Delasoie, J.; Schiel, P.; Vojnovic, S.; Nikodinovic-Runic, J.; Zobi, F. Photoactivatable Surface-Functionalized Diatom Microalgae for Colorectal Cancer Targeted Delivery and Enhanced Cytotoxicity of Anticancer Complexes. Pharmaceutics 2020, 12, 480. [Google Scholar] [CrossRef] [PubMed]
- Santoro, G.; Zlateva, T.; Ruggi, A.; Quaroni, L.; Zobi, F. Synthesis, characterization and cellular location of cytotoxic constitutional organometallic isomers of rhenium delivered on a cyanocobalmin scaffold. Dalton Trans. 2015, 44, 6999–7008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossier, J.; Hauser, D.; Kottelat, E.; Rothen-Rutishauser, B.; Zobi, F. Organometallic cobalamin anticancer derivatives for targeted prodrug delivery via transcobalamin-mediated uptake. Dalton Trans. 2017, 46, 2159–2164. [Google Scholar] [CrossRef] [Green Version]
- Sovari, S.N.; Vojnovic, S.; Bogojevic, S.S.; Crochet, A.; Pavic, A.; Nikodinovic-Runic, J.; Zobi, F. Design, synthesis and in vivo evaluation of 3-arylcoumarin derivatives of rhenium(I) tricarbonyl complexes as potent antibacterial agents against methicillin-resistant Staphylococcus aureus (MRSA). Eur. J. Med. Chem. 2020, 205, 112533. [Google Scholar] [CrossRef]
- Sovari, S.N.; Radakovic, N.; Roch, P.; Crochet, A.; Pavic, A.; Zobi, F. Combatting AMR: A molecular approach to the discovery of potent and non-toxic rhenium complexes active against C. albicans-MRSA co-infection. ChemRxiv 2021. [Google Scholar] [CrossRef]
- Epand, R.M.; Epand, R.F. Lipid domains in bacterial membranes and the action of antimicrobial agents. Biochim. Biophys. Acta 2009, 1788, 289–294. [Google Scholar] [CrossRef] [Green Version]
- Epand, R.M.; Epand, R.F. Domains in bacterial membranes and the action of antimicrobial agents. Mol. Biosyst. 2009, 5, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Romão, C.C.; Blättler, W.A.; Seixas, J.D.; Bernardes, G.J.L. Developing drug molecules for therapy with carbon monoxide. Chem. Soc. Rev. 2012, 41, 3571–3583. [Google Scholar] [CrossRef]
- Schibli, R.; Marti, N.; Maurer, P.; Spingler, B.; Lehaire, M.-L.; Gramlich, V.; Barnes, C.L. Syntheses and Characterization of Dicarbonyl−Nitrosyl Complexes of Technetium(I) and Rhenium(I) in Aqueous Media: Spectroscopic, Structural, and DFT Analyses. Inorg. Chem. 2005, 44, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Marti, N.; Spingler, B.; Breher, F.; Schibli, R. Comparative Studies of Substitution Reactions of Rhenium(I) Dicarbonyl−Nitrosyl and Tricarbonyl Complexes in Aqueous Media. Inorg. Chem. 2005, 44, 6082–6091. [Google Scholar] [CrossRef] [PubMed]
- Veghini, D.; Berke, H. The Nitrosyl Ligand and the Rhenium−Triflate Bond in Rhenium(I) Complexes. Inorg. Chem. 1996, 35, 4770–4778. [Google Scholar] [CrossRef]
- Kurz, P.; Rattat, D.; Angst, D.; Schmalle, H.; Spingler, B.; Alberto, R.; Berke, H.; Beck, W. The chemistry of the fac-[Re(CO)2(NO)]2+ fragment in aqueous solution. Dalton Trans. 2005, 4, 804–810. [Google Scholar] [CrossRef]
- Veghini, D.; Nefedov, S.E.; Schmalle, H.; Berke, H. Synthesis of nitrosyl rhenium(I) complexes bearing bidentate ligands. J. Organomet. Chem. 1996, 526, 117–134. [Google Scholar] [CrossRef]
- Casey, C.P.; Andrews, M.A.; McAlister, D.R.; Rinz, J.E. Reduction of coordinated carbon monoxide. Synthesis of neutral metal formyl and hydroxymethyl derivatives of the (cyclopentadienyl)dicarbonyl(nitrosyl)rhenium(1+) cation. J. Am. Chem. Soc. 1980, 102, 1927–1933. [Google Scholar] [CrossRef]
- Fischer, E.O.; Strametz, H. Aromatic Complexes of Metals. 105. Cyclopentadienyl-Rhenium-Dicarbonyl-Nitrosyl Cation. Z. Naturforsch. B 1968, 23, 278–279. [Google Scholar] [CrossRef]
- Sweet, J.R.; Graham, W.A.G. Stepwise reduction of coordinated carbon monoxide. J. Am. Chem. Soc. 1982, 104, 2811–2815. [Google Scholar] [CrossRef]
- Agbossou, F.; O’Connor, E.J.; Garner, C.M.; Méndez, N.Q.; Fernández, J.M.; Patton, A.T.; Ramsden, J.A.; Gladysz, J.A.; O’Connor, J.M.; Tajima, T.; et al. Cyclopentadienyl Rhenium Complexes. Inorg. Synth. 1992, 29, 211–225. [Google Scholar] [CrossRef]
- Zobi, F. Ligand Electronic Parameters as a Measure of the Polarization of the CO Bond in [M(CO)xLy](n) Complexes and of the Relative Stabilization of [M(CO)xLy](n/n+1) Species. Inorg. Chem. 2010, 49, 10370–10377. [Google Scholar] [CrossRef]
- Zobi, F. Parametrization of the Contribution of Mono- and Bidentate Ligands on the Symmetric CO Stretching Frequency of fac-[Re(CO)3]+ Complexes. Inorg. Chem. 2009, 48, 10845–10855. [Google Scholar] [CrossRef] [PubMed]
- Zobi, F.; Blacque, O.; Steyl, G.; Spingler, B.; Alberto, R. Formation and Reactivity of [(tacn)-N-CO-(ReBr)-Br-III(CO)2]+ in Water: A Theoretical and Experimental Study. Inorg. Chem. 2009, 48, 4963–4970. [Google Scholar] [CrossRef] [PubMed]
- Abram, U.; Hübener, R.; Alberto, R.; Schibli, R. Darstellung und Strukturen von (Et4N)2[Re(CO)3(NCS)3] und (Et4N)[Re(CO)2Br4]. Z. Anorg. Allg. Chem. 1996, 622, 813–818. [Google Scholar] [CrossRef]
- Schindler, K.; Crochet, A.; Zobi, F. Aerobically stable and substitutionally labile α-diimine rhenium dicarbonyl complexes. RSC Adv. 2021, 11, 7511–7520. [Google Scholar] [CrossRef]
- Evans, J.C.; Rinn, H.W.; Kuhn, S.J.; Olah, G.A. The Structures of Nitrogen Oxide-Boron Trifluoride Complexes. Inorg. Chem. 1964, 3, 857–861. [Google Scholar] [CrossRef]
- Griffiths, J.E.; Sunder, W.A. Raman spectrum of the hexafluoroaurate(V) anion. Spectrochim. Acta A 1979, 35, 1329–1331. [Google Scholar] [CrossRef]
- Millen, D.J. 509. Vibrational spectra of ionic forms of oxides and oxy-acids of nitrogen. Part IV. Raman spectral evidence of ionisation in crystalline nitronium salts. The constitution of solid dinitrogen pentoxide. Note on the spectrum of the perchlorate ion. J. Chem. Soc. 1950, 2606–2612. [Google Scholar] [CrossRef]
- Mesmer, R.E.; Palen, K.M.; Baes, C.F. Fluoroborate equilibriums in aqueous solutions. Inorg. Chem. 1973, 12, 89–95. [Google Scholar] [CrossRef]
- Hansch, C.; Leo, A.; Taft, R.W. A survey of Hammett substituent constants and resonance and field parameters. Chem. Rev. 1991, 91, 165–195. [Google Scholar] [CrossRef]
- Rattat, D.; Verbruggen, A.; Berke, H. Rhenium-Dicarbonyl-Nitrosyl-Komplexe mit Imidazol. Z. Anorg. Allg. Chem. 2006, 632, 1351–1355. [Google Scholar] [CrossRef]
- Rattat, D.; Verbruggen, A.; Berke, H.; Alberto, R. Exploring the nitrosyl-approach: “Re(CO)2(NO)”- and “Tc(CO)2(NO)”-complexes provide new pathways for bioorganometallic chemistry. J. Organomet. Chem. 2004, 689, 4833–4836. [Google Scholar] [CrossRef]
- Hayes, T.R.; Bottorff, S.C.; Slocumb, W.S.; Barnes, C.L.; Clark, A.E.; Benny, P.D. Influence of bidentate ligand donor types on the formation and stability in 2 + 1 fac-[MI(CO)3]+ (M = Re, 99mTc) complexes. Dalton Trans. 2017, 46, 1134–1144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pitchumony, T.S.; Banevicius, L.; Janzen, N.; Zubieta, J.; Valliant, J.F. Isostructural Nuclear and Luminescent Probes Derived From Stabilized [2 + 1] Rhenium(I)/Technetium(I) Organometallic Complexes. Inorg. Chem. 2013, 52, 13521–13528. [Google Scholar] [CrossRef] [PubMed]
- Choualeb, A.; Maccaroni, E.; Blacque, O.; Schmalle, H.W.; Berke, H. Rhenium Nitrosyl Complexes for Hydrogenations and Hydrosilylations. Organometallics 2008, 27, 3474–3481. [Google Scholar] [CrossRef]
- Yang, C.S.; Horng, H.C.; Liao, F.L.; Cheng, C.P. Novel coordination mode of boron tetrafluoride anion: Structure of a BF4—Capped trirhenium cluster: [NEt4]+2[Re3H2(CO)9BF4]2−. J. Chem. Soc. Chem. Commun. 1994, 14, 1637–1638. [Google Scholar] [CrossRef]
- Carballo, R.; Castiñeiras, A.; García-Fontán, S.; Losada-González, P.; Abram, U.; Vázquez-López, E.M. Synthesis, structure and reactivity of bromo- and aquatricarbonylrhenium(I) phosphinite and phosphonite derivatives. Polyhedron 2001, 20, 2371–2383. [Google Scholar] [CrossRef]
- Andreev, R.V.; Borodkin, G.I.; Gatilov, Y.V.; Shubin, V.G. First X-ray diffraction study of nitrosonium complexes of nitrogen-containing organic compounds: A complex of 1,10-phenanthroline with NO+BF4−. Russ. Chem. Bull. Int. Ed. 2001, 50, 2477–2478. [Google Scholar] [CrossRef]
- Andreev, R.V.; Borodkin, G.I.; Gatilov, Y.V.; Shubin, V.G. Molecular and crystal structure of 1,10-phenanthroline complex with nitrosonium cation. Russ. J. Org. Chem. 2002, 38, 845–850. [Google Scholar] [CrossRef]
- Kubacek, P.; Hoffmann, R. Deformations from octahedral geometry in d4 transition-metal complexes. J. Am. Chem. Soc. 1981, 103, 4320–4332. [Google Scholar] [CrossRef]
- Bessette, A.; Nag, S.; Pal, A.K.; Derossi, S.; Hanan, G.S. Neutral Re(I) complexes for anion sensing. Supramol. Chem. 2012, 24, 595–603. [Google Scholar] [CrossRef]
- Zobi, F.; Quaroni, L.; Santoro, G.; Zlateva, T.; Blacque, O.; Sarafimov, B.; Schaub, M.C.; Bogdanova, A.Y. Live-Fibroblast IR Imaging of a Cytoprotective PhotoCORM Activated with Visible Light. J. Med. Chem. 2013, 56, 6719–6731. [Google Scholar] [CrossRef] [Green Version]
- Zobi, F.; Blacque, O.; Jacobs, R.A.; Schaub, M.C.; Bogdanova, A.Y. 17 e(-) rhenium dicarbonyl CO-releasing molecules on a cobalamin scaffold for biological application. Dalton Trans. 2012, 41, 370–378. [Google Scholar] [CrossRef]
- Zobi, F.; Blacque, O. Reactivity of 17 e(-) Complex [(ReBr4)-Br-II(CO)2]2- with Bridging Aromatic Ligands. Characterization and CO-Releasing Properties. Dalton Trans. 2011, 40, 4994–5001. [Google Scholar] [CrossRef] [PubMed]
- Zobi, F.; Degonda, A.; Schaub, M.C.; Bogdanova, A.Y. CO Releasing Properties and Cytoprotective Effect of cis-trans- [Re-II(CO)2Br2L2](n) Complexes. Inorg. Chem. 2010, 49, 7313–7322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pierri, A.E.; Pallaoro, A.; Wu, G.; Ford, P.C. A Luminescent and Biocompatible PhotoCORM. J. Am. Chem. Soc. 2012, 134, 18197–18200. [Google Scholar] [CrossRef]
- Chakraborty, I.; Carrington, S.J.; Roseman, G.; Mascharak, P.K. Synthesis, Structures, and CO Release Capacity of a Family of Water-Soluble PhotoCORMs: Assessment of the Biocompatibility and Their Phototoxicity toward Human Breast Cancer Cells. Inorg. Chem. 2017, 56, 1534–1545. [Google Scholar] [CrossRef]
- Marker, S.C.; MacMillan, S.N.; Zipfel, W.R.; Li, Z.; Ford, P.C.; Wilson, J.J. Photoactivated in Vitro Anticancer Activity of Rhenium(I) Tricarbonyl Complexes Bearing Water-Soluble Phosphines. Inorg. Chem. 2018, 57, 1311–1331. [Google Scholar] [CrossRef]
- Jiménez-Pulido, S.B.; Illán-Cabeza, N.A.; Hueso-Ureña, F.; Maldonado, C.R.; Sánchez-Sánchez, P.; Fernández-Liencres, M.P.; Fernández-Gómez, M.; Moreno-Carretero, M.N. A combined experimental and DFT investigation on the structure and CO-releasing properties of mono and binuclear fac-ReI(CO)3 complexes with 5-pyridin-2-ylmethylene-amino uracils. Dalton Trans. 2016, 45, 15142–15154. [Google Scholar] [CrossRef] [PubMed]
- Kianfar, E.; Schäfer, C.; Lornejad-Schäfer, M.R.; Portenkirchner, E.; Knör, G. New photo-CORMs: Deeply-coloured biocompatible rhenium complexes for the controlled release of carbon monoxide. Inorg. Chim. Acta 2015, 435, 174–177. [Google Scholar] [CrossRef]
- Vaughan, J.G.; Reid, B.L.; Wright, P.J.; Ramchandani, S.; Skelton, B.W.; Raiteri, P.; Muzzioli, S.; Brown, D.H.; Stagni, S.; Massi, M. Photophysical and Photochemical Trends in Tricarbonyl Rhenium(I) N-Heterocyclic Carbene Complexes. Inorg. Chem. 2014, 53, 3629–3641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrington, S.J.; Chakraborty, I.; Bernard, J.M.L.; Mascharak, P.K. A Theranostic Two-Tone Luminescent PhotoCORM Derived from Re(I) and (2-Pyridyl)-benzothiazole: Trackable CO Delivery to Malignant Cells. Inorg. Chem. 2016, 55, 7852–7858. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, I.; Jimenez, J.; Sameera, W.M.C.; Kato, M.; Mascharak, P.K. Luminescent Re(I) Carbonyl Complexes as Trackable PhotoCORMs for CO delivery to Cellular Targets. Inorg. Chem. 2017, 56, 2863–2873. [Google Scholar] [CrossRef] [Green Version]
- Takeda, H.; Koike, K.; Morimoto, T.; Inumaru, H.; Ishitani, O. Photochemistry and photocatalysis of rhenium(I) diimine complexes. Adv. Inorg. Chem. 2011, 63, 137–186. [Google Scholar]
- Sato, S.; Sekine, A.; Ohashi, Y.; Ishitani, O.; Blanco-Rodríguez, A.M.; Vlček, A.; Unno, T.; Koike, K. Photochemical Ligand Substitution Reactions of fac-[Re(bpy)(CO)3Cl] and Derivatives. Inorg. Chem. 2007, 46, 3531–3540. [Google Scholar] [CrossRef]
- Gerbino, D.C.; Hevia, E.; Morales, D.; Clemente, M.E.N.; Pérez, J.; Riera, L.; Riera, V.; Miguel, D. A new reactivity pattern of low-valent transition-metal hydroxo complexes: Straightforward synthesis of hydrosulfido complexes via reaction with carbon disulfide. Chem. Commun. 2003, 3, 328–329. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skiba, J.; Kowalczyk, A.; Stączek, P.; Bernaś, T.; Trzybiński, D.; Woźniak, K.; Schatzschneider, U.; Czerwieniec, R.; Kowalski, K. Luminescent fac-[Re(CO)3(phen)] carboxylato complexes with non-steroidal anti-inflammatory drugs: Synthesis and mechanistic insights into the in vitro anticancer activity of fac-[Re(CO)3(phen)(aspirin)]. New J. Chem. 2019, 43, 573–583. [Google Scholar] [CrossRef]
- Motterlini, R.; Clark, J.E.; Foresti, R.; Sarathchandra, P.; Mann, B.E.; Green, C.J. Carbon Monoxide-Releasing Molecules. Circ. Res. 2002, 90, e17–e24. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| Complex | ν(CO) [cm−1] a | ν(NO) [cm−1] a | λmax [nm (M−1cm−1)] b |
|---|---|---|---|
| 1 | 2112, 2050 | 1801 | 312, 321 |
| 2 | 2118, 2057 | 1790 | 304, 339, 375 |
| 3 | 2104, 2040 | 1770 | 342, 357 |
| 4 | 2114, 2053 | 1797 | 309, 318 |
| 5 | 2114, 2052 | 1795 | 297, 336, 375 |
| 6 | 2113, 2051 | 1779 | 307, 316 |
| 7 | 2106, 2033 | 1784 | 342, 352 |
| 12 | 2127, 2069 | 1820 | 320, 330 |
| 14 | 2114, 2050 | 1796 | 323, 332 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasiri Sovari, S.; Kolly, I.; Schindler, K.; Cortat, Y.; Liu, S.-C.; Crochet, A.; Pavic, A.; Zobi, F. Efficient Direct Nitrosylation of α-Diimine Rhenium Tricarbonyl Complexes to Structurally Nearly Identical Higher Charge Congeners Activable towards Photo-CO Release. Molecules 2021, 26, 5302. https://doi.org/10.3390/molecules26175302
Nasiri Sovari S, Kolly I, Schindler K, Cortat Y, Liu S-C, Crochet A, Pavic A, Zobi F. Efficient Direct Nitrosylation of α-Diimine Rhenium Tricarbonyl Complexes to Structurally Nearly Identical Higher Charge Congeners Activable towards Photo-CO Release. Molecules. 2021; 26(17):5302. https://doi.org/10.3390/molecules26175302
Chicago/Turabian StyleNasiri Sovari, Sara, Isabelle Kolly, Kevin Schindler, Youri Cortat, Shing-Chi Liu, Aurelien Crochet, Aleksandar Pavic, and Fabio Zobi. 2021. "Efficient Direct Nitrosylation of α-Diimine Rhenium Tricarbonyl Complexes to Structurally Nearly Identical Higher Charge Congeners Activable towards Photo-CO Release" Molecules 26, no. 17: 5302. https://doi.org/10.3390/molecules26175302






