Anticancer and Antibiotic Rhenium Tri- and Dicarbonyl Complexes: Current Research and Future Perspectives
Abstract
:1. Introduction
2. Anticancer Complexes
2.1. Mononuclear Complexes
| Compound | IC50 (µM) | IC50 (µM) of Ref. Drug 1 | Cell Line | Ref. |
|---|---|---|---|---|
| 1a | 3 ± 2.5 | 5 ± 2.5 | HTB-132 | [19] |
| 1a | 5 ± 2.5 | 1 ± 2.5 | Balb/c | [19] |
| 1b | 2 ± 1.5 | 5 ± 2.5 | HTB-132 | [19] |
| 1b | 5 ± 3.5 | 1 ± 2.5 | Balb/c | [19] |
| 1c | 3 ± 2.5 | 5 ± 2.5 | HTB-132 | [19] |
| 1c | 4 ± 2.5 | 1 ± 2.5 | Balb/c | [19] |
| 1d | 3 ± 4.5 | 5 ± 2.5 | HTB-132 | [19] |
| 1d | 4 ± 2.5 | 1 ± 2.5 | Balb/c | [19] |
| 1e | 2 ± 2.5 | 5 ± 2.5 | HTB-132 | [19] |
| 1e | 5 ± 5.5 | 1 ± 2.5 | Balb/c | [19] |
| 1f | 4 ± 6.5 | 5 ± 2.5 | HTB-132 | [19] |
| 1f | 5 ± 1.5 | 1 ± 2.5 | Balb/c | [19] |
| 2a | 0.34 ± 0.30 | n.i. | MCF-7A | [20] |
| 2a | 0.25 ± 0.34 | n.i. | MDA-MB-231 | [20] |
| 2b | 0.85 ± 0.26 | n.i. | MCF-7A | [20] |
| 2b | 0.72 ± 0.24 | n.i. | MDA-MB-231 | [20] |
| 2c | 0.43 ± 0.23 | n.i. | MCF-7A | [20] |
| 2c | 0.02 ± 0.002 | n.i. | MCF-10A | [20] |
| 2d | 0.59 ± 0.15 | n.i. | MDA-MB-231 | [20] |
| 2e | 0.96 ± 0.26 | n.i. | MCF-7A | [20] |
| 3a | 3.9 ± 0.7 | 21.5 ± 2.5 | A549 | [21] |
| 3a | 1.2 ± 0.5 | 65.6 ± 1.6 | A549R | [21] |
| 3a | 0.95 ± 0.11 | 8.9 ± 1.0 | HeLa | [21] |
| 3a | 3.1 ± 0.5 | 29.9 ±2.1 | LO2 | [21] |
| 3b | 2.7 ± 0.5 | 65.6 ± 1.6 | A549R | [21] |
| 3b | 1.7 ± 0.4 | 8.9 ± 1.0 | HeLa | [21] |
| 3c | 3.4 ± 0.6 | 21.5 ± 2.5 | A549 | [21] |
| 3c | 0.75 ± 0.12 | 65.6 ± 1.6 | A549R | [21] |
| 3c | 0.52 ± 0.07 | 8.9 ± 1.0 | HeLa | [21] |
| 4a | 3.7 ± 0.2 | >50 | NCI-H1229 | [22] |
| 4b | 0.8 ± 0.1 | >50 | NCI-H1229 | [22] |
| 4b | 2.2 ± 0.2 | >50 | RKO | [22] |
| 4b | 4.0 ± 1.2 | >50 | MCF-7 | [22] |
| 4b | 4.1 ± 0.9 | >50 | A549 | [22] |
| 4b | 4.3 ± 0.7 | >50 | A549R | [22] |
| 4c | 2.9 ± 0.1 | >50 | NCI-H1229 | [22] |
| 5a | 0.7 ± 0.8 | 40.5 ± 2.2 | MDA-MB-231 | [23] |
| 5b | 0.4 ± 0.1 | 40.5 ± 2.2 | MDA-MB-231 | [23] |
| 6a | 4.8 ± 0.2 | 20.0 ± 2.1 | HeLa | [24] |
| 6a | 5.1 ± 0.3 | 18.2 ± 1.5 | A549 | [24] |
| 6b | 0.8 ± 0.1 | 20.0 ± 2.1 | HeLa | [24] |
| 6b | 1.1 ± 0.2 | 18.2 ± 1.5 | A549 | [24] |
| 6b | 1.5 ± 0.2 | 86.5 ± 9.0 | A549R | [24] |
| 6b | 1.0 ± 0.1 | 22.4 ± 2.0 | HepG2 | [24] |
| 7a * | 0.27 ± 0.02 | n.i. | HeLa | [25] |
| 7b * | 2.21 ± 0.12 | n.i. | HeLa | [25] |
| 7c * | 1.51 ± 0.01 | n.i. | HeLa | [25] |
| 8a | 4.3 ± 1.6 | 1.0 ± 0.3 | KB-3-1 | [26] |
| 8a | 3.5 ± 2.8 | 0.23 ± 0.07 | A2780 | [26] |
| 8a | 4.7 ± 1.4 | 8.2 ± 1.8 | A2780CP70 | [26] |
| 8a | 3.9 ± 4.6 | 12.4 ± 8.5 | A549 CisR | [26] |
| 8b | 0.77 ± 0.17 | 1.0 ± 0.3 | KB-3-1 | [26] |
| 8b | 2.2 ± 1.8 | 0.23 ± 0.07 | A2780 | [26] |
| 8b | 2.8 ± 2.5 | 8.2 ± 1.8 | A2780CP70 | [26] |
| 8c | 0.92 ± 0.20 | 1.0 ± 0.3 | KB-3-1 | [26] |
| 8c | 2.2 ± 0.2 | 0.23 ± 0.07 | A2780 | [26] |
| 8c | 3.0 ± 0.7 | 8.2 ± 1.8 | A2780CP70 | [26] |
| 8c | 4.5 ± 0.7 | 0.75 ± 0.43 | H460 | [26] |
| 8c | 4.1 ± 0.9 | 0.43 ± 0.14 | MRC-5 | [26] |
| 9 | 1.7 ± 0.7 | 1.3 ± 0.1 | A2780 | [27] |
| 9 | 1.9 ± 1 | 12 ± 3 | A2780CP70 | [27] |
| 9 | 1.4 ± 0.2 | 6.6 ± 0.7 | HeLa | [27] |
| 9 | 1.4 ± 0.6 | 5.6 ± 0.5 | A549 | [27] |
| 9 | 1.9 ± 0.2 | 1.7 ± 0.2 | HEK293 | [27] |
| 10a | 0.34 ± 0.03 | 0.11 ± 0.02 doxorubicin | HeLa | [28] |
| 10b | 1.65 ± 0.26 | 0.11 ± 0.02 doxorubicin | HeLa | [28] |
| 11a | 4.0 ± 1.2 | 6.8 ± 2.0 | ASPC1 | [29] |
| 11b | 4.8 ± 0.8 | 8.7 ± 4.3 | HPAF-II | [29] |
| 12 | 5 | n.i. | MDA-MB231 | [8] |
| 13a * | 0.9 ± 0.1 | n.i. | HeLa | [30] |
| 13b * | 3.3 ± 2.3 | n.i. | HeLa | [30] |
| 14 | 1 − 2.5 | n.i. | BJAB | [31] |
| 15a | 5.1 ± 0.5 | 1.1 ± 0.4 | A2780 | [32] |
| 15a | 3.7 ± 0.6 | 14.3 ± 1.3 | A2780CP70 | [32] |
| 15b | 4.3 ± 1.3 | 1.1 ± 0.4 | A2780 | [32] |
| 15b | 4.1 ± 1.7 | 14.3 ± 1.3 | A2780CP70 | [32] |
| 15c | 3.2 ± 0.3 | 1.1 ± 0.4 | A2780 | [32] |
| 15c | 3.6 ± 0.2 | 14.3 ± 1.3 | A2780CP70 | [32] |
| 16a | 4.3 ± 0.4 | 8.2 ± 0.7 | A549 | [33] |
| 16a | 3.0 ± 0.2 | 41.5 ± 5.2 | A549R | [33] |
| 16a | 2.2 ± 0.3 | 7.7 ± 0.8 | HeLa | [33] |
| 16a | 2.4 ± 0.4 | 9.4 ± 1.0 | MCF-7 | [33] |
| 16b | 2.2 ± 0.2 | 8.2 ± 0.7 | A549 | [33] |
| 16b | 2.1 ± 0.1 | 41.5 ± 5.2 | A549R | [33] |
| 16b | 1.8 ± 0.2 | 7.7 ± 0.8 | HeLa | [33] |
| 16b | 2.2 ± 0.3 | 9.4 ± 1.0 | MCF-7 | [33] |
| 17 | 4.5 ± 0.5 | 18.0 ± 2.0 | HCT116 | [34] |
| 18 * | 5.3 ± 1.0 | n.i. | HeLa | [35] |
| 19 | 1.1 ± 0.1 | 10.7 ± 0.9 | U2SO | [36] |
| 19 | 1.8 ± 0.1 | 9.2 ± 0.1 | HeLa | [36] |
| 19 | 0.8 ± 0.1 | 9.6 ± 0.8 | A549 | [36] |
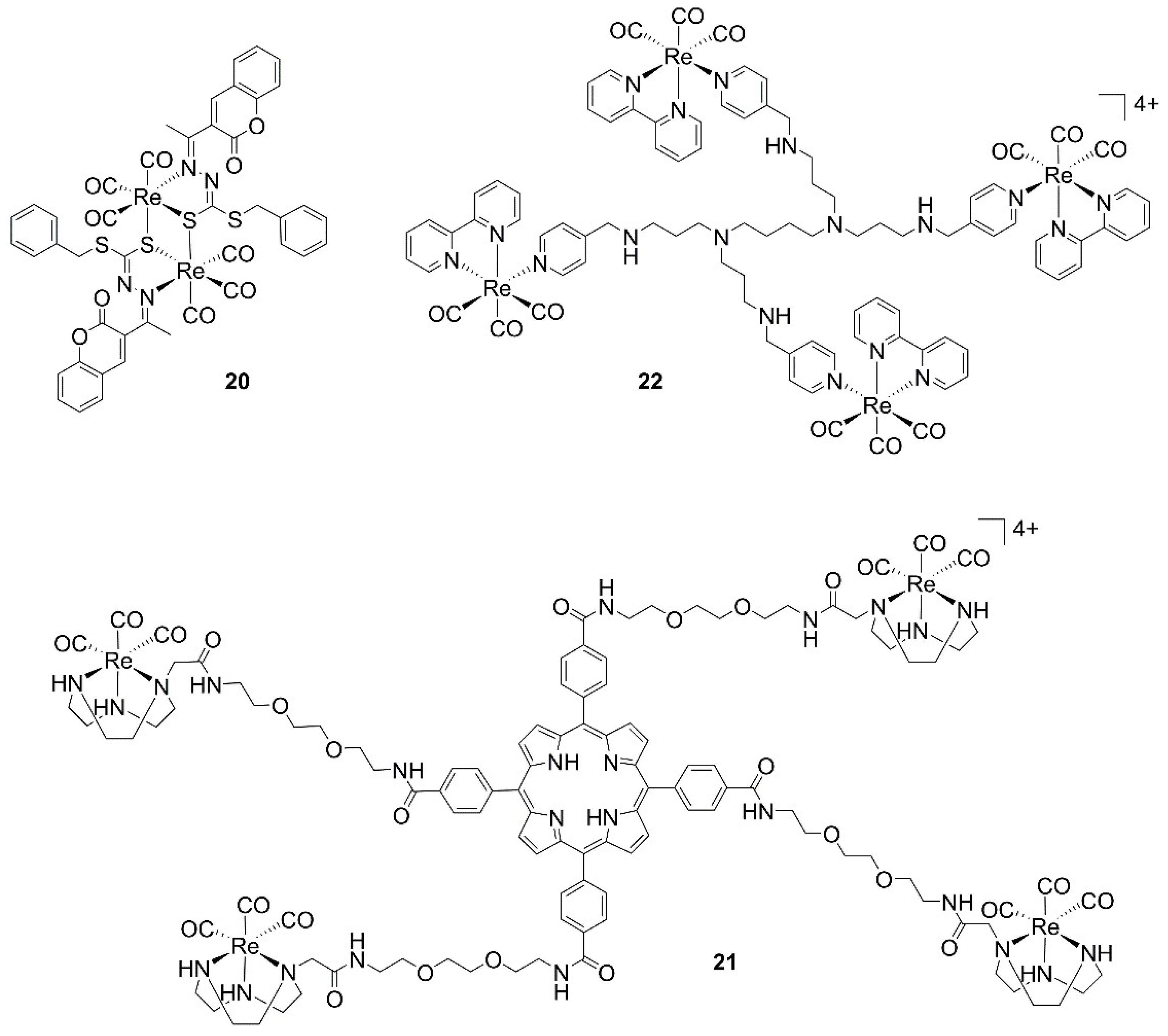
| 20 | 8.61 | n.i. | MDA-MB-231 | [37] |
| 21 * | 1.4 ± 1.3 | n.i. | HeLa | [38] |
| 21 * | 0.5 ± 0.2 | n.i. | H460M2 | [38] |
| 21 * | 0.5 ± 0.1 | n.i. | HBL-100 | [38] |
| 22 | 6.38 ± 1.18 | 37.30 ± 5.69 | A2780 | [39] |
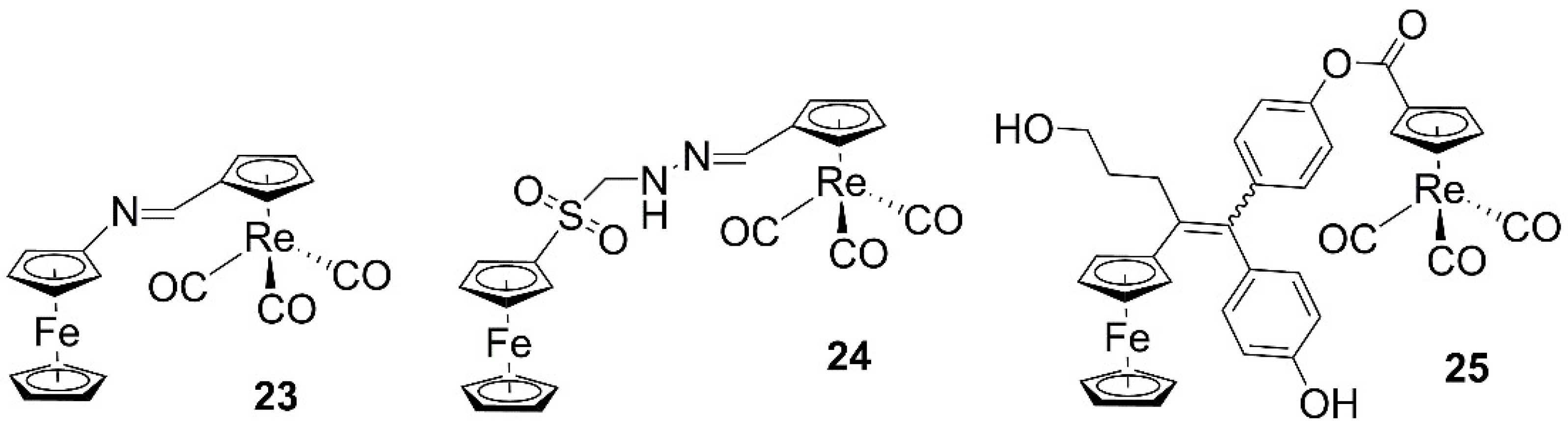
| 23 | 7.4 ± 1.5 | 13 ± 1.8 | MDA-MB-231 | [40] |
| 23 | 7.8 ± 3.3 | 14 ± 1.0 | HCT-116 | [40] |
| 26 | 4.5 ± 0.1 | 0.7 ± 0.2 | C6 | [41] |
| 27 | 1.7 ± 0.3 | 20.4 ± 3.4 | MDA-MB-231 | [42] |
| 27 | 1.1 ± 0.4 | 14 ± 3.5 | MCF-7 | [42] |
| 27 | 1.3 ± 0.2 | 1.0 ± 0.2 | A2780 | [42] |
| 27 | 3.3 ± 0.3 | 2.9 ± 0.8 | MCF-10A | [42] |
| 28 * | 13.5 ± 4.1 | n.i. | HeLa | [43] |
| 29 * | 4.8 ± 1.3 | n.i. | A2780CP70 | [44] |
2.2. Homonuclear Complexes
2.3. Heteronuclear Complexes
3. Antibiotic Complexes
4. Rhenium Dicarbonyl Complexes: Is There a Future for These Species?
5. Preparation cis-[Re(CO)2]+ Complexes via Decarbonylation Reactions
5.1. Trimethylamine N-Oxide Decarbonylation
5.2. Photo-Decarbonylation
5.3. Redox-Mediated Decarbonylation
5.4. Thermal Decarbonylation
5.5. Nitrosylation
6. Anticancer and Antibiotic Dicarbonyl Complexes
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Marston, H.D.; Dixon, D.M.; Knisely, J.M.; Palmore, T.N.; Fauci, A.S. Antimicrobial Resistance. JAMA 2016, 316, 1193–1204. [Google Scholar] [CrossRef] [Green Version]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The side effects of platinum-based chemotherapy drugs: A review for chemists. Dalton Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef]
- Garbutcheon-Singh, K.B.; Grant, P.M.; Harper, B.W.; Krause-Heuer, A.M.; Manohar, M.; Orkey, N.; Aldrich-Wright, J.R. Transition Metal Based Anticancer Drugs. Curr. Top. Med. Chem. 2011, 11, 521–542. [Google Scholar] [CrossRef]
- Leonidova, A.; Gasser, G. Underestimated potential of organometallic rhenium complexes as anticancer agents. ACS Chem. Biol. 2014, 9, 2180–2193. [Google Scholar] [CrossRef]
- Jürgens, S.; Herrmann, W.A.; Kühn, F.E. Rhenium and technetium based radiopharmaceuticals: Development and recent advances. J. Organomet. Chem. 2014, 751, 83–89. [Google Scholar] [CrossRef]
- Lee, L.C.; Leung, K.K.; Lo, K.K. Recent development of luminescent rhenium(I) tricarbonyl polypyridine complexes as cellular imaging reagents, anticancer drugs, and antibacterial agents. Dalton Trans. 2017, 46, 16357–16380. [Google Scholar] [CrossRef]
- Collery, P.; Desmaele, D.; Vijaykumar, V. Design of Rhenium Compounds in Targeted Anticancer Therapeutics. Curr. Pharm. Des. 2019, 25, 3306–3322. [Google Scholar] [CrossRef]
- Bauer, E.B.; Haase, A.A.; Reich, R.M.; Crans, D.C.; Kühn, F.E. Organometallic and coordination rhenium compounds and their potential in cancer therapy. Coord. Chem. Rev. 2019, 393, 79–117. [Google Scholar] [CrossRef]
- Huang, Z.; Wilson, J.J. Therapeutic and Diagnostic Applications of Multimetallic Rhenium(I) Tricarbonyl Complexes. Eur. J. Inorg. Chem. 2021, 2021, 1312–1324. [Google Scholar] [CrossRef]
- Konkankit, C.C.; Marker, S.C.; Knopf, K.M.; Wilson, J.J. Anticancer activity of complexes of the third row transition metals, rhenium, osmium, and iridium. Dalton Trans. 2018, 47, 9934–9974. [Google Scholar] [CrossRef]
- Liew, H.S.; Mai, C.-W.; Zulkefeli, M.; Madheswaran, T.; Kiew, L.V.; Delsuc, N.; Low, M.L. Recent Emergence of Rhenium(I) Tricarbonyl Complexes as Photosensitisers for Cancer Therapy. Molecules 2020, 25, 4176. [Google Scholar] [CrossRef]
- Mkhatshwa, M.; Moremi, J.M.; Makgopa, K.; Manicum, A.-L.E. Nanoparticles Functionalised with Re(I) Tricarbonyl Complexes for Cancer Theranostics. Int. J. Mol. Sci. 2021, 22, 6546. [Google Scholar] [CrossRef]
- Haley, T.J.; Cartwright, F.D. Pharmacology and toxicology of potassium perrhenate and rhenium trichloride. J. Pharm. Sci. 1968, 57, 321–323. [Google Scholar] [CrossRef]
- Vucina, J.; Han, R. Production and therapeutic application of rhenium isotopes, rhenium-186 and rhenium-188: Radioactive pharmaceuticals of the future. Med. Pregl. 2003, 56, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, E.; Brodack, J.W.; Deutsch, K.F. Radiation synovectomy revisited. Eur. J. Nucl. Med. 1993, 20, 1113–1127. [Google Scholar] [CrossRef]
- Li, S.; Liu, J.; Zhang, H.; Tian, M.; Wang, J.; Zheng, X. Rhenium-188 HEDP to treat painful bone metastases. Clin. Nucl. Med. 2001, 26, 919–922. [Google Scholar] [CrossRef]
- Wang, S.J.; Lin, W.Y.; Chen, M.N.; Hsieh, B.T.; Shen, L.H.; Tsai, Z.T.; Ting, G.; Chen, J.T.; Ho, W.L.; Mirzadeh, S.; et al. Rhenium-188 microspheres: A new radiation synovectomy agent. Nucl. Med. Commun. 1998, 19, 427–433. [Google Scholar] [CrossRef]
- Parson, C.; Smith, V.; Krauss, C.; Banerjee, H.N.; Reilly, C.; Krause, J.A.; Wachira, J.M.; Giri, D.; Winstead, A.; Mandal, S.K. Anticancer Properties of Novel Rhenium Pentylcarbanato Compounds against MDA-MB-468(HTB-132) Triple Node Negative Human Breast Cancer Cell Lines. Br. J. Pharm. Res. 2015, 4, 362–367. [Google Scholar] [CrossRef]
- Wilder, P.T.; Weber, D.J.; Winstead, A.; Parnell, S.; Hinton, T.V.; Stevenson, M.; Giri, D.; Azemati, S.; Olczak, P.; Powell, B.V.; et al. Unprecedented anticancer activities of organorhenium sulfonato and carboxylato complexes against hormone-dependent MCF-7 and hormone-independent triple-negative MDA-MB-231 breast cancer cells. Mol. Cell. Biochem. 2018, 441, 151–163. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, J.X.; Cao, Q.; Hao, L.; Zhou, D.X.; Gan, Z.J.; Ji, L.N.; Mao, Z.W. Simultaneously Inducing and Tracking Cancer Cell Metabolism Repression by Mitochondria-Immobilized Rhenium(I) Complex. ACS Appl. Mater. Interfaces 2017, 9, 13900–13912. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Cao, Q.; Zhang, H.; Hao, L.; Zhou, D.; Gan, Z.; Li, Z.; Tong, Y.-X.; Ji, L.-N.; Mao, Z.-W. Targeted reversal and phosphorescence lifetime imaging of cancer cell metabolism via a theranostic rhenium(I)-DCA conjugate. Biomaterials 2018, 176, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.-Y.; Tan, C.-P.; Rao, L.-S.; Zhang, H.; Zheng, Y.; Hao, L.; Ji, L.-N.; Mao, Z.-W. Recoding the Cancer Epigenome by Intervening in Metabolism and Iron Homeostasis with Mitochondria-Targeted Rhenium(I) Complexes. Angew. Chem. Int. Ed. 2020, 59, 18755–18762. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.-R.; Chen, B.-C.; Lu, J.-J.; Ma, X.-R.; Li, R.-T. Phosphorescent rhenium(I) complexes conjugated with artesunate: Mitochondrial targeting and apoptosis-ferroptosis dual induction. J. Inorg. Biochem. 2021, 223, 111537. [Google Scholar] [CrossRef] [PubMed]
- Yip, A.M.-H.; Shum, J.; Liu, H.-W.; Zhou, H.; Jia, M.; Niu, N.; Li, Y.; Yu, C.; Lo, K.K.-W. Luminescent Rhenium(I)–Polypyridine Complexes Appended with a Perylene Diimide or Benzoperylene Monoimide Moiety: Photophysics, Intracellular Sensing, and Photocytotoxic Activity. Chem. Eur. J. 2019, 25, 8970–8974. [Google Scholar] [CrossRef] [PubMed]
- Knopf, K.M.; Murphy, B.L.; MacMillan, S.N.; Baskin, J.M.; Barr, M.P.; Boros, E.; Wilson, J.J. In Vitro Anticancer Activity and in Vivo Biodistribution of Rhenium(I) Tricarbonyl Aqua Complexes. J. Am. Chem. Soc. 2017, 139, 14302–14314. [Google Scholar] [CrossRef]
- King, A.P.; Marker, S.C.; Swanda, R.V.; Woods, J.J.; Qian, S.-B.; Wilson, J.J. A Rhenium Isonitrile Complex Induces Unfolded Protein Response-Mediated Apoptosis in Cancer Cells. Chem. Eur. J. 2019, 25, 9206–9210. [Google Scholar] [CrossRef]
- Imstepf, S.; Pierroz, V.; Rubbiani, R.; Felber, M.; Fox, T.; Gasser, G.; Alberto, R. Organometallic Rhenium Complexes Divert Doxorubicin to the Mitochondria. Angew. Chem. Int. Ed. 2016, 55, 2792–2795. [Google Scholar] [CrossRef]
- Simpson, P.V.; Casari, I.; Paternoster, S.; Skelton, B.W.; Falasca, M.; Massi, M. Defining the Anti-Cancer Activity of Tricarbonyl Rhenium Complexes: Induction of G2/M Cell Cycle Arrest and Blockade of Aurora-A Kinase Phosphorylation. Chem. Eur. J. 2017, 23, 6518–6521. [Google Scholar] [CrossRef] [Green Version]
- Gianferrara, T.; Spagnul, C.; Alberto, R.; Gasser, G.; Ferrari, S.; Pierroz, V.; Bergamo, A.; Alessio, E. Towards Matched Pairs of Porphyrin-ReI/99mTcI Conjugates that Combine Photodynamic Activity with Fluorescence and Radio Imaging. Chemmedchem 2014, 9, 1231–1237. [Google Scholar] [CrossRef]
- König, M.; Siegmund, D.; Raszeja, L.J.; Prokop, A.; Metzler-Nolte, N. Resistance-breaking profiling and gene expression analysis on an organometallic ReI–phenanthridine complex reveal parallel activation of two apoptotic pathways. Med. Chem. Commun. 2018, 9, 173–180. [Google Scholar] [CrossRef]
- Konkankit, C.C.; Vaughn, B.A.; MacMillan, S.N.; Boros, E.; Wilson, J.J. Combinatorial Synthesis to Identify a Potent, Necrosis-Inducing Rhenium Anticancer Agent. Inorg. Chem. 2019, 58, 3895–3909. [Google Scholar] [CrossRef]
- He, L.; Pan, Z.-Y.; Qin, W.-W.; Li, Y.; Tan, C.-P.; Mao, Z.-W. Impairment of the autophagy-related lysosomal degradation pathway by an anticancer rhenium(i) complex. Dalton Trans. 2019, 48, 4398–4404. [Google Scholar] [CrossRef]
- Aleksanyan, D.V.; Churusova, S.G.; Brunova, V.V.; Rybalkina, E.Y.; Susova, O.Y.; Peregudov, A.S.; Klemenkova, Z.S.; Denisov, G.L.; Kozlov, V.A. Synthesis, characterization, and cytotoxic activity of N-metallated rhenium(I) pincer complexes with (thio)phosphoryl pendant arms. J. Organomet. Chem. 2020, 926, 121498. [Google Scholar] [CrossRef]
- Leonidova, A.; Pierroz, V.; Rubbiani, R.; Heier, J.; Ferrari, S.; Gasser, G. Towards cancer cell-specific phototoxic organometallic rhenium(i) complexes. Dalton Trans. 2014, 43, 4287–4294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.X.; Liang, J.H.; Zhang, H.; Wang, Z.H.; Wan, Q.; Tan, C.P.; Ji, L.N.; Mao, Z.W. Mitochondria-Accumulating Rhenium(I) Tricarbonyl Complexes Induce Cell Death via Irreversible Oxidative Stress and Glutathione Metabolism Disturbance. ACS Appl. Mater. Interfaces 2019, 11, 13123–13133. [Google Scholar] [CrossRef]
- Low, M.L.; Paulus, G.; Dorlet, P.; Guillot, R.; Rosli, R.; Delsuc, N.; Crouse, K.A.; Policar, C. Synthesis, characterization and biological activity of Cu(II), Zn(II) and Re(I) complexes derived from S-benzyldithiocarbazate and 3-acetylcoumarin. BioMetals 2015, 28, 553–566. [Google Scholar] [CrossRef] [PubMed]
- Mion, G.; Gianferrara, T.; Bergamo, A.; Gasser, G.; Pierroz, V.; Rubbiani, R.; Vilar, R.; Leczkowska, A.; Alessio, E. Phototoxic Activity and DNA Interactions of Water-Soluble Porphyrins and Their Rhenium(I) Conjugates. Chemmedchem 2015, 10, 1901–1914. [Google Scholar] [CrossRef]
- Giffard, D.; Fischer-Fodor, E.; Vlad, C.; Achimas-Cadariu, P.; Smith, G.S. Synthesis and antitumour evaluation of mono- and multinuclear [2+1] tricarbonylrhenium(I) complexes. Eur. J. Med. Chem. 2018, 157, 773–781. [Google Scholar] [CrossRef]
- Oyarzo, J.; Acuña, A.; Klahn, H.; Arancibia, R.; Silva, C.P.; Bosque, R.; López, C.; Font-Bardía, M.; Calvis, C.; Messeguer, R. Isomeric and hybrid ferrocenyl/cyrhetrenyl aldimines: A new family of multifunctional compounds. Dalton Trans. 2018, 47, 1635–1649. [Google Scholar] [CrossRef] [PubMed]
- Margiotta, N.; Denora, N.; Piccinonna, S.; Laquintana, V.; Lasorsa, F.M.; Franco, M.; Natile, G. Synthesis, characterization, and in vitro evaluation of new coordination complexes of platinum(ii) and rhenium(i) with a ligand targeting the translocator protein (TSPO). Dalton Trans. 2014, 43, 16252–16264. [Google Scholar] [CrossRef]
- Bertrand, B.; Botuha, C.; Forté, J.; Dossmann, H.; Salmain, M. A Bis-Chelating ONO/NN Ligand for the Synthesis of Heterobimetallic Platinum(II)/Rhenium(I) Complexes: Tools for the Optimization of a New Class of Platinum(II) Anticancer Agents. Chem. Eur. J. 2020, 26, 12846–12861. [Google Scholar] [CrossRef]
- Quental, L.; Raposinho, P.; Mendes, F.; Santos, I.; Navarro-Ranninger, C.; Alvarez-Valdes, A.; Huang, H.; Chao, H.; Rubbiani, R.; Gasser, G.; et al. Combining imaging and anticancer properties with new heterobimetallic Pt(ii)/M(i) (M = Re, 99mTc) complexes. Dalton Trans. 2017, 46, 14523–14536. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; King, A.P.; Lovett, J.; Lai, B.; Woods, J.J.; Harris, H.H.; Wilson, J.J. Photochemistry and in vitro anticancer activity of Pt(IV)Re(I) conjugates. Chem. Commun. 2021, 57, 11189–11192. [Google Scholar] [CrossRef]
- Amoroso, A.J.; Arthur, R.J.; Coogan, M.P.; Court, J.B.; Fernández-Moreira, V.; Hayes, A.J.; Lloyd, D.; Millet, C.; Pope, S.J.A. 3-Chloromethylpyridyl bipyridine fac-tricarbonyl rhenium: A thiol-reactive luminophore for fluorescence microscopy accumulates in mitochondria. New J. Chem. 2008, 32, 1097–1102. [Google Scholar] [CrossRef]
- Santoro, G.; Zlateva, T.; Ruggi, A.; Quaroni, L.; Zobi, F. Synthesis, characterization and cellular location of cytotoxic constitutional organometallic isomers of rhenium delivered on a cyanocobalmin scaffold. Dalton Trans. 2015, 44, 6999–7008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konkankit, C.C.; King, A.P.; Knopf, K.M.; Southard, T.L.; Wilson, J.J. In Vivo Anticancer Activity of a Rhenium(I) Tricarbonyl Complex. ACS Med. Chem. Lett. 2019, 10, 822–827. [Google Scholar] [CrossRef] [PubMed]
- Marker, S.C.; King, A.P.; Granja, S.; Vaughn, B.; Woods, J.J.; Boros, E.; Wilson, J.J. Exploring the In Vivo and In Vitro Anticancer Activity of Rhenium Isonitrile Complexes. Inorg. Chem. 2020, 59, 10285–10303. [Google Scholar] [CrossRef]
- Konkankit, C.C.; Lovett, J.; Harris, H.H.; Wilson, J.J. X-Ray fluorescence microscopy reveals that rhenium(i) tricarbonyl isonitrile complexes remain intact in vitro. Chem. Commun. 2020, 56, 6515–6518. [Google Scholar] [CrossRef]
- Delasoie, J.; Pavic, A.; Voutier, N.; Vojnovic, S.; Crochet, A.; Nikodinovic-Runic, J.; Zobi, F. Identification of novel potent and non-toxic anticancer, anti-angiogenic and antimetastatic rhenium complexes against colorectal carcinoma. Eur. J. Med. Chem. 2020, 204, 112583. [Google Scholar] [CrossRef]
- Delasoie, J.; Schiel, P.; Vojnovic, S.; Nikodinovic-Runic, J.; Zobi, F. Photoactivatable Surface-Functionalized Diatom Microalgae for Colorectal Cancer Targeted Delivery and Enhanced Cytotoxicity of Anticancer Complexes. Pharmaceutics 2020, 12, 480. [Google Scholar] [CrossRef] [PubMed]
- Delasoie, J.; Zobi, F. Natural Diatom Biosilica as Microshuttles in Drug Delivery Systems. Pharmaceutics 2019, 11, 537. [Google Scholar] [CrossRef] [Green Version]
- Delasoie, J.; Rossier, J.; Haeni, L.; Rothen-Rutishauser, B.; Zobi, F. Slow-targeted release of a ruthenium anticancer agent from vitamin B-12 functionalized marine diatom microalgae. Dalton Trans. 2018, 47, 17221–17232. [Google Scholar] [CrossRef] [PubMed]
- Domenichini, A.; Casari, I.; Simpson, P.V.; Desai, N.M.; Chen, L.; Dustin, C.; Edmands, J.S.; van der Vliet, A.; Mohammadi, M.; Massi, M.; et al. Rhenium N-heterocyclic carbene complexes block growth of aggressive cancers by inhibiting FGFR- and SRC-mediated signalling. J. Exp. Clin. Cancer Res. 2020, 39, 276. [Google Scholar] [CrossRef]
- Collery, P.; Veena, V.; Harikrishnan, A.; Desmaele, D. The rhenium(I)-diselenoether anticancer drug targets ROS, TGF-β1, VEGF-A, and IGF-1 in an in vitro experimental model of triple-negative breast cancers. Investig. New Drugs 2019, 37, 973–983. [Google Scholar] [CrossRef]
- Collery, P.; Santoni, F.; Ciccolini, J.; Tran, T.N.; Mohsen, A.; Desmaele, D. Dose Effect of Rhenium (I)-diselenoether as Anticancer Drug in Resistant Breast Tumor-bearing Mice After Repeated Administrations. Anticancer Res. 2016, 36, 6051–6057. [Google Scholar] [CrossRef] [Green Version]
- Collery, P.; Mohsen, A.; Kermagoret, A.; Corre, S.; Bastian, G.; Tomas, A.; Wei, M.; Santoni, F.; Guerra, N.; Desmaele, D.; et al. Antitumor activity of a rhenium (I)-diselenoether complex in experimental models of human breast cancer. Investig. New Drugs 2015, 33, 848–860. [Google Scholar] [CrossRef] [Green Version]
- Collery, P.; Bastian, G.; Santoni, F.; Mohsen, A.; Wei, M.; Collery, T.; Tomas, A.; Desmaele, D.; D’Angelo, J. Uptake and efflux of rhenium in cells exposed to rhenium diseleno-ether and tissue distribution of rhenium and selenium after rhenium diseleno-ether treatment in mice. Anticancer Res. 2014, 34, 1679–1689. [Google Scholar]
- Veena, V.; Harikrishnan, A.; Lakshmi, B.; Khanna, S.; Desmaele, D.; Collery, P. A New Model Applied for Evaluating a Rhenium-diselenium Drug: Breast Cancer Cells Stimulated by Cytokines Induced from Polynuclear Cells by LPS. Anticancer Res. 2020, 40, 1915–1920. [Google Scholar] [CrossRef]
- Balasingham, R.G.; Thorp-Greenwood, F.L.; Williams, C.F.; Coogan, M.P.; Pope, S.J.A. Biologically Compatible, Phosphorescent Dimetallic Rhenium Complexes Linked through Functionalized Alkyl Chains: Syntheses, Spectroscopic Properties, and Applications in Imaging Microscopy. Inorg. Chem. 2012, 51, 1419–1426. [Google Scholar] [CrossRef] [PubMed]
- Clede, S.; Lambert, F.; Saint-Fort, R.; Plamont, M.A.; Bertrand, H.; Vessieres, A.; Policar, C. Influence of the Side-Chain Length on the Cellular Uptake and the Cytotoxicity of Rhenium Triscarbonyl Derivatives: A Bimodal Infrared and Luminescence Quantitative Study. Chem. Eur. J. 2014, 20, 8714–8722. [Google Scholar] [CrossRef]
- Ashok Kumar, C.; Karthikeyan, S.; Varghese, B.; Veena, V.; Sakthivel, N.; Manimaran, B. Synthesis, characterisation and cytotoxicity evaluation of rhenium(I) based ester functionalised dinuclear metallacyclophanes. J. Organomet. Chem. 2014, 766, 86–94. [Google Scholar] [CrossRef]
- Ashok Kumar, C.; Divya, D.; Nagarajaprakash, R.; Veena, V.; Vidhyapriya, P.; Sakthivel, N.; Manimaran, B. Self-assembly of manganese(I) and rhenium(I) based semi-rigid ester functionalized M2L2-type metallacyclophanes: Synthesis, characterization and cytotoxicity evaluation. J. Organomet. Chem. 2017, 846, 152–160. [Google Scholar] [CrossRef]
- Govindarajan, R.; Nagarajaprakash, R.; Veena, V.; Sakthivel, N.; Manimaran, B. One-pot reaction of amide functionalized Re(I) based dinuclear metallacycles: Synthesis, characterization and evaluation for anticancer potential. Polyhedron 2018, 139, 229–236. [Google Scholar] [CrossRef]
- Ramakrishna, B.; Nagarajaprakash, R.; Veena, V.; Sakthivel, N.; Manimaran, B. Self-assembly of oxamidato bridged ester functionalised dirhenium metallastirrups: Synthesis, characterisation and cytotoxicity studies. Dalton Trans. 2015, 44, 17629–17638. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, M.; Govindarajan, R.; Duraisamy, E.; Veena, V.; Sakthivel, N.; Manimaran, B. Self-Assembly of Chalcogenolato-Bridged Ester and Amide Functionalized Dinuclear Re(I) Metallacycles: Synthesis, Structural Characterization and Preliminary Cytotoxicity Studies. ChemistrySelect 2017, 2, 3362–3368. [Google Scholar] [CrossRef]
- Ye, R.R.; Tan, C.P.; Chen, M.H.; Hao, L.; Ji, L.N.; Mao, Z.W. Mono- and Dinuclear Phosphorescent Rhenium(I) Complexes: Impact of Subcellular Localization on Anticancer Mechanisms. Chem. Eur. J. 2016, 22, 7800–7809. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.-Y.; Cai, D.-H.; He, L. Dinuclear phosphorescent rhenium(I) complexes as potential anticancer and photodynamic therapy agents. Dalton Trans. 2020, 49, 11583–11590. [Google Scholar] [CrossRef]
- Santoro, G.; Beltrami, R.; Kottelat, E.; Blacque, O.; Bogdanova, A.Y.; Zobi, F. N-Nitrosamine-{cis-Re[CO](2))(2+) cobalamin conjugates as mixed CO/NO-releasing molecules. Dalton Trans. 2016, 45, 1504–1513. [Google Scholar] [CrossRef]
- François, A.; Auzanneau, C.; Le Morvan, V.; Galaup, C.; Godfrey, H.S.; Marty, L.; Boulay, A.; Artigau, M.; Mestre-Voegtlé, B.; Leygue, N.; et al. A functionalized heterobimetallic 99mTc/Re complex as a potential dual-modality imaging probe: Synthesis, photophysical properties, cytotoxicity and cellular imaging investigations. Dalton Trans. 2014, 43, 439–450. [Google Scholar] [CrossRef]
- Jarman, P.J.; Noakes, F.; Fairbanks, S.; Smitten, K.; Griffiths, I.K.; Saeed, H.K.; Thomas, J.A.; Smythe, C. Exploring the Cytotoxicity, Uptake, Cellular Response, and Proteomics of Mono- and Dinuclear DNA Light-Switch Complexes. J. Am. Chem. Soc. 2019, 141, 2925–2937. [Google Scholar] [CrossRef]
- Zheng, Z.-B.; Wu, Y.-Q.; Wang, K.-Z.; Li, F. pH luminescence switching, dihydrogen phosphate sensing, and cellular uptake of a heterobimetallic ruthenium(ii)–rhenium(i) complex. Dalton Trans. 2014, 43, 3273–3284. [Google Scholar] [CrossRef]
- Huentupil, Y.; Chung, P.; Novoa, N.; Arancibia, R.; Roussel, P.; Oyarzo, J.; Klahn, A.H.; Silva, C.; Calvis, C.; Messeguer, R.; et al. Novel multifunctional and multitarget homo- (Fe2) and heterobimetallic [(Fe,M) with M = Re or Mn] sulfonyl hydrazones. Dalton Trans. 2020, 49, 12249–12265. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Heinemann, F.; Top, S.; Dazzi, A.; Policar, C.; Henry, L.; Lambert, F.; Jaouen, G.; Salmain, M.; Vessieres, A. Ferrocifens labelled with an infrared rhenium tricarbonyl tag: Synthesis, antiproliferative activity, quantification and nano IR mapping in cancer cells. Dalton Trans. 2018, 47, 9824–9833. [Google Scholar] [CrossRef] [Green Version]
- Gabano, E.; Do Quental, L.; Perin, E.; Silva, F.; Raposinho, P.; Paulo, A.; Ravera, M. Pt(IV)/Re(I) Chitosan Conjugates as a Flexible Platform for the Transport of Therapeutic and/or Diagnostic Anticancer Agents. Inorganics 2018, 6, 4. [Google Scholar] [CrossRef] [Green Version]
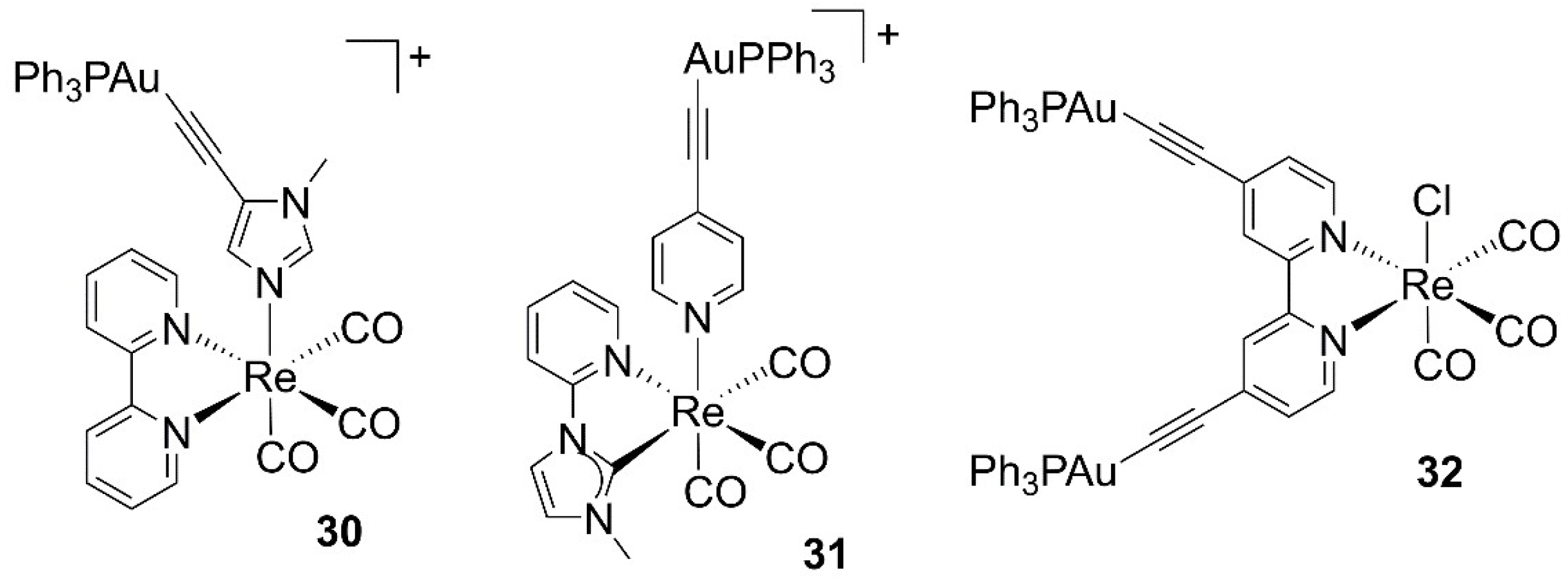
- Fernández-Moreira, V.; Marzo, I.; Gimeno, M.C. Luminescent Re(I) and Re(I)/Au(I) complexes as cooperative partners in cell imaging and cancer therapy. Chem. Sci. 2014, 5, 4434–4446. [Google Scholar] [CrossRef]
- Luengo, A.; Fernández-Moreira, V.; Marzo, I.; Gimeno, M.C. Trackable Metallodrugs Combining Luminescent Re(I) and Bioactive Au(I) Fragments. Inorg. Chem. 2017, 56, 15159–15170. [Google Scholar] [CrossRef] [PubMed]
- Luengo, A.; Fernández-Moreira, V.; Marzo, I.; Gimeno, M.C. Bioactive Heterobimetallic Re(I)/Au(I) Complexes Containing Bidentate N-Heterocyclic Carbenes. Organometallics 2018, 37, 3993–4001. [Google Scholar] [CrossRef] [Green Version]
- Luengo, A.; Redrado, M.; Marzo, I.; Fernández-Moreira, V.; Gimeno, M.C. Luminescent Re(I)/Au(I) Species As Selective Anticancer Agents for HeLa Cells. Inorg. Chem. 2020, 59, 8960–8970. [Google Scholar] [CrossRef] [PubMed]
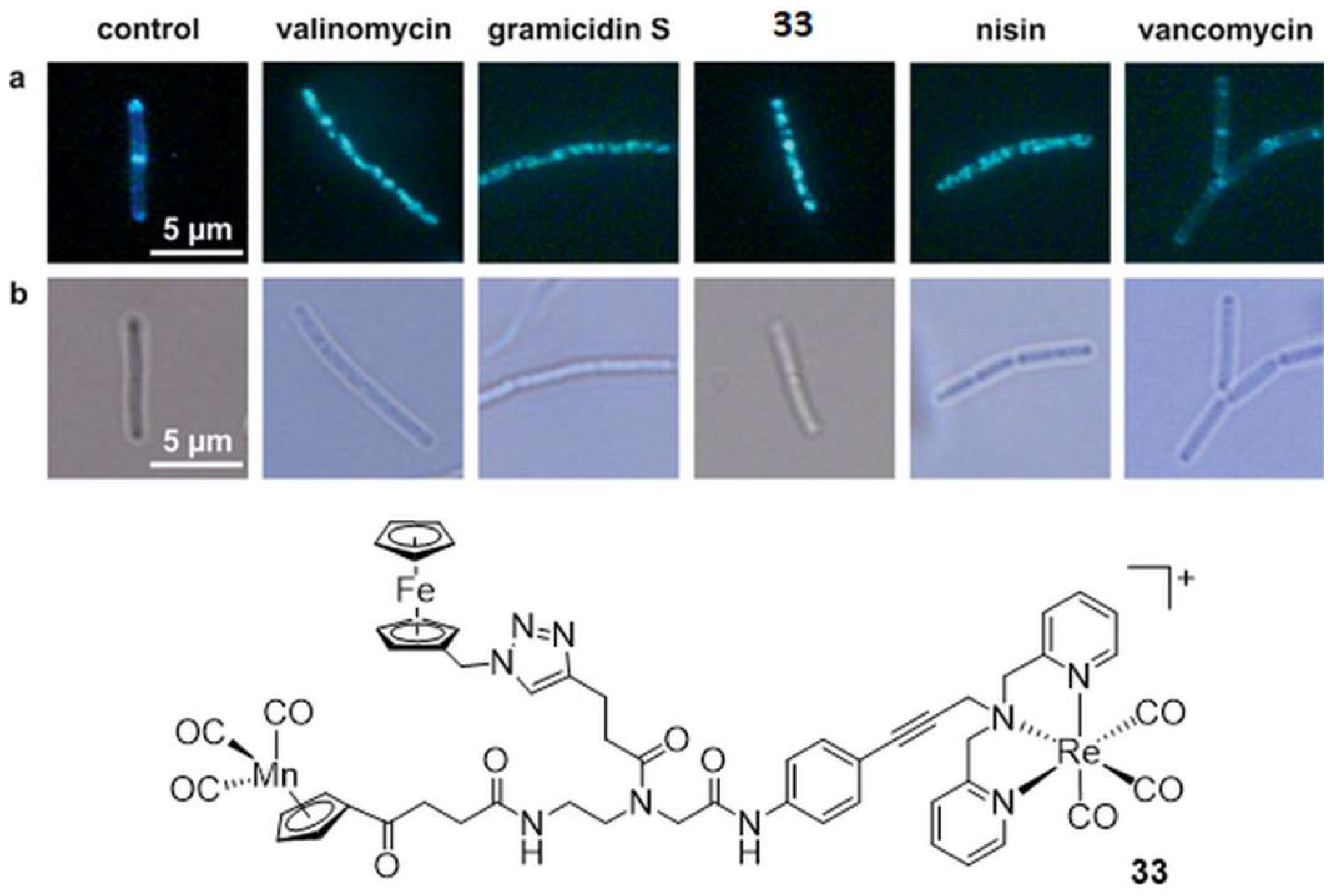
- Wenzel, M.; Patra, M.; Senges, C.H.; Ott, I.; Stepanek, J.J.; Pinto, A.; Prochnow, P.; Vuong, C.; Langklotz, S.; Metzler-Nolte, N.; et al. Analysis of the mechanism of action of potent antibacterial hetero-tri-organometallic compounds: A structurally new class of antibiotics. ACS Chem. Biol. 2013, 8, 1442–1450. [Google Scholar] [CrossRef]
- Patra, M.; Wenzel, M.; Prochnow, P.; Pierroz, V.; Gasser, G.; Bandow, J.E.; Metzler-Nolte, N. An organometallic structure-activity relationship study reveals the essential role of a Re(CO)3 moiety in the activity against gram-positive pathogens including MRSA. Chem. Sci. 2015, 6, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Kottelat, E.; Chabert, V.; Crochet, A.; Fromm, K.M.; Zobi, F. Towards Cardiolite-Inspired Carbon Monoxide Releasing Molecules—Reactivity of d(4), d(5) Rhenium and d(6) Manganese Carbonyl Complexes with Isocyanide Ligands. Eur. J. Inorg. Chem. 2015, 2015, 5628–5638. [Google Scholar] [CrossRef]
- Nasiri Sovari, S.; Kolly, I.; Schindler, K.; Cortat, Y.; Liu, S.C.; Crochet, A.; Pavic, A.; Zobi, F. Efficient Direct Nitrosylation of alpha-Diimine Rhenium Tricarbonyl Complexes to Structurally Nearly Identical Higher Charge Congeners Activable towards Photo-CO Release. Molecules 2021, 26, 5302. [Google Scholar] [CrossRef]
- Noor, A.; Huff, G.S.; Kumar, S.V.; Lewis, J.E.M.; Paterson, B.M.; Schieber, C.; Donnelly, P.S.; Brooks, H.J.L.; Gordon, K.C.; Moratti, S.C.; et al. [Re(CO)3]+ Complexes of exo-Functionalized Tridentate “Click” Macrocycles: Synthesis, Stability, Photophysical Properties, Bioconjugation, and Antibacterial Activity. Organometallics 2014, 33, 7031–7043. [Google Scholar] [CrossRef]
- Carreño, A.; Solís-Céspedes, E.; Zúñiga, C.; Nevermann, J.; Rivera-Zaldívar, M.M.; Gacitúa, M.; Ramírez-Osorio, A.; Páez-Hernández, D.; Arratia-Pérez, R.; Fuentes, J.A. Cyclic voltammetry, relativistic DFT calculations and biological test of cytotoxicity in walled-cell models of two classical rhenium (I) tricarbonyl complexes with 5-amine-1,10-phenanthroline. Chem. Phys. Lett. 2019, 715, 231–238. [Google Scholar] [CrossRef]
- Kydonaki, T.E.; Tsoukas, E.; Mendes, F.; Hatzidimitriou, A.G.; Paulo, A.; Papadopoulou, L.C.; Papagiannopoulou, D.; Psomas, G. Synthesis, characterization and biological evaluation of 99mTc/Re–tricarbonyl quinolone complexes. J. Inorg. Biochem. 2016, 160, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.G.; Vázquez-Hernández, M.; Prochnow, P.; Bandow, J.E.; Metzler-Nolte, N. A CuAAC Click Approach for the Introduction of Bidentate Metal Complexes to a Sulfanilamide-Derived Antibiotic Fragment. Inorg Chem 2019, 58, 9404–9413. [Google Scholar] [CrossRef]
- Pagoni, C.-C.; Xylouri, V.-S.; Kaiafas, G.C.; Lazou, M.; Bompola, G.; Tsoukas, E.; Papadopoulou, L.C.; Psomas, G.; Papagiannopoulou, D. Organometallic rhenium tricarbonyl–enrofloxacin and –levofloxacin complexes: Synthesis, albumin-binding, DNA-interaction and cell viability studies. J. Biol. Inorg. Chem. 2019, 24, 609–619. [Google Scholar] [CrossRef]
- Kumar, S.V.; Lo, W.K.C.; Brooks, H.J.L.; Hanton, L.R.; Crowley, J.D. Antimicrobial Properties of Mono- and Di-fac-rhenium Tricarbonyl 2-Pyridyl-1,2,3-triazole Complexes. Aust. J. Chem. 2016, 69, 489–498. [Google Scholar] [CrossRef] [Green Version]
- Nasiri Sovari, S.; Zobi, F. Recent Studies on the Antimicrobial Activity of Transition Metal Complexes of Groups 6–12. Chemistry 2020, 2, 418–452. [Google Scholar] [CrossRef]
- Varma, R.R.; Pandya, J.G.; Vaidya, F.U.; Pathak, C.; Dabhi, R.A.; Dhaduk, M.P.; Bhatt, B.S.; Patel, M.N. DNA interaction, anticancer, antibacterial, ROS and lipid peroxidation studies of quinoxaline based organometallic Re(I) carbonyls. J. Mol. Struct. 2021, 1240, 130529. [Google Scholar] [CrossRef]
- Carreño, A.; Páez-Hernández, D.; Zúñiga, C.; Ramírez-Osorio, A.; Pizarro, N.; Vega, A.; Solis-Céspedes, E.; Rivera-Zaldívar, M.M.; Silva, A.; Fuentes, J.A. Exploring rhenium (I) complexes as potential fluorophores for walled-cells (yeasts and bacteria): Photophysics, biocompatibility, and confocal microscopy. Dyes Pigm. 2021, 184, 108876. [Google Scholar] [CrossRef]
- Acosta, A.; Antipán, J.; Fernández, M.; Prado, G.; Sandoval-Altamirano, C.; Günther, G.; Gutiérrez-Urrutia, I.; Poblete-Castro, I.; Vega, A.; Pizarro, N. Photochemistry of P,N-bidentate rhenium(i) tricarbonyl complexes: Reactive species generation and potential application for antibacterial photodynamic therapy. RSC Adv. 2021, 11, 31959–31966. [Google Scholar] [CrossRef]
- Siegmund, D.; Lorenz, N.; Gothe, Y.; Spies, C.; Geissler, B.; Prochnow, P.; Nuernberger, P.; Bandow, J.E.; Metzler-Nolte, N. Benzannulated Re(i)–NHC complexes: Synthesis, photophysical properties and antimicrobial activity. Dalton Trans. 2017, 46, 15269–15279. [Google Scholar] [CrossRef]
- Frei, A.; Amado, M.; Cooper, M.A.; Blaskovich, M.A.T. Light-Activated Rhenium Complexes with Dual Mode of Action against Bacteria. Chem. Eur. J. 2020, 26, 2852–2858. [Google Scholar] [CrossRef]
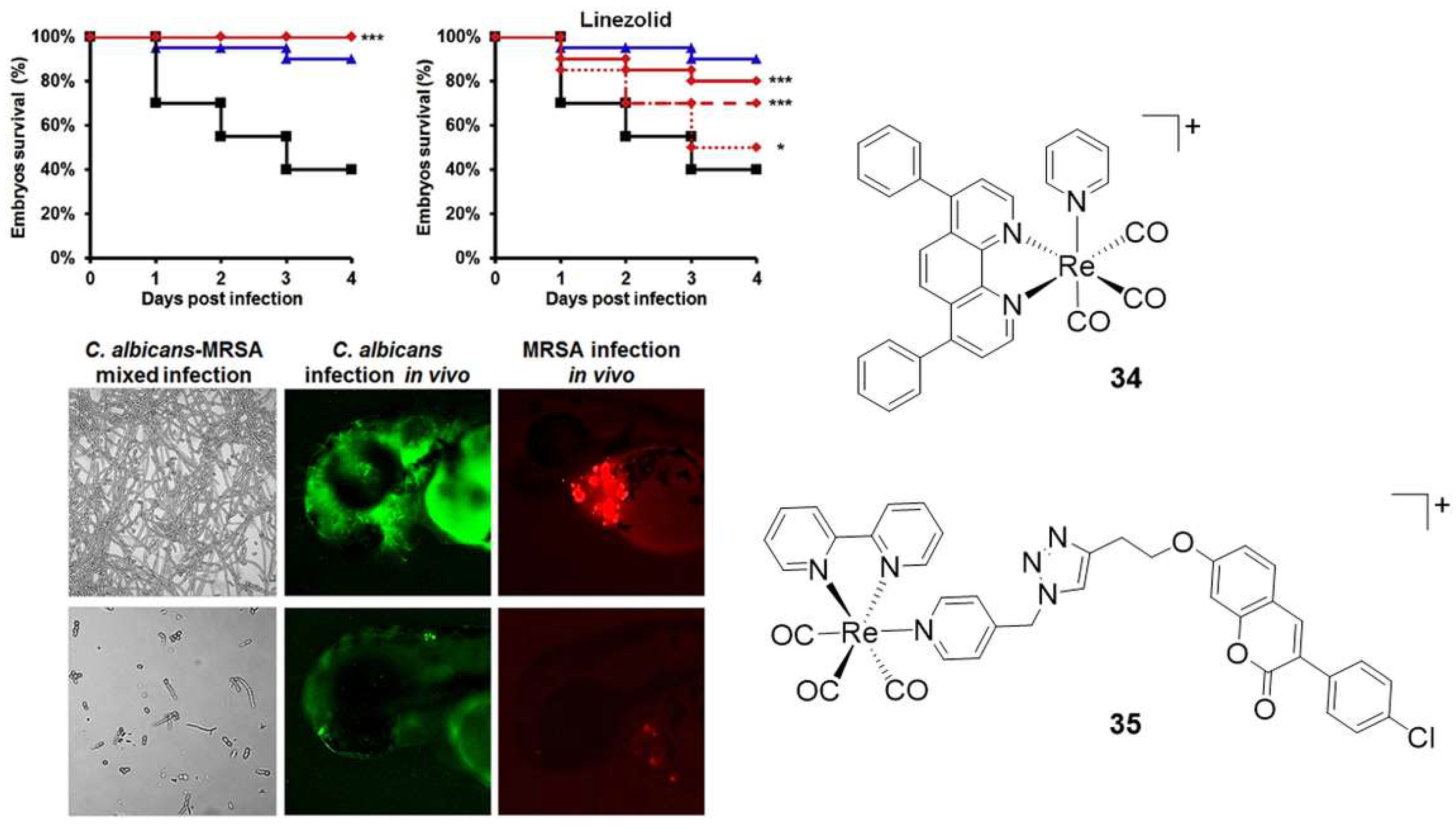
- Sovari, S.N.; Vojnovic, S.; Bogojevic, S.S.; Crochet, A.; Pavic, A.; Nikodinovic-Runic, J.; Zobi, F. Design, synthesis and in vivo evaluation of 3-arylcoumarin derivatives of rhenium(I) tricarbonyl complexes as potent antibacterial agents against methicillin-resistant Staphylococcus aureus (MRSA). Eur. J. Med. Chem. 2020, 205, 112533. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, R.; Hu, Y.; Zhou, H.; Cao, J.; Lv, H.; Chen, S.; Ding, S.; Chen, G. Epidemiology and risk factors of methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci infections in Zhejiang China from 2015 to 2017. Antimicrob. Resist. Infect. Control 2019, 8, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sovari, S.N.; Radakovic, N.; Roch, P.; Crochet, A.; Pavic, A.; Zobi, F. Combatting AMR: A molecular approach to the discovery of potent and non-toxic rhenium complexes active against C. albicans-MRSA co-infection. Eur. J. Med. Chem. 2021, 226, 113858. [Google Scholar] [CrossRef]
- Zobi, F. Parametrization of the Contribution of Mono- and Bidentate Ligands on the Symmetric C O Stretching Frequency of fac-[Re(CO)(3)](+) Complexes. Inorg. Chem. 2009, 48, 10845–10855. [Google Scholar] [CrossRef]
- Zobi, F. Ligand Electronic Parameters as a Measure of the Polarization of the C O Bond in [M(CO)(x)L-y](n) Complexes and of the Relative Stabilization of [M(CO)(x)L-y](n/n+1) Species. Inorg. Chem. 2010, 49, 10370–10377. [Google Scholar] [CrossRef]
- Schindler, K.; Zobi, F. Photochemistry of Rhenium(I) Diimine Tricarbonyl Complexes in Biological Applications. Chimia 2021, 75, 837–844. [Google Scholar] [CrossRef]
- Lorkovic, I.M.; Wrighton, M.S.; Davis, W.M. Use of a Redox-Active Ligand to Reversibly Alter Metal Carbonyl Electrophilicity. J. Am. Chem. Soc. 1994, 116, 6220–6228. [Google Scholar] [CrossRef]
- Xiao, Y.; Cheung, A.W.-Y.; Lai, S.-W.; Cheng, S.-C.; Yiu, S.-M.; Leung, C.-F.; Ko, C.-C. Electronic Communication in Luminescent Dicyanorhenate-Bridged Homotrinuclear Rhenium(I) Complexes. Inorg. Chem. 2019, 58, 6696–6705. [Google Scholar] [CrossRef]
- Ko, C.-C.; Ng, C.-O.; Yiu, S.-M. Luminescent Rhenium(I) Phenanthroline Complexes with a Benzoxazol-2-ylidene Ligand: Synthesis, Characterization, and Photophysical Study. Organometallics 2012, 31, 7074–7084. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Rohacova, J.; Ohtsu, H.; Kawano, M.; Ishitani, O. Synthesis of Re(I) Rings Comprising Different Re(I) Units and Their Light-Harvesting Abilities. Inorg. Chem. 2018, 57, 15158–15171. [Google Scholar] [CrossRef]
- Koike, K.; Tanabe, J.; Toyama, S.; Tsubaki, H.; Sakamoto, K.; Westwell, J.R.; Johnson, F.P.A.; Hori, H.; Saitoh, H.; Ishitani, O. New Synthetic Routes to Biscarbonylbipyridinerhenium(I) Complexes cis,trans-[Re(X2bpy)(CO)2(PR3)(Y)]n+ (X2bpy = 4,4’-X2-2,2’-bipyridine) via Photochemical Ligand Substitution Reactions, and Their Photophysical and Electrochemical Properties. Inorg. Chem. 2000, 39, 2777–2783. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, D.A.; Dhakal, B.; Donovan, E.S.; Nichol, G.S.; Felton, G.A.N. Non-photochemical synthesis of Re(diimine)(CO)2(L)Cl (L=phosphine or phosphite) compounds. Inorg. Chem. Commun. 2015, 59, 80–83. [Google Scholar] [CrossRef] [Green Version]
- Schutte-Smith, M.; Marker, S.C.; Wilson, J.J.; Visser, H.G. Aquation and Anation Kinetics of Rhenium(I) Dicarbonyl Complexes: Relation to Cell Toxicity and Bioavailability. Inorg. Chem. 2020, 59, 15888–15897. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, D.A.; Brereton, K.R.; Ruoff, K.P.; Tang, H.M.; Felton, G.A.N.; Miller, A.J.M.; Dempsey, J.L. Bathochromic Shifts in Rhenium Carbonyl Dyes Induced through Destabilization of Occupied Orbitals. Inorg. Chem. 2018, 57, 5389–5399. [Google Scholar] [CrossRef]
- Jana, M.S.; Pramanik, A.K.; Sarkar, D.; Biswas, S.; Mondal, T.K. Rhenium(I) complexes with NNS donor thioarylazoimidazole ligands with the cis-{Re(CO)2}+ core: Synthesis, characterization, electrochemical study and DFT calculation. J. Mol. Struct. 2013, 1047, 73–79. [Google Scholar] [CrossRef]
- Sato, S.; Matubara, Y.; Koike, K.; Falkenström, M.; Katayama, T.; Ishibashi, Y.; Miyasaka, H.; Taniguchi, S.; Chosrowjan, H.; Mataga, N.; et al. Photochemistry of fac-[Re(bpy)(CO)3Cl]. Chem. Eur. J. 2012, 18, 15722–15734. [Google Scholar] [CrossRef] [Green Version]
- Morimoto, T.; Ito, M.; Koike, K.; Kojima, T.; Ozeki, T.; Ishitani, O. Dual Emission from Rhenium(I) Complexes Induced by an Interligand Aromatic Interaction. Chem. Eur. J. 2012, 18, 3292–3304. [Google Scholar] [CrossRef]
- Tso-Lun Lo, L.; Lai, S.-W.; Yiu, S.-M.; Ko, C.-C. A new class of highly solvatochromic dicyano rhenate(i) diimine complexes—Synthesis, photophysics and photocatalysis. Chem. Commun. 2013, 49, 2311–2313. [Google Scholar] [CrossRef]
- Ng, C.-O.; Yiu, S.-M.; Ko, C.-C. Synthesis, Characterization, and Photophysical Study of Luminescent Rhenium(I) Diimine Complexes with Various Types of N-Heterocyclic Carbene Ligands. Inorg Chem 2014, 53, 3022–3031. [Google Scholar] [CrossRef] [PubMed]
- Marker, S.C.; MacMillan, S.N.; Zipfel, W.R.; Li, Z.; Ford, P.C.; Wilson, J.J. Photoactivated in Vitro Anticancer Activity of Rhenium(I) Tricarbonyl Complexes Bearing Water-Soluble Phosphines. Inorg. Chem. 2018, 57, 1311–1331. [Google Scholar] [CrossRef]
- Shakirova, J.R.; Nayeri, S.; Jamali, S.; Porsev, V.V.; Gurzhiy, V.V.; Levin, O.V.; Koshevoy, I.O.; Tunik, S.P. Targeted Synthesis of NIR Luminescent Rhenium Diimine cis,trans-[Re()(CO)2(L)2]n+ Complexes Containing N-Donor Axial Ligands: Photophysical, Electrochemical, and Theoretical Studies. ChemPlusChem 2020, 85, 2518–2527. [Google Scholar] [CrossRef]
- Ackroyd, N.C.; Katzenellenbogen, J.A. Pyridyl-Cyclopentadiene Re(CO)2+ Complexes as a Compact Core System for SPECT Ligand Development. Organometallics 2010, 29, 3669–3671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, R.D.; Hill, A.F.; Cavigliasso, G.E.; Stranger, R. [(μ-C){Re(CO)2(η-C5H5)}2]: A Surprisingly Simple Bimetallic Carbido Complex. Angew. Chem. Int. Ed. 2013, 52, 3699–3702. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Osses, M.; Siegmund, D.; Gómez, A.; Godoy, F.; Fierro, A.; Llanos, L.; Aravena, D.; Metzler-Nolte, N. Influence of the substituent on the phosphine ligand in novel rhenium(i) aldehydes. Synthesis, computational studies and first insights into the antiproliferative activity. Dalton Trans. 2018, 47, 13861–13869. [Google Scholar] [CrossRef]
- Abram, U.; Hübener, R.; Alberto, R.; Schibli, R. Darstellung und Strukturen von (Et4N)2[Re(CO)3(NCS)3] und (Et4N)[Re(CO)2Br4]. Z. Anorg. Allg. Chem. 1996, 622, 813–818. [Google Scholar] [CrossRef]
- Zobi, F.; Kromer, L.; Spingler, B.; Alberto, R. Synthesis and Reactivity of the 17 e− Complex [ReIIBr4(CO)2]2−: A Convenient Entry into Rhenium(II) Chemistry. Inorg. Chem. 2009, 48, 8965–8970. [Google Scholar] [CrossRef] [PubMed]
- Kromer, L.; Spingler, B.; Alberto, R. Substitution reactions with [ReBr2(CO)2(NCCH3)2]−: A convenient route to complexes with the cis-[Re(CO)2]+ core. Dalton Trans. 2008, 5800–5806. [Google Scholar] [CrossRef] [PubMed]
- Kromer, L.; Spingler, B.; Alberto, R. Synthesis and reactivity of [ReBr2(NCCH3)2(CO)2]−: A new precursor for bioorganometallic chemistry. J. Organomet. Chem. 2007, 692, 1372–1376. [Google Scholar] [CrossRef]
- Zobi, F.; Degonda, A.; Schaub, M.C.; Bogdanova, A.Y. CO Releasing Properties and Cytoprotective Effect of cis-trans- [ReII(CO)2Br2L2]n Complexes. Inorg. Chem. 2010, 49, 7313–7322. [Google Scholar] [CrossRef] [Green Version]
- Zobi, F.; Blacque, O. Reactivity of 17 e(-) Complex [(ReBr4)-Br-II(CO)(2)](2-) with Bridging Aromatic Ligands. Characterization and CO-Releasing Properties. Dalton Trans. 2011, 40, 4994–5001. [Google Scholar] [CrossRef]
- Zobi, F.; Blacque, O.; Jacobs, R.A.; Schaub, M.C.; Bogdanova, A.Y. 17 e−rhenium dicarbonyl CO-releasing molecules on a cobalamin scaffold for biological application. Dalton Trans. 2012, 41, 370–378. [Google Scholar] [CrossRef]
- Prieto, L.; Rossier, J.; Derszniak, K.; Dybas, J.; Oetterli, R.M.; Kottelat, E.; Chlopicki, S.; Zelder, F.; Zobi, F. Modified biovectors for the tuneable activation of anti-platelet carbon monoxide release. Chem. Commun. 2017, 53, 6840–6843. [Google Scholar] [CrossRef] [Green Version]
- Suliman, H.B.; Zobi, F.; Piantadosi, C.A. Heme Oxygenase-1/Carbon Monoxide System and Embryonic Stem Cell Differentiation and Maturation into Cardiomyocytes. Antiox. Redox Sign. 2016, 24, 345–360. [Google Scholar] [CrossRef] [Green Version]
- Schindler, K.; Crochet, A.; Zobi, F. Aerobically stable and substitutionally labile α-diimine rhenium dicarbonyl complexes. RSC Adv. 2021, 11, 7511–7520. [Google Scholar] [CrossRef]
- Triantis, C.; Shegani, A.; Kiritsis, C.; Ischyropoulou, M.; Roupa, I.; Psycharis, V.; Raptopoulou, C.; Kyprianidou, P.; Pelecanou, M.; Pirmettis, I.; et al. Dicarbonyl cis-[M(CO)2(N,O)(C)(P)] (M = Re, 99mTc) Complexes with a New [2 + 1 + 1] Donor Atom Combination. Inorg. Chem. 2018, 57, 8354–8363. [Google Scholar] [CrossRef]
- Manicum, A.-L.E.; Schutte-Smith, M.; Alexander, O.T.; Twigge, L.; Roodt, A.; Visser, H.G. First kinetic data of the CO substitution in fac-[Re(L,L’-Bid)(CO)3(X)] complexes (L,L’-Bid = acacetylacetonate or tropolonate) by tertiary phosphines PTA and PPh3: Synthesis and crystal structures of water-soluble rhenium(I) tri- and dicarbonyl complexes with 1,3,5-triaza-7-phosphaadamantane (PTA). Inorg. Chem. Commun. 2019, 101, 93–98. [Google Scholar] [CrossRef]
- Atallah, H.; Taliaferro, C.M.; Wells, K.A.; Castellano, F.N. Photophysics and ultrafast processes in rhenium(i) diimine dicarbonyls. Dalton Trans. 2020, 49, 11565–11576. [Google Scholar] [CrossRef] [PubMed]
- Black, D.R.; Hightower, S.E. Preparation and characterization of rhenium(I) dicarbonyl complexes based on the meridionally-coordinated terpyridine ligand. Inorg. Chem. Commun. 2012, 24, 16–19. [Google Scholar] [CrossRef]
- Frenzel, B.A.; Schumaker, J.E.; Black, D.R.; Hightower, S.E. Synthesis, spectroscopic, electrochemical and computational studies of rhenium(i) dicarbonyl complexes based on meridionally-coordinated 2,2’:6’,2’’-terpyridine. Dalton Trans. 2013, 42, 12440–12451. [Google Scholar] [CrossRef]
- Fernández-Terán, R.J.; Sévery, L. Coordination Environment Prevents Access to Intraligand Charge-Transfer States through Remote Substitution in Rhenium(I) Terpyridinedicarbonyl Complexes. Inorg. Chem. 2021, 60, 1325–1333. [Google Scholar] [CrossRef]
- Laramée-Milette, B.; Zaccheroni, N.; Palomba, F.; Hanan, G.S. Visible and Near-IR Emissions from k2N- and k3N-Terpyridine Rhenium(I) Assemblies Obtained by an [n × 1] Head-to-Tail Bonding Strategy. Chem. Eur. J. 2017, 23, 6370–6379. [Google Scholar] [CrossRef]
- Auvray, T.; Del Secco, B.; Dubreuil, A.; Zaccheroni, N.; Hanan, G.S. In-Depth Study of the Electronic Properties of NIR-Emissive κ3N Terpyridine Rhenium(I) Dicarbonyl Complexes. Inorg. Chem. 2021, 60, 70–79. [Google Scholar] [CrossRef]
- Jurca, T.; Chen, W.-C.; Michel, S.; Korobkov, I.; Ong, T.-G.; Richeson, D.S. Solid-State Thermolysis of a fac-Rhenium(I) Carbonyl Complex with a Redox Non-Innocent Pincer Ligand. Chem. Eur. J. 2013, 19, 4278–4286. [Google Scholar] [CrossRef]
- Bulsink, P.; Al-Ghamdi, A.; Joshi, P.; Korobkov, I.; Woo, T.; Richeson, D. Capturing Re(i) in an neutral N,N,N pincer Scaffold and resulting enhanced absorption of visible light. Dalton Trans. 2016, 45, 8885–8896. [Google Scholar] [CrossRef]
- Pichaandi, K.R.; Mazzotta, M.G.; Harwood, J.S.; Fanwick, P.E.; Abu-Omar, M.M. Synthesis, Dynamics, and DFT Studies of Rhenium Dicarbonyl PNN Pincer Complexes in Three Different Oxidation States. Organometallics 2014, 33, 1672–1677. [Google Scholar] [CrossRef]
- Teets, T.S.; Labinger, J.A.; Bercaw, J.E. Guanidine-Functionalized Rhenium Cyclopentadienyl Carbonyl Complexes: Synthesis and Cooperative Activation of H–H and O–H Bonds. Organometallics 2014, 33, 4107–4117. [Google Scholar] [CrossRef] [Green Version]
- Godoy, F.; Gómez, A.; Segura, R.; Doctorovich, F.; Pellegrino, J.; Gaviglio, C.; Guerrero, P.; Klahn, A.H.; Fuentealba, M.; Garland, M.T. Synthesis, structure, and reactivity of (η5:η1-C5Me4(CH2)2NMe2)Re(CO)2. Electron transfer behavior of a nitrosyl derivative. J. Organomet. Chem. 2014, 765, 8–16. [Google Scholar] [CrossRef]
- Pruitt, D.G.; Baumann, S.M.; Place, G.J.; Oyeamalu, A.N.; Sinn, E.; Jelliss, P.A. Synthesis and functionalization of nitrosyl rhenacarboranes towards their use as drug delivery vehicles. J. Organomet. Chem. 2015, 798, 60–69. [Google Scholar] [CrossRef] [Green Version]
- Pruitt, D.G.; Bullock, K.M.; Banks, W.A.; Jelliss, P.A. Development of rhenacarborane complexes as central nervous system (CNS) drug delivery agents. Inorg. Chim. Acta 2017, 466, 139–144. [Google Scholar] [CrossRef]
- Rossier, J.; Hauser, D.; Kottelat, E.; Rothen-Rutishauser, B.; Zobi, F. Organometallic cobalamin anticancer derivatives for targeted prodrug delivery via transcobalamin-mediated uptake. Dalton Trans. 2017, 46, 2159–2164. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schindler, K.; Zobi, F. Anticancer and Antibiotic Rhenium Tri- and Dicarbonyl Complexes: Current Research and Future Perspectives. Molecules 2022, 27, 539. https://doi.org/10.3390/molecules27020539
Schindler K, Zobi F. Anticancer and Antibiotic Rhenium Tri- and Dicarbonyl Complexes: Current Research and Future Perspectives. Molecules. 2022; 27(2):539. https://doi.org/10.3390/molecules27020539
Chicago/Turabian StyleSchindler, Kevin, and Fabio Zobi. 2022. "Anticancer and Antibiotic Rhenium Tri- and Dicarbonyl Complexes: Current Research and Future Perspectives" Molecules 27, no. 2: 539. https://doi.org/10.3390/molecules27020539
APA StyleSchindler, K., & Zobi, F. (2022). Anticancer and Antibiotic Rhenium Tri- and Dicarbonyl Complexes: Current Research and Future Perspectives. Molecules, 27(2), 539. https://doi.org/10.3390/molecules27020539







