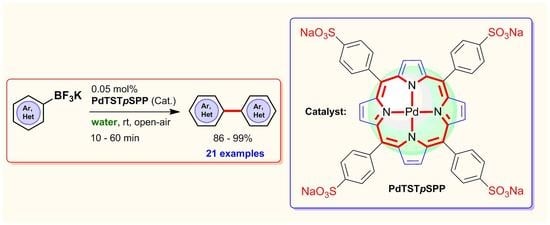
Porphyrin N-Pincer Pd(II)-Complexes in Water: A Base-Free and Nature-Inspired Protocol for the Oxidative Self-Coupling of Potassium Aryltrifluoroborates in Open-Air
Abstract
:1. Introduction
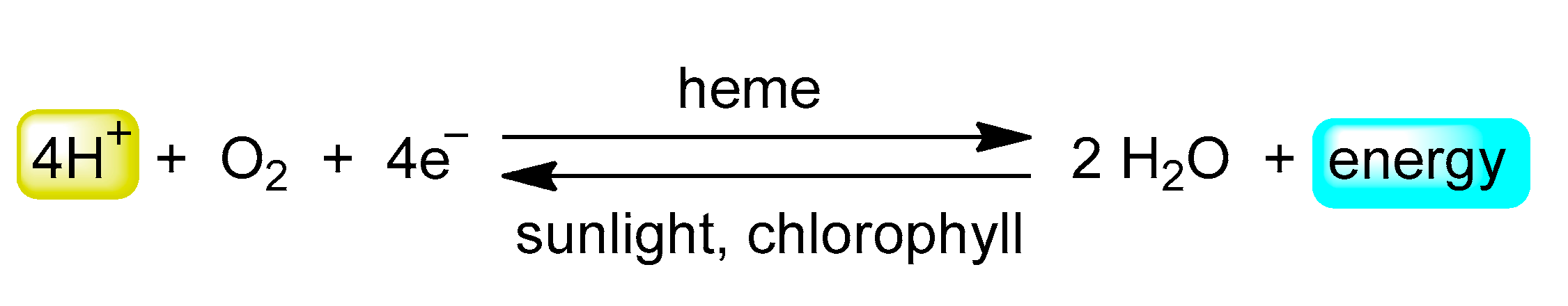
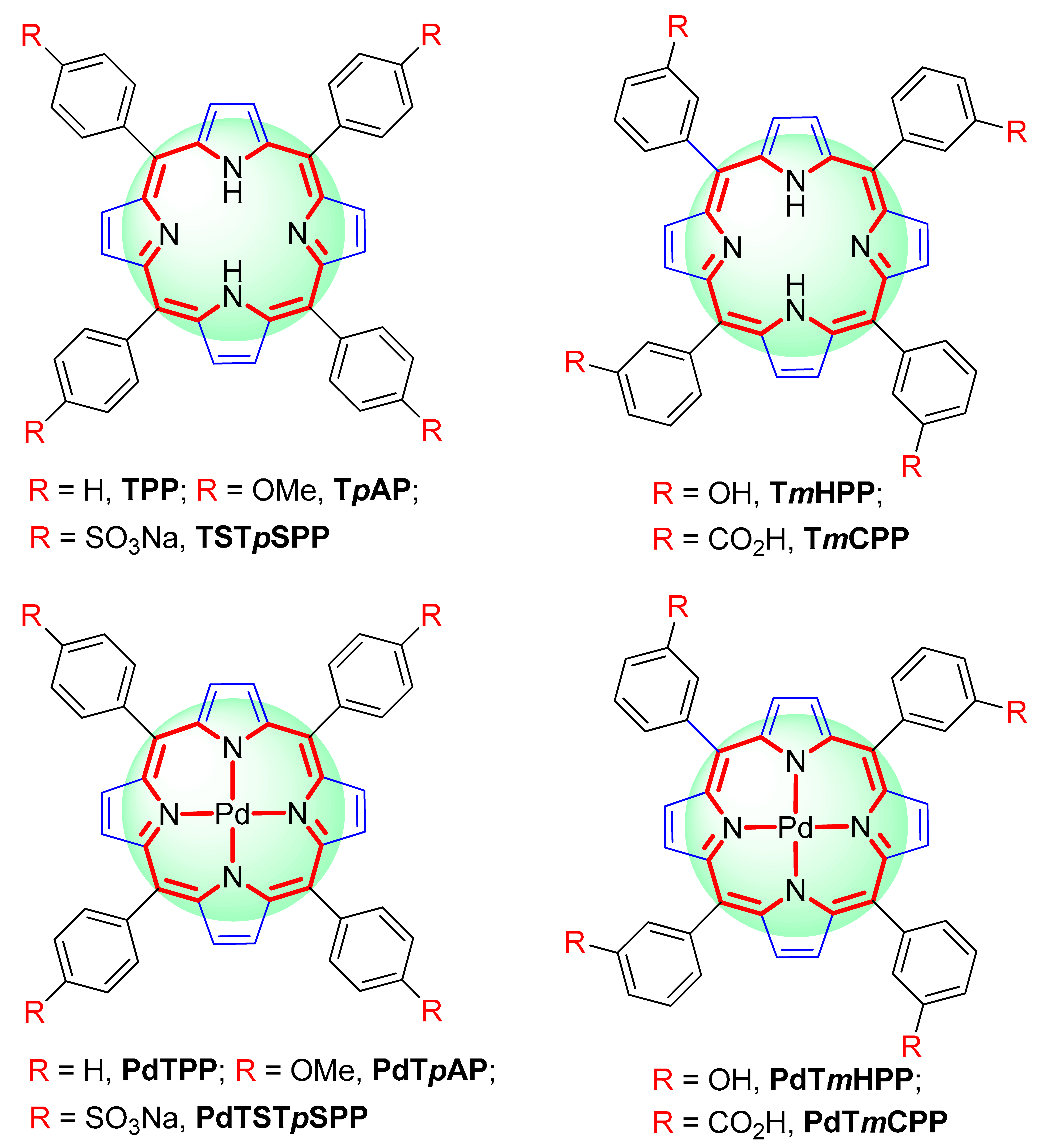
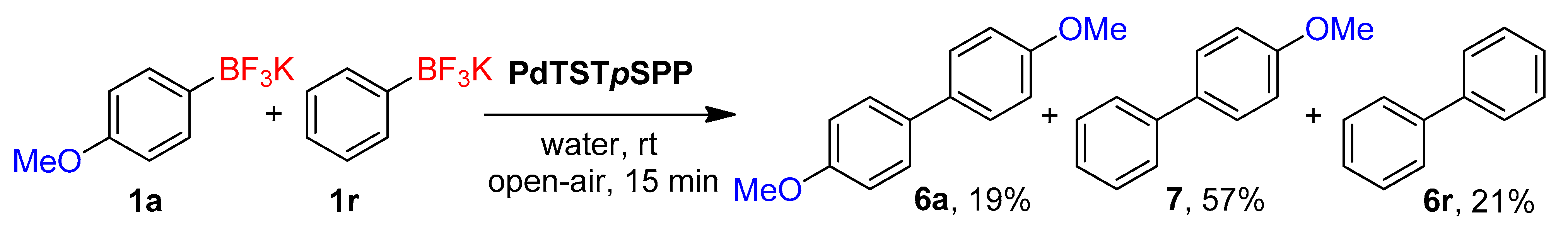
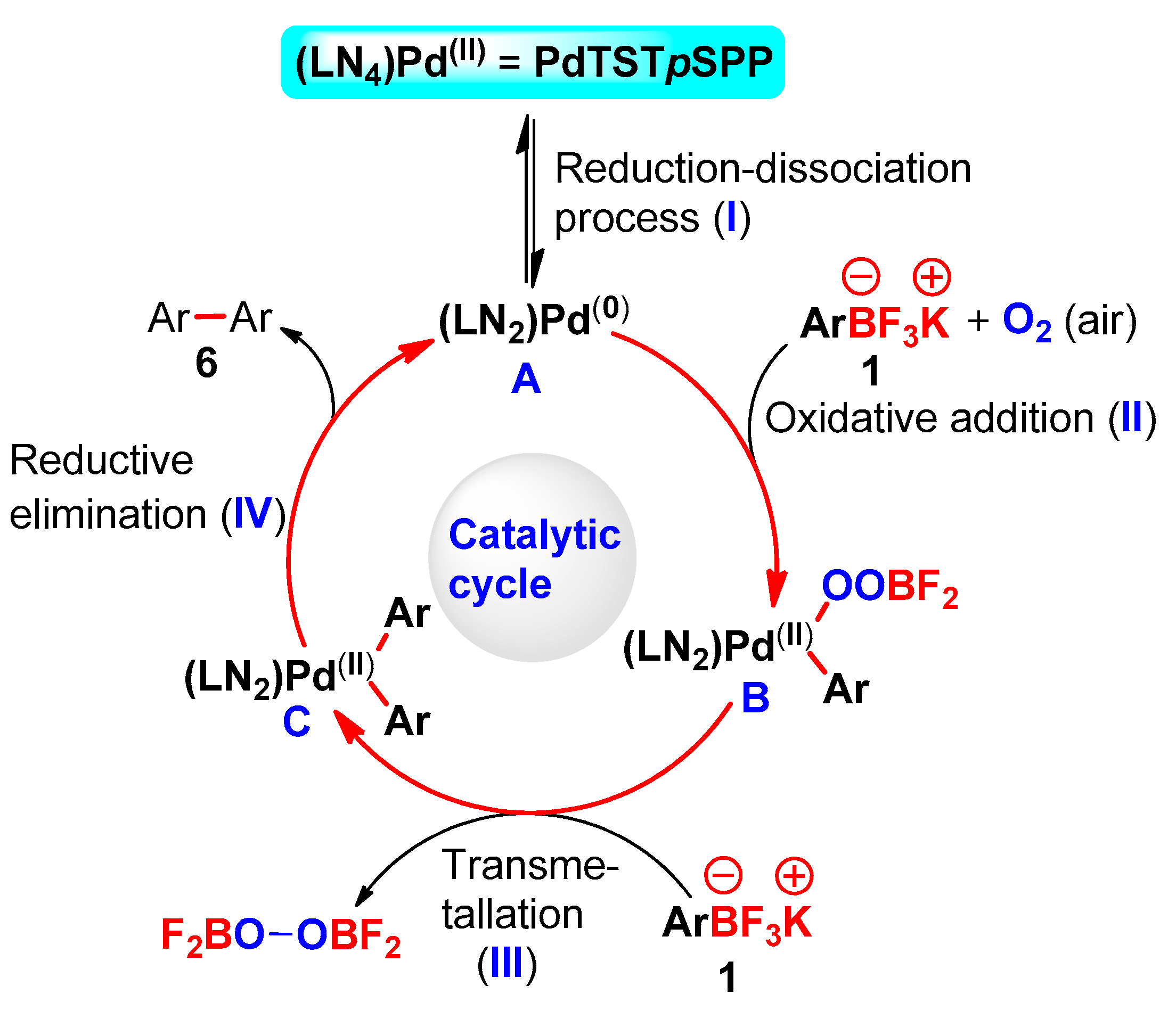
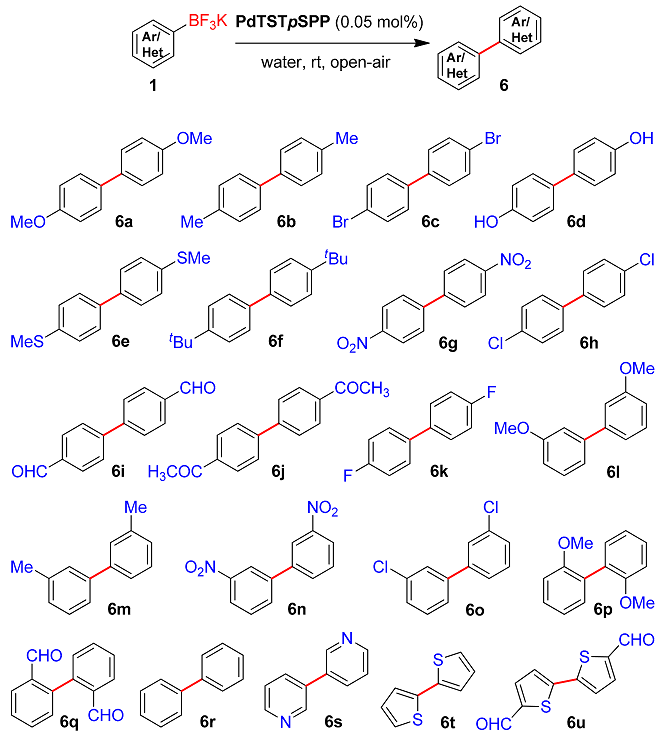
2. Results and Discussion
3. Materials, Methods and Characterization Data
3.1. Materials and Methods
3.1.1. General
3.1.2. Synthesis of Porphyrins
3.1.3. Synthesis of N-pincer Pd(II)-porphyrin Complexes
3.1.4. PABs Homocoupling Procedure
3.2. Characterization Data of Symmetrical Biaryls
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Feng, L.; Wang, K.-Y.; Joseph, E.; Zhou, H.-C. Catalytic porphyrin framework compounds. Trends Chem. 2020, 2, 555–568. [Google Scholar] [CrossRef]
- Venkateswarlu, K.; Rao, K.U. Cu(OAc)2-porphyrins as an efficient catalytic system for base-free, nature mimicking Chan-Lam coupling in water. Appl. Organometal. Chem. 2021, 35, e6223. [Google Scholar] [CrossRef]
- Mauzerall, D. Porphyrins, chlorophyll, and photosynthesis. In Photosynthesis I. Encyclopedia of Plant Physiology (New Series); Trebst, A., Avron, M., Eds.; Springer: Berlin/Heidelberg, Germany, 1997; Volume 117. [Google Scholar]
- Bonkovsky, H.L.; Guo, J.-T.; Hou, W.; Li, T.; Narang, T.; Thapar, M. Porphyrin and heme metabolism and the porphyrias. Compr. Physiol. 2013, 3, 365–401. [Google Scholar] [PubMed]
- Dolgopolova, E.A.; Rice, A.M.; Martin, C.R.; Shustova, N.B. Photochemistry and photophysics of MOFs: Steps towards MOF-based sensing enhancements. Chem. Soc. Rev. 2018, 47, 4710–4728. [Google Scholar] [CrossRef]
- Kirchon, A.; Feng, L.; Drake, H.F.; Joseph, E.A.; Zhou, H.-C. From fundamentals to applications: A toolbox for robust and multifunctional MOF materials. Chem. Soc. Rev. 2018, 47, 8611–8638. [Google Scholar] [CrossRef]
- Barona-Castaño, J.C.; Carmona-Vargas, C.C.; Brocksom, T.J.; de Oliveira, K.T. Porphyrins as catalysts in scalable organic reactions. Molecules 2016, 21, 310. [Google Scholar] [CrossRef] [Green Version]
- Kostas, I.D.; Coutsolelos, A.G.; Charalambidis, G.; Skondra, A. The first use of porphyrins as catalysts in cross-coupling reactions: A water-soluble palladium complex with a porphyrin ligand as an efficient catalyst precursor for Suzuki-Miyaura reaction in aqueous media under aerobic conditions. Tetrahedron Lett. 2007, 48, 6688. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, J.; Zhu, D.; Li, T. Porphyrin-based polymer-supported palladium as an excellent and recyclable catalyst for Suzuki-Miyaura coupling reaction in water. Appl. Orgamometal. Chem. 2018, 32, e3996. [Google Scholar] [CrossRef]
- Rao, K.U.; Appa, R.M.; Lakshmidevi, J.; Vijitha, R.; Rao, K.S.V.K.; Narasimhulu, M.; Venkateswarlu, K. C(sp2)–C(sp2) coupling in water: Palladium(II) complexes of N-pincer tetradentate porphyrins as effective catalysts. Asian J. Org. Chem. 2017, 6, 751–757. [Google Scholar] [CrossRef]
- Rao, K.U.; Lakshmidevi, J.; Appa, R.M.; Prasad, S.S.; Narasimhulu, M.; Vijitha, R.; Rao, K.S.V.K.; Venkateswarlu, K. Palladium(II)-porphyrin complexes as efficient and eco-friendly catalysts for Mizoroki-Heck coupling. ChemistrySelect 2017, 2, 7394–7398. [Google Scholar] [CrossRef]
- Rao, K.U.; Venkateswarlu, K. PdII-porphyrin complexe—The first use as safer and efficient catalysts for Miyaura borylation. Synlett 2018, 29, 1055–1060. [Google Scholar]
- Grigoropoulou, G.; Clark, J.H.; Elings, J.A. Recent developments on the epoxidation of alkenes using hydrogen peroxide as an oxidant. Green Chem. 2003, 5, 1–7. [Google Scholar] [CrossRef]
- Masteri-Farahani, M.; Rahimi, M.; Hosseini, M.-S. Heterogenization of porphyrin complexes within the nanocages of SBA-16: New efficient and stable catalysts for the epoxidation of olefins. Colloids Surf. A 2020, 603, 125229. [Google Scholar] [CrossRef]
- Mahmoudi, B.; Rostami, A.; Kazemnejadi, M.; Hamah-Ameen, B.A. Oxidation/MCR domino protocol for direct transformation of methyl benzene, alcohol, and nitro compounds to the corresponding tetrazole using a three-functional redox catalytic system bearing TEMPO/Co(III)-porphyrin/Ni(II) complex. Mol. Catal. 2021, 499, 111311. [Google Scholar] [CrossRef]
- Rebelo, S.L.H.; Simões, M.M.Q.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S. Oxidation of alkylaromatics with halogen peroxide catalyzed by manganese(III) porphyrins in the presence of ammonium acetate. J. Mol. Catal. A Chem. 2003, 201, 9–22. [Google Scholar] [CrossRef]
- Ji, H.-B.; Yuan, Q.-L.; Zhou, X.-T.; Pei, L.-X.; Wang, L.-F. Highly efficient selective oxidation of alcohols to carbonyl compounds catalyzed by ruthenium (III) meso-tetraphenylporphyrin chloride in the presence of molecular oxygen. Bioorg. Med. Chem. Lett. 2007, 17, 6364–6368. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, R.; Kurahashi, T.; Matsubara, S. Cobalt(III) porphyrin catalyzed aza-Diels-Alder reaction. Org. Lett. 2012, 14, 4794–4797. [Google Scholar] [CrossRef]
- Jiang, X.; Gou, F.; Chen, F.; Jing, H. Cycloaddition of epoxides and CO2 catalyzed by bisimidazole-functionalized porphyrin cobalt(III) complexes. Green Chem. 2016, 18, 3567–3576. [Google Scholar] [CrossRef]
- Enthaler, S.; Spilker, B.; Erre, G.; Junge, K.; Tse, M.K.; Beller, M. Biomimetic transfer hydrogenation of 2-alkoxy- and 2-aryloxyketones with iron-porphyrin catalysts. Tetrahedron 2008, 64, 3867–3876. [Google Scholar] [CrossRef]
- Stangel, C.; Charalambidis, G.; Varda, V.; Goutsolelos, A.G.; Kostas, I.D. Aqueous-organic biphasic hydrogenation of trans-cinnamaldehyde catalyzed by rhodium and ruthenium phosphane-free porphyrin complexes. Eur. J. Inorg. Chem. 2011, 4709–4716. [Google Scholar] [CrossRef]
- Fantauzzi, S.; Gallo, E.; Caselli, A.; Ragaini, F.; Macchi, P.; Casati, N.; Cenini, S. Origin of the deactivation in styrene aziridination by aryl azides, catalyzed by ruthenium porphyrin complexes. Structural characterization of Δ2-1,2,3-triazoline RuII(TPP)CO complex. Organometallics 2005, 24, 4710–4713. [Google Scholar] [CrossRef]
- Hopmann, K.H.; Ghosh, A. Mechanism of cobalt-porphyrin-catalyzed aziridination. ACS Catal. 2011, 1, 597–600. [Google Scholar] [CrossRef]
- Torrent-Sucarrat, M.; Arrastia, I.; Arrieta, A.; Cossio, F.P. Stereoselectivity, different oxidation states, and multiple spin states in the cyclopropanation of olefins catalyzed by Fe-porphyrin complexes. ACS Catal. 2018, 8, 11140–11153. [Google Scholar] [CrossRef]
- Anding, B.J.; Ellern, A.; Woo, L.K. Olefin cyclopropanation catalyzed by iridium(III) porphyrin complexes. Organometallics 2012, 31, 3628–3635. [Google Scholar] [CrossRef] [Green Version]
- Vasconcelos, S.N.S.; Reis, J.S.; de Oliveira, I.M.; Balfour, M.N.; Stefani, H.A. Synthesis of symmetrical biaryl compounds by homocoupling reaction. Tetrahedron 2019, 75, 1865–1959. [Google Scholar] [CrossRef]
- Appa, R.M.; Lakshmidevi, J.; Naidu, B.R.; Venkateswarlu, K. Pd-catalyzed oxidative homocoupling of arylboronic acids in WEPA: A sustainable access to symmetrical biaryls under added base and ligand-free ambient conditions. Mol. Catal. 2021, 501, 111366. [Google Scholar] [CrossRef]
- Cepanec, I.; Litvić, M.; Udiković, J.; Pogorelić, I.; Lovrić, M. Copper(I)-catalysed homo-coupling of aryldiazonium salts: Synthesis of symmetrical biaryls. Tetrahedron 2007, 63, 5614–5621. [Google Scholar] [CrossRef]
- Savanur, H.M.; Kalkhambkar, R.G.; Laali, K.K. Pd(OAc)2 catalyzed homocoupling of arenediazonium salts in ionic liquids: Synthesis of symmetrical biaryls. Tetrahedron Lett. 2016, 57, 663–667. [Google Scholar] [CrossRef]
- Lakshmidevi, J.; Appa, R.M.; Naidu, B.R.; Prasad, S.S.; Sarma, L.S.; Venkateswarlu, K. WEPA: A bio-derived medium for added base, π-acid and ligand free Ullmann coupling of aryl halides using Pd(OAc)2. Chem. Commun. 2018, 54, 12333–12336. [Google Scholar] [CrossRef]
- Dubey, A.V.; Kumar, A.V. A bio-inspired magnetically recoverable palladium nanocatalyst for the Ullmann coupling reaction of aryl halides and arylboronic acids in aqueous media. Appl. Organometal. Chem. 2020, 34, e5570. [Google Scholar] [CrossRef]
- Puthiaraj, P.; Ahn, W.-S. Ullmann coupling of aryl chlorides in water catalyzed by palladium nanoparticles supported on amine-grafted porous aromatic polymer. Mol. Catal. 2017, 437, 73–79. [Google Scholar] [CrossRef]
- Cheng, K.; Xin, B.; Zhang, Y. The Pd(OAc)2-catalyzed homocoupling of arylboronic acids in water and ionic liquid. J. Mol. Catal. A Chem. 2007, 273, 240–243. [Google Scholar] [CrossRef]
- Xia, J.; Cheng, M.; Chen, Q.; Cai, M. Recyclable and reusable Pd(OAc)2/PPh3/PEG-2000 system for homocoupling reaction of arylboronic acids under air without base. Appl. Organometal. Chem. 2015, 29, 113–116. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Suzuki, R.; Hattori, K.; Nishiyama, H. Base- and phosphine-free palladium-catalyzed homocoupling of arylboronic acid derivatives under air. Synlett 2006, 1027–1030. [Google Scholar] [CrossRef]
- Darzi, E.R.; White, B.M.; Loventhal, L.K.; Zakharov, L.N.; Jasti, R. An operationally simple and mild oxidative homocoupling of aryl boronic esters to access conformationally constrained macrocycles. J. Am. Chem. Soc. 2017, 139, 3106–3114. [Google Scholar] [CrossRef] [PubMed]
- Punna, S.; Díaz, D.D.; Finn, M.G. Palladium-catalyzed homocoupling of arylboronic acids and esters using fluoride in aqueous solvents. Synlett 2004, 2004, 2351–2354. [Google Scholar] [CrossRef]
- Yoshida, H.; Yamaryo, Y.; Ohshita, J.; Kunai, A. Base-free oxidative homocoupling of arylboronic esters. Tetrahedron Lett. 2003, 44, 1541–1544. [Google Scholar] [CrossRef]
- Prastaro, A.; Ceci, P.; Chiancone, E.; Boffi, A.; Fabrizi, G.; Cacchi, S. Homocoupling of arylboronic acids and potassium aryltrifluoroborates catalyzed by protein-stabilized palladium nanoparticles under air in water. Tetrahedron Lett. 2010, 51, 2550–2551. [Google Scholar] [CrossRef]
- Sakurai, H.; Tsunoyama, H.; Tsukuda, T. Oxidative homo-coupling of potassium aryltrifluoroborates catalyzed by gold nanocluster under aerobic conditions. J. Organomet. Chem. 2007, 692, 368–374. [Google Scholar] [CrossRef]
- Amatore, C.; Cammoun, C.; Jutand, A. Pd(OAc)2/p-benzoquinone-catalyzed anaerobic electrooxidative homocoupling of arylboronic acids, arylboronates and aryltrifluoroborates in DMF and/or water. Eur. J. Org. Chem. 2008, 4567–4570. [Google Scholar] [CrossRef]
- Musolino, B.; Quinn, M.; Hall, K.; Coltuclu, V.; Kabalka, G.W. Ultrasound induced, copper mediated homocoupling using polymer supported aryltrifluoroborates. Tetrahedron Lett. 2013, 54, 4080–4082. [Google Scholar] [CrossRef]
- Li, X.; Li, D.; Bai, Y.; Zhang, C.; Chang, H.; Gao, W.; Wei, W. Homocoupling reactions of terminal alkynes and alylboronic compounds catalyzed by in situ formed Al(OH)3-supported palladium nanoparticles. Tetrahedron 2016, 72, 6996–7002. [Google Scholar] [CrossRef]
- Cahiez, G.; Moyeux, A.; Buendia, J.; Duplais, C. Manganese- or iron-catalyzed homocoupling of Grignard reagents using atmospheric oxygen as an oxidant. J. Am. Chem. Soc. 2007, 129, 13788–13789. [Google Scholar] [CrossRef] [PubMed]
- Nagano, T.; Hayashi, T. Iron-catalyzed oxidative homo-coupling of aryl Grignard reagents. Org. Lett. 2005, 7, 491–493. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Peng, X.-S.; Wong, H.N.C. Ligand-free iron-catalyzed homo-coupling of aryllithium reagents. Asian J. Org. Chem. 2020, 9, 1834–1840. [Google Scholar] [CrossRef]
- Lu, F. Vanadium (IV) tetrachloride catalyzed oxidative homo-coupling of aryl lithium under mild reaction conditions. Tetrahedron Lett. 2012, 53, 2444–2446. [Google Scholar] [CrossRef]
- Fagnou, K.; Lautens, M. Rhodium-catalyzed carbon-carbon bond forming reactions of organometallic compounds. Chem. Rev. 2003, 103, 169–196. [Google Scholar] [CrossRef] [PubMed]
- Larock, R.C.; Bernhardt, J.C. Mercury in organic chemistry. 11. Synthesis of symmetrical 1,3-dienes and biaryls via rhodium catalyzed dimerization of vinyl- and arylmercurials. J. Org. Chem. 1977, 42, 1680–1684. [Google Scholar] [CrossRef]
- Percec, V.; Bae, J.Y.; Hill, D.H. Aryl mesylates in metal catalyzed homo- and cross-coupling reactions. 4. Scope and limitations of aryl mesylates in nickel catalyzed cross-coupling reactions. J. Org. Chem. 1995, 60, 6895–6903. [Google Scholar] [CrossRef]
- Percec, V.; Bae, J.-Y.; Zhao, M.; Hill, D.H. Aryl mesylates in metal-catalyzed homocoupling and cross-coupling reactions. 1. Functional symmetrical biaryls from phenols via nickel-catalyzed homocoupling of their mesylates. J. Org. Chem. 1995, 60, 176–185. [Google Scholar] [CrossRef]
- Maddaluno, J.; Durandetti, M. Dimerization of aryl sulfonates by in situ generated nickel(0). Synlett 2015, 26, 2385–2388. [Google Scholar]
- Jutand, A.; Mosleh, A. Nickel- and palladium-catalyzed homocoupling of aryl triflates. Scope, limitations, and mechanistic aspects. J. Org. Chem. 1997, 62, 261–274. [Google Scholar] [CrossRef]
- Shibata, M.; Ito, H.; Itami, K. Oxidative homocoupling reaction of aryltrimethylsilanes by Pd/o-chloranil catalyst. Chem. Lett. 2017, 46, 1701–1704. [Google Scholar] [CrossRef]
- Luo, H.-Q.; Dong, W. AgF-mediated homocoupling reaction of trialkoxy aryl silanes. Synth. Commun. 2013, 43, 2733–2738. [Google Scholar] [CrossRef]
- Rodríguez, N.; Goossen, L.J. Decarboxylative coupling reactions: A modern strategy for C–C-bond formation. Chem. Soc. Rev. 2011, 40, 5030–5048. [Google Scholar] [CrossRef] [Green Version]
- Cornella, J.; Lahlali, H.; Larrosa, I. Decarboxylative homocoupling of (hetero)aromatic carboxylic acids. Chem. Commun. 2010, 46, 8276–8278. [Google Scholar] [CrossRef]
- Geske, D.H. Evidence for the formation of biphenyl by intramolecular dimerization in the electroöxidation of tetraphenylborate ion. J. Phys. Chem. 1962, 66, 1743–1744. [Google Scholar] [CrossRef]
- Beil, S.B.; Möhle, S.; Enders, P.; Waldvogel, S.R. Electrochemical instability of highly fluorinated tetraphenyl borates and syntheses of their respective. Chem. Commun. 2018, 54, 6128–6131. [Google Scholar] [CrossRef] [PubMed]
- Music, A.; Baumann, A.N.; Spieß, P.; Plantefol, A.; Jagau, T.C.; Didier, D. Electrochemical synthesis of biaryls via oxidative intramolecular coupling of tetra(hetero)arylborates. J. Am. Chem. Soc. 2020, 142, 4341–4348. [Google Scholar] [CrossRef] [PubMed]
- Gerleve, C.; Studer, A. Transition-metal-free oxidative cross-coupling of tetraarylborates to biaryls using organic oxidants. Angew. Chem. Int. Ed. 2020, 59, 15468–15473. [Google Scholar] [CrossRef] [PubMed]
- Dhital, R.N.; Murugadoss, A.; Sakurai, H. Duel role of polyhydroxy matrices in the homocoupling of arylboronic acids catalyzed by gold nanoclusters under acidic conditions. Chem. Asian J. 2012, 7, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Lin, S.; Zhu, X.; Jiang, B.; Yang, Z.; Pan, Z. Reductant-directed formation of PS-PAMAM-supported gold nanoparticles for use as highly active and recyclable catalysts for the aerobic oxidation of alcohols and the homocoupling of phenylboronic acids. Chem. Commun. 2012, 48, 6235–6237. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, T.; Asai, T.; Shiose, S.; Kato, K. Homocoupling of arylboronic acids catalyzed by simple gold salts. Tetrahedron Lett. 2011, 52, 4779–4781. [Google Scholar] [CrossRef]
- Puthiaraj, P.; Suresh, P.; Pitchumani, K. Aerobic homocoupling of arylboronic acids catalyzed by copper terephthalate metal-organic frameworks. Green Chem. 2014, 16, 2865–2875. [Google Scholar] [CrossRef]
- Demir, A.S.; Reis, Ö.; Emrullahoglu, M. Role of copper specie in the oxidative dimerization of arylboronic acids: Synthesis of symmetrical biaryls. J. Org. Chem. 2003, 68, 10130–10134. [Google Scholar] [CrossRef]
- Kaboudin, B.; Abedi, Y.; Yokomatsu, T. CuII-β-cyclodextrin complex as a nanocatalyst for the homo- and cross-coupling of arylboronic acids under ligand- and base-free conditions in air: Chemoselective cross-coupling of arylboronic acids in water. Eur. J. Org. Chem. 2011, 6656–6662. [Google Scholar] [CrossRef]
- Cao, Y.-N.; Tian, X.-C.; Chen, X.-X.; Yao, Y.-X.; Gao, F.; Zhou, X.-L. Rapid ligand-free base-accelerated copper-catalyzed homocoupling reaction of arylboronic acids. Synlett 2017, 28, 601–606. [Google Scholar]
- Vogler, T.; Studer, A. Rhodium-catalyzed oxidative homocoupling of boronic acids. Adv. Synth. Catal. 2008, 350, 1963–1967. [Google Scholar] [CrossRef]
- Tyagi, D.; Binnani, C.; Rai, R.K.; Dwivedi, A.D.; Gupta, K.; Li, P.-Z.; Zhao, Y.; Singh, S.K. Ruthenium-catalyzed oxidative homocoupling of arylboronic acids in water: Ligand tuned reactivity and mechanistic study. Inorg. Chem. 2016, 55, 6332–6343. [Google Scholar] [CrossRef]
- Luque, R.; Baruwati, B.; Varma, R.S. Magnetically separable nanoferrite-anchored glutathione: Aqueous homocoupling of arylboronic acids under microwave irradiation. Green Chem. 2010, 12, 1540–1543. [Google Scholar] [CrossRef]
- Venkateswarlu, K. Ashes from organic waste as reagent in synthetic chemistry: A review. Environ. Chem. Lett. 2021, in press. [Google Scholar] [CrossRef]
- Adamo, C.; Amatore, C.; Ciofini, I.; Jutand, A.; Lakmini, H. Mechanism of the palladium-catalyzed homocoupling of arylboronic acids: Key involvement of a palladium peroxo complex. J. Am. Chem. Soc. 2006, 128, 6829–6836. [Google Scholar] [CrossRef] [PubMed]
- Aziz, N.I.H.A.; Hanafiah, M.M.; Ali, M.Y.M. Sustainable biogas production from agrowaste and effluents—A promising step for small-scale industry income. Renew. Energy 2019, 132, 363–369. [Google Scholar] [CrossRef]
- Marella, R.K.; Madduluri, V.R.; Lakkaboyana, S.K.; Hanafiah, M.M.; Yaaratha, S. Hydrogen-free hydrogenation of nitrobenzene via direct coupling with cyclohexanol dehydrogenation over ordered mesoporous MgO/SBA-15 supported Cu nanoparticles. RSC Adv. 2020, 10, 38755–38766. [Google Scholar] [CrossRef]
- Madduluri, V.R.; Marella, R.K.; Hanafiah, M.M.; Lakkaboyana, S.K.; Suresh babu, G. CO2 utilization as a soft oxidant for the synthesis of styrene from ethylbenzene over Co3O4 supported on magnesium aluminium spinel: Role of spinel activation temperature. Sci. Rep. 2020, 10, 22170. [Google Scholar] [CrossRef]
- Shaikh, M.M.; AlSuhaimi, A.; Hanafiah, M.M.; Alshahateet, S.F. Release of organic contaminants migrating from polyvinyl chloride polymeric into drinking water under three successive stagnant periods of time. Desal. Wat. Treat. 2019, 149, 105–116. [Google Scholar] [CrossRef] [Green Version]
- ‘Ainaa’ Idris, S.A.; Hanafiah, M.M.; Khan, M.F.; Hamid, H.H.A. Indoor generated PM2.5 compositions and volatile organic compounds: Potential sources and health risk implications. Chemosphere 2020, 255, 126932. [Google Scholar] [CrossRef]
- Adler, A.D.; Longo, F.R.; Finarelli, J.D.; Goldmacher, J.; Assour, J.; Korsakoff, L. A simplified synthesis for meso-tetraphenylporphine. J. Org. Chem. 1967, 32, 476. [Google Scholar] [CrossRef]

 | |||||
|---|---|---|---|---|---|
| Entry | Arylboron Compound | Catalyst (mol%) | Solvent (mL) | Time (min) | Isolated Yield (%) |
| 1 | 1a | PdTSTpSPP (0.1) | Water (4) | 15 | 98 |
| 2 | 1a | PdTPP (0.1) | Water (2) + DMF (2) | 240 | 21 |
| 3 | 1a | PdTpAP (0.1) | Water (2) + DMF (2) | 240 | 39 |
| 4 | 1a | PdTmHPP (0.1) | Water (2) + DMF (2) | 240 | 58 |
| 5 | 1a | PdTmCPP (0.1) | Water (2) + DMF (2) | 240 | 67 |
| 6 | 1a | PdTSTpSPP (0.07) | Water | 15 | 98 |
| 7 | 1a | PdTSTpSPP (0.05) | Water | 15 | 98 |
| 8 | 1a | PdTSTpSPP (0.03) | Water | 60 | 82 |
| 9 | 2 | PdTSTpSPP (0.05) | Water | 15 | 92 |
| 10 | 3 | PdTSTpSPP (0.05) | Water (2) + DMF (2) | 180 | 14 |
| 11 | 4 | PdTSTpSPP (0.05) | Water (2) + DMF (2) | 180 | 17 |
| 12 | 5 | PdTSTpSPP (0.05) | Water | 60 | 73 |
 | ||||||
|---|---|---|---|---|---|---|
| Entry | Aryltrifluoroborate (1) | Time (min) | Product (6) | Yield (%) 2 | TON | TOF |
| 1 | 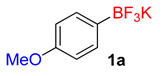 | 15 | 6a | 98 | 1960 | 7840 |
| 2 | 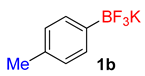 | 25 | 6b | 94 | 1880 | 4512 |
| 3 |  | 25 | 6c | 92 | 1840 | 4416 |
| 4 | 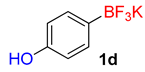 | 35 | 6d | 89 | 1780 | 3051 |
| 5 | 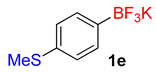 | 20 | 6e | 94 | 1880 | 5640 |
| 6 | 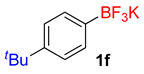 | 20 | 6f | 94 | 1880 | 5640 |
| 7 |  | 10 | 6g | 99 | 1980 | 11,880 |
| 8 |  | 15 | 6h | 97 | 1940 | 7760 |
| 9 | 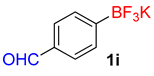 | 10 | 6i | 99 | 1980 | 11,880 |
| 10 | 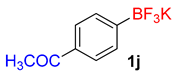 | 20 | 6j | 97 | 1940 | 5820 |
| 11 | 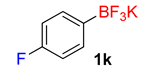 | 10 | 6k | 98 | 1960 | 11,760 |
| 12 | 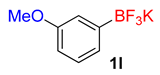 | 30 | 6l | 93 | 1860 | 3720 |
| 13 | 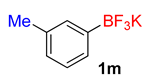 | 30 | 6m | 88 | 1760 | 3520 |
| 14 |  | 20 | 6n | 95 | 1900 | 5700 |
| 15 | 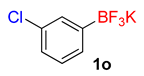 | 30 | 6o | 92 | 1840 | 3680 |
| 16 | 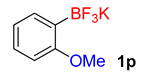 | 45 | 6p | 88 | 1760 | 2347 |
| 17 |  | 40 | 6q | 91 | 1820 | 2730 |
| 18 | 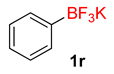 | 15 | 6r | 96 | 1920 | 7680 |
| 19 | 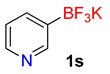 | 60 | 6s | 91 | 1820 | 1820 |
| 20 | 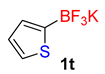 | 60 | 6t | 86 | 1720 | 1720 |
| 21 | 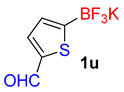 | 50 | 6u | 90 | 1800 | 2160 |
| Entry | Catalyst | Solvent | Base/Additive | Temp. | Time (h) | Yield (%) | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Pd NPs/Te-Dps 1 | Water | Tris-HCl buffer | 100 °C | 10–24 | 60–87 | [39] |
| 2 | Au nanoclusters:poly(N-vinyl-2-pyrrolidine) | Water | pH 6.86 buffer | 47 °C | 24 | 14–quant. | [40] |
| 3 | Pd(OAc)2–electrolysis | DMF | p-Benzoquinone | 80 °C | 0.24–0.40 | 41–99 | [41] |
| 4 | Cu(OAc)2–ultra sound | Aq. EtOH | Dowex polymer support | Ultrasound | 6 | 0–98 | [42] |
| 5 | Pd NPs@Al(OH)3 | Water | KOAc, Ag2O | 50 °C | 16–48 | 42–98 | [43] |
| 6 | PdTSTpSPP | Water | - | rt | 0.17–1.0 | 86–99 | Present |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prasad, S.S.; Naidu, B.R.; Hanafiah, M.M.; Lakshmidevi, J.; Marella, R.K.; Lakkaboyana, S.K.; Venkateswarlu, K. Porphyrin N-Pincer Pd(II)-Complexes in Water: A Base-Free and Nature-Inspired Protocol for the Oxidative Self-Coupling of Potassium Aryltrifluoroborates in Open-Air. Molecules 2021, 26, 5390. https://doi.org/10.3390/molecules26175390
Prasad SS, Naidu BR, Hanafiah MM, Lakshmidevi J, Marella RK, Lakkaboyana SK, Venkateswarlu K. Porphyrin N-Pincer Pd(II)-Complexes in Water: A Base-Free and Nature-Inspired Protocol for the Oxidative Self-Coupling of Potassium Aryltrifluoroborates in Open-Air. Molecules. 2021; 26(17):5390. https://doi.org/10.3390/molecules26175390
Chicago/Turabian StylePrasad, Sana Siva, Bandameeda Ramesh Naidu, Marlia M. Hanafiah, Jangam Lakshmidevi, Ravi Kumar Marella, Sivarama Krishna Lakkaboyana, and Katta Venkateswarlu. 2021. "Porphyrin N-Pincer Pd(II)-Complexes in Water: A Base-Free and Nature-Inspired Protocol for the Oxidative Self-Coupling of Potassium Aryltrifluoroborates in Open-Air" Molecules 26, no. 17: 5390. https://doi.org/10.3390/molecules26175390
APA StylePrasad, S. S., Naidu, B. R., Hanafiah, M. M., Lakshmidevi, J., Marella, R. K., Lakkaboyana, S. K., & Venkateswarlu, K. (2021). Porphyrin N-Pincer Pd(II)-Complexes in Water: A Base-Free and Nature-Inspired Protocol for the Oxidative Self-Coupling of Potassium Aryltrifluoroborates in Open-Air. Molecules, 26(17), 5390. https://doi.org/10.3390/molecules26175390