HS–SPME–GC–MS and Electronic Nose Reveal Differences in the Volatile Profiles of Hedychium Flowers
Abstract
:1. Introduction
2. Results
2.1. Chemical Composition of Floral Volatiles Analyzed via HS–SPME–GC–MS
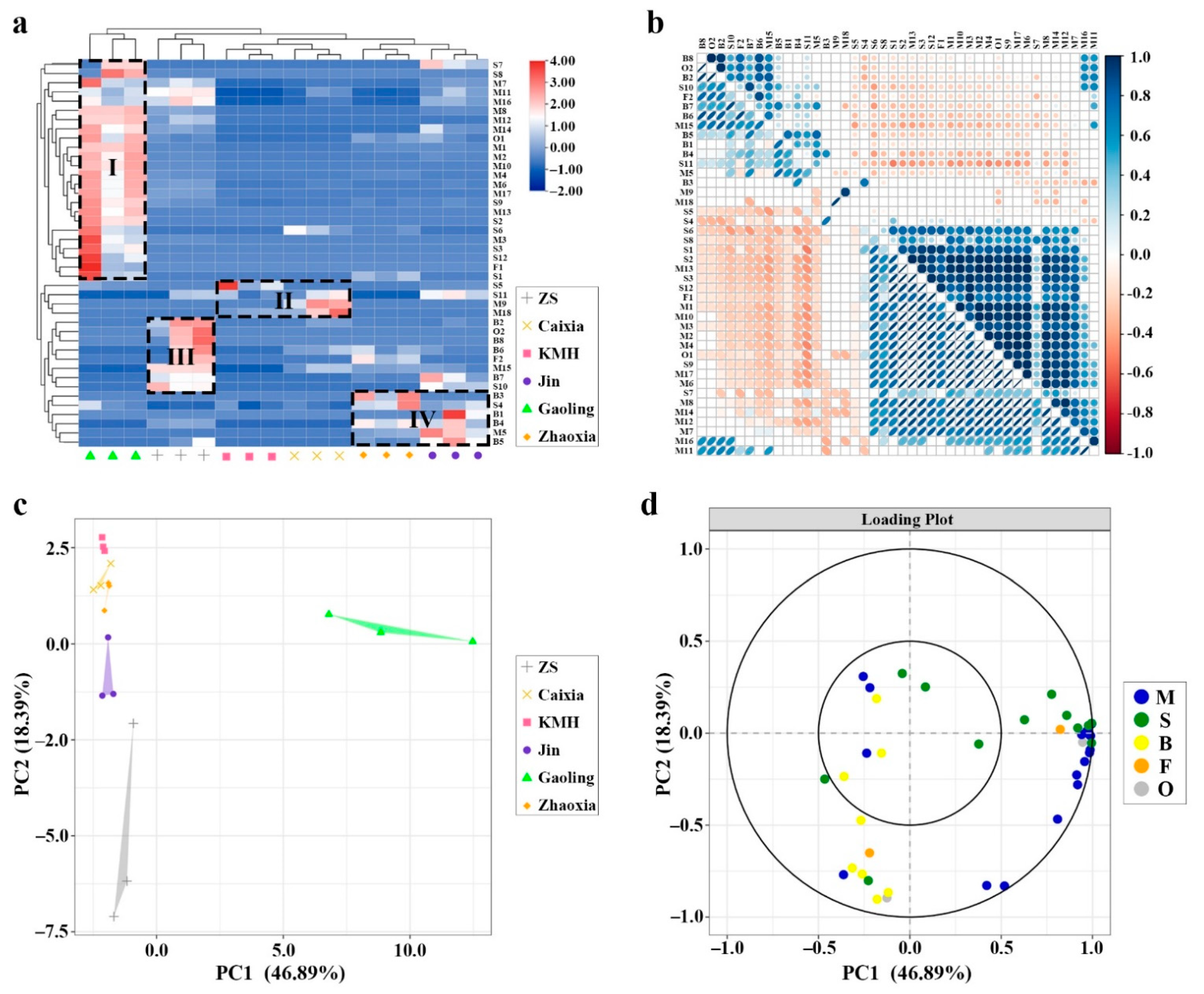
2.2. Hierarchical Clustering Analysis (HCA) Based on GC–MS Data
2.3. Principal Component Analysis Based on GC–MS Data
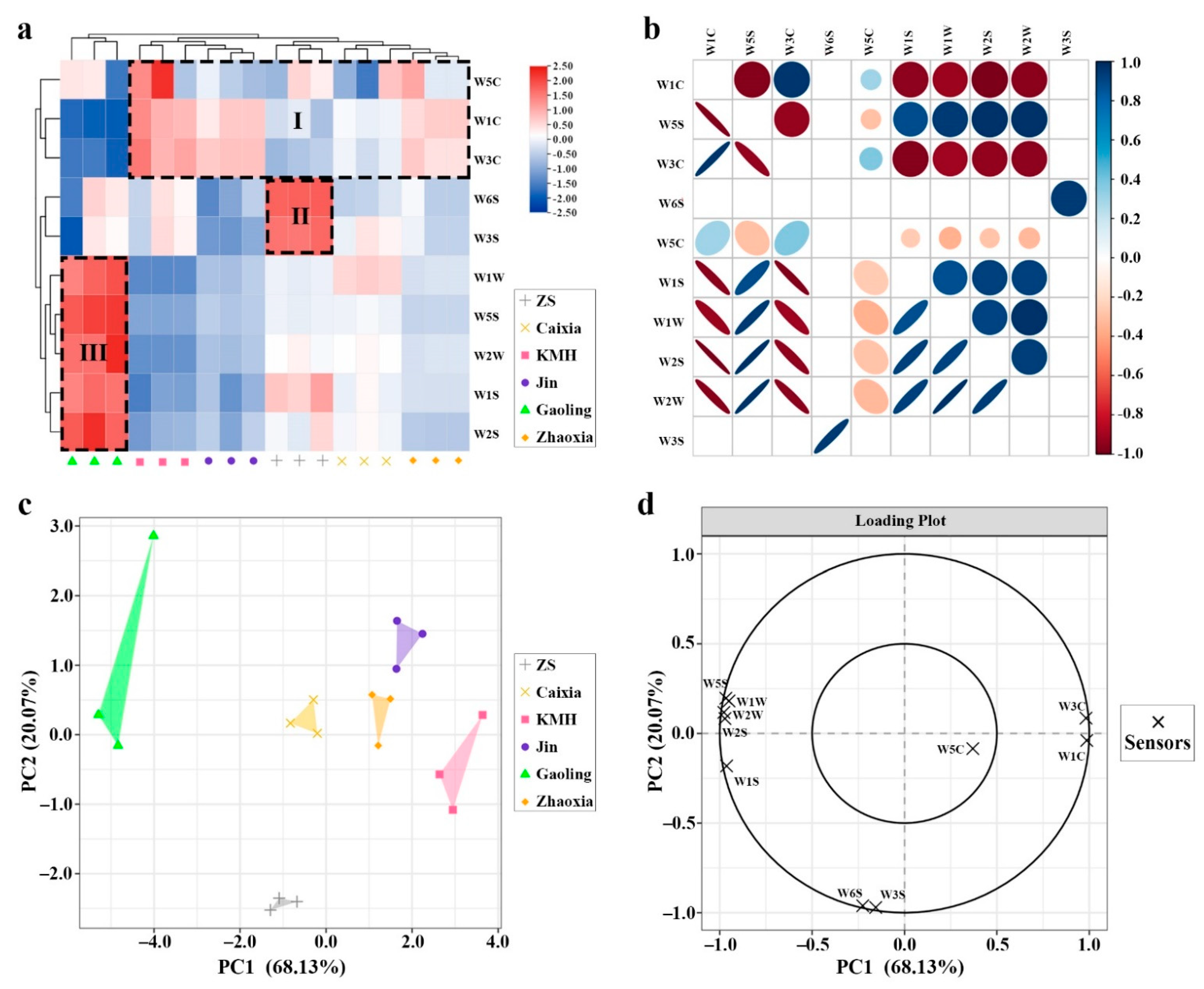
2.4. Discrimination of the Different Taxa Using the E-Nose
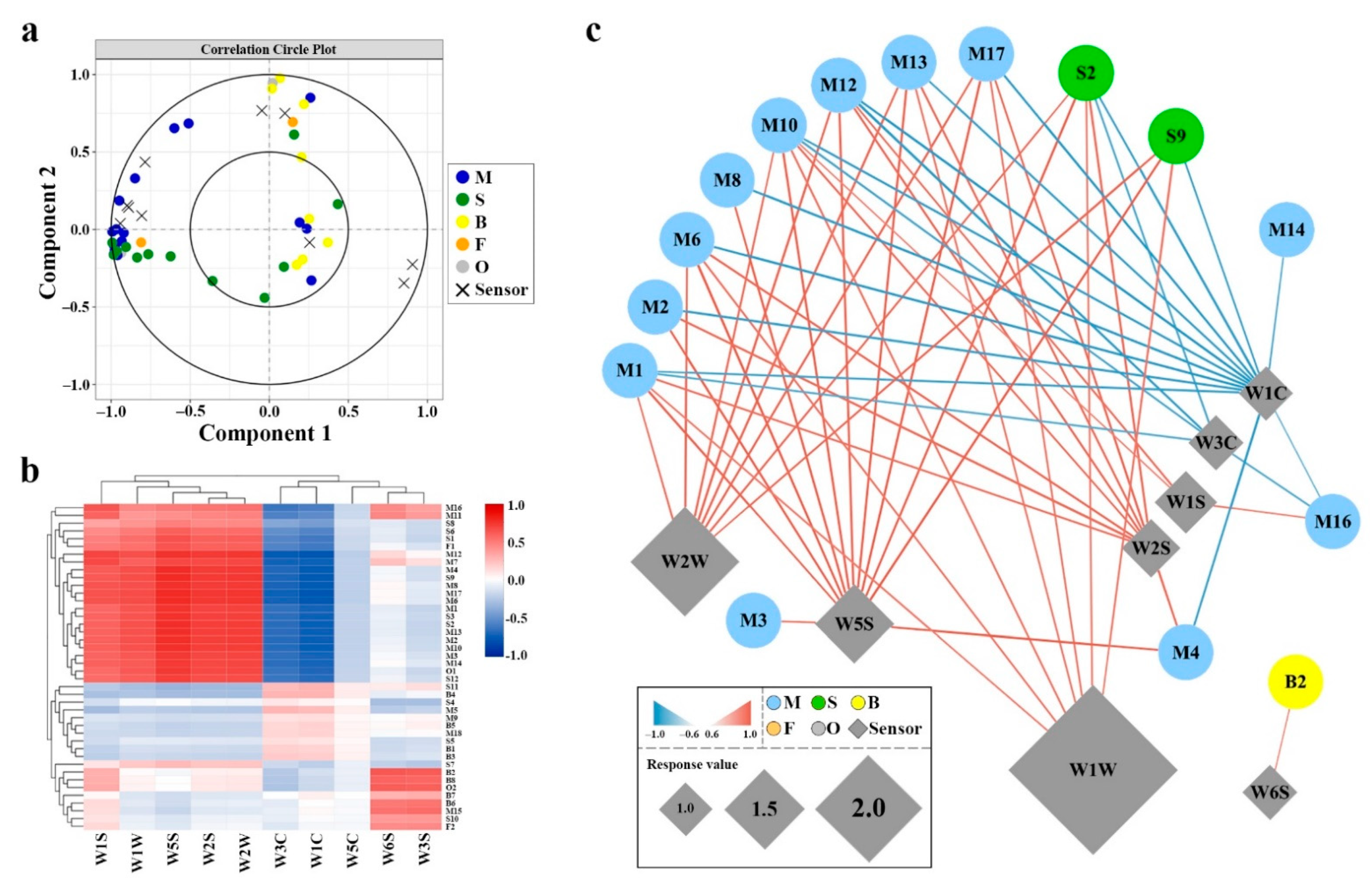
2.5. Correlation between GC–MS and E-Nose Sensors
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Sample Preparation and HS-SPME-GC–MS Analysis
4.3. E-Nose Analysis
4.4. Identification of VOCs
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Abbreviations
| E-nose | Electronic nose |
| GC–MS | Gas chromatography–mass spectrometry |
| HCA | Hierarchical clustering analysis |
| MOS | Metal oxide semiconductor |
| PCA | Principal component analysis |
| PDMS | Polydimethylsiloxane |
| PLS | Partial least squares |
| SPME | Solid-phase microextraction |
| SSR | Simple sequence repeat |
| VOCs | Volatile organic compounds |
References
- Sakhanokho, H.F.; Sampson, B.J.; Tabanca, N.; Wedge, D.E.; Demirci, B.; Baser, K.H.C.; Bernier, U.R.; Tsikolia, M.; Agramonte, N.M.; Becnel, J.J. Chemical composition, antifungal and insecticidal activities of Hedychium essential oils. Molecules 2013, 18, 4308–4327. [Google Scholar] [CrossRef] [Green Version]
- Chan, E.W.C.; Wong, S.K. Phytochemistry and pharmacology of ornamental gingers, Hedychium coronarium and Alpinia purpurata: A review. J. Integr. Med. 2015, 13, 368–379. [Google Scholar] [CrossRef]
- Joshi, S.; Chanotiya, C.S.; Agarwal, G.; Prakash, O.; Pant, A.K.; Mathela, C.S. Terpenoid compositions, and antioxidant and antimicrobial properties of the rhizome essential oils of different Hedychium species. Chem. Biodivers. 2008, 5, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.-B.; Yang, B.; Zhou, S.-S.; Li, R.; Maw, M.B.; Kyaw, W.M.; Tan, Y.-H. Hedychium putaoense (Zingiberaceae), a new species from Putao, Kachin state, northern Myanmar. PhytoKeys 2018, 94, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Ashokan, A.; Gowda, V. Hedychium ziroense (Zingiberaceae), a new species of ginger lily from Northeast India. PhytoKeys 2019, 117, 73–84. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Wei, X.; Abbas, F.; Yu, Y.; Yu, R.; Fan, Y. Genome-wide identification of simple sequence repeats and assessment of genetic diversity in Hedychium. J. Appl. Res. Med. Aromat. Plants 2021, 24, 100312. [Google Scholar]
- Fan, Y.-P.; Wang, X.-R.; Yu, R.-C.; Yang, P. Analysis on the aroma components in several species of Hedychium. Acta Hortic. Sin. 2007, 34, 231. [Google Scholar]
- Báez, D.; Pino, J.A.; Morales, D. Floral scent composition in Hedychium coronarium J. Koenig analyzed by SPME. J. Essent. Oil Res. 2011, 23, 64–67. [Google Scholar] [CrossRef]
- Ke, Y.; Abbas, F.; Zhou, Y.; Yu, R.; Yue, Y.; Li, X.; Yu, Y.; Fan, Y. Genome-wide analysis and characterization of the Aux/IAA Family genes related to floral scent formation in Hedychium coronarium. Int. J. Mol. Sci. 2019, 20, 3235. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.; Yu, R.; Huang, Y.; Chen, Y. Studies on the essential constituent of Hedychium flavum and H. coronarium. Acta Horti. Sin. 2003, 30, 475. [Google Scholar]
- Abbas, F.; Ke, Y.; Zhou, Y.; Yu, Y.; Waseem, M.; Ashraf, D.; Wang, C.; Wang, X.; Li, X.; Yue, Y. Genome-wide analysis reveals the potential role of MYB transcription factors in floral scent formation in Hedychium coronarium. Front. Plant Sci. 2021, 12, 58. [Google Scholar] [CrossRef]
- Abbas, F.; Ke, Y.; Zhou, Y.; Yu, Y.; Waseem, M.; Ashraf, U.; Li, X.; Yu, R.; Fan, Y. Genome-wide analysis of ARF transcription factors reveals HcARF5 expression profile associated with the biosynthesis of β-ocimene synthase in Hedychium coronarium. Plant Cell Rep. 2021, 40, 1–16. [Google Scholar] [CrossRef]
- Abbas, F.; Ke, Y.; Yu, R.; Yue, Y.; Amanullah, S.; Jahangir, M.M.; Fan, Y. Volatile terpenoids: Multiple functions, biosynthesis, modulation and manipulation by genetic engineering. Planta 2017, 246, 803–816. [Google Scholar] [CrossRef]
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Muhlemann, J.K.; Klempien, A.; Dudareva, N. Floral volatiles: From biosynthesis to function. Plant Cell Environ. 2014, 37, 1936–1949. [Google Scholar] [CrossRef] [PubMed]
- Gershenzon, J.; Dudareva, N. The function of terpene natural products in the natural world. Nat. Chem. Biol. 2007, 3, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Pichersky, E.; Dudareva, N. Scent engineering: Toward the goal of controlling how flowers smell. Trends Biotechnol. 2007, 25, 105–110. [Google Scholar] [CrossRef] [Green Version]
- Pichersky, E.; Noel, J.P.; Dudareva, N. Biosynthesis of plant volatiles: Nature’s diversity and ingenuity. Science 2006, 311, 808–811. [Google Scholar] [CrossRef] [Green Version]
- Nagegowda, D.A. Plant volatile terpenoid metabolism: Biosynthetic genes, transcriptional regulation and subcellular compartmentation. FEBS Lett. 2010, 584, 2965–2973. [Google Scholar] [CrossRef] [Green Version]
- Tholl, D. Biosynthesis and biological functions of terpenoids in plants. In Biotechnology of Isoprenoids; Springer: Berlin/Heidelberg, Germany, 2015; pp. 63–106. [Google Scholar]
- Knudsen, J.T.; Eriksson, R.; Gershenzon, J.; Ståhl, B. Diversity and distribution of floral scent. Bot. Rev. 2006, 72, 1–120. [Google Scholar] [CrossRef]
- Corley, J. Fragrances for natural and certified organic personal care products: The link between fragrance and health in personal care product development. Perfum. Flavorist 2007, 32, 24–28. [Google Scholar]
- Dötterl, S.; Wolfe, L.M.; Jürgens, A. Qualitative and quantitative analyses of flower scent in Silene latifolia. Phytochemistry 2005, 66, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Pino, J.A.; Quijano-Celis, C.E.; Peralta-Bohórquez, A.F. Qualitative and Quantitative Analyses of Flower Scent in Lantana canescens Kunth. J. Essent. Oil Bear. Plants 2011, 14, 30–37. [Google Scholar] [CrossRef]
- Rusanov, K.; Kovacheva, N.; Rusanova, M.; Atanassov, I. Traditional Rosa damascena flower harvesting practices evaluated through GC/MS metabolite profiling of flower volatiles. Food Chem. 2011, 129, 1851–1859. [Google Scholar] [CrossRef]
- Wang, C.; Abbas, F.; Zhou, Y.; Ke, Y.; Li, X.; Yue, Y.; Yu, Y.; Yu, R.; Fan, Y. Genome-wide identification and expression pattern of SnRK gene family under several hormone treatments and its role in floral scent emission in Hedychium coronarium. PeerJ 2021, 9, e10883. [Google Scholar] [CrossRef]
- Yue, Y.; Yu, R.; Fan, Y. Transcriptome profiling provides new insights into the formation of floral scent in Hedychium coronarium. BMC Genom. 2015, 16, 470. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Ma, H.; Wan, Y.; Li, T.; Liu, X.; Sun, Z.; Li, Z. Volatile organic compounds emissions from Luculia pinceana flower and its changes at different stages of flower development. Molecules 2016, 21, 531. [Google Scholar] [CrossRef] [Green Version]
- Abbas, F.; Ke, Y.; Yu, R.; Fan, Y. Functional characterization and expression analysis of two terpene synthases involved in floral scent formation in Lilium ‘Siberia’. Planta 2019, 249, 71–93. [Google Scholar] [CrossRef] [PubMed]
- Abbas, F.; Ke, Y.; Zhou, Y.; Ashraf, U.; Li, X.; Yu, Y.; Yue, Y.; Ahmad, K.W.; Yu, R.; Fan, Y. Molecular cloning, characterization and expression analysis of LoTPS2 and LoTPS4 involved in floral scent formation in oriental hybrid Lilium variety ‘Siberia’. Phytochemistry 2020, 173, 112294. [Google Scholar] [CrossRef] [PubMed]
- Abbas, F.; Ke, Y.; Zhou, Y.; Waseem, M.; Yu, Y.; Ashraf, U.; Li, X.; Wang, C.; Yue, Y.; Yu, R. Cloning, functional characterization and expression analysis of LoTPS5 from Lilium ‘Siberia’. Gene 2020, 756, 144921. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Mai, R.-Z.; Zou, J.-J.; Zhang, H.-Y.; Zeng, X.-L.; Zheng, R.-R.; Wang, C.-Y. Analysis of aroma-active compounds in three sweet osmanthus (Osmanthus fragrans) cultivars by GC-olfactometry and GC-MS. J. Zhejiang Univ. -Sci. B 2014, 15, 638–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, R.; Fan, Y. Molecular cloning and expression analysis of a terpene synthase gene, HcTPS2, in Hedychium coronarium. Plant Mol. Biol. Report. 2011, 29, 35–42. [Google Scholar] [CrossRef]
- Li, X.-Y.; Zheng, S.-Y.; Yu, R.-C.; Fan, Y.-P. Promoters of HcTPS1 and HcTPS2 genes from Hedychium coronarium direct floral-specific, developmental-regulated and stress-inducible gene expression in transgenic tobacco. Plant Mol. Biol. Report. 2014, 32, 864–880. [Google Scholar] [CrossRef]
- Chen, H.; Yue, Y.; Yu, R.; Fan, Y. A Hedychium coronarium short chain alcohol dehydrogenase is a player in allo-ocimene biosynthesis. Plant Mol. Biol. 2019, 101, 297–313. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.D. Application of electronic-nose technologies and VOC-biomarkers for the noninvasive early diagnosis of gastrointestinal diseases. Sensors 2018, 18, 2613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, H.; Zhang, M.; Adhikari, B. Advances of electronic nose and its application in fresh foods: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 2700–2710. [Google Scholar] [CrossRef]
- Capelli, L.; Sironi, S.; Del Rosso, R. Electronic noses for environmental monitoring applications. Sensors 2014, 14, 19979–20007. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Gao, L.; Wang, J. Classification and regression of ELM, LVQ and SVM for E-nose data of strawberry juice. J. Food Eng. 2015, 144, 77–85. [Google Scholar] [CrossRef]
- Fujioka, K.; Shirasu, M.; Manome, Y.; Ito, N.; Kakishima, S.; Minami, T.; Tominaga, T.; Shimozono, F.; Iwamoto, T.; Ikeda, K. Objective display and discrimination of floral odors from Amorphophallus titanum, bloomed on different dates and at different locations, using an electronic nose. Sensors 2012, 12, 2152–2161. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Huang, Y.; Zhang, Q.; Liu, X.; Li, F.; Chen, K. Fragrance discrimination of Chinese Cymbidium species and cultivars using an electronic nose. Sci. Hortic. 2014, 172, 271–277. [Google Scholar] [CrossRef]
- Srinivasan, A.; Ahn, M.S.; Jo, G.S.; Suh, J.N.; Seo, K.H.; Kim, W.H.; Kang, Y.I.; Lee, Y.R.; Choi, Y.J. Analysis of relative scent intensity, volatile compounds and gene expression in Freesia “Shiny Gold”. Plants 2020, 9, 1597. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Yu, R.; Fan, Y. Characterization of two monoterpene synthases involved in floral scent formation in Hedychium coronarium. Planta 2014, 240, 745–762. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Wang, T.; Fan, J.; Liu, Z.; Zong, J.; Fan, W.; Han, Y.; Grierson, D. Volatile composition and classification of Lilium flower aroma types and identification, polymorphisms, and alternative splicing of their monoterpene synthase genes. Hortic. Res. 2019, 6, 110. [Google Scholar] [CrossRef] [Green Version]
- Wei, Q.; Xia, Q.; Wang, Y.; Chen, W.; Liu, C.; Zeng, R.; Xie, L.; Yi, M.; Guo, H. Profiling of volatile compounds and associated gene expression in two Anthurium cultivars and their F1 hybrid progenies. Molecules 2021, 26, 2902. [Google Scholar] [CrossRef]
- Yue, Y.; Wang, L.; Yu, R.; Chen, F.; He, J.; Li, X.; Yu, Y.; Fan, Y. Coordinated and high-level expression of biosynthetic pathway genes is responsible for the production of a major floral scent compound methyl benzoate in Hedychium coronarium. Front. Plant Sci. 2021, 12, 650582. [Google Scholar] [CrossRef]
- Gutiérrez, J.; Horrillo, M.C. Advances in artificial olfaction: Sensors and applications. Talanta 2014, 124, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Wang, J.; Gao, L. Qualification and quantification of processed strawberry juice based on electronic nose and tongue. LWT-Food Sci. Technol. 2015, 60, 115–123. [Google Scholar] [CrossRef]
- Li, D.; Lei, T.; Zhang, S.; Shao, X.; Xie, C. A novel headspace integrated E-nose and its application in discrimination of Chinese medical herbs. Sens. Actuators B Chem. 2015, 221, 556–563. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, Y.; Wang, Y.; Kong, B.; Chen, Q. Evaluation of the flavour properties of cooked chicken drumsticks as affected by sugar smoking times using an electronic nose, electronic tongue, and HS-SPME/GC-MS. LWT 2021, 140, 110764. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, F.; Wu, W.; Wang, P.; Ye, N. Comparison of volatiles in different jasmine tea grade samples using electronic nose and automatic thermal desorption-gas chromatography-mass spectrometry followed by multivariate statistical analysis. Molecules 2020, 25, 380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, D.; Xu, M.; Wang, J.; Gu, S.; Zhu, L.; Hong, X. Tracing internal quality and aroma of a red-fleshed kiwifruit during ripening by means of GC-MS and E-nose. RSC Adv. 2019, 9, 21164–21174. [Google Scholar] [CrossRef] [Green Version]
- Xin, R.; Liu, X.; Wei, C.; Yang, C.; Liu, H.; Cao, X.; Wu, D.; Zhang, B.; Chen, K. E-Nose and GC-MS reveal a difference in the volatile profiles of white- and red-fleshed peach fruit. Sensors 2018, 18, 765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, J.; Zhang, W.; Zhou, T.; Zhang, D.; Zhang, D.; Zhang, L.; Wang, G.; Cao, F. Discrimination of Malus taxa with different scent intensities using electronic nose and gas chromatography–mass spectrometry. Sensors 2018, 18, 3429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ray, H.; Bhattacharyya, N.; Ghosh, A.; Tudu, B.; Bandyopadhyay, R.; Ghosh, A.; Biswas, S.P.; Majumdar, S. Fragrance profiling of Jasminum sambac Ait. flowers using electronic nose. IEEE Sens. J. 2016, 17, 160–168. [Google Scholar] [CrossRef]
- Schuhfried, E.; Betta, E.; Cappellin, L.; Aprea, E.; Gasperi, F.; Märk, T.D.; Biasioli, F. Withering of plucked Trachelospermum jasminoides (star jasmine) flowers–time-dependent volatile compound profile obtained with SPME/GC–MS and proton transfer reaction-mass spectrometry (PTR-MS). Postharvest Biol. Technol. 2017, 123, 1–11. [Google Scholar] [CrossRef]
- Tholl, D.; Hossain, O.; Weinhold, A.; Röse, U.S.; Wei, Q. Trends and applications in plant volatile sampling and analysis. Plant J. 2021, 106, 314–325. [Google Scholar] [CrossRef]
- Chaparro-Torres, L.A.; Bueso, M.C.; Fernández-Trujillo, J.P. Aroma volatiles obtained at harvest by HS-SPME/GC-MS and INDEX/MS-E-nose fingerprint discriminate climacteric behaviour in melon fruit. J. Sci. Food Agric. 2016, 96, 2352–2365. [Google Scholar] [CrossRef]
- Farneti, B.; Khomenko, I.; Grisenti, M.; Ajelli, M.; Betta, E.; Algarra, A.A.; Cappellin, L.; Aprea, E.; Gasperi, F.; Biasioli, F. Exploring blueberry aroma complexity by chromatographic and direct-injection spectrometric techniques. Front. Plant Sci. 2017, 8, 617. [Google Scholar] [CrossRef]
- Oyama-Okubo, N.; Tsuji, T. Analysis of floral scent compounds and classification by scent quality in tulip cultivars. J. Jpn. Soc. Hortic. Sci. 2013, 82, 344–353. [Google Scholar] [CrossRef] [Green Version]
- Cline, M.S.; Smoot, M.; Cerami, E.; Kuchinsky, A.; Landys, N.; Workman, C.; Christmas, R.; Avila-Campilo, I.; Creech, M.; Gross, B. Integration of biological networks and gene expression data using Cytoscape. Nat. Protoc. 2007, 2, 2366. [Google Scholar] [CrossRef] [Green Version]
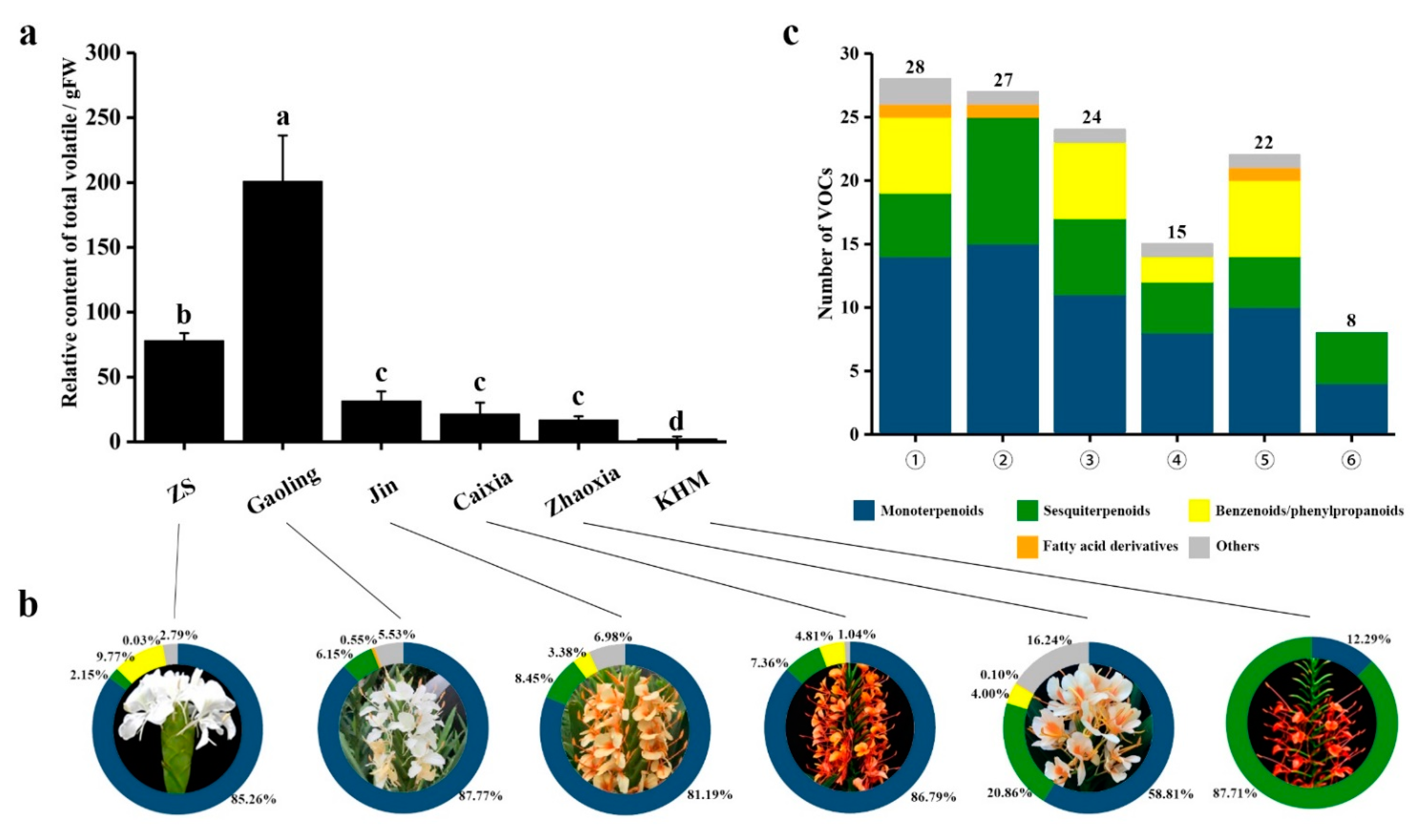
| Name | ID | RT 1 | LRI Calc 2 | LRI Nist 3 | MS 4 | Relative Content/% | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ZS | Gaoling | Jin | Caixia | Zhaoxia | KMH | ||||||
| Monoterpenoids | - | - | - | - | - | - | - | - | - | - | - |
| α-Thujene | M1 | 8.90 | 925 | 923 | 90 | 0.09 ± 0.02 b | 1.27 ± 0.09 a | 0.11 ± 0.02 b | - | 0.10 ± 0.03 b | - |
| α-Pinene | M2 | 9.05 | 932 | 937 | 91 | 0.26 ± 0.02 b | 3.67 ± 0.74 a | 0.23 ± 0.1 b | - | 0.08 ± 0.01 b | 0.03 ± 0.00 b |
| Camphene | M3 | 9.50 | 949 | 953 | 91 | 0.02 ± 0 b | 0.28 ± 0.18 a | - | - | - | - |
| β-Thujene | M4 | 10.30 | 972 | 966 | 91 | 0.83 ± 0.14 b | 8.38 ± 2.25 a | - | - | 0.48 ± 0.10 b | - |
| β-Pinene | M5 | 10.64 | 977 | 979 | 91 | - | - | 0.99 ± 0.38 a | - | 0.24 ± 0.05 b | - |
| β-Myrcene | M6 | 10.87 | 989 | 990 | 91 | 1.45 ± 0.12 b | 7.64 ± 1.87 a | 0.45 ± 0.16 b | 0.38 ± 0.18 b | 0.18 ± 0.02 b | - |
| α-Phellandrene | M7 | 11.35 | 1005 | 1004 | 80 | 0.54 ± 0.15 ab | 0.86 ± 0.52 a | 0.25 ± 0.04 b | 0.08 ± 0.03 bc | 0.07 ± 0.01 bc | - |
| α-Terpinene | M8 | 11.66 | 1017 | 1017 | 96 | 0.14 ± 0.02 b | 0.62 ± 0.03 a | 0.07 ± 0.01 c | - | - | - |
| Limonene | M9 | 12.26 | 1029 | 1028 | 91 | - | - | - | 0.08 ± 0.03 | - | - |
| Eucalyptol | M10 | 12.37 | 1032 | 1033 | 94 | 8.36 ± 0.48 b | 121.03 ± 17.91 a | 0.59 ± 0.12 cc | 0.12 ± 0.05 d | 0.46 ± 0.11 c | - |
| (E)-β-Ocimene | M11 | 12.87 | 1047 | 1040 | 96 | 35.58 ± 1.71 a | 25.15 ± 5.77 b | 16.63 ± 6.25 c | 9.62 ± 5.27 cd | 2.68 ± 0.62 d | 0.04 ± 0.06 e |
| Cyclopentene, 3-isopropenyl-5,5-dimethyl- | M12 | 12.98 | 1057 | - | 95 | 0.59 ± 0.26 b | 1.67 ± 0.09 a | - | - | - | - |
| (Z)-β-Terpineol | M13 | 13.56 | 1071 | 1145 | 92 | - | 0.90 ± 0.24 | - | - | - | - |
| Terpinolene | M14 | 13.67 | 1086 | 1085 | 93 | 0.14 ± 0.02 b | 0.55 ± 0.12 a | 0.19 ± 0.15 b | - | - | - |
| Linalool | M15 | 14.35 | 1099 | 1102 | 97 | 14.98 ± 0.24 a | 0.61 ± 0.23 c | 4.45 ± 1.32 b | 6.98 ± 2.4 b | 5.43 ± 0.35 b | 0.02 ± 0.04 c |
| 2,4,6-Octatriene, 2,6-dimethyl-, (E,Z)- | M16 | 15.04 | 1131 | 1131 | 97 | 3.33 ± 0.78 a | 2.68 ± 0.71 a | 1.57 ± 0.29 b | 0.47 ± 0.24 c | 0.17 ± 0.02 c | - |
| α-Terpineol | M17 | 16.78 | 1196 | 1199 | 86 | 0.16 ± 0.01 b | 0.94 ± 0.24 a | - | - | - | - |
| Dihydro-β-ionone | M18 | 20.83 | 1437 | 1433 | 98 | - | - | - | 0.75 ± 0.49 a | - | 0.18 ± 0.06 b |
| Sesquiterpenoids | - | - | - | - | - | - | - | - | - | - | - |
| α-Cubebene | S1 | 19.86 | 1371 | 1349 | 96 | - | 0.05 ± 0.04 a | - | - | 0.02 ± 0.01 a | - |
| Calarene | S2 | 20.04 | 1383 | 1388 | 87 | - | 0.3 ± 0.07 | - | - | - | - |
| 2-Norpinene | S3 | 20.40 | 1438 | 1436 | 98 | - | 0.15 ± 0.08 | - | - | - | - |
| Caryophyllene | S4 | 20.71 | 1429 | 1420 | 99 | 0.19 ± 0.08 c | 1.2 ± 0.72 bc | 1.52 ± 0.52 b | 0.49 ± 0.26 bc | 2.68 ± 1.2 a | 0.45 ± 0.47 bc |
| (E)-β-Famesene | S5 | 20.93 | 1454 | 1456 | 86 | 0.06 ± 0.01 b | 0.24 ± 0.1 ab | 0.14 ± 0.01 b | - | - | 1.3 ± 1.12 a |
| Humulene | S6 | 21.02 | 1466 | 1453 | 98 | - | 1.2 ± 0.72 a | - | 0.7 ± 0.42 a | 0.16 ± 0.07 b | - |
| Alloaromadendrene | S7 | 21.13 | 1470 | 1461 | 97 | - | 0.05 ± 0.04 a | 0.05 ± 0.02 a | - | - | - |
| β-Himachalene | S8 | 21.30 | 1471 | 1500 | 90 | - | 0.33 ± 0.29 | - | - | - | - |
| α-Farnesene | S9 | 21.75 | 1505 | 1524 | 91 | 1.28 ± 0.24 b | 8.2 ± 1.72 a | 0.77 ± 0.25 b | 0.29 ± 0.05 b | 0.65 ± 0.29 b | 0.13 ± 0.22 b |
| α-Amorphene | S10 | 21.82 | 1521 | 1519 | 94 | 0.1 ± 0.02 a | - | 0.06 ± 0.02 a | - | - | - |
| δ-Cadinene | S11 | 21.91 | 1525 | 1525 | 91 | 0.05 ± 0.04 b | - | 0.11 ± 0.02 a | 0.09 ± 0.05 ab | - | 0.04 ± 0.03 b |
| Nerolidol | S12 | 22.33 | 1563 | 1562 | 91 | - | 0.63 ± 0.5 | - | - | - | - |
| Benzenolds/phenylpropanoids | - | - | - | - | - | - | - | - | - | - | - |
| Anisole | B1 | 12.16 | 1019 | 1020 | 83 | - | - | 0.02 ± 0.02 | - | - | - |
| Methyl benzoate | B2 | 14.11 | 1093 | 1095 | 95 | 6.92 ± 3.26 a | - | 0.41 ± 0.44 b | 0.91 ± 0.67 b | 0.2 ± 0.04 c | - |
| Phenylethyl alcohol | B3 | 14.75 | 1112 | 1110 | 91 | - | - | - | - | 0.07 ± 0.04 | - |
| Benzyl nitrile | B4 | 15.41 | 1140 | 1150 | 93 | 0.09 ± 0.01 b | - | 0.19 ± 0.09 a | - | 0.22 ± 0.05 a | - |
| Eugenol | B5 | 19.59 | 1354 | 1356 | 95 | 0.04 ± 0.05 b | - | 0.11 ± 0.03 a | - | 0.02 ± 0.01 b | - |
| Phenol, 2-methoxy-4-(1-propenyl)- | B6 | 20.37 | 1450 | 1448 | 97 | 0.26 ± 0.24 a | - | 0.06 ± 0.03 b | 0.12 ± 0.05 ab | 0.13 ± 0.08 ab | - |
| 1-Butanol, 3-methyl-, benzoate | B7 | 20.84 | 1442 | 1441 | 83 | 0.28 ± 0.03 a | - | 0.28 ± 0.18 a | - | 0.04 ± 0.01 b | - |
| Benzyl benzoate | B8 | 24.33 | 1780 | 1760 | 96 | 0.03 ± 0.03 | - | - | - | - | - |
| Fatty acid derivatives | - | - | - | - | - | - | - | - | - | - | - |
| Isobornyl acetate | F1 | 18.32 | 1277 | - | 99 | - | 1.11 ± 1.27 | - | - | - | - |
| Methyl jasmonate | F2 | 23.42 | 1652 | 1638 | 92 | 0.02 ± 0.02 a | - | - | - | 0.02 ± 0.01 a | - |
| Others | - | - | - | - | - | - | - | - | - | - | - |
| Butyl aldoxime, 3-methyl-, syn- | O1 | 7.38 | 850 | - | 87 | 1.63 ± 0.43 b | 11.1 ± 3.71 a | 2.19 ± 1.8 b | 0.22 ± 0.14 c | 2.73 ± 0.68 b | - |
| Indole | O2 | 18.60 | 1292 | 1290 | 81 | 0.55 ± 0.37 | - | - | - | - | - |
| Sensors | Response Values | |||||
|---|---|---|---|---|---|---|
| ZS | Gaoling | Jin | Caixia | Zhaoxia | KMH | |
| W1C | 0.9802 ± 0.0025 d | 0.9668 ± 0.0007 e | 0.9887 ± 0.0017 b | 0.9835 ± 0.0006 c | 0.9885 ± 0.0008 b | 0.9928 ± 0.0023 a |
| W5S | 1.4154 ± 0.0100 b | 2.0529 ± 0.0498 a | 1.2621 ± 0.0176 d | 1.4315 ± 0.0137 b | 1.3074 ± 0.0062 c | 1.1173 ± 0.0049 e |
| W3C | 0.9849 ± 0.0009 d | 0.9788 ± 0.0011 e | 0.9929 ± 0.0003 b | 0.9878 ± 0.0009 c | 0.9924 ± 0.0012 b | 0.9953 ± 0.0018 a |
| W6S | 1.0082 ± 0.0001 a | 1.0024 ± 0.0035 bc | 1.0001 ± 0.0014 bc | 1.0018 ± 0.0005 bc | 1.0018 ± 0.0011 bc | 1.0032 ± 0.0013 b |
| W5C | 0.9955 ± 0.0022 a | 0.9945 ± 0.0035 a | 0.9941 ± 0.0010 a | 0.9936 ± 0.0037 a | 0.9990 ± 0.0043 a | 0.9984 ± 0.0045 a |
| W1S | 1.1539 ± 0.0114 b | 1.1864 ± 0.0058 a | 1.0777 ± 0.0142 d | 1.1193 ± 0.0065 c | 1.1054 ± 0.0016 c | 1.0645 ± 0.0111 d |
| W1W | 2.9863 ± 0.0414 c | 4.7909 ± 0.2573 a | 2.4171 ± 0.1133 d | 3.6650 ± 0.0539 b | 2.8194 ± 0.0465 c | 1.6414 ± 0.0091 e |
| W2S | 1.0535 ± 0.0094 b | 1.0998 ± 0.0071 a | 1.0323 ± 0.0081 c | 1.0517 ± 0.0026 b | 1.0351 ± 0.0004 c | 1.0255 ± 0.0053 c |
| W2W | 2.0548 ± 0.0514 b | 2.8989 ± 0.1979 a | 1.6443 ± 0.0362 d | 2.0564 ± 0.0238 b | 1.8660 ± 0.0220 c | 1.3316 ± 0.0070 e |
| W3S | 1.0382 ± 0.0009 a | 1.0146 ± 0.0151 bc | 1.0074 ± 0.0016 c | 1.0223 ± 0.0027 b | 1.0168 ± 0.0026 bc | 1.0212 ± 0.0057 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Abbas, F.; Wang, Z.; Yu, Y.; Yue, Y.; Li, X.; Yu, R.; Fan, Y. HS–SPME–GC–MS and Electronic Nose Reveal Differences in the Volatile Profiles of Hedychium Flowers. Molecules 2021, 26, 5425. https://doi.org/10.3390/molecules26175425
Zhou Y, Abbas F, Wang Z, Yu Y, Yue Y, Li X, Yu R, Fan Y. HS–SPME–GC–MS and Electronic Nose Reveal Differences in the Volatile Profiles of Hedychium Flowers. Molecules. 2021; 26(17):5425. https://doi.org/10.3390/molecules26175425
Chicago/Turabian StyleZhou, Yiwei, Farhat Abbas, Zhidong Wang, Yunyi Yu, Yuechong Yue, Xinyue Li, Rangcai Yu, and Yanping Fan. 2021. "HS–SPME–GC–MS and Electronic Nose Reveal Differences in the Volatile Profiles of Hedychium Flowers" Molecules 26, no. 17: 5425. https://doi.org/10.3390/molecules26175425
APA StyleZhou, Y., Abbas, F., Wang, Z., Yu, Y., Yue, Y., Li, X., Yu, R., & Fan, Y. (2021). HS–SPME–GC–MS and Electronic Nose Reveal Differences in the Volatile Profiles of Hedychium Flowers. Molecules, 26(17), 5425. https://doi.org/10.3390/molecules26175425








