Synthesis of Ammonium-Based Ionic Liquids for the Extraction Process of a Natural Pigment (Betanin)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Ammonium-Based ILs
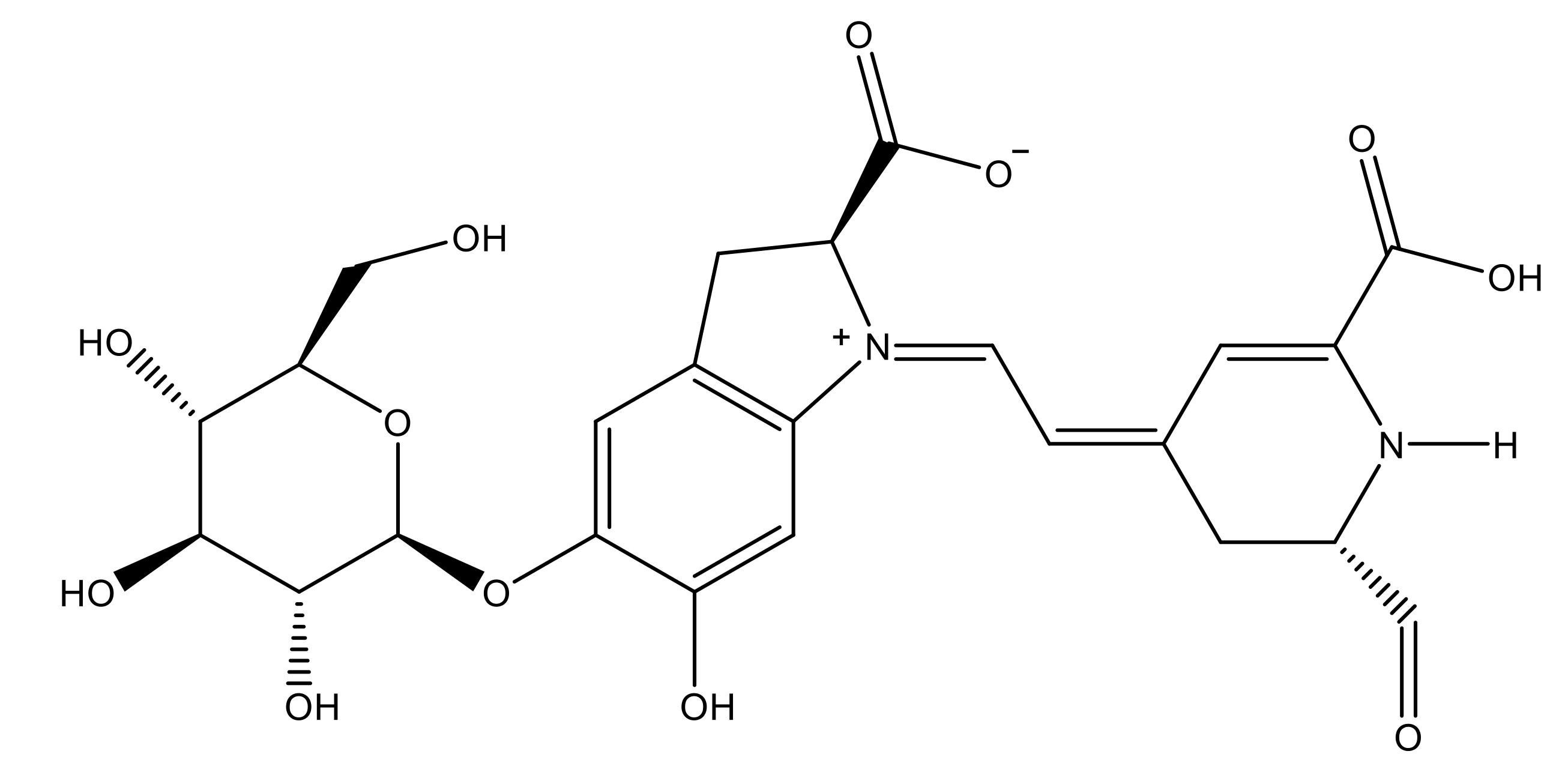
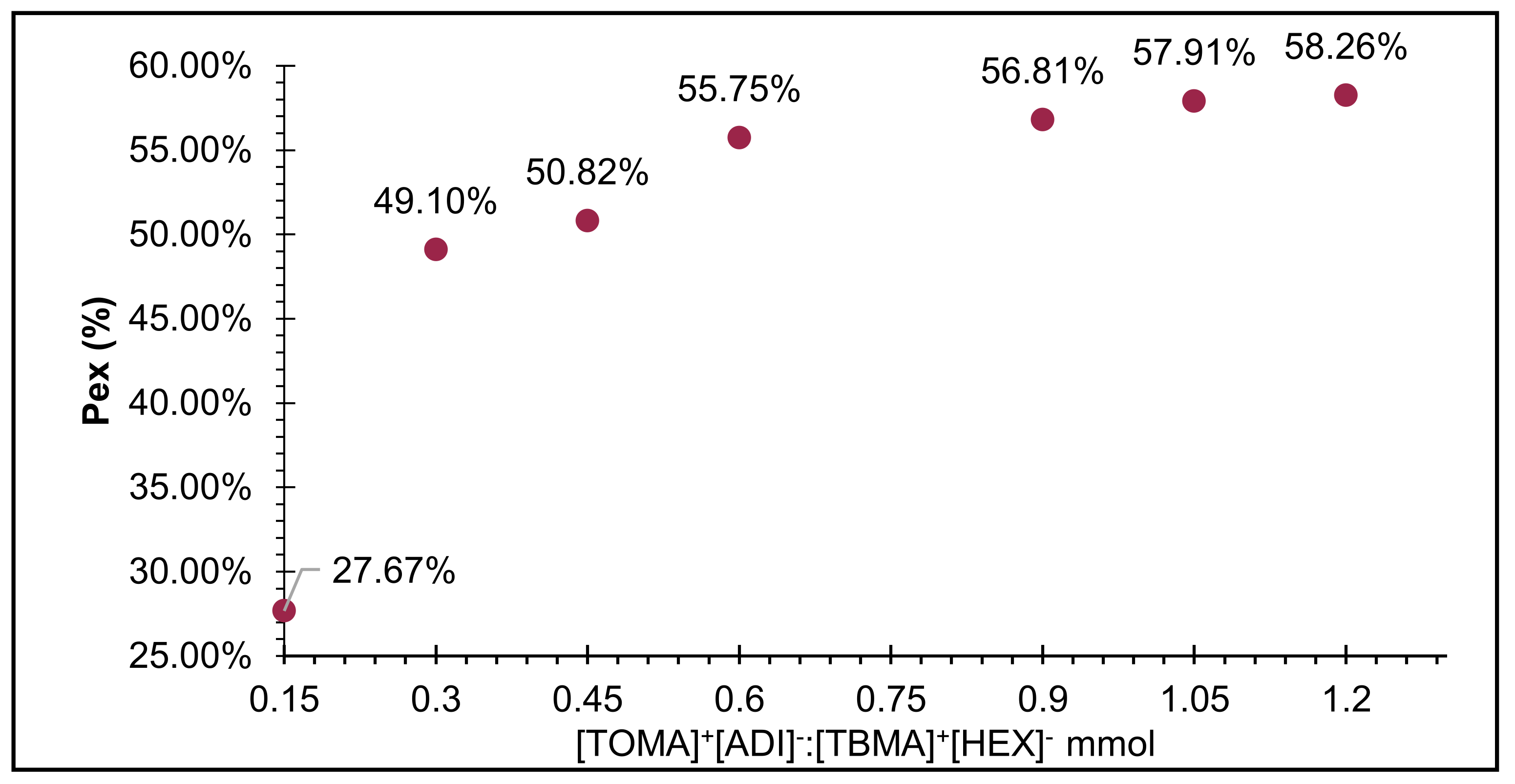
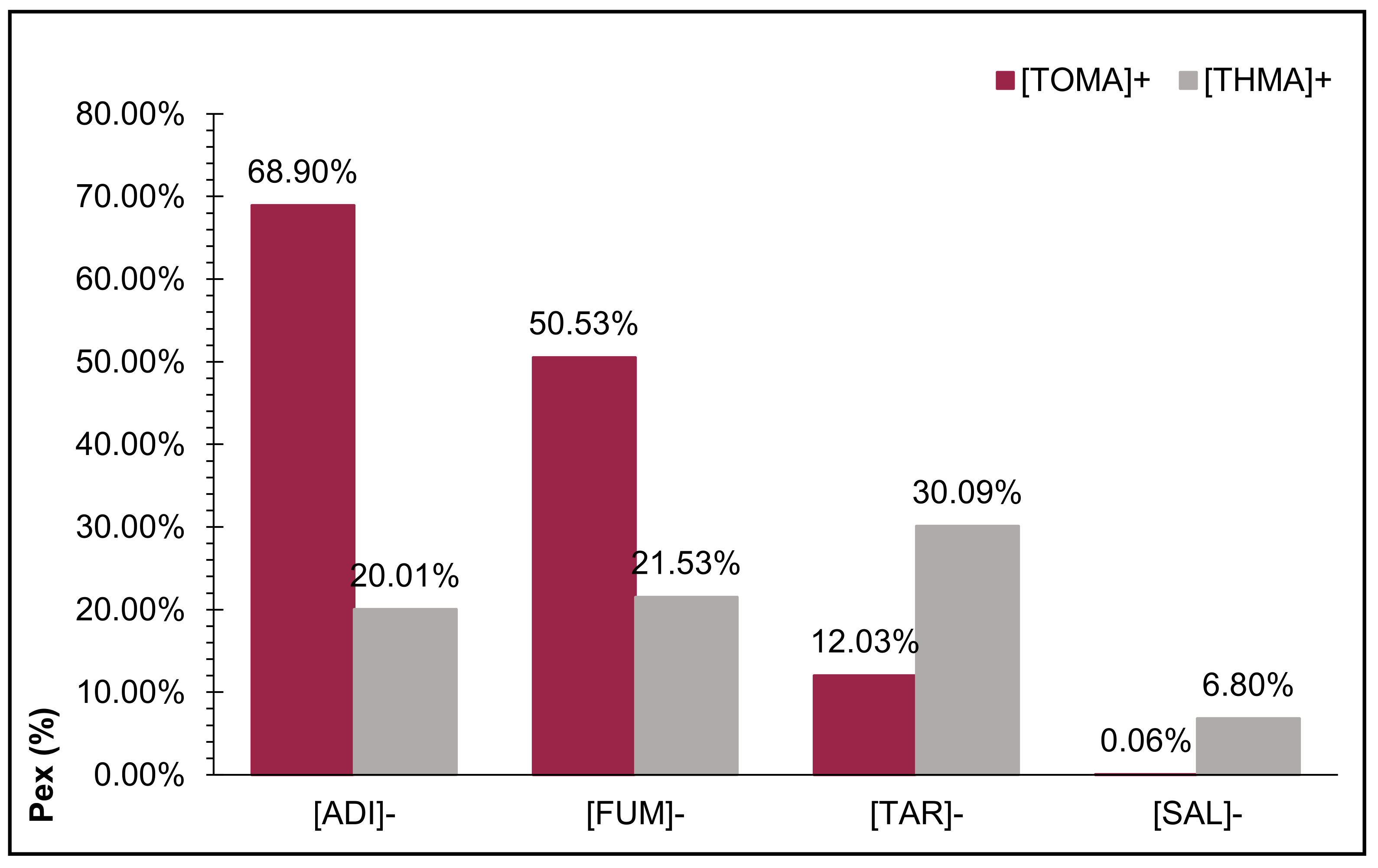
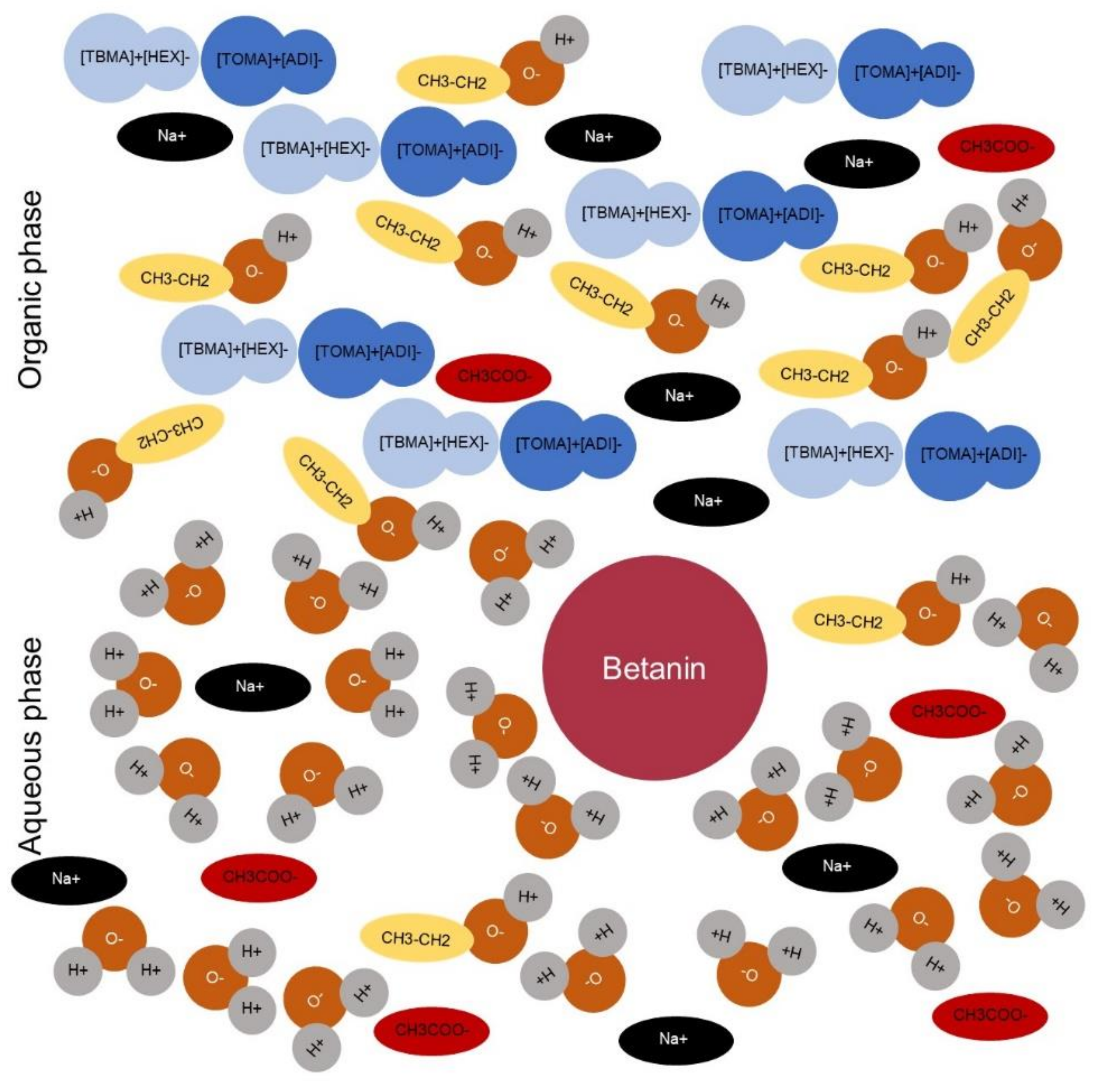
2.2. Selection of the Best IL System to Absorb the Molecular Structure of Betanin from an Aqueous Solution
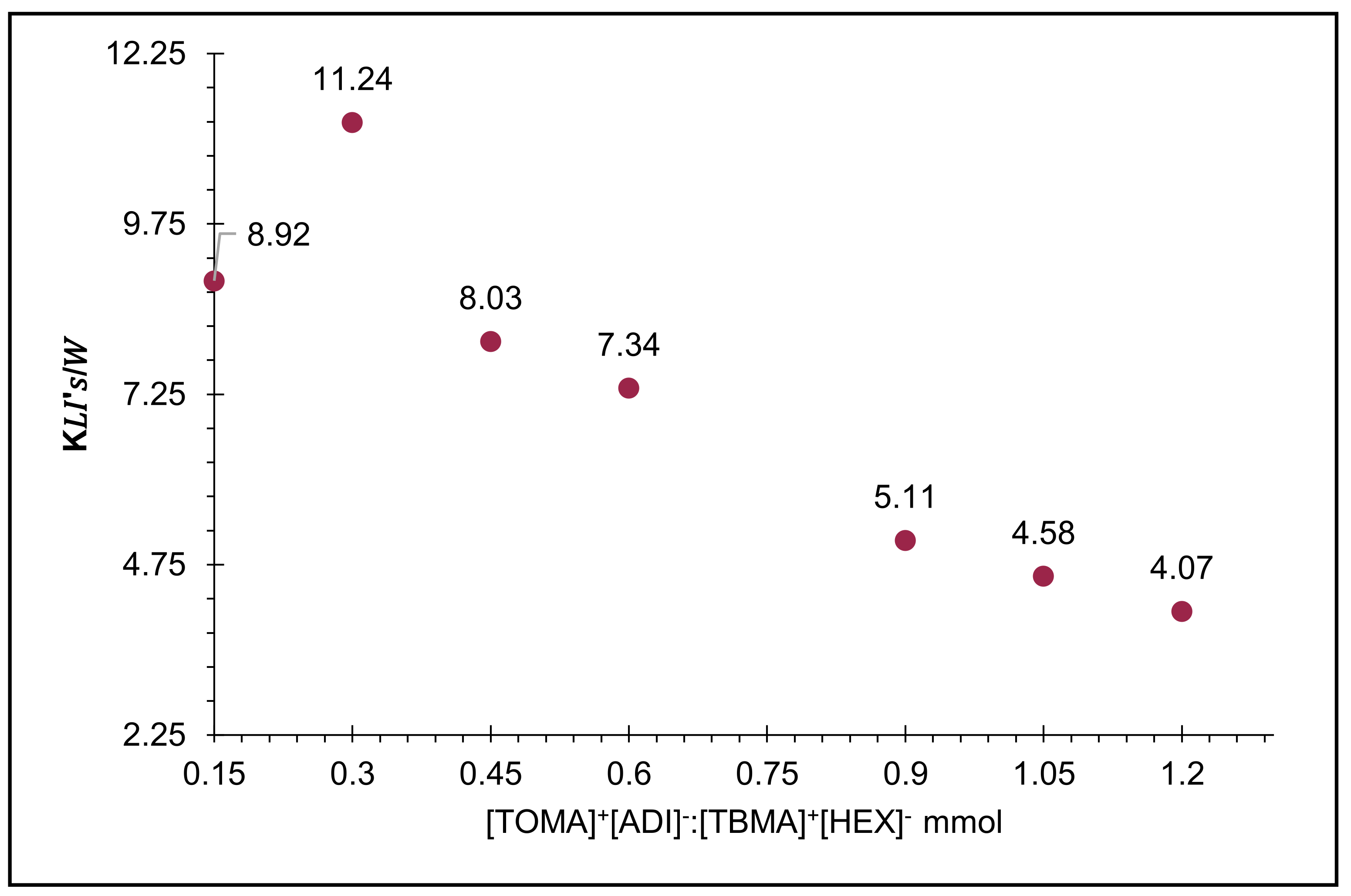
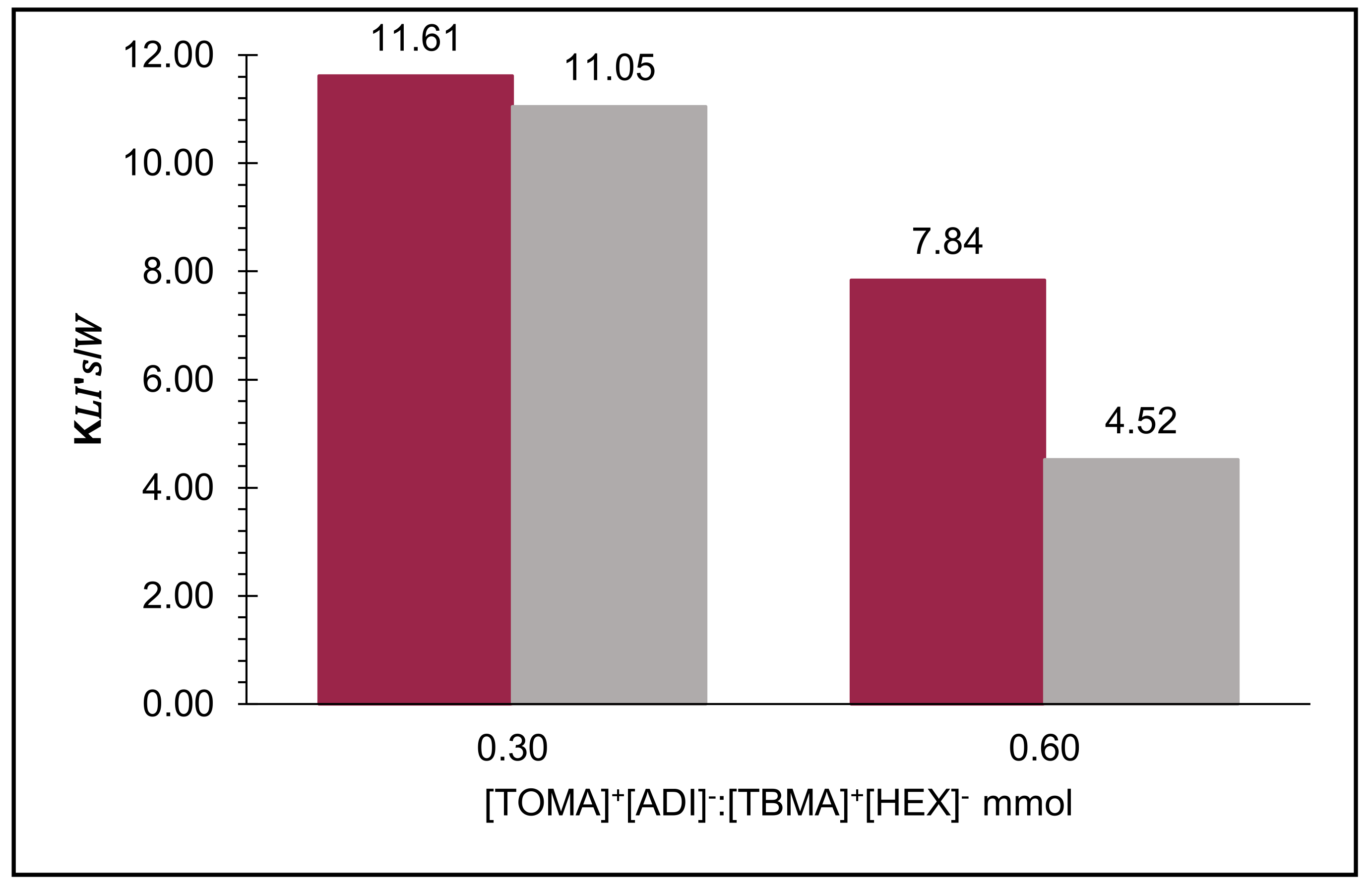
2.3. Calculation of Distribution Coefficients in the Biphasic Systems (KIL’s/W)
2.4. Aqueous-Based Biphasic System (ILs and Kosmotropic Salts) in the Desorption Process
3. Materials and Methods
3.1. General
3.2. IL synthesis and Characterization
3.3. Extraction Tests of Banin
3.4. Desorption Process
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodrigues, R.D.P.; de Lima, P.F.; Santiago-Aguiar, R.S.; Rocha, M.V.P. Evaluation of protic ionic liquids as potential solvents for the heating extraction of phycobiliproteins from Spirulina (Arthrospira) platensis. Algal Res. 2019, 38, 101391. [Google Scholar] [CrossRef]
- Xu, J.-J.; Li, Q.; Cao, J.; Warner, E.; An, M.; Tan, Z.; Wang, S.-L.; Peng, L.-Q.; Liu, X.-G. Extraction and enrichment of natural pigments from solid samples using ionic liquids and chitosan nanoparticles. J. Chromatogr. A 2016, 1463, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Lima, Á.S.; Soares, C.M.F.; Paltram, R.; Halbwirth, H.; Bica, K. Extraction and consecutive purification of anthocyanins from grape pomace using ionic liquid solutions. Fluid Phase Equilibria 2017, 451, 68–78. [Google Scholar] [CrossRef]
- Oliveira, M.V.S.; Vidal, B.T.; Melo, C.M.; de Miranda, R.C.M.; Soares, C.M.F.; Coutinho, J.A.P.; Ventura, S.P.M.; Mattedi, S.; Lima, Á.S. (Eco)toxicity and biodegradability of protic ionic liquids. Chemosphere 2016, 147, 460–466. [Google Scholar] [CrossRef]
- Pham, T.P.; Cho, C.-W.; Yun, Y.-S. Environmental fate and toxicity of ionic liquids: A review. Water Res. 2010, 44, 352–372. [Google Scholar] [CrossRef]
- Toledo Hijo, A.A.C.; Maximo, G.J.; Costa, M.C.; Batista, E.A.C.; Meirelles, A.J.A. Applications of Ionic Liquids in the Food and Bioproducts Industries. ACS Sustain. Chem. Eng. 2016, 4, 5347–5369. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Carle, R. Functional properties of anthocyanins and betalains in plants, food, and in human nutrition. Trends Food Sci. Technol. 2004, 15, 19–38. [Google Scholar] [CrossRef]
- Pasquet, V.; Chérouvrier, J.-R.; Farhat, F.; Thiéry, V.; Piot, J.-M.; Bérard, J.-B.; Kaas, R.; Serive, B.; Patrice, T.; Cadoret, J.-P.; et al. Study on the microalgal pigments extraction process: Performance of microwave assisted extraction. Process Biochem. 2011, 46, 59–67. [Google Scholar] [CrossRef] [Green Version]
- Zvitov, R.; Schwartz, A.; Zamski, E.; Nussinovitch, A. Direct Current Electrical Field Effects on Intact Plant Organs. Biotechnol. Prog. 2003, 19, 965–971. [Google Scholar] [CrossRef]
- Chalermchat, Y.; Fincan, M.; Dejmek, P. Pulsed electric field treatment for solid–liquid extraction of red beetroot pigment: Mathematical modelling of mass transfer. J. Food Eng. 2004, 64, 229–236. [Google Scholar] [CrossRef]
- López, N.; Puértolas, E.; Condón, S.; Raso, J.; Alvarez, I. Enhancement of the extraction of betanine from red beetroot by pulsed electric fields. J. Food Eng. 2009, 90, 60–66. [Google Scholar] [CrossRef]
- Nayak, C.A.; Chethana, S.; Rastogi, N.K.; Raghavarao, K.S.M.S. Enhanced mass transfer during solid–liquid extraction of gamma-irradiated red beetroot. Radiat. Phys. Chem. 2006, 75, 173–178. [Google Scholar] [CrossRef]
- Latorre, M.E.; Narvaiz, P.; Rojas, A.M.; Gerschenson, L.N. Effects of gamma irradiation on bio-chemical and physico-chemical parameters of fresh-cut red beet (Beta vulgaris L. var. conditiva) root. J. Food Eng. 2010, 98, 178–191. [Google Scholar] [CrossRef]
- Wiley, R.C.; Lee, Y.-N. Recovery of betalaines from red beets by a diffusion-extraction procedure. J. Food Sci. 1978, 43, 1056–1058. [Google Scholar] [CrossRef]
- Matyás, Z. Enterococcal L-tirosine decarboxylase (author’s transl). Ceskoslovenska Epidemiol. Mikrobiol. Imunol. 1977, 26, 89–94. [Google Scholar]
- King, B.D.; Lassiter, J.W.; Neathery, M.W.; Miller, W.J.; Gentry, R.P. Manganese Retention in Rats Fed Different Diets and Chemical Forms of Manganese. J. Anim. Sci. 1979, 49, 1235–1241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lebeau, J.; Petit, T.; Fouillaud, M.; Dufossé, L.; Caro, Y. Aqueous Two-Phase System Extraction of Polyketide-Based Fungal Pigments Using Ammonium- or Imidazolium-Based Ionic Liquids for Detection Purpose: A Case Study. J. Fungi 2020, 6, 375. [Google Scholar] [CrossRef]
- Rito-Palomares, M.; Benavides, J. Aqueous Two-Phase Systems for Bioprocess Development for the Recovery of Biological Products; Springer: Monterrey, Mexico, 2017; ISBN 978-3-319-59309-8. [Google Scholar]
- Ball, P.; Hallsworth, J.E. Water structure and chaotropicity: Their uses, abuses and biological implications. Phys. Chem. Chem. Phys. 2015, 17, 8297–8305. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Liao, Y.; Zhang, Z. Toxicity of Ionic Liquids. CLEAN—Soil Air Water 2007, 35, 42–48. [Google Scholar] [CrossRef]
- Clare, B.; Sirwardana, A.; MacFarlane, D.R. Synthesis, Purification and Characterization of Ionic Liquids. Top. Curr. Chem. 2009, 1–40. [Google Scholar] [CrossRef]
- Dalmau, M.E.; Eim, V.; Rosselló, C.; Cárcel, J.A.; Simal, S. Effects of convective drying and freeze-drying on the release of bioactive compounds from beetroot during in vitro gastric digestion. Food Funct. 2019, 10, 3209–3223. [Google Scholar] [CrossRef] [PubMed]
- Betageri, G.V.; Rogers, J.A. Thermodynamics of partitioning of β-blockers in the n-octanol- buffer and liposome systems. Int. J. Pharm. 1987, 36, 165–173. [Google Scholar] [CrossRef]
- Polturak, G.; Aharoni, A. “La Vie en Rose”: Biosynthesis, Sources, and Applications of Betalain Pigments. Mol. Plant 2019, 11, 7–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zangi, R.; Berne, B.J. Aggregation and Dispersion of Small Hydrophobic Particles in Aqueous Electrolyte Solutions. J. Phys. Chem. B 2006, 110, 22736–22741. [Google Scholar] [CrossRef] [Green Version]
- Fu, H.; Yang, S.-T.; Xiu, Z. Phase separation in a salting-out extraction system of ethanol–ammonium sulfate. Sep. Purif. Technol. 2015, 148, 32–37. [Google Scholar] [CrossRef]
- Chaplin, M. Structure and Properties of Water in Its Various States; Wiley, London South Bank University: London, UK, 2019; Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/9781119300762.wsts0002. (accessed on 27 August 2021).

| Structure | Name | Abbreviation | Yields |
|---|---|---|---|
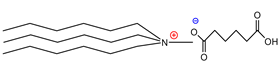 | Trihexylmethylammoniumadipate | [THMA]+[ADI]− | 85% |
 | Trioctylmethylammoniumadipate | [TOMA]+[ADI]− | 89% |
 | Trihexylmethylammoniumfumarate | [THMA]+[FUM]− | 84% |
 | Trioctylmethylammoniumfumarate | [TOMA]+[FUM]− | 86% |
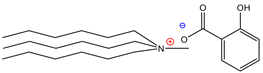 | Trihexylmethylammoniumsalicylate | [THMA]+[SAL]− | 85% |
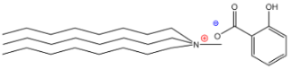 | Trioctylmethylammoniumsalicylate | [TOMA]+[SAL]− | 88% |
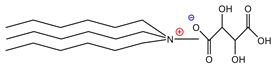 | Trihexylmethylammoniumtartarate | [THMA]+[TAR]− | 84% |
 | Trioctylmethylammoniumtartarate | [TOMA]+[TAR]− | 87% |
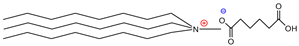
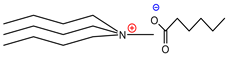 | Tributylmethylammoniumhexanoate | [TBMA]+[HEX]− | 84% |
| [TOMA]+[ADI]−/[TBMA]+[HEX]− Per Step (mmol) | Steps | Pex | Betanin Recovery (mmol) (10−3) | Total, Betanin Recovery (mmol) (10−3) Ptex (%) |
|---|---|---|---|---|
| 0.30 | 1 | 49.91% | 0.70 | |
| 2 | 48.67% | 0.36 | 1.04 74.30% | |
| 0.60 | 1 | 57.35% | 0.60 | |
| 2 | 43.67% | 0.25 | 1.15 82.40% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales-García, P.; Calvillo-Muñoz, E.Y.; Lijanova, I.V.; Likhanova, N.V.; Olivares-Xometl, O.; Arellanes-Lozada, P. Synthesis of Ammonium-Based Ionic Liquids for the Extraction Process of a Natural Pigment (Betanin). Molecules 2021, 26, 5458. https://doi.org/10.3390/molecules26185458
Morales-García P, Calvillo-Muñoz EY, Lijanova IV, Likhanova NV, Olivares-Xometl O, Arellanes-Lozada P. Synthesis of Ammonium-Based Ionic Liquids for the Extraction Process of a Natural Pigment (Betanin). Molecules. 2021; 26(18):5458. https://doi.org/10.3390/molecules26185458
Chicago/Turabian StyleMorales-García, Pedro, Evelyn Y. Calvillo-Muñoz, Irina V. Lijanova, Natalya V. Likhanova, Octavio Olivares-Xometl, and Paulina Arellanes-Lozada. 2021. "Synthesis of Ammonium-Based Ionic Liquids for the Extraction Process of a Natural Pigment (Betanin)" Molecules 26, no. 18: 5458. https://doi.org/10.3390/molecules26185458






