The Antifungal Action Mode of N-Phenacyldibromobenzimidazoles
Abstract
:1. Introduction
2. Results
2.1. Synthesis of N-Phenacyldibromobenzimidazoles
2.2. The Antifungal Effect of Dibromobenzimidazole Derivatives
2.3. Cytotoxicity of N-Phenacyldibromobenzimidazole Derivatives
2.4. Antifungal Activity of 5h in Combination with Osmoprotectant
2.5. Chitinolytic Activity of 5h
2.6. Efflux Disorder under 5h
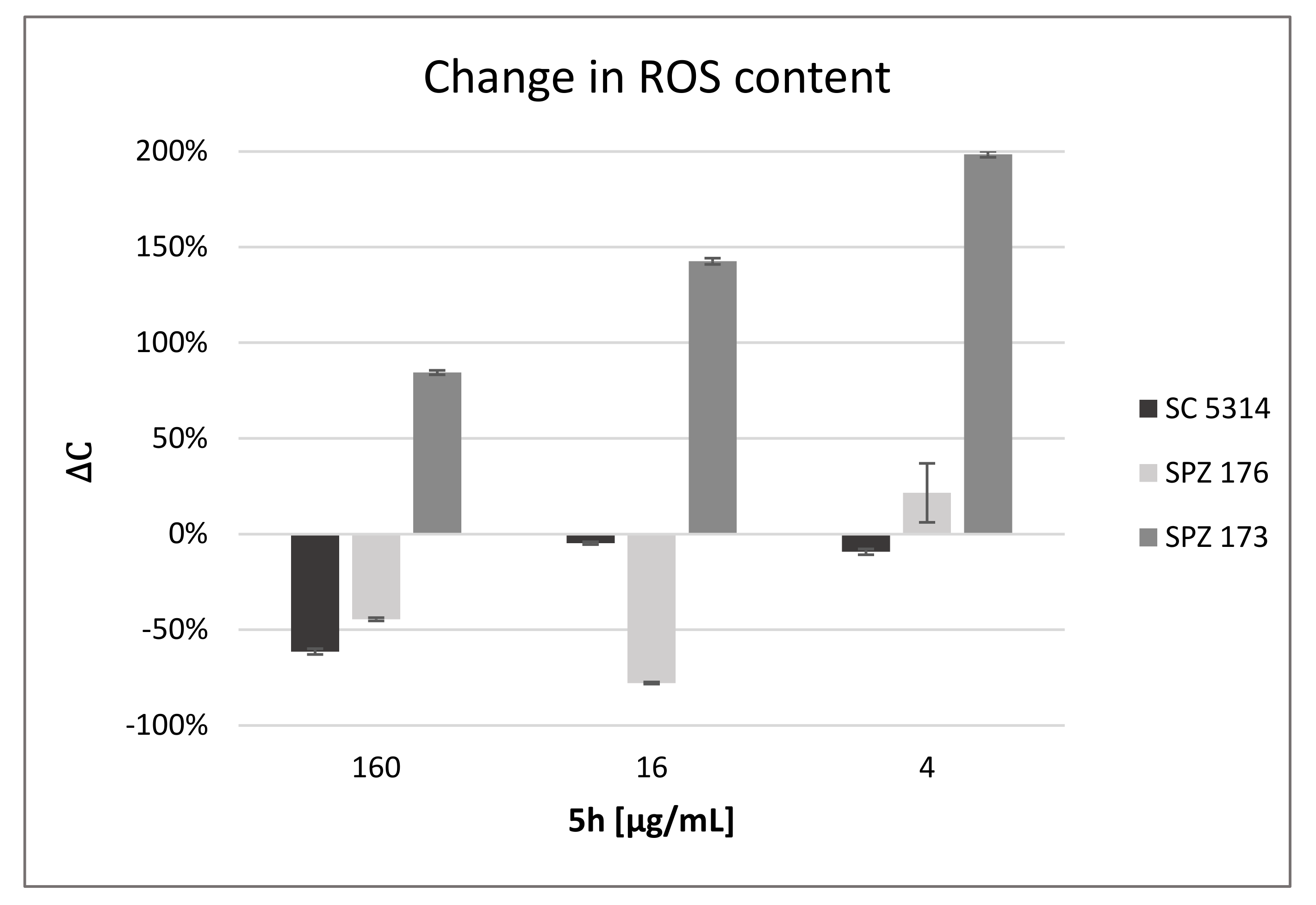
2.7. Compound 5h Induces ROS Generation
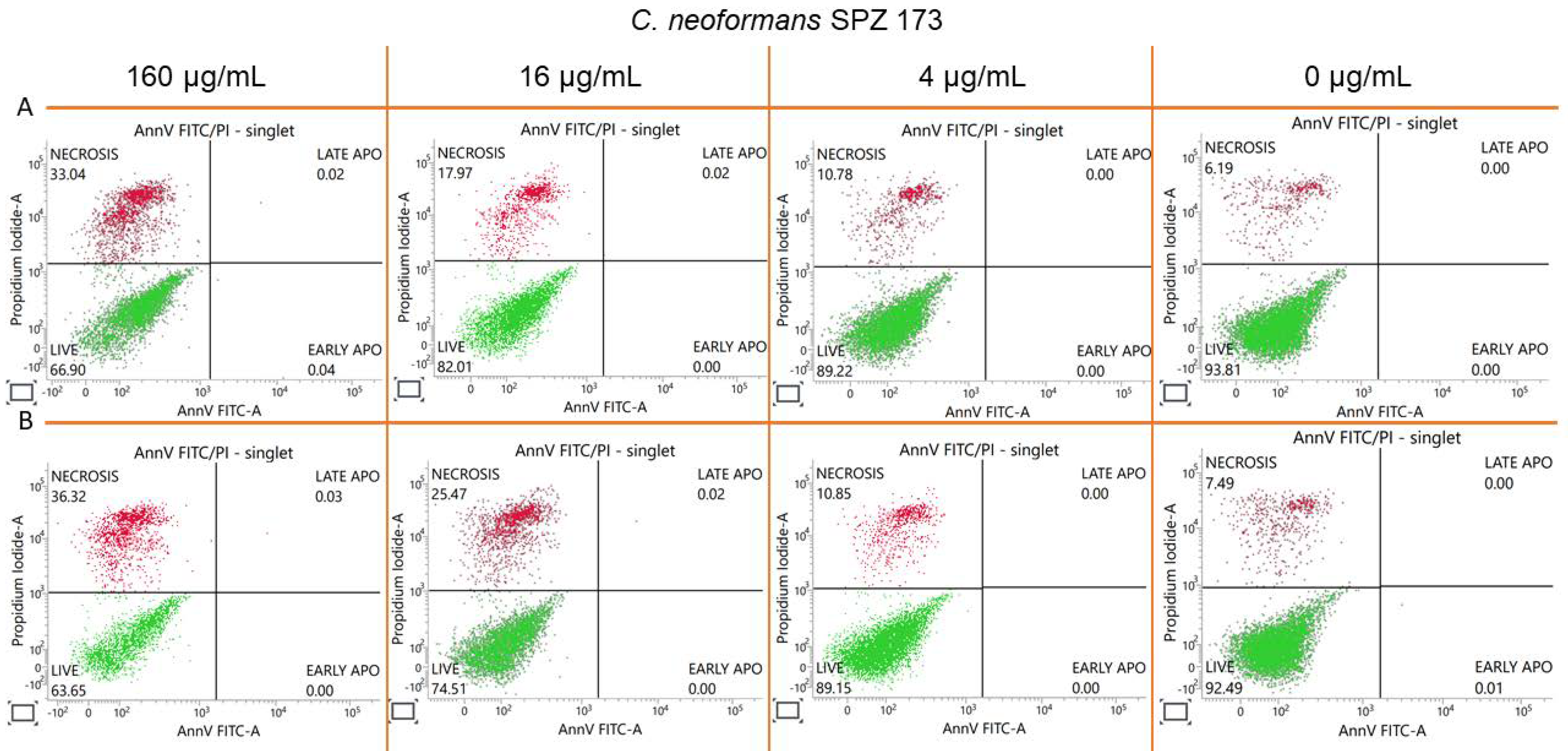
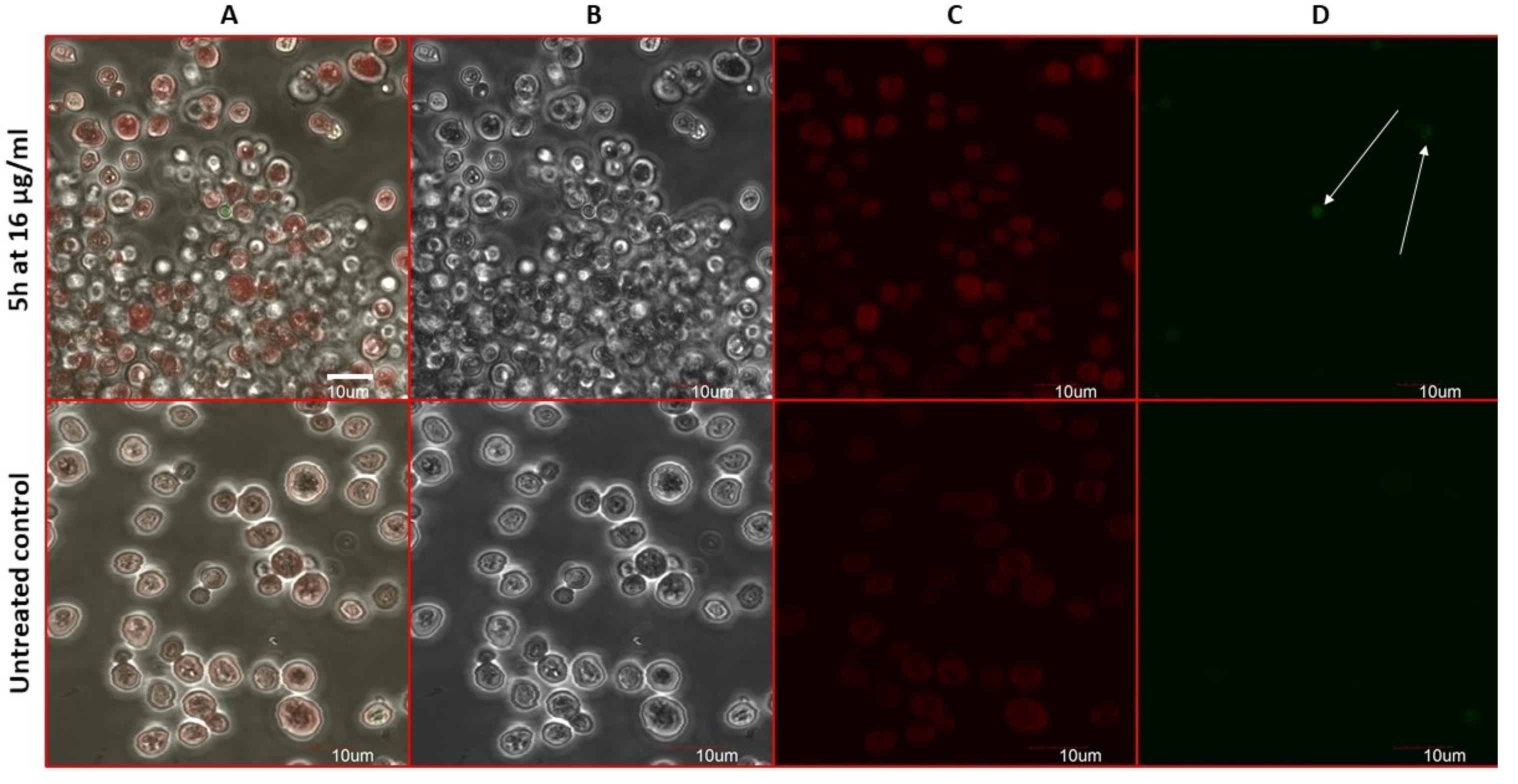
2.8. Estimation of Accidental Cell Death in the 5h-Treated Fungi
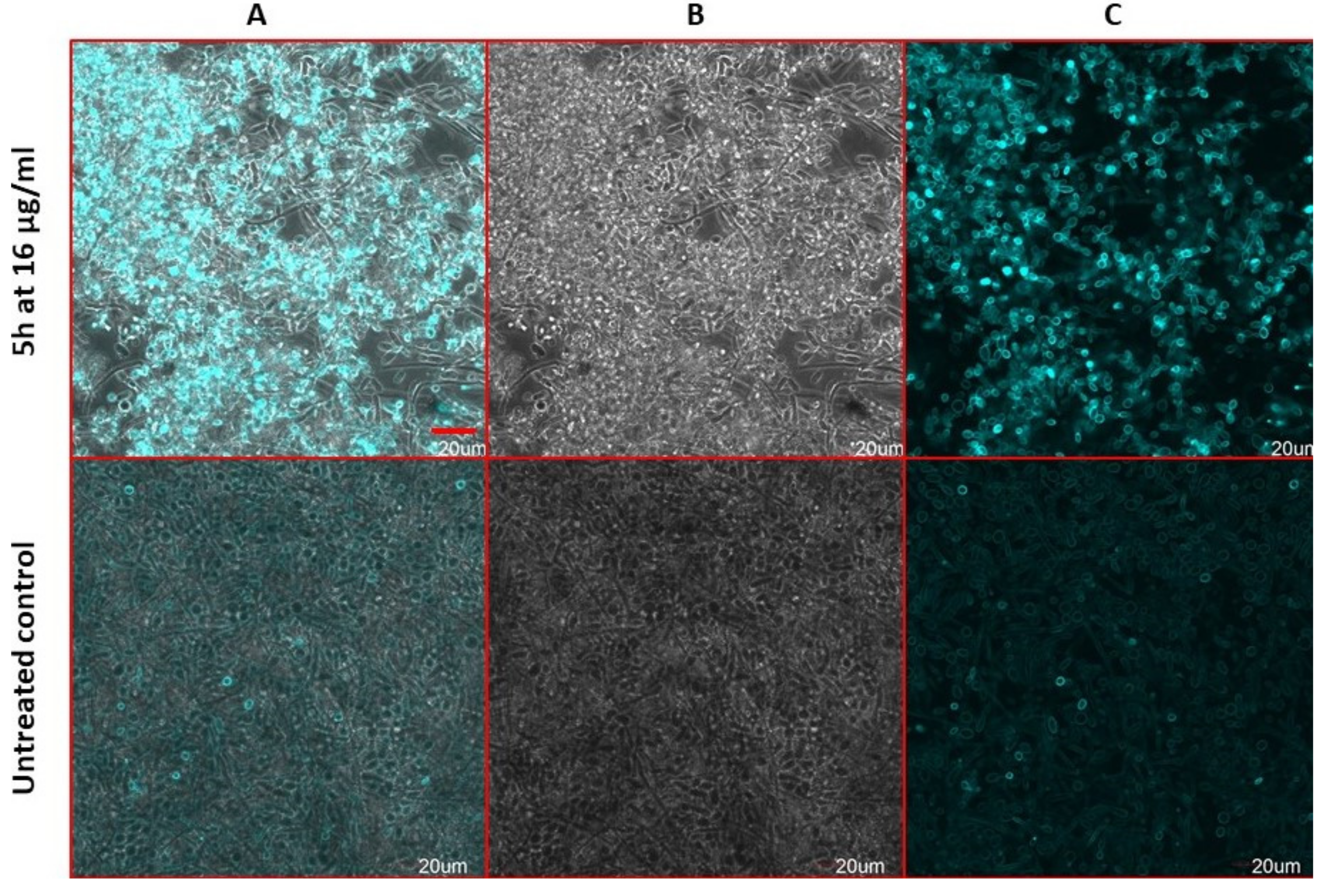
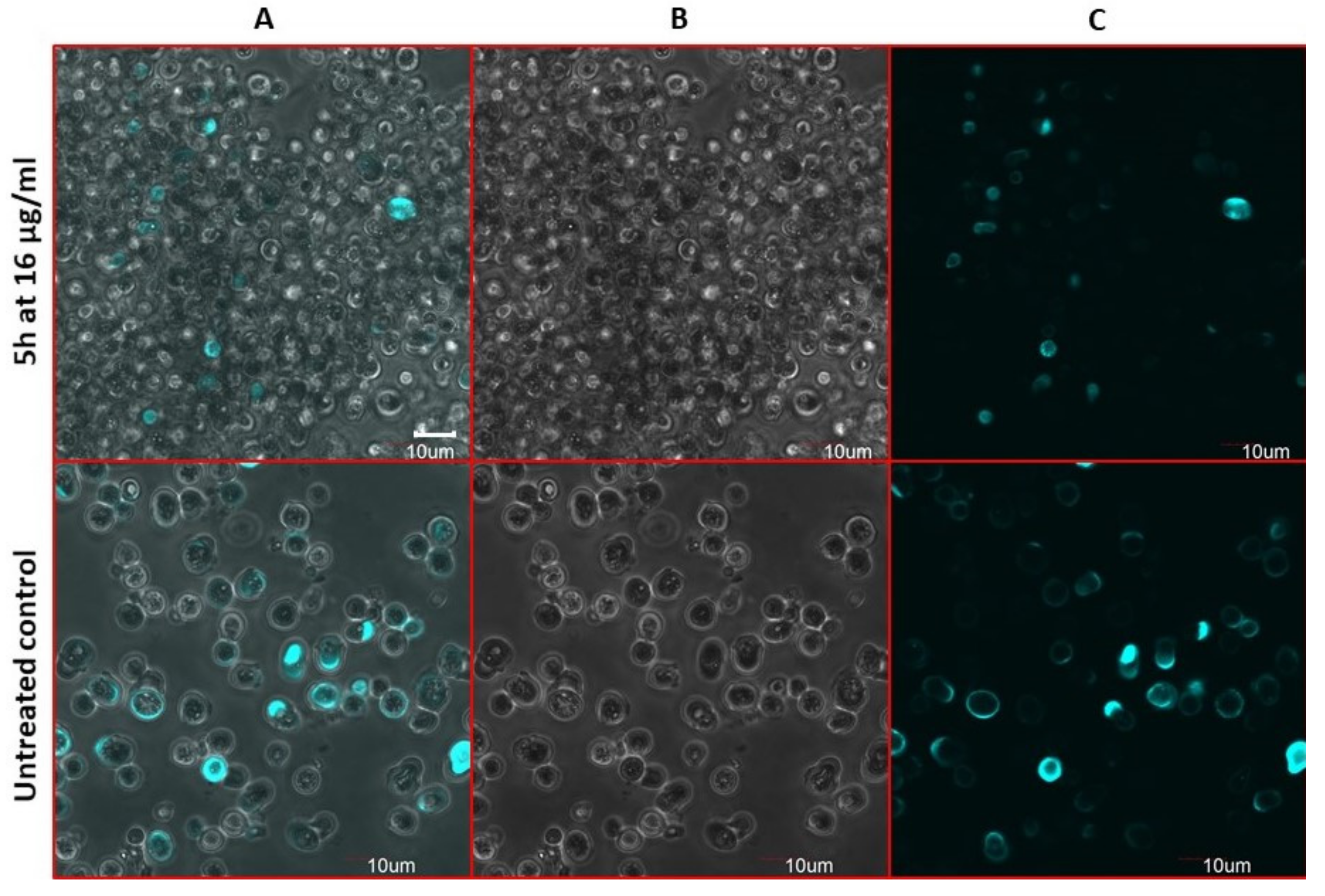
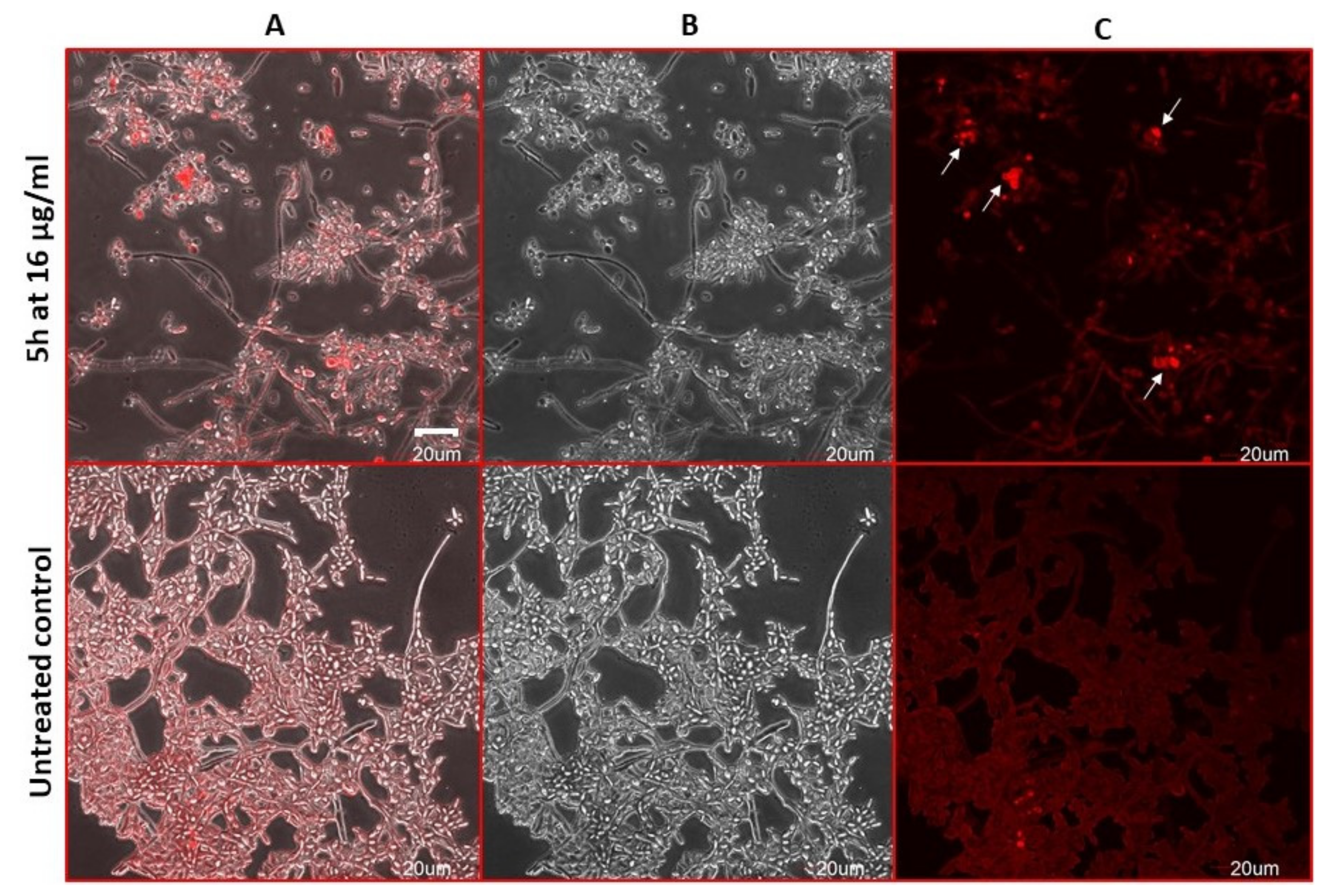
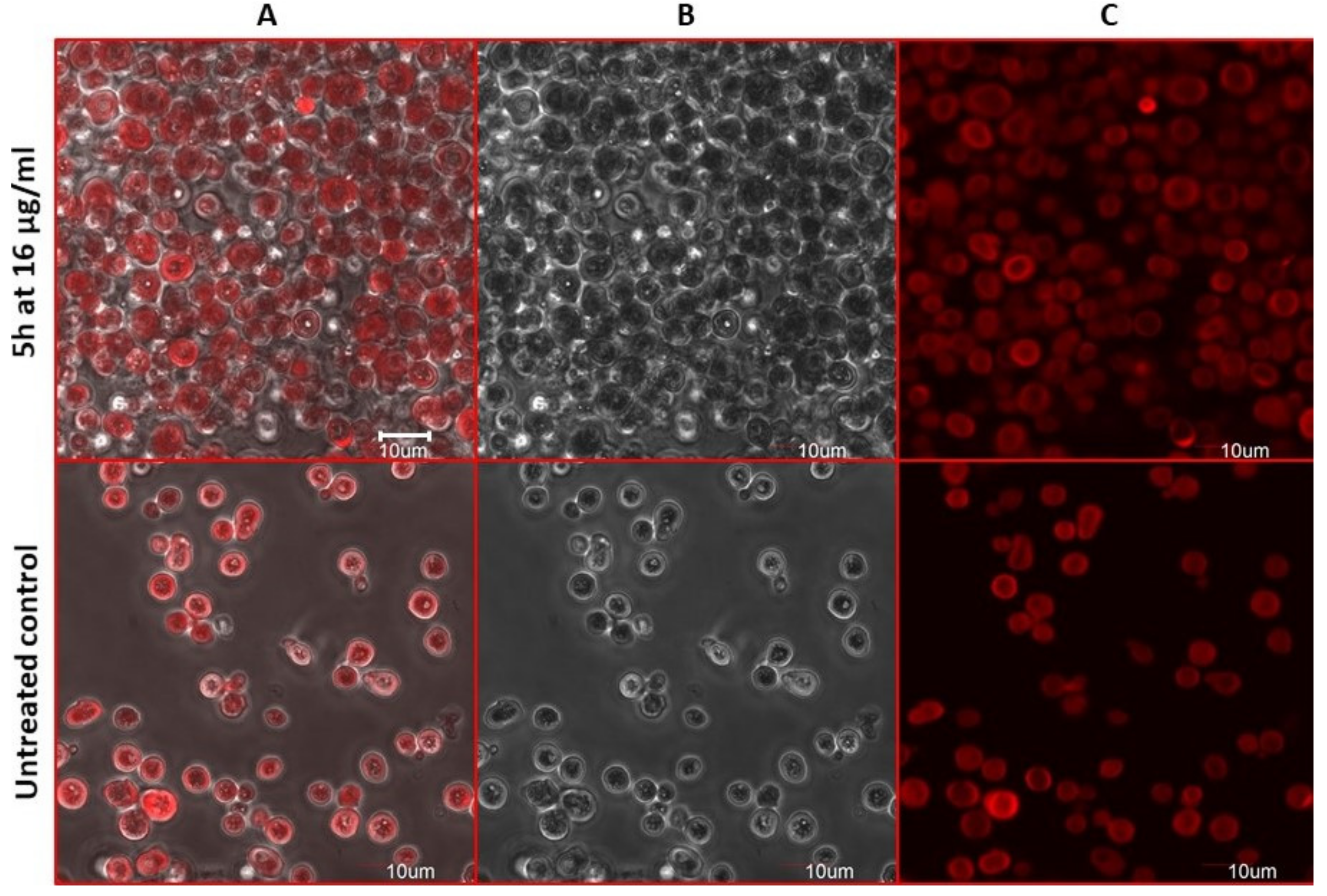
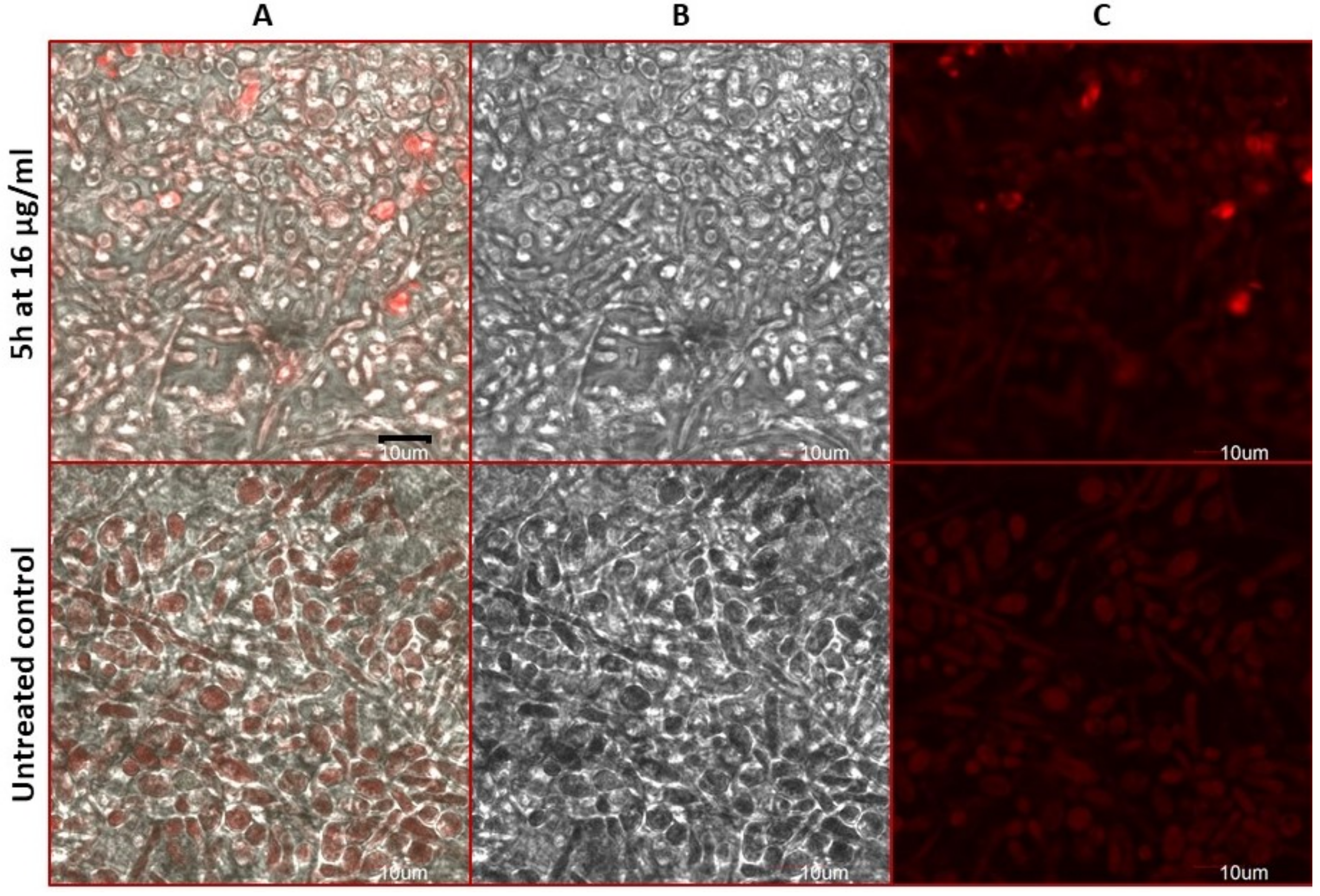
2.9. Antifungal Action and Accidental Cell Death by Fluorescent Structural Staining Techniques
3. Discussion
4. Materials and Methods
4.1. General Remarks of the N-Phenacyl Dibromobenzimidazole Derivatives Synthesis
4.1.1. Synthesis of 4a–d and 5a–d
4.1.2. Synthesis of 4e–i and 5e–i
4.2. Biological Studies
4.2.1. Yeast Cultures
4.2.2. Broth Microdilution Assay: MIC and MFC Determination
4.2.3. Determination of 5e–f and 5h Cytotoxicity
4.2.4. Broth Microdilution Assay: Activity of 5h Accompanied by Osmotic Protector
4.2.5. Examination of Chitinolytic Activity of 5h
4.2.6. Determination of the Rhodamine 123 Efflux from the Cells Treated with 5h
4.2.7. Determination of Reactive Oxygen Species (ROS) Concentration after Incubation with 5h
4.2.8. Cytometric Analysis of Cell Death Type
4.2.9. Confocal Laser Scanning Microscopy (CLSM) Analyses of the C. albicans and C. neoformans Biofilms Treated with 5h
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Pierce, C.G.; Lopez-Ribot, J.L. Candidiasis drug discovery and development: New approaches targeting virulence for discovering and identifying new drugs. Expert Opin. Drug Discov. 2013, 8, 1117–1126. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Su, C.; Liu, H. Candida albicans hyphal initiation and elongation. Trends Microbiol. 2014, 22, 707–714. [Google Scholar] [CrossRef] [Green Version]
- Leite, M.C.; Bezerra, A.P.; de Sousa, J.P.; Guerra, F.Q.; Lima Ede, O. Evaluation of Antifungal Activity and Mechanism of Action of Citral against Candida albicans. Evid. Based Complement. Altern. Med. 2014, 2014, 378280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shafiei, M.; Peyton, L.; Hashemzadeh, M.; Foroumadi, A. History of the development of antifungal azoles: A review on structures, SAR, and mechanism of action. Bioorg. Chem. 2020, 104, 104240. [Google Scholar] [CrossRef] [PubMed]
- Emami, S.; Foroumadi, A.; Falahati, M.; Lotfali, E.; Rajabalian, S.; Ebrahimi, S.A.; Farahyar, S.; Shafiee, A. 2-Hydroxyphenacyl azoles and related azolium derivatives as antifungal agents. Bioorg. Med. Chem. Lett. 2008, 18, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Olender, D.; Żwawiak, J.; Lukianchuk, V.; Lesyk, R.; Kropacz, A.; Fojutowski, A.; Zaprutko, L. Synthesis of some N-substituted nitroimidazole derivatives as potential antioxidant and antifungal agents. Eur. J. Med. Chem. 2009, 44, 645–652. [Google Scholar] [CrossRef]
- Nelson, R.; Kesternich, V.; Pérez-Fehrmann, M.; Salazar, F.; Marcourt, L.; Christen, P.; Godoy, P. Synthesis and Antifungal activity of phenacyl azoles. J. Chem. Res. 2014, 38, 549–552. [Google Scholar] [CrossRef]
- Olender, D.; Zaprutko, L.; Mertas, A.; Szliszka, E.; Wyrozumski, D.; Król, W. Anti-Candida Activity of 4-Morpholino-5-Nitro- and 4,5-Dinitro-Imidazole Derivatives. Pharm. Chem. J. 2018, 51, 1063–1067. [Google Scholar] [CrossRef]
- Elejalde, N.R.; Macías, M.; Castillo, J.C.; Sortino, M.; Svetaz, L.; Zacchino, S.; Portilla, J. Synthesis and in vitro Antifungal Evaluation of Novel N-Substituted 4-Aryl-2-methylimidazoles. Chem. Sel. 2018, 3, 5220–5227. [Google Scholar] [CrossRef]
- Sari, S.; Kart, D.; Öztürk, N.; Kaynak, F.B.; Gencel, M.; Taşkor, G.; Karakurt, A.; Saraç, S.; Eşsiz, Ş.; Dalkara, S. Discovery of new azoles with potent activity against Candida spp. and Candida albicans biofilms through virtual screening. Eur. J. Med. Chem. 2019, 179, 634–648. [Google Scholar] [CrossRef]
- Shaker, Y.M.; Omar, M.A.; Mahmoud, K.; Elhallouty, S.M.; El-Senousy, W.M.; Ali, M.M.; Mahmoud, A.E.; Abdel-Halim, A.H.; Soliman, S.M.; El Diwani, H.I. Synthesis, in vitro and in vivo antitumor and antiviral activity of novel 1-substituted ben-zimidazole derivatives. J. Enzym. Inhib. Med. Chem. 2015, 30, 826–845. [Google Scholar] [CrossRef] [Green Version]
- Kamil, A.; Akhter, S.; Ahmed, M.; Rizwani, G.H.; Hassan, S.; Naeem, S.; Jahan, S.; Khursheed, R.; Zahid, H. Antimalarial and insecticidal activities of newly synthesized derivatives of Benzimidazole. Pak. J. Pharm. Sci. 2015, 28, 2179–2184. [Google Scholar]
- Panchal, S.N.; Vekariya, R.H.; Patel, K.D.; Prajapati, S.M.; Rajani, D.P.; Rajani, S.D.; Patel, H.D. An efficient synthesis of novel carbohydrate and thiosemicarbazone hybrid benzimidazole derivatives and their antimicrobial evaluation. Indian J. Chem. 2016, 55B, 604–612. [Google Scholar]
- Vargas-Oviedo, D.; Butassi, E.; Zacchino, S.; Portilla, J. Eco friendly synthesis and antifungal evaluation of N substituted benzimidazoles. Mon. Chem.-Chem. Mon. 2020, 151, 575–588. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, J.; Rasheed, S.; Zhou, C. Design, synthesis, and biological evaluation of novel benzimidazole derivatives and their interaction with calf thymus DNA and synergistic effects with clinical drugs. Bioorg. Med. Chem. Lett. 2012, 22, 5363–5366. [Google Scholar] [CrossRef]
- Kumar, V.; Kaur, K.; Karelia, D.N.; Beniwal, V.; Gupta, G.K.; Sharma, A.K.; Gupta, A.K. Synthesis and biological evaluation of some 2-(3,5-dimethyl-1H-pyrazol-1-yl)-1-arylethanones: Antibacterial, DNA photocleavage, and anticancer activities. Eur. J. Med. Chem. 2014, 81, 267–276. [Google Scholar] [CrossRef]
- Jacob, K.S.; Ganguly, S. Synthesis, antimicrobial screening and cytotoxic studies of some novel pyrazole analogs. J. Appl. Pharm. Sci. 2016, 6, 135–141. [Google Scholar] [CrossRef] [Green Version]
- Karki, R.G.; Gokhale, V.M.; Kharkar, P.S.; Kulkarni, V.M. Azole compounds designed by molecular modelling show antifungal activity as predicted. Indian J. Chem. 2003, 42B, 372–381. [Google Scholar] [CrossRef]
- Gaikwad, N.D.; Patil, S.V.; Bobade, V.D. Hybrids of ravuconazole: Synthesis and biological evaluation. Eur. J. Med. Chem. 2012, 54, 295–302. [Google Scholar] [CrossRef]
- Gaikwad, N.D.; Patil, S.V.; Bobade, V.D. Synthesis and biological evaluation of some novel thiazole substituted benzotriazole derivatives. Bioorg. Med. Chem. Lett. 2012, 22, 3449–3454. [Google Scholar] [CrossRef]
- Meggio, F.; Shugar, D.; Pinna, L.A. Ribofuranosyl-benzimidazole derivatives as inhibitors of casein kinase-2 and casein kinase-1. Eur. J. Biochem. 1990, 187, 89–94. [Google Scholar] [CrossRef]
- Genieser, H.G.; Winkler, E.; Butt, E.; Zorn, M.; Schulz, S.; Iwitzki, F.; Störmann, R.; Jastorff, B.; Døskeland, S.O.; Øgreid, D.; et al. Derivatives of 1-β-d-ribofuranosylbenzimidazole 3′,5′-phosphate that mimic the actions of adenosine 3′,5′-phosphate (cAMP) and guanosine 3′,5′-phosphate (cGMP). Carbohydr. Res. 1992, 234, 217–235. [Google Scholar] [CrossRef]
- Zou, R.; Drach, J.C.; Townsend, L.B. Interaction of the putative human cytomegalovirus portal protein pUL104 with the large terminase subunit pUL56 and its inhibition by benzimidazole-D-ribonucleosides. J. Med. Chem. 1997, 40, 811–818. [Google Scholar] [CrossRef]
- Mancebo, H.S.Y.; Lee, G.; Flygare, J.; Tomassini, J.; Luu, P.; Zhu, Y.; Peng, J.; Blau, C.; Hazuda, D.; Price, D.; et al. P-TEFb kinase is required for HIV Tat transcriptional activation in vivo and in vitro. Gene. Dev. 1997, 11, 2633–2644. [Google Scholar] [CrossRef] [Green Version]
- Kowalkowska, A.; Chojnacki, K.; Wińska, P.; Mierzejewska, J.; Lewiński, R. Optimization of N-phenacyldibromobenzimidazole synthesis. in preparation.
- Stover, K.R.; Cleary, J.D. The Eagle-Like Effect of the Echinocandins: Is It Relevant for Clinical Decisions? Curr. Fungal Infect. Rep. 2015, 9, 88–93. [Google Scholar] [CrossRef]
- Rex, J.H.; Alexander, B.D.; Andes, D.; Arthington-Skaggs, B.; Brown, S.D.; Chaturvedi, V.; Ghannoum, M.A.; Espinel-Ingroff, A.; Knapp, C.C.; Ostrosky-Zeichner, L.; et al. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeast, 3rd ed.; Approved Standard M27-A3; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Carmona-Gutierrez, D.; Bauer, M.A.; Zimmermann, A.; Aguilera, A.; Austriaco, N.; Ayscough, K.; Balzan, R.; Bar-Nun, S.; Barrientos, A.; Belenky, P.; et al. Guidelines and recommendations on yeast cell death nomenclature. Microb. Cell 2018, 5, 4–31. [Google Scholar] [CrossRef] [Green Version]
- Borowiecki, P.; Wińska, P.; Bretner, M.; Gizińska, M.; Koronkiewicz, M.; Staniszewska, M. Synthesis of Novel Proxyphylline Derivatives with Dual Anti-Candida albicans and Anticancer Activity. Eur. J. Med. Chem. 2018, 150, 307–333. [Google Scholar] [CrossRef]
- Technical Bulletin: Chitinase Assay Kit (CS0980); Sigma-Aldrich: St. Louis, MO, USA, 2017; Available online: https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/product/documents/105/220/cs0980bul.pdf (accessed on 20 June 2021).
- Cantón, E.; Pemán, J.; Viudes, A.; Quindós, G.; Gobernado, M.; Espinel-Ingroff, A. Minimum fungicidal concentrations of amphotericin B for bloodstream Candida species. Diagn. Microbiol. Infect. Dis. 2003, 45, 203–206. [Google Scholar] [CrossRef]
- Staniszewska, M.; Bondaryk, M.; Kazek, M.; Gliniewicz, A.; Braunsdorf, C.; Schaller, M.; Mora-Montes, H.M.; Ochal, Z. Effect of serine protease KEX2 on Candida albicans virulence under halogenated methyl sulfones. Future Microbiol. 2017, 12, 285–306. [Google Scholar] [CrossRef] [PubMed]
- França, F.; Tagliati, C.; Ferreira, A.; Chaves, M.M. Amphotericin B nephrotoxicity in vitro: Differential profile of PKC signaling in VERO and MDCK cell lines. Curr. Top. Toxicol. 2014, 9, 15–19. [Google Scholar]
- Pereira, J.V.; Freires, I.A.; Castilho, A.R.; da Cunha, M.G.; Alves Had, S.; Rosalen, P.L. Antifungal potential of Sideroxylon obtusifolium and Syzygium cumini and their mode of action against Candida albicans. Pharm. Biol. 2016, 54, 2312–2319. [Google Scholar] [CrossRef] [Green Version]
- Górka-Nieć, W.; Perlińska-Lenart, U.; Zembek, P.; Palamarczyk, G.; Kruszewska, J.S. Influence of sorbitol on protein production and glycosylation and cell wall formation in Trichoderma reesei. Fungal Biol. 2010, 114, 855–862. [Google Scholar] [CrossRef]
- Makarasen, A.; Reukngam, N.; Khlaychan, P.; Chuysinuan, P.; Isobe, M.; Techasakul, S. Mode of action and synergistic effect of valinomycin and cereulide with amphotericin B against Candida albicans and Cryptococcus albidus. J. Mycol. Méd. 2018, 28, 112–121. [Google Scholar] [CrossRef]
- Brand, A. Hyphal growth in human fungal pathogens and its role in virulence. Int. J. Microbiol. 2012, 2012, 517529. [Google Scholar] [CrossRef] [Green Version]
- Tabata, E.; Wakita, S.; Kashimura, A.; Sugahara, Y.; Matoska, V.; Bauer, P.O.; Oyama, F. Residues of acidic chitinase cause chitinolytic activity degrading chitosan in porcine pepsin preparations. Sci. Rep. 2019, 9, 15609. [Google Scholar] [CrossRef]
- Nielsen, M.N.; Sørensen, J. Chitinolytic activity of Pseudomonas fluorescens isolates from barley and sugar beet rhizosphere. FEMS Microbiol. Ecol. 1999, 30, 217–227. [Google Scholar] [CrossRef]
- Shamly, V.; Kali, A.; Srirangaraj, S.; Umadevi, S. Comparison of Microscopic Morphology of Fungi Using Lactophenol Cotton Blue (LPCB), Iodine Glycerol and Congo Red Formaldehyde Staining. J. Clin. Diagn. Res. JCDR 2014, 8, DL01–DL02. [Google Scholar] [CrossRef] [PubMed]
- Staniszewska, M.; Sobiepanek, A.; Gizińska, M.; Peña-Cabrera, E.; Arroyo-Córdoba, I.J.; Kazek, M.; Kuryk, Ł.; Wieczorek, M.; Koronkiewicz, M.; Kobiela, T.; et al. Sulfone derivatives enter the cytoplasm of Candida albicans sessile cells. Eur. J. Med. Chem. 2020, 191, 1121139. [Google Scholar] [CrossRef]
- Eisenberg, T.; Carmona-Gutierrez, D.; Büttner, S.; Tavernarakis, N.; Madeo, F. Necrosis in yeast. Apoptosis 2010, 15, 257–268. [Google Scholar] [CrossRef]
- Bahmed, K.; Bonaly, R.; Coulon, J. Relation between cell wall chitin content and susceptibility to amphotericin B in Kluyveromyces, Candida and Schizosaccharomyces species. Res. Microbiol. 2003, 154, 215–222. [Google Scholar] [CrossRef]
- Mesa-Arango, A.C.; Trevijano-Contador, N.; Román, E.; Sánchez-Fresneda, R.; Casas, C.; Herrero, E.; Argüelles, J.C.; Pla, J.; Cuenca-Estrella, M.; Zaragoza, O. The production of reactive oxygen species is a universal action mechanism of Amphotericin B against pathogenic yeasts and contributes to the fungicidal effect of this drug. Antimicrob. Agents Chemother. 2014, 58, 6627–6638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saibabu, V.; Fatima, Z.; Khan, L.A.; Hameed, S. Vanillin Confers Antifungal Drug Synergism in Candida albicans by Impeding CaCdr2p Driven Efflux. J. Mycol. Med. 2020, 30, 100921. [Google Scholar] [CrossRef] [PubMed]
- Product Information Sheet: Reactive Oxygen Species (ROS) Detection Reagents; Thermo Fisher Scientific: Waltham, MA, USA, 2005; Available online: https://www.thermofisher.com/order/catalog/product/C369?SID=srch-srp-C369?query=DCFDA#/C369?SID=srch-srp-C369%3Fqueryassets.thermofisher.com (accessed on 20 June 2021).



| Lp. | 3, Ar COCH2, X | 3/1 or 3/2 [mol/mol] | Time of the Reaction [h] | 4 [%] | 5 [%] |
|---|---|---|---|---|---|
| 1 | 3a, Ph, Br | 1/1 | 24 | 4a, 94 | 5a, 74 |
| 2 | 3b, 4-FC6H4, Cl | 1/1 | 24 | 4b, 81 | 5b, 79 |
| 3 | 3c, 4-ClC6H4, Br | 1/1 | 24 | 4c, 83 | 5c, 72 |
| 4 | 3d, 4-BrC6H4, Cl | 1/1 | 24 | 4d, 79 | 5d, 73 |
| 5 | 3e, 2,4-Cl2C6H3, Cl | 2/1 | 3 | 4e, 17 | 5e, 21 |
| 6 | 3f, 3,4-Cl2C6H3, Cl | 2/1 | 3 | 4f, 13 | 5f, 15 |
| 7 | 3g, 2,4,6-Cl3C6H2, Cl | 2/1 | 96 | 4g, 14 | 5g, 15 |
| 8 | 3h, 2,4-F2C6H3, Cl | 2/1 | 3 | 4h, 21 | 5h, 22 |
| 9 | 3i, 2,5-F2C6H3, Cl | 2/1 | 3 | 4i, 16 | 5i, 17 |
| 10 | 3j, 2,4,6-F3C6H2, Cl | 2/1 | 3 | 4j, 23 | 5j, 24 |
| Candida albicans | Comp. [µg/mL] | Cell Growth Inhibition (%I) | ||
|---|---|---|---|---|
| 4 | 8 | 16 | ||
| ATCC SC5314 | 4a | 0 ± 0 | 0 ± 0 | 18 ± 1 |
| 4j | 0 ± 1 | 20 ± 2 | 85 ± 8 a | |
| 5b | 0 ± 2 | 95 ± 8 a | 53 ± 8 a | |
| 5d | 0 ± 10 | 0 ± 1 | 76 ± 1 a | |
| 5e | 71 ± 1 a | 58 ± 1 a | 51 ± 1 a | |
| 5f | 0 ± 1 | 46 ± 2 | 37 ± 1 | |
| 5h | 0 ± 5 | 81 ± 4 a | 75 ± 11 a | |
| 5j | 0 ± 3 | 83 ± 1 a | 0 ± 3 | |
| AmB | 100 ± 1 | 100 ± 2 | 100 ± 1 | |
| Isolate SPZ176 | 5e | 0 ± 0.0 | 17 ± 7 | 80 ± 8 a |
| 5f | 0 ± 10 | 0 ± 4 | 94 ± 5 a | |
| 5h | 0 ± 7 | 0 ± 2 | 100 ± 3 a | |
| AmB | 100 ± 2 | 100 ± 1 | 100 ± 1 | |
| Derivatives | Conc. [µg/mL] | a Dibromobenzimidazole Treated Cells [cfu × 10−7] | b Growth Control Cells [cfu × 10−7] | Logarithm Reduction R |
|---|---|---|---|---|
| 4a | 16 | 4.55 | 3.59 | −0.10 |
| 8 | 4.01 | −0.05 | ||
| 4 | 4.97 | −0.14 | ||
| 4j | 16 | 3.25 | 0.04 | |
| 8 | 2.65 | 0.13 | ||
| 4 | 4.51 | −0.10 | ||
| 5b | 16 | 5.70 | −0.20 | |
| 8 | 1.13 | 0.50 | ||
| 4 | 3.47 | 0.01 | ||
| 5d | 16 | 1.39 | 0.41 | |
| 8 | 3.82 | −0.03 | ||
| 4 | 2.06 | 0.24 | ||
| 5e | 16 | 3.10 | 0.06 | |
| 8 | 0.23 | 1.19 | ||
| 4 | 0.30 | 1.08 | ||
| 5f | 16 | 3.97 | −0.04 | |
| 8 | 0.36 | 1.00 | ||
| 4 | 0.95 | 0.58 | ||
| 5j | 16 | 7.65 | −0.33 | |
| 8 | 1.34 | 0.43 | ||
| 4 | 3.45 | 0.02 | ||
| 5h | 16 | 1.32 | 0.43 | |
| 8 | 2.21 | 0.21 | ||
| 4 | 7.50 | −0.32 |
| Comp. | Conc. [µg/mL] | C. albicans [cfu/mL] | C. neoformans [cfu/mL] |
|---|---|---|---|
| 5e | 4 | 105 | 105 |
| 8 | 7 × 102 | ||
| 16 | 105 | ||
| 5f | 4 | 104 | |
| 8 | 104 | ||
| 16 | 104 | ||
| 5h | 4 | 104 | |
| 8 | 0 | ||
| 16 | 0 | ||
| AmB | 4 | 0 | |
| 8 | 0 | ||
| 16 | 0 |
| Strains | Conc. [μg/mL] | Growth Inhibition [% ± SD] | |
|---|---|---|---|
| 96 h | 120 h | ||
| C. neoformans SPZ173 | 4 | 0 | 0 |
| 8 | 93 ± 15 | 79 ± 9 | |
| 16 | 95 ± 26 | 86 ± 15 | |
| C. albicans SPZ176 | 4 | 0 | 0 |
| 8 | 0 | 0 | |
| 16 | 0 | 8 ± 18 | |
| Substrate | U/mL × 105 | ||
|---|---|---|---|
| A | B | C | |
| 5h | 21 | 0 | 21 |
| Chitinase | 3140 | 3560 | 3360 |
| 5h [μg/mL] | C. albicans SC5314 | C. albicans SPZ176 | C. neoformans SPZ173 |
|---|---|---|---|
| 160 | 4 ± 9 | 0 ± 0 | 15 ± 2 |
| 16 | 6 ± 2 | 6 ± 1 | 14 ± 4 |
| 4 | 0 ± 1 | 0 ± 5 | 18 ± 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Staniszewska, M.; Kuryk, Ł.; Gryciuk, A.; Kawalec, J.; Rogalska, M.; Baran, J.; Kowalkowska, A. The Antifungal Action Mode of N-Phenacyldibromobenzimidazoles. Molecules 2021, 26, 5463. https://doi.org/10.3390/molecules26185463
Staniszewska M, Kuryk Ł, Gryciuk A, Kawalec J, Rogalska M, Baran J, Kowalkowska A. The Antifungal Action Mode of N-Phenacyldibromobenzimidazoles. Molecules. 2021; 26(18):5463. https://doi.org/10.3390/molecules26185463
Chicago/Turabian StyleStaniszewska, Monika, Łukasz Kuryk, Aleksander Gryciuk, Joanna Kawalec, Marta Rogalska, Joanna Baran, and Anna Kowalkowska. 2021. "The Antifungal Action Mode of N-Phenacyldibromobenzimidazoles" Molecules 26, no. 18: 5463. https://doi.org/10.3390/molecules26185463
APA StyleStaniszewska, M., Kuryk, Ł., Gryciuk, A., Kawalec, J., Rogalska, M., Baran, J., & Kowalkowska, A. (2021). The Antifungal Action Mode of N-Phenacyldibromobenzimidazoles. Molecules, 26(18), 5463. https://doi.org/10.3390/molecules26185463







