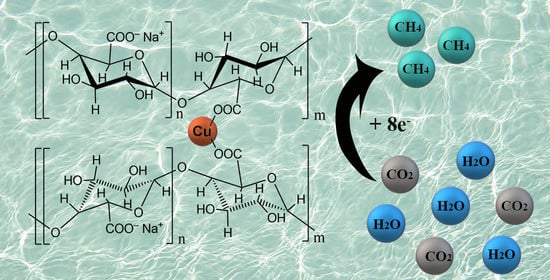
A Water-Soluble Sodium Pectate Complex with Copper as an Electrochemical Catalyst for Carbon Dioxide Reduction
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of the Sodium Pectate Complex with Copper
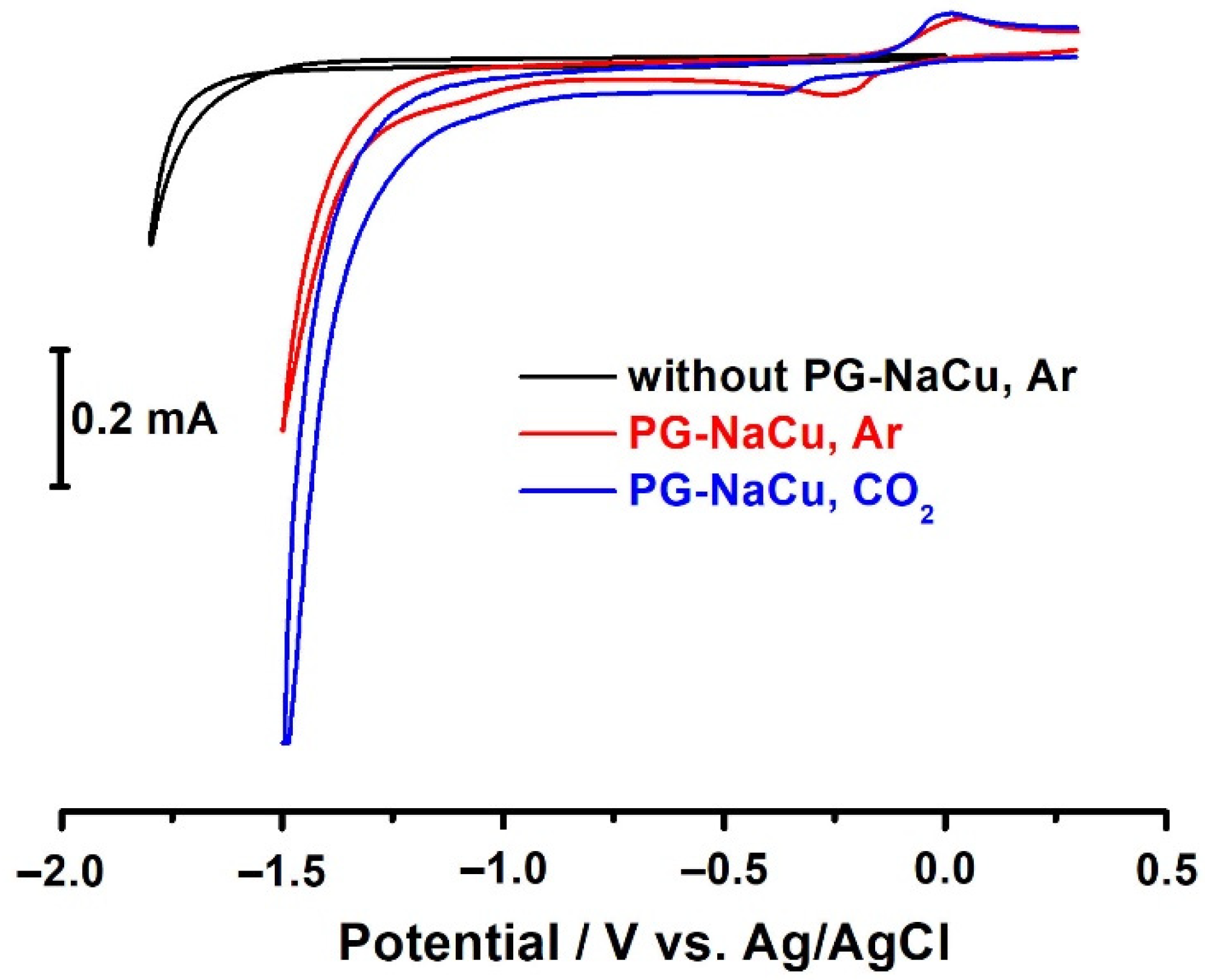
2.2. Electrocatalytic CO2RR Tests Using the PG-NaCu Catalyst
2.3. Infrared Spectroscopy
2.4. Inductively Coupled Plasma Atomic Emission Spectroscopy
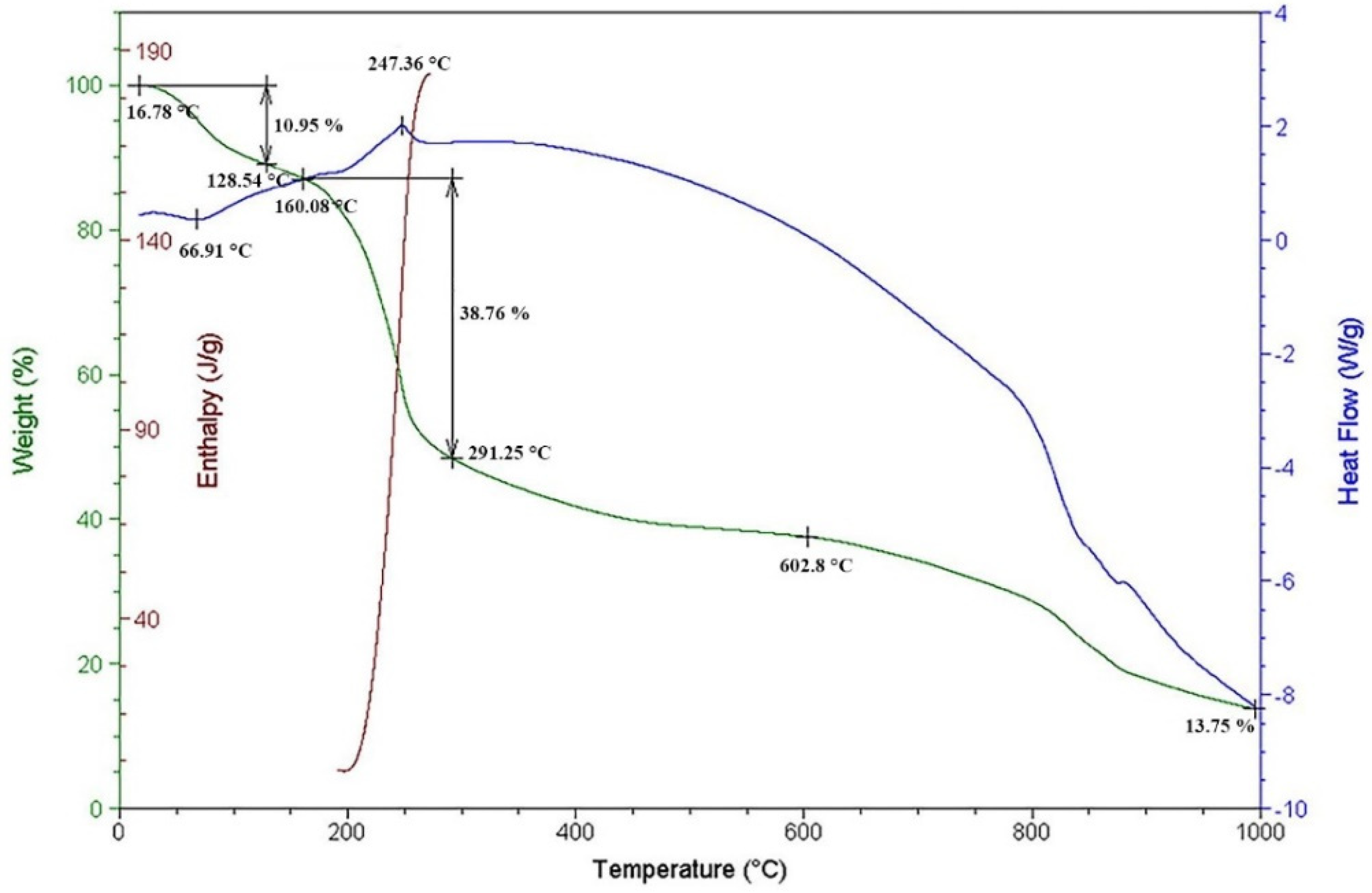
2.5. Thermal Analysis
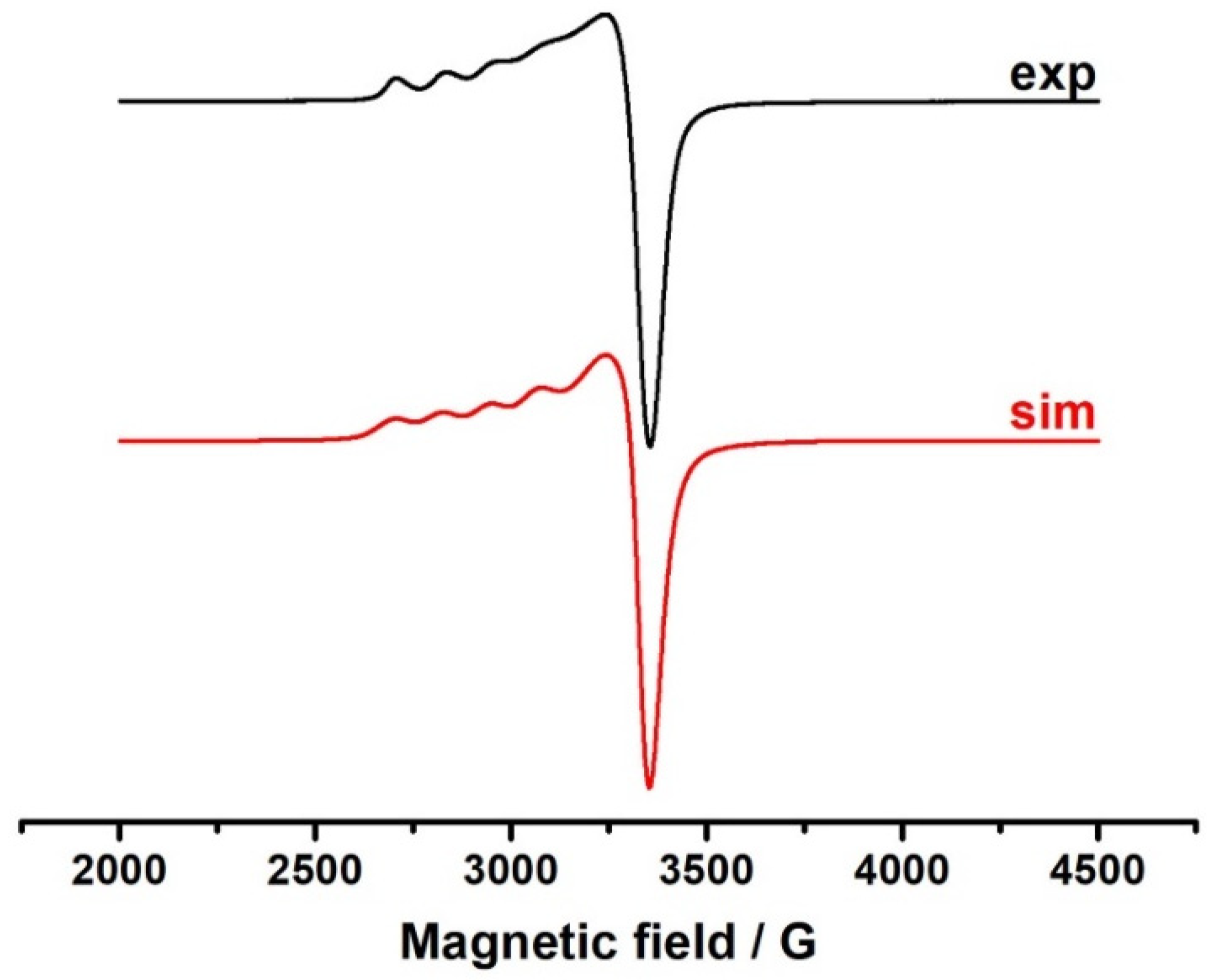
2.6. Electron Spin Resonance Spectroscopy
- g1 = 2.395; aCu = 122 G; ∆H = 80 G
- g2 = 2.096; ∆H = 130 G
- g3 = 2.073; ∆H = 40 G.
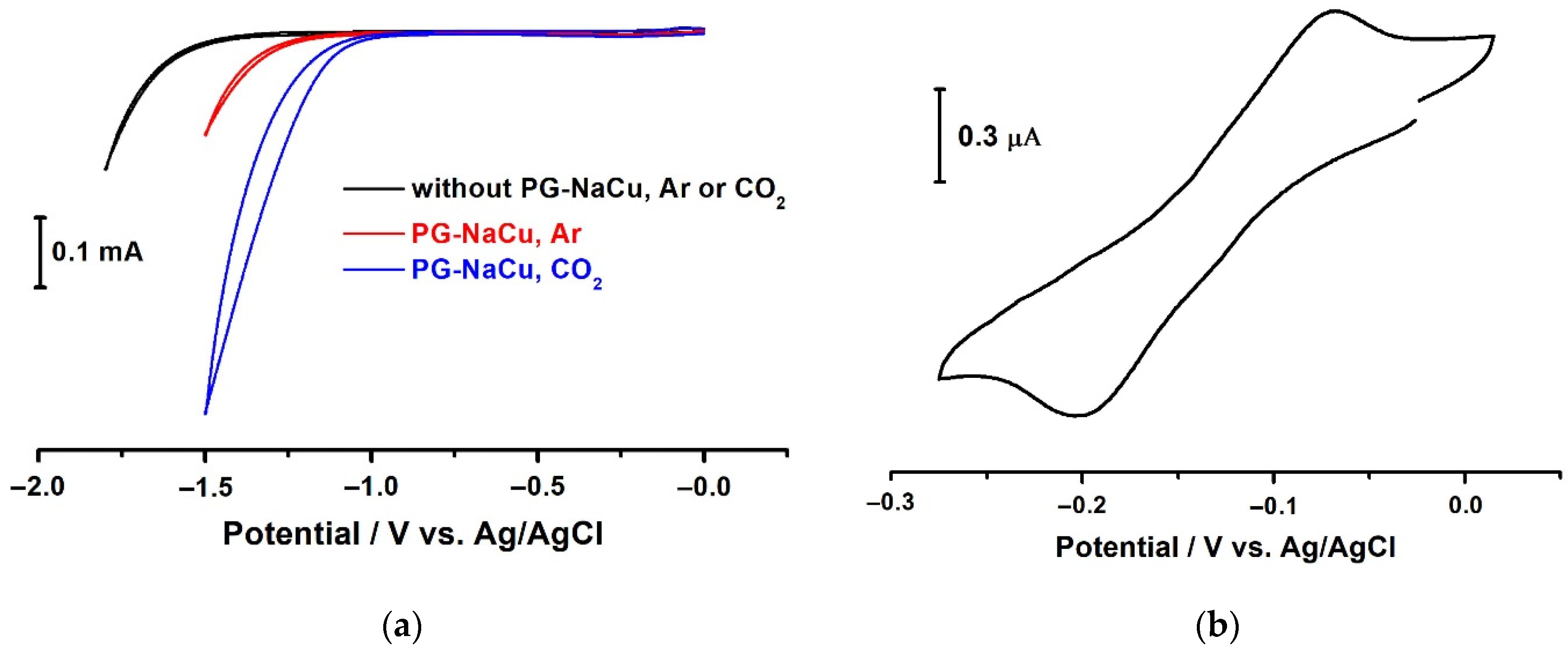
2.7. Electrochemistry in Homogeneous Conditions (PG-NaCu in Water Solution)
- −
- Firstly, we do not observe any adsorption peaks on the cyclic voltammograms, which would indicate copper electrodeposition on the electrode.
- −
- Secondly, we carried out a study of the electrode surface before electrolysis, after 30 min and after 12.5 h of electrolysis in the presence of PG-NaCu (Figure S4) using scanning electron microscopy. The electrode was gently washed with deionized water after electrolysis to remove electrolyte residues and only then microscopy was performed. In cases before electrolysis and after 30 min of electrolysis, the surface turned out to be identical without any particles or films. In the case of 12.5 h electrolysis, the presence of a very small number of nanoparticles on the electrode was found. Moreover, energy-dispersive X-ray spectroscopy showed no copper on the electrode surface in all the cases (in the case of 12.5 h of electrolysis, the amount of copper may have been below the sensitivity threshold) (Figure S5).
- −
- Thirdly, we observe a similar catalytic activity of the copper complex under heterogeneous conditions (as will be shown below), where the formation of copper or copper oxide particles or films is unlikely.
2.8. Electrochemistry in Heterogeneous Conditions (PG-NaCu in Solid Composite of Carbon Paste Electrode)
3. Materials and Methods
3.1. Synthesis of the Sodium Pectate Complex with Copper
3.2. Fourier-Transform Infrared Spectroscopy
3.3. Inductively Coupled Plasma Optical Emission Spectroscopy
3.4. Thermal Analysis
3.5. Electron Spin Resonance
3.6. Electrochemistry
3.7. Scanning Electron Microscopy
3.8. Gas Chromatography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Hori, Y.; Wakebe, H.; Tsukamoto, T.; Koga, O. Electrocatalytic process of CO selectivity in electrochemical reduction of CO2 at metal electrodes in aqueous media. Electrochim. Acta 1994, 39, 1833–1839. [Google Scholar] [CrossRef]
- Jitaru, M.; Lowy, D.; Toma, M.; Toma, B.C.; Oniciu, L. Electrochemical reduction of carbon dioxide on flat metallic cathodes. J. Appl. Electrochem. 1997, 27, 875–889. [Google Scholar] [CrossRef]
- Hori, Y.; Suzuki, S. Electrolytic Reduction of Carbon Dioxide at Mercury Electrode in Aqueous Solution. Bull. Chem. Soc. Jpn. 1982, 55, 660–665. [Google Scholar] [CrossRef] [Green Version]
- Hori, Y. Electrochemical CO2 reduction on metal electrodes. In Modern Aspects of Electrochemistry; Vayenas, C.G., White, R.E., Gamboa-Aldeco, M.E., Eds.; Springer: New York, NY, USA, 2008; Volume 42, pp. 89–189. [Google Scholar] [CrossRef]
- Benson, E.E.; Kubiak, C.P.; Sathrum, A.J.; Smieja, J.M. Electrocatalytic and homogeneous approaches to conversion of CO2 to liquid fuels. Chem. Soc. Rev. 2008, 38, 89–99. [Google Scholar] [CrossRef]
- Elouarzaki, K.; Kannan, V.; Jose, V.; Sabharwal, H.S.; Lee, J. Recent Trends, Benchmarking, and Challenges of Electrochemical Reduction of CO2 by Molecular Catalysts. Adv. Energy Mater. 2019, 9, 1900090. [Google Scholar] [CrossRef]
- Liu, D.-C.; Zhong, D.-C.; Lu, T.-B. Non-noble metal-based molecular complexes for CO2 reduction: From the ligand design perspective. EnergyChem 2020, 2, 100034. [Google Scholar] [CrossRef]
- Takeda, H.; Cometto, C.; Ishitani, O.; Robert, M. Electrons, Photons, Protons and Earth-Abundant Metal Complexes for Molecular Catalysis of CO2 Reduction. ACS Catal. 2016, 7, 70–88. [Google Scholar] [CrossRef]
- Walsh, J.J.; Neri, G.; Smith, C.L.; Cowan, A.J. Water-Soluble Manganese Complex for Selective Electrocatalytic CO2 Reduction to CO. Organometallics 2018, 38, 1224–1229. [Google Scholar] [CrossRef]
- Nakada, A.; Ishitani, O. Selective Electrocatalysis of a Water-Soluble Rhenium(I) Complex for CO2 Reduction Using Water as an Electron Donor. ACS Catal. 2017, 8, 354–363. [Google Scholar] [CrossRef]
- Wang, J.; Gan, L.; Zhang, Q.; Reddu, V.; Peng, Y.; Liu, Z.; Xia, X.; Wang, C.; Wang, X. A Water-Soluble Cu Complex as Molecular Catalyst for Electrocatalytic CO2 Reduction on Graphene-Based Electrodes. Adv. Energy Mater. 2018, 9, 1803151. [Google Scholar] [CrossRef]
- Costentin, C.; Robert, M.; Savéant, J.-M.; Tatin, A. Efficient and selective molecular catalyst for the CO2-to-CO electrochemical conversion in water. Proc. Natl. Acad. Sci. USA 2015, 112, 6882–6886. [Google Scholar] [CrossRef] [Green Version]
- Beley, M.; Collin, J.P.; Ruppert, R.; Sauvage, J.P. Electrocatalytic reduction of carbon dioxide by nickel cyclam2+ in water: Study of the factors affecting the efficiency and the selectivity of the process. J. Am. Chem. Soc. 1986, 108, 7461–7467. [Google Scholar] [CrossRef] [PubMed]
- Kang, P.; Meyer, T.J.; Brookhart, M. Selective electrocatalytic reduction of carbon dioxide to formate by a water-soluble iridium pincer catalyst. Chem. Sci. 2013, 4, 3497–3502. [Google Scholar] [CrossRef]
- Minzanova, S.; Mironov, V.; Vyshtakalyuk, A.; Tsepaeva, O.; Mironova, L.; Mindubaev, A.; Nizameev, I.; Kholin, K.; Milyukov, V. Complexation of pectin with macro- and microelements. Antianemic activity of Na, Fe and Na, Ca, Fe complexes. Carbohydr. Polym. 2015, 134, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Selvarengan, P.; Kubicki, J.; Guégan, J.-P.; Châtellier, X. Complexation of carboxyl groups in bacterial lipopolysaccharides: Interactions of H+, Mg2+, Ca2+, Cd2+, and UO22+ with Kdo and galacturonate molecules via quantum mechanical calculations and NMR spectroscopy. Chem. Geol. 2010, 273, 55–75. [Google Scholar] [CrossRef]
- Minzanova, S.T.; Mironov, V.F.; Mironova, L.G.; Nizameev, I.R.; Kholin, K.; Voloshina, A.D.; Kulik, N.V.; Nazarov, N.G.; Milyukov, V.A. Synthesis, Properties, and Antimicrobial Activity of Pectin Complexes with Cobalt and Nickel. Chem. Nat. Compd. 2016, 52, 26–31. [Google Scholar] [CrossRef]
- Cescutti, P.; Rizzo, R. Divalent cation interactions with oligogalacturonides. J. Agric. Food Chem. 2001, 49, 3262–3267. [Google Scholar] [CrossRef] [PubMed]
- Kuzmann, E.; Garg, V.; De Oliveira, A.; Klencsar, Z.; Szentmihályi, K.; Fodor, J.; May, Z.; Homonnay, Z. Mössbauer study of the effect of pH on Fe valence in iron–polygalacturonate as a medicine for human anaemia. Radiat. Phys. Chem. 2014, 107, 195–198. [Google Scholar] [CrossRef]
- Assifaoui, A.; Lerbret, A.; Huynh, U.T.D.; Neiers, F.; Chambin, O.; Loupiac, C.; Cousin, F.; Uyen, H.T.D. Structural behaviour differences in low methoxy pectin solutions in the presence of divalent cations (Ca2+ and Zn2+): A process driven by the binding mechanism of the cation with the galacturonate unit. Soft Matter 2015, 11, 551–560. [Google Scholar] [CrossRef]
- Strmečki, S.; Dautović, J.; Plavšić, M. Constant current chronopotentiometric stripping characterisation of organic matter in seawater from the northern Adriatic, Croatia. Environ. Chem. 2014, 11, 158. [Google Scholar] [CrossRef]
- Strmečki, S.; Plavšić, M. Adsorptive transfer chronopotentiometric stripping of sulphated polysaccharides. Electrochem. Commun. 2012, 18, 100–103. [Google Scholar] [CrossRef]
- Strmečki, S.; Plavšić, M.; Ćosović, B.; Ostatná, V.; Paleček, E. Constant current chronopotentiometric stripping of sulphated polysaccharides. Electrochem. Commun. 2009, 11, 2032–2035. [Google Scholar] [CrossRef]
- Paleček, E.; Římánková, L. Chitosan catalyzes hydrogen evolution at mercury electrodes. Electrochem. Commun. 2014, 44, 59–62. [Google Scholar] [CrossRef]
- Wang, X.; Klingan, K.; Klingenhof, M.; Möller, T.; de Araújo, J.F.; Martens, I.; Bagger, A.; Jiang, S.; Rossmeisl, J.; Dau, H.; et al. Morphology and mechanism of highly selective Cu(II) oxide nanosheet catalysts for carbon dioxide electroreduction. Nat. Commun. 2021, 12, 794. [Google Scholar] [CrossRef] [PubMed]
- De Luna, P.; Quintero-Bermudez, R.; Dinh, C.-T.; Ross, M.B.; Bushuyev, O.S.; Todorović, P.; Regier, T.; Kelley, S.O.; Yang, P.; Sargent, E.H. Catalyst electro-redeposition controls morphology and oxidation state for selective carbon dioxide reduction. Nat. Catal. 2018, 1, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Van Oversteeg, C.H.; Rosales, M.T.; Helfferich, K.H.; Ghiasi, M.; Meeldijk, J.D.; Firet, N.J.; Ngene, P.; Donegá, C.D.M.; de Jongh, P.E. Copper sulfide derived nanoparticles supported on carbon for the electrochemical reduction of carbon dioxide. Catal. Today 2020, 377, 157–165. [Google Scholar] [CrossRef]
- Guan, A.; Chen, Z.; Quan, Y.; Peng, C.; Wang, Z.; Sham, T.-K.; Yang, C.; Ji, Y.; Qian, L.; Xu, X.; et al. Boosting CO2 Electroreduction to CH4 via Tuning Neighboring Single-Copper Sites. ACS Energy Lett. 2020, 5, 1044–1053. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, L.; Gu, Y.; Zhang, W.; Pan, Y.; Fang, W.; Ma, J.; Lan, Y.-Q.; Bai, J. Formation of a mixed-valence Cu(i)/Cu(ii) metal–organic framework with the full light spectrum and high selectivity of CO2 photoreduction into CH4. Chem. Sci. 2020, 11, 10143–10148. [Google Scholar] [CrossRef] [PubMed]
- Angamuthu, R.; Byers, P.; Lutz, M.; Spek, A.L.; Bouwman, E. Electrocatalytic CO2 Conversion to Oxalate by a Copper Complex. Science 2010, 327, 313–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weng, Z.; Jiang, J.; Wu, Y.; Wu, Z.; Guo, X.; Materna, K.L.; Liu, W.; Batista, V.S.; Brudvig, G.W.; Wang, H. Electrochemical CO2 Reduction to Hydrocarbons on a Heterogeneous Molecular Cu Catalyst in Aqueous Solution. J. Am. Chem. Soc. 2016, 138, 8076–8079. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Z.; Han, P.; Du, Y.; Gu, Z.; Xu, X.; Zheng, G. Single-Atomic Cu with Multiple Oxygen Vacancies on Ceria for Electrocatalytic CO2 Reduction to CH4. ACS Catal. 2018, 8, 7113–7119. [Google Scholar] [CrossRef]
- Francke, R.; Schille, B.; Roemelt, M. Homogeneously Catalyzed Electroreduction of Carbon Dioxide—Methods, Mechanisms, and Catalysts. Chem. Rev. 2018, 118, 4631–4701. [Google Scholar] [CrossRef] [PubMed]
- De Gregorio, G.L.; Burdyny, T.; Loiudice, A.; Iyengar, P.; Smith, W.A.; Buonsanti, R. Facet-Dependent Selectivity of Cu Catalysts in Electrochemical CO2 Reduction at Commercially Viable Current Densities. ACS Catal. 2020, 10, 4854–4862. [Google Scholar] [CrossRef] [Green Version]
- Assifaoui, A.; Loupiac, C.; Chambin, O.; Cayot, P. Structure of calcium and zinc pectinate films investigated by FTIR spectroscopy. Carbohydr. Res. 2010, 345, 929–933. [Google Scholar] [CrossRef]
- Chatjigakis, A.; Pappas, C.; Proxenia, N.; Kalantzi, O.; Rodis, P.; Polissiou, M. FT-IR spectroscopic determination of the degree of esterification of cell wall pectins from stored peaches and correlation to textural changes. Carbohydr. Polym. 1998, 37, 395–408. [Google Scholar] [CrossRef]
- Synytsya, A. Fourier transform Raman and infrared spectroscopy of pectins. Carbohydr. Polym. 2003, 54, 97–106. [Google Scholar] [CrossRef]
- Minzanova, S.T.; Khamatgalimov, A.R.; Ryzhkina, I.S.; Murtazina, L.I.; Mironova, L.G.; Kadirov, M.K.; Vyshtakalyuk, A.B.; Milyukov, V.A.; Mironov, V.F. Synthesis and physicochemical properties of antianemic iron and calcium complexes with sodium polygalacturonate. Dokl. Phys. Chem. 2016, 467, 45–48. [Google Scholar] [CrossRef]
- Aburto, J.; Moran, M.; Galano, A.; Torres-García, E. Non-isothermal pyrolysis of pectin: A thermochemical and kinetic approach. J. Anal. Appl. Pyrolysis 2015, 112, 94–104. [Google Scholar] [CrossRef]
- Rezvanian, M.; Ahmad, N.; Amin, M.C.I.M.; Ng, S.-F. Optimization, characterization, and in vitro assessment of alginate-pectin ionic cross-linked hydrogel film for wound dressing applications. Int. J. Biol. Macromol. 2017, 97, 131–140. [Google Scholar] [CrossRef]
- Gryaznova, T.V.; Kholin, K.V.; Nikanshina, E.O.; Khrizanforova, V.; Strekalova, S.O.; Fayzullin, R.R.; Budnikova, Y.H. Copper or Silver-Mediated Oxidative C(sp2)–H/N–H Cross-Coupling of Phthalimide and Heterocyclic Arenes: Access to N-Arylphthalimides. Organometallics 2019, 38, 3617–3628. [Google Scholar] [CrossRef]
- Walsh, J.J.; Smith, C.L.; Neri, G.; Whitehead, G.; Robertson, C.; Cowan, A.J. Improving the efficiency of electrochemical CO2 reduction using immobilized manganese complexes. Faraday Discuss. 2015, 183, 147–160. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Vilar, R.; Eugénio, S.; Grondin, J.; Danten, Y. Electrodeposition of copper thin films from 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide. J. Appl. Electrochem. 2014, 45, 87–93. [Google Scholar] [CrossRef]
- Weng, Z.; Wu, Y.; Wang, M.; Jiang, J.; Yang, K.; Huo, S.; Wang, X.-F.; Ma, Q.; Brudvig, G.W.; Batista, V.S.; et al. Active sites of copper-complex catalytic materials for electrochemical carbon dioxide reduction. Nat. Commun. 2018, 9, 415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Z.; Yu, F.; Yang, Y.; Leung, C.-F.; Ng, S.-M.; Ko, C.-C.; Cometto, C.; Lau, T.; Robert, M. Photocatalytic Conversion of CO2 to CO by a Copper(II) Quaterpyridine Complex. ChemSusChem 2017, 10, 4009–4013. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.-X.; Liu, W.-X.; Wang, C.-L.; Zhan, S.-Z.; Wu, S.-P. A bis(thiosemicarbazonato)-copper complex, a new catalyst for electro- and photo-reduction of CO2 to methanol. New J. Chem. 2020, 44, 2721–2726. [Google Scholar] [CrossRef]
- Khrizanforov, M.N.; Arkhipova, D.M.; Shekurov, R.; Gerasimova, T.; Ermolaev, V.; Islamov, D.R.; Miluykov, V.A.; Kataeva, O.N.; Khrizanforova, V.; Sinyashin, O.; et al. Novel paste electrodes based on phosphonium salt room temperature ionic liquids for studying the redox properties of insoluble compounds. J. Solid State Electrochem. 2015, 19, 2883–2890. [Google Scholar] [CrossRef]
- Gryaznova, T.; Dudkina, Y.; Khrizanforov, M.; Sinyashin, O.; Kataeva, O.; Budnikova, Y. Electrochemical properties of diphosphonate-bridged palladacycles and their reactivity in arene phosphonation. J. Solid State Electrochem. 2015, 19, 2665–2672. [Google Scholar] [CrossRef]
- Fedorenko, S.; Grechkina, S.L.; Mustafina, A.R.; Kholin, K.; Stepanov, A.S.; Nizameev, I.R.; Ismaev, I.E.; Kadirov, M.K.; Zairov, R.R.; Fattakhova, A.N.; et al. Tuning the non-covalent confinement of Gd(III) complexes in silica nanoparticles for high T1-weighted MR imaging capability. Colloids Surf. B Biointerfaces 2017, 149, 243–249. [Google Scholar] [CrossRef]
- Kadirov, M.K.; Budnikova, Y.G.; Kholin, K.; Valitov, M.I.; Krasnov, S.A.; Gryaznova, T.V.; Sinyashin, O. Spin-adduct of the P4·− radical anion during the electrochemical reduction of white phosphorus. Russ. Chem. Bull. 2010, 59, 466–468. [Google Scholar] [CrossRef]
- Khrizanforova, V.V.; Kholin, K.V.; Khrizanforov, M.N.; Kadirov, M.K.; Budnikova, Y.H. Electrooxidative CH/PH functionalization as a novel way to synthesize benzo[b]phosphole oxides mediated by catalytic amounts of silver acetate. New J. Chem. 2017, 42, 930–935. [Google Scholar] [CrossRef] [Green Version]
- Budnikova, Y.G.; Gryaznova, T.V.; Kadirov, M.K.; Tret’yakov, E.V.; Kholin, K.; Ovcharenko, V.I.; Sagdeev, R.Z.; Sinyashin, O. Electrochemistry of nitronyl and imino nitroxides. Russ. J. Phys. Chem. A 2009, 83, 1976–1980. [Google Scholar] [CrossRef]
- Kadirov, M.; Tretyakov, E.; Budnikova, Y.; Valitov, M.; Holin, K.; Gryaznova, T.; Ovcharenko, V.; Sinyashin, O. Electrochemistry of the sterically hindered imidazolidine zwitterion and its paramagnetic derivative. J. Electroanal. Chem. 2008, 624, 69–72. [Google Scholar] [CrossRef]
- Kadirov, M.K.; Tret’yakov, E.V.; Budnikova, Y.G.; Kholin, K.; Valitov, M.I.; Vavilova, V.N.; Ovcharenko, V.I.; Sagdeev, R.Z.; Sinyashin, O. Cyclic voltammetry of nitronyl- and iminonitroxyls detected by electron spin resonance. Russ. J. Phys. Chem. A 2009, 83, 2163–2169. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kholin, K.V.; Khrizanforov, M.N.; Babaev, V.M.; Nizameeva, G.R.; Minzanova, S.T.; Kadirov, M.K.; Budnikova, Y.H. A Water-Soluble Sodium Pectate Complex with Copper as an Electrochemical Catalyst for Carbon Dioxide Reduction. Molecules 2021, 26, 5524. https://doi.org/10.3390/molecules26185524
Kholin KV, Khrizanforov MN, Babaev VM, Nizameeva GR, Minzanova ST, Kadirov MK, Budnikova YH. A Water-Soluble Sodium Pectate Complex with Copper as an Electrochemical Catalyst for Carbon Dioxide Reduction. Molecules. 2021; 26(18):5524. https://doi.org/10.3390/molecules26185524
Chicago/Turabian StyleKholin, Kirill V., Mikhail N. Khrizanforov, Vasily M. Babaev, Guliya R. Nizameeva, Salima T. Minzanova, Marsil K. Kadirov, and Yulia H. Budnikova. 2021. "A Water-Soluble Sodium Pectate Complex with Copper as an Electrochemical Catalyst for Carbon Dioxide Reduction" Molecules 26, no. 18: 5524. https://doi.org/10.3390/molecules26185524
APA StyleKholin, K. V., Khrizanforov, M. N., Babaev, V. M., Nizameeva, G. R., Minzanova, S. T., Kadirov, M. K., & Budnikova, Y. H. (2021). A Water-Soluble Sodium Pectate Complex with Copper as an Electrochemical Catalyst for Carbon Dioxide Reduction. Molecules, 26(18), 5524. https://doi.org/10.3390/molecules26185524