Bile Salts in Chiral Micellar Electrokinetic Chromatography: 2000–2020
Abstract
1. Introduction
2. Aggregation Behavior and Mechanism of Chiral Recognition of Bile Salts
3. General Considerations on Chiral MEKC with Bile Salts
4. Method Development
4.1. Bile Salts as Sole CS
4.2. Bile Salts with Another CS
4.3. Advanced Techniques
5. Applications to Real Sample Analyses
6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bernardo-Bermejo, S.; Sánchez-López, E.; Castro-Puyana, M.; Marina, M.L. Chiral capillary electrophoresis. Trends Anal. Chem. 2020, 124, 115807. [Google Scholar] [CrossRef]
- Deeb, S.E.; Silva, C.F.; Junior, C.S.N.; Hanafi, R.S.; Borges, K.B. Chiral capillary electrokinetic chromatography: Principle and applications, detection and identification, design of experiment, and exploration of chiral recognition using molecular modeling. Molecules 2021, 26, 2841. [Google Scholar] [CrossRef]
- Guo, C.; Xiao, Y. Negatively charged cyclodextrins: Synthesis and applications in chiral analysis—A review. Carbohydr. Polym. 2021, 256, 117517. [Google Scholar] [CrossRef] [PubMed]
- Hancu, G.; Papp, L.A.; Tóth, G.; Kelemen, H. The use of dual cyclodextrin chiral selector systems in the enantioseparation of pharmaceuticals by capillary electrophoresis: An overview. Molecules 2021, 26, 2261. [Google Scholar] [CrossRef]
- Peluso, P.; Chankvetadze, B. Native and substituted cyclodextrins as chiral selectors for capillary electrophoresis enantioseparations: Structures, features, application, and molecular modeling. Electrophoresis 2021. [Google Scholar] [CrossRef]
- Ranasinghe, M.; Quirino, J.P. Can we replace liquid chromatography with the greener capillary electrophoresis? Curr. Opin. Green Sustain. Chem. 2021, 31, 100515. [Google Scholar] [CrossRef]
- Yu, R.B.; Quirino, J.P. Chiral selectors in capillary electrophoresis: Trends during 2017–2018. Molecules 2019, 24, 1135. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.B.; Quirino, J.P. Ionic liquids in electrokinetic chromatography. J. Chromatogr. A 2021, 1637. [Google Scholar] [CrossRef] [PubMed]
- Terabe, S.; Otsuka, K.; Ichikawa, K.; Tsuchiya, A.; Ando, T. Electrokinetic Separations with Micellar Solutions and Open-Tubular Capillaries. Anal. Chem. 1984, 56, 111–113. [Google Scholar] [CrossRef]
- Terabe, S.; Shibata, M.; Miyashita, Y. Chiral separation by electrokinetic chromatography with bile salt micelles. J. Chromatogr. A 1989, 480, 403–411. [Google Scholar] [CrossRef]
- Nishi, H.; Fukuyama, T.; Matsuo, M.; Terabe, S. Chiral separation of optical isomeric drugs using micellar electrokinetic chromatography and bile salts. J. Microcolumn Sep. 1989, 1, 234–241. [Google Scholar] [CrossRef]
- Coreta-Gomes, F.M.; Vaz, W.L.C.; Wasielewski, E.; Geraldes, C.F.G.; Moreno, M.J. Quantification of Cholesterol Solubilized in Dietary Micelles: Dependence on Human Bile Salt Variability and the Presence of Dietary Food Ingredients. Langmuir 2016, 32, 4564–4574. [Google Scholar] [CrossRef] [PubMed]
- Haustein, M.; Wahab, M.; Mögel, H.J.; Schiller, P. Vesicle Solubilization by Bile Salts: Comparison of Macroscopic Theory and Simulation. Langmuir 2015, 31, 4078–4086. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Yang, K.; Fan, L.; Lv, Y.; Jin, Z.; Zhu, S.; Qin, C.; Wang, Y.; Yin, L. Denatured globular protein and bile salt-coated nanoparticles for poorly water-soluble drugs: Penetration across the intestinal epithelial barrier into the circulation system and enhanced oral bioavailability. Int. J. Pharm. 2015, 495, 9–18. [Google Scholar] [CrossRef]
- Pavlović, N.; Goločorbin-Kon, S.; Ðanić, M.; Stanimirov, B.; Al-Salami, H.; Stankov, K.; Mikov, M. Bile Acids and Their Derivatives as Potential Modifiers of Drug Release and Pharmacokinetic Profiles. Front. Pharmacol. 2018, 9, 1283. [Google Scholar] [CrossRef]
- Eckenroad, K.W.; Manley, G.A.; Yehl, J.B.; Pirnie, R.T.; Strein, T.G.; Rovnyak, D. An Edge Selection Mechanism for Chirally Selective Solubilization of Binaphthyl Atropisomeric Guests by Cholate and Deoxycholate Micelles. Chirality 2016, 28, 525–533. [Google Scholar] [CrossRef]
- Meier, A.R.; Yehl, J.B.; Eckenroad, K.W.; Manley, G.A.; Strein, T.G.; Rovnyak, D. Stepwise Aggregation of Cholate and Deoxycholate Dictates the Formation and Loss of Surface-Available Chirally Selective Binding Sites. Langmuir 2018, 34, 6489–6501. [Google Scholar] [CrossRef]
- Patel, V.; Shamsi, S.A. Carbohydrate-based polymeric surfactants for chiral micellar electrokinetic chromatography (CMEKC) coupled to mass spectrometry. In Methods in Molecular Biology; Humana Press, Inc.: New York, NY, USA, 2019; Volume 1985, pp. 417–444. [Google Scholar]
- He, J.; Shamsi, S.A. Application of polymeric surfactants in chiral micellar electrokinetic chromatography (CMEKC) and cmekc coupled to mass spectrometry. In Methods in Molecular Biology; Humana Press, Inc.: Totowa, NJ, USA, 2013; Volume 970, pp. 319–348. [Google Scholar]
- Palmer, C.P. Recent progress in the development, characterization and application of polymeric pseudophases for electrokinetic chromatography. Electrophoresis 2002, 23, 3993–4004. [Google Scholar] [CrossRef]
- Palmer, C.P. Electrokinetic chromatography with polymeric pseudostationary phases. J. Sep. Sci. 2008, 31, 783–793. [Google Scholar] [CrossRef]
- Yarabe, H.H.; Billiot, E.; Warner, I.M. Enantiomeric separations by use of polymeric surfactant electrokinetic chromatography. J. Chromatogr. A 2000, 875, 179–206. [Google Scholar] [CrossRef]
- El Rassi, Z. Chiral glycosidic surfactants for enantiomeric separation in capillary electrophoresis. J. Chromatogr. A 2000, 875, 207–233. [Google Scholar] [CrossRef]
- Otsuka, K.; Terabe, S. Enantiomer separation of drugs by micellar electrokinetic chromatography using chiral surfactants. J. Chromatogr. A 2000, 875, 163–178. [Google Scholar] [CrossRef]
- Madenci, D.; Egelhaaf, S.U. Self-assembly in aqueous bile salt solutions. Curr. Opin. Colloid Interface Sci. 2010, 15, 109–115. [Google Scholar] [CrossRef]
- Ninomiya, R.; Matsuoka, K.; Moroi, Y. Micelle formation of sodium chenodeoxycholate and solubilization into the micelles: Comparison with other unconjugated bile salts. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2003, 1634, 116–125. [Google Scholar] [CrossRef]
- Kumar, K.; Patial, B.S.; Chauhan, S. Conductivity and fluorescence studies on the micellization properties of sodium cholate and sodium deoxycholate in aqueous medium at different temperatures: Effect of selected amino acids. J. Chem. Thermodyn. 2015, 82, 25–33. [Google Scholar] [CrossRef]
- Meyerhoffer, S.M.; McGown, L.B. Critical micelle concentration behavior of sodium taurocholate in water. Langmuir 1990, 6, 187–191. [Google Scholar] [CrossRef]
- Matsuoka, K.; Yamamoto, A. Study on Micelle Formation of Bile Salt Using Nuclear Magnetic Resonance Spectroscopy. J. Oleo Sci. 2017, 66, 1129–1137. [Google Scholar] [CrossRef]
- Qiu, S.M.; Wen, G.; Hirakawa, N.; Soloway, R.D.; Hong, N.K.; Crowther, R.S. Glycochenodeoxycholic acid inhibits calcium phosphate precipitation in vitro by preventing the transformation of amorphous calcium phosphate to calcium hydroxyapatite. J. Clin. Investig. 1991, 88, 1265–1271. [Google Scholar] [CrossRef] [PubMed]
- Small, D.M. Size and Structure of Bile Salt Micelles. In Molecular Association in Biological and Related Systems; American Chemical Society: Washington, DC, USA, 1968; Volume 84, pp. 31–52. [Google Scholar]
- Hebling, C.M.; Thompson, L.E.; Eckenroad, K.W.; Manley, G.A.; Fry, R.A.; Mueller, K.T.; Strein, T.G.; Rovnyak, D. Sodium Cholate Aggregation and Chiral Recognition of the Probe Molecule (R,S)-1,1′-Binaphthyl-2,2′-diylhydrogenphosphate (BNDHP) Observed by 1H and 31P NMR Spectroscopy. Langmuir 2008, 24, 13866–13874. [Google Scholar] [CrossRef][Green Version]
- Eckenroad, K.W.; Thompson, L.E.; Strein, T.G.; Rovnyak, D. Proton NMR assignments for R,S-1,1′-binaphthol (BN) and R,S-1,1′-binaphthyl-2,2′-diyl hydrogen phosphate (BNDHP) interacting with bile salt micelles. Magn. Reson. Chem. 2007, 45, 72–75. [Google Scholar] [CrossRef]
- Hu, S.-Q.; Guo, X.-M.; Shi, H.-J.; Luo, R.-J. Separation mechanisms for palonosetron stereoisomers at different chiral selector concentrations in MEKC. Electrophoresis 2015, 36, 825–829. [Google Scholar] [CrossRef] [PubMed]
- Cabral, D.J.; Hamilton, J.A.; Small, D.M. The ionization behavior of bile acids in different aqueous environments. J. Lipid Res. 1986, 27, 334–343. [Google Scholar] [CrossRef]
- Asztemborska, M.; Miśkiewicz, M.; Sybilska, D. Separation of some chiral flavanones by micellar electrokinetic chromatography. Electrophoresis 2003, 24, 2527–2531. [Google Scholar] [CrossRef] [PubMed]
- Tian, K.; Chen, H.; Tang, J.; Chen, X.; Hu, Z. Enantioseparation of palonosetron hydrochloride by micellar electrokinetic chromatography with sodium cholate as chiral selector. J. Chromatogr. A 2006, 1132, 333–336. [Google Scholar] [CrossRef]
- Guo, X.; Liu, Q.; Hu, S.; Guo, W.; Yang, Z.; Zhang, Y. Thermodynamic models to elucidate the enantioseparation of drugs with two stereogenic centers by micellar electrokinetic chromatography. J. Chromatogr. A 2017, 1512, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Trapp, O. A dynamic molecular probe to investigate catalytic effects and Joule heating in enantioselective MEKC. Electrophoresis 2007, 28, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Schoetz, G.; Trapp, O.; Schurig, V. Dynamic Micellar Electrokinetic Chromatography. Determination of the Enantiomerization Barriers of Oxazepam, Temazepam, and Lorazepam. Anal. Chem. 2000, 72, 2758–2764. [Google Scholar] [CrossRef]
- Wistuba, D.; Trapp, O.; Gel-Moreto, N.; Galensa, R.; Schurig, V. Stereoisomeric Separation of Flavanones and Flavanone-7-O-glycosides by Capillary Electrophoresis and Determination of Interconversion Barriers. Anal. Chem. 2006, 78, 3424–3433. [Google Scholar] [CrossRef]
- Xu, Z.; Xue, T.; He, T. Investigation on the chiral recognition mechanism between verteporfin and cholate salts by capillary electrophoresis. J. Sep. Sci. 2020, 43, 2905–2913. [Google Scholar] [CrossRef]
- Peng, X.; Sternberg, E.; Dolphin, D. Chiral separation of benzoporphyrin derivative mono- and diacids by laser induced fluorescence-capillary electrophoresis. Electrophoresis 2002, 23, 93–101. [Google Scholar] [CrossRef]
- Hu, S.-Q.; Yang, H.-B.; Shi, H.-J.; Zhang, Y.-H.; Yang, Z. Separation of palonosetron stereoisomers by electrokinetic chromatography using sodium cholate as chiral selector: Comparison of separation modes and elucidation of migration orders. Electrophoresis 2013, 34, 3086–3090. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.-Q.; Shi, H.-J.; Yu, H.; Dong, J.; Guo, X.-M. Solvent-modified MEKC for the enantioseparation of palonosetron hydrochloride and related enantiomeric impurities. Electrophoresis 2015, 36, 2762–2767. [Google Scholar] [CrossRef]
- Hu, S.-Q.; Wang, G.-X.; Guo, W.-B.; Guo, X.-M.; Zhao, M. Effect of low concentration sodium dodecyl sulfate on the electromigration of palonosetron hydrochloride stereoisomers in micellar electrokinetic chromatography. J. Chromatogr. A 2014, 1342, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Lin, J.; Xu, L.; Chen, G. Nonaqueous and aqueous-organic media for the enantiomeric separations of neutral organophosphorus pesticides by CE. Electrophoresis 2007, 28, 2758–2764. [Google Scholar] [CrossRef]
- Hefnawy, M.M. Optimization of the Chiral Resolution of Metyrosine by Capillary Electrophoresis and/or Micellar Electrokinetic Capillary Chromatography. J. Liquid Chromatogr. Relat. Technol. 2005, 28, 439–452. [Google Scholar] [CrossRef]
- Hefnawy, M.M. Micellar electrokinetic capillary chromatography determination of (+)S and (−)R arotinolol in serum using UV detection and solid phase extraction. Chirality 2002, 14, 67–71. [Google Scholar] [CrossRef]
- Elshihabi, S.; Black, K.D.; Sutton, J.K.; Woody, K.A.; Burke, J.A., Jr.; Bushey, M.M. Micellar electrokinetic chromatography of tri aza aromatic ligand compounds of iron (II): Influence of bile salt type on enantiomeric separation. Electrophoresis 2001, 22, 3771–3777. [Google Scholar] [CrossRef]
- Okerberg, E.S.; Elshihabi, S.; Carmichael, P.T.; Woody, K.A.; Barckholtz, T.A.; Burke, J.A.; Bushey, M.M. MEKC with bile salt micelles for the enantiomeric separation of bis(8-((pyridine-2-methylene)amino)quinoline)iron(II) hexafluorophosphate: Kinetics and mechanism of the racemization. J. Microcolumn Sep. 2000, 12, 391–397. [Google Scholar] [CrossRef]
- Choy, T.M.H.; Chan, W.-H.; Lee, A.W.M.; Huie, C.W. Stacking and separation of enantiomers by acetonitrile-salt mixtures in micellar electrokinetic chromatography. Electrophoresis 2003, 24, 3116–3123. [Google Scholar] [CrossRef]
- Xu, Z.; Li, A.; Wang, Y.; Chen, Z.; Hirokawa, T. Pressure-assisted electrokinetic injection stacking for verteporfin drug to achieve highly sensitive enantioseparation and detection in artificial urine by capillary electrophoresis. J. Chromatogr. A 2014, 1355, 284–290. [Google Scholar] [CrossRef]
- Lucangioli, S.E.; Hermida, L.G.; Tripodi, V.P.; Rodrĺguez, V.G.; López, E.E.; Rouge, P.D.; Carducci, C.N. Analysis of cis–trans isomers and enantiomers of sertraline by cyclodextrin-modified micellar electrokinetic chromatography. J.Chromatogr. A 2000, 871, 207–215. [Google Scholar] [CrossRef]
- Pérez-Fernández, V.; García, M.Á.; Marina, M.L. Enantiomeric separation of cis-bifenthrin by CD-MEKC: Quantitative analysis in a commercial insecticide formulation. Electrophoresis 2010, 31, 1533–1539. [Google Scholar] [CrossRef]
- Wang, S.; Fan, L.; Cui, S. CE-LIF chiral separation of aspartic acid and glutamic acid enantiomers using human serum albumin and sodium cholate as dual selectors. J. Sep. Sci. 2009, 32, 3184–3190. [Google Scholar] [CrossRef]
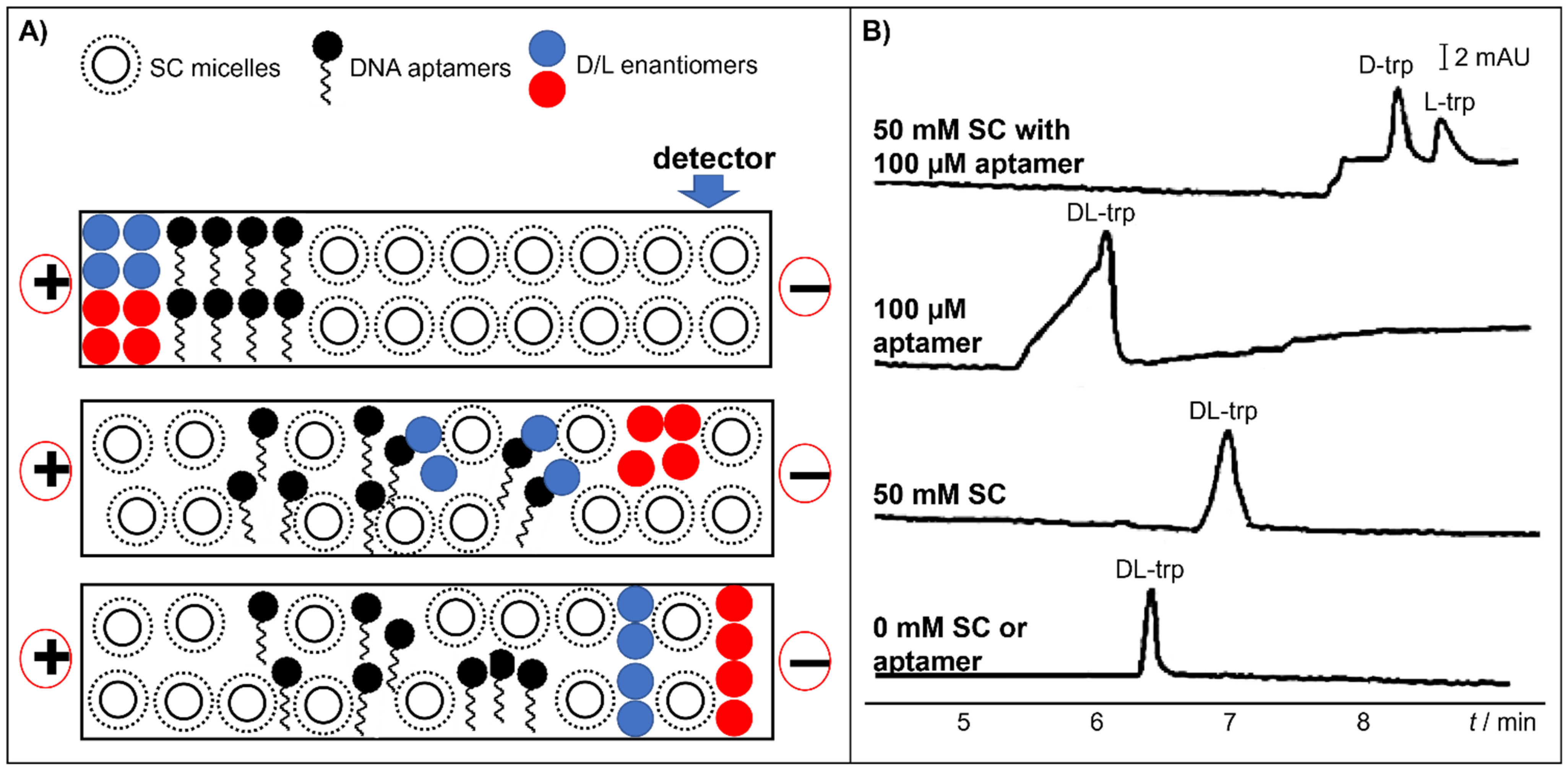
- Huang, R.; Xiong, W.; Wang, D.; Guo, L.; Lin, Z.; Yu, L.; Chu, K.; Qiu, B.; Chen, G. Label-free aptamer-based partial filling technique for enantioseparation and determination of dl-tryptophan with micellar electrokinetic chromatography. Electrophoresis 2013, 34, 254–259. [Google Scholar] [CrossRef]
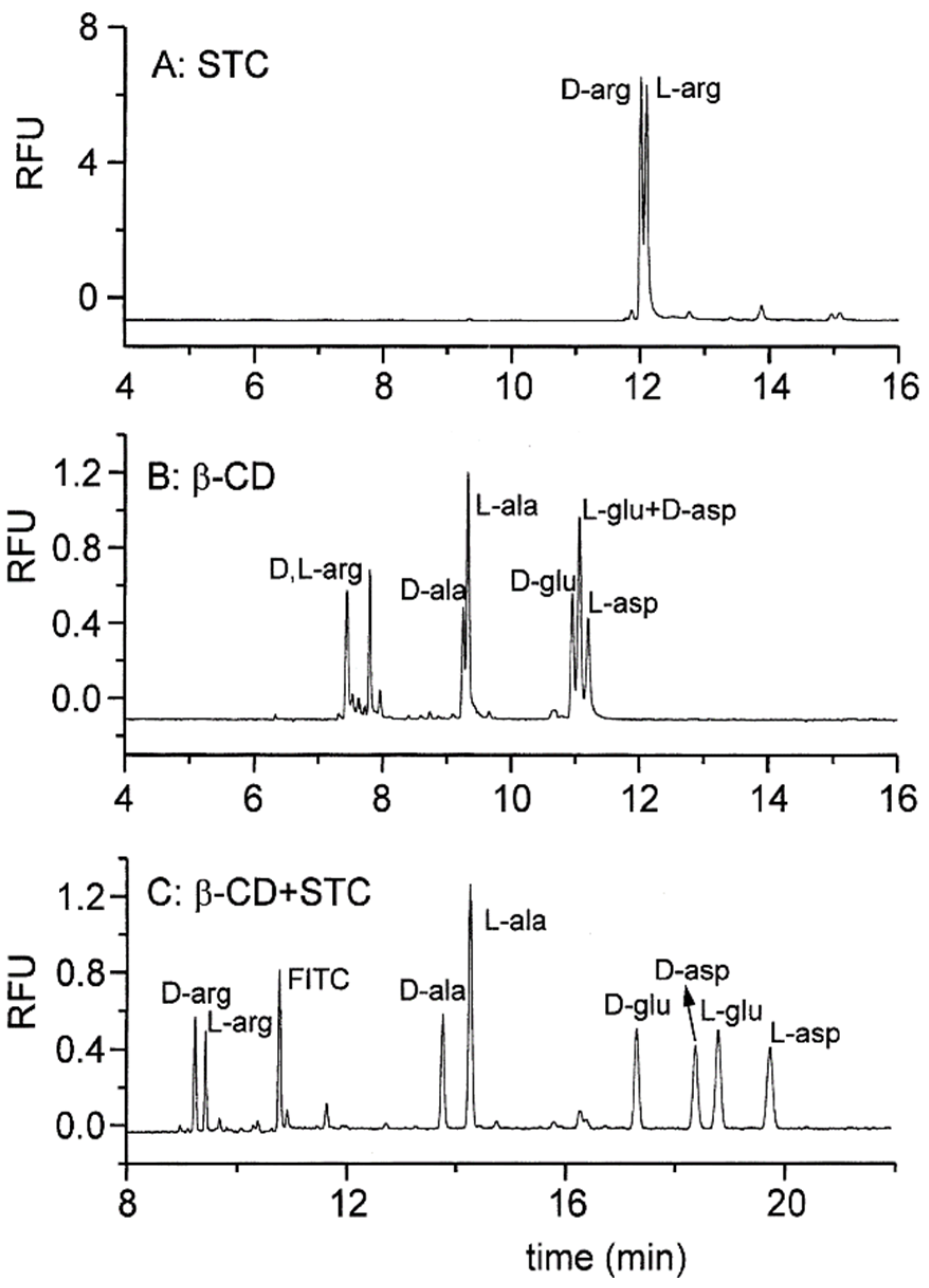
- Lu, X.; Chen, Y. Chiral separation of amino acids derivatized with fluoresceine-5-isothiocyanate by capillary electrophoresis and laser-induced fluorescence detection using mixed selectors of β-cyclodextrin and sodium taurocholate. J. Chromatogr. A 2002, 955, 133–140. [Google Scholar] [CrossRef]
- Yang, L.-l.; Zhang, D.-q.; Yuan, Z.-b. Enantioseparation of o-phthaldiadehyde derivatized amino acids using β-CD-modified micellar electrokinetic chromatography in the mixed aqueous-organic media. Anal. Chim. Acta 2001, 433, 23–30. [Google Scholar] [CrossRef]
- Zhang, T.; Fang, Q.; Du, W.-B.; Fu, J.-L. Microfluidic Picoliter-Scale Translational Spontaneous Sample Introduction for High-Speed Capillary Electrophoresis. Anal. Chem. 2009, 81, 3693–3698. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.Y.; Yang, J.; Gong, M.; Flachsbart, B.R.; Shannon, M.A.; Bohn, P.W.; Sweedler, J.V. Multidimensional Separation of Chiral Amino Acid Mixtures in a Multilayered Three-Dimensional Hybrid Microfluidic/Nanofluidic Device. Anal. Chem. 2009, 81, 2715–2722. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Choi, K.; Ahmed, A.Y.B.H.; Alothman, Z.A.; Chung, D.S. Highly sensitive chiral analysis of amino acids by in-line single drop microextraction and capillary electrophoresis with laser-induced fluorescence detection. Anal. Chim. Acta 2010, 677, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Creamer, J.S.; Mora, M.F.; Willis, P.A. Enhanced Resolution of Chiral Amino Acids with Capillary Electrophoresis for Biosignature Detection in Extraterrestrial Samples. Anal. Chem. 2017, 89, 1329–1337. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Song, Y.; Liu, Y.-M. A novel capillary electrophoresis method for the determination of d-serine in neural samples. Talanta 2005, 67, 212–216. [Google Scholar] [CrossRef]
- Cheng, H.; Zhang, Q.; Tu, Y. Separation of Fat-soluble Isoquinoline Enantiomers using β-Cyclodextrin-modified Micellar Capillary Electrokinetic Chromatography. Curr. Pharm. Anal. 2012, 8, 37–43. [Google Scholar] [CrossRef]
- Chen, D.; Chen, Y.; Hu, Y. Optimized Separation of cis-trans Isomers and Enantiomers of Sertraline Using Cyclodextrin-Modified Micellar Electrokinetic Chromatography. Chromatographia 2004, 60, 469–473. [Google Scholar] [CrossRef]
- García, M.Á.; Menéndez-López, N.; Boltes, K.; Castro-Puyana, M.; Marina, M.L. A capillary micellar electrokinetic chromatography method for the stereoselective quantitation of bioallethrin in biotic and abiotic samples. J. Chromatogr. A 2017, 1510, 108–116. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.; Zhang, L.; Chen, G. Separation of dipeptides with two chiral centers using 2-hydroxypropyl-β-CD-modified MEKC. Electrophoresis 2010, 31, 1493–1497. [Google Scholar] [CrossRef]
- Jabor, V.A.P.; Bonato, P.S. Enantiomeric determination of praziquantel and its main metabolite trans-4-hydroxypraziquantel in human plasma by cyclodextrin-modified micellar electrokinetic chromatography. Electrophoresis 2001, 22, 1399–1405. [Google Scholar] [CrossRef]
- Lin, E.-P.; Lin, K.-C.; Chang, C.-W.; Hsieh, M.-M. On-line sample preconcentration by sweeping and poly(ethylene oxide)-mediated stacking for simultaneous analysis of nine pairs of amino acid enantiomers in capillary electrophoresis. Talanta 2013, 114, 297–303. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, S.; Qi, L.; Chen, Y. Chiral capillary electrophoretic separation of amino acids derivatized with 9-fluorenylmethylchloroformate using mixed chiral selectors of β-cyclodextrin and sodium taurodeoxycholate. Electrophoresis 2006, 27, 2896–2904. [Google Scholar] [CrossRef]
- Crego, A.L.; García, M.A.; Marina, M.L. Enantiomeric separation of chiral polychlorinated biphenyls by micellar electrokinetic chromatography using mixtures of bile salts and sodium dodecyl sulphate with and without γ-cyclodextrin in the separation buffer. J. Microcolumn Sep. 2000, 12, 33–40. [Google Scholar] [CrossRef]
- Méndez, S.P.; González, E.B.; Sanz-Medel, A. Enantiomeric separation of selenoaminoacid derivatives by cyclodextrin-modified micellar electrokinetic chromatography using a mixed micellar system of sodium dodecyl sulphate and taurodeoxycholic acid. Anal. Chim. Acta 2000, 416, 1–7. [Google Scholar] [CrossRef]
- Giuffrida, A.; Tabera, L.; González, R.; Cucinotta, V.; Cifuentes, A. Chiral analysis of amino acids from conventional and transgenic yeasts. J. Chromatogr. B 2008, 875, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Bielejewska, A.; Duszczyk, K.; Kwaterczak, A.; Sybilska, D. Comparative study on the enantiomer separation of 1,1′-binaphthyl-2,2′diyl hydrogenphosphate and 1,1′-bi-2-naphthol by liquid chromatography and capillary electrophoresis using single and combined chiral selector systems. J. Chromatogr. A 2002, 977, 225–237. [Google Scholar] [CrossRef]
- Kowalski, P.; Olędzka, I.; Plenis, A.; Miękus, N.; Pieckowski, M.; Bączek, T. Combination of field amplified sample injection and hydrophobic interaction electrokinetic chromatography (FASI-HIEKC) as a signal amplification method for the determination of selected macrocyclic antibiotics. Anal. Chim. Acta 2019, 1046, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Quirino, J.P. Micelle to solvent stacking of organic cations in capillary zone electrophoresis with electrospray ionization mass spectrometry. J. Chromatogr. A 2009, 1216, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Quirino, J.P.; Terabe, S. Exceeding 5000-fold concentration of dilute analytes in micellar electrokinetic chromatography. Science 1998, 282, 465–468. [Google Scholar] [CrossRef]
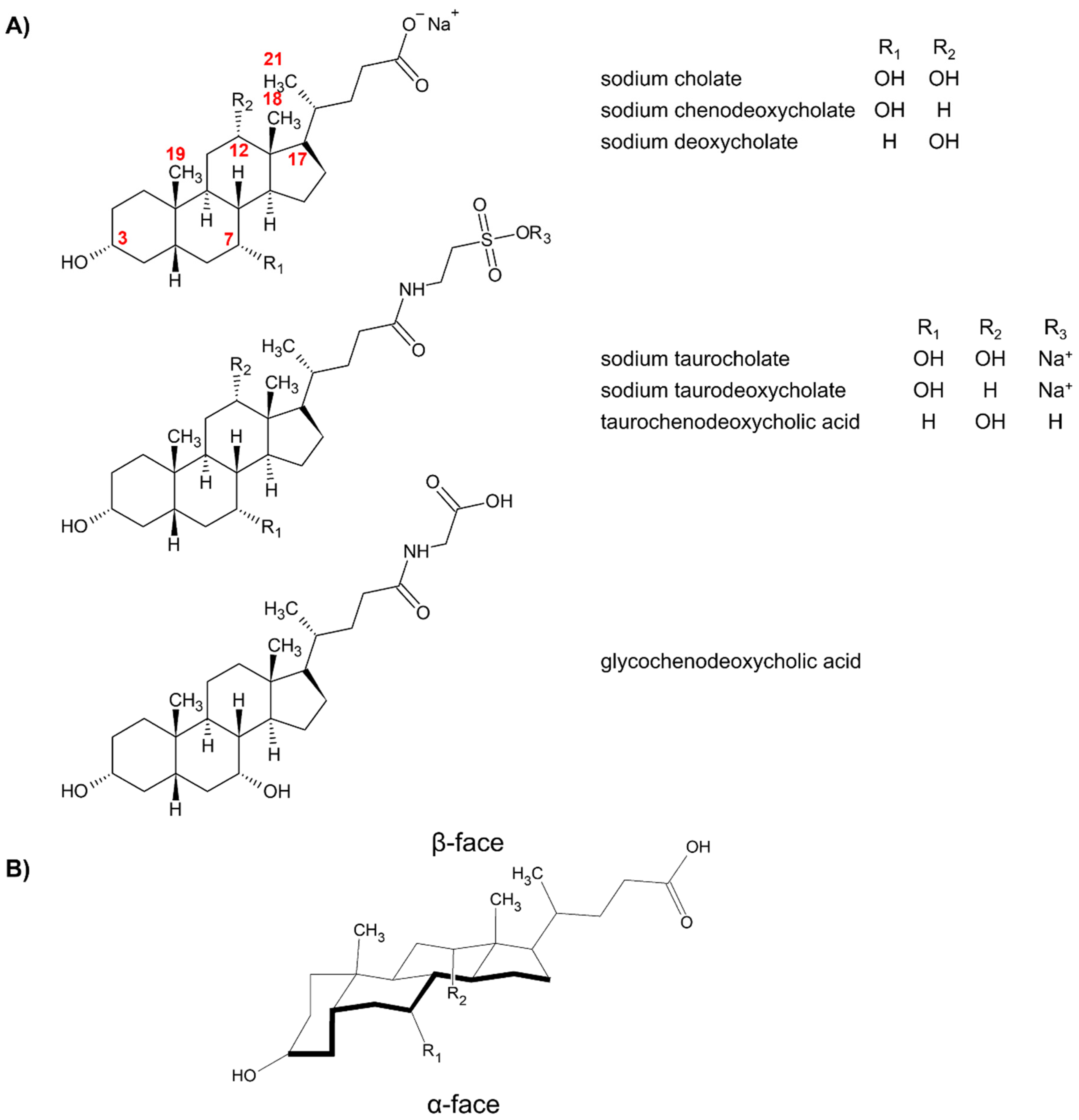
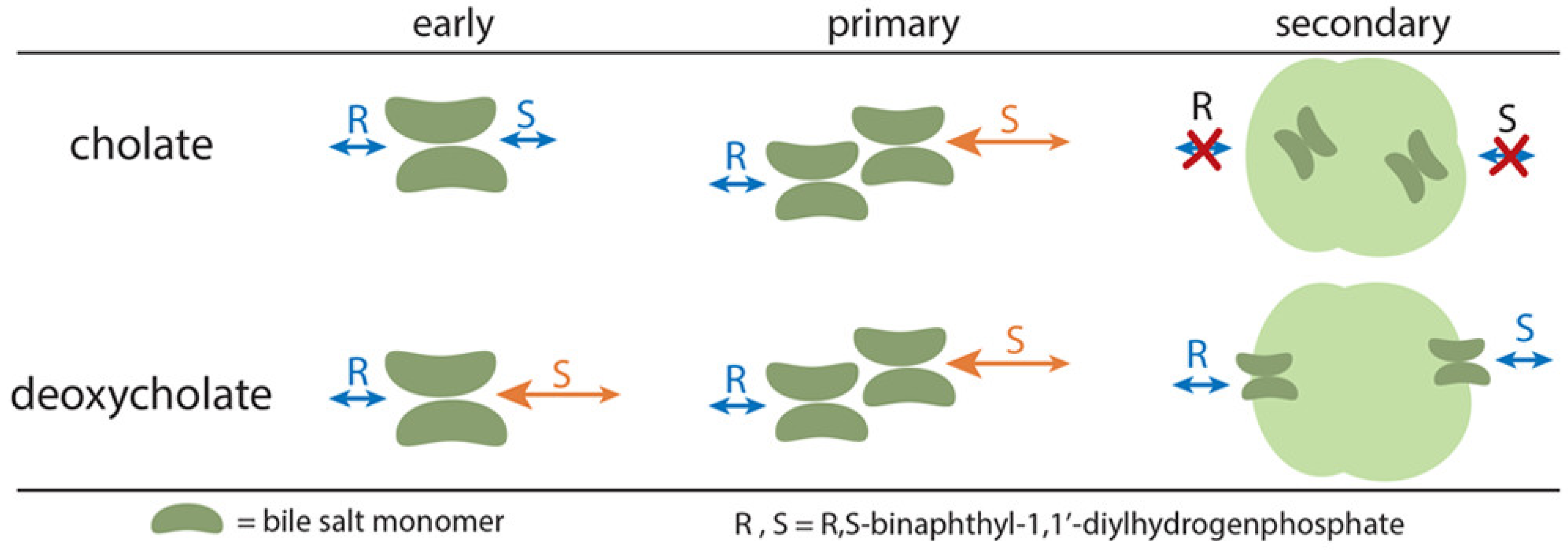

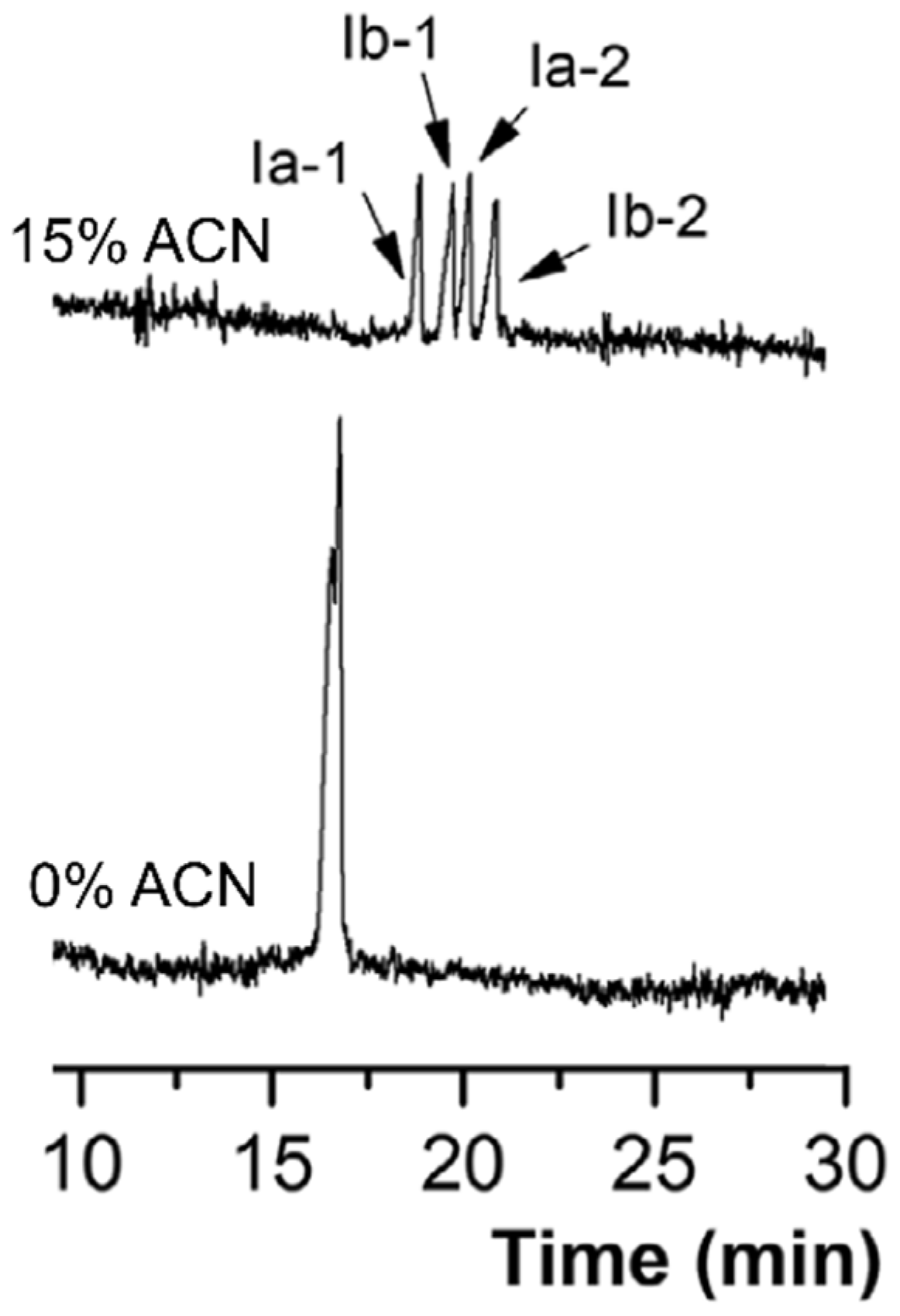
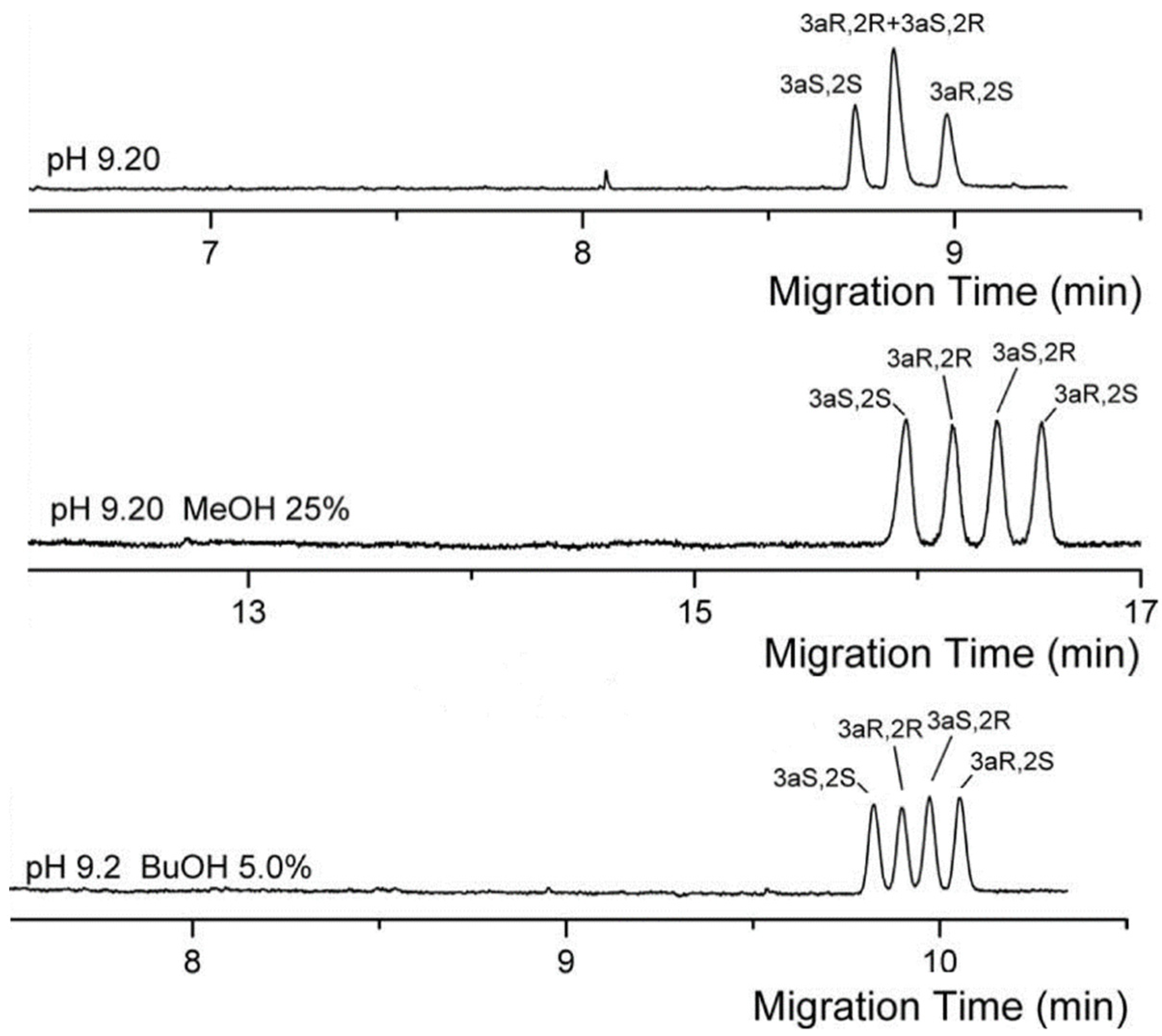
| Bile Salt | CMC, mM a | Ref. |
|---|---|---|
| Sodium cholate | 13–14 (pH 12, 25 °C) | [17] |
| Sodium chenodeoxycholate | 3 (pure water, 25 °C) | [26] |
| Sodium deoxycholate | 5.4 (pure water, 25 °C) | [27] |
| Sodium taurocholate | 8–12 (pure water, 25 °C) | [28] |
| Sodium taurodeoxycholate | 3.5–6 (pure water, 25 °C) | [29] |
| Taurochenodeoxycholic acid | 7 (pure water, 25 °C) | [29] |
| Glycochenodeoxycholic aicd | 2 (pH 7.5, 25 °C) | [30] |
| Surfactant | Other Chiral Selector | MEKC Conditions | Summary of Outcomes | Analytes | Ref. |
|---|---|---|---|---|---|
| As Sole Chiral Selector | |||||
| Sodium Cholate | N/A | capillary dimension: 65 cm (59 cm effective length) × 75 μm BGS: 100 mM sodium cholate in 50 mM Tris and 50 mM borate buffer (pH 8.3) injection: 0.8 psi for 3 s capillary temperature: 20 °C separation voltage: 20 kV detection: 220 nm | - | naringenin, neohesperidin | [36] |
| capillary dimension: 60 cm (52.5 cm effective length) × 50 μm BGS: 70 mM sodium cholate in 30 mM borate buffer (pH 9.4) with 20% (v/v) MeOH injection: 5 sec at a height of 10 cm (anodic) capillary temperature: 25 °C separation voltage: 20 kV detection: 254 nm | - | palonosetron hydrochloride | [37] | ||
| capillary dimension: 80.5 cm (72 cm effective length) × 50 μm BGS: 46.5 mM sodium cholate in 50 mM sodium borate/sodium dihydrogen phosphate buffer (pH 8) injection: 50 mbar for 1 s capillary temperature: 10–30 °C separation voltage: 15–25 kV detection: 210 ± 5 nm | - | ferroin (tris(1,10-phenantroline)-iron(II) complex | [39] | ||
| capillary dimension: 112 cm (95 cm effective length) × 50 μm BGS: 60 mM sodium cholate in 20 mM borate/phosphate buffer (pH 8) injection: 20 mbar for 2 s capillary temperature: 0–30 °C separation voltage: 25 kV detection: 230 nm | - | lorazepam, oxazepam, temazepam | [40] | ||
| capillary dimension: 60 cm (51.5 cm effective length) × 50 μm BGS: 50 mg/mL sodium cholate in 25 mM borate/phosphate (pH 7 or 9) or 25 mM borate (pH 10) injection: 30 mbar for 3 s capillary temperature: 15 °C separation voltage: 20 kV detection: 210 + 40 nm | - | naringin, neohesperidin | [41] | ||
| capillary dimension: 50 cm (40 cm effective length) × 75 μm BGS: 25 mM sodium cholate in 150 mM Tris and 15% DMF (pH 7.5) injection: 0.5 psi for 5 s capillary temperature: 25 °C separation voltage: 15 kV detection: 428 nm | - | verteporfin | [42] | ||
| capillary dimension: 37 cm (30 cm effective length) × 50 μm BGS: 25 mM sodium cholate in 300 mM borate and 10% ACN (pH 9.2) injection: +10 kV for 2 s capillary temperature: 20 °C separation voltage: 20 kV detection: 488 nm (λex)/694 nm (λem) | linear range: 0.05–50 mg/L LOD: 3.09–4.88 µM | benzoporphyrin derivative mono- and diacids | [43] | ||
| capillary dimension: 60 cm (52.5 cm effective length) × 50 μm BGS: 70 mM sodium cholate in 30 mM sodium tetraborate buffer (pH 9.2) with 25% (v/v) MeOH or 5.0% (v/v) n-BuOH injection: 5 kPa for 1 s capillary temperature: 25 °C separation voltage: 25 kV detection: 214 nm | - | palonosetron hydrochloride | [44] | ||
| capillary dimension: 60 cm (50 cm effective length) × 50 μm BGS: 30 mM sodium cholate in 30 mM sodium tetraborate buffer (pH 9.2) with 14% MeOH, 14% EtOH, 9% n-PrOH, 11% i-PrOH, 10% t-BuOH, 11% acetone, 11% ACN, 6.5% DMF, or 15% DMSO injection: 6 kPa for 1 s capillary temperature: 25 °C separation voltage: 25 kV detection: 214 or 254 nm | linear range: 5–50 µg/mL LOD: 0.3 µg/mL LOQ: 1.0 µg/mL | palonosetron hydrochloride | [45] | ||
| capillary dimension: 60 cm (50 cm effective length) × 50 μm BGS: 30 mM sodium cholate with 1 mM SDS in 30 mM sodium tetraborate (pH 9.2) injection: 10 kPa for 1 or 5 s capillary temperature: 20 °C separation voltage: 25 kV detection: 214 nm | linear range: 0.5–50 µg/mL LOD: 0.08–0.09 µg/mL LOQ: 0.28–0.31 µg/mL | palonosetron hydrochloride | [46] | ||
| capillary dimension: 60 cm (50 cm effective length) × 50 μm BGS: 50 mM sodium cholate and 100 mM SDS in MeOH/ACN (4:1 v/v) sample injection: 0.5 psi for 5 s capillary temperature: 25 °C separation voltage: 30 kV detection: 200 nm | - | pyraclofos | [47] | ||
| capillary dimension: 30–50 cm (19.5–39.5 cm effective length) × 50 μm BGS: 60 mM sodium cholate in 20 mM CAPS (pH 9.0) and 20% ACN injection: 0.5 psi for 5–90 s separation voltage: 25 kV detection: 220 nm | SEF: ~18 | Binaphthyl enantiomers, DL-trp | [52] | ||
| capillary dimension: 50 cm (40 cm effective length) × 75 μm BGS: 25 mM sodium cholate in 150 mM Tris (pH 7.5) and 15% (v/v) DMF sample injection: 0.8 psi vs. −10.3 kV for 120 s capillary temperature: 25 °C separation voltage: 20 kV detection: 428 nm | LOD: 10.3 µg/L | verteporfin | [53] | ||
| Sodium Taurocholate | capillary dimension: 72 cm effective length × 50 μm BGS: 5 mM sodium taurocholate in 50 mM acetate buffer (pH 2.5) capillary temperature: 30 °C separation voltage: 20 kV detection: 220 nm | - | metyrosine | [48] | |
| capillary dimension: 72 cm (50 cm effective length) × 50 μm BGS: 5 mM sodium taurocholate in 50 mM sodium dihydrogen phosphate (pH 2.5) capillary temperature: 30 °C separation voltage: 15 kV detection: 220 nm | linear range: 50–500 ng/mL LOD: 25 ng/mL LOQ: 50 ng/mL | arotinolol | [49] | ||
| Taurochenodeoxycholic Acid or Glycochenodeoxycholic Acid | capillary dimension: 63 cm (49 cm effective length) × 50 μm BGS: 35 mM taurochenodeoxycholic or glycochenodeoxycholic acid in 0.005 mM phosphate/borate buffer with 10% acetone (pH 9) injection: 5 kV for 7 s separation voltage: 20 kV detection: 232 nm | - | Iron (II) triaza aromatic ligand | [50] | |
| capillary dimension: 43 cm (29 cm effective length) × 50 μm BGS: 20 mM sodium taurochenodeoxycholate or glycochenodeoxycholate in 0.005 mM phosphate/borate buffer with 10% (v/v) acetone (pH 3–9) injection: 5 kV for 7 s separation voltage: 15–20 kV detection: 232 nm | - | bis(8-((pyridine-2-methylene)amino)quinoline) iron(II) hexafluorophosphate | [51] | ||
| With Another Chiral Selector | |||||
| Sodium Cholate | γ-CD | capillary dimension: 60 cm (50 cm effective length) × 50 μm BGS: 50–75 mM sodium cholate and 10–20 mM γ-CD in MeOH/H2O/ACN (5:4:1 v/v) sample injection: 0.5 psi for 5 s capillary temperature: 25 °C separation voltage: 30 kV detection: 200 nm | - | profenofos, prothiofos, sulprofos | [47] |
| sulfated β-CD and hydroxypropyl β-CD | capillary dimension: 60 cm (53 cm effective length) × 50 μm BGS: 50 mM sodium cholate with 15 mM sulfated β-CD and 5 mM hydroxypropyl β-CD in 20 mM sodium borate (pH 9.0) sample injection: 18 s at a height of 10 cm capillary temperature: 10 °C separation voltage: 30 kV detection: 214 nm | linear range: 0.7–400 µg/mL LOD: 0.2 µg/mL LOQ: 0.7 µg/mL | sertraline | [54] | |
| heptakis (2,3,6-tri-O-methyl)- β-CD | capillary temperature: 58.5 cm (50 cm effective length) × 50 μm BGS: 100 mM sodium cholate with 20 mM heptakis (2,3,6-tri-O-methyl)- β-CD in 100 mM borate (pH 8) and 2 M urea injection: 50 mbar for 2 s capillary temperature: 15 °C separation voltage: 30 kV detection: 210 nm | linear range: 10–150 mg/L LOD: 3.9 (E2), 4.8 (E1) LOQ: 11.8 (E2), 11.8 (E1) | cis-bifentrhin | [55] | |
| human serum albumin | capillary temperature: 60.2 cm (50 cm effective length) × 50 μm BGS: (asp) 12 mM sodium cholate with 0.5% (v/v) HSA in 12 mM sodium (pH 8.9) and 10% (v/v) MeOH (glu) 12 mM sodium cholate with 1.6% (v/v) HSA in 12 mM sodium (pH 9.1) and 5% (v/v) MeOH capillary temperature: 25 °C separation voltage: −25 kV detection: 488 nm (λex)/520 nm (λem) | linear range: 0.60–160 ng/mL LOD: 0.022–0.038 ng/mL LOQ: 0.60−1.20 ng/mL | DL-asp, DL-glu | [56] | |
| 34-mer single stranded DNA aptamer | capillary dimension: 60 cm (50 cm effective length) × 75 μm BGS: 50 mM sodium cholate with 10 mM NaCl, 1 mM MgCl2, 2.5 mM KCl, 2 mM KH2PO4 and 10 mM Na2HPO4 (pH 7.4) injection: 0.5 psi for 6 s capillary temperature: 25 °C separation voltage: 20 kV detection: 280 nm | linear range: 0.0625–2 mM LOD: 0.0125 mM (D), 0.0153 mM (L) | DL-trp | [57] | |
| Sodium Taurocholate | β-CD | capillary dimension: 57 cm (50 cm effective length) × 50 μm BGS: 30 mM sodium taurocholate with 20 mM β-CD in 80 mM sodium borate buffer (pH 9.3) injection: 0.5 psi for 1 s capillary temperature: 20 °C separation voltage: 20 kV detection: 488 nm (λex) / 520 nm (λem) | - | 20 FITC-derivatized amino acids | [58] |
| capillary dimesnion: 67 cm (50 cm effective length) × 50 μm BGS: 40 mM sodium taurocholate with 30 mM β-CD in 40 mM borax (pH 9) and 15% (v/v) i-PrOH injection: 10 kV for 10 s capillary temperature: 22 °C separation voltage: 20 kV detection: 214 nm | - | o-phthaldiadehyde derivatized amino acids | [59] | ||
| BGS: 12 mM sodium taurocholate with 8 mM β-CD in 5 mM borate (pH 9.2) | - | DL-asp, DL-leu | [60] | ||
| BGS: 6 mM sodium taurocholate with 18 mM β-CD in 50 mM sodium borate buffer and 20% (v/v) MeOH (pH 9.3) | - | DL-asp, DL-ser | [61] | ||
| capillary temperature: 60 cm (50 cm effective length) × 25 μm BGS: 18 mM sodium taurocholate with 12 mM β-CD in 80 mM sodium borate (pH 9.3) injection: 0.3 psi for 2 s capillary temperature: 25 °C separation voltage: 25 kV detection: 520 nm (LIF) | linear range: 0.8–50 pM (DL-ala, DL-asp, DL-leu); 1.2–50 (DL-glu) LOD: 30 pM (DL-ala, DL-leu); 60 pM (DL-asp, DL-glu) SEF: 330–400 | DL-ala, DL-asp, DL-glu, DL-leu | [62] | ||
| γ-CD | capillary dimension: 30–60 cm (20–50 cm effective length) × 50 μm BGS: 30 mM sodium taurocholate with 30 mM γ-CD in 80 mM sodium tetraborate (pH 9.2) with 5% ACN injection: 0.5 psi for 4 s capillary temperature: 20 °C separation voltage: 0.5 kV/cm detection: 488 nm (LIF) | LOD: 5–25 nM | DL-his, DL-leu, DL-val, Dl-ser, DL-ala, DL-glu, DL-asp | [63] | |
| Sodium Deoxycholate | β-CD | capillary dimension: 50 cm effective length × 50 μm BGS: 60 mM sodium deoxycholate with 30 mM β-CD in 100 mM borate (pH 9.5) injection: at a height differential of 20 cm for 10 s separation voltage: 15 kV detection: 457.9 nm (LIF) | LOD: 0.3 nM | D-ser | [64] |
| β-CD | capillary dimension: 40 cm effective length × 25 μm BGS: 30 mM sodium deoxycholate with 20 mM β-CD in 35 mM phosphate buffered saline (pH 7.85) and 20% (v/v) acetonitrile injection: 20 kV for 4 s separation voltage: 20 kV detection: 1.3 V (vs. AgCl/Ag) | - | (RS)-1-phenyl tetrahydrodgen isoquinoline | [65] | |
| hydroxypropyl-β-CD | capillary dimension: 57 cm (50 cm effective length) × 50 μm BGS: 30 mM sodium deoxycholate with 20 mg/mL HP-β-CD in 35 mM borate (pH 11.5) capillary temperature: 20 °C separation voltage: 25 kV detection: 210 nm | - | sertraline | [66] | |
| acetyl-β-CD | capillary dimension: 58.5 cm (50 cm effective length) × 50 μm BGS: 75 mM sodium deoxycholate with 15 mM acetyl-β-CD in 100 mM borate (pH 8) and 2 mM urea injection: 50 mbar for 2 s capillary temperature: 25 °C separation voltage: 30 kV detection: 220 nm | linear range: (external calibration) 0.5–150 mg/L; (standard addition) 0–110 mg/L LOD: (R) 0.2 mg/L; (S) 0.3 mg/L LOQ: (R) 0.7 mg/L; (S) 0.9 mg/L | bioallenthrin | [67] | |
| 2-hydroxypropyl- β-CD | capillary dimension: 50 cm (40 cm effective length) × 50 μm BGS: 15–25 mM sodium deoxycholate with 40–50 mM HP-β-CD in 100 mM Tris-H3PO4 (pH 8–9.2) injection: 0.5 psi for 5 s capillary temperature: 25 °C separation voltage: 20 kV detection: 200 nm | - | derivatized dipeptides (ala-gln, tyr-leu, tyr-phe) | [68] | |
| sulfated β-CD | capillary dimension: 42 cm (36 cm effective length) × 50 μm BGS: 20 mM sodium deoxycholate with 2% (w/v) sulfated β-CD in 20 mM sodium borate (pH 10) injection: 0.8 psi for 5 s capillary temperature: 20 °C separation voltage: 18 kV detection: 210 nm | LOD: (R/S-praziquantel) 50 ng/mL (R/S-trans-4-hydroxypraziquantel) 62.5 ng/mL | praziquantel, trans-4-hydroxy-praziquantel | [69] | |
| Sodium Taurodeoxycholate | β-CD | capillary temperature: 50 cm (40 cm effective length) × 75 μm conditioning buffer: 35 mM sodium taurodeoxycholate and 35 mM β-CD in 1.5 M Tris-borate (pH 8.5) with 12.5% (v/v) IPA BGS: 35 mM sodium taurodeoxycholate with 35 mM β-CD and 0.5% w/v PEO in 150 mM Tris-borate (pH 8.5) with 12.5% (v/v) i-PrOH injection: 240 s at 20 cm height separation voltage: 15–18 kV detection: 260 nm | SEF: 106–219 | FMOC -derivatized amino acids | [70] |
| β-CD | capillary temperature: 60 cm (50 cm effective length) × 75 μm BGS: 35 mM sodium taurodeoxycholate with 35 mM β-CD in 150 mM borate (pH 8.5) with 18% (v/v) i-PrOH injection: 0.3 psi for 2 s capillary temperature: 25 °C separation voltage: 25 kV detection: 214 nm | - | FMOC -derivatized amino acids | [71] | |
| With an Achiral Surfactant and a CS | |||||
| Sodium Taurodeoxycholate | SDS and γ-CD | capillary dimension: 65 cm (50 cm effective length) × 50 μm BGS: 235 nm/0.05 M sodium cholate, 0.05 M SDS and 0.05 γ-CD in 0.1 M CHES buffer (pH 9) and 2 M urea injection: 20 mbar for 1.2 s separation voltage: 15 kV detection: 235 nm | - | chiral polychlorinated biphenyls | [72] |
| Taurodeoxycholic acid | SDS and β-CD | capillary dimension: 67 cm (50 cm effective length) × 50 μm BGS: 50 mM taurodeoxycholic acid, 50 mM SDS and 30 mM β-CD in 30 mM phosphate buffer/10 mM boric acid (pH 7) injection: 70 mbar for 0.6 s separation voltage: 12 kV detection: 230 nm | LOD: 0.06 mM | DL-selenomethionine, DL-selenoethionine | [73] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, R.B.; Quirino, J.P. Bile Salts in Chiral Micellar Electrokinetic Chromatography: 2000–2020. Molecules 2021, 26, 5531. https://doi.org/10.3390/molecules26185531
Yu RB, Quirino JP. Bile Salts in Chiral Micellar Electrokinetic Chromatography: 2000–2020. Molecules. 2021; 26(18):5531. https://doi.org/10.3390/molecules26185531
Chicago/Turabian StyleYu, Raymond B., and Joselito P. Quirino. 2021. "Bile Salts in Chiral Micellar Electrokinetic Chromatography: 2000–2020" Molecules 26, no. 18: 5531. https://doi.org/10.3390/molecules26185531
APA StyleYu, R. B., & Quirino, J. P. (2021). Bile Salts in Chiral Micellar Electrokinetic Chromatography: 2000–2020. Molecules, 26(18), 5531. https://doi.org/10.3390/molecules26185531






