Evaluation of Benzaldehyde as an Antibiotic Modulator and Its Toxic Effect against Drosophila melanogaster
Abstract
:1. Introduction
2. Results
2.1. MIC Tests
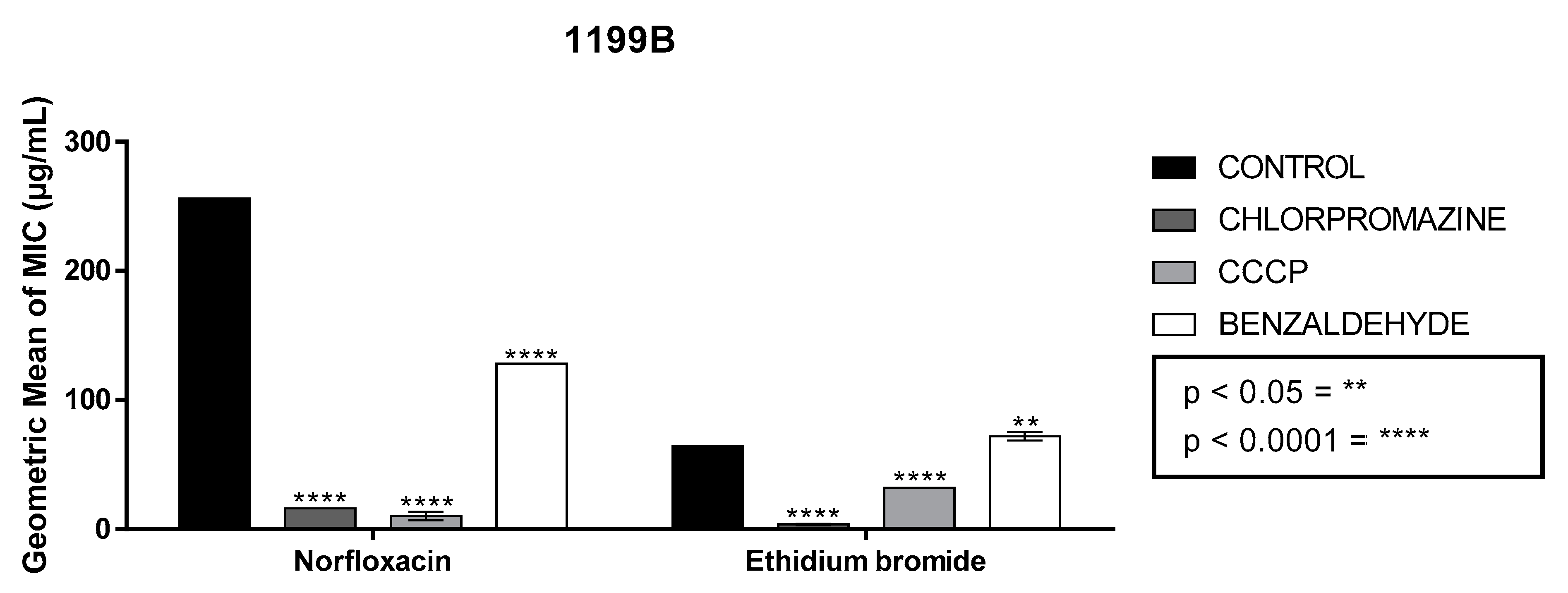
2.2. Antibiotic and Efflux Pump Inhibition Tests by Reducing the MIC of Ethidium Bromide
2.3. Toxicity
2.3.1. Mortality
2.3.2. Damage to the Locomotor Apparatus
3. Discussion
4. Material and Methods
4.1. Culture Media
4.2. Microorganisms
4.3. Origin and Preparation of Substances
4.4. Preparation and Standardization of the Inoculum
4.5. MIC Tests
4.6. Efflux Pump Inhibition Tests by Modifying the MIC of the Antibiotic and Ethidium Bromide
4.7. Toxicity Test
4.7.1. Breeding and Stocking of Drosophila melanogaster
4.7.2. Mortality Test
4.7.3. Negative Geotaxis Test
4.8. Statistical Analysis
4.8.1. Statistical Analysis of Microbiological Results
4.8.2. Statistical Analysis of Toxicity Results
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Hussain, A.I.; Anwar, F.; Nigam, P.S.; Ashraf, M.; Gilani, A.H. Seasonal variation in content, chemical composition and antimicrobial and cytotoxic activities of essential oils from four Mentha species. J. Sci. Food Agric. 2010, 90, 1827–1836. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.H.; Dias, H.J.; Vieira, T.M.; de Souza, M.G.M.; Cruz, A.F.F.; Badoco, F.R.; Nicolella, H.D.; Cunha, W.R.; Groppo, M.; Martins, C.H.G.; et al. Chemical Composition, Antibacterial, Schistosomicidal, and Cytotoxic Activities of the Essential Oil of Dysphania ambrosioides (L.) Mosyakin & Clemants (Chenopodiaceae). Chem. Biodivers. 2017, 14, e1700149. [Google Scholar] [CrossRef]
- Soares, J.J.; Gonçalves, M.B.; Gayer, M.C.; Bianchini, M.C.; Caurio, A.C.; Soares, S.J.; Puntel, R.L.; Roehrs, R.; Denardin, E.L.G. Continuous liquid feeding: New method to study pesticides toxicity in Drosophila melanogaster. Anal. Biochem. 2017, 537, 60–62. [Google Scholar] [CrossRef]
- Tariq, S.; Wani, S.; Rasool, W.; Shafi, K.; Bhat, M.A.; Prabhakar, A.; Shalla, A.H.; Rather, M.A. A comprehensive review of the antibacterial, antifungal and antiviral potential of essential oils and their chemical constituents against drug-resistant microbial pathogens. Microb. Pathog. 2019, 134, 103580. [Google Scholar] [CrossRef]
- Leite, G.d.O.; Ecker, A.; Seeger, R.L.; Krum, B.N.; Lugokenski, T.H.; Fachinetto, R.; Sudati, J.H.; Barbosa, N.V.; Wagner, C. Protective effect of (−)-α-bisabolol on rotenone-induced toxicity in Drosophila melanogaster. Can. J. Physiol. Pharmacol. 2018, 96, 359–365. [Google Scholar] [CrossRef] [Green Version]
- Cavanagh, H.M.A.; Wilkinson, J.M. Biological activities of lavender essential oil. Phyther. Res. 2002, 16, 301–308. [Google Scholar] [CrossRef]
- De Morais Oliveira-Tintino, C.D.; Tintino, S.R.; Limaverde, P.W.; Figueredo, F.G.; Campina, F.F.; da Cunha, F.A.B.; da Costa, R.H.S.; Pereira, P.S.; Lima, L.F.; de Matos, Y.M.L.S.; et al. Inhibition of the essential oil from Chenopodium ambrosioides L. and α-terpinene on the NorA efflux-pump of Staphylococcus aureus. Food Chem. 2018, 262, 72–77. [Google Scholar] [CrossRef]
- Patridge, E.; Gareiss, P.; Kinch, M.S.; Hoyer, D. An analysis of FDA-approved drugs: Natural products and their derivatives. Drug Discov. Today 2016, 21, 204–207. [Google Scholar] [CrossRef] [PubMed]
- De Lacerda Neto, L.J.; Ramos, A.G.B.; Kerntopf, M.R.; Coutinho, H.D.M.; Quintans-Junior, L.J.; Almeida, J.R.G.S.; Ribeiro-Filho, J.; Menezes, I.R.A. Modulation of antibiotic activity by the hydroalcoholic extract from leaves of Caryocar coriaceum WITTM. Nat. Prod. Res. 2018, 32, 477–480. [Google Scholar] [CrossRef]
- Céspedes, C.L.; Salazar, J.R.; Ariza-Castolo, A.; Yamaguchi, L.; Avila, J.G.; Aqueveque, P.; Kubo, I.; Alarcón, J.; Céspedes, C.L.; Aqueveque, P.; et al. Biopesticides from plants: Calceolaria integrifolia s.l. Environ. Res. 2014, 132, 391–406. [Google Scholar] [CrossRef] [PubMed]
- Brahmachari, G. Natural products in drug discovery: Impacts and opportunities—An assessment. In Bioactive Natural Products: Opportunities and Challenges in Medicinal Chemistry; Brahmachari, G., Ed.; World Scientific Publishing Co.: Singapore, 2011; pp. 1–199. ISBN 9789814335386. [Google Scholar]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.; Ademiluyi, A.; et al. Biological Activities of Essential Oils: From Plant Chemoecology to Traditional Healing Systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef] [PubMed]
- Zolfaghari Emameh, R.; Syrjänen, L.; Barker, H.; Supuran, C.T.; Parkkila, S. Drosophila melanogaster: A model organism for controlling Dipteran vectors and pests. J. Enzyme Inhib. Med. Chem. 2015, 30, 505–513. [Google Scholar] [CrossRef]
- Abdelgaleil, S.A.M.; El-Sabrout, A.M. Anti-nutritional, antifeedant, growth-disrupting and insecticidal effects of four plant essential oils on Spodoptera littoralis (Lepidoptera: Noctuidae). J. Crop Prot. 2018, 7, 135–150. [Google Scholar]
- Taylor, M.J.; Tuxworth, R.I. Continuous tracking of startled Drosophila as an alternative to the negative geotaxis climbing assay. J. Neurogenet. 2019, 33, 190–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.F.; Wang, H.; Zheng, L.; Yang, F.; Li, H.Z.; Li, J.X.; Cheng, D.; Lu, K.; Liu, Y. Effects of Modest Hypoxia and Exercise on Cardiac Function, Sleep-Activity, Negative Geotaxis Behavior of Aged Female Drosophila. Front. Physiol. 2020, 10, 1610. [Google Scholar] [CrossRef] [Green Version]
- Dilliane, C.C.; Suelen, F.; Jaqueline, V.; Welligton, L.B. Valeriana officinalis and melatonin: Evaluation of the effects in Drosophila melanogaster rapid iterative negative geotaxis (RING) test. J. Med. Plants Res. 2017, 11, 703–712. [Google Scholar] [CrossRef] [Green Version]
- Chaudhary, P.; Dhande, S. Evaluation of Neuroprotective effect of medicinal plants in Drosophila melanogaster model. Indian J. Pharm. Biol. Res. 2018, 6, 09–12. [Google Scholar] [CrossRef]
- Murphey, R.M.; Hall, C.F. Some correlates of negative geotaxis in Drosophila melanogaster. Anim. Behav. 1969, 17, 181–185. [Google Scholar] [CrossRef]
- Bainton, R.J.; Tsai, L.T.Y.; Singh, C.M.; Moore, M.S.; Neckameyer, W.S.; Heberlein, U. Dopamine modulates acute responses to cocaine, nicotine and ethanol in Drosophila. Curr. Biol. 2000, 10, 187–194. [Google Scholar] [CrossRef] [Green Version]
- Gillings, M.R.; Paulsen, I.T.; Tetu, S.G. Genomics and the evolution of antibiotic resistance. Ann. N. Y. Acad. Sci. 2017, 1388, 92–107. [Google Scholar] [CrossRef]
- Floyd, J.T.; Kumar, S.; Mukherjee, M.M.; He, G.; Varela, M.F. A review of the molecular mechanisms of drug efflux in pathogenic bacteria: A structure-function perspective. In Recent Research Developments in Membrane Biology; Research Signpost: Trivandrum, India, 2013; Volume 37661, pp. 15–66. ISBN 978-81-308-0529-0. [Google Scholar]
- Stavri, M.; Piddock, L.J.V.; Gibbons, S. Bacterial efflux pump inhibitors from natural sources. J. Antimicrob. Chemother. 2007, 59, 1247–1260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Venter, H.; Ma, S. Efflux Pump Inhibitors: A Novel Approach to Combat Efflux-Mediated Drug Resistance in Bacteria. Curr. Drug Targets 2015, 17, 702–719. [Google Scholar] [CrossRef] [PubMed]
- Chitsaz, M.; Brown, M.H. The role played by drug efflux pumps in bacterial multidrug resistance. Essays Biochem. 2017, 61, 127–139. [Google Scholar] [PubMed]
- Meena, H.; Siddhardha, B. Microbial Efflux Pump -Specific Determinant to Fight Against the Antimicrobial Resistance in Pathogenic Strains. Curr. Chem. Biol. 2018, 12, 135–149. [Google Scholar] [CrossRef]
- Gupta, A.; Mumtaz, S.; Li, C.H.; Hussain, I.; Rotello, V.M. Combatting antibiotic-resistant bacteria using nanomaterials. Chem. Soc. Rev. 2019, 48, 415–427. [Google Scholar] [CrossRef]
- Kaatz, G.W.; McAleese, F.; Seo, S.M. Multidrug resistance in Staphylococcus aureus due to overexpression of a novel multidrug and toxin extrusion (MATE) transport protein. Antimicrob. Agents Chemother. 2005, 49, 1857–1864. [Google Scholar] [CrossRef] [Green Version]
- Lei, G.; Wang, L.; Liu, X.; Zhang, A. Chemical composition of essential oils and hydrosols from fresh flowers of Cerasus subhirtella and Cerasus serrulata from East China. Nat. Prod. Res. 2014, 28, 1923–1925. [Google Scholar] [CrossRef]
- CROTEAU, R. Biosynthesis of benzaldehyde, benzyl alcohol and benzyl benzoate from benzoic acid in cranberry (Vaccinium macrocarpon). J. Food Biochem. 1978, 1, 317–326. [Google Scholar] [CrossRef]
- Lee, B.H.; Choi, W.S.; Lee, S.E.; Park, B.S. Fumigant toxicity of essential oils and their constituent compounds towards the rice weevil, Sitophilus oryzae (L.). Crop Prot. 2001, 20, 317–320. [Google Scholar] [CrossRef]
- Ullah, I.; Khan, A.L.; Ali, L.; Khan, A.R.; Waqas, M.; Hussain, J.; Lee, I.J.; Shin, J.H. Benzaldehyde as an insecticidal, antimicrobial, and antioxidant compound produced by Photorhabdus temperata M1021. J. Microbiol. 2015, 53, 127–133. [Google Scholar] [CrossRef]
- Verma, R.S.; Padalia, R.C.; Singh, V.R.; Goswami, P.; Chauhan, A.; Bhukya, B. Natural benzaldehyde from Prunus persica (L.) Batsch. Int. J. Food Prop. 2017, 20, 1–5. [Google Scholar] [CrossRef]
- Barros, A.F.; Campos, V.P.; De Oliveira, D.F.; De Jesus Silva, F.; Jardim, I.N.; Costa, V.A.; Matrangolo, C.A.R.; Ribeiro, R.C.F.; Silva, G.H. Activities of essential oils from three Brazilian plants and benzaldehyde analogues against Meloidogyne incognita. Nematology 2019, 21, 1081–1089. [Google Scholar] [CrossRef]
- Auezova, L.; Najjar, A.; Kfoury, M.; Fourmentin, S.; Greige-Gerges, H. Antibacterial activity of free or encapsulated selected phenylpropanoids against Escherichia coli and Staphylococcus epidermidis. J. Appl. Microbiol. 2020, 128, 710–720. [Google Scholar] [CrossRef] [PubMed]
- Alamri, A.; El-Newehy, M.H.; Al-Deyab, S.S. Biocidal polymers: Synthesis and antimicrobial properties of benzaldehyde derivatives immobilized onto amine-terminated polyacrylonitrile. Chem. Cent. J. 2012, 6, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badawy, M.E.I.; Marei, G.I.K.; Rabea, E.I.; Taktak, N.E.M. Antimicrobial and antioxidant activities of hydrocarbon and oxygenated monoterpenes against some foodborne pathogens through in vitro and in silico studies. Pestic. Biochem. Physiol. 2019, 158, 185–200. [Google Scholar] [CrossRef]
- Coêlho, M.L.; Ferreira, J.H.L.; de Siqueira Júnior, J.P.; Kaatz, G.W.; Barreto, H.M.; de Carvalho Melo Cavalcante, A.A. Inhibition of the NorA multi-drug transporter by oxygenated monoterpenes. Microb. Pathog. 2016, 99, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, A.H.; Bezerra, S.R.; Macêdo, N.S.; de Sousa Silveira, Z.; dos Santos Barbosa, C.R.; de Freitas, T.S.; Muniz, D.F.; de Sousa Júnior, D.L.; Júnior, J.P.S.; Donato, I.A.; et al. Effect of estragole over the RN4220 Staphylococcus aureus strain and its toxicity in Drosophila melanogaster. Life Sci. 2020, 118675. [Google Scholar] [CrossRef]
- Friedman, M.; Henika, P.R.; Mandrell, R.E. Antibacterial Activities of Phenolic Benzaldehydes and Benzoic Acids against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes, and Salmonella enterica. J. Food Prot. 2003, 66, 1811–1821. [Google Scholar] [CrossRef]
- Bezerra, C.F.; Camilo, C.J.; do Nascimento Silva, M.K.; de Freitas, T.S.; Ribeiro-Filho, J.; Coutinho, H.D.M. Vanillin selectively modulates the action of antibiotics against resistant bacteria. Microb. Pathog. 2017, 113, 265–268. [Google Scholar] [CrossRef]
- Lima, W.G.; Ramos-Alves, M.C.; Soares, A.C. Dos distúrbios psiquiátricos à antibioticoterapia: Reposicionamento da clorpromazina como agente antibacteriano. Rev. Colomb. Ciencias Químico-Farmacéuticas 2019, 48, 5–28. [Google Scholar] [CrossRef]
- Grimsey, E.M.; Fais, C.; Marshall, R.L.; Ricci, V.; Ciusa, M.L.; Stone, J.W.; Ivens, A.; Malloci, G.; Ruggerone, P.; Vargiu, A.V.; et al. Chlorpromazine and amitriptyline are substrates and inhibitors of the acrb multidrug efflux pump. MBio 2020, 11. [Google Scholar] [CrossRef]
- Pagès, J.M.; Amaral, L. Mechanisms of drug efflux and strategies to combat them: Challenging the efflux pump of Gram-negative bacteria. Biochim. Biophys. Acta—Proteins Proteom. 2009, 1794, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Schindler, B.D.; Jacinto, P.; Kaatz, G.W. Inhibition of drug efflux pumps in Staphylococcus aureus: Current status of potentiating existing antibiotics. Future Microbiol. 2013, 8, 491–507. [Google Scholar] [CrossRef]
- Freitas, P.; Araújo, A.C.; Barbosa, C.; Muniz, D.; Tintino, S.; Ribeiro-Filho, J.; Júnior, J.S.; Barbosa, J.M.; Sousa, G.; Coutinho, H. Inhibition of Efflux Pumps by Monoterpene (α-Pinene) and impact on Staphylococcus aureus Resistance to Tetracycline and Erythromycin. Curr. Drug Metab. 2020, 21. [Google Scholar] [CrossRef]
- Secoli, S.R. Drugs interactions: Fundamental aspects for clinical practicing nursing. Rev. Esc. Enferm. USP 2001, 35, 28–34. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Chu, C.; Zhang, Y.-H.; Zheng, M.; Zhu, L.; Kong, X.; Huang, T. Identification of Drug-Drug Interactions Using Chemical Interactions. Curr. Bioinform. 2016, 12. [Google Scholar] [CrossRef]
- Alissa, E.M. Medicinal herbs and therapeutic drugs interactions. Ther. Drug Monit. 2014, 36, 413–422. [Google Scholar] [CrossRef]
- Paulraj, M.G.; Reegan, A.D.; Ignacimuthu, S. Toxicity of benzaldehyde and propionic acid against immature and adult stages of Aedes aegypti (Linn.) and Culex quinquefasciatus (Say) (Diptera: Culicidae). J. Entomol. 2011, 8, 539–547. [Google Scholar] [CrossRef]
- Mishra, P.; Tripathi, A.; Dikshit, A.; Pandey, A. Insecticides Derived from Natural Products: Diversity and Potential Applications. In Natural Bioactive Products in Sustainable Agriculture; Springer: Singapore, 2020; pp. 83–99. [Google Scholar]
- Boyko, A.A.; Brygadyrenko, V.V. Changes in the viability of Strongyloides ransomi larvae (Nematoda, Rhabditida) under the influence of synthetic flavourings. Regul. Mech. Biosyst. 2017, 8, 36–40. [Google Scholar] [CrossRef] [Green Version]
- Da Cunha, F.A.B.; Waczuk, E.P.; Duarte, A.E.; Barros, L.M.; Elekofehinti, O.O.; Matias, E.F.F.; da Costa, J.G.M.; Sanmi, A.A.; Boligon, A.A.; da Rocha, J.B.T.; et al. Cytotoxic and antioxidative potentials of ethanolic extract of Eugenia uniflora L. (Myrtaceae) leaves on human blood cells. Biomed. Pharmacother. 2016, 84, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Da Cunha, F.A.B.; Wallau, G.L.; Pinho, A.I.; Nunes, M.E.M.; Leite, N.F.; Tintino, S.R.; da Costa, G.M.; Athayde, M.L.; Boligon, A.A.; Coutinho, H.D.M.; et al. Eugenia uniflora leaves essential oil induces toxicity in Drosophila melanogaster: Involvement of oxidative stress mechanisms. Toxicol. Res. 2015, 4, 634–644. [Google Scholar] [CrossRef]
- Störtkuhl, K.F.; Kettler, R.; Fischer, S.; Hovemann, B.T. An increased receptive field of olfactory receptor Or43a in the antennal lobe of Drosophila reduces benzaldehyde-driven avoidance behavior. Chem. Senses 2005, 30, 81–87. [Google Scholar] [CrossRef]
- Fernandes, N.d.S.; Silva, F.A.N.; de Aragão, F.A.S.; Zocolo, G.J.; Freitas, B.M. Volatile Organic Compounds Role in Selective Pollinator Visits to Commercial Melon Types. J. Agric. Sci. 2019, 11, 93. [Google Scholar] [CrossRef]
- El-Sayed, A.M.; Unelius, C.R.; Suckling, D.M. Honey Norisoprenoids Attract Bumble Bee, Bombus terrestris, in New Zealand Mountain Beech Forests. J. Agric. Food Chem. 2018, 66, 13065–13072. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, T.; Zhang, Y.; Wang, L.; Xie, Y. Fumigant toxicity of monoterpenes against fruitfly, Drosophila melanogaster. Ind. Crops Prod. 2016, 81, 147–151. [Google Scholar] [CrossRef]
- Javadpour, M.M.; Juban, M.M.; Lo, W.C.J.; Bishop, S.M.; Alberty, J.B.; Cowell, S.M.; Becker, C.L.; McLaughlin, M.L. De novo antimicrobial peptides with low mammalian cell toxicity. J. Med. Chem. 1996, 39, 3107–3113. [Google Scholar] [CrossRef] [PubMed]
- Elshikh, M.; Ahmed, S.; Funston, S.; Dunlop, P.; McGaw, M.; Marchant, R.; Banat, I.M. Resazurin-based 96-well plate microdilution method for the determination of minimum inhibitory concentration of biosurfactants. Biotechnol. Lett. 2016, 38, 1015–1019. [Google Scholar] [CrossRef] [Green Version]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48, 5–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coulom, H.; Birman, S. Chronic exposure to rotenone models sporadic Parkinson’s disease in Drosophila melanogaster. J. Neurosci. 2004, 24, 10993–10998. [Google Scholar] [CrossRef] [Green Version]



| Strain | Efflux Pump | MIC of Antibiotics | MIC of Benzaldehyde | Association (Antibiotic + MIC/8 of Benzaldehyde) |
|---|---|---|---|---|
| 1199 | - | Norfloxacin 287 μg/mL | ≥1024 μg/mL | 256 μg/mL |
| 1199B | NorA | Norfloxacin 256 μg/mL | ≥1024 μg/mL | 128 μg/mL |
| IS-58 | TetK | Tetracycline 128 μg/mL | ≥1024 μg/mL | 128 μg/mL |
| K2068 | MepA | Ciprofloxacin 64 μg/mL | ≥1024 μg/mL | 32 μg/mL |
| RN4220 | QacC | Erythromycin ≥ 1024 μg/mL | ≥1024 μg/mL | 1024 μg/mL |
| K4414 | QacA/B | Penicillin 362 μg/mL | ≥1024 μg/mL | 1024 μg/mL |
| K4100 | MsrA | Oxacillin 162 μg/mL | ≥1024 μg/mL | 143 μg/mL |
| Strain | Pump | Family | Gene | Antibiotic |
|---|---|---|---|---|
| 1199 | - | - | - | Norfloxacin |
| 1199B | NorA | MFS | norA Smal D | Norfloxacin |
| IS-58 | TetK | MFS | Plasmid PT181 | Tetracycline |
| K2068 | MepA | MATE | MepA | Ciprofloxacin |
| K4100 | QacC | SMR | qacC | Oxacilin |
| K4414 | QacA/B | SMR | qacA/B | Penicillin |
| RN4220 | MsrA | MRSA | msrA pUL5054 | Erythromycin |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neto, L.J.d.L.; Ramos, A.G.B.; Freitas, T.S.d.; Barbosa, C.R.d.S.; de Sousa Júnior, D.L.; Siyadatpanah, A.; Nejat, M.; Wilairatana, P.; Coutinho, H.D.M.; da Cunha, F.A.B. Evaluation of Benzaldehyde as an Antibiotic Modulator and Its Toxic Effect against Drosophila melanogaster. Molecules 2021, 26, 5570. https://doi.org/10.3390/molecules26185570
Neto LJdL, Ramos AGB, Freitas TSd, Barbosa CRdS, de Sousa Júnior DL, Siyadatpanah A, Nejat M, Wilairatana P, Coutinho HDM, da Cunha FAB. Evaluation of Benzaldehyde as an Antibiotic Modulator and Its Toxic Effect against Drosophila melanogaster. Molecules. 2021; 26(18):5570. https://doi.org/10.3390/molecules26185570
Chicago/Turabian StyleNeto, Luiz Jardelino de Lacerda, Andreza Guedes Barbosa Ramos, Thiago Sampaio de Freitas, Cristina Rodrigues dos Santos Barbosa, Dárcio Luiz de Sousa Júnior, Abolghasem Siyadatpanah, Morteza Nejat, Polrat Wilairatana, Henrique Douglas Melo Coutinho, and Francisco Assis Bezerra da Cunha. 2021. "Evaluation of Benzaldehyde as an Antibiotic Modulator and Its Toxic Effect against Drosophila melanogaster" Molecules 26, no. 18: 5570. https://doi.org/10.3390/molecules26185570






