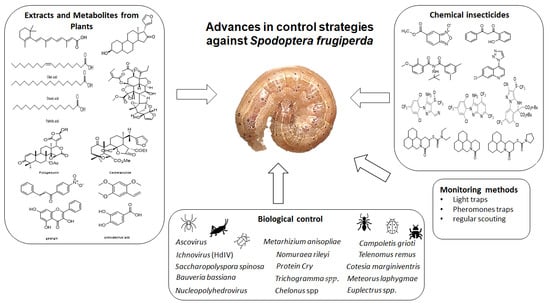
Advances in Control Strategies against Spodoptera frugiperda. A Review
Abstract
:1. Introduction
2. Chemical Insecticides
3. Extracts and Metabolites from Plants
4. Biological Control
5. Monitoring Methods
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- He, H.; Qin, X.; Dong, F.; Ye, J.; Xu, C.; Zhang, H.; Liu, Z.; Lv, X.; Wu, Y.; Jiang, X.; et al. Synthesis, characterization of two matrine derivatives and their cytotoxic effect on Sf9 cell of Spodoptera frugiperda. Sci. Rep. 2020, 10, 17999. [Google Scholar] [CrossRef] [PubMed]
- Casmuz, A.; Juárez, M.L.; Socías, M.G.; Murúa, M.G.; Prieto, S.; Medina, S.; Willink, E.; Gastaminza, G. Review of the host plants of fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae). Rev. Soc. Entomol. Argent. 2010, 69, 209–231. [Google Scholar]
- Montezano, D.G.; Specht, A.; Sosa-Gómez, D.R.; Roque-Specht, V.F.; Sousa-Silva, J.C.; Paula-Moraes, S.V.; Peterson, J.A.; Hunt, T.E. Host plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr. Entomol. 2018, 26, 286–301. [Google Scholar] [CrossRef] [Green Version]
- Bolzan, A.; Padovez, F.E.O.; Nascimento, A.R.B.; Kaiser, I.S.; Lira, E.C.; Amaral, F.S.A.; Kanno, R.H.; Malaquias, J.B.; Omoto, C. Selection and characterization of the inheritance of resistance of Spodoptera frugiperda (Lepidoptera: Noctuidae) to chlorantraniliprole and cross-resistance to other diamide insecticides. Pest Manag. Sci. 2019, 75, 2682–2689. [Google Scholar] [CrossRef] [PubMed]
- Lira, E.C.; Bolzan, A.; Nascimento, A.R.; Amaral, F.S.; Kanno, R.H.; Kaiser, I.S.; Omoto, C. Resistance of Spodoptera frugiperda (Lepidoptera:Noctuidae) to spinetoram: Inheritance and cross-resistance to spinosad. Pest Manag. Sci. 2020, 76, 2674–2680. [Google Scholar] [CrossRef] [PubMed]
- Mink, J.S.; Luttrell, R.G. Mortality of fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae) eggs, larvae and adults exposed to several insecticides on cotton. J. Entomol. Sci. 1989, 24, 563–571. [Google Scholar] [CrossRef]
- Adamczyk, J.J.; Leonard, B.R.; Graves, J.B. Toxicity of selected insecticides to fall armyworms (Lepidoptera: Noctuidae) in laboratory bioassay studies. Fla. Entomol. 1999, 82, 230–236. [Google Scholar] [CrossRef]
- Dres, M.; Mallet, J. Host races in plant-feeding insects and their importance sympatric speciation. Philos. Trans. R. Soc. Sci. 2002, 35, 471–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vélez-Arango, A.M.; Arango, R.E.; Villanueva, D.; Aguilera, E.; Saldamando, C.I. Identification of Spodoptera frugiperda bi-otypes (Lepidoptera: Noctuidae) through using mitochondrial and nuclear markers. Rev. Colom. Entom. 2008, 34, 145–150. [Google Scholar]
- Montezano, D.G.; Specht, A.; Sosa-Gómez, D.R.; Roque-Specht, V.F.; de Paula-Moraes, S.V.; Peterson, J.A.; Hunt, T.E. De-velopmental parameters of Spodoptera frugiperda (Lepidoptera: Noctuidae) immature stages under controlled and standardized conditions. J. Agric. Sci. 2019, 11, 76–89. [Google Scholar]
- Nagoshi, R.N.; Meagher, R.L. Behavior and distribution of the two fall armyworm host strain in Florida. Fla. Entomol. 2004, 87, 440–449. [Google Scholar] [CrossRef]
- Salinas-Hernandez, H.; Saldamando-Benjumea, C.I. Haplotype identification within Spodoptera frugiperda (J.E. Smith) (Lep-idoptera: Noctuidae) corn and rice strains from Colombia. Neotrop. Entomol. 2011, 40, 421–430. [Google Scholar] [PubMed]
- EPA. DDT-A Brief History and Status. Available online: https://www.epa.gov/ingredients-used-pesticide-products/ddt-brief-history-and-status (accessed on 1 September 2021).
- Carvalho, R.A.; Omoto, C.; Field, L.M.; Williamson, M.S.; Bass, C. Investigating the molecular mechanisms of organo-phosphate and pyrethroid resistance in the fall armyworm Spodoptera frugiperda. PLoS ONE 2013, 8, e62268. [Google Scholar] [CrossRef] [Green Version]
- Okuma, D.M.; Bernardi, D.; Horikoshi, R.J.; Bernardi, O.; Silva, A.P.; Omoto, C. Inheritance and fitness costs of Spodoptera frugiperda (Lepidoptera: Noctuidae) resistance to spinosad in Brazil. Pest Manag. Sci. 2018, 74, 1441–1448. [Google Scholar] [CrossRef]
- Guan, F.; Zhang, J.; Shen, H.; Wang, X.; Padovan, A.; Walsh, T.K.; Tay, W.T.; Gordon, K.H.J.; James, W.; Czepak, C.; et al. Whole-genome sequencing to detect mutations associated with resistance to insecticides and Bt proteins in Spodoptera frugiperda. Insect Sci. 2020, 28, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.X.; Huang, J.M.; Ni, H.; Guo, D.; Yang, F.X.; Wang, X.; Wu, S.F.; Gao, C.F. Susceptibility of fall armyworm, Spodoptera frugiperda (J.E. Smmith), to eight insecticides in China, with special reference to lambda-cyhalothrin. Pestic. Biochem. Physiol. 2020, 168, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, B.R.; Cordeiro, A.K.; Shigeo, T.F.; Czepak, C.; Fernandes, P.; Radomille, T.G. Efficiency of insecticides in the control of Spodoptera frugiperda (J. E. Smith 1797) (Lepidoptera: Noctuidae) on cotton crop. Pesqui. Agropecu. Trop. 2005, 35, 179–182. [Google Scholar]
- Zamora, M.C.; Martínez, A.M.; Nieto, M.S.; Schneider, M.I.; Figueroa, J.I.; Pineda, S. Activity of several biorational insecticides against the fall armyworm. Rev. Fitotec. Mex. 2008, 31, 35–357. [Google Scholar]
- Patidar, A.K.; Jeyakandan, M.; Mobiya, A.K.; Selvam, G. Exploring the potential of quinoxaline moiety. Int. J. PharmTech Res. 2011, 3, 386–392. [Google Scholar]
- Gupta, B.; Rani, M.; Kumar, R.; Dureja, P. Decay profile and metabolic pathways of quinalphos in water, soil, and plants. Chemosphere 2011, 85, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Yu, W.; Min, L.J.; Wedge, D.E.; Tan, C.X.; Weng, J.Q.; Wu, H.K.; Cantrell, C.L.; Bajsa-Hirschel, J.; Hua, X.W.; et al. Synthesis and pesticidal activities of new quinoxalines. J. Agric. Food Chem. 2020, 15, 7324–7332. [Google Scholar] [CrossRef] [PubMed]
- Rosas-García, N.; Herrera-Mayorga, V.; Mireles-Martínez, M.; Villegas-Mendoza, J.M.; Rivera, G. Toxic activity of n-oxide derivatives against three mexican populations of Spodoptera frugiperda. Southwest. Entomol. 2014, 39, 717–726. [Google Scholar] [CrossRef]
- Niknam, K.; Saberi, D.; Mohagheghnejad, M. Silica bonded s-sulfonic acid: A recyclable catalyst for the synthesis of quinoxalines at room temperature. Molecules 2009, 14, 1915–1926. [Google Scholar] [CrossRef] [Green Version]
- Xia, R.; Guo, T.; Chen, M.; Su, S.; He, J.; Tang, X.; Jiang, S.; Xue, W. Synthesis, antiviral and antibacterial activities and action mechanism of penta-1,4-dien-3-one oxime ether derivatives containing a quinoxaline moiety. New J. Chem. 2019, 43, 16461–16467. [Google Scholar] [CrossRef]
- Khatoon, H.; Abdulmalek, E. Novel synthetic routes to prepare biologically active quinoxalines and their derivatives: A synthetic review for the last two decades. Molecules 2021, 18, 1055. [Google Scholar] [CrossRef]
- Romanelli, G.; Virla, E.; Duchowicz, P.; Gaddi, A.; Ruiz, D.; Bernardi, D.; Del Valle, E.; Autino, J. Sustainable synthesis of flavonoid derivatives, QSAR study and insecticidal activity against the fall armyworm, Spodoptera frugiperda (Lep.: Noctuidae). J. Agric. Food Chem. 2010, 58, 6290–6295. [Google Scholar] [CrossRef]
- Dent, W.H.; Pobanz, M.A.; Geng, C.; Sparks, T.C.; Watson, G.B.; Letherer, T.J.; Beavers, K.W.; Young, C.D.; Adelfinskaya, Y.A.; Ross, R.R.; et al. Discovery of the aryl heterocyclic amine insecticides: Synthesis, insecticidal activity, field results, mode of action and bioavailability of a leading field candidate. Pest Manag. Sci. 2016, 73, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Yang, S.; Yan, Y.; Lin, F.; Zhang, L.; Zhao, W.; Zhao, C.; Xu, H. Design, synthesis, and insecticidal activity of 5,5-disubstituted 4,5-dihydropyrazolo [1,5-a] quinazolines as novel antagonists of GABA receptors. J. Agric. Food. Chem. 2020, 68, 15005–15014. [Google Scholar] [CrossRef]
- Rosado-Solano, D.N.; Barón-Rodríguez, M.A.; Sanabria Florez, P.L.; Luna-Parada, L.K.; Puerto-Galvis, C.E.; Zorro-González, A.F.; Kouznetsov, V.V.; Vargas-Méndez, L.Y. Synthesis, biological evaluation and in silico computational studies of 7-chloro-4-(1h-1,2,3-triazol-1-yl) quinoline derivatives: Search for new controlling agents against Spodoptera frugiperda (Lepidop-tera: Noctuidae) larvae. J. Agric. Food Chem. 2019, 67, 9210–9219. [Google Scholar] [CrossRef]
- Alves, D.S.; Machado, A.R.T.; Campos, V.A.C.; Oliveira, D.F.; Carvalho, G.A. Selection of annonaceae species for the control of Spodoptera frugiperda (Lepidoptera: Noctuidae) and metabolic profiling of Duguetia lanceolata using nuclear magnetic resonance spectroscopy. J. Econ. Entomol. 2016, 109, 649–659. [Google Scholar] [CrossRef]
- Zaynab, M.; Fatima, M.; Sharif, Y.; Zafar, M.H.; Ali, H.; Khan, K.A. Role of primary metabolites in plant defense against pathogens. Microb. Pathog. 2019, 137, 1–4. [Google Scholar] [CrossRef]
- Lizarazo, H.K.; Mendoza, F.C.; Carrero, S.R. Effect of plant extracts of Polygonum hydropiperoides, Solanum nigrum and Calliandra pittieri in Spodoptera frugiperda. Agron. Colomb. 2008, 26, 427–434. [Google Scholar]
- Franco, A.S.L.; Jiménez, P.A.; Luna, L.C.; Figueroa, B.R. Toxic effect of seeds of four varieties of Carica papaya (Caricaceae) en Spodoptera frugiperda (Lepidoptera: Noctuidae). Fol. Entomol. Mex. 2006, 45, 171–177. [Google Scholar]
- Pérez-Gutiérrez, S.; Zavala-Sánchez, M.A.; González-Chávez, M.M.; Cárdenas-Ortega, N.C.; Ramos-López, M.A. Bioactivity of Carica papaya (Caricaceae) against Spodoptera frugiperda (Lepidoptera: Noctuidae). Molecules 2011, 16, 7502–7509. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, A.A.; Rodríguez, L.D.; Murillo, W.; Méndez, J.J.; Rueda, E.A. Antifeedant activity of secondary metabolites of citrus waste on Spodoptera frugiperda (Lepidoptera: Noctuidae). Rev. Colomb. Entomol. 2013, 39, 113–119. [Google Scholar]
- Braga, T.M.; Rocha, L.; Chung, T.Y.; Oliveira, R.F.; Pinho, C.; Oliveira, A.I.; Morgado, J.; Cruz, A. Biological activities of gedunin—A limonoid from the Meliaceae Family. Molecules 2020, 25, 493. [Google Scholar] [CrossRef] [Green Version]
- Ruberto, G.; Renda, A.; Tringali, C.; Napoli, E.; Simmonds, M. Citrus limonoids and their semisynthetic derivatives as anti-feedant agents against larvae. A structure-activity relationship study. J. Agric. Food Chem. 2002, 50, 6766–6774. [Google Scholar] [CrossRef]
- Trujillo-Ruiz, P.A.; Zapata-Restrepo, L.N.; Hoyos-Sánchez, R.A.; Yepes-Rodríguez, F.C.; Capataz-Tafur, J.; Orozco-Sánchez, F. Determination of the DL50 y TL50 ethanol’s extracts of celular suspensions from Azadirachta indica over Spodoptera frugiperda. Revista Facultad Nacional de Agronomía Medellín 2008, 61, 4564–4575. [Google Scholar]
- Céspedes, C.L.; Calderon, J.S.; Lina, L.; Aranda, E. Growth inhibitory effects on fall armyworm Spodoptera frugiperda of some limonoids isolated from Cedrela spp. (Meliaceae). J. Agric. Food Chem. 2000, 48, 1903–1908. [Google Scholar] [CrossRef]
- Ávila, M.C.; Cuca, S.L.E.; Cerón, S.J.A. Insecticidal activity against Spodoptera frugiperda (Lepidoptera: Noctuidae) of metab-olites isolated from the aerial part of Piper septuplinervium (Miq.) c. dc. and inflorescences of Piper subtomentosum Trel. & Yunck. (Piperaceae). Quím. Nova. 2015, 37, 442–446. [Google Scholar]
- Batista-Pereira, L.G.; Castral, T.C.; Silva, M.T.M.; Amaral, B.R.; Fernandes, J.B.; Vieira, P.C.; Silva, M.F.; Correa, A.G. Insec-ticidal activity of synthetic amides on Spodoptera frugiperda. Z. Naturforsch. 2006, 61, 196–202. [Google Scholar] [CrossRef]
- Castral, T.; Matos, A.; Monteiro, J.; Araujo, F.; Bondancia, T.; Batista-Pereira, L.; Fernandes, J.; Vieira, P.; Da Silva, F.; Correa, A. Synthesis of a combinatorial library of amides and its evaluation against the fall armyworm, Spodoptera frugiperda. J. Agric. Food Chem. 2011, 59, 4822–4827. [Google Scholar] [CrossRef] [PubMed]
- Phambala, K.; Tembo, Y.; Kasambala, T.; Kabambe, V.H.; Stevenson, P.C.; Belmain, S.R. Bioactivity of common pesticidal plants on fall armyworm larvae (Spodoptera frugiperda). Plants 2020, 9, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carpinella, M.; Defago, M.; Valladares, G.; Palacios, S. Antifeedant and insecticide properties of a limonoid from Melia azedarach (Meliaceae) with potential use for pest management. J. Agric. Food Chem. 2003, 51, 369–374. [Google Scholar] [CrossRef]
- Dyer, L.A.; Dodson, C.D.; Stireman, J.O.; Tobler, M.A.; Smilanich, A.M.; Fincher, R.M.; Letourneau, D.K. Synergistic effects of three Piper amides on generalist and specialist herbivores. J. Chem. Ecol. 2003, 29, 2499–2514. [Google Scholar] [CrossRef] [PubMed]
- De Castro, M.D.P.; da Silva, P.H.S.; Padua, L.E.D. Piper tuberculatum Jacq. (Piperaceae) extract activity against Spodoptera frugiperda (J. E. Smith). Rev. Cienc. Agron. 2008, 39, 437–442. [Google Scholar]
- Fontes, E.M.G.; Laumann, R. Special section on biological control. Neotrop. Entomol. 2019, 48, 873–874. [Google Scholar] [CrossRef] [Green Version]
- Stenberg, J.A.; Sundh, I.; Becher, P.G.; Björkman, C.; Dubey, M.; Egan, P.A.; Friberg, H.; Gil, J.F.; Jensen, D.F.; Jonsson, M.; et al. When is it biological control? A framework of definitions, mechanisms, and classifications. J. Pest Sci. 2021, 94, 665–676. [Google Scholar] [CrossRef]
- Ochoa, G.; Arrivillaga, J. Bacillus thuringiensis: Advances and perspectives in the biological control of Aedes aegypti. Boletin de Malariologóa y Salud Ambiental 2009, 59, 181–191. [Google Scholar]
- Machado, E.P.; Rodrigues, J.D.; Führ, F.M.; Zago, S.L.; Marques, L.H.; Santos, A.C.; Nowatzki, T.; Dahmer, M.L.; Omoto, C.; Bernardi, O. Cross-crop resistance of Spodoptera frugiperda selected on Bt maize to genetically-modified soybean expressing Cry1Ac and Cry1F proteins in Brazil. Sci. Rep. 2020, 10, 10080. [Google Scholar] [CrossRef]
- Santos-Amaya, O.; Delgado-Restrepo, E.; Arguelles-Cárdenas, O.; Aguilera-Garramuño, J.H. Evaluation of Spodoptera com-plex behavior with the introduction of transgenic cotton in Tolima, Colombia. Cienc. Tecnol. Agropecuaria 2009, 10, 24–32. [Google Scholar]
- Ingber, D.A.; Mason, C.E.; Flexner, L. Cry1 Bt susceptibilities of fall armyworm (Lepidoptera: Noctuidae) host strains. J. Econ. Entomol. 2018, 111, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Boaventura, D.; Ulrich, J.; Lueke, B.; Bolzan, A.; Okuma, D.; Gutbrod, O.; Geibel, S.; Zeng, Q.; Dourado, P.M.; Martinelli, S.; et al. Molecular characterization of Cry1F resistance in the fall armyworm, Spodoptera frugiperda from Brazil. Insect Biochem. Mol. Biol. 2020, 116, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lemes, A.R.N.; Chiaradia, D.C.; Brunini, C.L.P.C.; Aparecido, F.O.; Ferré, J.; Franco, L.M.V.; Apparecida, D.J. Synergism and antagonism between Bacillus thuringiensis Vip3a and Cry1 proteins in Heliothis virescens, Diatraea saccharalis and Spodoptera frugiperda. PLoS ONE 2014, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Soares, F.C.; Nunes, L.A.R.; Sebastião, I.; Desidério, J.A. Synergism of the Bacillus thuringiensis Cry1, Cry2, and Vip3 proteins in Spodoptera frugiperda control. Appl. Biochem. Biotechnol. 2019, 188, 798–809. [Google Scholar] [CrossRef]
- Zhang, J.; Jin, M.; Yang, Y.; Liu, L.; Yang, Y.; Gómez, I.; Bravo, A.; Soberón, M.; Xiao, Y.; Liu, K. The cadherin protein is not involved in susceptibility to Bacillus thuringiensis Cry1Ab or Cry1Fa Toxins in Spodoptera frugiperda. Toxinas 2020, 12, 375. [Google Scholar] [CrossRef]
- Miles, M. The effects of spinosad, a naturally derived insect control agent to the honeybee. Bull. Insectology. 2003, 56, 119–124. [Google Scholar]
- Williams, T.; Cisneros, J.; Penagos, D.I.; Valle, J.; Tamez-Guerra, P. Ultralow rates of spinosad in phagostimulant granules provide control of Spodoptera frugiperda (Lepidoptera: Noctuidae). J. Econ. Entomol. 2004, 97, 422–428. [Google Scholar] [CrossRef]
- Sari, D.; Gupta, K.; Thimiri, G.R.D.B.; Aubert, A.; Drncová, P.; Garzoni, F.; Fitzgerald, D.; Berger, I. The multiBac baculovi-rus/insect cell expression vector system for producing complex protein biologics. Adv. Exp. Med. Biol. 2016, 896, 199–215. [Google Scholar]
- Schroeder, L.; Mar, T.B.; Haynes, J.R.; Wang, R.; Wempe, L.; Goodin, M.M. Host range and population survey of Spodoptera frugiperda rhabdovirus. J. Virol. 2019, 93, 1–30. [Google Scholar] [CrossRef] [Green Version]
- Cuartas, P.; Barrera, G.; Barreto, E.; Villamizar, L. Characterization of a Colombian granulovirus (Baculoviridae: Betaba-culovirus) isolated from Spodoptera frugiperda (Lepidoptera: Noctuidae) larvae. Biocontrol Sci. Technol. 2014, 24, 1265–1285. [Google Scholar] [CrossRef]
- Pidre, M.L.; Sabalette, K.B.; Romanowski, V.; Ferrelli, M.L. Identification of an Argentinean isolate of Spodoptera frugiperda granulovirus. Rev. Argent. Microbiol. 2019, 51, 381–385. [Google Scholar] [CrossRef]
- Zaghloul, H.A.H.; Hice, R.; Arensburger, P.; Federici, B.A. Transcriptome analysis of the Spodoptera frugiperda ascovirus in vivo provides insights into how its apoptosis inhibitors and caspase promote the increased synthesis of viral vesicles and virion progeny. J. Virol. 2017, 91, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Visconti, V.; Eychenne, M.; Darboux, I. Modulation of antiviral immunity by the ichnovirus HdIV in Spodoptera frugiperda. Mol. Immunol. 2019, 108, 89–101. [Google Scholar] [CrossRef]
- Bentivenha, J.P.F.; Rodrigues, J.G.; Lima, M.F.; Marçon, P.; Popham, H.J.R.; Omoto, C. Baseline susceptibility of Spodoptera frugiperda (Lepidoptera: Noctuidae) to SfMNPV and evaluation of cross-resistance to major insecticides and Bt proteins. J. Econ. Entomol. 2019, 12, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.M.; Uribe, L.A.; Ruiz, J.C.; Tabima, L.; Gómez, J.A.; Villamizar, L.F. Spodoptera frugiperda nucleopolyhedrovirus SfNPV003: Compatibility with agrochemicals and storage stability. Corpoica Cienc. Tecnol. Agropecu. 2014, 15, 219–228. [Google Scholar] [CrossRef] [Green Version]
- Peteira, B.; González, I.; Arias, Y.; Fernández, T.A.; Miranda, I.; Martínez, B. Biochemical characterization of six isolates of Beauveria bassiana (balsamo) Vuillemin. Rev. Protección Veg. 2011, 26, 16–22. [Google Scholar]
- Gurrola-Reyes, J.N.; González-Maldonado, M.B.; Correa-Ramírez, M.M. Evaluation of the toxicity of bioinsecticides and biorationals in the control of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the state of Durango. Entomol. Mex. 2014, 1, 196–201. [Google Scholar]
- Lezama, R.; Molina, J.; López, M.; Pescador, A.; Galindo, E.; Angel, C.A.; Michel, A.C. Effect of entomopathogen fungus Metarhizium anisopliae on the control of the cogollero corn worm in the field. Av. Investig. Agropecu. 2005, 9, 1–6. [Google Scholar]
- Rivero-Borja, M.; Guzmán-Franco, A.W.; Rodríguez-Leyva, E.; Santillán-Ortega, C.; Pérez-Panduro, A. Interaction of Beauveria bassiana and Metarhizium anisopliae with chlorpyrifos ethyl and spinosad in Spodoptera frugiperda larvae. Pest Manag. Sci. 2018, 74, 2047–2052. [Google Scholar] [CrossRef]
- Villamizar, L.; Arriero, C.; Bosa, C.F.; Cotes, A.M. Development of pre formulated products based on Nomuraea rileyi for control of Spodoptera frugiperda (Lepidoptera: Moctuidae). Rev. Col. Ento. 2004, 30, 1–11. [Google Scholar]
- Camacho, J.E.; Gómez, M.I.; Villamizar, L.F. Effect of temperature and two drying processes on the insecticidal activity of a nucleopolyhedrovirus of Spodoptera frugiperda. Rev. Mex. Ing. Quim. 2013, 12, 437–450. [Google Scholar]
- Bosa, O.C.F.; Chávez, D.; Torres, L.; París, A.; Villamizar, L.; Cotes, A.M. Evaluation of natíve isolates of Nomuraea rileyi for the control of Spodoptera frugiperda (Lepidoptera: Noctuidae). Rev. Colomb. Entomol. 2004, 30, 1–7. [Google Scholar]
- Arthurs, S.; Dara, S.K. Microbial biopesticides for invertebrate pests and their markets in the United States. J. Invertebr. Pathol. 2019, 165, 13–21. [Google Scholar] [CrossRef]
- García-Gutiérrez, C.; González-Maldonado, M.B.; González-Hernández, A. Natural parasitism of Braconidae and Ichneu-monidae (Hymenoptera) on Spodoptera frugiperda (Lepidoptera: Noctuidae). Rev. Colomb. Entomol. 2013, 39, 212–215. [Google Scholar]
- Jaraleño-Teniente, J.; Lomeli-Flores, J.R.; Rodríguez-Leyva, E.; Bujanos-Muñiz, R.; Rodríguez-Rodríguez, S.E. Egg Parasitoids Survey of Spodoptera frugiperda (Smith) (Lepidoptera: Noctuidae) in maize and sorghum in Central Mexico. Insects 2020, 11, 157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murillo, H. Predation of Spodoptera frugiperda (Lepidoptera: Noctuidae) eggs in cotton and corn in El Espinal, Tolima, Co-lombia. Rev. Colomb. Entomol. 2014, 40, 63–66. [Google Scholar]
- Molina-Ochoa, J.; Carpenter, J.E.; Heinrichsc, E.A.; Foster, J.E. Parasitoids and parasites of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas and Caribbean basin: An inventory. Fla. Entomol. 2003, 86, 254–289. [Google Scholar] [CrossRef]
- Meagher Jr, R.L.; Nuessly, G.S.; Nagoshi, R.N.; Hay-Roe, M.M. Parasitoids attacking fall armyworm (Lepidoptera: Noctuidae) in sweet corn habitats. Biol. Control 2016, 95, 66–72. [Google Scholar] [CrossRef] [Green Version]
- Canico, A.; Mexia, A.; Santos, L. First report of native parasitoids of fall armyworm Spodoptera frugiperda Smith (Lepidoptera: Noctuidae) in Mozambique. Insects 2020, 11, 615. [Google Scholar] [CrossRef] [PubMed]
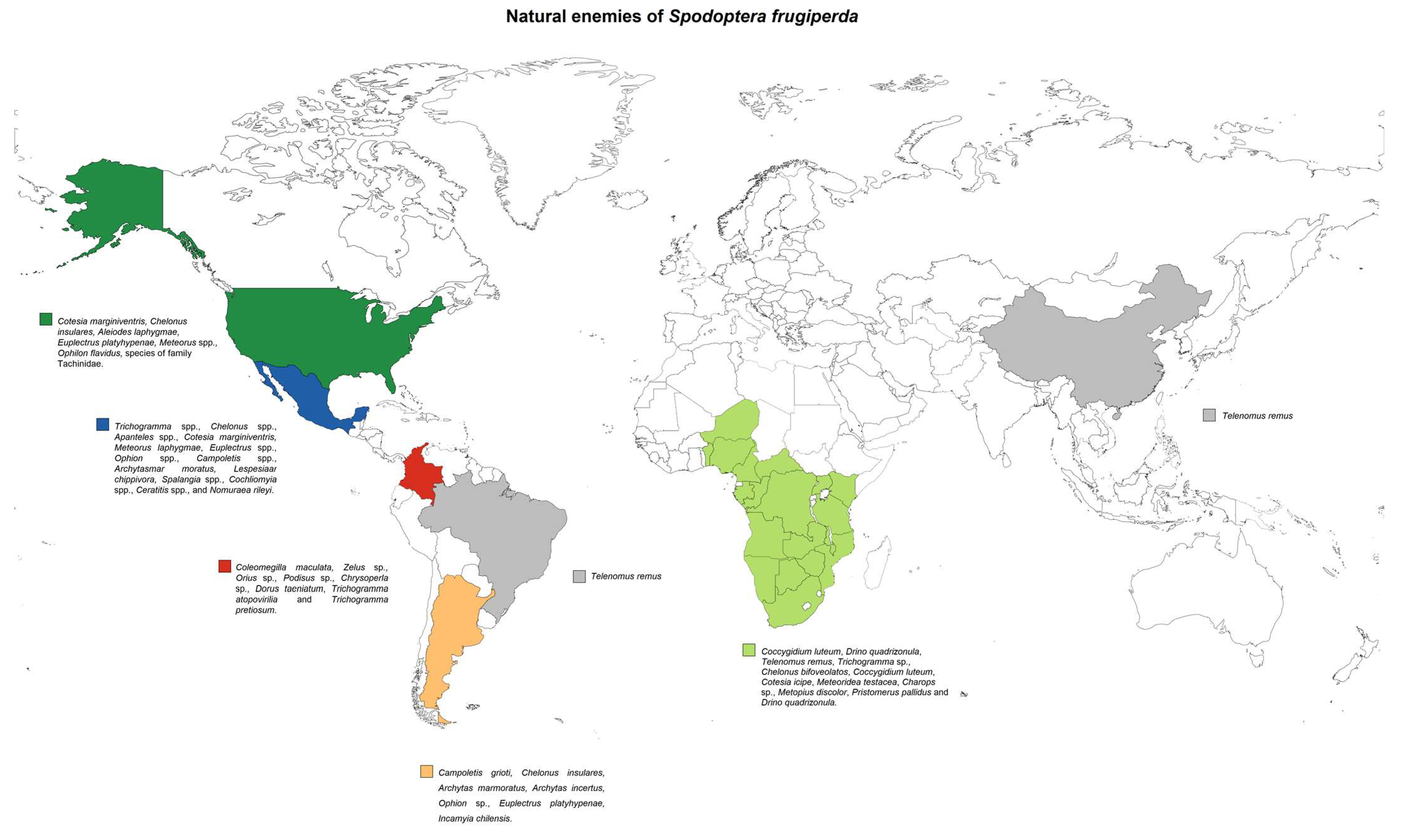
- Kenis, M.; du Plessis, H.; van den Berg, J.; Ba, M.N.; Goergen, G.; Kwadjo, K.E.; Baoua, I.; Tefera, T.; Buddie, A.; Cafà, G.; et al. Telenomus remus, a candidate parasitoid for the biological control of Spodoptera frugiperda in Africa, is already present on the continent. Insects 2019, 10, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agboyi, L.K.; Goergen, G.; Beseh, P.; Mensah, S.A.; Clottey, V.A.; Glikpo, R.; Buddie, A.; Cafà, G.; Offord, L.; Day, R.; et al. Parasitoid complex of fall armyworm, Spodoptera frugiperda, in Ghana and Benin. Insects 2020, 11, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murúa, M.G.; Molina-Ochoa, J.; Fidalgo, P. Natural distribution of parasitoids of larvae of the fall armyworm, Spodoptera frugiperda, in Argentina. J. Insect Sci. 2009, 9, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Li, Y.; Wang, M.; Mao, J.; Zhang, L. Evaluating the potential of using Spodoptera litura eggs for mass-rearing Telenomus remus, a promising egg parasitoid of Spodoptera frugiperda. Insects 2021, 12, 384. [Google Scholar] [CrossRef]
- Prasanna, B.M.; Huesing, J.E.; Eddy, R.; Peschke, V.M. Fall Armyworm in Africa: A Guide for Integrated Pest Management, 1st ed.; CIMMYT: Mexico City, Mexico, 2018; pp. 63–88. [Google Scholar]
- Tepa-Yotto, G.T.; Tonnang, H.E.Z.; Goergen, G.; Subramanian, S.; Kimathi, E.; Abdel-Rahman, E.M.; Flø, D.; Thunes, K.H.; Fiaboe, K.K.M.; Niassy, S.; et al. Global habitat suitability of Spodoptera frugiperda (JE Smith) (Lepidoptera, Noctuidae): Key parasitoids considered for its biological control. Insects 2021, 12, 273. [Google Scholar] [CrossRef]
- Harrison, R.D.; Thierfelder, C.; Baudron, F.; Chinwada, P.; Midega, C.; Schaffner, U.; van den Berg, J. Agro-ecological options for fall armyworm (Spodoptera frugiperda JE Smith) management: Providing low-cost, smallholder friendly solutions to an invasive pest. J. Environ. Manag. 2019, 243, 318–330. [Google Scholar] [CrossRef] [PubMed]
- Gebreziher, H.G. Review on management methods of fall armyworm (Spodoptera frugiperda JE Smith) in Sub-Saharan Africa. Int. J. Entomol. Res. 2020, 5, 9–14. [Google Scholar]
- Liu, Y.J.; Zhang, D.D.; Yang, L.Y.; Dong, Y.H.; Liang, G.M.; Philip, D.; Ren, G.; Xu, P.J.; Wu, K. Analysis of phototactic responses in Spodoptera frugiperda using Helicoverpa armigera as control. J. Integr. Agric. 2021, 20, 821–828. [Google Scholar] [CrossRef]
- Gebreziher, H.G.; Gebreziher, F.G. Effect of integrating night-time light traps and push-pull method on monitoring and deterring adult fall armyworm (Spodoptera frugiperda). Int. J. Entomol. Res. 2019, 5, 28–32. [Google Scholar]
- Wyatt, T.D. Pheromones. Curr. Biol. 2017, 27, R739–R743. [Google Scholar] [CrossRef]
- Cruz-Esteban, S.; Rojas, J.C.; Malo, E.A. A pheromone lure for catching fall armyworm males (Lepidoptera: Noctuidae) in Mexico. Acta Zool. Mex. 2020, 36, e3612271. [Google Scholar] [CrossRef] [Green Version]
- Batista-Pereira, L.G.; Stein, K.; de Paula, A.F.; Moreira, J.A.; Cruz, I.; Corrêa-Figueiredo, M.L.; Perri, J.; Correa, A.G. Isolation, identification, synthesis, and field evaluation of the sex pheromone of the Brazilian population of Spodoptera frugiperda. J. Chem. Ecol. 2006, 32, 1085–1099. [Google Scholar] [CrossRef]
- Bratovich, C.; Saluso, A.; Murua, M.G.; Guerenstein, P.G. Evaluation of sex pheromone formulations to attract Spodoptera frugiperda (Lepidoptera: Noctuidae) adult males in Argentina. Revista de la Sociedad Entomológica Argentina 2019, 78, 7–14. [Google Scholar] [CrossRef]
- Guerrero, A.; Malo, E.A.; Coll, J.; Quero, C. Semiochemical and natural product-based approaches to control Spodoptera spp. (Lepidoptera: Noctuidae). J. Pest Sci. 2014, 87, 231–247. [Google Scholar] [CrossRef]
- Cruz, I.; Figueiredo, M.; Silva, R.; Silva, I.; Paula, C.; Foster, J. Using sex pheromone traps in the decision-making process for pesticide application against fall armyworm (Spodoptera frugiperda [Smith] [Lepidoptera: Noctuidae]) larvae in maize. Int. J. Pest Manag. 2012, 58, 83–90. [Google Scholar] [CrossRef] [Green Version]
- Tepa-Yotto, G.; Winsou, J.K.; Dahoueto, B.; Tamò, M. Assessing new scouting approaches for field sampling of Spodoptera frugiperda and its parasitoids. Proceedings 2021, 68, 1–8. [Google Scholar]
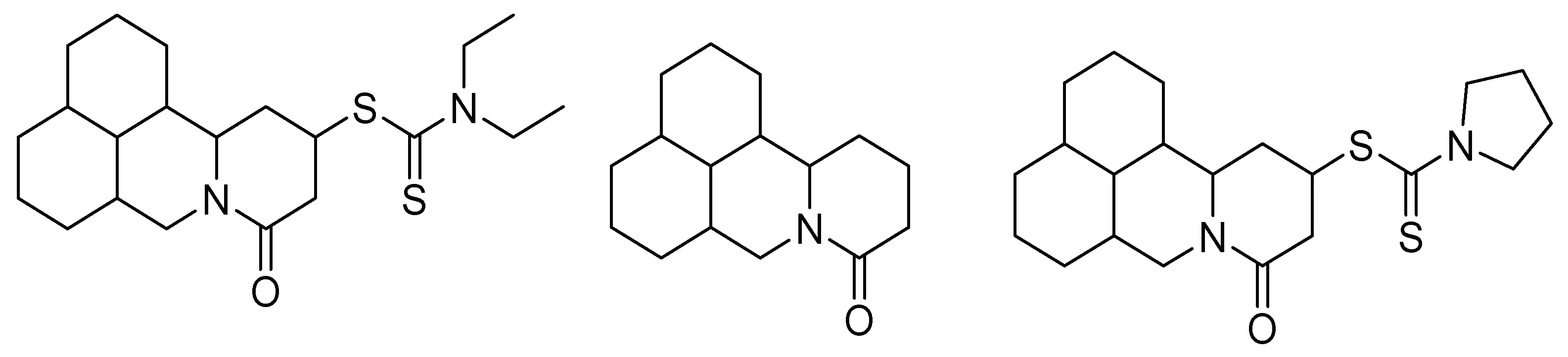
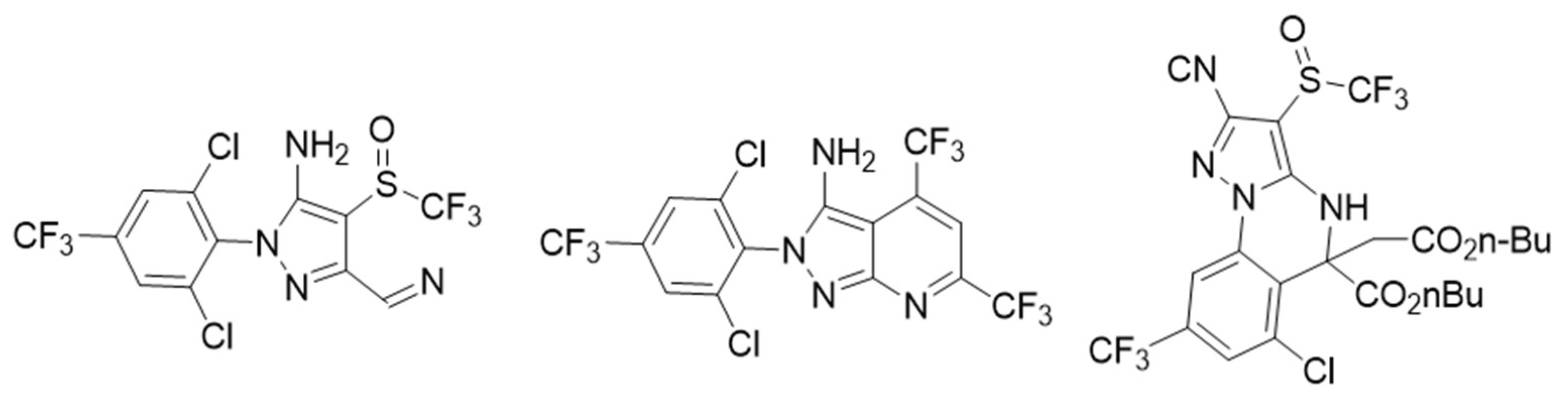
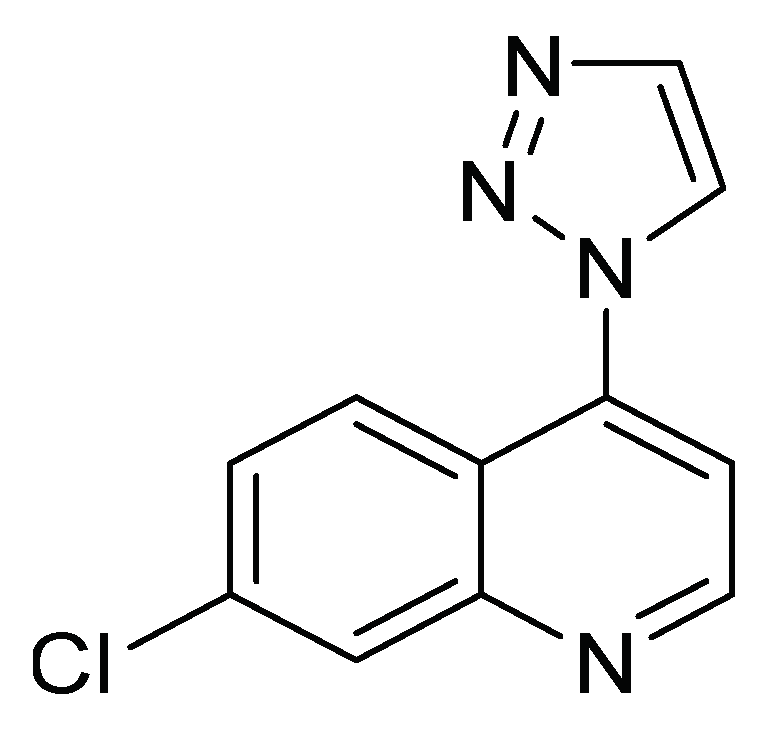

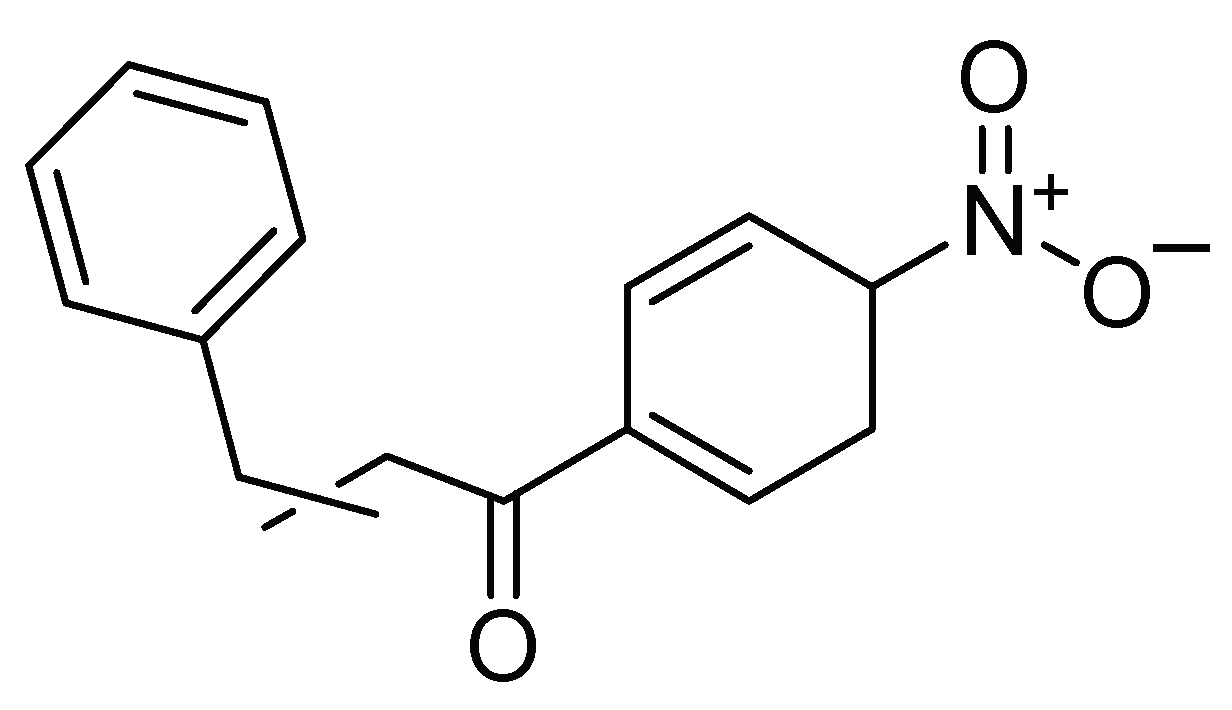

| Control Agents | Application Method | Dose | Activity | Reference | Country |
|---|---|---|---|---|---|
| Matrine and derivatives | In vivo | 0.648 mmol/L 1.13 mmol/L | Apoptosis induction | [1] | China |
| Emamectin benzoate | Ingestion | 0.025 mg/L | Acetylcholinesterase inhibitors | [16] | China |
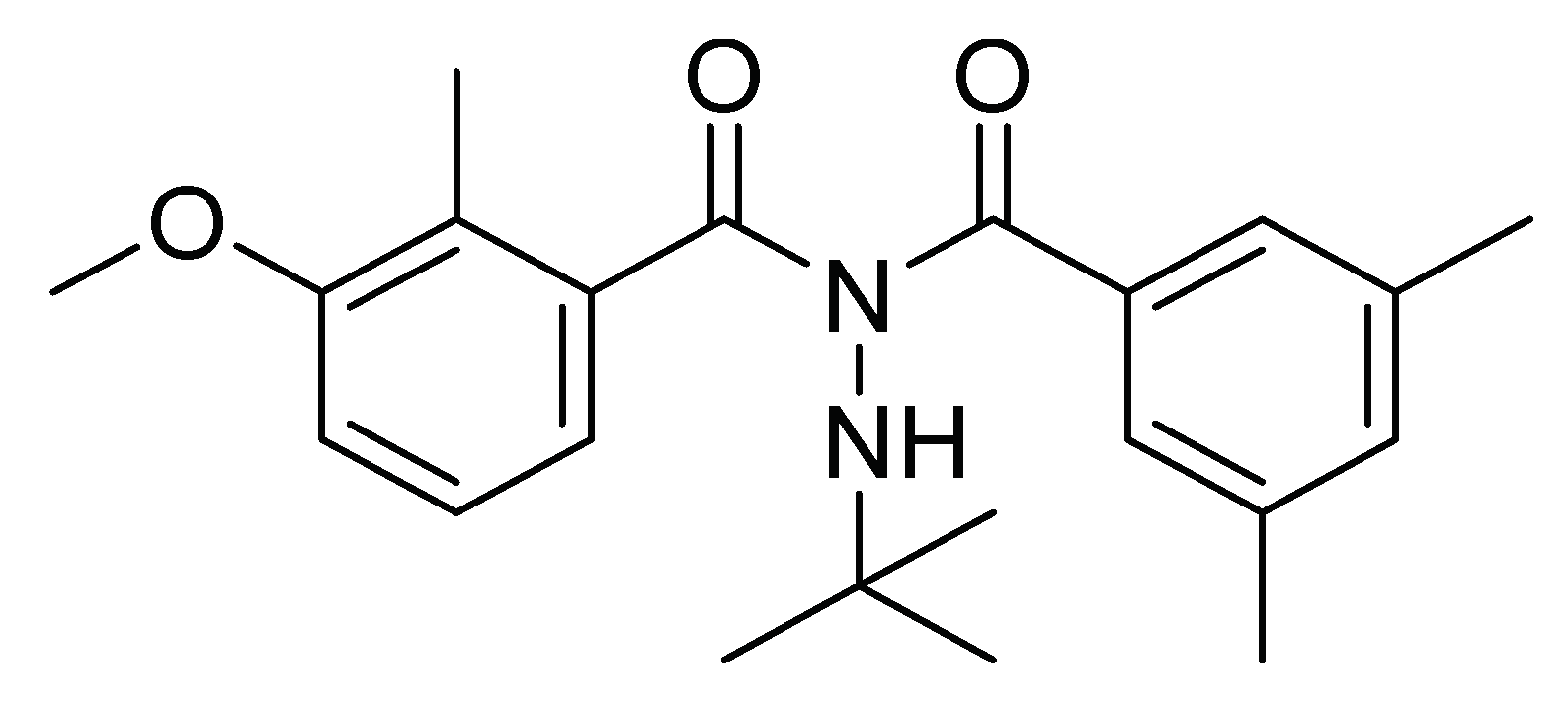
| N′-tert-butyl-N′-(3,5-dimethylbenzoyl)-3-methoxy-2-methyl benzohydrazide | Ingestion | 500 mL/ha | Insecticide Induce premature molting and cause death | [18] | Brazil |
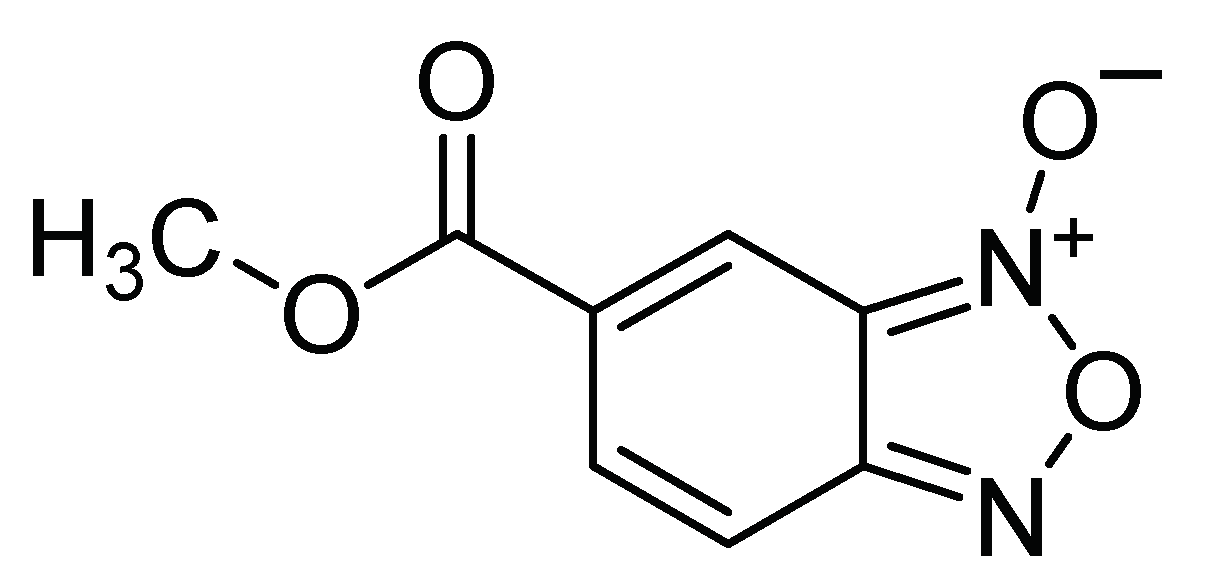
| N-oxide benzofuroxan methyl-5-carboxylate N-oxide derivatives | Ingestion | 0.328 mg/mL 0.229 mg/mL 0.289 mg/mL | Insecticide esterase inhibitor | [23] | Mexico |
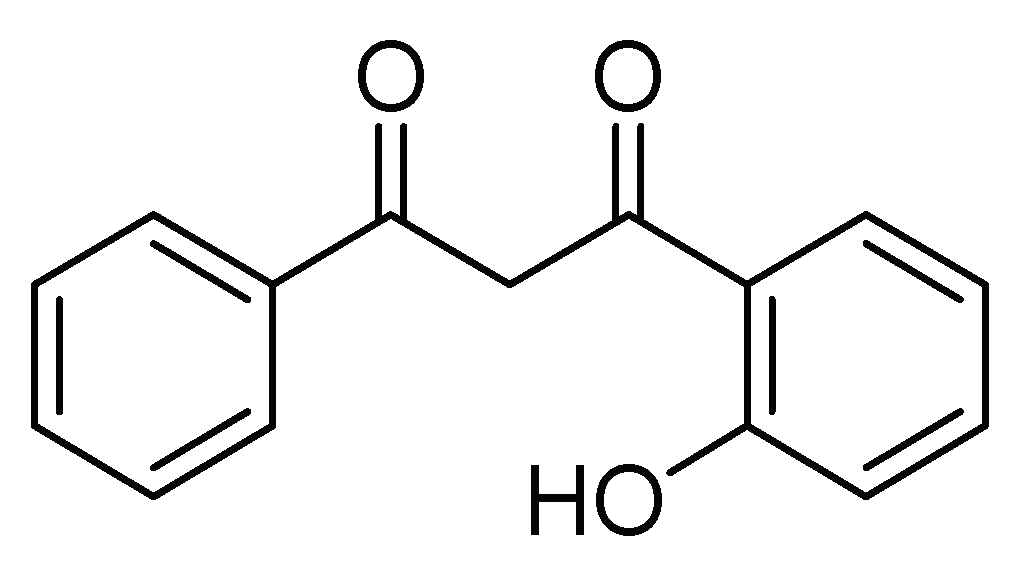
| Flavone derivative of 1-(2-hydroxyphenyl)-3-aryl-1,3-propanedione | Topical | 200 mg/kg | Insecticide Modulation of feeding and oviposition of the insect | [27] | Argentina |
| Aril amine heterocyclic-7-pyrazolo pyridine | Ingestion | 0.85 µg/cm2 | Insecticide GABA antagonists | [28] | United States of America |
| 5-acetyl-8-chloro-5-(3-hydroxypropyl) -7-(trifluoromethyl)-3-((trifluoromethyl)sulfinyl)-4,5-dihydropyrazolo [1,5] quinazoline-2-carbonitrile | Ingestion | 100 mg/L | Insecticide antagonists GABA | [29] | China |
| 4-(4-methyl phenyl)-1H-1,2,3-triazolyl-quinoline | Ingestion | 0.65 mg/g insect | Acetylcholinesterase inhibitors | [30] | Colombia |
| Control Agents | Application Method | Effective Dose | Effectivity/Stage | Reference | Country |
|---|---|---|---|---|---|
| Azadirachta indica extract | Ingestion Topical | 14.79 mg i.a. kg−1 diet 7.06 µg i.a. g−1 larvae | Ovicidal | [19] | Mexico |
| Duguetia lanceolata extract | Ingestion | 946.5 µg/mL | Insecticidal | [31] | Brazil |
| Polygonum hydropiperoides extract | Ingestion | 2.5 mg/L | Insecticidal and antifeedant | [33] | Colombia |
| Carica papaya extract | Ingestion | 10–15% | Larvicidal | [34] | Mexico |
| Citrus sinensis and C. limonia extract | Ingestion | 0.75–1.0% | Antifeedant and antinutritional | [36] | Colombia |
| Citrus limon limonoids (limonina and obacunona) | Ingestion | 0.05 M | Antifeedant | [38] | Italy |
| Citrus limon limonoids (limonol, liomonin, 7-oxime limonin, and methoxime) | Ingestion | 0.05 M | Antifeedant | [38] | Italy |
| Azadirachta indica extract | Ingestion | 2.256 ppm 3.928 ppm 2.818 ppm 1.064 ppm | Antifeedant and repellent | [39] | Colombia |
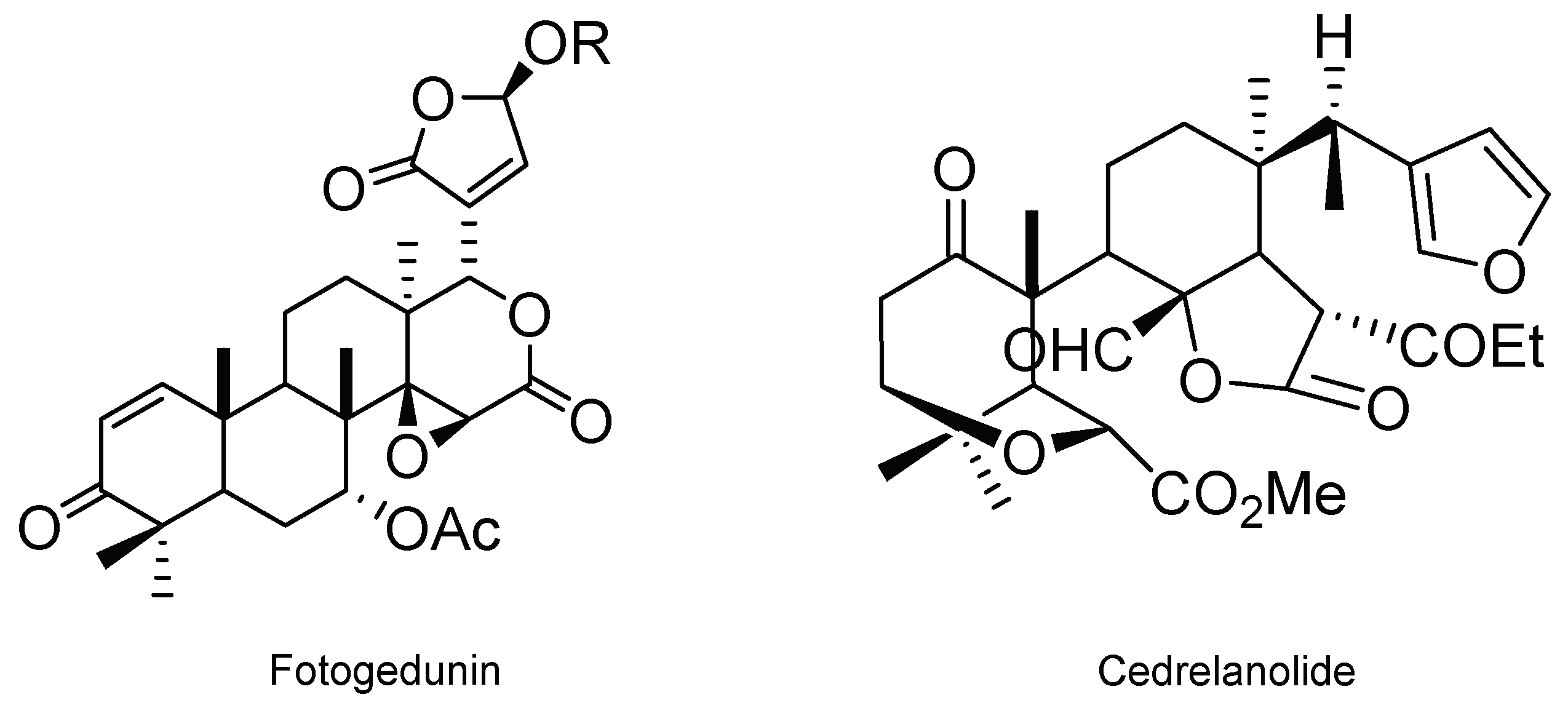
| Cedrela salvadorensis and C. dugessi metabolites (fotogedunin, gedunin, and cedrelanolide) | 39.0 ppm 10.0 ppm 8.0 ppm | Insecticidal | [40] | Mexico | |
| Piper piressi amide (N-[3-(3′,4′-methylenedioxyphenyl)-2-€-propenoyl] piperidine) | Ingestion | 1.07 µg/mg larvae | Insecticidal | [42] | Brazil |
| Natural and synthetic amides of Piper (E)-1-(1-Piperidinyl)-3-[4-(trifluoro methoxy)phenyl]-2-propen-1-one) | Ingestion | 0.793 µg/mg larvae | Insecticidal | [43] | Brazil |
| Lippia javanica, Nicotiana tabacum | Ingestion Contact | 10% | Insecticidal | [44] | Africa |
| Melia azedarach extract | Ingestion | 2000 µg/cm2 | Antifeedant | [45] | Argentina |
| Piper cenacladum amides (piplartine, 4′-desmethylpiplartine) | Ingestion | Piplartine: 0.203 g 4′-desmethyl piplartine: 0.1575 g | Antifeedant | [46] | United States of America |
| Piper tuberculatum extract | Ingestion | 219 mg/insect | Insecticidal | [47] | Brazil |
| Control Agents | Application Method | Effective Dose | Effectivity/Stage | Reference | Country |
|---|---|---|---|---|---|
| Protein Cry and Vip B. thuringiensis Proportion Vip3Aa:Cry1Ca 1:0 0:1 1:2 | Ingestion | 0.44 µg/cm2 0.052 µg/cm2 0.30 µg/cm2 | Insecticidal | [55] | Brazil |
| Saccharopolyspora spinosa | Ingestion | 0.3 and 1.0 g IA/ha | Insecticidal | [59] | Mexico |
| Granulovirus SfGV (VG008) | Ingestion | 4.5 × 105 OB/mL for 29 days | Insecticidal | [62] | Colombia |
| Granulovirus | Ingestion | 1.0 × 108 OB/mL for 14 days | Insecticidal | [63] | Argentina |
| Ascovirus 1a (SfAV-1a), | Topical | 1 × 108/mL for 7 days | Insecticidal | [64] | United States |
| Ichnovirus (HdIV) | Topical | 4 × 105 | Insecticidal | [65] | France |
| Bauveria bassiana | Topical | 1.3 × 108 spores/mL | Insecticidal | [69] | Mexico |
| Metarhizium anisopliae | Topical | 1×1012 conidia/ha | Insecticidal | [70] | Mexico |
| Nucleopolyhedrovirus NVP009 NVP011 | Ingestion | 2.2×105 CI/mL 7.0×105 CI/mL | Insecticidal | [73] | Colombia |
| Nomuraea rileyi | Ingestion | 1.0× 107 conidia/mL | Insecticidal | [74] | Colombia |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paredes-Sánchez, F.A.; Rivera, G.; Bocanegra-García, V.; Martínez-Padrón, H.Y.; Berrones-Morales, M.; Niño-García, N.; Herrera-Mayorga, V. Advances in Control Strategies against Spodoptera frugiperda. A Review. Molecules 2021, 26, 5587. https://doi.org/10.3390/molecules26185587
Paredes-Sánchez FA, Rivera G, Bocanegra-García V, Martínez-Padrón HY, Berrones-Morales M, Niño-García N, Herrera-Mayorga V. Advances in Control Strategies against Spodoptera frugiperda. A Review. Molecules. 2021; 26(18):5587. https://doi.org/10.3390/molecules26185587
Chicago/Turabian StyleParedes-Sánchez, Francisco A., Gildardo Rivera, Virgilio Bocanegra-García, Hadassa Y. Martínez-Padrón, Martín Berrones-Morales, Nohemí Niño-García, and Verónica Herrera-Mayorga. 2021. "Advances in Control Strategies against Spodoptera frugiperda. A Review" Molecules 26, no. 18: 5587. https://doi.org/10.3390/molecules26185587
APA StyleParedes-Sánchez, F. A., Rivera, G., Bocanegra-García, V., Martínez-Padrón, H. Y., Berrones-Morales, M., Niño-García, N., & Herrera-Mayorga, V. (2021). Advances in Control Strategies against Spodoptera frugiperda. A Review. Molecules, 26(18), 5587. https://doi.org/10.3390/molecules26185587