Soapwort (Saponaria officinalis L.) Extract vs. Synthetic Surfactants—Effect on Skin-Mimetic Models
Abstract
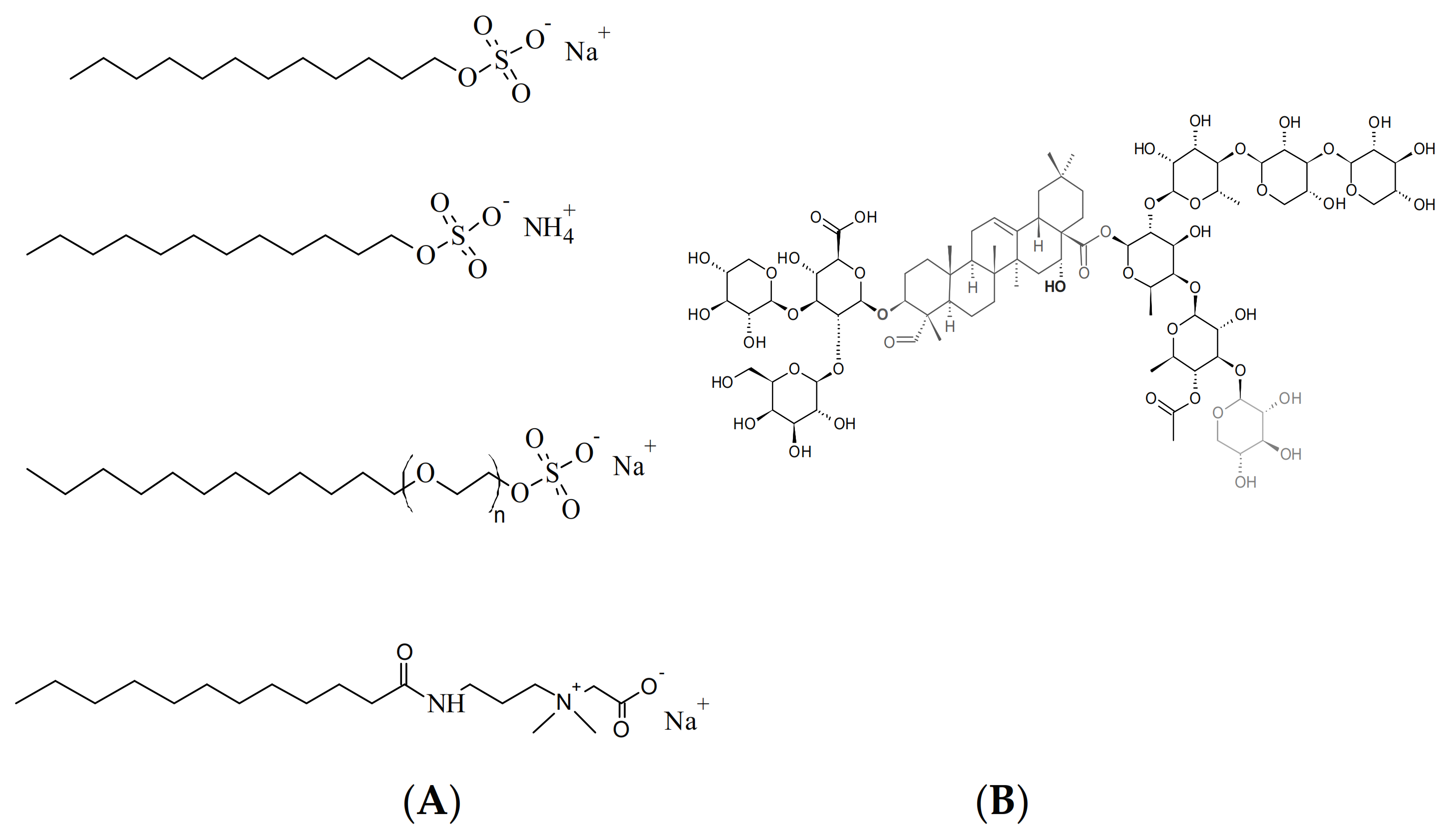
1. Introduction
2. Results and Discussion
- (a)
- Model liposomes composed of dipalmitoylphosphatidylcholine (DPPC) and cholesterol mixed in a 7:3 mol/mol ratio, which corresponds to a typical phospholipid/cholesterol ratio in mammalian cells, including keratinocytes [37].
- (b)
- Albumin denaturation, by measuring the increase in pH of the bovine serum albumin upon contact with (bio)surfactants.
- (c)
- Zein solubilization, by measuring the percentage of corn zein solubilized by (bio)surfactants.
- (d)
- Oil emulsification capacity using olive oil and engine oil which probes the ability of (bio)surfactants to emulsify lipids.
- (1)
- Anionic (SLS, ALS, SLES), which solubilizes well with the monolayers but not the bilayers.
- (2)
- Nonionic (CAPB), which solubilizes well with both mono- and bilayers.
- (3)
- Biosurfactant (SAP), which penetrates without solubilization the monolayers at 1% dry mass, and probably penetrates bilayers, increasing their size (without solubilization).
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Baroni, A.; Buommino, E.; De Gregorio, V.; Ruocco, E.; Ruocco, V.; Wolf, R. Structure and function of the epidermis related to barrier properties. Clin. Dermatol. 2012, 30, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Mathes, S.H.; Ruffner, H.; Graf-hausner, U. The use of skin models in drug development. Adv. Drug Deliv. Rev. 2014, 69, 81–102. [Google Scholar] [CrossRef] [PubMed]
- Braun, K.M.; Prowse, D.M. Distinct epidermal stem cell compartments are maintained by independent niche microenvironments. Stem Cell Rev. 2006, 2, 221–232. [Google Scholar] [CrossRef] [PubMed]
- El Maghraby, G.M.; Barry, B.W.; Williams, A.C. Liposomes and skin: From drug delivery to model membranes. Eur. J. Pharm. Sci. 2008, 203–222. [Google Scholar] [CrossRef]
- Fartasch, M.; Ponec, M. Improved barrier structure formation in air-exposed human keratinocyte culture systems. J. Investig. Dermatol. 1994, 102, 366–374. [Google Scholar] [CrossRef][Green Version]
- Lee, W.; Debasitis, J.C.; Lee, V.K.; Lee, J.H.; Fischer, K.; Edminster, K.; Park, J.K.; Yoo, S.S. Multi-layered culture of human skin fibroblasts and keratinocytes through three-dimensional freeform fabrication. Biomaterials 2009, 30, 1587–1595. [Google Scholar] [CrossRef]
- Tomasek, J.J.; Gabbiani, G.; Hinz, B.; Chaponnier, C.; Brown, R.A. Myofibroblasts and mechano: Regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 2002, 3, 349–363. [Google Scholar] [CrossRef]
- Parsonage, G.; Filer, A.D.; Haworth, O.; Nash, G.B.; Rainger, G.E.; Salmon, M.; Buckley, C.D. A stromal address code defined by fibroblasts. Trends Immunol. 2005, 26, 150–156. [Google Scholar] [CrossRef]
- Del Rosso, J.Q. Repair and maintenance of the epidermal barrier in patients diagnosed with atopic dermatitis: An evaluation of the components of a body wash-moisturizer skin care regimen directed at management of atopic skin. J. Clin. Aesthet. Dermatol. 2011, 4, 45–55. [Google Scholar]
- Corazza, M.; Lauriola, M.; Zappaterra, M.; Bianchi, A.; Virgili, A. Surfactants, skin cleansing protagonists. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 1–6. [Google Scholar] [CrossRef]
- Lu, G.; Moore, D.J. Study of surfactant-skin interactions by skin impedance measurements. Int. J. Cosmet. Sci. 2012, 34, 74–80. [Google Scholar] [CrossRef]
- Mehling, A.; Kleber, M.; Hensen, H. Comparative studies on the ocular and dermal irritation potential of surfactants. Food Chem. Toxicol. 2007, 45, 747–758. [Google Scholar] [CrossRef]
- Corazza, M.; Lauriola, M.M.; Bianchi, A.; Zappaterra, M.; Virgili, A. Irritant and sensitizing potential of eight surfactants commonly used in skin cleansers: An evaluation of 105 patients. Dermatitis 2010, 21, 262–268. [Google Scholar] [CrossRef]
- Triglia, D.; Braa, S.S.; Yonan, C.; Naughton, G.K. In vitro toxicity of various classes of test agents using the neutral red assay on a human three-dimensional physiologic skin model. Vitr. Cell. Dev. Biol.—Anim. 1991, 27, 239–244. [Google Scholar] [CrossRef]
- Ananthapadmanabhan, K.P.; Moore, D.J.; Subramanyan, K.; Misra, M.; Meyer, F. Cleansing without compromise: The impact of cleansers on the skin barrier and the technology of mild cleansing. Dermatol. Ther. 2004, 17, 16–25. [Google Scholar] [CrossRef]
- Yu, Y.; Zhao, J.; Bayly, A.E. Development of Surfactants and Builders in Detergent Formulations. Chin. J. Chem. Eng. 2008, 16, 517–527. [Google Scholar] [CrossRef]
- Góral, I.; Wojciechowski, K. Surface activity and foaming properties of saponin-rich plants extracts. Adv. Colloid Interface Sci. 2020, 279, 102145. [Google Scholar] [CrossRef]
- Martín, R.S.; Briones, R. Industrial uses and sustainable supply of Quillaja saponaria (Rosaceae) saponins. Econ. Bot. 1999, 53, 302–311. [Google Scholar] [CrossRef]
- Böttger, S.; Melzig, M.F. Triterpenoid saponins of the Caryophyllaceae and Illecebraceae family. Phytochem. Lett. 2011, 4, 59–68. [Google Scholar] [CrossRef]
- Smułek, W.; Zdarta, A.; Pacholak, A.; Zgoła-Grześkowiak, A.; Marczak, Ł.; Jarzębski, M.; Kaczorek, E. Saponaria officinalis L. extract: Surface active properties and impact on environmental bacterial strains. Colloids Surf. B Biointerfaces 2017, 150, 209–215. [Google Scholar] [CrossRef]
- Jurek, I.; Góral, I.; Mierzyńska, Z.; Moniuszko-Szajwaj, B.; Wojciechowski, K. Effect of synthetic surfactants and soapwort (Saponaria officinalis L.) extract on skin-mimetic model lipid monolayers. Biochim. Biophys. Acta—Biomembr. 2019, 1861, 556–564. [Google Scholar] [CrossRef]
- Lu, Y.; Van, D.; Deibert, L.; Bishop, G.; Balsevich, J. Antiproliferative quillaic acid and gypsogenin saponins from Saponaria officinalis L. roots. Phytochemistry 2015, 113, 108–120. [Google Scholar] [CrossRef]
- Moniuszko-Szajwaj, B.; Masullo, M.; Kowalczyk, M.; Pecio, Ł.; Szumacher-Strabel, M.; Cieślak, A.; Piacente, S.; Oleszek, W.; Stochmal, A. Highly Polar Triterpenoid Saponins from the Roots of Saponaria officinalis L. Helv. Chim. Acta 2016, 99, 347–354. [Google Scholar] [CrossRef]
- Pavela, R. Acaricidal properties of extracts of some medicinal and culinary plants against Tetranychus urticae Koch. Plant Prot. Sci. 2016, 52, 54–63. [Google Scholar] [CrossRef]
- Francis, G.; Kerem, Z.; Makkar, H.P.S.; Becker, K. The biological action of saponins in animal systems: A review. Br. J. Nutr. 2002, 88, 587. [Google Scholar] [CrossRef] [PubMed]
- Jurek, I.; Góral, I.; Gęsiński, K.; Wojciechowski, K. Effect of saponins from quinoa on a skin-mimetic lipid monolayer containing cholesterol. Steroids 2019, 147, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Osbourn, A.; Goss, R.J.M.; Field, R.A. The saponins-polar isoprenoids with important and diverse biological activities. Nat. Prod. Rep. 2011, 28, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Orczyk, M.; Wojciechowski, K. Comparison of the effect of two Quillaja bark saponin extracts on DPPC and DPPC/cholesterol Langmuir monolayers. Colloids Surf. B Biointerfaces 2015, 136, 291–299. [Google Scholar] [CrossRef]
- Wojciechowski, K.; Orczyk, M.; Gutberlet, T.; Brezesinski, G.; Geue, T.; Fontaine, P. On the Interaction between Digitonin and Cholesterol in Langmuir Monolayers. Langmuir 2016, 32, 9064–9073. [Google Scholar] [CrossRef]
- Wojciechowski, K.; Orczyk, M.; Gutberlet, T.; Geue, T. Complexation of phospholipids and cholesterol by triterpenic saponins in bulk and in monolayers. Biochim. Biophys. Acta—Biomembr. 2016, 1858, 363–373. [Google Scholar] [CrossRef]
- Orczyk, M.; Wojciechowski, K.; Brezesinski, G. Disordering Effects of Digitonin on Phospholipid Monolayers. Langmuir 2017, 33, 3871–3881. [Google Scholar] [CrossRef]
- Wojciechowski, K.; Orczyk, M.; Gutberlet, T.; Trapp, M.; Marcinkowski, K.; Kobiela, T.; Geue, T. Unusual penetration of phospholipid mono- and bilayers by Quillaja bark saponin biosurfactant. Biochim. Biophys. Acta—Biomembr. 2014, 1838, 1931–1940. [Google Scholar] [CrossRef][Green Version]
- Perkins, M.A.; Osborne, R.; Rana, F.R.; Ghassemi, A.; Robinson, M.K. Comparison of in vitro and in vivo human skin responses to consumer products and ingredients with a range of irritancy potential. Toxicol. Sci. 1999, 48, 218–229. [Google Scholar] [CrossRef]
- Törmä, H.; Lindberg, M.; Berne, B. Skin barrier disruption by sodium lauryl sulfate-exposure alters the expressions of involucrin, transglutaminase 1, profilaggrin, and kallikreins during the repair phase in human skin in vivo. J. Investig. Dermatol. 2008, 128, 1212–1219. [Google Scholar] [CrossRef]
- Almeida, J.A.S.; Faneca, H.; Carvalho, R.A.; Marques, E.F.; Pais, A.A.C.C. Dicationic alkylammonium bromide gemini surfactants. membrane perturbation and skin irritation. PLoS ONE 2011, 6, e26965. [Google Scholar] [CrossRef]
- Kapoor, Y.; Howell, B.A.; Chauhan, A. Liposome assay for evaluating ocular toxicity of surfactants. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2727–2735. [Google Scholar] [CrossRef]
- Elias, P.M. Advances in Lipid Research: Skin Lipids; Elsevier: Amsterdam, The Netherlands, 2016; Volume 24, ISBN 9781483215457. [Google Scholar]
- Góral, I.; Stochmal, A.; Wojciechowski, K. Surface activity of the oat, horse chestnut, cowherb, soybean, quinoa and soapwort extracts—Is it only due to saponins? Colloid Interface Sci. Commun. 2021, 42, 100400. [Google Scholar] [CrossRef]
- Seddon, A.M.; Curnow, P.; Booth, P.J. Membrane proteins, lipids and detergents: Not just a soap opera. Biochim. Biophys. Acta—Biomembr. 2004, 1666, 105–117. [Google Scholar] [CrossRef]
- Lasch, J. Interaction of detergents with lipid vesicles. Biochim. Biophys. Acta—Rev. Biomembr. 1995, 1241, 269–292. [Google Scholar] [CrossRef]
- Lichtenberg, D.; Ahyayauch, H.; Alonso, A.; Goñi, F.M. Detergent solubilization of lipid bilayers: A balance of driving forces. Trends Biochem. Sci. 2013, 38, 85–93. [Google Scholar] [CrossRef]
- Ahyayauch, H.; Larijani, B.; Alonso, A.; Goñi, F.M. Detergent solubilization of phosphatidylcholine bilayers in the fluid state: Influence of the acyl chain structure. Biochim. Biophys. Acta—Biomembr. 2006, 1758, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Sreij, R.; Dargel, C.; Hannappel, Y.; Jestin, J.; Prévost, S.; Dattani, R.; Wrede, O.; Hellweg, T. Temperature dependent self-organization of DMPC membranes promoted by intermediate amounts of the saponin aescin. Biochim. Biophys. Acta—Biomembr. 2019, 1861, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Dargel, C.; Geisler, R.; Hannappel, Y.; Kemker, I.; Sewald, N.; Hellweg, T. Self-Assembly of the Bio-Surfactant Aescin in Solution: A Small-Angle X-ray Scattering and Fluorescence Study. Colloids Interfaces 2019, 3, 47. [Google Scholar] [CrossRef]
- Viola, P.; Viola, M. Virgin olive oil as a fundamental nutritional component and skin protector. Clin. Dermatol. 2009, 27, 159–165. [Google Scholar] [CrossRef]
- Mehta, S.S.; Reddy, B.S.N. Cosmetic dermatitis—current perspectives. Int. J. Dermatol. 2003, 42, 533–542. [Google Scholar] [CrossRef]
- Tavss, E.A.; Eigen, E.; Kligman, A.M. Anionic detergent-induced skin irritation and anionic detergent-induced pH rise of bovine serum albumin. J. Soc. Cosmet. Chem. 1988, 39, 267–272. [Google Scholar]
- Kelley, D.; McClements, D.J. Interactions of bovine serum albumin with ionic surfactants in aqueous solutions. Food Hydrocoll. 2003, 17, 73–85. [Google Scholar] [CrossRef]
- Pezron, I.; Galet, L.; Clausse, D. Surface interaction between a protein monolayer and surfactants and its correlation with skin irritation by surfactants. J. Colloid Interface Sci. 1996, 180, 285–289. [Google Scholar] [CrossRef]
- Cohen, L.; Martin, M.; Soto, F.; Trujillo, F.; Sanchez, E. The Effect of Counterions of Linear Alkylbenzene Sulfonate on Skin Compatibility. J. Surfactants Deterg. 2016, 19, 219–222. [Google Scholar] [CrossRef]
- Moore, P.N.; Puvvada, S.; Blankschtein, D. Role of the surfactant polar head structure in protein-surfactant complexation: Zein protein solubilization by SDS and by SDS/C12En surfactant solutions. Langmuir 2003, 19, 1009–1016. [Google Scholar] [CrossRef]
- Erfani, A.; Khosharay, S.; Flynn, N.H.; Ramsey, J.D.; Aichele, C.P. Effect of zwitterionic betaine surfactant on interfacial behavior of bovine serum albumin (BSA). J. Mol. Liq. 2020, 318, 114067. [Google Scholar] [CrossRef]
- Nizioł-łukaszewska, Z.; Osika, P.; Wasilewski, T.; Bujak, T. Hydrophilic dogwood extracts as materials for reducing the skin irritation potential of body wash cosmetics. Molecules 2017, 22, 320. [Google Scholar] [CrossRef]
- Wojciechowski, K. Surface activity of saponin from Quillaja bark at the air/water and oil/water interfaces. Colloids Surf. B Biointerfaces 2013, 108, 95–102. [Google Scholar] [CrossRef]
- Mir, M.A.; Gull, N.; Khan, J.M.; Khan, R.H.; Dar, A.A.; Rather, G.M. Interaction of bovine serum albumin with cationic single chain+nonionic and cationic gemini+nonionic binary surfactant mixtures. J. Phys. Chem. B 2010, 114, 3197–3204. [Google Scholar] [CrossRef]
- Ward, R.K.; Hubbard, A.W.; Sulley, H.; Garle, M.J.; Clothier, R.H. Human keratinocyte cultures in an in vitro approach for the assessment of surfactant-induced irritation. Toxicol. Vitr. 1998, 12, 163–165. [Google Scholar] [CrossRef]
- Bangham, A.D.; Standish, M.M.; Watkins, J.C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 1965, 13, 238–252. [Google Scholar] [CrossRef]
- Kabashima, K. Immunology of the Skin: Basic and Clinical Sciences in Skin Immune Responses; Springer: Kyoto, Japan, 2018; ISBN 9784431567127. [Google Scholar]
- Babick, F. Dynamic light scattering (DLS). In Characterization of Nanoparticles; Hodoroaba, V.D., Unger, W.E.S., Shard, A.G., Eds.; Elsevier: Dresden, Germany, 2020; pp. 137–172. [Google Scholar]
- Tang, H.; Sun, X.; Yuan, G. Calculation method for particle mean diameter and particle size distribution function under dependent model algorithm. Chin. Opt. Lett. 2007, 5, 31–33. [Google Scholar]
- Götte, E. Chemistry and physics of surface active substances. In Proceedings of the Fourth International Congress on Surface Active Substances, Brussels, Belgium, 7–12 September 1964; Volume 3, p. 83. [Google Scholar]
- Seweryn, A. Interactions between surfactants and the skin—Theory and practice. Adv. Colloid Interface Sci. 2018, 256, 242–255. [Google Scholar] [CrossRef]
- Wang, P.; Cui, J.; Du, X.; Yang, Q.; Jia, C.; Xiong, M.; Yu, X.; Li, L.; Wang, W.; Chen, Y.; et al. Panax notoginseng saponins (PNS) inhibits breast cancer metastasis. J. Ethnopharmacol. 2014, 154, 663–671. [Google Scholar] [CrossRef]
- Man, S.; Gao, W.; Zhang, Y.; Huang, L.; Liu, C. Chemical study and medical application of saponins as anti-cancer agents. Fitoterapia 2010, 81, 703–714. [Google Scholar] [CrossRef]
- Rejinold, N.S.; Muthunarayanan, M.; Muthuchelian, K.; Chennazhi, K.P.; Nair, S.V.; Jayakumar, R. Saponin-loaded chitosan nanoparticles and their cytotoxicity to cancer cell lines in vitro. Carbohydr. Polym. 2011, 84, 407–416. [Google Scholar] [CrossRef]
- Kozińska, N.; Tokarska, K.; Chudy, M.; Wojciechowski, K. Cytotoxicity of Quillaja saponaria Saponins towards Lung Cells Is Higher for Cholesterol-Rich Cells. Biophysica 2021, 1, 126–136. [Google Scholar] [CrossRef]






| Cell Line | EC50 [Dry Mass Content, %] | ||||
|---|---|---|---|---|---|
| SAP | ALS | SLES | SLS | CAPB | |
| HaCaT | 0.3999 | 0.0210 | 0.0004 | 0.0012 | 0.0007 |
| A375 | 0.1348 | 0.0259 | 0.0016 | 0.0013 | 0.0011 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurek, I.; Szuplewska, A.; Chudy, M.; Wojciechowski, K. Soapwort (Saponaria officinalis L.) Extract vs. Synthetic Surfactants—Effect on Skin-Mimetic Models. Molecules 2021, 26, 5628. https://doi.org/10.3390/molecules26185628
Jurek I, Szuplewska A, Chudy M, Wojciechowski K. Soapwort (Saponaria officinalis L.) Extract vs. Synthetic Surfactants—Effect on Skin-Mimetic Models. Molecules. 2021; 26(18):5628. https://doi.org/10.3390/molecules26185628
Chicago/Turabian StyleJurek, Ilona, Aleksandra Szuplewska, Michał Chudy, and Kamil Wojciechowski. 2021. "Soapwort (Saponaria officinalis L.) Extract vs. Synthetic Surfactants—Effect on Skin-Mimetic Models" Molecules 26, no. 18: 5628. https://doi.org/10.3390/molecules26185628
APA StyleJurek, I., Szuplewska, A., Chudy, M., & Wojciechowski, K. (2021). Soapwort (Saponaria officinalis L.) Extract vs. Synthetic Surfactants—Effect on Skin-Mimetic Models. Molecules, 26(18), 5628. https://doi.org/10.3390/molecules26185628








