Development and Validation of QuEChERS Followed by UHPLC-ToF-MS Method for Determination of Multi-Mycotoxins in Pistachio Nuts
Abstract
:1. Introduction
2. Results and Discussion
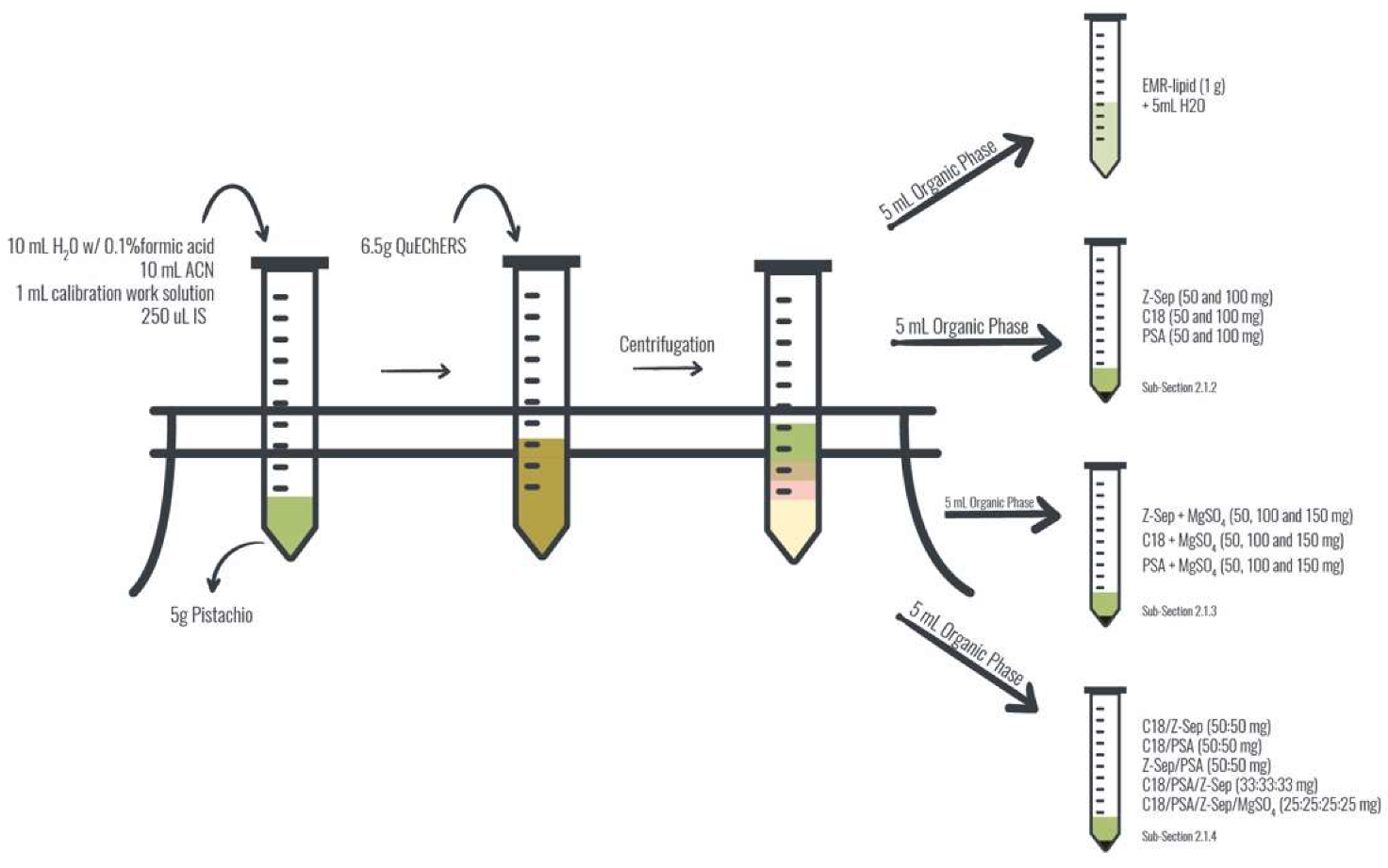
2.1. Extraction and Clean-up Optimization
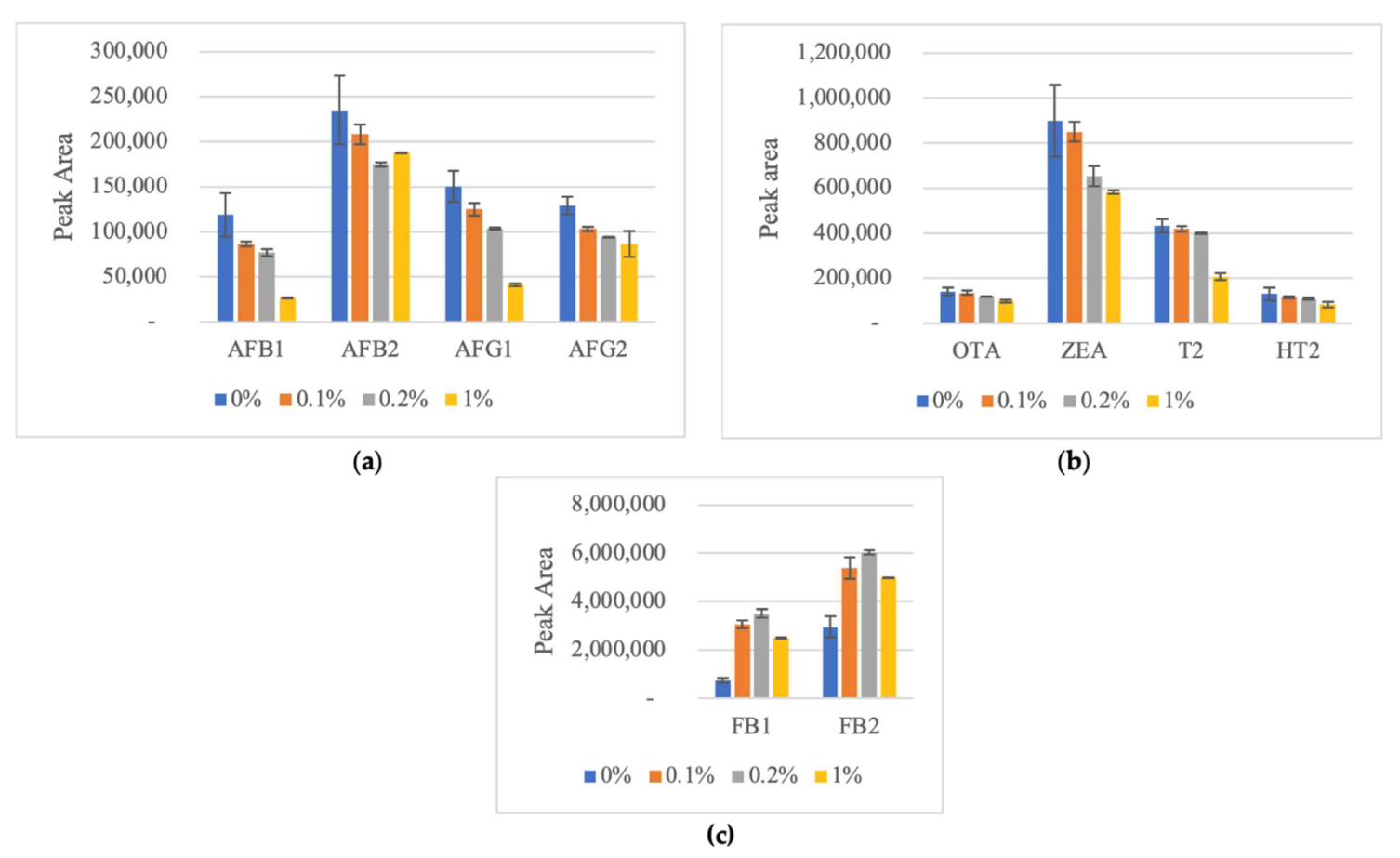
2.1.1. Optimization of Acidification of Water
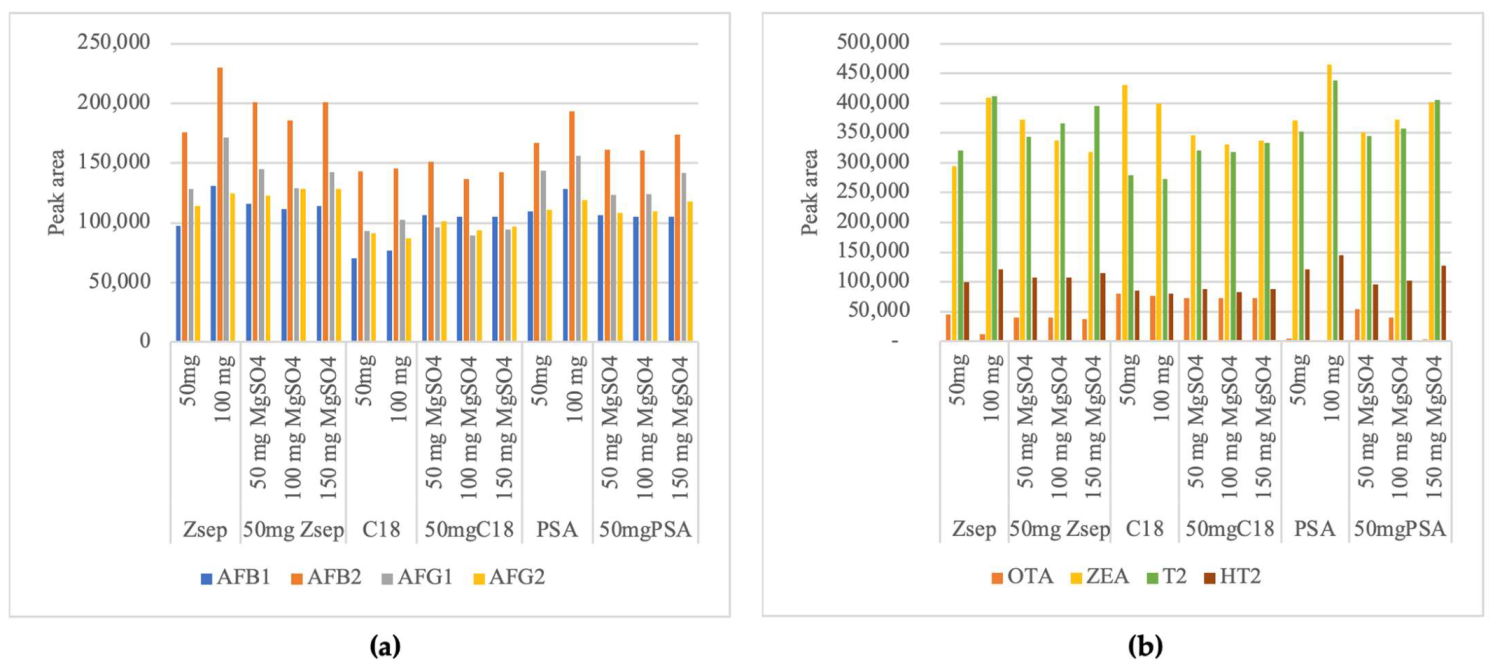
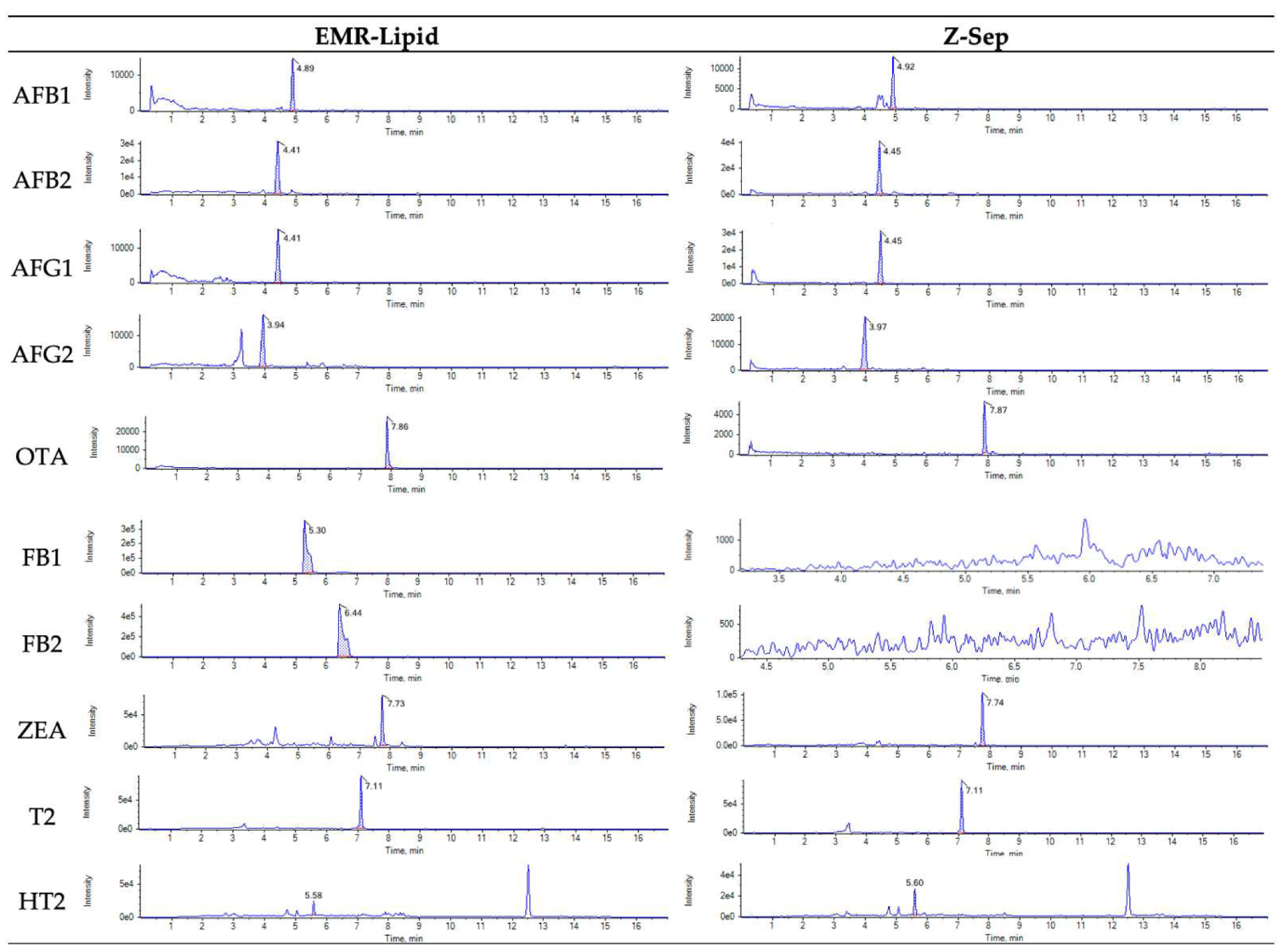
2.1.2. Influence of C18, PSA and Z-Sep Sorbents
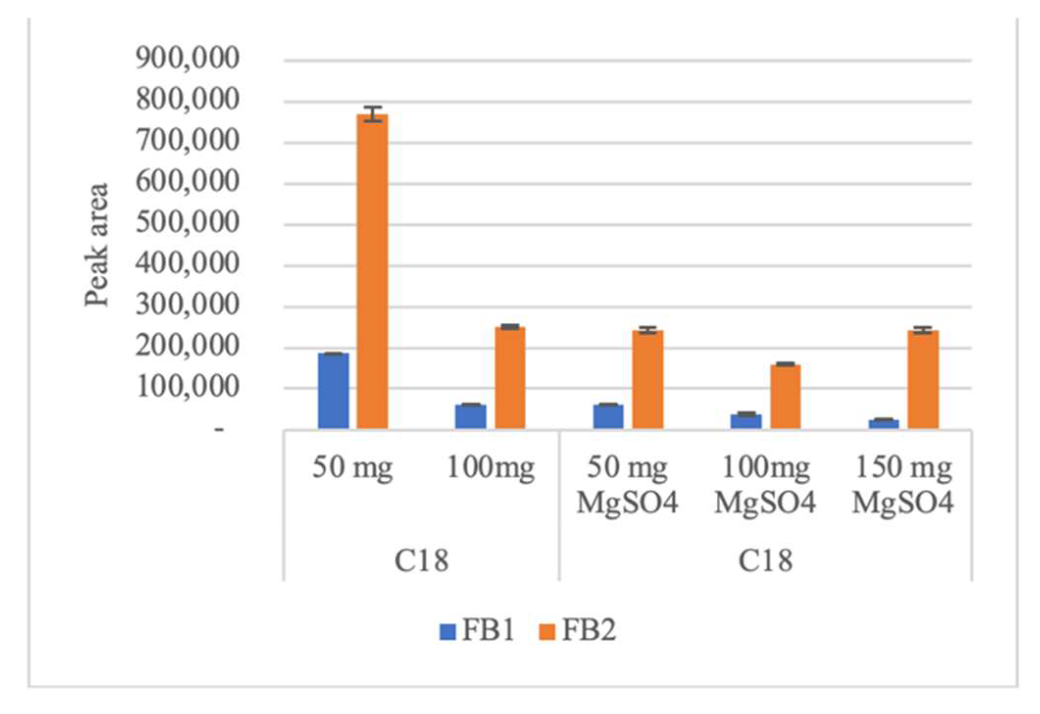
2.1.3. Influence of Addition of Magnesium Sulfate with C18, PSA and Z-Sep Sorbents
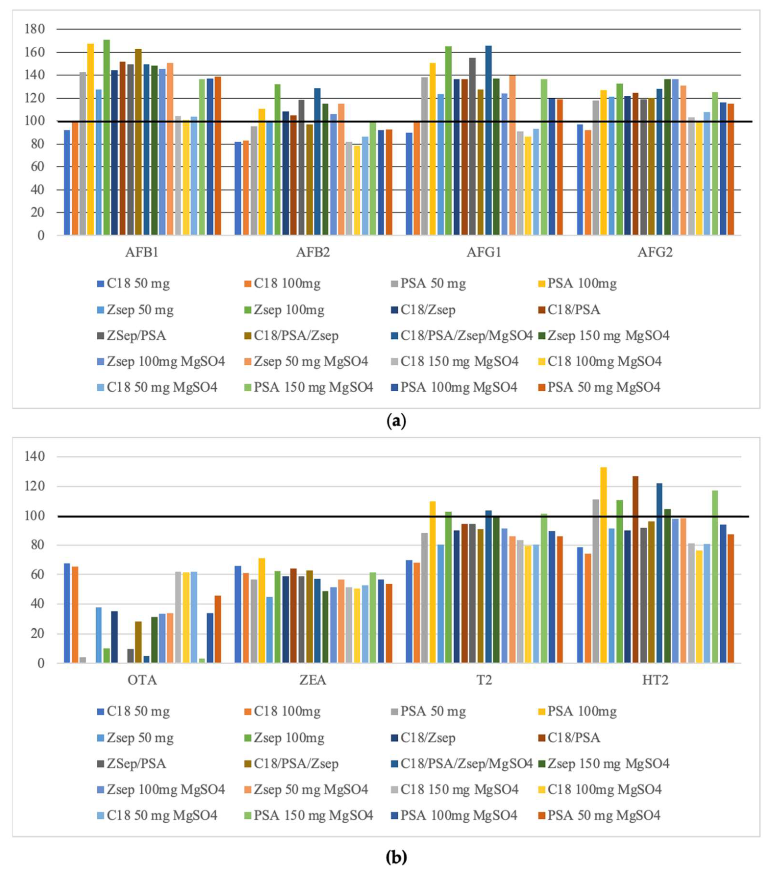
2.1.4. Influence of the Combination of Different Sorbents
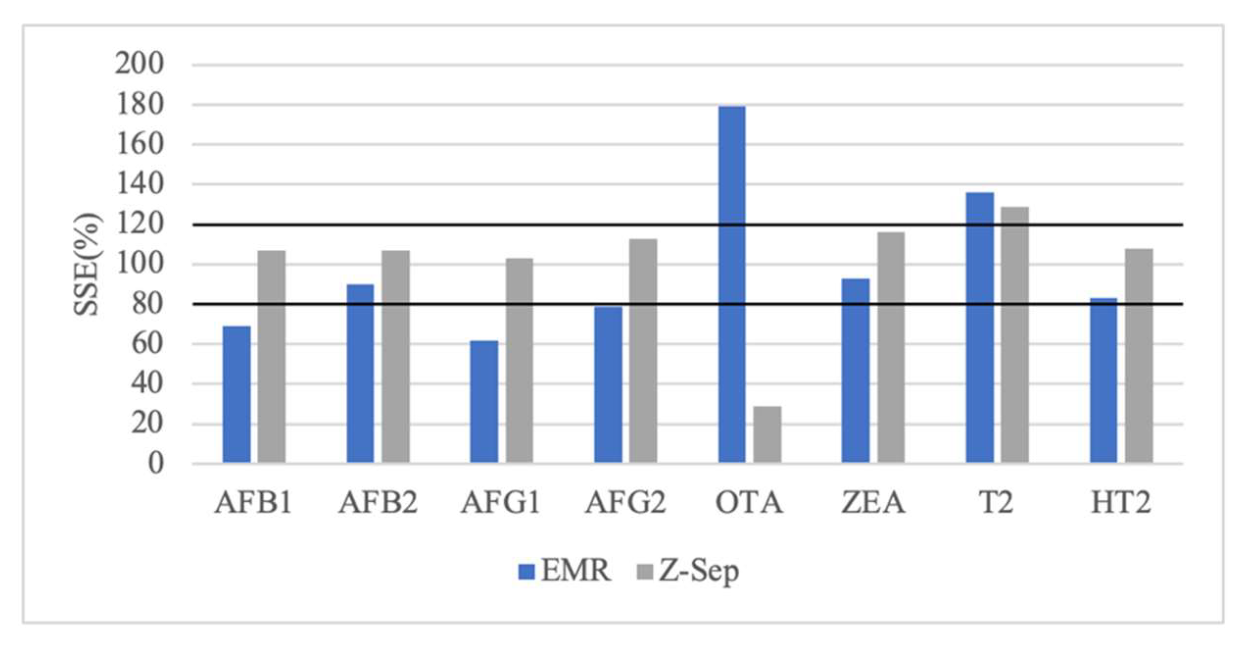
2.2. Validation of Analytical Method
2.3. Occurrence of Mycotoxins in Pistachio
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Samples and Sampling Procedure
3.3. Extraction Procedure
3.3.1. Clean-up Experiments
3.3.2. Spiking Experiments
3.3.3. Matrix Effect
3.4. UHPLC–ToF-MS Parameters
3.5. Identification of Mycotoxins
3.6. Validation of the UHPLC-ToF-MS Method
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Widmer, R.J.; Flammer, A.J.; Lerman, L.O.; Lerman, A. The Mediterranean Diet, its Components, and Cardiovascular Disease. Am. J. Med. 2015, 128, 229–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashaninejad, M.; Tabil, L.G. Pistachio (Pistacia vera L.). Postharvest Biol. Technol. Trop. Subtrop. Fruits 2011, 4. [Google Scholar] [CrossRef]
- Dreher, M.L. Pistachio nuts: Composition and potential health benefits. Nutr. Rev. 2012, 70, 234–240. [Google Scholar] [CrossRef]
- FAO STAT Pistachio World Production. Available online: http://www.fao.org/faostat/en/?#data/QC (accessed on 3 March 2021).
- Bui-Klimke, T.R.; Guclu, H.; Kensler, T.W.; Yuan, J.M.; Wu, F. Aflatoxin regulations and global pistachio trade: Insights from social network analysis. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Council for Agricultural Science. Mycotoxins: Risks in Plant, Animal, and Human Systems Council for Agricultural Science and Technology; Council for Agricultural Science: Ames, IA, USA, 2003; ISBN 1887383220. [Google Scholar]
- Yang, Y.; Li, G.; Wu, D.; Liu, J.; Li, X.; Luo, P.; Hu, N.; Wang, H.; Wu, Y. Recent advances on toxicity and determination methods of mycotoxins in foodstuffs. Trends Food Sci. Technol. 2020, 96, 233–252. [Google Scholar] [CrossRef]
- Ismaiel, A.; Papenbrock, J. Mycotoxins: Producing Fungi and Mechanisms of Phytotoxicity. Agriculture 2015, 5, 492–537. [Google Scholar] [CrossRef] [Green Version]
- Boutrif, E.; Canet, C. Mycotoxin prevention and control FAO programmes. Rev. Med. Vet. (France) 1998, 6, 681–694. [Google Scholar]
- Silva, A.S.; Brites, C.; Pouca, A.V.; Barbosa, J.; Freitas, A. UHPLC-ToF-MS method for determination of multi-mycotoxins in maize: Development and validation. Curr. Res. Food Sci. 2019, 1, 1–7. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer Some Naturally Occurring Substances: Food Items and Constituents, Heterocyclic Aromatic Amines and Mycotoxins. Who/Iarc Monogr. Eval. Carcinog. Risks Hum. 1993, 56, 245–522. [CrossRef] [Green Version]
- Ostry, V.; Malir, F.; Toman, J.; Grosse, Y. Mycotoxins as human carcinogens—The IARC Monographs classification. Mycotoxin Res. 2017, 33, 65–73. [Google Scholar] [CrossRef]
- Cheraghali, A.M.; Yazdanpanah, H.; Doraki, N.; Abouhossain, G.; Hassibi, M.; Ali-abadi, S.; Aliakbarpoor, M.; Amirahmadi, M.; Askarian, A.; Fallah, N.; et al. Incidence of aflatoxins in Iran pistachio nuts. Food Chem. Toxicol. 2007, 45, 812–816. [Google Scholar] [CrossRef]
- Varga, E.; Glauner, T.; Berthiller, F.; Krska, R.; Schuhmacher, R.; Sulyok, M. Development and validation of a (semi-)quantitative UHPLC-MS/MS method for the determination of 191 mycotoxins and other fungal metabolites in almonds, hazelnuts, peanuts and pistachios. Anal. Bioanal. Chem. 2013, 405, 5087–5104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Commission. Commission Regulation (EC) No. 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstufs. Off. J. Eur. Union 2006, 364, 5–24. [Google Scholar]
- FAO/WHO Codex Alimentarius Comission, J. Codex Alimentarius; Food and Agriculture Organization of the United Nations: Rome, Ialy, 1995. [Google Scholar]
- PortFIR Pistachio Nutrional Composition. Available online: http://portfir.insa.pt/foodcomp/food?21237 (accessed on 11 May 2021).
- Cunha, S.C.; Sá, S.V.M.; Fernandes, J.O. Multiple mycotoxin analysis in nut products: Occurrence and risk characterization. Food Chem. Toxicol. 2018, 114, 260–269. [Google Scholar] [CrossRef]
- Mbundi, L.; Gallar-ayala, H.; Khan, M.R.; Barber, J.L.; Losada, S.; Busquets, R. Advances in the Analysis of Challenging Food Contaminants: Nanoparticles, Bisphenols, Mycotoxins, and Brominated Flame Retardants. Adv. Mol. Toxicol. 2014, 8, 35–105. [Google Scholar]
- Rejczak, T.; Tuzimski, T. A review of recent developments and trends in the QuEChERS sample preparation approach. Open Chem. 2015, 13, 980–1010. [Google Scholar] [CrossRef]
- Parrilla Vázquez, P.; Hakme, E.; Uclés, S.; Cutillas, V.; Martínez Galera, M.; Mughari, A.R.; Fernández-Alba, A.R. Large multiresidue analysis of pesticides in edible vegetable oils by using efficient solid-phase extraction sorbents based on quick, easy, cheap, effective, rugged and safe methodology followed by gas chromatography–tandem mass spectrometry. J. Chromatogr. A 2016, 1463, 20–31. [Google Scholar] [CrossRef]
- Kaczynski, P.; Hrynko, I.; Lozowicka, B. Evolution of novel sorbents for effective clean-up of honeybee matrix in highly toxic insecticide LC/MS/MS analysis. Ecotoxicol. Env. Saf. 2017, 139, 124–131. [Google Scholar] [CrossRef]
- Hernández-Mesa, M.; García-Campaña, A.M. Determination of sulfonylurea pesticide residues in edible seeds used as nutraceuticals by QuEChERS in combination with ultra-high-performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2020, 1617, 460831. [Google Scholar] [CrossRef]
- Alcántara-Durán, J.; Moreno-González, D.; García-Reyes, J.F.; Molina-Díaz, A. Use of a modified QuEChERS method for the determination of mycotoxin residues in edible nuts by nano flow liquid chromatography high resolution mass spectrometry. Food Chem. 2019, 279, 144–149. [Google Scholar] [CrossRef]
- Sinha, K.K. Testing methods for aflatoxins in foods. Food Nutr. Bull. 1999, 20, 458–464. [Google Scholar] [CrossRef]
- European Comission. The Rapid Alert System for Food and Feed 2019; Publications Office of the European Union: Luxembourg, 2020; ISBN 978-92-76-17508-7.
- European Comission RASFF Window-Pistachios Notiffications. Available online: https://webgate.ec.europa.eu/rasff-window/screen/list (accessed on 22 July 2021).
- European Commission Notification 2020.5604: Ochratoxin A in Pistachios from USA. Available online: https://webgate.ec.europa.eu/rasff-window/screen/notification/453652 (accessed on 22 July 2021).
- Spanjer, M.C.; Rensen, P.M.; Scholten, J.M. LC–MS/MS multi-method for mycotoxins after single extraction, with validation data for peanut, pistachio, wheat, maize, cornflakes, raisins and figs. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2008, 25, 472–489. [Google Scholar] [CrossRef]
- Alsharif, A.M.A.; Choo, Y.M.; Tan, G.H. Detection of five mycotoxins in different food matrices in the malaysian market by using validated liquid chromatography electrospray ionization triple quadrupole mass spectrometry. Toxins (Basel) 2019, 11, 196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narváez, A.; Rodríguez-Carrasco, Y.; Castaldo, L.; Izzo, L.; Graziani, G.; Ritieni, A. Occurrence and exposure assessment of mycotoxins in ready-to-eat tree nut products through ultra-high performance liquid chromatography coupled with high resolution q-orbitrap mass spectrometry. Metabolites 2020, 10, 344. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Chen, X.; Shen, C.; Qu, B. Determination of 16 mycotoxins in vegetable oils using a QuEChERS method combined with high-performance liquid chromatography-tandem mass spectrometry. Food Addit. Contam. Part A 2016, 34, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.-W.; Park, M.-K.; Heo, H.; Jeon, H.-J.; Choi, S.-D.; Lee, S.-E.; Moon, J.-K. Simultaneous determination of 13 mycotoxins in feedstuffs using QuEChERS extraction. Appl. Biol. Chem. 2021, 64, 34. [Google Scholar] [CrossRef]
- European Commission Commission Regulation (EC) No 401/2006 of 23 February 2006 laying down the methods of sampling and analysis for the official control of the levels of mycotoxins in foodstuffs. Off. J. Eur. Union 2006, 70, 12–34.
- Zhou, W.; Yang, S.; Wang, P.G. Matrix effects and application of matrix effect factor. Bioanalysis 2017, 9, 1839–1844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hidalgo-Ruiz, J.L.; Romero-González, R.; Martínez Vidal, J.L.; Garrido Frenich, A. Determination of mycotoxins in nuts by ultra high-performance liquid chromatography-tandem mass spectrometry: Looking for a representative matrix. J. Food Compos. Anal. 2019, 82. [Google Scholar] [CrossRef]
- Arroyo-Manzanares, N.; Huertas-Pérez, J.F.; Gámiz-Gracia, L.; García-Campaña, A.M. A new approach in sample treatment combined with UHPLC-MS/MS for the determination of multiclass mycotoxins in edible nuts and seeds. Talanta 2013, 115, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Tebele, S.M.; Gbashi, S.; Adebo, O.; Changwa, R.; Naidu, K.; Njobeh, P.B. Quantification of multi-mycotoxin in cereals (maize, maize porridge, sorghum and wheat) from Limpopo province of South Africa. Food Addit. Contam. Part A 2020, 37, 1922–1938. [Google Scholar] [CrossRef] [PubMed]
- Jettanajit, A.; Nhujak, T. Determination of Mycotoxins in Brown Rice Using QuEChERS Sample Preparation and UHPLC–MS-MS. J. Chromatogr. Sci. 2016, 54, 720–729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abreu, D.C.P.; da Silva Oliveira, F.A.; Vargas, E.A.; Madureira, F.D.; Magalhães, E.J.; da Silva, L.P.; Saczk, A.A. Methodology development based on “dilute and shoot” and QuEChERS for determination of multiple mycotoxins in cocoa by LC-MS/MS. Anal. Bioanal. Chem. 2020, 412, 1757–1767. [Google Scholar] [CrossRef]
- Pantano, L.; La Scala, L.; Olibrio, F.; Galluzzo, F.G.; Bongiorno, C.; Buscemi, M.D.; Macaluso, A.; Vella, A. QuEChERS LC–MS/MS Screening Method for Mycotoxin Detection in Cereal Products and Spices. Int. J. Environ. Res. Public Health 2021, 18, 3774. [Google Scholar] [CrossRef] [PubMed]
- Diella, G.; Caggiano, G.; Ferrieri, F.; Ventrella, A.; Palma, M.; Napoli, C.; Rutigliano, S.; Lopuzzo, M.; Lovero, G.; Montagna, M.T. Aflatoxin contamination in nuts marketed in Italy: Preliminary results. Ann. Di Ig. 2018, 30, 401–409. [Google Scholar] [CrossRef]
- Fernane, F.; Cano-Sancho, G.; Sanchis, V.; Marin, S.; Ramos, A.J. Aflatoxins and ochratoxin A in pistachios sampled in Spain: Occurrence and presence of mycotoxigenic fungi. Food Addit. Contam. Part B Surveill. 2010, 3, 185–192. [Google Scholar] [CrossRef]
- Ulca, P.; Evcimen, M.K.; Senyuva, H.Z. Surveys of aflatoxin B 1 contamination of retail Turkish foods and of products intended for export between 2007 and 2009. Food Addit. Contam. Part B 2010, 3, 120–125. [Google Scholar] [CrossRef]
- Shadbad, S.; Reza, M.; Masoud, A.; Ali, T.; Faranak, G.; Mahboob, N. Determination of aflatoxins in nuts of Tabriz confectionaries by ELISA and HPLC methods. Adv. Pharm. Bull. 2012, 2, 123–126. [Google Scholar] [CrossRef]
- El tawila, M.M.; Neamatallah, A.; Serdar, S.A. Incidence of aflatoxins in commercial nuts in the holy city of Mekkah. Food Control 2013, 29, 121–124. [Google Scholar] [CrossRef]
- Kumar, P.; Mahato, D.K.; Kamle, M.; Mohanta, T.K.; Kang, S.G. Aflatoxins: A Global Concern for Food Safety, Human Health and Their Management. Front. Microbiol. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, K.; Banerjee, K. A Review: Sample Preparation and Chromatographic Technologies for Detection of Aflatoxins in Foods. Toxins (Basel) 2020, 12, 539. [Google Scholar] [CrossRef]
- Wacoo, A.P.; Wendiro, D.; Vuzi, P.C.; Hawumba, J.F. Methods for Detection of Aflatoxins in Agricultural Food Crops. J. Appl. Chem. 2014, 2014, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Marroquín-Cardona, A.G.; Johnson, N.M.; Phillips, T.D.; Hayes, A.W. Mycotoxins in a changing global environment—A review. Food Chem. Toxicol. 2014, 69, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Medina, Á.; Rodríguez, A.; Magan, N. Climate change and mycotoxigenic fungi: Impacts on mycotoxin production. Curr. Opin. Food Sci. 2015, 5, 99–104. [Google Scholar] [CrossRef]
- Baazeem, A.; Rodríguez, A.; Medina, A.; Magan, N. Impacts of Climate Change Interacting Abiotic Factors on Growth, aflD and aflR Gene Expression and Aflatoxin B1 Pistachio Nuts. Toxins (Basel) 2021, 13, 385. [Google Scholar] [CrossRef] [PubMed]
- Peter Mshelia, L.; Selamat, J.; Iskandar Putra Samsudin, N.; Rafii, M.Y.; Abdul Mutalib, N.-A.; Nordin, N.; Berthiller, F. Effect of Temperature, Water Activity and Carbon Dioxide on Fungal Growth and Mycotoxin Production of Acclimatised Isolates of Fusarium verticillioides and F. graminearum. Toxins (Basel) 2020, 12, 478. [Google Scholar] [CrossRef]
- Liao, C.-D.; Wong, J.W.; Zhang, K.; Yang, P.; Wittenberg, J.B.; Trucksess, M.W.; Hayward, D.G.; Lee, N.S.; Chang, J.S. Multi-mycotoxin Analysis of Finished Grain and Nut Products Using Ultrahigh-Performance Liquid Chromatography and Positive Electrospray Ionization–Quadrupole Orbital Ion Trap High-Resolution Mass Spectrometry. J. Agric. Food Chem. 2015, 63, 8314–8332. [Google Scholar] [CrossRef]
- De Ruyck, K.; De Boevre, M.; Huybrechts, I.; De Saeger, S. Dietary mycotoxins, co-exposure, and carcinogenesis in humans: Short review. Rev. Mutat. Res. 2015, 766, 32–41. [Google Scholar] [CrossRef] [Green Version]
- Coronel, M.B.; Marín, S.; Cano-Sancho, G.; Ramos, A.J.; Sanchis, V. Exposure assessment to ochratoxin A in Catalonia (Spain) based on the consumption of cereals, nuts, coffee, wine, and beer. Food Addit. Contam. Part A 2012, 29, 979–993. [Google Scholar] [CrossRef]
- European Commission Commission Recomendations of 27 March 2013 on the presence of T-2 and HT-2 toxin in cereals and cereal products. Off. J. Eur. Union 2013, 56, 12–15. [CrossRef]
- USDA Database Pistachio Nuts, Pistachio Nuts, Raw. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/170184/nutrients (accessed on 27 August 2021).
- USDA Database Pistachio Oats, Raw. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/1101825/nutrients (accessed on 27 August 2021).
- PortFIR Rye. Available online: http://portfir.insa.pt/foodcomp/food?21255 (accessed on 27 August 2021).
- PortFIR Maize, Raw Dry Grain. Available online: http://portfir.insa.pt/foodcomp/food?20196 (accessed on 27 August 2021).
- PortFIR Barley. Available online: http://portfir.insa.pt/foodcomp/food?21239 (accessed on 27 August 2021).
- PortFIR Rice, Raw. Available online: http://portfir.insa.pt/foodcomp/food?21166 (accessed on 27 August 2021).
- Ferrer Amate, C.; Unterluggauer, H.; Fischer, R.J.; Fernández-Alba, A.R.; Masselter, S. Development and validation of a LC–MS/MS method for the simultaneous determination of aflatoxins, dyes and pesticides in spices. Anal. Bioanal. Chem. 2010, 397, 93–107. [Google Scholar] [CrossRef] [PubMed]
- European Commission Commission Decision 657 of 12 August 2002 implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. Off. J. Eur. Communities 2002, 221, 8–36.

| Mycotoxin | LOD (μg/kg) | LOQ (μg/kg) | Linear Range (μg/kg) | Calibration Curve Parameters | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r2 | Slope | Interception | ||||||||||
| EMR | Z-Sep | EMR | Z-Sep | EMR | Z-Sep | EMR | Z-Sep | EMR | Z-Sep | EMR | Z-Sep | |
| AFB1 | 0.125 | 0.125 | 0.5 | 0.125 | 0.5−4.0 | 0.125−2.0 | 0.9901 | 0.9993 | 32491.9 | 60259.9 | 4765.9 | 571.7 |
| AFB2 | 0.25 | 0.25 | 0.5 | 0.25 | 0.50−8.0 | 0.25−4.0 | 0.9989 | 0.9973 | 42436.2 | 49476.5 | −2191.6 | 1238.5 |
| AFG1 | 0.50 | 0.25 | 1.0 | 0.25 | 1.0−8.0 | 0.25−4.0 | 0.9929 | 0.9974 | 17067.9 | 38603.0 | 11613.0 | −967.9 |
| AFG2 | 0.50 | 0.25 | 1.0 | 0.25 | 1.0−4.0 | 0.5−8.0 | 0.9931 | 0.9976 | 22299.4 | 30307.8 | 6944.3 | 2158.1 |
| OTA | 0.19 | 0.75 | 0.38 | 1.50 | 0.38−3.0 | 1.5−6.0 | 0.9934 | 0.9914 | 32398.2 | 6792.0 | 1900.9 | −2529.1 |
| ZEA | 12.5 | 12.5 | 25 | 12.5 | 25−200 | 12.5−400 | 0.9958 | 0.9994 | 1504.5 | 1910.7 | −3896.5 | −1000.2 |
| T2 | 12.5 | 12.5 | 25 | 25 | 25−200 | 25−400 | 0.9938 | 0.9979 | 1644.7 | 1570.9 | 12400.9 | 3811.1 |
| HT2 | 25 | 25 | 25 | 25 | 25−400 | 25−400 | 0.9976 | 0.9979 | 287.6 | 403.1 | 7879.4 | 7252.4 |
| FB1 | 12.5 | * | 25 | * | 25−200 | * | 0.9961 | * | 16960.4 | * | −33602.9 | * |
| FB2 | 12.5 | * | 12.5 | * | 12.5−200 | * | 0.9983 | * | 28671.6 | * | −1339.8 | * |
| EMR-Lipid | Z-Sep | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mycotoxin | Ion | Retention Time (min) | Spiked Level (μg/kg) | Rec (%) (n = 6) | RSDr (%) | RSDR (%) | Spiked Level (μg/kg) | Rec (%) (n = 6) | RSDr (%) | RSDR (%) |
| AFB1 | 313.07066 [M + H]+ | 5.00 | 0.50 1.0 1.5 2.0 4.0 | 119.1 89.1 101.9 101.9 100.2 | 10.68 8.27 10.73 10.35 11.90 | 3.70 8.80 | 0.125 0.250 0.50 1.0 1.5 2.0 | 93.3 98.1 98.9 100.7 97.3 101.4 | 3.59 6.99 4.97 5.29 5.06 3.59 | 3.77 2.56 |
| AFB2 | 315.08631 [M + H]+ | 4.52 | 0.50 1.0 2.0 3.0 4.0 8.0 | 111.4 102.6 100.5 100.4 97.9 97.5 | 4.85 6.16 6.90 2.97 4.83 2.43 | 3.54 3.56 2.79 | 0.25 0.50 1.0 2.0 3.0 4.0 | 102.8 98.0 95.3 99.9 100.3 96.5 | 8.69 6.09 6.65 5.56 6.68 5.64 | 5.67 4.12 |
| AFG1 | 329.06558 [M + H]+ | 4.53 | 1.0 2.0 3.0 4.0 8.0 | 77.3 76.9 97.9 104.6 101.7 | 25.25 24.74 7.70 6.68 12.98 | 6.76 3.25 | 0.25 0.50 1.0 2.0 3.0 4.0 | 105.7 112.0 92.1 101.8 99.3 97.6 | 5.71 6.83 9.62 6.72 5.04 6.01 | 8.17 5.62 |
| AFG2 | 331.08123 [M + H]+ | 4.04 | 1.0 2.0 3.0 4.0 | 86.0 95.4 100.1 104.3 | 20.66 11.78 12.04 7.02 | 26.75 9.37 | 0.50 1.0 2.0 3.0 4.0 8.0 | 119.7 100.4 103.6 96.3 98.0 99.4 | 5.37 4.82 5.83 9.92 4.10 9.44 | 9.69 2.96 2.21 |
| OTA | 404.08954 [M + H]+ | 7.97 | 0.38 0.75 1.50 2.25 3.0 | 95.0 97.5 100.4 108.5 102.3 | 10.30 11.95 14.74 4.81 10.04 | 9.85 9.01 | 1.50 2.25 3.0 6.0 | 105.9 88.7 109.4 82.6 | 9.26 6.97 7.31 3.99 | 7.85 6.35 |
| ZEA | 319.154 [M + H]+ | 7.83 | 25 50.0 100 150 200 | 93.9 96.3 112.9 92.1 99.6 | 1.61 10.21 16.38 14.25 5.43 | 10.05 7.41 | 12.5 25 50.0 100 150 200 400 | 106.0 102.4 97.1 94.7 97.1 98.8 98.8 | 2.56 7.18 4.77 8.54 4.13 5.48 2.74 | 4.47 2.49 3.97 |
| T2 | 489.2095 [M + Na]+ | 7.21 | 25 50.0 100 150 200 | 80.8 99.1 100.6 106.9 96.7 | 2.40 1.30 3.41 6.98 3.33 | 9.42 6.33 | 25 50.0 100 150 200 400 | 100.2 97.3 105.6 98.3 98.86 97.9 | 5.89 3.75 3.38 1.85 5.29 7.24 | 7.82 7.92 1.77 |
| HT2 | 425.217 [M + H]+ | 5.69 | 50.0 100 150 200 400 | 118.9 90.7 108.3 109.8 102.2 | 15.23 9.12 11.35 10.79 2.69 | 10.81 7.42 | 25 50.0 100 150 200 400 | 75.2 91.2 105.0 108.2 103.8 99.8 | 3.89 7.42 6.88 1.21 2.12 3.70 | 8.89 6.82 7.75 |
| FB1 | 722.396 [M + H]+ | 5.32 | 25 50.0 100 150 200 | 104.0 94.4 99.3 101.9 99.2 | 4.01 5.36 2.18 5.66 4.65 | 2.58 4.56 | na | na | na | na |
| FB2 | 706.401 [M + H]+ | 6.48 | 12.5 25 50.0 100 150 200 | 101.3 100.1 91.1 103.5 100.0 99.4 | 4.67 3.17 8.28 1.02 3.56 2.98 | 3.66 3.43 | na | na | na | na |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mateus, A.R.S.; Barros, S.; Pena, A.; Silva, A.S. Development and Validation of QuEChERS Followed by UHPLC-ToF-MS Method for Determination of Multi-Mycotoxins in Pistachio Nuts. Molecules 2021, 26, 5754. https://doi.org/10.3390/molecules26195754
Mateus ARS, Barros S, Pena A, Silva AS. Development and Validation of QuEChERS Followed by UHPLC-ToF-MS Method for Determination of Multi-Mycotoxins in Pistachio Nuts. Molecules. 2021; 26(19):5754. https://doi.org/10.3390/molecules26195754
Chicago/Turabian StyleMateus, Ana Rita Soares, Sílvia Barros, Angelina Pena, and Ana Sanches Silva. 2021. "Development and Validation of QuEChERS Followed by UHPLC-ToF-MS Method for Determination of Multi-Mycotoxins in Pistachio Nuts" Molecules 26, no. 19: 5754. https://doi.org/10.3390/molecules26195754
APA StyleMateus, A. R. S., Barros, S., Pena, A., & Silva, A. S. (2021). Development and Validation of QuEChERS Followed by UHPLC-ToF-MS Method for Determination of Multi-Mycotoxins in Pistachio Nuts. Molecules, 26(19), 5754. https://doi.org/10.3390/molecules26195754









