Micropropagation of Rare Scutellaria havanensis Jacq. and Preliminary Studies on Antioxidant Capacity and Anti-Cancer Potential
Abstract
:1. Introduction
2. Materials and Methods
2.1. Micropropagation
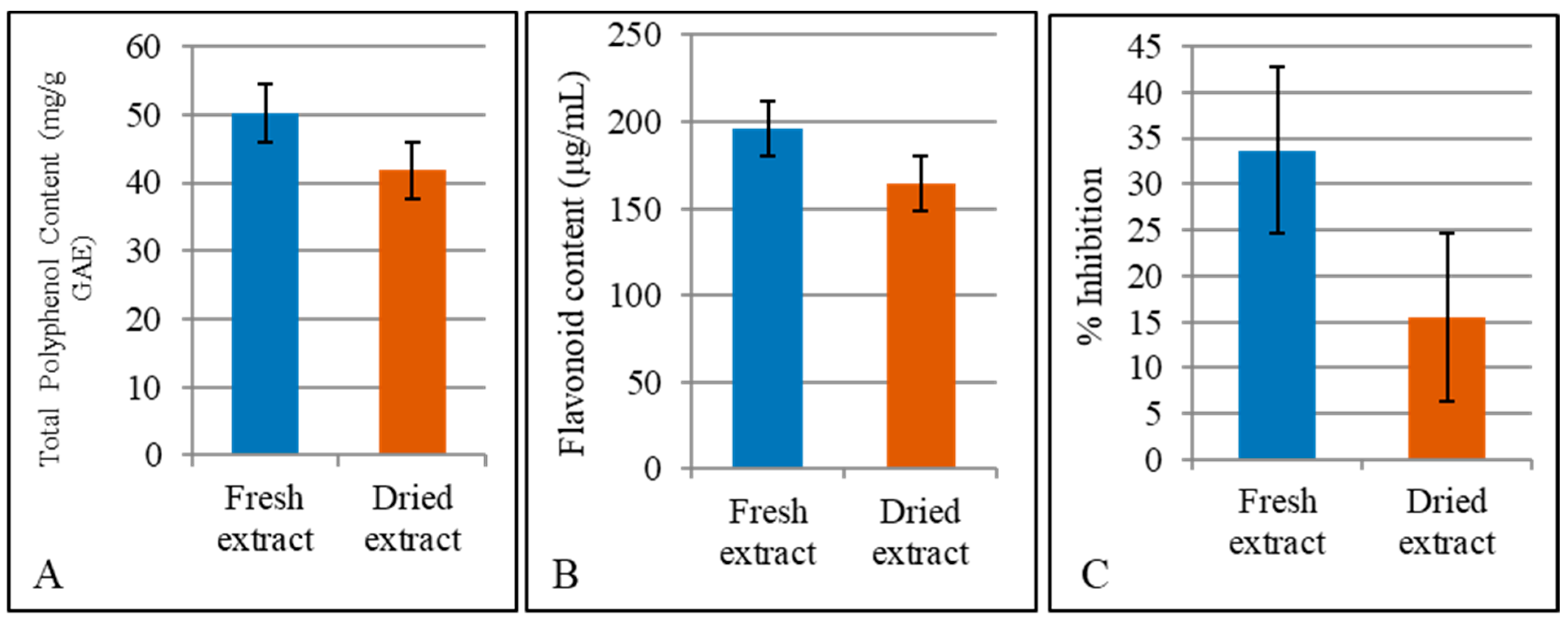
2.2. Preparation of Leaf Extracts
2.3. Biochemical Assays
2.4. Culture of HCT 116 Cell Line
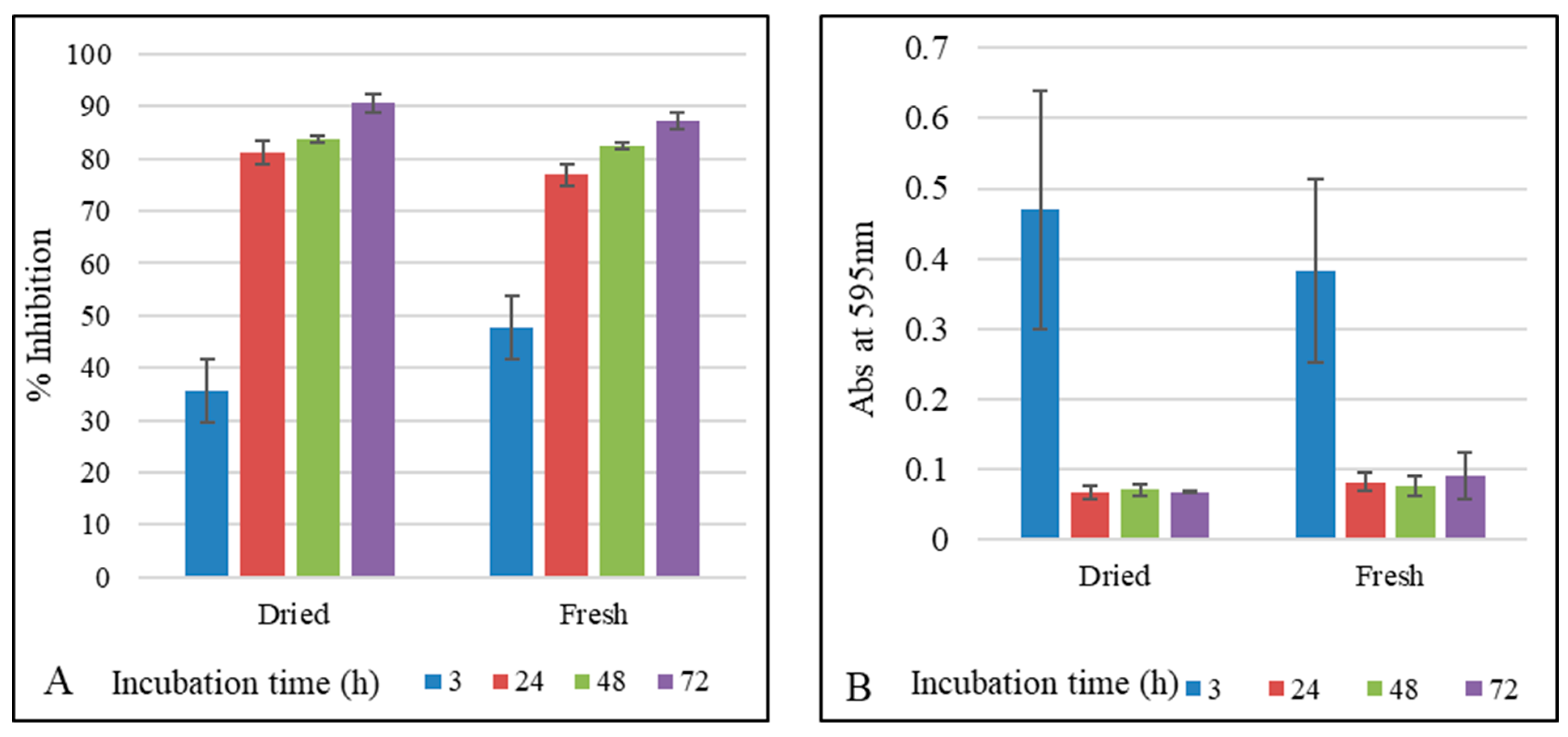
2.5. Cell Viability by MTT Colorimetric Method
3. Results and Discussion
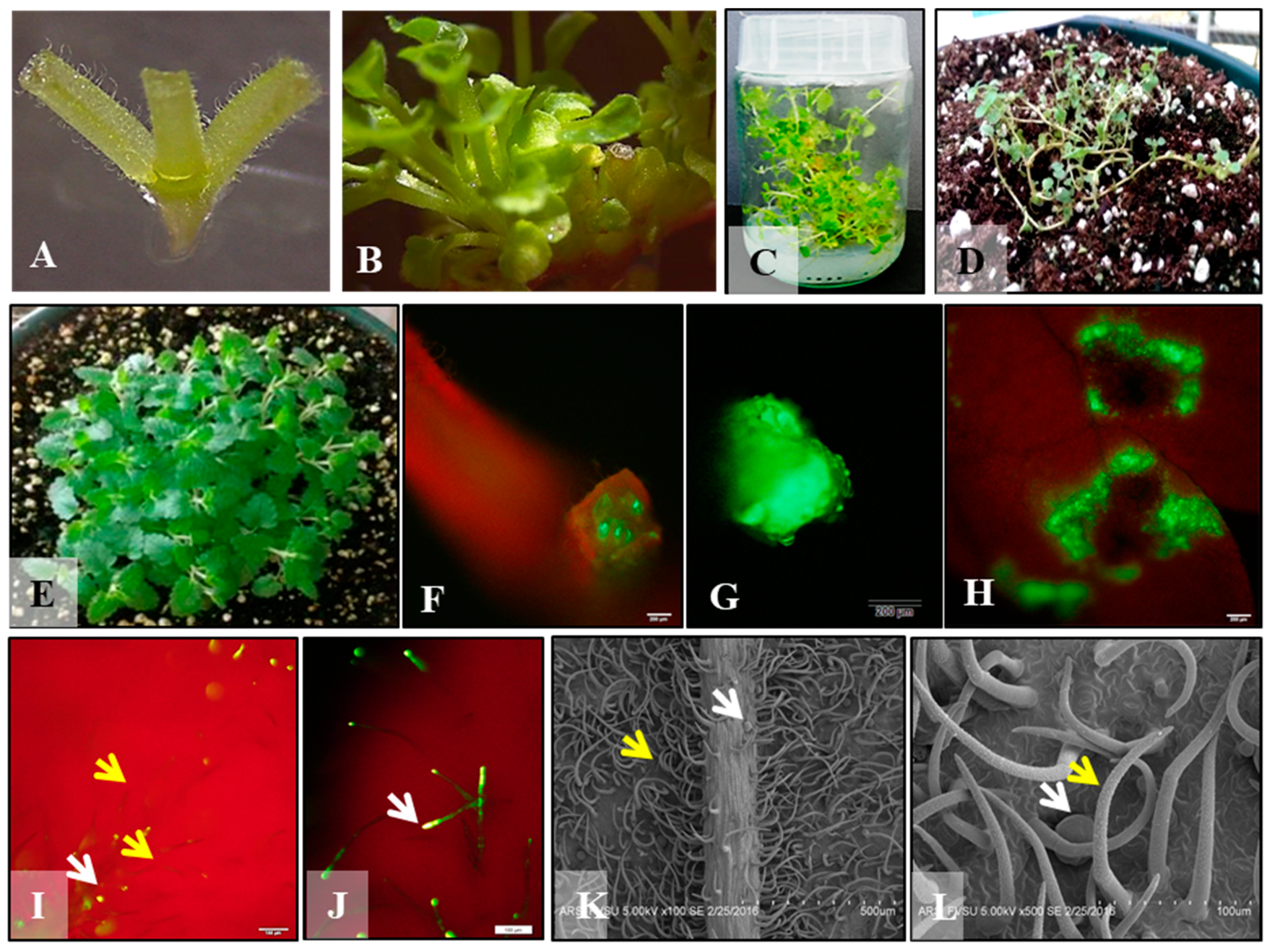
3.1. Micropropagation
3.2. Optimization of Agroinfection and Co-Cultivation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Paton, A. A Global Taxonomic Investigation of Scutellaria (Labiatae). Kew Bull. 1990, 45, 399. [Google Scholar] [CrossRef]
- Shang, X.; Hea, X.; Hea, X.; Li, M.; Zhanga, R.; Fana, P. The genus Scutellaria an ethnopharmacological and phytochemical review. J. Ethnopharmacol. 2010, 128, 279–313. [Google Scholar] [CrossRef]
- Lee, H.; Kim, Y.O.; Kim, H.; Kim, S.Y.; Noh, H.S.; Kang, S.S.; Cho, G.J.; Choi, W.S.; Suk, K. Flavonoid wogonin from medicinal herb is neuroprotective by inhibiting inflammatory activation of microglia. FASEB J. 2003, 17, 1–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li-Weber, M. New therapeutic aspects of flavones: The anticancer properties of Scutellaria and its main active constituents Wogonin, Baicalein and Baicalin. Cancer Treat. Rev. 2009, 35, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Parajuli, P.; Joshee, N.; Chinni, S.R.; Rimando, A.M.; Mittal, S.; Sethi, S.; Yadav, A.K. Delayed growth of glioma by Scutellaria flavonoids involve inhibition of Akt, GSK-3 and NF-κB signaling. J. Neuro-Oncol. 2010, 101, 15–24. [Google Scholar] [CrossRef] [Green Version]
- Dandawate, S.; Williams, L.; Joshee, N.; Rimando, A.M.; Mittal, S.; Thakur, A.; Lum, L.G.; Parajuli, P. Scutellaria extract and wogonin inhibit tumor-mediated induction of Treg cells via inhibition of TGF-β1 activity. Cancer Immunol. Immunother. 2011, 61, 701–711. [Google Scholar] [CrossRef] [Green Version]
- Patel, P.; Joshee, N.; Rimando, A.; Parajuli, P. Anti-cancer Scopes and Associated Mechanisms of Scutellaria Extract and Flavonoid Wogonin. Curr. Cancer Ther. Rev. 2013, 9, 34–42. [Google Scholar]
- Gaire, B.P.; Young Ock, K.; Zhen Hua, J.; Juyeon Jin, P.; Juyeon, P.; Hoyoung, C. Bioactivity-guided isolation of baicalein and wogonin from the root of Scutellaria baicalensis Georgi, and their neuroprotective effect against 4-VO induced global ischemic model in rat. SONSIK J. 2012, 4, 11–15. [Google Scholar]
- Huang, W.-H.; Lee, A.-R.; Yang, C.-H. Antioxidative and Anti-Inflammatory Activities of Polyhydroxyflavonoids ofScutellaria baicalensisGEORGI. Biosci. Biotechnol. Biochem. 2006, 70, 2371–2380. [Google Scholar] [CrossRef] [Green Version]
- Vaidya, B.N.; Brearley, T.A.; Joshee, N. Antioxidant capacity of fresh and dry leaf extracts of sixteen Scutellaria species. J. Med. Act. Plants 2013, 2, 42–49. [Google Scholar]
- Tayarani-Najarani, Z.; Asili, J.; Parsaee, H.; Mousavi, S.H.; Mashhadian, N.V.; Mirzaee, A.; Emami, S.A. Wogonin and neobaicalein from Scutellaria litwinowii roots are apoptotic for HeLa cells. Rev. Bras. Farm. 2012, 22, 268–276. [Google Scholar] [CrossRef] [Green Version]
- Irvin, L.; Jackson, C.; Hill, A.L.; Bajaj, R.; Mahmoudi, C.; Vaidya, B.N.; Joshee, N. Skullcaps (Scutellaria spp.): Ethnobotany and current research. In Medicinal Plants; Dheckney, S.A., Parajuli, P., Eds.; Springer: Berlin, Germany, 2019; pp. 141–168. [Google Scholar]
- Wu, J.-Y.; Tsai, K.-W.; Li, Y.-Z.; Chang, Y.-S.; Lai, Y.-C.; Laio, Y.-H.; Wu, J.-D.; Liu, Y.-W. Anti-Bladder-Tumor Effect of Baicalein fromScutellaria baicalensisGeorgi and Its ApplicationIn Vivo. Evid.-Based Complement. Altern. Med. 2013, 2013, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.H.; Hossain, M.A.; Kang, Y.J.; Jang, J.Y.; Lee, Y.J.; Im, E.; Yoon, J.-H.; Kim, H.S.; Chung, H.Y.; Kim, N.D. Baicalein, an active component of Scutellaria baicalensis Georgi, induces apoptosis in human colon cancer cells and prevents AOM/DSS-induced colon cancer in mice. Int. J. Oncol. 2013, 43, 1652–1658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Havermann, S.; Rohrig, R.; Chovolou, Y.; Humpf, H.-U.; Wätjen, W. Molecular Effects of Baicalein in Hct116 Cells and Caenorhabditis elegans: Activation of the Nrf2 Signaling Pathway and Prolongation of Lifespan. J. Agric. Food Chem. 2013, 61, 2158–2164. [Google Scholar] [CrossRef]
- Wang, T.-S.; Wang, S.-Q.; Xiao, D.-L. A review of phytochemistry and antitumor activity of a valuable medicinal species: Scutellaria barbata. J. Med. Plants Res. 2012, 6, 4259–4275. [Google Scholar]
- Wright, R.E., III; Shahin, L.; Gogineni, V.; Hussain, Z.; Naeem, A.; Sadasivan, S.; Sinha, I.; Neely, M.; Michellhaugh, S.K.; Mittal, S.; et al. Scutellaria extract inhibits proliferation and migration of brain- metastatic lung cancer cells via regulation of multiple signaling pathways. J. Med. Act. Plants 2021, 10, 32–41. [Google Scholar] [CrossRef]
- Liogier, A. Melastomataceae to Lentibulariaceae, Puerto Rico: La Editorial. In Descriptive Flora of Puerto Rico and Adjacent Islands: Spermatophyta—Dicotyledoneae; Universidad de Puerto Rico: San Juan, Spain, 1995; Volume 4. [Google Scholar]
- Delange, D.M.; Rico, C.L.M.; Canavaciolo, V.L.G.; Oliver, E.S.; De la Sierra, R.; Pérez, R.D.L.C.S.; Leyes, E.A.R.; Murillo, R.V. Phytochemical screening of Scutellaria havanensis Jacq. Rev. Cuba. Plantas Med. 2012, 17, 402–407. [Google Scholar]
- Austin, D. Florida Ethnobotany, 1st ed.; CRC: Boca Raton, FL, USA, 2004. [Google Scholar]
- Marrero, D.; Morales, C.; González, V.; Rodríguez, E.; Sierra, R. Volatile Constituents from Leaves of Endemic Scutellaria havanensis Jacq. in Cuba. J. Essent. Oil Bear. Plants 2013, 16, 368–371. [Google Scholar] [CrossRef]
- Scheck, A.C.; Perry, K.; Hank, N.C.; Clark, W.D. Anticancer activity of extracts derived from the mature roots of Scutellaria baicalensis on human malignant brain tumor cells. BMC Complement. Altern. Med. 2006, 6, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsieh, C.-J.; Hall, K.; Ha, T.; Li, C.; Krishnaswamy, G.; Chi, D. Baicalein inhibits IL-1β- and TNF-α-induced inflammatory cytokine production from human mast cells via regulation of the NF-κB pathway. Clin. Mol. Allergy 2007, 5, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bardakci, H.; Skaltsa, H.; Milosevic-Ifantis, T.; Lazari, D.; Hadjipavlou-Litina, D.; Yeşilada, E.; Kırmızıbekmez, H. Antioxidant activities of several Scutellaria taxa and bioactive phytoconstituents from Scutellaria hastifolia L. Ind. Crops Prod. 2015, 77, 196–203. [Google Scholar] [CrossRef]
- Valdés, A.F.-C.; Fidalgo, L.M.; Ramos, I.S.; Delange, D.M.; Rico, C.L.M.; Martínez, J.M.; Cuéllar, A.C. Antiprotozoal screening of the Cuban native plant Scutellaria havanensis. Pharm. Biol. 2016, 54, 3197–3202. [Google Scholar] [CrossRef] [PubMed]
- Sherman, S.H.; Vaidya, B.N.; Joshee, N. Micromorphological studies in an anxiolytic medicinal plant, Scutellaria lateriflora L. J. Agric. Food Res. 2021, 1, 1–14. [Google Scholar]
- Cole, I.B.; Saxena, P.K.; Murch, S.J. Medicinal biotechnology in the genus scutellaria. In Vitro Cell. Dev. Biol.-Plant 2007, 43, 318–327. [Google Scholar] [CrossRef]
- Joshee, N.; Mentreddy, S.; Yadav, A.K. Mycorrhizal fungi and growth and development of micropropagated Scutellaria integrifolia plants. Ind. Crops Prod. 2007, 25, 169–177. [Google Scholar] [CrossRef]
- Tascan, A.; Adelberg, J.; Tascan, M.; Rimando, A.; Joshee, N.; Yadav, A.K. Hyperhydricity and Flavonoid Content of Scutellaria Species In Vitro on Polyester-supported Liquid Culture Systems. HortScience 2010, 45, 1723–1728. [Google Scholar] [CrossRef] [Green Version]
- Brearley, T.A.; Vaidya, B.N.; Joshee, N. Cytokinin, Carbon Source, and Acclimatization Requirements for in Vitro Propagation of Scutellaria barbata D. Don and Scutellaria racemosa Pers. Am. J. Plant. Sci. 2014, 05, 3662–3672. [Google Scholar] [CrossRef] [Green Version]
- Marsh, Z.; Yang, T.; Nopo-Olazabal, L.; Wu, S.; Ingle, T.; Joshee, N.; Medina-Bolivar, F. Effect of light, methyl jasmonate and cyclodextrin on production of phenolic compounds in hairy root cultures of Scutellaria lateriflora. Phytochemistry 2014, 107, 50–60. [Google Scholar] [CrossRef]
- Kowalczyk, T.; Wieczfinska, J.; Skała, E.; Śliwiński, T.; Sitarek, P. Transgenesis as a Tool for the Efficient Production of Selected Secondary Metabolites from Plant in Vitro Cultures. Plants 2020, 9, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaidya, B.N.; Jackson, C.L.; Perry, Z.D.; Dhekney, S.A.; Joshee, N. Agrobacterium-mediated transformation of thin cell layer explants of Scutellaria ocmulgee small: A rare plant with anti-tumor properties. Plant. Cell Tissue Organ. Cult. (PCTOC) 2016, 127, 57–69. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Li, Z.T.; Jayasankar, S.; Gray, D. Bi-directional duplex promoters with duplicated enhancers significantly increase transgene expression in grape and tobacco. Transgenic Res. 2004, 13, 143–154. [Google Scholar] [CrossRef]
- Einrich, G.H.; Pfeifhofer, H.W.; Stabentheiner, E.; Sawidis, T. Glandular Hairs of Sigesbeckia jorullensis Kunth (Asteraceae): Morphology, Histochemistry and Composition of Essential Oil. Ann. Bot. 2002, 89, 459–469. [Google Scholar] [CrossRef] [Green Version]
- Combrinck, S.; Du Plooy, G.W.; McCrindle, R.I.; Botha, B.M. Morphology and Histochemistry of the Glandular Trichomes of Lippia scaberrima (Verbenaceae). Ann. Bot. 2007, 99, 1111–1119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, H.; Bladt, S. Plant Drug Analysis: A Thin Layer Chromatographic Atlas, 2nd ed.; Springer: Heidelberg, Germany, 1996. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Yi, W.; Wetzstein, H.Y. Effects of Drying and Extraction Conditions on the Biochemical Activity of Selected Herbs. HortScience 2011, 46, 70–73. [Google Scholar] [CrossRef]
- Vaidya, B. Antioxidant Potential, Conservation, and Reproductive Biology of Medicinal Scutellaria. Master’s Thesis, Fort Valley State University, Fort Valley, GA, USA, 2013. [Google Scholar]
- Singleton, V.L.; Rossi, J.A., Jr. Colorimetry of total phenolics with phosphomolybdic phosphotunstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Davies, M.J.; Fornit, L.G.; Willson, R.L. Vitamin E analogue Trolox C E.s.r. and pulse radiolysis studies of free-radical reactions. Biochem. J. 1988, 255, 513–522. [Google Scholar]
- Louis, K.S.; Siegel, A.C. Cell Viability Analysis Using Trypan Blue: Manual and Automated Methods. In Mammalian Cell Viability; Humana Press: Totowa, NJ, USA, 2011; Volume 740, pp. 7–12. [Google Scholar] [CrossRef]
- Felicity, C.; Hoye, C.; Fernandez, V. Influence of Heating on the Polyphenolic Content and Antioxidant Activity of Grape Seed Flour. J. Food Sci. 2011, 76, 884–890. [Google Scholar]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Marrero, D.; Morales, C.; González, V.; Cuellar, A.; Salas, E. Selective and High Yield Isolation of Pure Wogonin from Aerial Parts of Scutellaria havanensis Jacq. Int. J. Pharm. Sci. Rev. Res. 2015, 30, 104–108. [Google Scholar]
- Genena, A.K.; Hense, H.; Junior, A.S.; De Souza, S.M. Rosemary (Rosmarinus officinalis): A study of the composition, antioxidant and antimicrobial activities of extracts obtained with supercritical carbon dioxide. Food Sci. Technol. 2008, 28, 463–469. [Google Scholar] [CrossRef] [Green Version]
- Ghasemzadeh, A.; Jaafar, H.Z.E.; Rahmat, A. Antioxidant Activities, Total Phenolics and Flavonoids Content in Two Varieties of Malaysia Young Ginger (Zingiber officinale Roscoe). Molecules 2010, 15, 4324–4333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demiroglu, A.; Basara, B.; Kilic, E.; Yanikkaya, G. The Investigation of effects of quercetin and its combination with cisplatin on malignant mesothelioma cells in vitro. J. Biomed. Biotechnol. 2010. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Otsuyama, K.-I.; Liu, S.; Abroun, S.; Ishikawa, H.; Tsuyama, N.; Obata, M.; Li, F.-J.; Zheng, X.; Maki, Y.; et al. Baicalein, a component of Scutellaria radix from Huang-Lian-Jie-Du-Tang (HLJDT), leads to suppression of proliferation and induction of apoptosis in human myeloma cells. Blood 2005, 105, 3312–3318. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.-H.; Kim, C.; Zhang, L.; Lee, Y.J. Role of p53, PUMA, and Bax in wogonin-induced apoptosis in human cancer cells. Biochem. Pharmacol. 2008, 75, 2020–2033. [Google Scholar] [CrossRef] [Green Version]
| Treatment | Number of Days in the Shoot Induction Medium and Number of Shoots | ||||
|---|---|---|---|---|---|
| 3 | 5 | 7 | 10 | 14 | |
| MS (Control) | Number of elongated adventitious shoots | ||||
| 0 | 0 | 0 | 0 | 0 | |
| MS + 0.05 µM BAP | 2.67 ± 1.25 c | 5.33 ± 1.25 c | 2.67 ± 0.94 c | 2.67 ± 0.47 c | 2.67 ± 1.25 cd |
| MS + 0.5 µM BAP | 2.33 ± 0.94 d | 0.0 | 4.0 ± 2.83 c | 9.33 ± 4.78 b | 3.67 ± 1.89 c |
| MS + 5.0 µM BAP | 0.67 ± 0.94 d | 3.33 ± 2.87 c | 4.33 ± 0.47 c | 4.0 ± 2.94 c | 5.33 ± 1.25 c |
| MS + 10.0 µM BAP | 5.0 ± 0.82 c | 2.0 ± 0.82 d | 8.0 ± 4.55 bc | 14.67 ± 1.25 a | * 1.0 ± 0.82 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Irvin, L.; Zavala Ortiz, Y.; Rivera, K.R.; Nanda Vaidya, B.; Sherman, S.H.; Batista, R.A.; Negrón Berríos, J.A.; Joshee, N.; Arun, A. Micropropagation of Rare Scutellaria havanensis Jacq. and Preliminary Studies on Antioxidant Capacity and Anti-Cancer Potential. Molecules 2021, 26, 5813. https://doi.org/10.3390/molecules26195813
Irvin L, Zavala Ortiz Y, Rivera KR, Nanda Vaidya B, Sherman SH, Batista RA, Negrón Berríos JA, Joshee N, Arun A. Micropropagation of Rare Scutellaria havanensis Jacq. and Preliminary Studies on Antioxidant Capacity and Anti-Cancer Potential. Molecules. 2021; 26(19):5813. https://doi.org/10.3390/molecules26195813
Chicago/Turabian StyleIrvin, Lani, Yarelia Zavala Ortiz, Kamila Rivera Rivera, Brajesh Nanda Vaidya, Samantha H Sherman, Rosalinda Aybar Batista, Juan A. Negrón Berríos, Nirmal Joshee, and Alok Arun. 2021. "Micropropagation of Rare Scutellaria havanensis Jacq. and Preliminary Studies on Antioxidant Capacity and Anti-Cancer Potential" Molecules 26, no. 19: 5813. https://doi.org/10.3390/molecules26195813
APA StyleIrvin, L., Zavala Ortiz, Y., Rivera, K. R., Nanda Vaidya, B., Sherman, S. H., Batista, R. A., Negrón Berríos, J. A., Joshee, N., & Arun, A. (2021). Micropropagation of Rare Scutellaria havanensis Jacq. and Preliminary Studies on Antioxidant Capacity and Anti-Cancer Potential. Molecules, 26(19), 5813. https://doi.org/10.3390/molecules26195813






