Synthesis, Chemical–Physical Characterization, and Biomedical Applications of Functional Gold Nanoparticles: A Review
Abstract
:1. Introduction
- -
- Sensors: AuNPs can be used for protein detection in Raman spectroscopy utilized as support for the implementation of the analysis of vibrational energies of chemical bonds [17].
- -
- Probes: used for biological imaging application. AuNPs can produce an array of colors employed in dark-field microscopy [18].
- -
- Diagnostics: AuNPs are able to detect biomarkers as a valid tool in the diagnosis of cancers, infectious agents, and heart diseases [19].
- -
- Treatment Agent Transport: AuNP surfaces can be functionalized with hundreds of biomolecules, which are delivered to target cells [20].
- -
- Photodynamic Therapy: AuNPs generate heat when they are irradiated by 700–800-nm wavelengths of light. The heat of these nanoparticles produced when they are inside cancer cells is then exploited to induce death [21].
2. Fabrication of Gold Nanoparticles by Chemical Routes
- the choice of proper reduction agents, which provide electrons to reduce gold cations—that is, Au3+ and Au+ to metallic gold. Nowadays, many reduction agents are used for the synthesis of AuNPs, such as citric acid and citrate, borohydrides, polyols, sulfites, etc. [22].
- the use of stabilization agents, which are crucial to manage the growth of AuNPs in terms of size and geometric shape. In fact, by attributing a repulsive force, they are able to prevent aggregation during the synthetic procedure in the chosen solvent. The most used stabilization agents are sulfur or phosphorous ligands, but polymers and surfactants are also employed; there is also the possibility of using the same molecule to operate as a reducing and stabilizing agent at the same time [23].
2.1. Current AuNP Physical and Chemical Synthetic Methods
- a destructive method: top-down approach
- a constructive method: bottom-up approach (Figure 1).
2.2. Turkevich Synthesis
2.3. Synthesis with NaBH4 with or without Citrate Addition
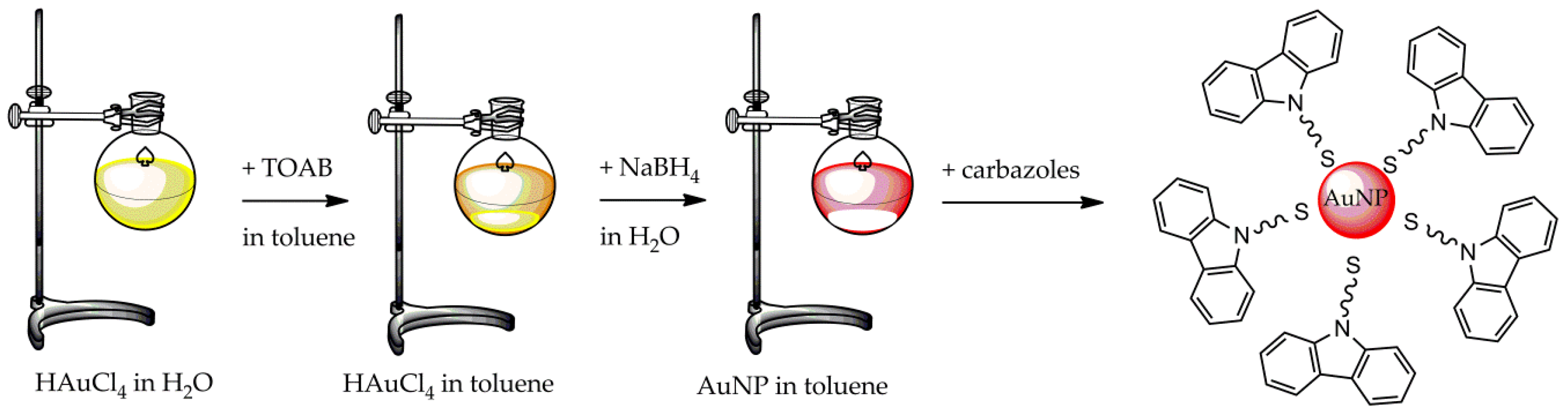
2.4. Brust–Schiffrin Synthesis
2.5. Synthesis by Seeding-Growth Technique
2.6. Synthesis by Ascorbic Acid
2.7. Green Synthesis Methods
| HAuCl4 Concentration | Chemicals Used | AuNPs Size | Reference |
|---|---|---|---|
| Turkevich synthesis | |||
| 0.15 mM | sodium citrate | 20 nm | [52] |
| 5.8 mM | sodium 3-mercaptopropionate, and sodium citrate | 10 nm | [53] |
| 0.5 M | sodium citrate | 40 nm | [54] |
| 24.3 mM | sodium citrate | 4 nm | [55] |
| 1 mM | sodium citrate | 10 nm | [56] |
| 0.25 mM | sodium citrate | 18 nm | [57] |
| 1 mM | sodium citrate | 13 nm | [58] |
| 0.25 mM | sodium citrate | 9 nm | [59] |
| Synthesis with NaBH4 with/without citrate | |||
| 0.25 mM | NaCl, NaBH4 and sodium citrate | 19 nm | [86] |
| 0.1 mM | sodium citrate, NaBH4 | 6 nm | [61] |
| 0.3 mM | NaBH4 | 30 nm | [87] |
| 0.25 mL | sodium citrate, NaBH4 | 3.5 nm | [88] |
| 0.1 mM | NaBH4 | 7 nm | [89] |
| 0.03 mM | sodium citrate, NaBH4 | 4 nm | [90] |
| 0.3 mM | sodium citrate, NaBH4 | 8 nm | [91] |
| Brust–Schiffrin method | |||
| 30 mM | tetraoctylammonium bromide, dodecanethiol, NaBH4 | 2.5 nm | [63] |
| 30 mM | tetraoctylammonium bromide, NaBH4 | 3.4 nm | [92] |
| 10 mM | tetraoctylammonium bromide, cetyltrimethylammonium chloride, cetyltrimethylammonium bromide, NaBH4 | 10 nm | [2] |
| 30 mM | tetraoctylammonium bromide, pentanethiol, NaBH4 | 5 nm | [93] |
| 10 mM | 3-mercaptopropionic acid, tetraoctylammonium bromide, NaBH4 | 2 nm | [64] |
| 30 mM | 4-(N,N-dimethylaminopyridine), NaBH4 | 20 nm | [65] |
| 0.45 mM | tetraoctylammonium bromide, dodecanethiol, NaBH4 | 1.8 nm | [94] |
| 34 mM | tetraoctylammonium bromide, NaBH4 | 1.8 nm | [95] |
| 30 mM | tetraoctylammonium bromide, NaBH4 | 10 nm | [96] |
| 0.1 mM | tetraoctylammonium bromide, 1-decanethiol, NaBH4 | 4 nm | [97] |
| 30 mM | tetraoctylammonium bromide, 4-dimethylaminopyridine, NaBH4 | 5.5 nm | [98] |
| 4.0 mM | tetraoctylammonium bromide, Chlorobenzenemethanethiol, NaBH4 | 3–4 nm | [67] |
| 50 mM | HCl, NaBH4, NaOH, dodecanethiol, n-hexane, | 4 nm | [99] |
| 5 mM | tetraoctylammonium bromide, 1-hexanethiol, NaBH4 | 2 nm | [100] |
| Synthesis by Seeding-Growth technique | |||
| 0.25 mM | sodium citrate, cetyltrimethylammonium bromide, NaBH4, ascorbic acid | 6 nm 17 nm 37 nm | [69] |
| 0.25 mM | cetyltrimethylammonium bromide, AgNO3, ascorbic acid | 10 nm | [70] |
| 10 mM | cetyltrimethylammonium bromide, NaBH4, AgNO3, ascorbic acid | 33 nm length 13 nm width | [101] |
| 0.25 mM | 1,2-Bis(10,12-tricosadiynoyl)-sn-glycero-3-phosphocholine, sodium citrate, NaBH4, ascorbic acid | 17 nm | [102] |
| Synthesis by ascorbic acid | |||
| 0.05 mM | cetyltrimethylammonium bromide, ascorbic acid | 30 nm | [71] |
| 20 mM | cetyltrimethylammonium bromide, ascorbic acid | 15 nm | [72] |
| 0.5 mM | ascorbic acid, chitosan | 18 nm | [73] |
| Green synthesis | |||
| 0.1 mM | Avena sativa biomass | 5–20 nm | [74] |
| 1 mM | Thyme | 6–26 nm | [75] |
| 10 mM | N-(4-imidazolyl)methylchitosan | 3 nm | [76] |
| 5 mM | Hibiscus sabdariffa L. | 9 nm | [77] |
| 1 mM | Chitosan | 10–15 nm | [78] |
| 0.2 mM | Guggulutiktham Kashayam | 15–50 nm | [79] |
| 0.3 mM | coconut oil | 25–45 nm | [80] |
| 0.25 mM | Anacardium occidentale | 15–40 nm | [81] |
| 0.1 M | Natural honey | 15 nm | [82] |
| 60 mM | Volvariella volvacea mushroom | 20–150 nm | [83] |
| 3–5% | Aqueous coffee pulp extract (Coffea arabica L.) | 5–22 nm | [84] |
| 1 mM | Garcinia kola pulp extract | 18–38 nm | [85] |
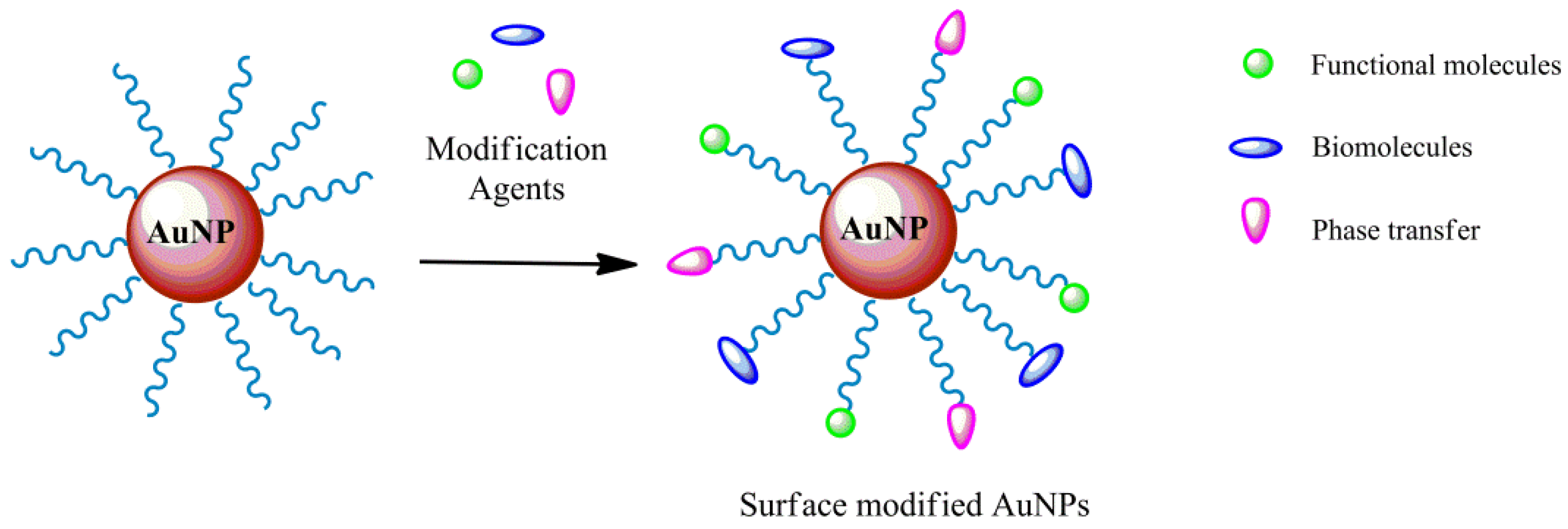
3. Gold Nanoparticles Surface Modification
3.1. Secondary Modification
3.2. Physical Sorption
3.3. Dative Bond and Formation of “Self-Assembled Monolayers” (SAMs)
3.4. Polymer Coating
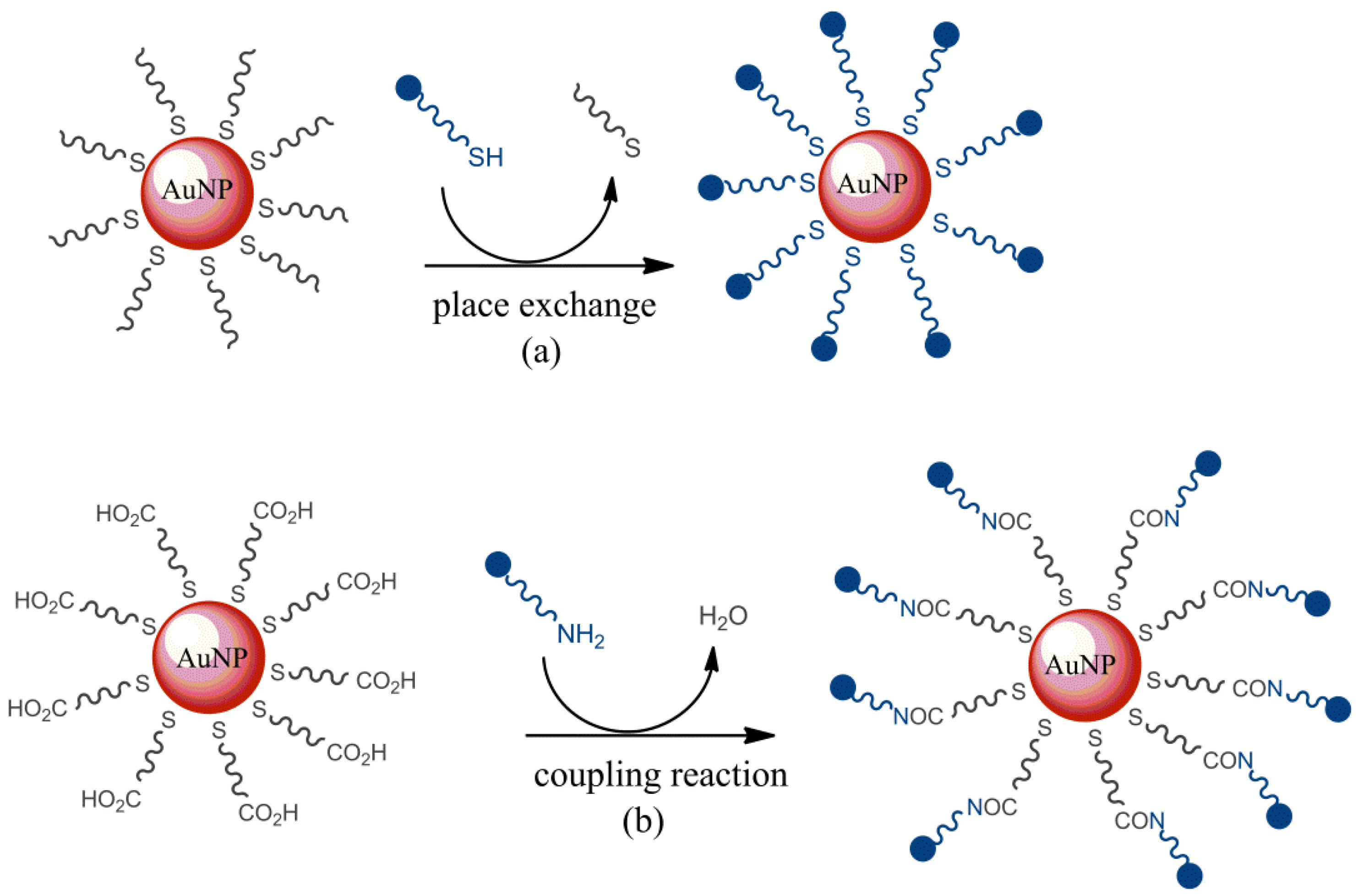
3.5. Covalent Immobilization of Ligands
3.6. Bioaffinity Immobilization of Ligands
4. Chemical–Physical Characterization of Gold Nanoparticles
4.1. Ultraviolet–Visible Spectroscopy (UV-VIS)
4.2. Dynamic Light Scattering (DLS)
4.3. Transmission Electron Microscopy (TEM)
4.4. Thermogravimetric Analysis (TGA)
4.5. X-ray Photoelectron Spectroscopy XPS
5. Surface Coating Determination
5.1. Indirect Methods
5.2. Direct Methods
6. Physical and Chemical Properties of AuNPs Depending on Particle Size
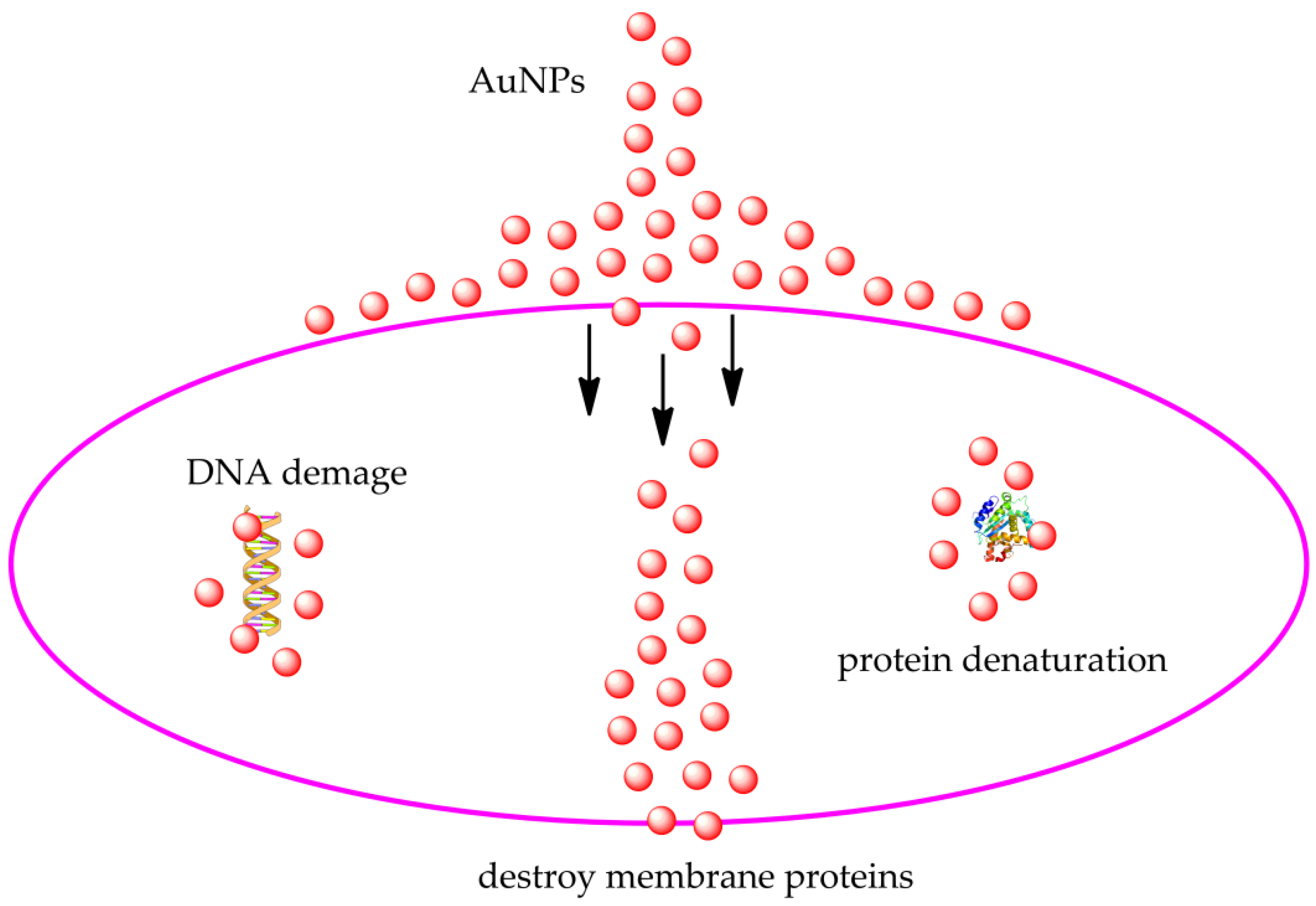
6.1. Antibacterial Activity
6.2. Localized Surface Plasmon Resonance in Nanoparticles (LSPR)
7. Applications
7.1. Hyperthermia and Photothermal Therapy
7.2. AuNP for Health Applications
7.2.1. Biodistribution and Cytotoxicity of AuNP
7.2.2. AuNPs as Delivery Carriers
7.3. Diagnostics
7.3.1. Enhanced Permeability and Retention Effect (EPR) and Tumor Targeting
7.3.2. Application of AuNPs for Small Molecule Detection
7.3.3. Application of AuNPs for Detection of Biological Molecules
7.4. Imaging
7.5. Application of AuNPs for the Biomarker Analysis
7.6. Application of AuNPs as Bio-Barcodes
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Daniel, M.C.; Astruc, D. Gold Nanoparticles: Assembly, Supramolecular Chemistry, Quantum-Size-Related Properties, and Applications toward Biology, Catalysis, and Nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef] [PubMed]
- Daruich De Souza, C.; Ribeiro Nogueira, B.; Rostelato, M.E.C.M. Review of the methodologies used in the synthesis gold nanoparticles by chemical reduction. J. Alloys Compd. 2019, 798, 714–740. [Google Scholar] [CrossRef]
- Čitaković, N. Physical Properties of Nanomaterials. Vojnotehnički Glasnik 2019, 67, 159–171. [Google Scholar] [CrossRef]
- Caballero-Calero, O.; D’Agosta, R. Review—Towards the Next Generation of Thermoelectric Materials: Tailoring Electronic and Phononic Properties of Nanomaterials. ECS J. Solid State Sci. Technol. 2017, 6, N3065–N3079. [Google Scholar] [CrossRef] [Green Version]
- Barattucci, A.; Plutino, M.R.; Faggi, C.; Bonaccorsi, P.; Monsù Scolaro, L.; Aversa, M.C. Mono- and trinuclear tripodal platinum(II) chelated complexes containing a pyridine/sulfoxide based anchoring framework. Eur. J. Inorg. Chem. 2013, 2013, 3412–3420. [Google Scholar] [CrossRef]
- Ray, M.; Basu, T.S.; Bandyopadhyay, N.R.; Klie, R.F.; Ghosh, S.; Raja, S.O.; Dasgupta, A.K. Highly lattice-mismatched semiconductor-metal hybrid nanostructures: Gold nanoparticle encapsulated luminescent silicon quantum dots. Nanoscale 2014, 6, 2201–2210. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Si, L.S.; Wu, F.; Ying Chan, S.; Yu, P.; Fei, B. Electrical and mechanical self-healing membrane using gold nanoparticles as localized “nano-heaters”. J. Mater. Chem. C 2016, 4, 10018–10025. [Google Scholar] [CrossRef]
- Kucherik, A.; Kutrovskaya, S.; Osipov, A.; Gerke, M.; Chestnov, I.; Arakelian, S. Nano-Antennas Based on Silicon-Gold Nanostructures. Sci. Rep. 2019, 9, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Kaminker, R.; Lahav, M.; Motiei, L.; Vartanian, M.; Popovitz-Biro, R.; Iron, M.A.; van der Boom, M.E. Molecular Structure-Function Relations of the Optical Properties and Dimensions of Gold Nanoparticle Assemblies. Angew. Chem. 2010, 122, 1240–1243. [Google Scholar] [CrossRef]
- Zou, C.; Yang, B.; Bin, D.; Wang, J.; Li, S.; Yang, P.; Wang, C.; Shiraishi, Y.; Du, Y. Electrochemical synthesis of gold nanoparticles decorated flower-like graphene for high sensitivity detection of nitrite. J. Colloid Interface Sci. 2017, 488, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Wostek-Wojciechowska, D.; Jeszka, J.K.; Uznanski, P.; Amiens, C.; Chaudret, B.; Lecante, P. Synthesis of gold nanoparticles in solid state by thermal decomposition of an organometallic precursor. Mater. Sci. Pol. 2004, 22, 407–413. [Google Scholar]
- Huang, X.; El-Sayed, I.H.; Qian, W.; El-Sayed, M.A. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J. Am. Chem. Soc. 2006, 128, 2115–2120. [Google Scholar] [CrossRef]
- Jain, P.K.; Lee, K.S.; El-Sayed, I.H.; El-Sayed, M.A. Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: Applications in biological imaging and biomedicine. J. Phys. Chem. B 2006, 110, 7238–7248. [Google Scholar] [CrossRef] [Green Version]
- Siddiqi, K.S.; Husen, A. Recent advances in plant-mediated engineered gold nanoparticles and their application in biological system. J. Trace Elem. Med. Biol. 2017, 40, 10–23. [Google Scholar] [CrossRef]
- Puoci, F.; Saturnino, C.; Trovato, V.; Iacopetta, D.; Piperopoulos, E.; Triolo, C.; Bonomo, M.G.; Drommi, D.; Parisi, O.I.; Milone, C.; et al. Sol-gel treatment of textiles for the entrapping of an antioxidant/anti-inflammatory molecule: Functional coating morphological characterization and drug release evaluation. Appl. Sci. 2020, 10, 2287. [Google Scholar] [CrossRef] [Green Version]
- Brown, S.D.; Nativo, P.; Smith, J.A.; Stirling, D.; Edwards, P.R.; Venugopal, B.; Flint, D.J.; Plumb, J.A.; Graham, D.; Wheate, N.J. Gold nanoparticles for the improved anticancer drug delivery of the active component of oxaliplatin. J. Am. Chem. Soc. 2010, 132, 4678–4684. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chu, W.; Foroushani, A.D.; Wang, H.; Li, D.; Liu, J.; Barrow, C.J.; Wang, X.; Yang, W. New gold nanostructures for sensor applications: A review. Materials 2014, 7, 5169–5201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, J.; Liu, Y.; Gao, P.F.; Zou, H.Y.; Huang, C.Z. Precision improvement in dark-field microscopy imaging by using gold nanoparticles as an internal reference: A combined theoretical and experimental study. Nanoscale 2016, 8, 8729–8736. [Google Scholar] [CrossRef]
- Cordeiro, M.; Carlos, F.F.; Pedrosa, P.; Lopez, A.; Baptista, P.V. Gold Nanoparticles for Diagnostics: Advances towards Points of Care. Diagnostics 2016, 6, 43. [Google Scholar] [CrossRef] [PubMed]
- Raliya, R.; Saha, D.; Chadha, T.S.; Raman, B.; Biswas, P. Non-invasive aerosol delivery and transport of gold nanoparticles to the brain. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef] [Green Version]
- García Calavia, P.; Bruce, G.; Pérez-García, L.; Russell, D.A. Photosensitiser-gold nanoparticle conjugates for photodynamic therapy of cancer. Photochem. Photobiol. Sci. 2018, 17, 1534–1552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, P.; Li, N.; Astruc, D. State of the art in gold nanoparticle synthesis. Coord. Chem. Rev. 2013, 257, 638–665. [Google Scholar] [CrossRef]
- Kimling, J.; Maier, M.; Okenve, B.; Kotaidis, V.; Ballot, H.; Plech, A. Turkevich method for gold nanoparticle synthesis revisited. J. Phys. Chem. B 2006, 110, 15700–15707. [Google Scholar] [CrossRef]
- Schleh, C.; Semmler-Behnke, M.; Lipka, J.; Wenk, A.; Hirn, S.; Schäffler, M.; Schmid, G.; Simon, U.; Kreyling, W.G. Size and surface charge of gold nanoparticles determine absorption across intestinal barriers and accumulation in secondary target organs after oral administration. Nanotoxicology 2012, 6, 36–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weirich, T.E.; Friedrich, B.G.; Pani, V.V. Structural and Electrochemical Properties of Nesting and Core/Shell Pt/TiO2 Spherical Particles Synthesized by Ultrasonic Spray Pyrolysis. Metals 2020, 10, 11. [Google Scholar]
- Yu, X.; Pham, J.T.; Subramani, C.; Creran, B.; Yeh, Y.C.; Du, K.; Patra, D.; Miranda, O.R.; Crosby, A.J.; Rotello, V.M. Direct patterning of engineered ionic gold nanoparticles via nanoimprint lithography. Adv. Mater. 2012, 24, 6330–6334. [Google Scholar] [CrossRef]
- Davies, G.L.; O’Brien, J.; Gun’ko, Y.K. Rare Earth Doped Silica Nanoparticles via Thermolysis of a Single Source Metallasilsesquioxane Precursor. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Mirzaei, A.; Neri, G. Microwave-assisted synthesis of metal oxide nanostructures for gas sensing application: A review. Sens. Actuators B Chem. 2016, 237, 749–775. [Google Scholar] [CrossRef]
- Medici, S.; Peana, M.; Nurchi, V.M.; Lachowicz, J.I.; Crisponi, G.; Zoroddu, M.A. Noble metals in medicine: Latest advances. Coord. Chem. Rev. 2015, 284, 329–350. [Google Scholar] [CrossRef]
- Hasan, S. A Review on Nanoparticles: Their Synthesis and Types. Res. J. Recent Sci. 2014, 4, 1–3. [Google Scholar]
- Booth, S.G.; Uehara, A.; Chang, S.Y.; Mosselmans, J.F.W.; Schroeder, S.L.M.; Dryfe, R.A.W. Gold Deposition at a Free-Standing Liquid/Liquid Interface: Evidence for the Formation of Au(I) by Microfocus X-ray Spectroscopy (μXRF and μXAFS) and Cyclic Voltammetry. J. Phys. Chem. C 2015, 119, 16785–16792. [Google Scholar] [CrossRef]
- Balachandramohan, J.; Sivasankar, T.; Sivakumar, M. Facile sonochemical synthesis of Ag2O-guar gum nanocomposite as a visible light photocatalyst for the organic transformation reactions. J. Hazard. Mater. 2020, 385, 121621. [Google Scholar] [CrossRef]
- Paiva-Santos, A.C.; Herdade, A.M.; Guerra, C.; Peixoto, D.; Pereira-Silva, M.; Zeinali, M.; Mascarenhas-Melo, F.; Paranhos, A.; Veiga, F. Plant-mediated green synthesis of metal-based nanoparticles for dermopharmaceutical and cosmetic applications. Int. J. Pharm. 2021, 597, 120311. [Google Scholar] [CrossRef]
- Ealias, A.M.; Saravanakumar, M.P. A review on the classification, characterisation, synthesis of nanoparticles and their application. IOP Conf. Ser. Mater. Sci. Eng. 2017, 263, 32019. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Yonezawa, T. Sputtering onto a liquid: Interesting physical preparation method for multi-metallic nanoparticles. Sci. Technol. Adv. Mater. 2018, 19, 883–898. [Google Scholar] [CrossRef] [Green Version]
- Habibullah, G.; Viktorova, J.; Ruml, T. Current Strategies for Noble Metal Nanoparticle Synthesis. Nanoscale Res. Lett. 2021, 16, 1–12. [Google Scholar] [CrossRef]
- Yesildag, C.; Ouyang, Z.; Zhang, Z.; Lensen, M.C. Micro-patterning of PEG-based hydrogels with gold nanoparticles using a reactive micro-contact-printing approach. Front. Chem. 2019, 7, 1–10. [Google Scholar] [CrossRef]
- Xu, C.; De, S.; Balu, A.M.; Ojeda, M.; Luque, R. Mechanochemical synthesis of advanced nanomaterials for catalytic applications. Chem. Commun. 2015, 51, 6698–6713. [Google Scholar] [CrossRef]
- Schreyer, H.; Eckert, R.; Immohr, S.; de Bellis, J.; Felderhoff, M.; Schüth, F. Milling Down to Nanometers: A General Process for the Direct Dry Synthesis of Supported Metal Catalysts. Angew. Chem. Int. Ed. 2019, 58, 11262–11265. [Google Scholar] [CrossRef]
- Korshed, P.; Li, L.; Ngo, D.T.; Wang, T. Effect of storage conditions on the long-term stability of bactericidal effects for laser generated silver nanoparticles. Nanomaterials 2018, 8, 218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sportelli, M.C.; Izzi, M.; Volpe, A.; Clemente, M.; Picca, R.A.; Ancona, A.; Lugarà, P.M.; Palazzo, G.; Cioffi, N. The pros and cons of the use of laser ablation synthesis for the production of silver nano-antimicrobials. Antibiotics 2018, 7, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.; Lai, J.; Lu, J.; Li, Z. Pulsed laser ablation of bulk target and particle products in liquid for nanomaterial fabrication. AIP Adv. 2019, 9, 15307. [Google Scholar] [CrossRef]
- Odularu, A.T. Metal Nanoparticles: Thermal Decomposition, Biomedicinal Applications to Cancer Treatment, and Future Perspectives. Bioinorg. Chem. Appl. 2018, 2018, 9354708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azharuddin, M.; Zhu, G.H.; Das, D.; Ozgur, E.; Uzun, L.; Turner, A.P.F.; Patra, H.K. A repertoire of biomedical applications of noble metal nanoparticles. Chem. Commun. 2019, 55, 6964–6996. [Google Scholar] [CrossRef]
- Malik, M.A.; Wani, M.Y.; Hashim, M.A. Microemulsion method: A novel route to synthesize organic and inorganic nanomaterials. 1st Nano Update. Arab. J. Chem. 2012, 5, 397–417. [Google Scholar] [CrossRef] [Green Version]
- Solanki, J.N.; Murthy, Z.V.P. Controlled size silver nanoparticles synthesis with water-in-oil microemulsion method: A topical review. Ind. Eng. Chem. Res. 2011, 50, 12311–12323. [Google Scholar] [CrossRef]
- Yanilkin, V.V.; Nasretdinova, G.R.; Kokorekin, V.A. Mediated electrochemical synthesis of metal nanoparticles. Russ. Chem. Rev. 2018, 87, 1080–1110. [Google Scholar] [CrossRef]
- Kuntyi, O.; Kytsya, R.; Mertsalo, I.P.; Mazur, S.; Zozula, G.; Bazylyak, L.I.; Тopchak, R.V. Electrochemical synthesis of silver nanoparticles by reversible current in solutions of sodium polyacrylate. Colloid Polym. Sci. 2019, 297, 689–695. [Google Scholar] [CrossRef]
- Flores-Rojas, G.G.; López-Saucedo, F.; Bucio, E. Gamma-irradiation applied in the synthesis of metallic and organic nanoparticles: A short review. Radiat. Phys. Chem. 2020, 169, 107962. [Google Scholar] [CrossRef]
- Čubová, K.; Čuba, V. Synthesis of inorganic nanoparticles by ionizing radiation—A review. Radiat. Phys. Chem. 2020, 169, 108774. [Google Scholar] [CrossRef]
- Kostyukhin, E.M.; Nissenbaum, V.D.; Abkhalimov, E.V.; Kustov, A.L.; Ershov, B.G.; Kustov, L.M. Microwave-assisted synthesis of water-dispersible humate-coated magnetite nanoparticles: Relation of coating process parameters to the properties of nanoparticles. Nanomaterials 2020, 10, 1558. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Yonezawa, T.; Kunitake, T. Practical preparation of anionic mercapto ligand-stabilized gold nanoparticles and their immobilization. Colloids Surf. A Physicochem. Eng. Asp. 1999, 149, 193–199. [Google Scholar] [CrossRef]
- Seitz, O.; Chehimi, M.M.; Cabet-Deliry, E.; Truong, S.; Felidj, N.; Perruchot, C.; Greaves, S.J.; Watts, J.F. Preparation and characterisation of gold nanoparticle assemblies on silanised glass plates. Colloids Surf. A Physicochem. Eng. Asp. 2003, 218, 225–239. [Google Scholar] [CrossRef]
- Yang, Y.; Shi, J.; Chen, H.; Dai, S.; Liu, Y. Enhanced off-resonance optical nonlinearities of Au@CdS core-shell nanoparticles embedded in BaTiO 3 thin films. Chem. Phys. Lett. 2003, 370, 1–6. [Google Scholar] [CrossRef]
- Huang, H.; Yang, X. Chitosan mediated assembly of gold nanoparticles multilayer. Colloids Surf. A Physicochem. Eng. Asp. 2003, 226, 77–86. [Google Scholar] [CrossRef]
- Akiyama, T.; Inoue, K.; Kuwahara, Y.; Terasaki, N.; Niidome, Y.; Yamada, S. Particle-size effects on the photocurrent efficiency of nanostructured assemblies consisting of gold nanoparticles and a ruthenium complex-viologen linked thiol. J. Electroanal. Chem. 2003, 550–551, 303–307. [Google Scholar] [CrossRef]
- Mayya, K.S.; Patil, V.; Sastry, M. Lamellar Multilayer Gold Cluster Films Deposited by the Langmuir–Blodgett Technique. Langmuir 1997, 13, 2575–2577. [Google Scholar] [CrossRef]
- Ojea-Jiménez, I.; Bastús, N.G.; Puntes, V. Influence of the sequence of the reagents addition in the citrate-mediated synthesis of gold nanoparticles. J. Phys. Chem. C 2011, 115, 15752–15757. [Google Scholar] [CrossRef]
- Zhao, L.; Jiang, D.; Cai, Y.; Ji, X.; Xie, R.; Yang, W. Tuning the size of gold nanoparticles in the citrate reduction by chloride ions. Nanoscale 2012, 4, 5071–5076. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Sun, C.; Wang, L.; Ji, X.; Bai, Y.; Li, T.; Li, J. Electrochemical detection of DNA immobilized on gold colloid particles modified self-assembled monolayer electrode with silver nanoparticle label. J. Pharm. Biomed. Anal. 2003, 33, 1117–1125. [Google Scholar] [CrossRef]
- Brust, M.; Walker, M.; Bethell, D.; Schiffrin, D.J.; Whyman, R. Synthesis of Thiol-derivatised Gold Nanoparticles in a two-phase Liquid–Liquid system. J. Chem. Soc. Chem. Commun. 1994, 7, 801–802. [Google Scholar] [CrossRef]
- Brust, M.; Gordillo, G.J. Electrocatalytic hydrogen redox chemistry on gold nanoparticles. J. Am. Chem. Soc. 2012, 134, 3318–3321. [Google Scholar] [CrossRef]
- Kuroda, Y.; Fukumoto, K.; Kuroda, K. Uniform and high dispersion of gold nanoparticles on imogolite nanotubes and assembly into morphologically controlled materials. Appl. Clay Sci. 2012, 55, 10–17. [Google Scholar] [CrossRef]
- Ghosh, S.K. Spectroscopic evaluation of 4-(dimethylamino)pyridine versus citrate as stabilizing ligand for gold nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2010, 371, 98–103. [Google Scholar] [CrossRef]
- Ke, W.H.; Hsia, C.F.; Chen, Y.J.; Huang, M.H. Synthesis of Ultrasmall Cu2O Nanocubes and Octahedra with Tunable Sizes for Facet-Dependent Optical Property Examination. Small 2016, 12, 3530–3534. [Google Scholar] [CrossRef] [PubMed]
- Duncan, B.; Kim, C.; Rotello, V.M. Gold nanoparticle platforms as drug and biomacromolecule delivery systems. J. Control. Release 2010, 148, 122–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uson, L.; Sebastian, V.; Arruebo, M.; Santamaria, J. Continuous microfluidic synthesis and functionalization of gold nanorods. Chem. Eng. J. 2016, 285, 286–292. [Google Scholar] [CrossRef] [Green Version]
- Jana, N.R.; Gearheart, L.; Murphy, C.J. Seeding growth for size control of 5–40 nm diameter gold nanoparticles. Langmuir 2001, 17, 6782–6786. [Google Scholar] [CrossRef]
- Sahoo, G.P.; Kumar Bhui, D.; Das, D.; Misra, A. Synthesis of anisotropic gold nanoparticles and their catalytic activities of breaking azo bond in sudan-1. J. Mol. Liq. 2014, 198, 215–222. [Google Scholar] [CrossRef]
- Khan, Z.; Singh, T.; Hussain, J.I.; Hashmi, A.A. Au(III)-CTAB reduction by ascorbic acid: Preparation and characterization of gold nanoparticles. Colloids Surf. B Biointerfaces 2013, 104, 11–17. [Google Scholar] [CrossRef]
- Firdhouse, M.J.; Lalitha, P. Biosynthesis of Cubic Gold nanoparticles. Int. J. Sci. Eng. Res. 2014, 5, 1832–1837. [Google Scholar]
- Boca, S.C.; Potara, M.; Toderas, F.; Stephan, O.; Baldeck, P.L.; Astilean, S. Uptake and biological effects of chitosan-capped gold nanoparticles on Chinese Hamster Ovary cells. Mater. Sci. Eng. C 2011, 31, 184–189. [Google Scholar] [CrossRef]
- Armendariz, V.; Herrera, I.; Peralta-Videa, J.R.; Jose-Yacaman, M.; Troiani, H.; Santiago, P.; Gardea-Torresdey, J.L. Size controlled gold nanoparticle formation by Avena sativa biomass: Use of plants in nanobiotechnology. J. Nanoparticle Res. 2004, 6, 377–382. [Google Scholar] [CrossRef]
- Hamelian, M.; Varmira, K.; Veisi, H. Green synthesis and characterizations of gold nanoparticles using Thyme and survey cytotoxic effect, antibacterial and antioxidant potential. J. Photochem. Photobiol. B Biol. 2018, 184, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Nazirov, A.; Pestov, A.; Privar, Y.; Ustinov, A.; Modin, E.; Bratskaya, S. One-pot green synthesis of luminescent gold nanoparticles using imidazole derivative of chitosan. Carbohydr. Polym. 2016, 151, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Mohd Taib, S.H.; Shameli, K.; Moozarm Nia, P.; Etesami, M.; Miyake, M.; Rasit Ali, R.; Abouzari-Lotf, E.; Izadiyan, Z. Electrooxidation of nitrite based on green synthesis of gold nanoparticles using Hibiscus sabdariffa leaves. J. Taiwan Inst. Chem. Eng. 2019, 95, 616–626. [Google Scholar] [CrossRef]
- Sonia; Komal; Kukreti, S.; Kaushik, M. Exploring the DNA damaging potential of chitosan and citrate-reduced gold nanoparticles: Physicochemical approach. Int. J. Biol. Macromol. 2018, 115, 801–810. [Google Scholar] [CrossRef]
- Suvith, V.S.; Philip, D. Catalytic degradation of methylene blue using biosynthesized gold and silver nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 118, 526–532. [Google Scholar] [CrossRef]
- Meena Kumari, M.; Philip, D. Facile one-pot synthesis of gold and silver nanocatalysts using edible coconut oil. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 111, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Sheny, D.S.; Mathew, J.; Philip, D. Synthesis characterization and catalytic action of hexagonal gold nanoparticles using essential oils extracted from Anacardium occidentale. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 97, 306–310. [Google Scholar] [CrossRef]
- Philip, D. Honey mediated green synthesis of gold nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009, 73, 650–653. [Google Scholar] [CrossRef]
- Philip, D. Biosynthesis of Au, Ag and Au-Ag nanoparticles using edible mushroom extract. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009, 73, 374–381. [Google Scholar] [CrossRef]
- Bonilla-Nepomuceno, G.; Ríos-Corripio, M.A.; Gómez-Merino, C.F.; Méndez-Rojas, M.Á.; Arcila-Lozano, L.S.; Hernández-Cázares, A.S.; Rojas-Lòpez, M. Analysis by response surface methodology of gold nanoparticles obtained by green chemical reduction using aqueous coffee pulp extract (Coffea arabica). Can. J. Chem. 2021, 99, 519–530. [Google Scholar] [CrossRef]
- Akintelu, S.A.; Yao, B.; Folorunso, A.S. Green Synthesis, Characterization, and Antibacterial Investigation of Synthesized Gold Nanoparticles (AuNPs) from Garcinia kola Pulp Extract. Plasmonics 2021, 16, 157–165. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, L.; Cheng, R.; Mao, L.; Arnold, R.D.; Howerth, E.W.; Chen, Z.G.; Platt, S. Magnetic nanoparticle-based hyperthermia for head & neck cancer in mouse models. Theranostics 2012, 2, 113–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iqbal, M.; Usanase, G.; Oulmi, K.; Aberkane, F.; Bendaikha, T.; Fessi, H.; Zine, N.; Agusti, G.; Errachid, E.S.; Elaissari, A. Preparation of gold nanoparticles and determination of their particles size via different methods. Mater. Res. Bull. 2016, 79, 97–104. [Google Scholar] [CrossRef]
- Kesik, M.; Kanik, F.E.; Hizalan, G.; Kozanoglu, D.; Esenturk, E.N.; Timur, S.; Toppare, L. A functional immobilization matrix based on a conducting polymer and functionalized gold nanoparticles: Synthesis and its application as an amperometric glucose biosensor. Polymer 2013, 54, 4463–4471. [Google Scholar] [CrossRef]
- Aryal, S.; Remant, B.K.C.; Dharmaraj, N.; Bhattarai, N.; Kim, C.H.; Kim, H.Y. Spectroscopic identification of SAu interaction in cysteine capped gold nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2006, 63, 160–163. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, M.; Han, L.; Zhao, Y.; Fan, A. Enhancement effect of p-iodophenol on gold nanoparticle-catalyzed chemiluminescence and its applications in detection of thiols and guanidine. Talanta 2018, 182, 523–528. [Google Scholar] [CrossRef]
- Shajkumar, A.; Nandan, B.; Sanwaria, S.; Albrecht, V.; Libera, M.; Lee, M.H.; Auffermann, G.; Stamm, M.; Horechyy, A. Silica-supported Au@hollow-SiO2 particles with outstanding catalytic activity prepared via block copolymer template approach. J. Colloid Interface Sci. 2017, 491, 246–254. [Google Scholar] [CrossRef]
- Isaacs, S.R.; Cutler, E.C.; Park, J.S.; Lee, T.R.; Shon, Y.S. Synthesis of tetraoctylammonium-protected gold nanoparticles with improved stability. Langmuir 2005, 21, 5689–5692. [Google Scholar] [CrossRef]
- Briñas, R.P.; Maetani, M.; Barchi, J.J. A survey of place-exchange reaction for the preparation of water-soluble gold nanoparticles. J. Colloid Interface Sci. 2013, 392, 415–421. [Google Scholar] [CrossRef] [Green Version]
- Sierra-Fernandez, A.; Gomez-Villalba, L.S.; Rabanal, M.E.; Fort, R. New nanomaterials for applications in conservation and restoration of stony materials: A review. Mater. Constr. 2017, 67, e107. [Google Scholar] [CrossRef]
- Vörös, N.M.; Patakfalvi, R.; Dékány, I. Alkylthiol-functionalized gold nanoparticles for sensing organic vapours: The connection between the adsorption isotherm and the sensor resistance. Colloids Surf. A Physicochem. Eng. Asp. 2008, 329, 205–210. [Google Scholar] [CrossRef]
- Zhang, L.; Yuan, R.; Chai, Y.; Li, X. Investigation of the electrochemical and electrocatalytic behavior of positively charged gold nanoparticle and l-cysteine film on an Au electrode. Anal. Chim. Acta 2007, 596, 99–105. [Google Scholar] [CrossRef]
- Liu, X.; Atwater, M.; Wang, J.; Huo, Q. Extinction coefficient of gold nanoparticles with different sizes and different capping ligands. Colloids Surf. B Biointerfaces 2007, 58, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.W.; Hong, J.; Park, J.H.; Mun, S.H.; Kim, J.H.; Cho, J.; Char, K.; Kang, Y.S. Nanocomposite membranes containing positively polarized gold nanoparticles for facilitated olefin transport. J. Memb. Sci. 2008, 321, 90–93. [Google Scholar] [CrossRef]
- Kyrychenko, A.; Karpushina, G.V.; Bogatyrenko, S.I.; Kryshtal, A.P.; Doroshenko, A.O. Preparation, structure, and a coarse-grained molecular dynamics model for dodecanethiol-stabilized gold nanoparticles. Comput. Theor. Chem. 2011, 977, 34–39. [Google Scholar] [CrossRef]
- Razzaq, H.; Qureshi, R.; Cabo-Fernandez, L.; Schiffrin, D.J. Synthesis of Au clusters-redox centre hybrids by diazonium chemistry employing double layer charged gold nanoparticles. J. Electroanal. Chem. 2018, 819, 9–15. [Google Scholar] [CrossRef]
- Spadavecchia, J.; Casale, S.; Boujday, S.; Pradier, C.M. Bioconjugated gold nanorods to enhance the sensitivity of FT-SPR-based biosensors. Colloids Surf. B Biointerfaces 2012, 100, 1–8. [Google Scholar] [CrossRef]
- He, P.; Zhu, X. Phospholipid-assisted synthesis of size-controlled gold nanoparticles. Mater. Res. Bull. 2007, 42, 1310–1315. [Google Scholar] [CrossRef]
- Cai, Y.; Qiu, Z.; Lin, X.; Zeng, W.; Cao, Y.; Liu, W.; Liu, Y. Self-assembled nanomaterials based on aggregation-induced emission of AuNCs: Fluorescence and colorimetric dual-mode biosensing of organophosphorus pesticides. Sens. Actuators B Chem. 2020, 321, 128481. [Google Scholar] [CrossRef]
- Ielo, I.; Giacobello, F.; Sfameni, S.; Rando, G.; Galletta, M.; Trovato, V.; Rosace, G.; Plutino, M.R. Nanostructured Surface Finishing and Coatings: Functional Properties and Applications. Materials 2021, 14, 2733. [Google Scholar] [CrossRef]
- De Luca, G.; Bonaccorsi, P.; Trovato, V.; Mancuso, A.; Papalia, T.; Pistone, A.; Casaletto, M.P.; Mezzi, A.; Brunetti, B.; Minuti, L.; et al. Tripodal tris-disulfides as capping agents for a controlled mixed functionalization of gold nanoparticles. New J. Chem. 2018, 42, 16436–16440. [Google Scholar] [CrossRef]
- Xiong, D.; Chen, M.; Li, H. Synthesis of para-sulfonatocalix[4]arene–modified silver nanoparticles as colorimetric histidine probes. Chem. Commun. 2008, 880–882. [Google Scholar] [CrossRef]
- Singh, R.; Thakur, P.; Thakur, A.; Kumar, H.; Chawla, P.; Rohit, J.V.; Kaushik, R.; Kumar, N. Colorimetric sensing approaches of surface-modified gold and silver nanoparticles for detection of residual pesticides: A review. Int. J. Environ. Anal. Chem. 2020, 1–17. [Google Scholar] [CrossRef]
- Krukowski, P.; Kowalczyk, D.A.; Piskorski, M.; Dabrowski, P.; Rogala, M.; Caban, P.; Ciepielewski, P.; Jung, J.; Baranowski, J.M.; Ulanski, J.; et al. Work Function Tunability of Graphene with Thermally Evaporated Rhenium Heptoxide for Transparent Electrode Applications. Adv. Eng. Mater. 2020, 22, 1–6. [Google Scholar] [CrossRef]
- Sabela, M.; Balme, S.; Bechelany, M.; Janot, J.M.; Bisetty, K. A Review of Gold and Silver Nanoparticle-Based Colorimetric Sensing Assays. Adv. Eng. Mater. 2017, 19, 1–24. [Google Scholar] [CrossRef]
- Sinicropi, M.S.; Iacopetta, D.; Rosano, C.; Randino, R.; Caruso, A.; Saturnino, C.; Muià, N.; Ceramella, J.; Puoci, F.; Rodriquez, M.; et al. N-thioalkylcarbazoles derivatives as new anti-proliferative agents: Synthesis, characterisation and molecular mechanism evaluation. J. Enzym. Inhib. Med. Chem. 2018, 33, 434–444. [Google Scholar] [CrossRef] [Green Version]
- Walter, E.C.; Murray, B.J.; Favier, F.; Kaltenpoth, G.; Grunze, M.; Penner, R.M. Noble and coinage metal nanowires by electrochemical step edge decoration. J. Phys. Chem. B 2002, 106, 11407–11411. [Google Scholar] [CrossRef]
- Braun, G.B.; Pallaoro, A.; Wu, G.; Missirlis, D.; Zasadzinski, J.A.; Tirrell, M.; Reich, N.O. Laser-activated gene silencing via gold nanoshell-siRNA conjugates. ACS Nano 2009, 3, 2007–2015. [Google Scholar] [CrossRef]
- Gibson, J.D.; Khanal, B.P.; Zubarev, E.R. Paclitaxel-functionalized gold nanoparticles. J. Am. Chem. Soc. 2007, 129, 11653–11661. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, Y.; Jiang, J.; Li, J. Electroactive gold nanoparticles protected by 4-ferrocene thiophenol monolayer. J. Colloid Interface Sci. 2003, 264, 109–113. [Google Scholar] [CrossRef]
- Liu, F.; Liu, X.; Astruc, D.; Gu, H. Dendronized triazolyl-containing ferrocenyl polymers as stabilizers of gold nanoparticles for recyclable two-phase reduction of 4-nitrophenol. J. Colloid Interface Sci. 2019, 533, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Wong, S.S. Functionalization of Carbon Nanotubes with a Metal-Containing Molecular Complex. Nano Lett. 2002, 2, 49–53. [Google Scholar] [CrossRef]
- Mandal, T.K.; Fleming, M.S.; Walt, D.R. Preparation of Polymer Coated Gold Nanoparticles by Surface-Confined Living Radical Polymerization at Ambient Temperature. Nano Lett. 2002, 2, 3–7. [Google Scholar] [CrossRef]
- Kolny, J.; Kornowski, A.; Weller, H. Self-Organization of Cadmium Sulfide and Gold Nanoparticles by Electrostatic Interaction. Nano Lett. 2002, 2, 361–364. [Google Scholar] [CrossRef]
- Xiao, W.; Xiong, J.; Zhang, S.; Xiong, Y.; Zhang, H.; Gao, H. Influence of ligands property and particle size of gold nanoparticles on the protein adsorption and corresponding targeting ability. Int. J. Pharm. 2018, 538, 105–111. [Google Scholar] [CrossRef]
- Zhang, G.; Yang, Z.; Lu, W.; Zhang, R.; Huang, Q.; Tian, M.; Li, L.; Liang, D.; Li, C. Influence of anchoring ligands and particle size on the colloidal stability and in vivo biodistribution of polyethylene glycol-coated gold nanoparticles in tumor-xenografted mice. Biomaterials 2009, 30, 1928–1936. [Google Scholar] [CrossRef] [Green Version]
- Rohit, J.V.; Basu, H.; Singhal, R.K.; Kailasa, S.K. Development of p-nitroaniline dithiocarbamate capped gold nanoparticles-based microvolume UV–vis spectrometric method for facile and selective detection of quinalphos insecticide in environmental samples. Sens. Actuators B Chem. 2016, 237, 826–835. [Google Scholar] [CrossRef]
- Johnson, B.J.; Russ Algar, W.; Malanoski, A.P.; Ancona, M.G.; Medintz, I.L. Understanding enzymatic acceleration at nanoparticle interfaces: Approaches and challenges. Nano Today 2014, 9, 102–131. [Google Scholar] [CrossRef]
- Dhanyalayam, D.; Scrivano, L.; Parisi, O.I.; Sinicropi, M.S.; Fazio, A.; Saturnino, C.; Plutino, M.R.; Di Cristo, F.; Puoci, F.; Cappello, A.R.; et al. Biopolymeric self-assembled nanoparticles for enhanced antibacterial activity of Ag-based compounds. Int. J. Pharm. 2017, 517, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Häkkinen, H. The gold-sulfur interface at the nanoscale. Nat. Chem. 2012, 4, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Saturnino, C.; Sinicropi, M.S.; Iacopettab, D.; Ceramella, J.; Caruso, A.; Muià, N.; Longo, P.; Rosace, G.; Galletta, M.; Ielo, I.; et al. N-Thiocarbazole-based gold nanoparticles: Synthesis, characterization and anti-proliferative activity evaluation. IOP Conf. Ser. Mater. Sci. Eng. 2018, 459, 12023. [Google Scholar] [CrossRef]
- Saturnino, C.; Caruso, A.; Longo, P.; Capasso, A.; Pingitore, A.; Caroleo, M.C.; Cione, E.; Perri, M.; Nicolo, F.; Nardo, V.M.; et al. Crystallographic Study and Biological Evaluation of 1,4-dimethyl-N-alkylcarbazoles. Curr. Top. Med. Chem. 2015, 15, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Höldrich, M.; Sievers-Engler, A.; Lämmerhofer, M. Gold nanoparticle-conjugated pepsin for efficient solution-like heterogeneous biocatalysis in analytical sample preparation protocols. Anal. Bioanal. Chem. 2016, 408, 5415–5427. [Google Scholar] [CrossRef] [PubMed]
- Jeong, M.L.; Hyun, K.P.; Jung, Y.; Jin, K.K.; Sun, O.J.; Bong, H.C. Direct immobilization of protein G variants with various numbers of cysteine residues on a gold surface. Anal. Chem. 2007, 79, 2680–2687. [Google Scholar] [CrossRef]
- Faccenda, A.; Bonham, C.A.; Vacratsis, P.O.; Zhang, X.; Mutus, B. Gold nanoparticle enrichment method for identifying S-nitrosylation and S-glutathionylation sites in proteins. J. Am. Chem. Soc. 2010, 132, 11392–11394. [Google Scholar] [CrossRef]
- Romeo, R.; Scolaro, L.M.; Plutino, M.R.; Albinati, A. Structural properties of the metallointercalator cationic complex (2,2′:6′,2″-terpyridine)methylplatinum(II) ion. J. Organomet. Chem. 2000, 593–594, 403–408. [Google Scholar] [CrossRef]
- Ielo, I.; Iacopetta, D.; Saturnino, C.; Longo, P.; Galletta, M.; Drommi, D.; Rosace, G.; Sinicropi, M.S.; Plutino, M.R. Gold Derivatives Development as Prospective Anticancer Drugs for Breast Cancer Treatment. Appl. Sci. 2021, 11, 2089. [Google Scholar] [CrossRef]
- Masereel, B.; Dinguizli, M.; Bouzin, C.; Moniotte, N.; Feron, O.; Gallez, B.; Vander Borght, T.; Michiels, C.; Lucas, S. Antibody immobilization on gold nanoparticles coated layer-by-layer with polyelectrolytes. J. Nanoparticle Res. 2011, 13, 1573–1580. [Google Scholar] [CrossRef]
- Liu, S.; Lämmerhofer, M. Functionalized gold nanoparticles for sample preparation: A review. Electrophoresis 2019, 40, 2438–2461. [Google Scholar] [CrossRef] [PubMed]
- Castellano, A.; Colleoni, C.; Iacono, G.; Mezzi, A.; Plutino, M.R.; Malucelli, G.; Rosace, G. Synthesis and characterization of a phosphorous/nitrogen based sol-gel coating as a novel halogen- and formaldehyde-free flame retardant finishing for cotton fabric. Polym. Degrad. Stab. 2019, 162, 148–159. [Google Scholar] [CrossRef]
- Trovato, V.; Colleoni, C.; Castellano, A.; Plutino, M.R. The key role of 3-glycidoxypropyltrimethoxysilane sol–gel precursor in the development of wearable sensors for health monitoring. J. Sol-Gel Sci. Technol. 2018, 87, 27–40. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Correa-Duarte, M.A.; Liz-Marzán, L.M. Sol-gel processing of silica-coated gold nanoparticles. Langmuir 2001, 17, 6375–6379. [Google Scholar] [CrossRef]
- Plutino, M.R.; Colleoni, C.; Donelli, I.; Freddi, G.; Guido, E.; Maschi, O.; Mezzi, A.; Rosace, G. Sol-gel 3-glycidoxypropyltriethoxysilane finishing on different fabrics: The role of precursor concentration and catalyst on the textile performances and cytotoxic activity. J. Colloid Interface Sci. 2017, 506, 504–517. [Google Scholar] [CrossRef] [PubMed]
- Saturnino, C.; Popolo, A.; Ramunno, A.; Adesso, S.; Pecoraro, M.; Plutino, M.R.; Rizzato, S.; Albinati, A.; Marzocco, S.; Sala, M.; et al. Anti-inflammatory, antioxidant and crystallographic studies of N-Palmitoyl-ethanol amine (PEA) derivatives. Molecules 2017, 22, 616. [Google Scholar] [CrossRef] [Green Version]
- Rosace, G.; Guido, E.; Colleoni, C.; Brucale, M.; Piperopoulos, E.; Milone, C.; Plutino, M.R. Halochromic resorufin-GPTMS hybrid sol-gel: Chemical-physical properties and use as pH sensor fabric coating. Sens. Actuators B Chem. 2017, 241, 85–95. [Google Scholar] [CrossRef]
- Hinterwirth, H.; Lindner, W.; Lämmerhofer, M. Bioconjugation of trypsin onto gold nanoparticles: Effect of surface chemistry on bioactivity. Anal. Chim. Acta 2012, 733, 90–97. [Google Scholar] [CrossRef]
- Liu, S.; Höldrich, M.; Sievers-Engler, A.; Horak, J.; Lämmerhofer, M. Papain-functionalized gold nanoparticles as heterogeneous biocatalyst for bioanalysis and biopharmaceuticals analysis. Anal. Chim. Acta 2017, 963, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Li, Z.; Yao, M.; Gao, C. Influence of Albumin Configuration by the Chiral Polymer-Grafted Gold Nanoparticles. Langmuir 2016, 32, 5608–5616. [Google Scholar] [CrossRef] [PubMed]
- Haller, E.; Lindner, W.; Lämmerhofer, M. Gold nanoparticle-antibody conjugates for specific extraction and subsequent analysis by liquid chromatography-tandem mass spectrometry of malondialdehyde-modified low density lipoprotein as biomarker for cardiovascular risk. Anal. Chim. Acta 2015, 857, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Yaseen, M.; Humayun, M.; Khan, A.; Usman, M.; Ullah, H.; Tahir, A.A.; Ullah, H. Preparation, Functionalization, Modification, and Applications of Nanostructured Gold: A Critical Review. Energies 2021, 14, 1278. [Google Scholar] [CrossRef]
- Koo, W.-T.; Kim, Y.; Savagatrup, S.; Yoon, B.; Jeon, I.; Choi, S.-J.; Kim, I.-D.; Swager, T.M. Porous Ion Exchange Polymer Matrix for Ultrasmall Au Nanoparticle-Decorated Carbon Nanotube Chemiresistors. Chem. Mater. 2019, 31, 5413–5420. [Google Scholar] [CrossRef]
- Deshmukh, S.P.; Dhodamani, A.G.; Patil, S.M.; Mullani, S.B.; More, K.V.; Delekar, S.D. Interfacially Interactive Ternary Silver-Supported Polyaniline/Multiwalled Carbon Nanotube Nanocomposites for Catalytic and Antibacterial Activity. ACS Omega 2020, 5, 219–227. [Google Scholar] [CrossRef]
- Presnova, G.V.; Rubtsova, M.Y.; Presnov, D.E.; Grigorenko, V.G.; Yaminsky, I.V.; Egorov, A.M. Streptavidin conjugates with gold nanoparticles for visualization of single DNA interactions on the silicon surface. Biochem. Suppl. Ser. B Biomed. Chem. 2014, 8, 164–167. [Google Scholar] [CrossRef]
- Urban, D.A.; Milosevic, A.M.; Bossert, D.; Crippa, F.; Moore, T.L.; Geers, C.; Balog, S.; Rothen-Rutishauser, B.; Petri-Fink, A. Taylor Dispersion of Inorganic Nanoparticles and Comparison to Dynamic Light Scattering and Transmission Electron Microscopy. Colloids Interface Sci. Commun. 2018, 22, 29–33. [Google Scholar] [CrossRef] [Green Version]
- Kondekar, U.R.; Walekar, L.S.; Gore, A.H.; Anbhule, P.V.; Han, S.H.; Patil, S.R.; Kolekar, G.B. Ultrasensitive, highly specific, colorimetric recognition of sulfide ions [S2-] in aqueous media: Applications to environmental analysis. Anal. Methods 2015, 7, 2547–2553. [Google Scholar] [CrossRef]
- Haiss, W.; Thanh, N.T.K.; Aveyard, J.; Fernig, D.G. Determination of size and concentration of gold nanoparticles from UV-Vis spectra. Anal. Chem. 2007, 79, 4215–4221. [Google Scholar] [CrossRef]
- Hinterwirth, H.; Wiedmer, S.K.; Moilanen, M.; Lehner, A.; Allmaier, G.; Waitz, T.; Lindner, W.; Lämmerhofer, M. Comparative method evaluation for size and size-distribution analysis of gold nanoparticles. J. Sep. Sci. 2013, 36, 2952–2961. [Google Scholar] [CrossRef]
- Sýkora, D.; Kašička, V.; Mikšík, I.; Řezanka, P.; Záruba, K.; Matějka, P.; Král, V. Application of gold nanoparticles in separation sciences. J. Sep. Sci. 2010, 33, 372–387. [Google Scholar] [CrossRef]
- Piella, J.; Bastús, N.G.; Puntes, V. Size-dependent protein-nanoparticle interactions in citrate-stabilized gold nanoparticles: The emergence of the protein corona. Bioconjug. Chem. 2017, 28, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Driskell, J.D.; Jones, C.A.; Tompkins, S.M.; Tripp, R.A. One-step assay for detecting influenza virus using dynamic light scattering and gold nanoparticles. Analyst 2011, 136, 3083–3090. [Google Scholar] [CrossRef] [PubMed]
- Goldberg-Oppenheimer, P.; Regev, O. Exploring a nanotube dispersion mechanism with gold-labeled proteins via cryo-TEM imaging. Small 2007, 3, 1894–1899. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, E.; Tyner, K.M.; Poling, C.M.; Blacklock, J.L. Determination of nanoparticle surface coatings and nanoparticle purity using microscale thermogravimetric analysis. Anal. Chem. 2014, 86, 1478–1484. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.S.K.; Saxby, J.D.; Chatfield, S.P. Thermogravimetric analysis of carbon nanotubes and nanoparticles. J. Phys. Chem. 1993, 97, 6941–6942. [Google Scholar] [CrossRef]
- Sublemontier, O.; Nicolas, C.; Aureau, D.; Patanen, M.; Kintz, H.; Liu, X.; Gaveau, M.A.; Le Garrec, J.L.; Robert, E.; Barreda, F.A.; et al. X-ray photoelectron spectroscopy of isolated nanoparticles. J. Phys. Chem. Lett. 2014, 5, 3399–3403. [Google Scholar] [CrossRef]
- Mahoney, J.; Monroe, C.; Swartley, A.M.; Ucak-Astarlioglu, M.G.; Zoto, C.A. Surface analysis using X-ray photoelectron spectroscopy. Spectrosc. Lett. 2020, 53, 726–736. [Google Scholar] [CrossRef]
- Techane, S.D.; Gamble, L.J.; Castner, D.G. Multitechnique characterization of self-assembled carboxylic acid-terminated alkanethiol monolayers on nanoparticle and flat gold surfaces. J. Phys. Chem. C 2011, 115, 9432–9441. [Google Scholar] [CrossRef] [Green Version]
- Mahato, K.; Nagpal, S.; Shah, M.A.; Srivastava, A.; Maurya, P.K.; Roy, S.; Jaiswal, A.; Singh, R.; Chandra, P. Gold nanoparticle surface engineering strategies and their applications in biomedicine and diagnostics. 3 Biotech 2019, 9, 57. [Google Scholar] [CrossRef]
- Tran, T.H.; Park, S.; Lee, H.; Park, S.; Kim, B.; Kim, O.H.; Oh, B.C.; Lee, D.; Lee, H. Ultrasmall gold nanoparticles for highly specific isolation/enrichment of N-linked glycosylated peptides. Analyst 2012, 137, 991–998. [Google Scholar] [CrossRef]
- Xia, X.; Yang, M.; Wang, Y.; Zheng, Y.; Li, Q.; Chen, J.; Xia, Y. Quantifying the coverage density of poly(ethylene glycol) chains on the surface of gold nanostructures. ACS Nano 2012, 6, 512–522. [Google Scholar] [CrossRef]
- Hinterwirth, H.; Kappel, S.; Waitz, T.; Prohaska, T.; Lindner, W.; Lämmerhofer, M. Quantifying thiol ligand density of self-assembled monolayers on gold nanoparticles by inductively coupled plasma-mass spectrometry. ACS Nano 2013, 7, 1129–1136. [Google Scholar] [CrossRef]
- Miller, J.T.; Kropf, A.J.; Zha, Y.; Regalbuto, J.R.; Delannoy, L.; Louis, C.; Bus, E.; van Bokhoven, J.A. The effect of gold particle size on Au–Au bond length and reactivity toward oxygen in supported catalysts. J. Catal. 2006, 240, 222–234. [Google Scholar] [CrossRef]
- Okazaki, K.; Ichikawa, S.; Maeda, Y.; Haruta, M.; Kohyama, M. Electronic structures of Au supported on TiO2. Appl. Catal. A Gen. 2005, 291, 45–54. [Google Scholar] [CrossRef]
- Delcour, A.H. Outer membrane permeability and antibiotic resistance. Biochim. Biophys. Acta Proteins Proteom. 2009, 1794, 808–816. [Google Scholar] [CrossRef] [Green Version]
- Lu, Z.; Zhang, J.; Yu, Z.; Liu, X.; Zhang, Z.; Wang, W.; Wang, X.; Wang, Y.; Wang, D. Vancomycin-hybrid bimetallic Au/Ag composite nanoparticles: Preparation of the nanoparticles and characterization of the antibacterial activity. New J. Chem. 2017, 41, 5276–5279. [Google Scholar] [CrossRef]
- Khandelwal, P.; Singh, D.K.; Sadhu, S.; Poddar, P. Study of the nucleation and growth of antibiotic labeled Au NPs and blue luminescent Au8 quantum clusters for Hg2+ ion sensing, cellular imaging and antibacterial applications. Nanoscale 2015, 7, 19985–20002. [Google Scholar] [CrossRef]
- Fiori-Duarte, A.T.; de Paiva, R.E.F.; Manzano, C.M.; Lustri, W.R.; Corbi, P.P. Silver(I) and gold(I) complexes with sulfasalazine: Spectroscopic characterization, theoretical studies and antiproliferative activities over Gram-positive and Gram-negative bacterial strains. J. Mol. Struct. 2020, 1214, 128158. [Google Scholar] [CrossRef]
- Wang, L.; Yamauchi, Y. Strategic synthesis of trimetallic au@pd@pt core-shell nanoparticles from poly(vinylpyrrolidone)-based aqueous solution toward highly active electrocatalysts. Chem. Mater. 2011, 23, 2457–2465. [Google Scholar] [CrossRef]
- Connor, E.E.; Mwamuka, J.; Gole, A.; Murphy, C.J.; Wyatt, M.D. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small 2005, 1, 325–327. [Google Scholar] [CrossRef]
- Dykman, L.A.; Khlebtsov, N.G. Gold Nanoparticles in Biology and Medicine: Recent Advances and Prospects. Acta Nat. 2011, 3, 34–55. [Google Scholar] [CrossRef] [Green Version]
- Zharov, V.P.; Mercer, K.E.; Galitovskaya, E.N.; Smeltzer, M.S. Photothermal nanotherapeutics and nanodiagnostics for selective killing of bacteria targeted with gold nanoparticles. Biophys. J. 2006, 90, 619–627. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.K.; Ghosh, P.; Rotello, V.M. Multimodal drug delivery using gold nanoparticles. Nanoscale 2009, 1, 61–67. [Google Scholar] [CrossRef] [Green Version]
- Gu, X.; Xu, Z.; Gu, L.; Xu, H.; Han, F.; Chen, B.; Pan, X. Preparation and antibacterial properties of gold nanoparticles: A review. Environ. Chem. Lett. 2020, 19, 167–187. [Google Scholar] [CrossRef]
- Vanaraj, S.; Jabastin, J.; Sathiskumar, S.; Preethi, K. Production and Characterization of Bio-AuNPs to Induce Synergistic Effect Against Multidrug Resistant Bacterial Biofilm. J. Clust. Sci. 2017, 28, 227–244. [Google Scholar] [CrossRef]
- Lanh, L.T.; Hoa, T.T.; Cuong, N.D.; Khieu, D.Q.; Quang, D.T.; Van Duy, N.; Hoa, N.D.; Van Hieu, N. Shape and size controlled synthesis of Au nanorods: H2S gas-sensing characterizations and antibacterial application. J. Alloys Compd. 2015, 635, 265–271. [Google Scholar] [CrossRef]
- Prashant, K.J.; El Ivan, H.; El-Sayed, M.A. Au nanoparticles target cancer. Nano Today 2007, 2, 18–29. [Google Scholar] [CrossRef]
- Farafonov, V.G.; Voshchinnikov, N.V. Light scattering by a multilayered spheroidal particle. Appl. Opt. 2012, 51, 1586–1597. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Juste, J.; Pastoriza-Santos, I.; Liz-Marzán, L.M.; Mulvaney, P. Gold nanorods: Synthesis, characterization and applications. Coord. Chem. Rev. 2005, 249, 1870–1901. [Google Scholar] [CrossRef]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The optical properties of metal nanoparticles: The influence of size, shape, and dielectric environment. J. Phys. Chem. B 2003, 107, 668–677. [Google Scholar] [CrossRef]
- Rashidi-Huyeh, M.; Palpant, B. Counterintuitive thermo-optical response of metal-dielectric nanocomposite materials as a result of local electromagnetic field enhancement. Phys. Rev. B Condens. Matter Mater. Phys. 2006, 74, 1–8. [Google Scholar] [CrossRef]
- Hutter, E.; Maysinger, D. Gold nanoparticles and quantum dots for bioimaging. Microsc. Res. Tech. 2011, 74, 592–604. [Google Scholar] [CrossRef]
- Rai, P.; Mallidi, S.; Zheng, X.; Rahmanzadeh, R.; Mir, Y.; Elrington, S.; Khurshid, A.; Hasan, T. Development and applications of photo-triggered theranostic agents. Adv. Drug Deliv. Rev. 2010, 62, 1094–1124. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, M.Z.; Akhter, S.; Rahman, Z.; Akhter, S.; Anwar, M.; Mallik, N.; Ahmad, F.J. Nanometric gold in cancer nanotechnology: Current status and future prospect. J. Pharm. Pharmacol. 2013, 65, 634–651. [Google Scholar] [CrossRef]
- Milane, L.; Ganesh, S.; Shah, S.; Duan, Z.F.; Amiji, M. Multi-modal strategies for overcoming tumor drug resistance: Hypoxia, the Warburg effect, stem cells, and multifunctional nanotechnology. J. Control. Release 2011, 155, 237–247. [Google Scholar] [CrossRef] [Green Version]
- Jang, B.; Park, J.Y.; Tung, C.H.; Kim, I.H.; Choi, Y. Gold nanorod-photosensitizer complex for near-infrared fluorescence imaging and photodynamic/photothermal therapy in vivo. ACS Nano 2011, 5, 1086–1094. [Google Scholar] [CrossRef]
- Huang, X.; Swierczewska, M.; Choi, K.Y.; Zhu, L.; Bhirde, A.; Park, J.; Kim, K.; Xie, J.; Niu, G.; Lee, K.C.; et al. Multiplex imaging of an intracellular proteolytic cascade by using a broad-spectrum nanoquencher. Angew. Chem. Int. Ed. 2012, 51, 1625–1630. [Google Scholar] [CrossRef]
- Mura, S.; Couvreur, P. Nanotheranostics for personalized medicine. Nanotheranostics Pers. Med. 2016, 13, 1–337. [Google Scholar] [CrossRef] [Green Version]
- Cafeo, G.; Carbotti, G.; Cuzzola, A.; Fabbi, M.; Ferrini, S.; Kohnke, F.H.; Papanikolaou, G.; Plutino, M.R.; Rosano, C.; White, A.J.P. Drug delivery with a calixpyrrole-trans-Pt(II) complex. J. Am. Chem. Soc. 2013, 135, 2544–2551. [Google Scholar] [CrossRef]
- Anderson, N.L. The clinical plasma proteome: A survey of clinical assays for proteins in plasma and serum. Clin. Chem. 2010, 56, 177–185. [Google Scholar] [CrossRef]
- Retèl, V.P.; Hummel, M.J.M.; van Harten, W.H. Review on early technology assessments of nanotechnologies in oncology. Mol. Oncol. 2009, 3, 394–401. [Google Scholar] [CrossRef]
- Levi-Polyachenko, N.H.; Stewart IV, J.H. Clinical relevance of nanoparticle induced hyperthermia for drug delivery and treatment of abdominal cancers. Open Nanomed. J. 2011, 3, 24–37. [Google Scholar] [CrossRef] [Green Version]
- Hirsch, L.R.; Stafford, R.J.; Bankson, J.A.; Sershen, S.R.; Rivera, B.; Price, R.E.; Hazle, J.D.; Halas, N.J.; West, J.L. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc. Natl. Acad. Sci. USA 2003, 100, 13549–13554. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Zhang, Y.; Ding, T.; Liu, J.; Zhao, H. Multifunctional Gold Nanoparticles: A Novel Nanomaterial for Various Medical Applications and Biological Activities. Front. Bioeng. Biotechnol. 2020, 8, 1–17. [Google Scholar] [CrossRef]
- Chenthamara, D.; Subramaniam, S.; Ramakrishnan, S.G.; Krishnaswamy, S.; Essa, M.M.; Lin, F.-H.; Qoronfleh, M.W. Therapeutic efficacy of nanoparticles and routes of administration. Biomater. Res. 2019, 23, 20. [Google Scholar] [CrossRef] [PubMed]
- Hillyer, J.F.; Albrecht, R.M. Gastrointestinal persorption and tissue distribution of differently sized colloidal gold nanoparticles. J. Pharm. Sci. 2001, 90, 1927–1936. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Yang, W.; Bao, Y.; Xu, H.; Qin, S.; Tu, Y. BSA capped Au nanoparticle as an efficient sensitizer for glioblastoma tumor radiation therapy. RSC Adv. 2015, 5, 40514–40520. [Google Scholar] [CrossRef]
- Hainfeld, J.F.; Dilmanian, F.A.; Slatkin, D.N.; Smilowitz, H.M. Radiotherapy enhancement with gold nanoparticles. J. Pharm. Pharmacol. 2010, 60, 977–985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ernsting, M.J.; Murakami, M.; Roy, A.; Li, S.-D. Factors controlling the pharmacokinetics, biodistribution and intratumoral penetration of nanoparticles. J. Control. Release 2013, 172, 782–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Jong, W.H.; Hagens, W.I.; Krystek, P.; Burger, M.C.; Sips, A.J.A.M.; Geertsma, R.E. Particle size-dependent organ distribution of gold nanoparticles after intravenous administration. Biomaterials 2008, 29, 1912–1919. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.R.K.; Rahman, M.A.; Wu, Y.; Han, T.; Peng, X.; Mackey, M.A.; Wang, D.; Shin, H.J.; Chen, Z.G.; Xiao, H.; et al. Efficacy, long-term toxicity, and mechanistic studies of gold nanorods photothermal therapy of cancer in xenograft mice. Proc. Natl. Acad. Sci. USA 2017, 114, E3110–E3118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanavi, M.R.; Asadi, S.; Ahmadieh, H. Ex vivo distribution of gold nanoparticles in choroidal melanoma. Int. J. Nanomed. 2017, 12, 8527–8529. [Google Scholar] [CrossRef] [Green Version]
- Ivask, A.; Titma, T.; Visnapuu, M.; Vija, H.; Kakinen, A.; Sihtmae, M.; Pokhrel, S.; Madler, L.; Heinlaan, M.; Kisand, V.; et al. Toxicity of 11 Metal Oxide Nanoparticles to Three Mammalian Cell Types In Vitro. Curr. Top. Med. Chem. 2015, 15, 1914–1929. [Google Scholar] [CrossRef]
- Bahadar, H.; Maqbool, F.; Niaz, K.; Abdollahi, M. Toxicity of nanoparticles and an overview of current experimental models. Iran. Biomed. J. 2016, 20, 1–11. [Google Scholar] [CrossRef]
- Boisselier, E.; Astruc, D. Gold nanoparticles in nanomedicine: Preparations, imaging, diagnostics, therapies and toxicity. Chem. Soc. Rev. 2009, 38, 1759–1782. [Google Scholar] [CrossRef]
- Patra, H.K.; Banerjee, S.; Chaudhuri, U.; Lahiri, P.; Dasgupta, A.K. Cell selective response to gold nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 111–119. [Google Scholar] [CrossRef]
- Carnovale, C.; Bryant, G.; Shukla, R.; Bansal, V. Identifying Trends in Gold Nanoparticle Toxicity and Uptake: Size, Shape, Capping Ligand, and Biological Corona. ACS Omega 2019, 4, 242–256. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Jia, J.; Jiang, C.; Zhai, S. Gold nanoparticle-induced cell death and potential applications in nanomedicine. Int. J. Mol. Sci. 2018, 19, 754. [Google Scholar] [CrossRef] [Green Version]
- Asadi, S.; Bianchi, L.; De Landro, M.; Korganbayev, S.; Schena, E.; Saccomandi, P. Laser-induced optothermal response of gold nanoparticles: From a physical viewpoint to cancer treatment application. J. Biophotonics 2021, 14, e202000161. [Google Scholar] [CrossRef] [PubMed]
- Arnida; Malugin, A.; Ghandehari, H. Cellular uptake and toxicity of gold nanoparticles in prostate cancer cells: A comparative study of rods and spheres. J. Appl. Toxicol. 2010, 30, 212–217. [Google Scholar] [CrossRef]
- Ali, M.R.K.; Wu, Y.; El-Sayed, M.A. Gold-Nanoparticle-Assisted Plasmonic Photothermal Therapy Advances Toward Clinical Application. J. Phys. Chem. C 2019, 123, 15375–15393. [Google Scholar] [CrossRef]
- Mooney, R.; Schena, E.; Saccomandi, P.; Zhumkhawala, A.; Aboody, K.; Berlin, J.M. Gold nanorod-mediated near-infrared laser ablation: In vivo experiments on mice and theoretical analysis at different settings. Int. J. Hyperth. 2017, 33, 150–159. [Google Scholar] [CrossRef] [Green Version]
- Phillips, W.T.; Bao, A.; Brenner, A.J.; Goins, B.A. Image-guided interventional therapy for cancer with radiotherapeutic nanoparticles. Adv. Drug Deliv. Rev. 2014, 76, 39–59. [Google Scholar] [CrossRef] [Green Version]
- Adewale, O.B.; Davids, H.; Cairncross, L.; Roux, S. Toxicological Behavior of Gold Nanoparticles on Various Models: Influence of Physicochemical Properties and Other Factors. Int. J. Toxicol. 2019, 38, 357–384. [Google Scholar] [CrossRef]
- Paciotti, G.F.; Kingston, D.G.I.; Tamarkin, L. Colloidal gold nanoparticles: A novel nanoparticle platform for developing multifunctional tumor-targeted drug delivery vectors. Drug Dev. Res. 2006, 67, 47–54. [Google Scholar] [CrossRef]
- Hu, G.; Guo, M.; Xu, J.; Wu, F.; Fan, J.; Huang, Q.; Yang, G.; Lv, Z.; Wang, X.; Jin, Y. Nanoparticles targeting macrophages as potential clinical therapeutic agents against cancer and inflammation. Front. Immunol. 2019, 10, 1998. [Google Scholar] [CrossRef] [PubMed]
- Amina, S.J.; Guo, B. A review on the synthesis and functionalization of gold nanoparticles as a drug delivery vehicle. Int. J. Nanomed. 2020, 15, 9823–9857. [Google Scholar] [CrossRef]
- Singh, P.; Pandit, S.; Mokkapati, V.R.S.S.; Garg, A.; Ravikumar, V.; Mijakovic, I. Gold nanoparticles in diagnostics and therapeutics for human cancer. Int. J. Mol. Sci. 2018, 19, 1979. [Google Scholar] [CrossRef]
- Zhao, X.; Huang, Q.; Jin, Y. Gold nanorod delivery of LSD1 siRNA induces human mesenchymal stem cell differentiation. Mater. Sci. Eng. C 2015, 54, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Wang, Z.; Liu, R.; Li, H.; Zhang, Z.; Su, T.; Yang, J.; Liu, H. The effect of phospho-peptide on the stability of gold nanoparticles and drug delivery. J. Nanobiotechnol. 2019, 17, 88. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Simard, J.M.; Worrall, J.W.E.; Rotello, V.M. Tunable Reactivation of Nanoparticle-Inhibited β-Galactosidase by Glutathione at Intracellular Concentrations. J. Am. Chem. Soc. 2004, 126, 13987–13991. [Google Scholar] [CrossRef] [PubMed]
- Bhumkar, D.R.; Joshi, H.M.; Sastry, M.; Pokharkar, V.B. Chitosan reduced gold nanoparticles as novel carriers for transmucosal delivery of insulin. Pharm. Res. 2007, 24, 1415–1426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, M.; Shim, K.H.; An, S.S.A.; Yi, D.K. Review on gold nanoparticles and their applications. Toxicol. Environ. Health Sci. 2011, 3, 193–205. [Google Scholar] [CrossRef]
- Gao, W.; Chan, J.M.; Farokhzad, O.C. reviews pH-Responsive Nanoparticles for Drug Delivery. Mol. Pharm. 2010, 7, 1913–1920. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, Y.-C.; Dou, S.; Xiong, M.-H.; Sun, T.-M.; Wang, J. Doxorubicin-Tethered Responsive Gold Nanoparticles Facilitate Intracellular Drug Delivery for Overcoming Multidrug Resistance in Cancer Cells. ACS Nano 2011, 5, 3679–3692. [Google Scholar] [CrossRef] [PubMed]
- Saito, G.; Swanson, J.A.; Lee, K.-D. Drug delivery strategy utilizing conjugation via reversible disulfide linkages: Role and site of cellular reducing activities. Adv. Drug Deliv. Rev. 2003, 55, 199–215. [Google Scholar] [CrossRef]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic. Bioeng. Transl. Med. 2016, 1, 10–29. [Google Scholar] [CrossRef]
- Nejati, K.; Dadashpour, M.; Gharibi, T.; Mellatyar, H.; Akbarzadeh, A. Biomedical Applications of Functionalized Gold Nanoparticles: A Review. J. Clust. Sci. 2021, 1–16. [Google Scholar] [CrossRef]
- Maeda, H. Tumor-selective delivery of macromolecular drugs via the EPR effect: Background and future prospects. Bioconjug. Chem. 2010, 21, 797–802. [Google Scholar] [CrossRef]
- Kommareddy, S.; Tiwari, S.B.; Amiji, M.M. Long-circulating polymeric nanovectors for tumor-selective gene delivery. Technol. Cancer Res. Treat. 2005, 4, 615–625. [Google Scholar] [CrossRef] [Green Version]
- Yuan, F.; Dellian, M.; Fukumura, D.; Leunig, M.; Berk, D.A.; Jain, R.K.; Torchilin, V.P. Vascular Permeability in a Human Tumor Xenograft: Molecular Size Dependence and Cutoff Size. Cancer Res. 1995, 55, 3752–3756. [Google Scholar]
- Perrault, S.D.; Walkey, C.; Jennings, T.; Fischer, H.C.; Chan, W.C.W. Mediating tumor targeting efficiency of nanoparticles through design. Nano Lett. 2009, 9, 1909–1915. [Google Scholar] [CrossRef]
- Levine, E.A.; Stewart IV, J.H.; Russell, G.B.; Geisinger, K.R.; Loggie, B.L.; Shen, P. Cytoreductive Surgery and Intraperitoneal Hyperthermic Chemotherapy for Peritoneal Surface Malignancy: Experience with 501 Procedures. J. Am. Coll. Surg. 2007, 204, 943–953. [Google Scholar] [CrossRef]
- Iacopetta, D.; Grande, F.; Caruso, A.; Mordocco, R.A.; Plutino, M.R.; Scrivano, L.; Ceramella, J.; Muià, N.; Saturnino, C.; Puoci, F.; et al. New insights for the use of quercetin analogs in cancer treatment. Future Med. Chem. 2017, 9, 2011–2028. [Google Scholar] [CrossRef] [PubMed]
- Chimento, A.; Saturnino, C.; Iacopetta, D.; Mazzotta, R.; Caruso, A.; Plutino, M.R.; Mariconda, A.; Ramunno, A.; Sinicropi, M.S.; Pezzi, V.; et al. Erratum: Inhibition of human topoisomerase I and II and anti-proliferative effects on MCF-7 cells by new titanocene complexes. Bioorg. Med. Chem. 2015, 23, 7785. [Google Scholar] [CrossRef]
- Altintas, I.; Kok, R.J.; Schiffelers, R.M. Targeting epidermal growth factor receptor in tumors: From conventional monoclonal antibodies via heavy chain-only antibodies to nanobodies. Eur. J. Pharm. Sci. 2012, 45, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Karimipour, G.; Ghaedi, M.; Sahraei, R.; Daneshfar, A.; Biyareh, M.N. Modification of gold nanoparticle loaded on activated carbon with bis(4-methoxysalicylaldehyde)-1,2-phenylenediamine as new sorbent for enrichment of some metal ions. Biol. Trace Elem. Res. 2012, 145, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshini, E.; Pradhan, N.; Panda, P.K.; Mishra, B.K. Biogenic unmodified gold nanoparticles for selective and quantitative detection of cerium using UV-vis spectroscopy and photon correlation spectroscopy (DLS). Biosens. Bioelectron. 2015, 68, 598–603. [Google Scholar] [CrossRef]
- Kiriakidou, K.; Plutino, M.R.; Prestopino, F.; Monari, M.; Johansson, M.; Elding, L.I.; Valls, E.; Gobetto, R.; Aime, S.; Nordlander, E. Detection of a novel intermediate in the addition of thiols to osmium carbonyl clusters. Chem. Commun. 1998, 1, 2721–2722. [Google Scholar] [CrossRef]
- Wang, H.; Campiglia, A.D. Direct determination of benzo[a]pyrene in water samples by a gold nanoparticle-based solid phase extraction method and laser-excited time-resolved Shpol’skii spectrometry. Talanta 2010, 83, 233–240. [Google Scholar] [CrossRef]
- Wilson, W.B.; Hewitt, U.; Miller, M.; Campiglia, A.D. Water analysis of the sixteen environmental protection agency-polycyclic aromatic hydrocarbons via solid-phase nanoextraction-gas chromatography/mass spectrometry. J. Chromatogr. A 2014, 1345, 1–8. [Google Scholar] [CrossRef]
- Huang, Y.F.; Chang, H.T. Nile red-adsorbed gold nanoparticle matrixes for determining aminothiols through surface-assisted laser desorption/ionization mass spectrometry. Anal. Chem. 2006, 78, 1485–1493. [Google Scholar] [CrossRef]
- Su, C.L.; Tseng, W.L. Gold nanoparticles as assisted matrix for determining neutral small carbohydrates through laser desorption/ionization time-of-flight mass spectrometry. Anal. Chem. 2007, 79, 1626–1633. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.W.; Chen, W.T.; Chang, H.T. Exploring the interactions between gold nanoparticles and analytes through surface-assisted laser desorption/ ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2010, 24, 933–938. [Google Scholar] [CrossRef]
- Lin, J.H.; Chang, C.W.; Tseng, W.L. Fluorescent sensing of homocysteine in urine: Using fluorosurfactant-capped gold nanoparticles and o-Phthaldialdehyde. Analyst 2010, 135, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.C.; Tseng, W.L.; Hsieh, M.M. Selective enrichment of aminothiols using polysorbate 20-capped gold nanoparticles followed by capillary electrophoresis with laser-induced fluorescence. J. Chromatogr. A 2009, 1216, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Huang, Q.; Fan, K.; Wu, L.; Nie, D.; Guo, W.; Wu, Y.; Han, Z. Reduced graphene oxide and gold nanoparticle composite-based solid-phase extraction coupled with ultra-high-performance liquid chromatography-tandem mass spectrometry for the determination of 9 mycotoxins in milk. Food Chem. 2018, 264, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Zare, F.; Ghaedi, M.; Daneshfar, A. Application of an ionic-liquid combined with ultrasonic-assisted dispersion ofgold nanoparticles for micro-solid phase extraction of unmetabolized pyridoxine and folic acid in biological fluids prior to high-performance liquid chromatography. RSC Adv. 2015, 5, 70064–70072. [Google Scholar] [CrossRef]
- Libertino, S.; Plutino, M.R.; Rosace, G. Design and development of wearable sensing nanomaterials for smart textiles. AIP Conf. Proc. 2018, 1990, 20016. [Google Scholar] [CrossRef]
- Delmulle, B.S.; De Saeger, S.M.D.G.; Sibanda, L.; Barna-Vetro, I.; Van Peteghem, C.H. Development of an immunoassay-based lateral flow dipstick for the rapid detection of aflatoxin B1 in pig feed. J. Agric. Food Chem. 2005, 53, 3364–3368. [Google Scholar] [CrossRef]
- Aveyard, J.; Nolan, P.; Wilson, R. Improving the sensitivity of immunoassays by tuning gold nanoparticles to the tipping point. Anal. Chem. 2008, 80, 6001–6005. [Google Scholar] [CrossRef]
- Fernández-Sánchez, C.; McNeil, C.J.; Rawson, K.; Nilsson, O.; Leung, H.Y.; Gnanapragasam, V. One-step immunostrip test for the simultaneous detection of free and total prostate specific antigen in serum. J. Immunol. Methods 2005, 307, 1–12. [Google Scholar] [CrossRef]
- Suzuki, T.; Tanaka, M.; Otani, S.; Matsuura, S.; Sakaguchi, Y.; Nishimura, T.; Ishizaka, A.; Hasegawa, N. New rapid detection test with a combination of polymerase chain reaction and immunochromatographic assay for Mycobacterium tuberculosis complex. Diagn. Microbiol. Infect. Dis. 2006, 56, 275–280. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, H.; Zhou, Z.; Huang, K.; Yang, S.; Han, G. Recent Advances on Magnetic Relaxation Switching Assay-Based Nanosensors. Bioconjug. Chem. 2017, 28, 869–879. [Google Scholar] [CrossRef]
- Blanco-Formoso, M.; Pazos-Perez, N.; Alvarez-Puebla, R.A. Fabrication and SERS properties of complex and organized nanoparticle plasmonic clusters stable in solution. Nanoscale 2020, 12, 14948–14956. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Huo, Y.; Ning, T.; Liu, R.; Zha, Z.; Shafi, M.; Li, C.; Li, S.; Xing, K.; Zhang, R.; et al. Composite Structure Based on Gold-Nanoparticle Layer and HMM for Surface-Enhanced Raman Spectroscopy Analysis. Nanomaterials 2021, 11, 587. [Google Scholar] [CrossRef] [PubMed]
- Hinterwirth, H.; Stübiger, G.; Lindner, W.; Lämmerhofer, M. Gold nanoparticle-conjugated anti-oxidized low-density lipoprotein antibodies for targeted lipidomics of oxidative stress biomarkers. Anal. Chem. 2013, 85, 8376–8384. [Google Scholar] [CrossRef] [PubMed]
- Haller, E.; Stübiger, G.; Lafitte, D.; Lindner, W.; Lämmerhofer, M. Chemical recognition of oxidation-specific epitopes in low-density lipoproteins by a nanoparticle based concept for trapping, enrichment, and liquid chromatography-tandem mass spectrometry analysis of oxidative stress biomarkers. Anal. Chem. 2014, 86, 9954–9961. [Google Scholar] [CrossRef] [PubMed]
- Nagahori, N.; Abe, M.; Nishimura, S.I. Structural and functional glycosphingolipidomics by glycoblotting with an aminooxy-functionalized gold nanoparticle. Biochemistry 2009, 48, 583–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sudhir, P.R.; Wu, H.F.; Zhou, Z.C. Identification of peptides using gold nanoparticle-assisted single-drop microextraction coupled with AP-MALDI mass spectrometry. Anal. Chem. 2005, 77, 7380–7385. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, K.; Hatai, Y.; Okada, A. Gold nanoparticle-based multivalent carbohydrate probes: Selective photoaffinity labeling of carbohydrate-binding proteins. Chem. Sci. 2016, 7, 702–705. [Google Scholar] [CrossRef] [Green Version]
- Liang, K.; Wu, H.; Li, Y. Immune-enrichment of insulin in bio-fluids on gold-nanoparticle decorated target plate and in situ detection by MALDI MS. Clin. Proteom. 2017, 14, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Rudd, P.M.; Wormald, M.R.; Dwek, R.A. Glycosylation and the immune system. J. Protein Chem. 1998, 17, 519. [Google Scholar] [CrossRef]
- Alwael, H.; Connolly, D.; Clarke, P.; Thompson, R.; Twamley, B.; O’Connor, B.; Paull, B. Pipette-tip selective extraction of glycoproteins with lectin modified gold nano-particles on a polymer monolithic phase. Analyst 2011, 136, 2619–2628. [Google Scholar] [CrossRef] [Green Version]
- Yao, G.; Zhang, H.; Deng, C.; Lu, H.; Zhang, X.; Yang, P. Facile synthesis of 4-mercaptophenylboronic acid functionalized gold nanoparticles for selective enrichment of glycopeptides. Rapid Commun. Mass Spectrom. 2009, 23, 3493–3500. [Google Scholar] [CrossRef]
- Liang, Y.; Wu, C.; Zhao, Q.; Wu, Q.; Jiang, B.; Weng, Y.; Liang, Z.; Zhang, L.; Zhang, Y. Gold nanoparticles immobilized hydrophilic monoliths with variable functional modification for highly selective enrichment and on-line deglycosylation of glycopeptides. Anal. Chim. Acta 2015, 900, 83–89. [Google Scholar] [CrossRef]
- Taton, T.A.; Mirkin, C.A.; Letsinger, R.L. Scanometric DNA array detection with nanoparticle probes. Science 2000, 289, 1757–1760. [Google Scholar] [CrossRef] [Green Version]
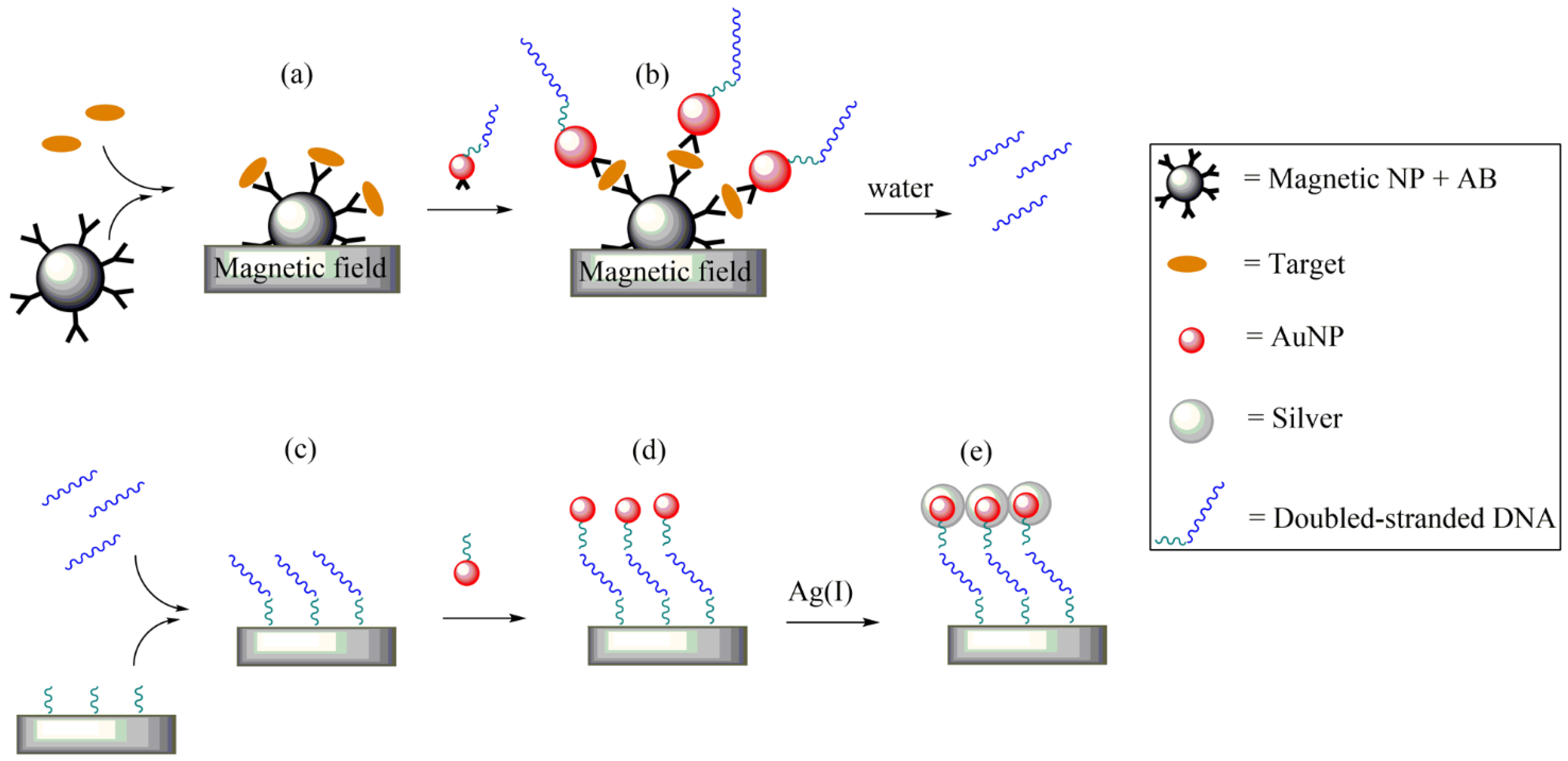
- Nam, J.; Thaxton, C.S.; Mirkin, C.A. Nanoparticle-Based Bio-Bar Codes for the Ultrasensitive. Science 2003, 301, 1884–1887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goluch, E.D.; Nam, J.M.; Georganopoulou, D.G.; Chiesl, T.N.; Shaikh, K.A.; Ryu, K.S.; Barron, A.E.; Mirkin, C.A.; Liu, C. A bio-barcode assay for on-chip attomolar-sensitivity protein detection. Lab Chip 2006, 6, 1293–1299. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ielo, I.; Rando, G.; Giacobello, F.; Sfameni, S.; Castellano, A.; Galletta, M.; Drommi, D.; Rosace, G.; Plutino, M.R. Synthesis, Chemical–Physical Characterization, and Biomedical Applications of Functional Gold Nanoparticles: A Review. Molecules 2021, 26, 5823. https://doi.org/10.3390/molecules26195823
Ielo I, Rando G, Giacobello F, Sfameni S, Castellano A, Galletta M, Drommi D, Rosace G, Plutino MR. Synthesis, Chemical–Physical Characterization, and Biomedical Applications of Functional Gold Nanoparticles: A Review. Molecules. 2021; 26(19):5823. https://doi.org/10.3390/molecules26195823
Chicago/Turabian StyleIelo, Ileana, Giulia Rando, Fausta Giacobello, Silvia Sfameni, Angela Castellano, Maurilio Galletta, Dario Drommi, Giuseppe Rosace, and Maria Rosaria Plutino. 2021. "Synthesis, Chemical–Physical Characterization, and Biomedical Applications of Functional Gold Nanoparticles: A Review" Molecules 26, no. 19: 5823. https://doi.org/10.3390/molecules26195823
APA StyleIelo, I., Rando, G., Giacobello, F., Sfameni, S., Castellano, A., Galletta, M., Drommi, D., Rosace, G., & Plutino, M. R. (2021). Synthesis, Chemical–Physical Characterization, and Biomedical Applications of Functional Gold Nanoparticles: A Review. Molecules, 26(19), 5823. https://doi.org/10.3390/molecules26195823











