The Different Facets of Triclocarban: A Review
Abstract
1. Introduction
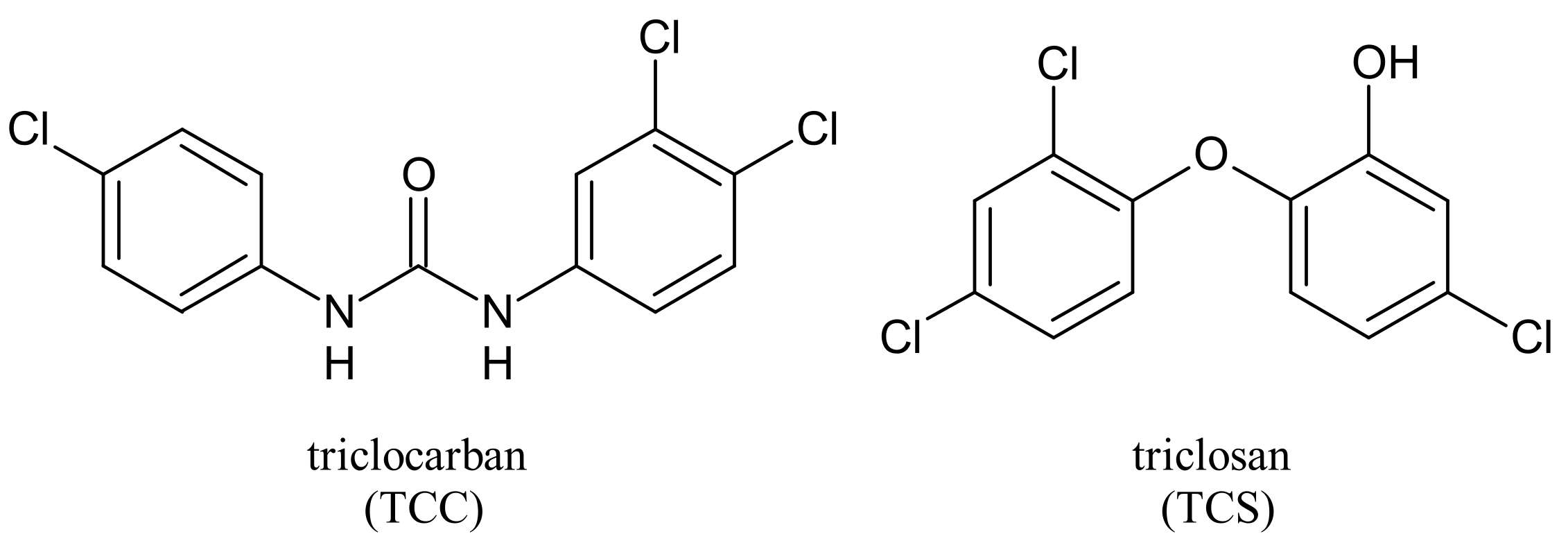
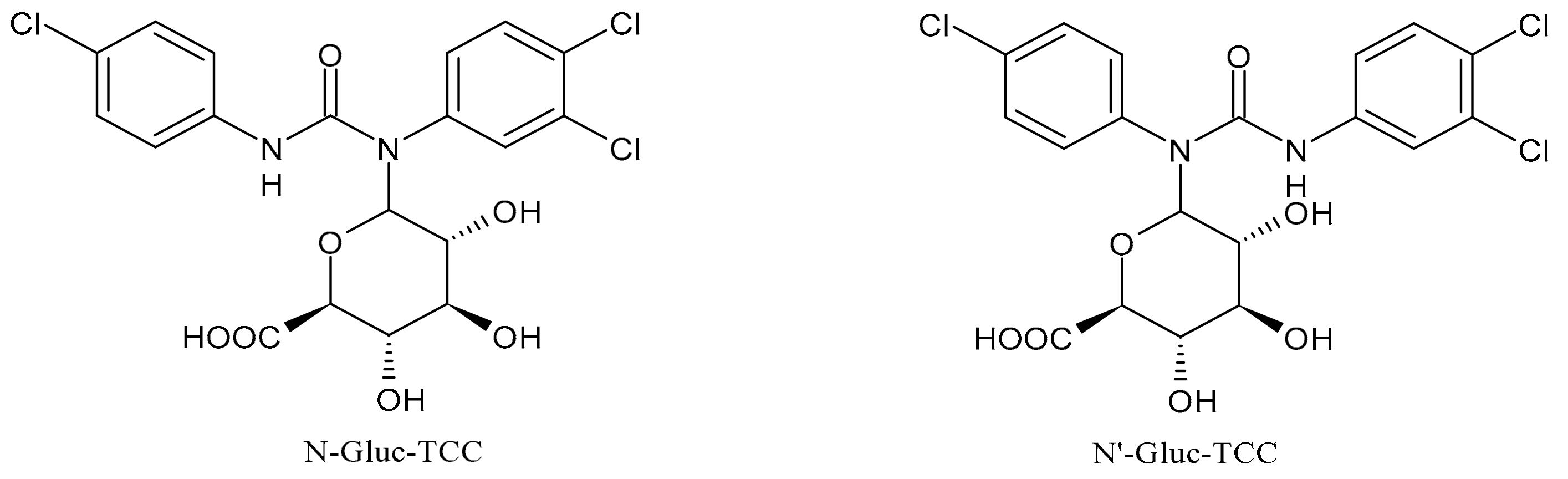
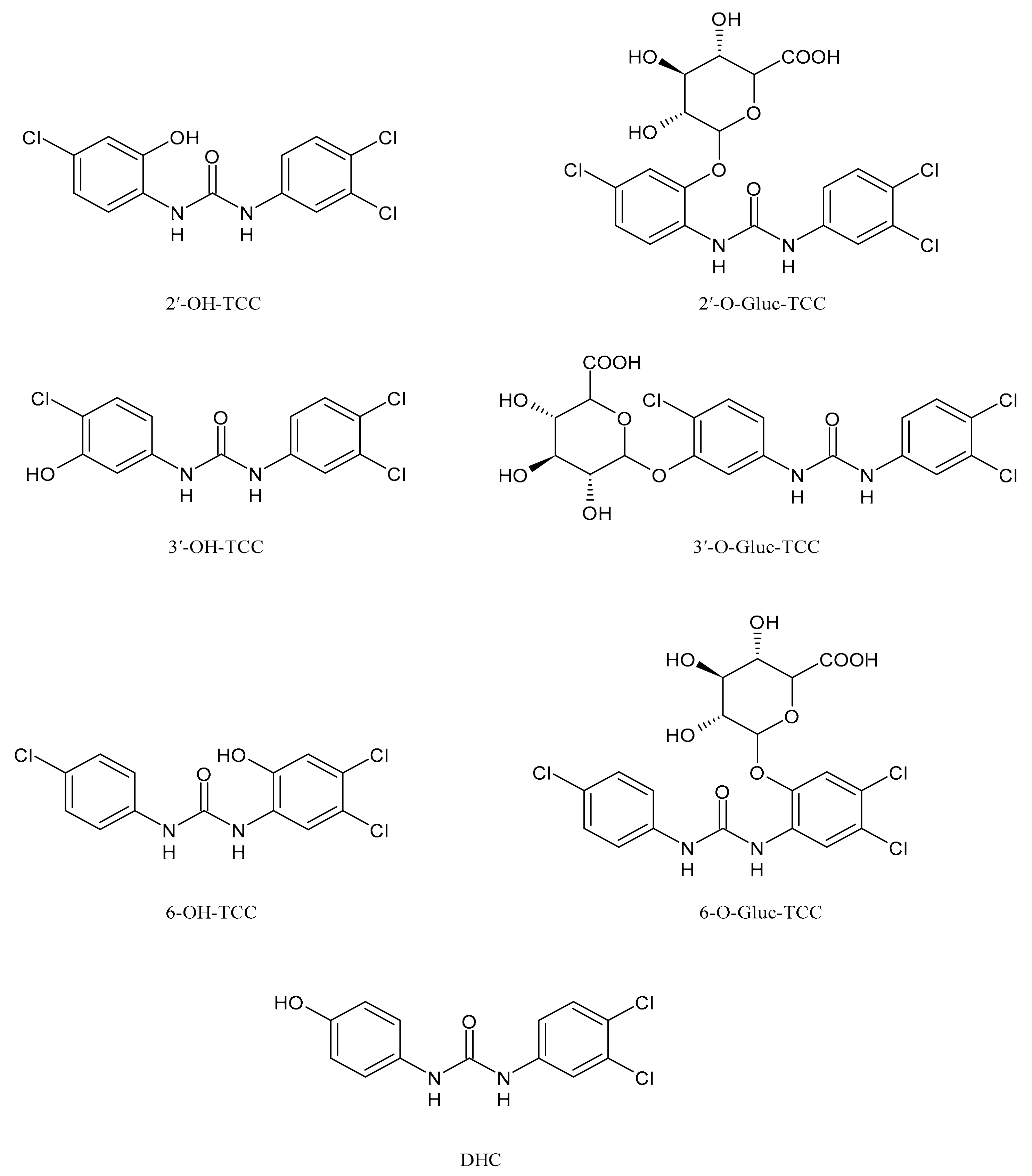
2. Metabolism and Transformation Products of TCC
3. Biological Activity of TCC
4. Ecotoxicity of Triclocarban
5. Analogues of TCC
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| B. subtilis | Bacillus subtilis |
| CAT | Acatalase |
| 3-CA | 3-Chloroaniline |
| 4-CA | 4-Chloroaniline |
| CFDA | Carboxyfluorescein diacetate |
| CEC | Contaminant of Emerging Concern |
| C. elegans | Caenorhabditis elegans |
| 3,4-DCA | 3,4-Dichloroaniline |
| DCC | 4,4′-Dichlorocarbanilide |
| DHC | 3′,4′-Dichloro-4-hydroxycarbanilide |
| E. coli | Escherichia coli |
| E. faecalis | Enterococcus faecalis |
| EOCs | Emerging Organic Contaminants |
| ERRγ | Estrogen-related receptor γ |
| EU | European Union |
| FDA | Food and Drug Administration |
| GJIC | Gap junctional intercellular communication |
| GSH | Glutathione |
| GST | GLUTATHIONE-S-TRANSFERASE |
| IE | INFECTIVE ENDOCARDITIS |
| LPO | LIPID PEROXIDATION |
| MCC | 1-(3-Chlorophenyl)-3-phenylurea |
| MICs | Minimum inhibitory concentrations |
| NCC | Carbanilide |
| NIS | Sodium/iodide symporter |
| NRU | Neutral red uptake |
| 2′-OH-TCC | 2′-Hydroxytriclocarban |
| 3′-OH-TCC | 3′-Hydroxytriclocarban |
| P. aeruginosa | Pseudomonas aeruginosa |
| P. falciparum | Plasmodium falciparum |
| P. mirabilis | Proteus mirabilis |
| PPCPs | Personal care product compounds |
| S. aureus | Staphylococcus aureus |
| S. epidermidis | Staphylococcus epidermidis |
| S. japonicum | Schistosoma japonicum |
| S. mansoni | Schistosoma mansoni |
| sEH | Soluble epoxide hydrolase |
| TCC | Triclocarban |
| TCS | Triclosan |
| TH | Thyroid hormone |
| Tjp1 | Tight junction protein 1 |
| TPO | Thyroid peroxidase |
| TRVP1 | Transient receptor potential vanilloid 1 |
| UGTs | UDP-glucuronosyltransferases |
| U.S. | United States |
| WRRF | Water resource recovery facility |
| WWT | Wastewater treatment |
| WWTPs | Wastewater treatment plants |
References
- Liao, C.; Kannan, K. A survey of alkylphenols, bisphenols, and triclosan in personal care products from China and the United States. Arch. Environ. Contam. Toxicol. 2014, 67, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Asimakopoulos, A.G.; Xue, J.; De Carvalho, B.P.; Iyer, A.; Abualnaja, K.O.; Yaghmoor, S.S.; Kumosani, T.A.; Kannan, K. Urinary biomarkers of exposure to 57 xenobiotics and its association with oxidative stress in a population in Jeddah, Saudi Arabia. Environ. Res. 2016, 150, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Lu, C.; Wu, M.; Liang, J.; Ying, Y.; Liu, K.; Huang, X.; Zheng, S.; Du, X.; Liu, D.; et al. Association between Triclocarban and Triclosan Exposures and the Risks of Type 2 Diabetes Mellitus and Impaired Glucose Tolerance in the National Health and Nutrition Examination Survey (NHANES 2013–2014). Environ. Int. 2020, 136, 105445. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.M.; Trinh, H.T.; Anh, H.Q.; Van Le, T.; Le, S.N.; Minh, T.B. Characterization of triclosan and triclocarban in indoor dust from home micro-environments in Vietnam and relevance of non-dietary exposure. Sci. Total Environ. 2020, 732, 139326. [Google Scholar] [CrossRef]
- Gomes, M.F.; de Paula, V.D.C.S.; Martins, L.R.R.; Garcia, J.R.E.; Yamamoto, F.Y.; de Freitas, A.M. Sublethal effects of triclosan and triclocarban at environmental concentrations in silver catfish (Rhamdia quelen) embryos. Chemosphere 2021, 263, 127985. [Google Scholar] [CrossRef]
- Catalano, A.; Iacopetta, D.; Sinicropi, M.S.; Franchini, C. Diarylureas as Antitumor Agents. Appl. Sci. 2021, 11, 374. [Google Scholar] [CrossRef]
- Qiao, L.; Hao, S. Novel trifluoromethylcoumarinyl urea derivatives: Synthesis, characterization, fluorescence, and bioactivity. Molecules 2018, 23, 600. [Google Scholar] [CrossRef]
- Xue, D.; Chen, W.; Neamati, N. Discovery, structure-activity relationship study and biological evaluation of 2-thioureidothiophene-3-carboxylates as a novel class of CXC chemokine receptor 2 (CXCR2) antagonists. Eur. J. Med. Chem. 2020, 204, 112387. [Google Scholar] [CrossRef]
- Aghcheli, A.; Toolabi, M.; Ayati, A.; Moghimi, S.; Firoozpour, L.; Bakhshaiesh, T.O.; Nazeri, E.; Norouzbahari, M.; Esmaeili, R.; Foroumadi, A. Design, synthesis, and biological evaluation of 1-(5-(benzylthio)-1, 3, 4-thiadiazol-2-yl)-3-phenylurea derivatives as anticancer agents. Med. Chem. Res. 2020, 29, 2000–2010. [Google Scholar] [CrossRef]
- Perrey, D.A.; Zhang, Y. Therapeutics development for addiction: Orexin-1 receptor antagonists. Brain Res. 2020, 1731, 145922. [Google Scholar] [CrossRef]
- Podlewska, S.; Bugno, R.; Lacivita, E.; Leopoldo, M.; Bojarski, A.J.; Handzlik, J. Low basicity as a characteristic for atypical ligands of serotonin receptor 5-HT2. Int. J. Mol. Sci. 2021, 22, 1035. [Google Scholar] [CrossRef]
- Catalano, A. COVID-19: Could Irisin Become the Handyman Myokine of the 21st Century? Coronaviruses 2020, 1, 32–41. [Google Scholar] [CrossRef]
- Catalano, A.; Iacopetta, D.; Pellegrino, M.; Aquaro, S.; Franchini, C.; Sinicropi, M.S. Diarylureas: Repositioning from antitumor to antimicrobials or multi-target agents against new pandemics. Antibiotics 2021, 10, 92. [Google Scholar] [CrossRef]
- Sreevidya, V.S.; Lenz, K.A.; Svoboda, K.R.; Ma, H. Benzalkonium chloride, benzethonium chloride, and chloroxylenol—Three replacement antimicrobials are more toxic than triclosan and triclocarban in two model organisms. Environ. Pollut. 2018, 235, 814–824. [Google Scholar] [CrossRef]
- Arifin, S.N.H.; Mohamed, R.; Al-Gheethi, A.; Lai, C.W.; Yashni, G. Heterogeneous photocatalysis of triclocarban and triclosan in greywater: A systematic and bibliometric review analysis. Int. J. Environ. Anal. Chem. 2021, 1–19. [Google Scholar] [CrossRef]
- Asimakopoulos, A.G.; Elangovan, M.; Kannan, K. Migration of parabens, bisphenols, benzophenone-type UV filters, triclosan, and triclocarban from teethers and its implications for infant exposure. Environ. Sci. Technol. 2016, 50, 13539–13547. [Google Scholar] [CrossRef]
- Moreta, C.; Tena, M.T.; Kannan, K. Analytical method for the determination and a survey of parabens and its derivatives in pharmaceuticals. Environ. Res. 2015, 142, 452–460. [Google Scholar] [CrossRef]
- Li, W.; Zhang, W.; Chang, M.; Ren, J.; Xie, W.; Chen, H.; Zhang, Z.; Zhuang, X.; Shen, G.; Li, H. Metabonomics reveals that triclocarban affects liver metabolism by affecting glucose metabolism, β-oxidation of fatty acids, and the TCA cycle in male mice. Toxicol. Lett. 2018, 299, 76–85. [Google Scholar] [CrossRef]
- European Commission, Scientific Committee on Consumer Products. Opinion on Triclocarban for Other Uses than as a Preservative; Colipa no. P29. SCCP/0851/04; European Commission: Brussels, Belgium, 2005. [Google Scholar]
- Musee, N. Environmental risk assessment of triclosan and triclocarban from personal care products in South Africa. Environ. Pollut. 2018, 242, 827–838. [Google Scholar] [CrossRef]
- Chaudhari, U.; Nemade, H.; Sureshkumar, P.; Vinken, M.; Ates, G.; Rogiers, V.; Hescheler, J.; Hengstler, J.G.; Sachinidis, A. Functional cardiotoxicity assessment of cosmetic compounds using human-induced pluripotent stem cell-derived cardiomyocytes. Arch. Toxicol. 2018, 92, 371–381. [Google Scholar] [CrossRef]
- Halden, R.U.; Paul, D.H. Co-occurence of triclocarban and triclosan in U.S. water resources. Environ. Sci. Technol. 2005, 39, 1420–1426. [Google Scholar] [CrossRef]
- Yang, H.; Sanidad, K.Z.; Wang, W.; Xie, M.; Gu, M.; Cao, X.; Xiao, H.; Zhang, G. Triclocarban exposure exaggerates colitis and colon tumorigenesis: Roles of gut microbiota involved. Gut Microbes 2020, 12, 1690364. [Google Scholar] [CrossRef]
- Ye, X.; Wong, L.Y.; Dwivedi, P.; Zhou, X.; Jia, T.; Calafat, A.M. Urinary concentrations of the antibacterial agent triclocarban in United States residents: 2013−2014 National health and nutrition examination survey. Environ. Sci. Technol. 2016, 50, 13548–13554. [Google Scholar] [CrossRef]
- Halden, R.U.; Lindeman, A.E.; Aiello, A.E.; Andrews, D.; Arnold, W.A.; Fair, P.; Fuoco, R.E.; Geer, L.A.; Johnson, P.I.; Lohmann, R.; et al. The Florence statement on triclosan and triclocarban. Environ. Health Perspect. 2017, 125, 064501. [Google Scholar] [CrossRef]
- CDC (Centers for Disease Control and Prevention). Recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC). 52(RR10). 2003. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5210a1.htm (accessed on 15 April 2021).
- Guedes-Alonso, R.; Montesdeoca-Esponda, S.; Herrera-Melián, J.A.; Rodríguez-Rodríguez, R.; Ojeda-González, Z.; Landívar-Andrade, V.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. Pharmaceutical and personal care product residues in a macrophyte pond-constructed wetland treating wastewater from a university campus: Presence, removal and ecological risk assessment. Sci. Total Environ. 2020, 703, 135596. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.C.; Zhao, B.; Chen, J.; Cherednichenko, G.; Sanmarti, E.; Denison, M.S.; Lasley, B.; Pessah, I.N.; Kultz, D.; Chang, D.P.; et al. In vitro biologic activities of the antimicrobials triclocarban, its analogs, and triclosan in bioassay screens: Receptor-based bioassay screens. Environ. Health Perspect. 2008, 116, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Tarnow, P.; Tralau, T.; Hunecke, D.; Luch, A. Effects of triclocarban on the transcription of estrogen, androgen and aryl hydrocarbon receptor responsive genes in human breast cancer cells. Toxicol. Vitro 2013, 27, 1467–1475. [Google Scholar] [CrossRef] [PubMed]
- Vimalkumar, K.; Arun, E.; Krishna-Kumar, S.; Poopal, R.K.; Nikhil, N.P.; Subramanian, A.; Ramaswamy, B.R. Occurrence of triclocarban and benzotriazole ultraviolet stabilizers in water, sediment, and fish from Indian rivers. Sci. Total Environ. 2018, 625, 1351–1360. [Google Scholar] [CrossRef]
- Subedi, B.; Lee, S.; Moon, H.B.; Kannan, K. Emission of artificial sweeteners, select pharmaceuticals, and personal care products through sewage sludge from wastewater treatment plants in Korea. Environ. Int. 2014, 68, 33–40. [Google Scholar] [CrossRef]
- Karthikraj, R.; Kannan, K.; Karthikraj, R. Mass loading and removal of benzotriazoles, benzothiazoles, benzophenones, and bisphenols in Indian sewage treatment plants. Chemosphere 2017, 181, 216–223. [Google Scholar] [CrossRef]
- Vimalkumar, K.; Seethappan, S.; Pugazhendhi, A. Fate of Triclocarban (TCC) in aquatic and terrestrial systems and human exposure. Chemosphere 2019, 230, 201–209. [Google Scholar] [CrossRef]
- Abbott, T.; Kor-Bicakci, G.; Islam, M.S.; Eskicioglu, C. A review on the fate of legacy and alternative antimicrobials and their metabolites during wastewater and sludge treatment. Int. J. Mol. Sci. 2020, 21, 9241. [Google Scholar] [CrossRef]
- Xia, K.; Bhandari, A.; Das, K.; Pillar, G. Occurrence and Fate of Pharmaceuticals and Personal Care Products (PPCPs) in Biosolids. J. Environ. Qual. 2005, 34, 91–104. [Google Scholar] [CrossRef]
- Onesios, K.M.; Yu, J.T.; Bouwer, E.J. Biodegradation and removal of pharmaceuticals and personal care products in treatment systems: A review. Biodegradation 2009, 20, 441–466. [Google Scholar] [CrossRef]
- Thelusmond, J.R.; Kawka, E.; Strathmann, T.J.; Cupples, A.M. Diclofenac, carbamazepine and triclocarban biodegradation in agricultural soils and the microorganisms and metabolic pathways affected. Sci. Total Environ. 2018, 640, 1393–1410. [Google Scholar] [CrossRef]
- Armstrong, D.L.; Rice, C.P.; Ramirez, M.; Torrents, A. Influence of thermal hydrolysis-anaerobic digestion treatment of wastewater solids on concentrations of triclosan, triclocarban, and their transformation products in biosolids. Chemosphere 2017, 171, 609–616. [Google Scholar] [CrossRef]
- Yun, H.; Liang, B.; Kong, D.; Li, X.; Wang, A. Fate, risk and removal of triclocarban: A critical review. J. Hazard. Mater. 2020, 387, 121944. [Google Scholar] [CrossRef]
- Yin, J.; Wei, L.; Shi, Y.; Zhang, J.; Wu, Q.; Shao, B. Chinese population exposure to triclosan and triclocarban as measured via human urine and nails. Environ. Geochem. Health 2016, 38, 1125–1135. [Google Scholar] [CrossRef]
- Wei, L.; Qiao, P.; Shi, Y.; Ruan, Y.; Yin, J.; Wu, Q.; Shao, B. Triclosan/triclocarban levels in maternal and umbilical blood samples and their association with fetal malformation. Clin. Chim. Acta 2017, 466, 133–137. [Google Scholar] [CrossRef]
- Buck Louis, G.M.; Smarr, M.M.; Sun, L.; Chen, Z.; Honda, M.; Wang, W.; Karthikraj, R.; Weck, J.; Kannan, K. Endocrine disrupting chemicals in seminal plasma and couple fecundity. Environ. Res. 2018, 163, 64–70. [Google Scholar] [CrossRef]
- Li, A.J.; Xue, J.; Lin, S.; Al-Malki, A.L.; Al-Ghamdi, M.A.; Kumosani, T.A.; Kannan, K. Urinary Concentrations of Environmental Phenols and Their Association with Type 2 Diabetes in a Population in Jeddah, Saudi Arabia. Environ. Res. 2018, 166, 544–552. [Google Scholar] [CrossRef]
- Rocha, B.A.; Asimakopoulos, A.G.; Honda, M.; da Costa, N.L.; Barbosa, R.M.; Barbosa, F., Jr.; Kannan, K. Advanced data mining approaches in the assessment of urinary concentrations of bisphenols, chlorophenols, parabens and benzophenones in Brazilian children and their association to DNA damage. Environ. Int. 2018, 116, 269–277. [Google Scholar] [CrossRef]
- Iyer, A.P.; Xue, J.; Honda, M.; Robinson, M.; Kumosani, T.A.; Abulnaja, K.; Kannan, K. Urinary levels of triclosan and triclocarban in several Asian countries, Greece and the USA: Association with oxidative stress. Environ. Res. 2018, 160, 91–96. [Google Scholar] [CrossRef]
- Brose, D.A.; Kumar, K.; Liao, A.; Hundal, L.S.; Tian, G.; Cox, A.; Zhang, H.; Podczerwinski, E.W. A reduction in triclosan and triclocarban in water resource recovery facilities’ influent, effluent, and biosolids following the US Food and Drug Administration’s 2013 proposed rulemaking on antibacterial products. Water Environ. Res. 2019, 91, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Safety and effectiveness of consumer antiseptics: Topical antimicrobial drug products for over-the-counter human use. Fed. Regist. 2016, 81, 61106–61130. [Google Scholar]
- Waidyanatha, S.; Black, S.R.; Patel, P.R.; Watson, S.L.; Snyder, R.W.; Sutherland, V.; Stanko, J.; Fennell, T.R. Disposition and metabolism of antibacterial agent, triclocarban, in rodents; a species and route comparison. Xenobiotica 2020, 50, 1469–1482. [Google Scholar] [CrossRef] [PubMed]
- Juncker, J.-C. (Ed.) Commission Impementing Decision not Approving Triclosan as an Existing Active Substance for Use in Biocidal Products for Product-Type 1, 528/2012; Union European: Brussels, Belgium, 2016. [Google Scholar]
- Chen, J.; Meng, X.Z.; Bergman, A.; Halden, R.U. Nationwide reconnaissance of five parabens, triclosan, triclocarban and its transformation products in sewage sludge from China. J. Hazard. Mater. 2019, 365, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Costa, N.O.; Forcato, S.; Cavichioli, A.M.; Pereira, M.R.F.; Gerardin, D.C.C. In utero and lactational exposure to triclocarban: Age-associated changes in reproductive parameters of male rat offspring. Toxicol. Appl. Pharmacol. 2020, 115077. [Google Scholar] [CrossRef]
- Ye, X.; Zhou, X.; Furr, J.; Ahn, K.C.; Hammock, B.D.; Gray, E.L.; Calafat, A.M. Biomarkers of exposure to triclocarban in urine and serum. Toxicology 2011, 286, 69–74. [Google Scholar] [CrossRef]
- Schebb, N.H.; Inceoglu, B.; Ahn, K.C.; Morisseau, C.; Gee, S.J.; Hammock, B.D. Investigation of human exposure to triclocarban after showering and preliminary evaluation of its biological effects. Environ. Sci. Technol. 2011, 45, 3109–3115. [Google Scholar] [CrossRef]
- Gao, C.-J.; Kannan, K. Phthalates, bisphenols, parabens, and triclocarban in feminine hygiene products from the United States and their implications for human exposure. Environ. Int. 2020, 136, 105465. [Google Scholar] [CrossRef]
- De Bellis, M.; De Luca, A.; Rana, F.; Cavalluzzi, M.M.; Catalano, A.; Lentini, G.; Franchini, C.; Tortorella, V.; Conte Camerino, D. Evaluation of the pharmacological activity of the major mexiletine metabolites on skeletal muscle sodium currents. Br. J. Pharmacol. 2006, 149, 300–310. [Google Scholar] [CrossRef]
- Schebb, N.H.; Franze, B.; Maul, R.; Ranganathan, A.; Hammock, B.D. In vitro glucuronidation of the antibacterial triclocarban and its oxidative metabolites. Drug Metab. Disposit. 2012, 40, 25–31. [Google Scholar] [CrossRef]
- Armstrong, D.L.; Lozano, N.; Rice, C.P.; Ramirez, M.; Torrents, A. Degradation of triclosan and triclocarban and formation of transformation products in activated sludge using benchtop bioreactors. Environ. Res. 2018, 161, 17–25. [Google Scholar] [CrossRef]
- Miller, T.R.; Colquhoun, D.R.; Halden, R.U. Identification of wastewater bacteria involved in the degradation of triclocarban and its non-chlorinated congener. J. Hazard. Mater. 2010, 183, 766–772. [Google Scholar] [CrossRef]
- Pycke, B.F.G.; Roll, I.B.; Brownawell, B.J.; Kinney, C.A.; Furlong, E.T.; Kolpin, D.W.; Halden, R.U. Transformation products and human metabolites of triclocarban and triclosan in sewage sludge across the United States. Environ. Sci. Technol. 2014, 48, 7881–7890. [Google Scholar] [CrossRef]
- Birch, C.G.; Hiles, R.A.; Eichhold, T.H.; Jeffcoat, A.R.; Handy, R.W.; Hill, J.M.; Willis, S.L.; Hess, T.R.; Wall, M.E. Biotransformation products of 3,4,4′-trichlorocarbanilide in rat, monkey, and man. Drug Metab. Dispos. 1978, 6, 169–176. [Google Scholar]
- Schebb, N.H.; Flores, I.; Kurobe, T.; Franze, B.; Ranganathan, A.; Hammock, B.D.; Teh, S. Bioconcentration, metabolism and excretion of triclocarban in larval Quart Medaka (Oryzias latipes). Aquat. Toxicol. 2011, 105, 448–454. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, Y.; Liang, Y.; Jiang, L.; Cai, Z. Triclocarban-induced responses of endogenous and xenobiotic metabolism in human hepatic cells: Toxicity assessment based on nontargeted metabolomics approach. J. Hazard. Mater. 2020, 392, 122475. [Google Scholar] [CrossRef]
- World Health Organization. Antimicrobial Resistance: Global Report on Surveillance; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- O’Neill, J. Antimicrobial resistance: Tackling a crisis for the health and wealth of nations. Rev. Antimicrob Resist. 2014, 20, 1–16. [Google Scholar]
- Kim, S.A.; Rhee, M.S. Microbicidal effects of plain soap vs. triclocarban-based antibacterial soap. J. Hosp. Infect. 2016, 94, 276–280. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Safety and Effectiveness of Consumer Antiseptics; Topical Antimicrobial Drug Products for Overthe-Counter Human Use; Proposed Amendment of the Tentative Final Monograph; Reopening of Administrative Record; US Food and Drug Administration: Silver Spring, MD, USA, 2013. Available online: https://www.federalregister.gov/articles/2013/12/17/2013-29814/safetyand-effectiveness-of-consumer-antiseptics-topical-antimicrobialdrug-products-for (accessed on 15 April 2021).
- Pozzi, C.; Ferrari, S.; Cortesi, D.; Luciani, R.; Stroud, R.M.; Catalano, A.; Costi, M.P.; Mangani, S. The structure of Enterococcus faecalis thymidylate synthase provides clues about folate bacterial metabolism. Acta Crystallogr. D Biol. Crystallogr. 2012, 68, 1232–1241. [Google Scholar] [CrossRef]
- Dahl, A.; Iversen, K.; Tonder, N.; Hoest, N.; Arpi, M.; Dalsgaard, M.; Chehri, M.; Soerensen, L.L.; Fanoe, S.; Junge, S. Prevalence of infective endocarditis in Enterococcus faecalis bacteremia. J. Am. Coll. Cardiol. 2019, 74, 193–201. [Google Scholar] [CrossRef]
- Yu, J.J.; Manus, M.B.; Mueller, O.; Windsor, S.C.; Horvath, J.E.; Nunn, C.L. Antibacterial soap use impacts skin microbial communities in rural Madagascar. PLoS ONE 2018, 13, e0199899. [Google Scholar]
- Pujol, E.; Blanco-Cabra, N.; Julián, E.; Leiva, R.; Torrents, E.; Vázquez, S. Pentafluorosulfanyl-containing triclocarban analogs with potent antimicrobial activity. Molecules 2018, 23, 2853. [Google Scholar] [CrossRef]
- Catalano, A.; Iacopetta, D.; Rosato, A.; Salvagno, L.; Ceramella, J.; Longo, F.; Sinicropi, M.S.; Franchini, C. Searching for small molecules as antibacterials: Non-cytotoxic diarylureas analogues of triclocarban. Antibiotics 2021, 10, 204. [Google Scholar] [CrossRef]
- Chen, J.; Ki, C.A.; Gee, N.A.; Ahmed, M.I.; Duleba, A.J.; Zhao, L.; Gee, S.J.; Hammock, B.D.; Lasley, B.L. Triclocarban enhances testosterone action: A new type of endocrine disruptor? Endocrinology 2008, 149, 1173–1179. [Google Scholar] [CrossRef]
- Duleba, A.J.; Ahmed, M.I.; Sun, M.; Gao, A.C.; Villanueva, J.; Conley, A.J.; Turgeon, J.L.; Benirschke, K.; Gee, N.A.; Chen, J.; et al. Effects of triclocarban on intact immature male rat: Augmentation of androgen action. Reprod. Sci. 2011, 18, 119–127. [Google Scholar] [CrossRef]
- Cao, L.Y.; Xu, Y.H.; He, S.; Ren, X.M.; Yang, Y.; Luo, S.; Xie, X.D.; Luo, L. Antimicrobial triclocarban exhibits higher agonistic activity on estrogen-related receptor γ than triclosan at human exposure levels: A novel estrogenic disruption mechanism. Environ. Sci. Technol. Lett. 2020, 7, 434–439. [Google Scholar] [CrossRef]
- Gálvez-Ontiveros, Y.; Páez, S.; Monteagudo-Sanchez, C.; Rivas, A. Endocrine disruptors in food: Impact on gut microbiota and metabolic diseases: A systematic review. Nutrients 2020, 12, 1158. [Google Scholar] [CrossRef] [PubMed]
- Rochester, J.R.; Bolden, A.L.; Pelch, K.E.; Kwiatkowski, C.F. Potential developmental and reproductive impacts of triclocarban: A scoping review. J. Toxicol. 2017, 2017, 9679738. [Google Scholar] [CrossRef] [PubMed]
- Aker, A.M.; Ferguson, K.K.; Rosario, Z.Y.; Mukherjee, B.; Alshawabkeh, A.N.; Cordero, J.F.; Meeker, J.D. The associations between prenatal exposure to triclocarban, phenols and parabens with gestational age and birth weight in northern Puerto Rico. Environ. Res. 2019, 169, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Watkins, D.J.; Ferguson, K.K.; Anzalota Del Toro, L.V.; Alshawabkeh, A.N.; Cordero, J.F.; Meeker, J.D. Associations between urinary phenol and paraben concentrations and markers of oxidative stress and inflammation among pregnant women in Puerto Rico. Int. J. Hyg. Environ. Health 2015, 218, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhou, T.; Hu, Z.; Li, Y.; Yuan, H.; Zhao, K.; Zhang, H.; Liu, C. Effects of triclocarban on oxidative stress and innate immune response in zebrafish embryos. Chemosphere 2018, 210, 93–101. [Google Scholar] [CrossRef]
- Ding, Z.M.; Ahmad, M.J.; Meng, F.; Chen, F.; Wang, Y.S.; Zhao, X.Z.; Zhang, S.X.; Miao, Y.L.; Xiong, J.J.; Huo, L.J. Triclocarban exposure affects mouse oocyte in vitro maturation through inducing mitochondrial dysfunction and oxidative stress. Environ. Pollut. 2020, 262, 114271. [Google Scholar] [CrossRef]
- Xie, M.; Zhang, H.; Wang, W.; Sherman, H.L.; Minter, L.M.; Cai, Z.; Zhang, G. Triclocarban Exposure Exaggerates Spontaneous Colonic Inflammation in Il-10−/− Mice. Toxicol. Sci. 2020, 174, 92–99. [Google Scholar] [CrossRef]
- Dong, M.; Yuan, P.; Song, Y.; Lei, H.; Chen, G.; Zhu, X.; Wu, F.; Chen, C.; Liu, C.; Shi, Z.; et al. In vitro effects of triclocarban on adipogenesis in murine preadipocyte and human hepatocyte. J. Hazard. Mater. 2020, 122829. [Google Scholar] [CrossRef]
- Li, H.; Zhao, Y.; Chen, L.; Su, Y.; Li, X.; Jin, L.; Ge, R.S. Triclocarban and triclosan inhibit human aromatase via different mechanisms. BioMed Res. Int. 2017, 2017, 8284097. [Google Scholar] [CrossRef]
- Taweetanawanit, P.; Ratpukdi, T.; Siripattanakul-Ratpukdi, S. Performance and kinetics of triclocarban removal by entrapped Pseudomonas fluorescens strain MC46. Bioresour. Technol. 2019, 274, 113–119. [Google Scholar] [CrossRef]
- Kennedy, R.C.; Fling, R.R.; Robeson, M.S.; Saxton, A.M.; Donnell, R.L.; Darcy, J.L.; Bemis, D.A.; Liu, J.; Zhao, L.; Chen, J. Temporal development of gut microbiota in triclocarban exposed pregnant and neonatal rats. Sci. Rep. 2016, 6, 33430. [Google Scholar] [CrossRef]
- Ribado, J.V.; Ley, C.; Haggerty, T.D.; Tkachenko, E.; Bhatt, A.S.; Parsonnet, J. Household triclosan and triclocarban exposure impacts the adult intestinal microbiome but not the infant intestinal microbiome. BioRxiv 2017, 126334. [Google Scholar]
- Pflughoeft, K.J.; Versalovic, J. Human microbiome in health and disease. Annu. Rev. Pathol. 2012, 7, 99–122. [Google Scholar] [CrossRef]
- Wu, X.; Ernst, F.; Conkle, J.L.; Gan, J. Comparative uptake and translocation of pharmaceutical and personal care products (PPCPs) by common vegetables. Environ. Int. 2013, 60, 15–22. [Google Scholar] [CrossRef]
- Macherius, A.; Lapen, D.R.; Reemtsma, T.; Römbke, J.; Topp, E.; Coors, A. Triclocarban, triclosan and its transformation product methyl triclosan in native earthworm species four years after a commercial-scale biosolids application. Sci. Total Environ. 2014, 472, 235–238. [Google Scholar] [CrossRef]
- Andrade, N.A.; Lozano, N.; McConnell, L.L.; Torrents, A.; Rice, C.P.; Ramirez, M. Long-term trends of PBDEs, triclosan, and triclocarban in biosolids from a wastewater treatment plant in the Mid-Atlantic region of the US. J. Hazard. Mater. 2015, 282, 68–74. [Google Scholar] [CrossRef]
- Chen, F.; Ying, G.G.; Ma, Y.B.; Chen, Z.F.; Lai, H.J.; Peng, F.J. Field dissipation and risk assessment of typical personal care products TCC, TCS, AHTN and HHCB in biosolid-amended soils. Sci. Total Environ. 2014, 470, 1078–1086. [Google Scholar] [CrossRef]
- Armstrong, D.L.; Lozano, N.; Rice, C.P.; Ramirez, M.; Torrents, A. Fate of triclosan, triclocarban, and their transformation products in wastewater under nitrifying conditions. J. Water Process Eng. 2019, 28, 144–151. [Google Scholar] [CrossRef]
- Bian, Y.; Wang, D.; Liu, X.; Yang, Q.; Liu, Y.; Wang, Q.; Ni, B.-J.; Li, H.; Zhang, Y. The fate and impact of TCC in nitrifying cultures. Water Res. 2020, 178, 115851. [Google Scholar] [CrossRef]
- Wang, D.; Tao, L.; Yang, J.; Xu, Z.; Yang, Q.; Zhang, Y.; Liu, X.; Liu, Q.; Huang, J. Understanding the interaction between triclocarban and denitrifiers. J. Hazard. Mater. 2021, 401, 123343. [Google Scholar] [CrossRef]
- Chalew, T.E.; Halden, R.U. Environmental exposure of aquatic and terrestrial biota to triclosan and triclocarban. J. Am. Water Works Assoc. 2009, 45, 4–13. [Google Scholar] [CrossRef]
- Brausch, J.M.; Rand, G.M. A review of personal care products in the aquatic environment: Environmental concentrations and toxicity. Chemosphere 2011, 82, 1518–1532. [Google Scholar] [CrossRef] [PubMed]
- Lenz, K.A.; Pattison, C.; Ma, H. Triclosan (TCS) and triclocarban (TCC) induce systemic toxic effects in a model organism the nematode Caenorhabditis elegans. Environ. Pollut. 2017, 231, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Patrick, L. Thyroid disruption: Mmechanism and clinical implications in human health. Altern. Med. Rev. 2009, 14, 326–346. [Google Scholar] [PubMed]
- Wu, Y.; Beland, F.A.; Fang, J.L. Effect of triclosan, triclocarban, 2,20,4,40-tetrabromodiphenyl ether, and bisphenol A on the iodide uptake, thyroid peroxidase activity, and expression of genes involved in thyroid hormone synthesis. Toxicology 2016, 32, 310–319. [Google Scholar]
- Junior, S.F.S.; Vallerie, Q.; de Farias Araujo, G.; Soares, L.O.S.; da Silva, E.O.; Correia, F.V.; Saggioro, E.M. Triclocarban affects earthworms during long-term exposure: Behavior, cytotoxicity, oxidative stress and genotoxicity assessments. Environ. Pollut. 2020, 267, 115570. [Google Scholar] [CrossRef]
- Yawer, A.; Sychrová, E.; Labohá, P.; Raška, J.; Jambor, T.; Babica, P.; Sovadinová, I. Endocrine-disrupting chemicals rapidly affect intercellular signaling in Leydig cells. Toxicol. Appl. Pharmacol. 2020, 404, 115177. [Google Scholar] [CrossRef]
- Sipahutar, M.K.; Vangnai, A.S. Role of plant growth-promoting Ochrobactrum sp. MC22 on triclocarban degradation and toxicity mitigation to legume plants. J. Hazard. Mater. 2017, 329, 38–48. [Google Scholar] [CrossRef]
- Hena, S.; Gutierrez, L.; Croué, J.-P. Removal of pharmaceutical and personal care products (PPCPs) from wastewater using microalgae: A review. J. Hazard. Mater. 2021, 403, 124041. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Kang, D.; Wu, C.; Wu, Y. Removal of pharmaceuticals and personal care products from wastewater using algae-based technologies: A review. Rev. Environ. Sci. Biotechnol. 2017, 16, 717–735. [Google Scholar] [CrossRef]
- Genchi, G.; Lauria, G.; Catalano, A.; Carocci, A.; Sinicropi, M.S. The double face of metals: The intriguing case of chromium. Appl. Sci. 2021, 11, 638. [Google Scholar] [CrossRef]
- Qu, H.; Barrett, H.; Wang, B.; Han, J.; Wang, F.; Gong, W.; Wu, J.; Wang, W.; Yu, G. Co-occurrence of antiseptic triclocarban and chiral anti-inflammatory ibuprofen in environment: Association between biological effect in sediment and risk to human health. J. Hazard. Mater. 2021, 407, 124871. [Google Scholar] [CrossRef]
- Karthikraj, R.; Lee, S.; Kannan, K. Biomonitoring of exposure to bisphenols, benzophenones, triclosan, and triclocarban in pet dogs and cats. Environ. Res. 2020, 180, 108821. [Google Scholar] [CrossRef]
- Tomlin, C.D.S. The Pesticide Manual, 10th ed.; Royal Society of Chemistry: Cambridge, UK; British Crop Protection Council: London, UK, 1995. [Google Scholar]
- Chang, H.C.; Huang, Y.T.; Chen, C.S.; Chen, Y.W.; Huang, Y.T.; Su, J.C.; Teng, L.J.; Shiau, C.W.; Chiu, H.C. In vitro and in vivo activity of a novel sorafenib derivative SC5005 against MRSA. J. Antimicrob. Chemother. 2016, 71, 449–459. [Google Scholar] [CrossRef]
- Le, P.; Kunold, E.; Macsics, R.; Rox, K.; Jennings, M.C.; Ugur, I.; Reinecke, M.; Chaves-Moreno, D.; Hackl, M.W.; Fetzer, C.; et al. Repurposing human kinase inhibitors to create an antibiotic active against drug-resistant Staphylococcus aureus, persisters and biofilms. Nat. Chem. 2020, 12, 145–158. [Google Scholar] [CrossRef]
- Hassan, M.A.; Sayed, G.H.; El-Nagar, A.M.; Hussien, A.M. A convenient synthesis of some diarylurea and thiourea derivatives as antimicrobial compounds. Chem. Process Eng. Res. 2014, 25, 1–11. [Google Scholar]
- Sarveswari, S.; Vijayakumar, V. A Facile Synthesis of diaylureas and their antimicrobial evaluation. Chiang Mai J. Sci. 2018, 45, 997–1007. [Google Scholar]
- Cowan, N.; Dätwyler, P.; Ernst, B.; Wang, C.; Vennerstro, J.L.; Spangenberg, T.; Keiser, J. Activities of N,N′-diarylurea MMV665852 analogs against Schistosoma mansoni. Antimicrob. Agents Chemother. 2015, 59, 1935–1941. [Google Scholar] [CrossRef]
- Wu, J.; Wang, C.; Leas, D.; Vargas, M.; White, K.L.; Shackleford, D.M.; Chen, G.; Sanford, A.G.; Hemsley, R.M.; Davis, P.H.; et al. Progress in antischistosomal N,N′-diaryl urea SAR. Bioorg. Med. Chem. Lett. 2018, 28, 244–248. [Google Scholar] [CrossRef]
- Yao, H.; Liu, F.; Chen, J.; Li, Y.; Cui, J.; Qiao, C. Antischistosomal activity of N,N′-arylurea analogs against Schistosoma japonicum. Bioorg. Med. Chem. Lett. 2016, 26, 1386–1390. [Google Scholar] [CrossRef] [PubMed]
- Ingram-Sieber, K.; Cowan, N.; Panic, G.; Vargas, M.; Mansour, N.R.; Bickle, Q.D.; Wells, T.N.C.; Spangenberg, T.; Keiser, J. Orally active antischistosomal early leads identified from the open access Malaria Box. PLoS Negl. Trop. Dis. 2014, 8, 30. [Google Scholar] [CrossRef]
- Khan, K.M.; Saeed, S.; Ali, M.; Gohar, M.; Zahid, J.; Khan, A.; Perveen, S.; Choudhary, M.I. Unsymmetrically disubstituted urea derivatives: A potent class of antiglycating agents. Bioorg. Med. Chem. 2009, 17, 2447–2451. [Google Scholar] [CrossRef]
- Obukowicz, M.G.; Welsch, D.J.; Salsgiver, W.J.; Martin-Berger, C.L.; Chinn, K.S.; Duffin, K.L.; Raz, A.; Needlemann, P. Novel, selective ∆6 or ∆5 fatty acid desaturase inhibitors as antiinflammatory agents in mice. J. Pharmacol. Exp. Ther. 1998, 287, 157–166. [Google Scholar]
- Rakesh, K.P.; Darshini, N.; Vidhya, S.L.; Mallesha, N. Synthesis and SAR studies of potent H+/K+-ATPase and anti-inflammatory activities of symmetrical and unsymmetrical urea analogues. Med. Chem. Res. 2017, 26, 1675–1681. [Google Scholar] [CrossRef]
- Feng, Z.; Pearce, L.V.; Zhang, Y.; Xing, C.; Herold, B.K.; Ma, S.; Hu, Z.; Turcios, N.A.; Yang, P.; Tong, Q.; et al. Multi-functional diarylurea small molecule inhibitors of TRPV1 with therapeutic potential for neuroinflammation. AAPS J. 2016, 18, 898–913. [Google Scholar] [CrossRef]

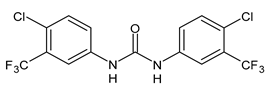 | Flucofuron | MIC = 0.25 mg/L (S. aureus; S. epidermidis) | Chang et al., 2016 [98] |
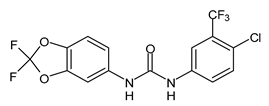 | PK150 | MIC = 0.3 µM (S. aureus) | Le et al., 2020 [110] |
 | 1 | MIC50 = 0.05 µg/mL (S. aureus) | Pujol et al., 2018 [70] |
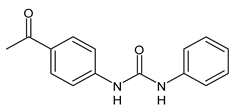 | 2 | Inhibition zones = 14.0 mm (B. subtilis P. aeruginosa. E. coli) | Hassan et al., 2014 [111] |
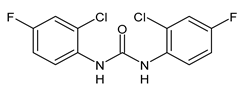 | 3 | Inhibition zone = 23 mm (P. mirabilis) | Sarveswari et al., 2018 [112] |
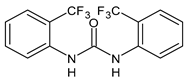 | 4 | Inhibition zone = 24 mm (P. mirabilis) | Sarveswari et al., 2018 [112] |
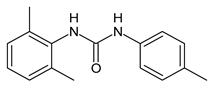 | 5 | MIC = 16 µM (S. aureus) MIC = 32 µM (E. faecalis) | Catalano et al., 2021 [71] |
 | 6 | MIC = 16 µM (S. aureus) MIC = 32 µM (E. faecalis) | Catalano et al., 2021 [71] |
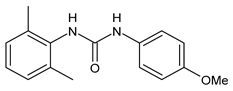 | 7 | MIC = 16 µM (S. aureus) | Catalano et al., 2021 [71] |
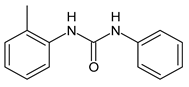 | 8 | MIC = 16 µM (S. aureus) | Catalano et al., 2021 [71] |
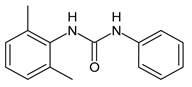 | 9 | MIC = 16 µM (S. aureus) | Catalano et al., 2021 [71] |
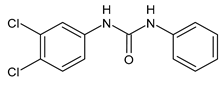 | 10 | MIC = 16 µM (S. aureus) | Catalano et al., 2021 [71] |
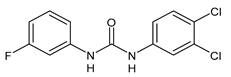 | 11 | IC50 = 1.3 µM (S. mansoni NTS) IC50 = 0.7 µM (adult S. mansoni) | Cowan et al., 2015 [113] |
 | 12 | IC50 = 0.2 µM (adult S. mansoni) | Cowan et al., 2015 [113] |
 | 13 | IC50 = 3.6 µM (adult S. mansoni) | Cowan et al., 2015 [113] |
 | 14 | IC50 = 7.0 µM (adult S. mansoni) | Cowan et al., 2015 [113] |
 | 15 | IC50 = 0.15 µM (S. mansoni NTS) IC50 = 0.19 µM (adult S. mansoni) | Wu et al., 2018 [114] |
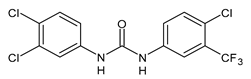 | 16 | IC50 = 2.5 µM (juvenile S. japonicum) IC50 = 1.5 µM (adult S. japonicum) | Yao et al., 2016 [115] |
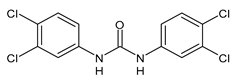 | MMV665852 | IC50 = 4.7 µM (S. mansoni NTS) IC50 = 0.8 µM (adult S. mansoni) IC50 = 4.4 µM (juvenile S. japonicum) IC50 = 2.2 µM (adult S. japonicum) EC50 = 1160 nM (P. falciparum) | Ingram-Sieber et al., 2014 [116] Yao et al., 2016 [115] |
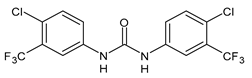 | Flucofuron | IC50 = 2.8 µM (juvenile S. japonicum) IC50 = 1.5 µM (adult S. japonicum) | Yao et al., 2016 [115] |
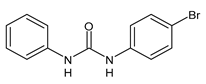 | 17 | C50 = 4.26 µM (antiglycating activity) | Khan et al., 2009 [117] |
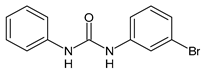 | 18 | IC50 = 4.26 µM (antiglycating activity) | Khan et al., 2009 [117] |
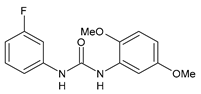 | CP-214339 | IC50 = 0.13 µM (Δ5-desaturase inhibitor in rodents) | Obukowicz et al., 1998 [118] |
 | 19 | IC50 = 18.4 µg/mL (proton pump inhibition) | Rakesh et al., 2017 [119] |
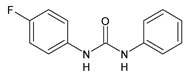 | 20 | IC50 = 20.3 µg/mL (anti-inflammatory activity in human blood) | Rakesh et al., 2017 [119] |
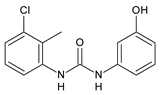 | 21 | Ki = 0.56 µM (TRPV1 capsaicin antagonism) | Feng et al., 2016 [120] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iacopetta, D.; Catalano, A.; Ceramella, J.; Saturnino, C.; Salvagno, L.; Ielo, I.; Drommi, D.; Scali, E.; Plutino, M.R.; Rosace, G.; et al. The Different Facets of Triclocarban: A Review. Molecules 2021, 26, 2811. https://doi.org/10.3390/molecules26092811
Iacopetta D, Catalano A, Ceramella J, Saturnino C, Salvagno L, Ielo I, Drommi D, Scali E, Plutino MR, Rosace G, et al. The Different Facets of Triclocarban: A Review. Molecules. 2021; 26(9):2811. https://doi.org/10.3390/molecules26092811
Chicago/Turabian StyleIacopetta, Domenico, Alessia Catalano, Jessica Ceramella, Carmela Saturnino, Lara Salvagno, Ileana Ielo, Dario Drommi, Elisabetta Scali, Maria Rosaria Plutino, Giuseppe Rosace, and et al. 2021. "The Different Facets of Triclocarban: A Review" Molecules 26, no. 9: 2811. https://doi.org/10.3390/molecules26092811
APA StyleIacopetta, D., Catalano, A., Ceramella, J., Saturnino, C., Salvagno, L., Ielo, I., Drommi, D., Scali, E., Plutino, M. R., Rosace, G., & Sinicropi, M. S. (2021). The Different Facets of Triclocarban: A Review. Molecules, 26(9), 2811. https://doi.org/10.3390/molecules26092811













