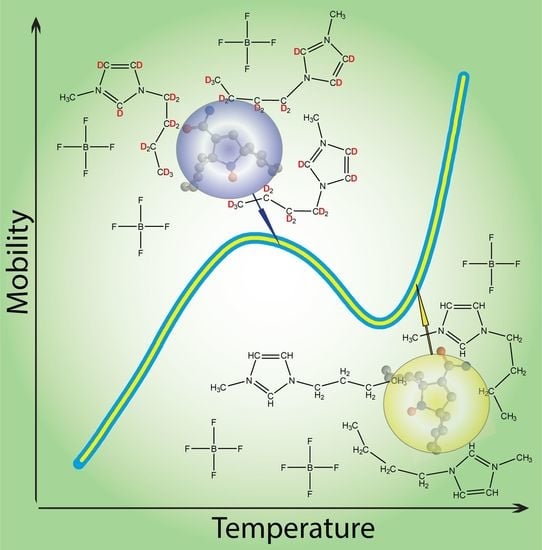
Validation of Structural Grounds for Anomalous Molecular Mobility in Ionic Liquid Glasses
Abstract
:1. Introduction
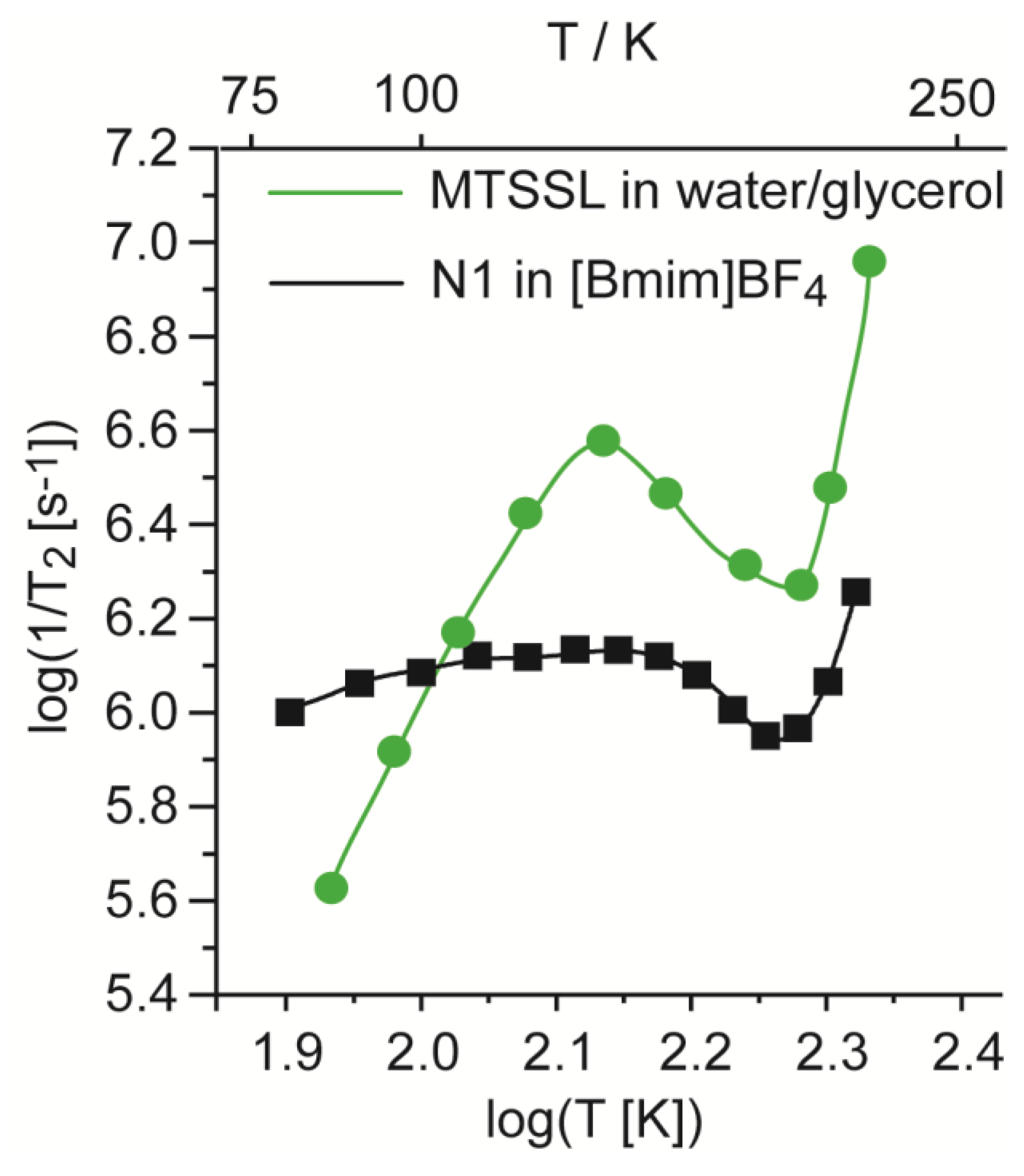
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Xu, C.; Cheng, Z. Thermal stability of ionic liquids: Current status and prospects for future development. Processes 2021, 9, 337. [Google Scholar] [CrossRef]
- Wu, H.B.; Zhang, B.; Liu, S.H.; Chen, C.C. Flammability estimation of 1-hexyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide. J. Loss Prev. Process. Ind. 2020, 66, 104196. [Google Scholar] [CrossRef]
- Barulli, L.; Mezzetta, A.; Brunetti, B.; Guazzelli, L.; Vecchio Ciprioti, S.; Ciccioli, A. Evaporation thermodynamics of the tetraoctylphosphonium bis(trifluoromethansulfonyl)imide([P8888]NTf2) and tetraoctylphosphonium nonafluorobutane-1-sulfonate ([P8888]NFBS) ionic liquids. J. Mol. Liq. 2021, 333. [Google Scholar] [CrossRef]
- Welton, T. Room-Temperature Ionic Liquids. Solvents for Synthesis and Catalysis. Chem. Rev. 1999, 99, 2071–2084. [Google Scholar] [CrossRef]
- Hallett, J.P.; Welton, T. Room-Temperature Ionic Liquids: Solvents for Synthesis and Catalysis. 2. Chem. Rev. 2011, 111, 3508–3576. [Google Scholar] [CrossRef] [PubMed]
- Weingärtner, H. Understanding Ionic Liquids at the Molecular Level: Facts, Problems, and Controversies. Angew. Chem. Int. Ed. 2008, 47, 654–670. [Google Scholar] [CrossRef] [PubMed]
- Welton, T. Ionic liquids: A brief history. Biophys. Rev. 2018, 10, 691–706. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.K.; Savoy, A.W. Ionic liquids synthesis and applications: An overview. J. Mol. Liq. 2020, 297, 112038. [Google Scholar] [CrossRef]
- Zhang, Q.; Cai, S.; Zhang, W.; Lan, Y.; Zhang, X. Density, viscosity, conductivity, refractive index and interaction study of binary mixtures of the ionic liquid 1–ethyl–3–methylimidazolium acetate with methyldiethanolamine. J. Mol. Liq. 2017, 233, 471–478. [Google Scholar] [CrossRef]
- Greaves, T.L.; Drummond, C.J. Protic ionic liquids: Properties and applications. Chem. Rev. 2008, 108, 206–237. [Google Scholar] [CrossRef] [PubMed]
- Nordness, O.; Brennecke, J.F. Ion Dissociation in Ionic Liquids and Ionic Liquid Solutions. Chem. Rev. 2020, 120, 12873–12902. [Google Scholar] [CrossRef]
- Martins, V.L.; Torresi, R.M. Ionic liquids in electrochemical energy storage. Curr. Opin. Electrochem. 2018, 9, 26–32. [Google Scholar] [CrossRef]
- Conrad Zhang, Z. Catalysis in Ionic Liquids. Adv. Catal. 2006, 49, 153–237. [Google Scholar] [CrossRef]
- Mezzetta, A.; Becherini, S.; Pretti, C.; Monni, G.; Casu, V.; Chiappe, C.; Guazzelli, L. Insights into the levulinate-based ionic liquid class: Synthesis, cellulose dissolution evaluation and ecotoxicity assessment. New, J. Chem. 2019, 43, 13010–13019. [Google Scholar] [CrossRef]
- Trujillo-Rodríguez, M.J.; Nan, H.; Varona, M.; Emaus, M.N.; Souza, I.D.; Anderson, J.L. Advances of Ionic Liquids in Analytical Chemistry. Anal. Chem. 2019, 91, 505–531. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, A.; Mukundan, R.; Yang, P.; Batista, E.R. Solubility model of metal complex in ionic liquids from first principle calculations. RSC Adv. 2019, 9, 18506–18526. [Google Scholar] [CrossRef] [Green Version]
- Santos, M.M.; Alves, C.; Silva, J.; Florindo, C.; Costa, A.; Petrovski, Ž.; Marrucho, I.M.; Pedrosa, R.; Branco, L.C. Antimicrobial activities of highly bioavailable organic salts and ionic liquids from fluoroquinolones. Pharmaceutics 2020, 12, 694. [Google Scholar] [CrossRef] [PubMed]
- Marrucho, I.M.; Branco, L.C.; Rebelo, L.P.N. Ionic liquids in pharmaceutical applications. Annu. Rev. Chem. Biomol. Eng. 2014, 5, 527–546. [Google Scholar] [CrossRef]
- Hough, W.L.; Rogers, R.D. Ionic liquids then and now: From solvents to materials to active pharmaceutical ingredients. Bull. Chem. Soc. Jpn. 2007, 80, 2262–2269. [Google Scholar] [CrossRef]
- Egorova, K.S.; Gordeev, E.G.; Ananikov, V.P. Biological Activity of Ionic Liquids and Their Application in Pharmaceutics and Medicine. Chem. Rev. 2017, 117, 7132–7189. [Google Scholar] [CrossRef]
- Wasserscheid, P.; Keim, W. Ionic Liquids—New “Solutions” for Transition Metal Catalysis. Angew. Chem. Int. Ed. 2000, 39, 3772–3789. [Google Scholar] [CrossRef]
- Zhao, D.; Wu, M.; Kou, Y.; Min, E. Ionic liquids: Applications in catalysis. Catal. Today 2002, 74, 157–189. [Google Scholar] [CrossRef]
- Dai, C.; Zhang, J.; Huang, C.; Lei, Z. Ionic Liquids in Selective Oxidation: Catalysts and Solvents. Chem. Rev. 2017, 117, 6929–6983. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Thomas, M.L.; Zhang, S.; Ueno, K.; Yasuda, T.; Dokko, K. Application of Ionic Liquids to Energy Storage and Conversion Materials and Devices. Chem. Rev. 2017, 117, 7190–7239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shang, D.; Liu, X.; Bai, L.; Zeng, S.; Xu, Q.; Gao, H.; Zhang, X. Ionic liquids in gas separation processing. Curr. Opin. Green Sustain. Chem. 2017, 5, 74–81. [Google Scholar] [CrossRef]
- Zhang, M.; Ettelaie, R.; Yan, T.; Zhang, S.; Cheng, F.; Binks, B.P.; Yang, H. Ionic Liquid Droplet Microreactor for Catalysis Reactions Not at Equilibrium. J. Am. Chem. Soc. 2017, 139, 17387–17396. [Google Scholar] [CrossRef] [PubMed]
- Chiappe, C.; Mezzetta, A.; Pomelli, C.S.; Puccini, M.; Seggiani, M. Product as Reaction Solvent: An Unconventional Approach for Ionic Liquid Synthesis. Org. Process. Res. Dev. 2016, 20, 2080–2084. [Google Scholar] [CrossRef]
- Wang, Y.-L.L.; Li, B.; Sarman, S.; Mocci, F.; Lu, Z.Y.; Yuan, J.; Laaksonen, A.; Fayer, M.D. Microstructural and Dynamical Heterogeneities in Ionic Liquids. Chem. Rev. 2020, 120, 5798–5877. [Google Scholar] [CrossRef] [Green Version]
- Hayes, R.; Warr, G.G.; Atkin, R. Structure and Nanostructure in Ionic Liquids. Chem. Rev. 2015, 115, 6357–6426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Gao, S.; Wang, J.; Tang, J. Aggregation of Ionic Liquids [ C n mim ] Br ( n ) 4, 6, 8, 10, 12 ) in D 2 O: A NMR Study. J. Phys. Chem. B 2008, 2031–2039. [Google Scholar]
- Li, S.; Bañuelos, J.L.; Zhang, P.; Feng, G.; Dai, S.; Rother, G.; Cummings, P.T. Toward understanding the structural heterogeneity and ion pair stability in dicationic ionic liquids. Soft Matter 2014, 10, 9193–9200. [Google Scholar] [CrossRef]
- Sha, M.; Liu, Y.; Dong, H.; Luo, F.; Jiang, F.; Tang, Z.; Zhu, G.; Wu, G. Origin of heterogeneous dynamics in local molecular structures of ionic liquids. Soft Matter 2016, 12, 8942–8949. [Google Scholar] [CrossRef]
- Zheng, Z.P.; Fan, W.H.; Roy, S.; Mazur, K.; Nazet, A.; Buchner, R.; Bonn, M.; Hunger, J. Ionic liquids: Not only structurally but also dynamically heterogeneous. Angew. Chem. Int. Ed. 2015, 54, 687–690. [Google Scholar] [CrossRef]
- Usui, K.; Hunger, J.; Bonn, M.; Sulpizi, M. Dynamical heterogeneities of rotational motion in room temperature ionic liquids evidenced by molecular dynamics simulations. J. Chem. Phys. 2018, 148. [Google Scholar] [CrossRef] [PubMed]
- Canongia Lopes, J.N.A.; Pádua, A.A.H. Nanostructural organization in ionic liquids. J. Phys. Chem. B 2006, 110, 3330–3335. [Google Scholar] [CrossRef] [PubMed]
- Urahata, S.M.; Ribeiro, M.C.C. Unraveling Dynamical Heterogeneity in the Ionic Liquid 1-Butyl-3-methylimidazolium Chloride. J. Phys. Chem. Lett. 2010, 1, 1738–1742. [Google Scholar] [CrossRef]
- Wang, Y.; Voth, G.A. Unique spatial heterogeneity in ionic liquids. J. Am. Chem. Soc. 2005, 127, 12192–12193. [Google Scholar] [CrossRef]
- Triolo, A.; Russina, O.; Bleif, H.-J.; Di Cola, E. Nanoscale Segregation in Room Temperature Ionic Liquids. J. Phys. Chem. B 2007, 111, 4641–4644. [Google Scholar] [CrossRef]
- Triolo, A.; Russina, O.; Fazio, B.; Appetecchi, G.B.; Carewska, M.; Passerini, S. Nanoscale organization in piperidinium-based room temperature ionic liquids. J. Chem. Phys. 2009, 130, 164521. [Google Scholar] [CrossRef]
- Russina, O.; Triolo, A. New experimental evidence supporting the mesoscopic segregation model in room temperature ionic liquids. Faraday Discuss. 2012, 154, 97–109. [Google Scholar] [CrossRef]
- Russina, O.; Triolo, A.; Gontrani, L.; Caminiti, R. Mesoscopic Structural Heterogeneities in Room-Temperature Ionic Liquids. J. Phys. Chem. Lett. 2012, 3, 27–33. [Google Scholar] [CrossRef]
- Triolo, A.; Russina, O.; Arrighi, V.; Juranyi, F.; Janssen, S.; Gordon, C.M. Quasielastic neutron scattering characterization of the relaxation processes in a room temperature ionic liquid. J. Chem. Phys. 2003, 119, 8549–8557. [Google Scholar] [CrossRef] [Green Version]
- Russina, O.; Triolo, A.; Aihara, Y.; Telling, M.T.F.; Grimm, H. Quasi-elastic neutron scattering investigation of dynamics in polymer electrolytes. Macromolecules 2004, 37, 8653–8660. [Google Scholar] [CrossRef] [Green Version]
- Seitkalieva, M.M.; Grachev, A.A.; Egorova, K.S.; Ananikov, V.P. Nanoscale organization of ionic liquids and their interaction with peptides probed by 13C NMR spectroscopy. Tetrahedron 2014, 70, 6075–6081. [Google Scholar] [CrossRef]
- Griffin, P.J.; Holt, A.P.; Tsunashima, K.; Sangoro, J.R.; Kremer, F.; Sokolov, A.P. Ion transport and structural dynamics in homologous ammonium and phosphonium-based room temperature ionic liquids. J. Chem. Phys. 2015, 142. [Google Scholar] [CrossRef]
- Marekha, B.A.; Kalugin, O.N.; Bria, M.; Idrissi, A. Probing structural patterns of ion association and solvation in mixtures of imidazolium ionic liquids with acetonitrile by means of relative 1H and 13C NMR chemical shifts. Phys. Chem. Chem. Phys. 2015, 17, 23183–23194. [Google Scholar] [CrossRef]
- Mladenova, B.Y.; Kattnig, D.R.; Grampp, G. Room-temperature ionic liquids discerned via nitroxyl spin probe dynamics. J. Phys. Chem. B 2011, 115, 8183–8198. [Google Scholar] [CrossRef] [PubMed]
- Mladenova, B.Y.; Chumakova, N.A.; Pergushov, V.I.; Kokorin, A.I.; Grampp, G.; Kattnig, D.R. Rotational and translational diffusion of spin probes in room-temperature ionic liquids. J. Phys. Chem. B 2012, 116, 12295–12305. [Google Scholar] [CrossRef] [PubMed]
- Kundu, K.; Kattnig, D.R.; Mladenova, B.Y.; Grampp, G.; Das, R. Electron spin-lattice relaxation mechanisms of nitroxyl radicals in ionic liquids and conventional organic liquids: Temperature dependence of a thermally activated process. J. Phys. Chem. B 2015, 119, 4501–4511. [Google Scholar] [CrossRef] [PubMed]
- Stoesser, R.; Herrmann, W.; Zehl, A.; Strehmel, V.; Laschewsky, A. ESR spin probes in ionic liquids. ChemPhysChem 2006, 7, 1106–1111. [Google Scholar] [CrossRef]
- Strehmel, V.; Laschewsky, A.; Stoesser, R.; Zehl, A.; Herrmann, W. Mobility of spin probes in ionic liquids. J. Phys. Org. Chem. 2006, 19, 318–325. [Google Scholar] [CrossRef]
- Strehmel, V. Radicals in Ionic Liquids. ChemPhysChem 2012, 13, 1649–1663. [Google Scholar] [CrossRef]
- Ivanov, M.Y.; Veber, S.L.; Prikhod’ko, S.A.; Adonin, N.Y.; Bagryanskaya, E.G.; Fedin, M.V.; Prikhod’ko, S.A.; Adonin, N.Y.; Bagryanskaya, E.G.; Fedin, M.V. Probing Microenvironment in Ionic Liquids by Time-Resolved EPR of Photoexcited Triplets. J. Phys. Chem. B 2015, 119, 13440–13449. [Google Scholar] [CrossRef]
- Ivanov, M.Y.; Krumkacheva, O.A.; Dzuba, S.A.; Fedin, M.V. Microscopic rigidity and heterogeneity of ionic liquids probed by stochastic molecular librations of the dissolved nitroxides. Phys. Chem. Chem. Phys. 2017, 19, 26158–26163. [Google Scholar] [CrossRef] [Green Version]
- Ivanov, M.Y.; Prikhod’Ko, S.A.; Adonin, N.Y.; Bagryanskaya, E.G.; Fedin, M.V. Influence of C2-Methylation of Imidazolium Based Ionic Liquids on Photoinduced Spin Dynamics of the Dissolved ZnTPP Studied by Time-Resolved EPR. Z. Phys. Chem. 2017, 231, 391–404. [Google Scholar] [CrossRef]
- Ivanov, M.Y.; Prikhod’ko, S.A.; Adonin, N.Y.; Kirilyuk, I.A.; Adichtchev, S.V.; Surovtsev, N.V.; Dzuba, S.A.; Fedin, M.V.; Prikhod’Ko, S.A.; Adonin, N.Y.; et al. Structural Anomalies in Ionic Liquids near the Glass Transition Revealed by Pulse EPR. J. Phys. Chem. Lett. 2018, 9, 4607–4612. [Google Scholar] [CrossRef]
- Ivanov, M.Y.; Fedin, M.V. Nanoscale heterogeneities in ionic liquids: Insights from EPR of spin probes. Mendeleev Commun. 2018, 28, 565–573. [Google Scholar] [CrossRef]
- Kuzhelev, A.A.; Krumkacheva, O.A.; Ivanov, M.Y.; Prikhod’Ko, S.A.; Adonin, N.Y.; Tormyshev, V.M.; Bowman, M.K.; Fedin, M.V.; Bagryanskaya, E.G.; Prikhod’ko, S.A.; et al. Pulse EPR of Triarylmethyl Probes: A New Approach for the Investigation of Molecular Motions in Soft Matter. J. Phys. Chem. B 2018, 122, 8624–8630. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, M.Y.; Prikhod’ko, S.A.; Adonin, N.Y.; Fedin, M.V.; Prikhod’Ko, S.A.; Adonin, N.Y.; Fedin, M.V. Structural Anomalies in Binary Mixtures of Ionic Liquid [Bmim]BF 4 with Water Studied by EPR. J. Phys. Chem. B 2019, 123, 9956–9962. [Google Scholar] [CrossRef]
- Ivanov, M.Y.; Poryvaev, A.S.; Polyukhov, D.M.; Prikhod’ko, S.A.; Adonin, N.Y.; Fedin, M.V. Nanoconfinement effects on structural anomalies in imidazolium ionic liquids. Nanoscale 2020, 12, 23480–23487. [Google Scholar] [CrossRef]
- Bakulina, O.D.; Ivanov, M.Y.; Prikhod’ko, S.A.; Pylaeva, S.; Zaytseva, I.V.; Surovtsev, N.V.; Adonin, N.Y.; Fedin, M.V. Nanocage formation and structural anomalies in imidazolium ionic liquid glasses governed by alkyl chains of cations. Nanoscale 2020, 12, 19982–19991. [Google Scholar] [CrossRef]
- Dzuba, S.A. Libration motion of guest spin probe molecules in organic glasses: CW EPR and electron spin echo study. Spectrochim. Acta. Part. A Mol. Biomol. Spectrosc. 2000, 56, 227–234. [Google Scholar] [CrossRef]
- Rajca, A.; Kathirvelu, V.; Roy, S.K.; Pink, M.; Rajca, S.; Sarkar, S.; Eaton, S.S.; Eaton, G.R. A Spirocyclohexyl Nitroxide Amino Acid Spin Label for Pulsed EPR Spectroscopy Distance Measurements. Chem. A Eur. J. 2010, 16, 5778–5782. [Google Scholar] [CrossRef] [Green Version]
- Zecevic, A.; Eaton, G.R.; Eaton, S.S.; Lindgren, M. Dephasing of electron spin echoes for nitroxyl radicals in glassy solvents by non-methyl and methyl protons. Mol. Phys. 1998, 95, 1255–1263. [Google Scholar] [CrossRef]
- Sato, H.; Kathirvelu, V.; Fielding, A.; Blinco, J.P.; Micallef, A.S.; Bottle, S.E.; Eaton, S.S.; Eaton, G.R. Impact of molecular size on electron spin relaxation rates of nitroxyl radicals in glassy solvents between 100 and 300 K. Mol. Phys. 2007, 105, 2137–2151. [Google Scholar] [CrossRef] [Green Version]
- Kirilyuk, I.A.; Polienko, Y.F.; Krumkacheva, O.A.; Strizhakov, R.K.; Gatilov, Y.V.; Grigor’ev, I.A.; Bagryanskaya, E.G. Synthesis of 2,5-bis(spirocyclohexane)-substituted nitroxides of pyrroline and pyrrolidine series, including thiol-specific spin label: An analogue of MTSSL with long relaxation time. J. Org. Chem. 2012, 77, 8016–8027. [Google Scholar] [CrossRef] [PubMed]
- Kathirvelu, V.; Smith, C.; Parks, C.; Mannan, M.A.; Miura, Y.; Takeshita, K.; Eaton, S.S.; Eaton, G.R. Relaxation rates for spirocyclohexyl nitroxyl radicals are suitable for interspin distance measurements at temperatures up to about 125 K. Chem. Commun. 2009, 454–456. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Paletta, J.T.; Elajaili, H.; Huber, K.; Pink, M.; Rajca, S.; Eaton, G.R.; Eaton, S.S.; Rajca, A. Synthesis and Electron Spin Relaxation of Tetracarboxylate Pyrroline Nitroxides. J. Org. Chem. 2017, 82, 1538–1544. [Google Scholar] [CrossRef] [PubMed]
- Eaton, S.S.; Eaton, G.R. Relaxation Times of Organic Radicals and Transition Metal Ions BT—Distance Measurements in Biological Systems by EPR; Berliner, L.J., Eaton, G.R., Eaton, S.S., Eds.; Springer US: Boston, MA, USA, 2000; Volume 19, pp. 29–154. ISBN 978-0-306-47109-4. [Google Scholar]
- Soetbeer, J.; Hülsmann, M.; Godt, A.; Polyhach, Y.; Jeschke, G. Dynamical decoupling of nitroxides in: O-terphenyl: A study of temperature, deuteration and concentration effects. Phys. Chem. Chem. Phys. 2018, 20, 1615–1628. [Google Scholar] [CrossRef]
- Goslar, J.; Hoffmann, S.K.; Lijewski, S. Dynamics of 4-oxo-TEMPO-d16-15N nitroxide-propylene glycol system studied by ESR and ESE in liquid and glassy state in temperature range 10-295 K. J. Magn. Reson. 2016, 269, 162–175. [Google Scholar] [CrossRef]
- Paschenko, S.V.; Toropov, Y.V.; Dzuba, S.A.; Tsvetkov, Y.D.; Vorobiev, A.K. Temperature dependence of amplitudes of libration motion of guest spin-probe molecules in organic glasses. J. Chem. Phys. 1999, 110, 8150–8154. [Google Scholar] [CrossRef]
- Dzuba, S.A.; Kirilina, E.P.; Salnikov, E.S. On the possible manifestation of harmonic-anharmonic dynamical transition in glassy media in electron paramagnetic resonance of nitroxide spin probes. J. Chem. Phys. 2006, 125, 054502. [Google Scholar] [CrossRef]
- Kirilina, E.P.; Grigoriev, I.A.; Dzuba, S.A. Orientational motion of nitroxides in molecular glasses: Dependence on the chemical structure, on the molecular size of the probe, and on the type of the matrix. J. Chem. Phys. 2004, 121, 12465–12471. [Google Scholar] [CrossRef]
- Golysheva, E.A.; Shevelev, G.Y.; Dzuba, S.A. Dynamical transition in molecular glasses and proteins observed by spin relaxation of nitroxide spin probes and labels. J. Chem. Phys. 2017, 147, 064501. [Google Scholar] [CrossRef]
- Isaev, N.P.; Dzuba, S.A. Fast Stochastic Librations and Slow Rotations of Spin Labeled Stearic Acids in a Model Phospholipid Bilayer at Cryogenic Temperatures. J. Phys. Chem. B 2008, 112, 13285–13291. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Lu, X.; Zhou, Q.; Li, X.; Zhang, X.; Li, S. Ionic Liquids; Elsevier: Amsterdam, The Netherlands, 2009; ISBN 9780444534279. [Google Scholar]
- Greaves, T.L.; Drummond, C.J. Solvent nanostructure, the solvophobic effect and amphiphile self-assembly in ionic liquids. Chem. Soc. Rev. 2013, 42, 1096–1120. [Google Scholar] [CrossRef] [PubMed]
- Golysheva, E.A.; Samoilova, R.I.; De Zotti, M.; Toniolo, C.; Formaggio, F.; Dzuba, S.A. Electron spin echo detection of stochastic molecular librations: Non-cooperative motions on solid surface. J. Magn. Reson. 2019, 309, 106621. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanov, M.Y.; Prikhod’ko, S.A.; Bakulina, O.D.; Kiryutin, A.S.; Adonin, N.Y.; Fedin, M.V. Validation of Structural Grounds for Anomalous Molecular Mobility in Ionic Liquid Glasses. Molecules 2021, 26, 5828. https://doi.org/10.3390/molecules26195828
Ivanov MY, Prikhod’ko SA, Bakulina OD, Kiryutin AS, Adonin NY, Fedin MV. Validation of Structural Grounds for Anomalous Molecular Mobility in Ionic Liquid Glasses. Molecules. 2021; 26(19):5828. https://doi.org/10.3390/molecules26195828
Chicago/Turabian StyleIvanov, Mikhail Yu., Sergey A. Prikhod’ko, Olga D. Bakulina, Alexey S. Kiryutin, Nicolay Yu. Adonin, and Matvey V. Fedin. 2021. "Validation of Structural Grounds for Anomalous Molecular Mobility in Ionic Liquid Glasses" Molecules 26, no. 19: 5828. https://doi.org/10.3390/molecules26195828
APA StyleIvanov, M. Y., Prikhod’ko, S. A., Bakulina, O. D., Kiryutin, A. S., Adonin, N. Y., & Fedin, M. V. (2021). Validation of Structural Grounds for Anomalous Molecular Mobility in Ionic Liquid Glasses. Molecules, 26(19), 5828. https://doi.org/10.3390/molecules26195828