A Non-Covalent Dimer Formation of Quaternary Ammonium Cation with Unusual Charge Neutralization in Electrospray-Ionization Mass Spectrometry
Abstract
:1. Introduction
2. Results and Discussion
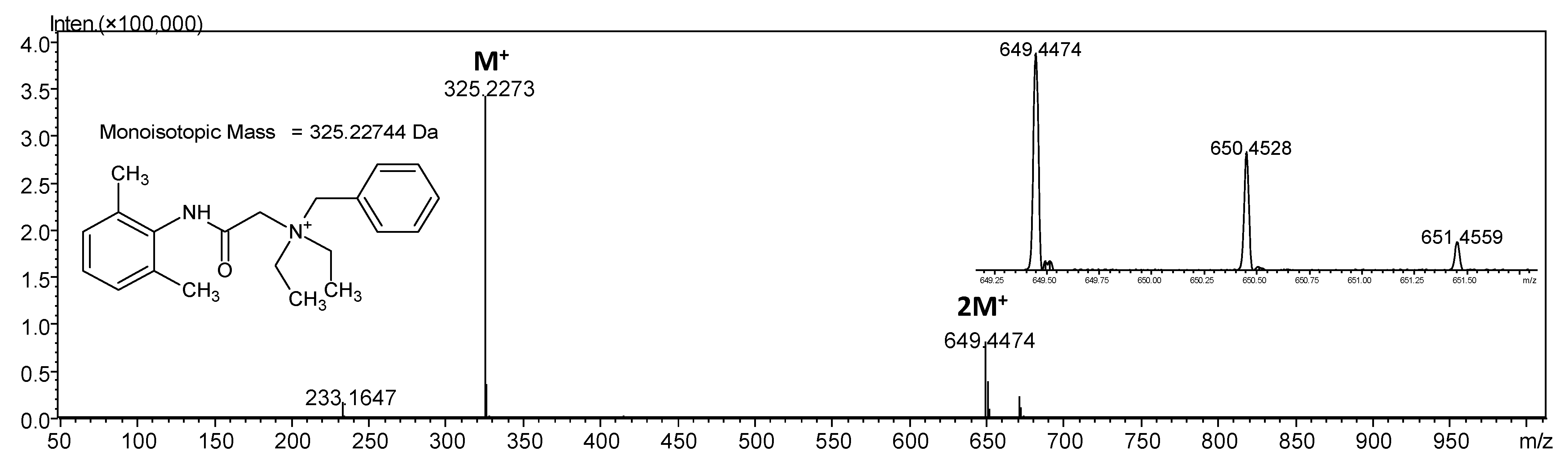
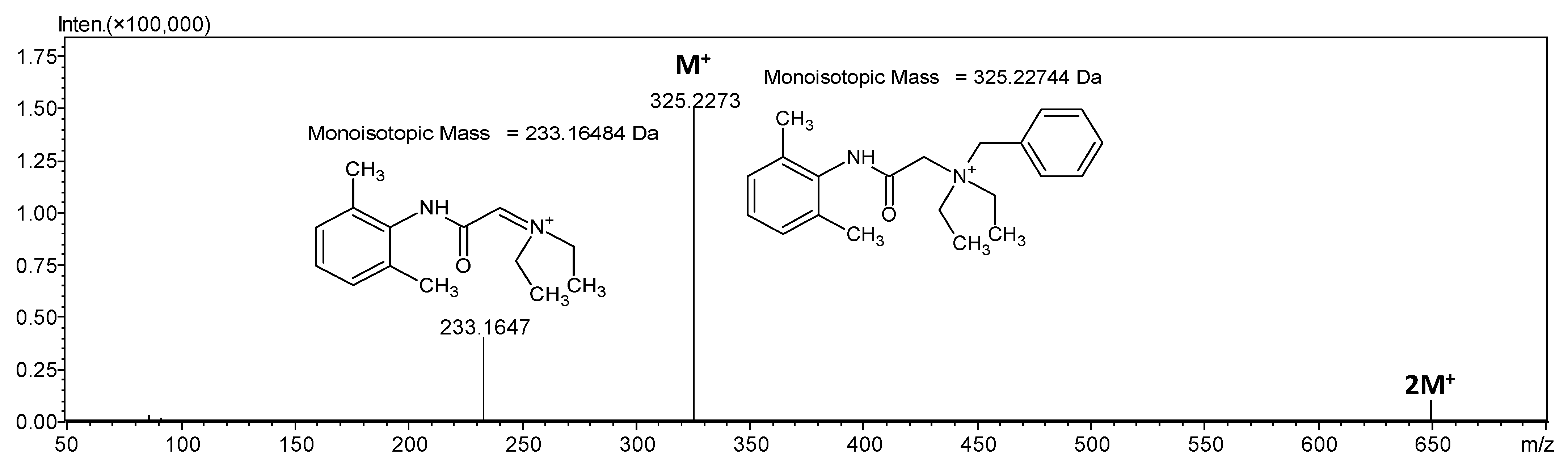
2.1. Mass Spectrometry Analysis
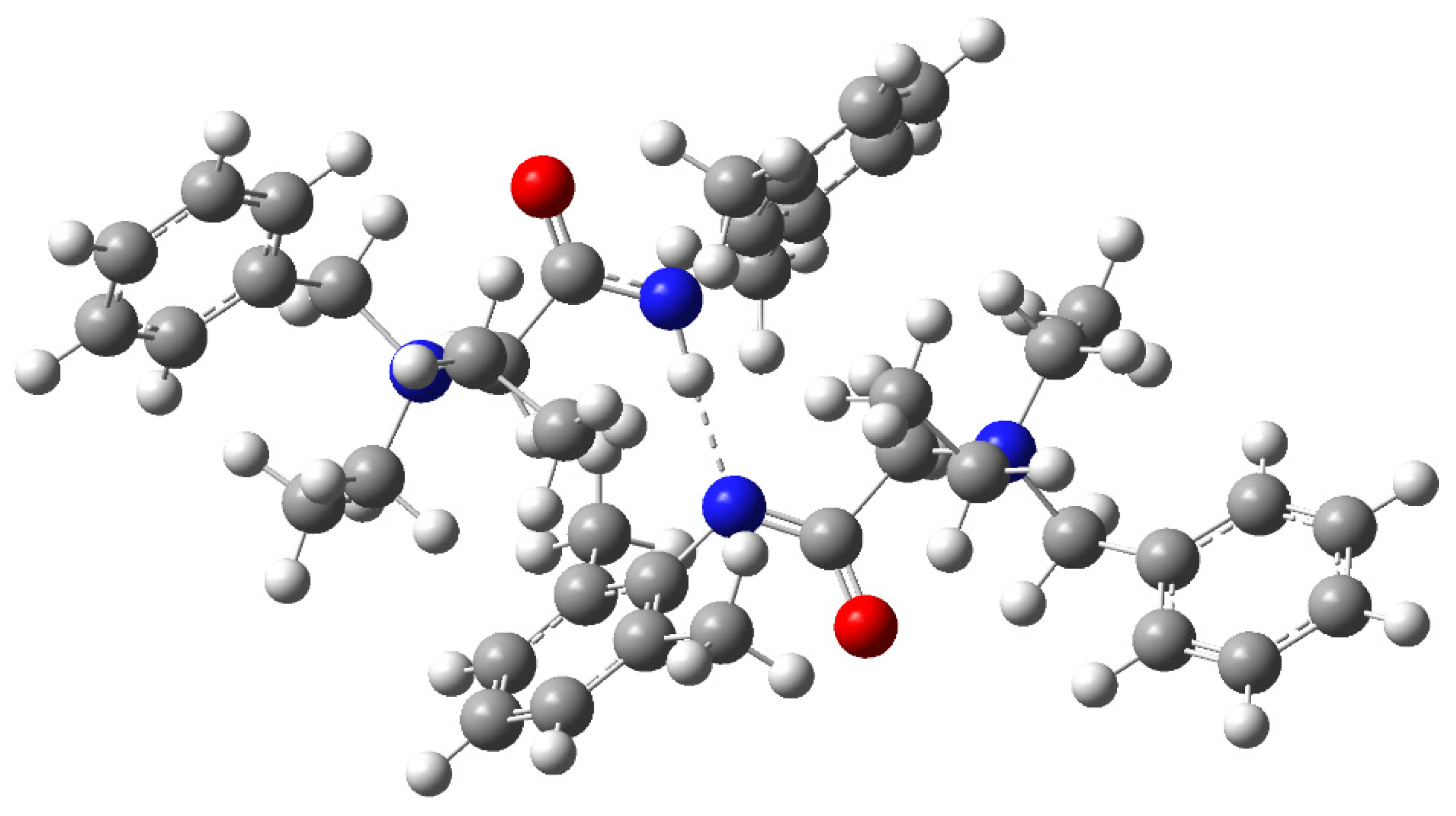
2.2. Computational Analysis
3. Materials and Methods
3.1. Chemicals
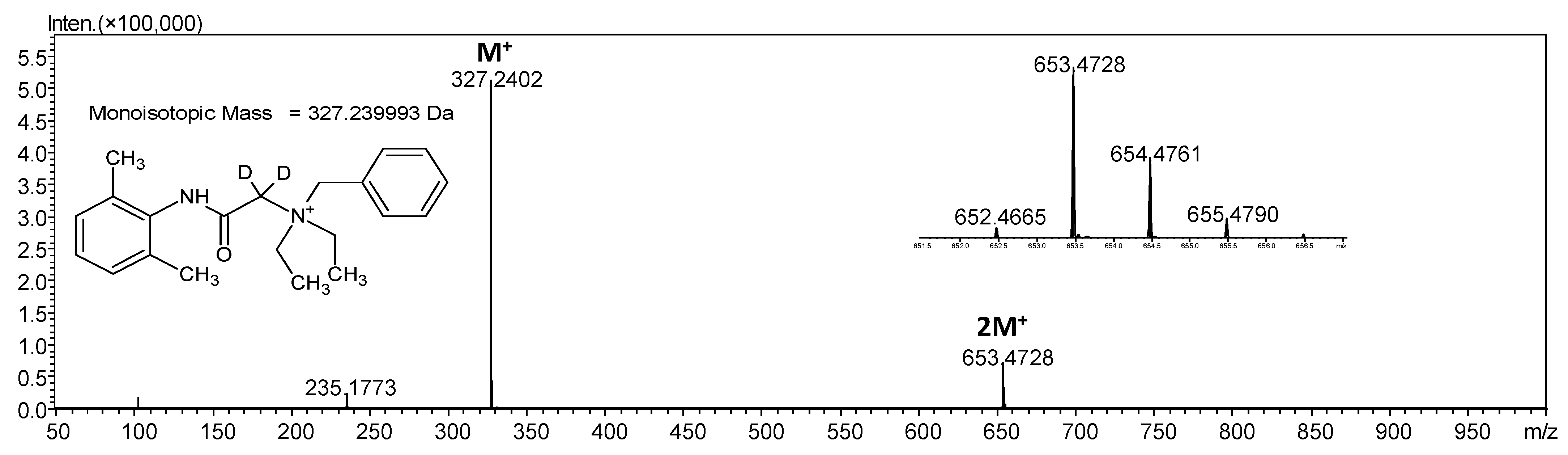
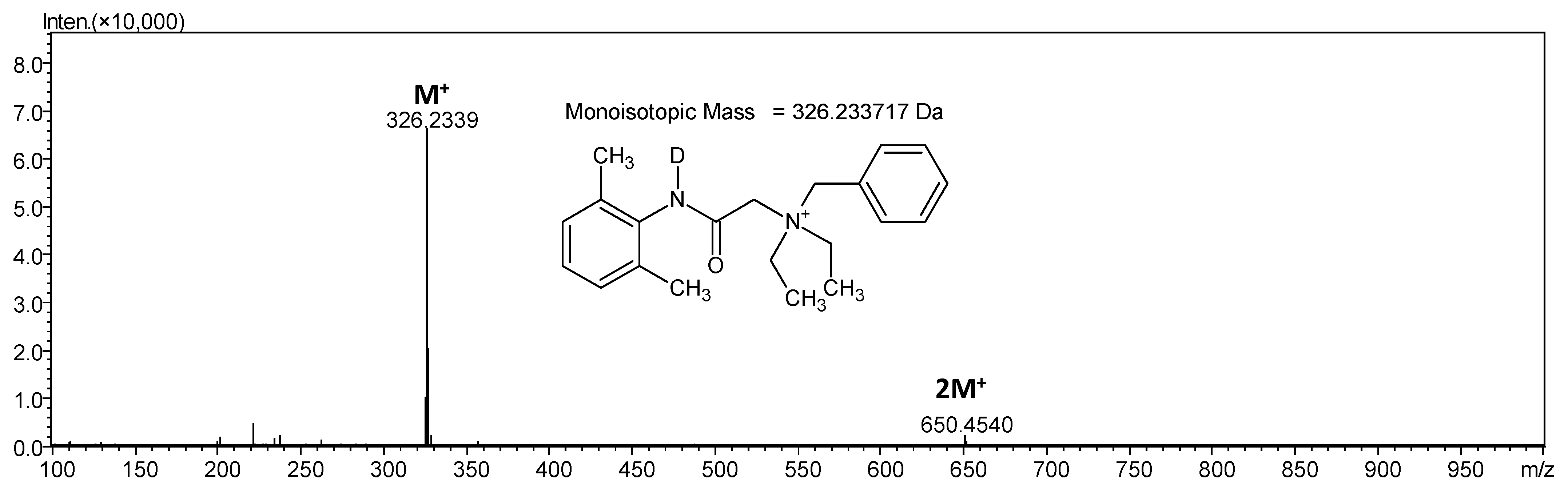
3.2. Isotopic Exchange
3.3. Mass Spectrometry
3.4. Computational Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Covey, T.R.; Bonner, R.F.; Shushan, B.I.; Henion, J.D. The determination of protein, oligonucleotide and peptide molecular weights by ion-spray mass spectrometry. Rapid Commun. Mass Spectrom. 1988, 2, 249–256. [Google Scholar] [CrossRef]
- Sallam, S.; Dolog, I.; Paik, B.A.; Jia, X.; Kiick, K.K.; Wesdemiotis, C. Sequence and conformational analysis of peptide–polymer bioconjugates by multidimensional mass spectrometry. Biomacromolecules 2018, 19, 1498–1507. [Google Scholar] [CrossRef] [PubMed]
- Fenn, J.B.; Mann, M.; Meng, C.K.; Wong, S.F.; Whitehouse, C.M. Electrospray ionization–principles and practice. Mass Spectrom. Rev. 1990, 9, 37–70. [Google Scholar] [CrossRef]
- Heck, A.J.R.; van del Heuvel, R.H.H. Investigation of intact protein complexes by mass spectrometry. Mass Spectrom. Rev. 2004, 23, 368–389. [Google Scholar] [CrossRef] [PubMed]
- Song, F. A Study of noncovalent protein complexes by matrix-assisted laser desorption/ionization. J. Am. Soc. Mass Spectrom. 2007, 18, 1286–1290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganguly, A.K.; Pramanik, B.N.; Tsarbopoulos, A.; Covey, T.R.; Huang, E.; Fuhrman, S.A. Mass spectrometric detection of the noncovalent GDP-bound conformational state of the human H-ras protein. J. Amer. Chem. Soc. 1992, 114, 6559–6560. [Google Scholar] [CrossRef]
- Ganem, B.; Li, Y.-T.; Henion, J.D. Detection of oligonucleotide duplex forms by ion-spray mass spectrometry. Tetrahedron Lett. 1993, 34, 1445–1448. [Google Scholar] [CrossRef]
- Loo, J.A.; Holsworth, D.D.; Root-Bernstein, R.S. Use of electrospray ionization mass spectrometry to probe antisense peptide interactions. Biol. Mass Spectrom. 1994, 23, 6–12. [Google Scholar] [CrossRef]
- Jiang, H.; Somogyi, A.; Timmermann, B.N.; Gang, D.R. Analysis of curcuminoids by positive and negative electrospray ionization and tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2006, 20, 1001–1012. [Google Scholar] [CrossRef]
- Milliet, A.; Rempp, M.; Sozzi, G. Formation of a stable ‘proton-bridged dimer’ by decomposition of CH3CH(OC2H5)CH(OC2H5)CH3+• in a mass spectrometer. J. Mass Spectrom. 1996, 31, 749–755. [Google Scholar] [CrossRef]
- Pen, H. A non-covalent dimer formed in electrospray ionisation mass spectrometry behaving as a precursor for fragmentations. Rapid Commun. Mass Spectrom. 2008, 22, 3555–3560. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, H.; Regnier, F. Enhancing electrospray ionization efficiency of peptides by derivatization. Anal. Chem. 2006, 78, 4175–4183. [Google Scholar] [CrossRef]
- Bąchor, R.; Mielczarek, P.; Rudowska, M.; Silberring, J.; Szewczuk, Z. Sensitive detection of charge derivatized peptides at the attomole level using nano-LC-ESI-MRM analysis. Int. J. Mass Spectrom. 2014, 362, 32–38. [Google Scholar] [CrossRef]
- Bąchor, R.; Waliczek, M.; Stefanowicz, P.; Szewczuk, Z. Trends in the design of new isobaric labeling reagents for quantitative proteomics. Molecules 2019, 24, 701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bąchor, R.; Gąszczyk, D.; Panek-Laszczyńska, K.; Konieczny, A.; Witkiewicz, W.; Stefanowicz, P.; Szewczuk, Z. Detection of podocin in human urine sediment samples by charge derivatization and LC-MS-MRM method. Int. J. Mol.Sci. 2020, 21, 3225. [Google Scholar] [CrossRef] [PubMed]
- Cydzik, M.; Rudowska, M.; Stefanowicz, P.; Szewczuk, Z. The competition of charge remote and charge directed fragmentation mechanisms in quaternary ammonium salt derivatized peptides—An isotopic exchange study. J. Am. Soc. Mass Spectrom. 2011, 22, 2103–2107. [Google Scholar] [CrossRef] [Green Version]
- D’Arcy, P.F.; Taylor, E.P. Quaternary Ammonium Compounds in Medicinal Chemistry. I. J. Pharm. Pharmacol. 1962, 14, 129–146. [Google Scholar] [CrossRef]
- Bąchor, R.; Kluczyk, A.; Stefanowicz, P.; Szewczuk, Z. Facile synthesis of deuterium-labeled denatonium cation and its application in the quantitative analysis of Bitrex by liquid chromatography-mass spectrometry. Anal. Bioanal. Chem. 2015, 407, 6557–6561. [Google Scholar] [CrossRef] [Green Version]
- Ng, L.-K.; Hupe, M.; Harnois, J.; Lawrence, A.H. Direct injection gas chromatographic/mass spectrometric analysis for denatonium benzoate in specific denatured alcohol formulations. Anal. Chem. 1998, 70, 4389–4393. [Google Scholar] [CrossRef]
- Rudowska, M.; Wojewska, D.; Kluczyk, A.; Bachor, R.; Stefanowicz, P.; Szewczuk, Z. The hydrogen-deuterium exchange at α-carbon atom in N,N,N-trialkylglycine residue: ESI-MS studies. J. Am. Soc. Mass. Spectrom. 2012, 23, 1024–1028. [Google Scholar] [CrossRef] [Green Version]
- Bąchor, R.; Rudowska, M.; Kluczyk, A.; Stefanowicz, P.; Szewczuk, Z. Hydrogen-deuterium exchange of α-carbon protons and fragmentation pathways in N-methylated glycine and alanine-containing peptides derivatized by quaternary ammonium salts. J. Mass Spectrom. 2014, 49, 529–536. [Google Scholar] [CrossRef]
- Grocholska, P.; Bąchor, R. Trends in the hydrogen−deuterium exchange at the carbon centers. Preparation of internal standards for quantitative analysis by LC-MS. Molecules 2021, 26, 2989. [Google Scholar] [CrossRef] [PubMed]
- Mielke, Z.; Latajka, Z.; Olbert-Majkut, A.; Wieczorek, R. Matrix infrared spectra and ab initio calculations of the nitrous acid complexes with nitrogen monoxide. J. Phys. Chem. A 2000, 104, 3764–3769. [Google Scholar] [CrossRef]
- Wieczorek, R.; Latajka, Z.; Lundell, J. Quantum chemical study of the bimolecular complex of HONO. J. Phys.Chem. A 1999, 103, 6234–6239. [Google Scholar] [CrossRef]
- Olszewski, T.K.; Wojaczyńska, E.; Wieczorek, R.; Bąkowicz, J. α-Hydroxyphosphonic acid derivatives of 2-azanorbornane: Synthesis, DFT calculations, and crystal structure analysis. Tetrahedron-Asymmetry 2015, 26, 601–607. [Google Scholar] [CrossRef]
- Salvador, P.; Wieczorek, R.; Dannenberg, J.J. Direct calculation oftranshydrogen-bond 13C-15N 3-bond J-couplings in entire polyalanine α-helices. A density functional theory study. J. Phys. Chem. B 2007, 111, 2398–2403. [Google Scholar] [CrossRef] [PubMed]
- Rudowska, M.; Wieczorek, R.; Kluczyk, A.; Stefanowicz, P.; Szewczuk, Z. Gas-phase fragmentaargtion of oligoproline peptide ions lacking easily mobilizable protons. J. Am. Soc. Mass Spectrom. 2013, 24, 846–856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desiraju, G.; Steiner, T. The Weak Hydrogen Bond: In Structural Chemistry and Biology; International Union of Crystallography; Reprint edition; Oxford University Press: Oxford, UK, 2001; ISBN 9780198509707. [Google Scholar]
- Eswar, N.; Ramakrishnan, C. Deterministic features of sidechain main-chain hydrogen bonds in globular protein structures. Protein Eng. 2000, 13, 227–238. [Google Scholar] [CrossRef] [Green Version]
- Krishna Deepak, R.N.V.; Sankararamakrishna, R. Unconventional N-H...N hydrogen bonds involving proline backbone nitrogen in protein structures. Biophys. J. 2016, 110, 1967–1979. [Google Scholar] [CrossRef] [Green Version]
- Steiner, T.; Koellner, G. Hydrogen bonds with π-acceptors in proteins: Frequencies and role in stabilizing local 3D structures. J. Mol. Biol. 2001, 305, 535–557. [Google Scholar] [CrossRef]
- Perrin, C.L.; Ohta, B.K. Symmetry of O-H-O and N-H-N hydrogen bonds in 6-hydroxy-2-formylfulvene and 6-aminofulvene-2-aldimines. Bioorg. Chem. 2002, 30, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Sigh, K.S.; Das, A. Competition between n→π(Ar)* and conventional hydrogen bonding (N-H...N) interactions: An ab initio study of the complexes of 7-azaindole and fluorosubstituted pyridines. Phys. Chem. Chem. Phys. 2014, 16, 8819–8827. [Google Scholar] [CrossRef]
- Toh, J.S.-S.; Jordon, M.J.T.; Husovitz, B.C.; Del Bene, J.E. Can protonshared or ion-pair N-H-N hydrogen bonds be produced in uncharged complexes? A systematic ab initio study of the structures and selected NMR and IR properties of complexes with N-H-N hydrogen bonds. J. Phys. Chem. A. 2001, 105, 10906–10914. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Chai, J.-D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom-atom dispersion corretions. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef] [Green Version]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef] [PubMed]
- Costentin, C.; Robert, M.; Saveant, J.M. Electrochemical and Homogeneous Proton-Coupled Electron Transfers: Concerted Pathways in the One-Electron Oxidation of a Phenol Coupled with an Intramolecular Amine-Driven Proton Transfer. J. Am. Chem. Soc. 2006, 128, 4552–4553. [Google Scholar] [CrossRef]
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView; Version 6; Semichem Inc.: Shawnee Mission, KS, USA, 2016. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grocholska, P.; Kowalska, M.; Wieczorek, R.; Bąchor, R. A Non-Covalent Dimer Formation of Quaternary Ammonium Cation with Unusual Charge Neutralization in Electrospray-Ionization Mass Spectrometry. Molecules 2021, 26, 5868. https://doi.org/10.3390/molecules26195868
Grocholska P, Kowalska M, Wieczorek R, Bąchor R. A Non-Covalent Dimer Formation of Quaternary Ammonium Cation with Unusual Charge Neutralization in Electrospray-Ionization Mass Spectrometry. Molecules. 2021; 26(19):5868. https://doi.org/10.3390/molecules26195868
Chicago/Turabian StyleGrocholska, Paulina, Marta Kowalska, Robert Wieczorek, and Remigiusz Bąchor. 2021. "A Non-Covalent Dimer Formation of Quaternary Ammonium Cation with Unusual Charge Neutralization in Electrospray-Ionization Mass Spectrometry" Molecules 26, no. 19: 5868. https://doi.org/10.3390/molecules26195868





