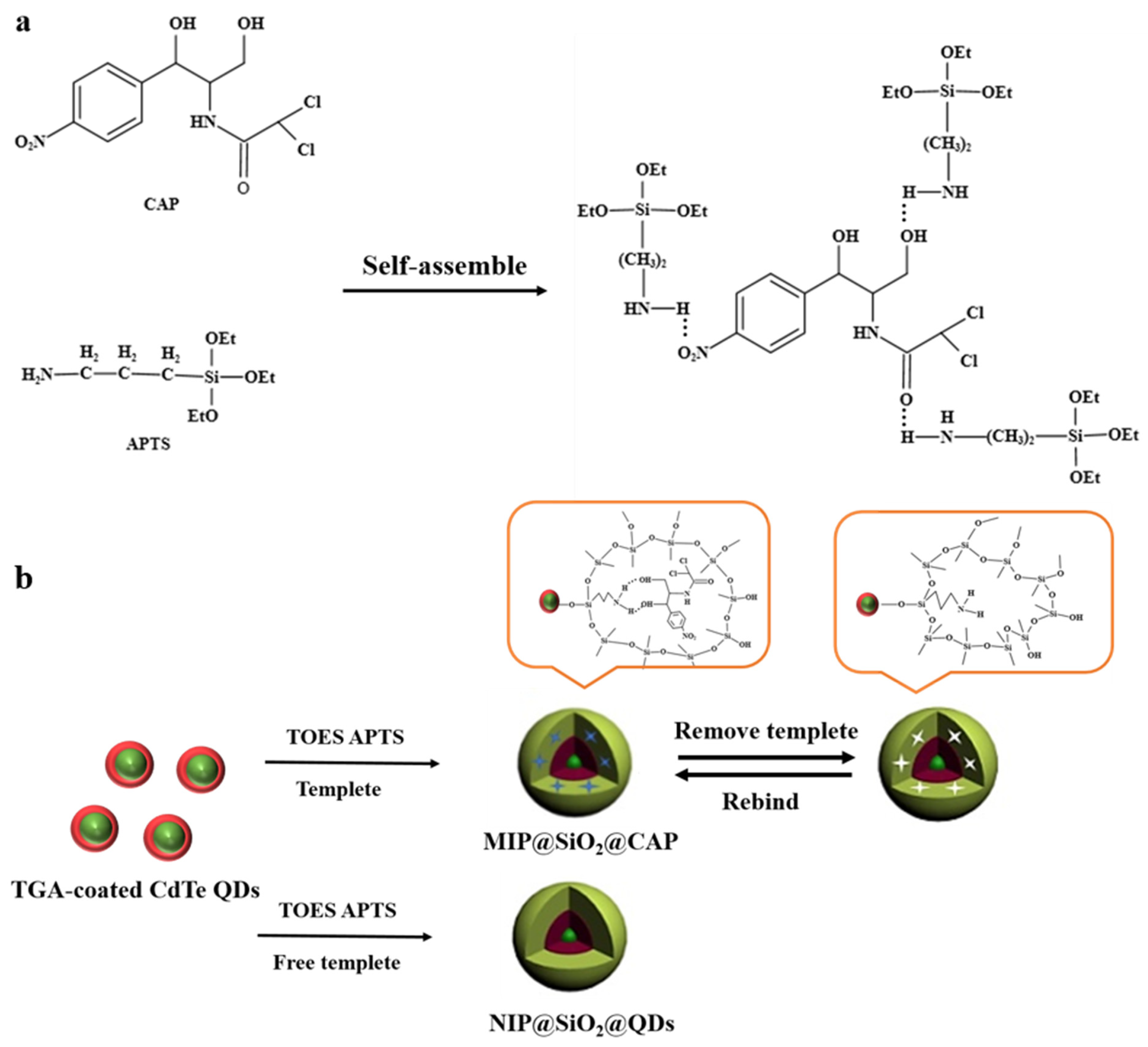
Molecularly Imprinted Silica-Coated CdTe Quantum Dots for Fluorometric Determination of Trace Chloramphenicol
Abstract
:1. Introduction
2. Result and Discussion
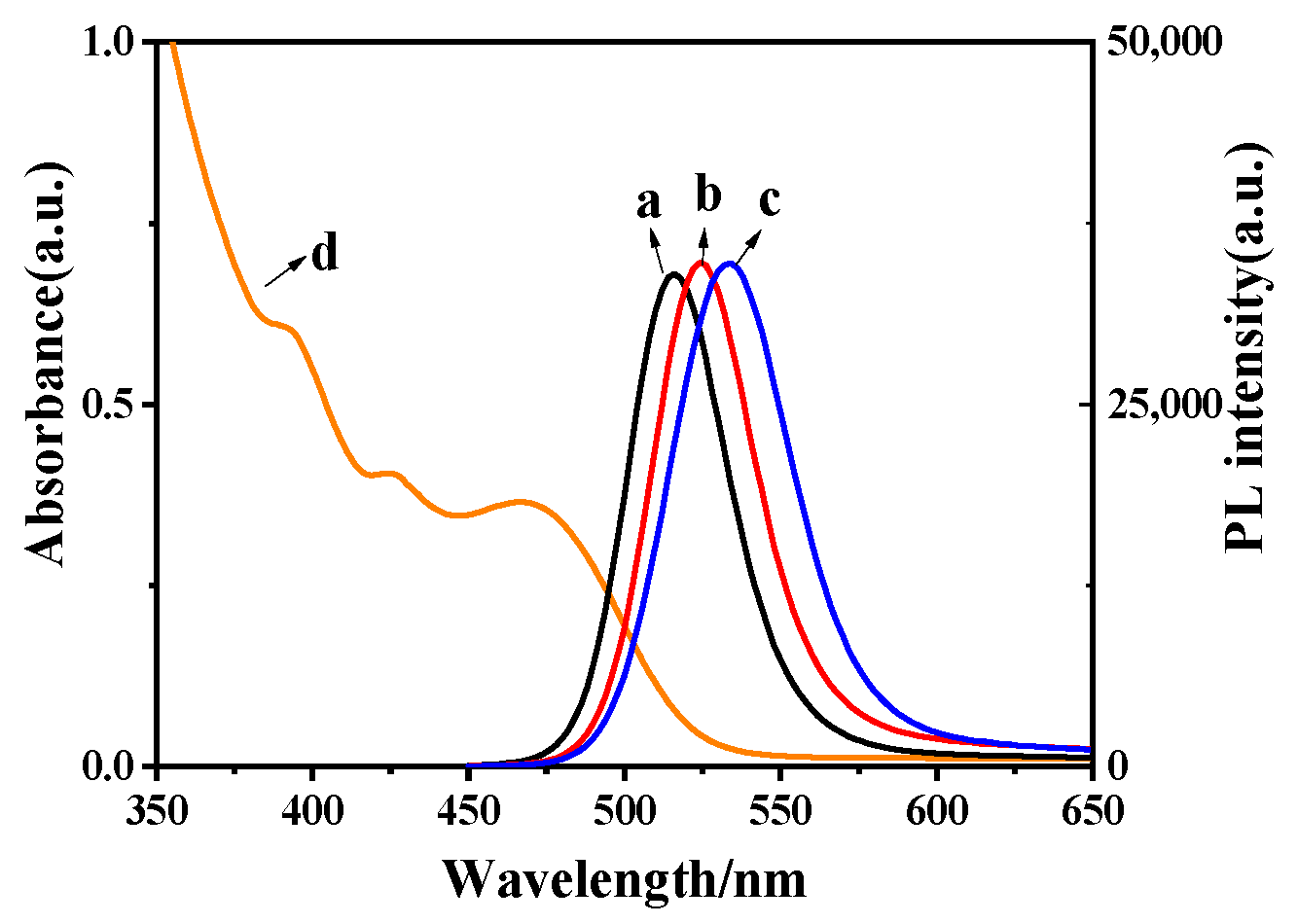
2.1. Characterization
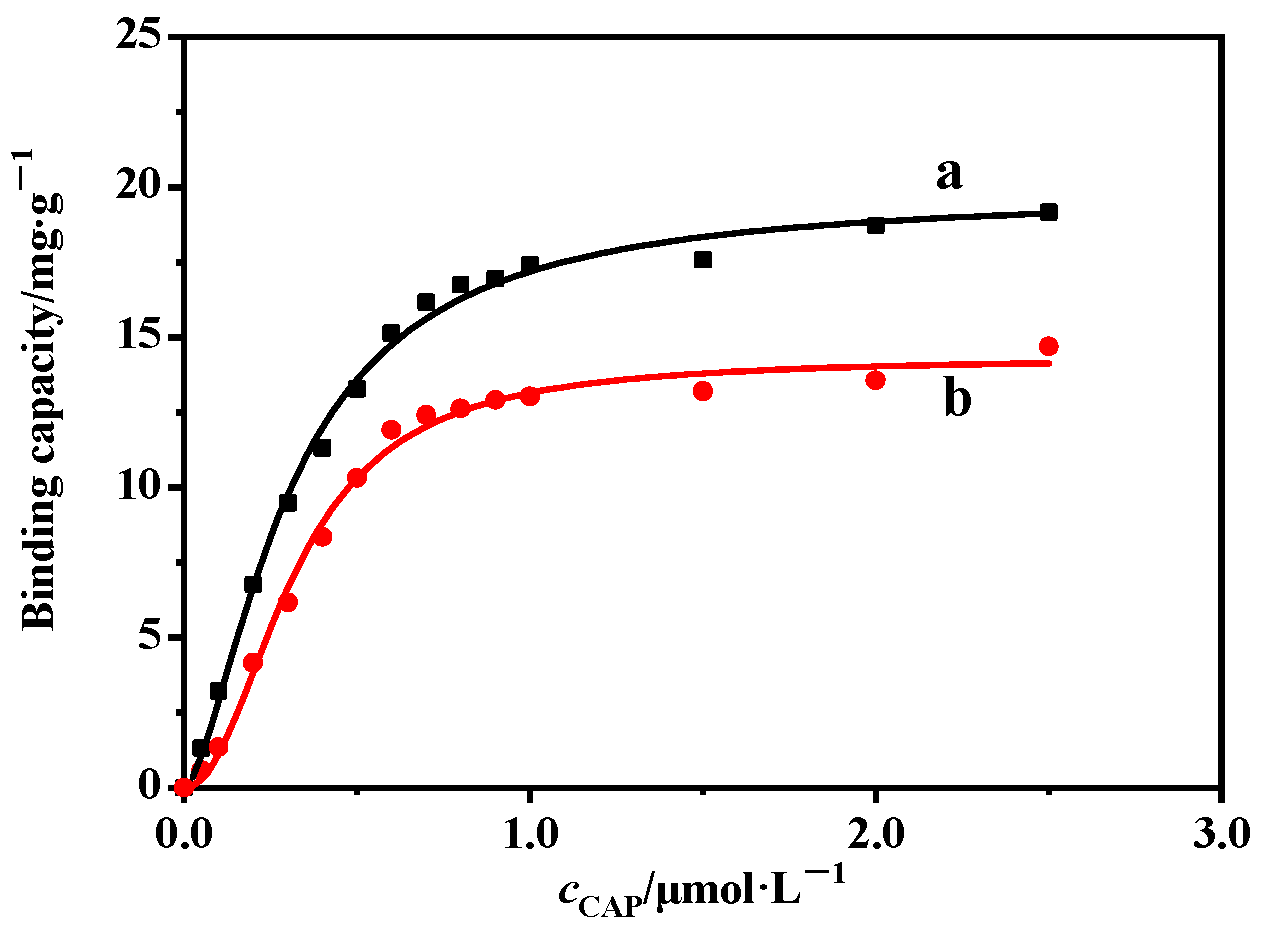
2.2. Adsorptive Property of MIP@SiO2@QDs
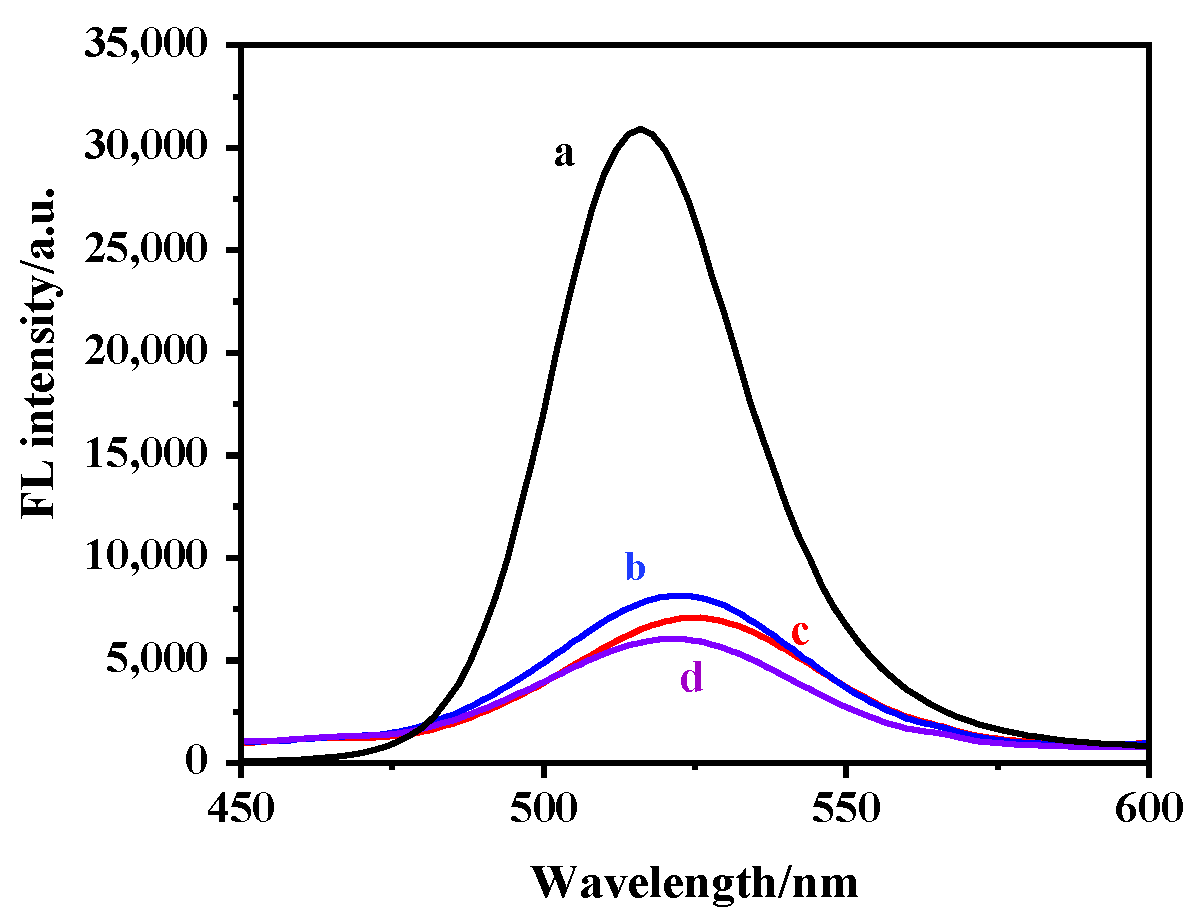
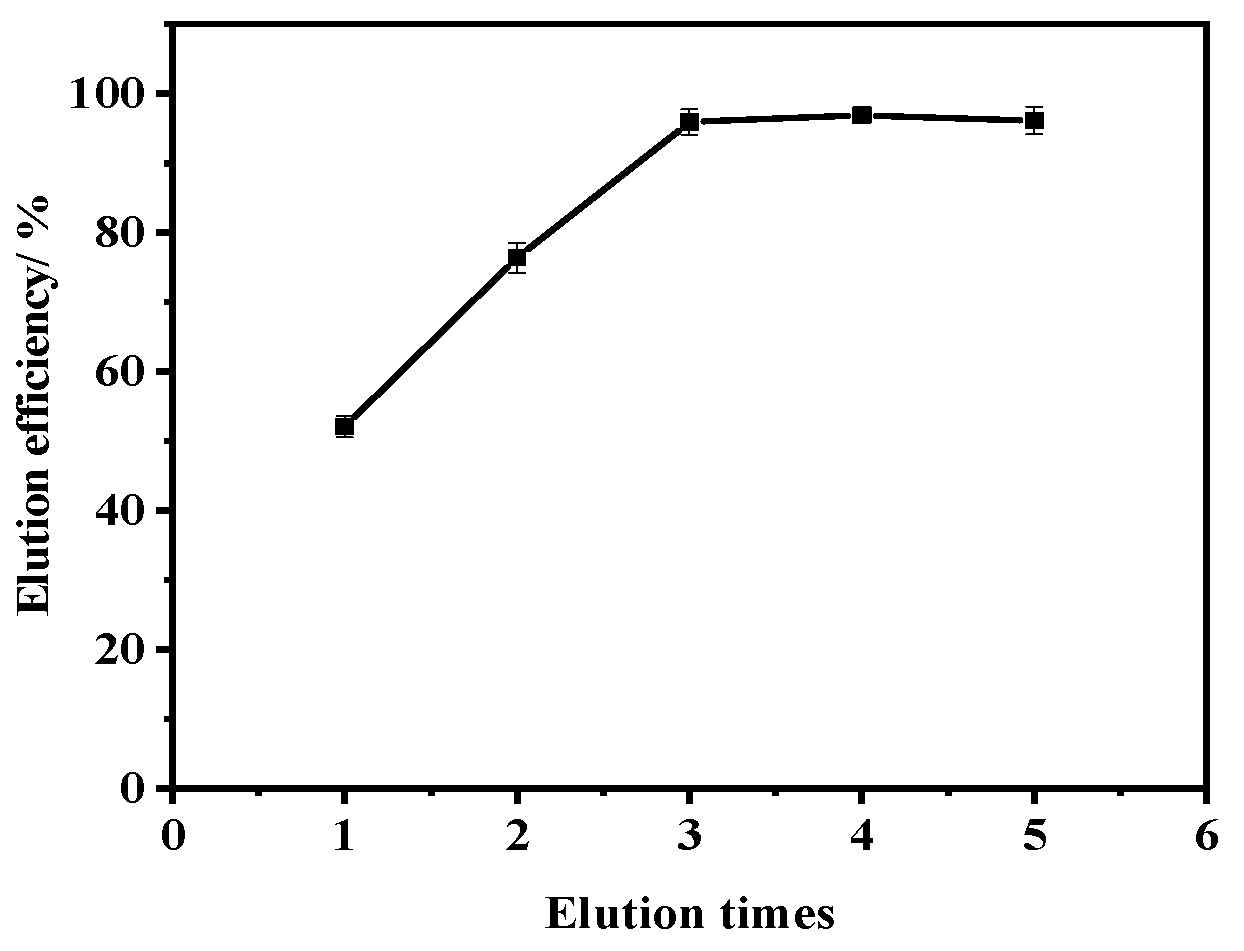
2.3. Optimization of Elution Times
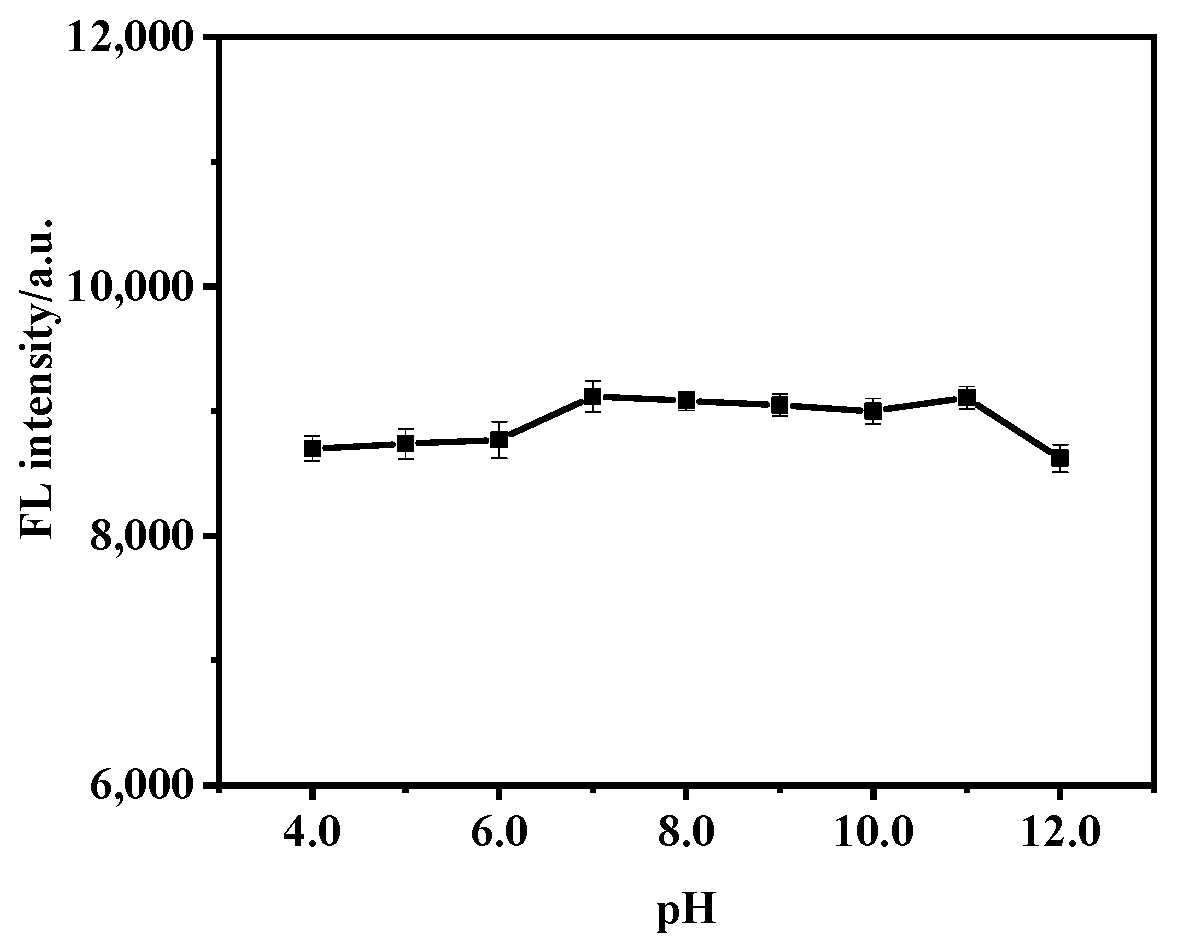
2.4. Effect of pH on Fluorescence of MIP@SiO2@QDs
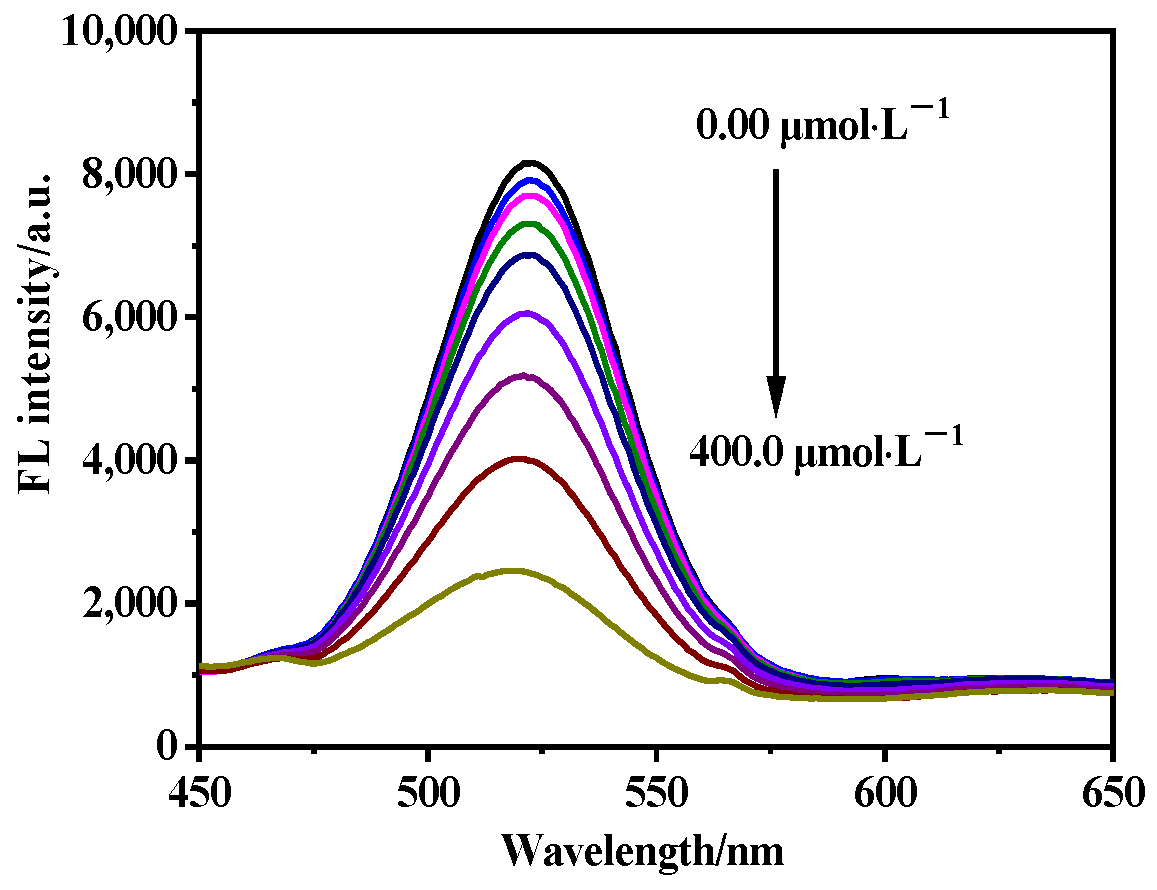
2.5. Sensitivity of MIP@SiO2@QDs
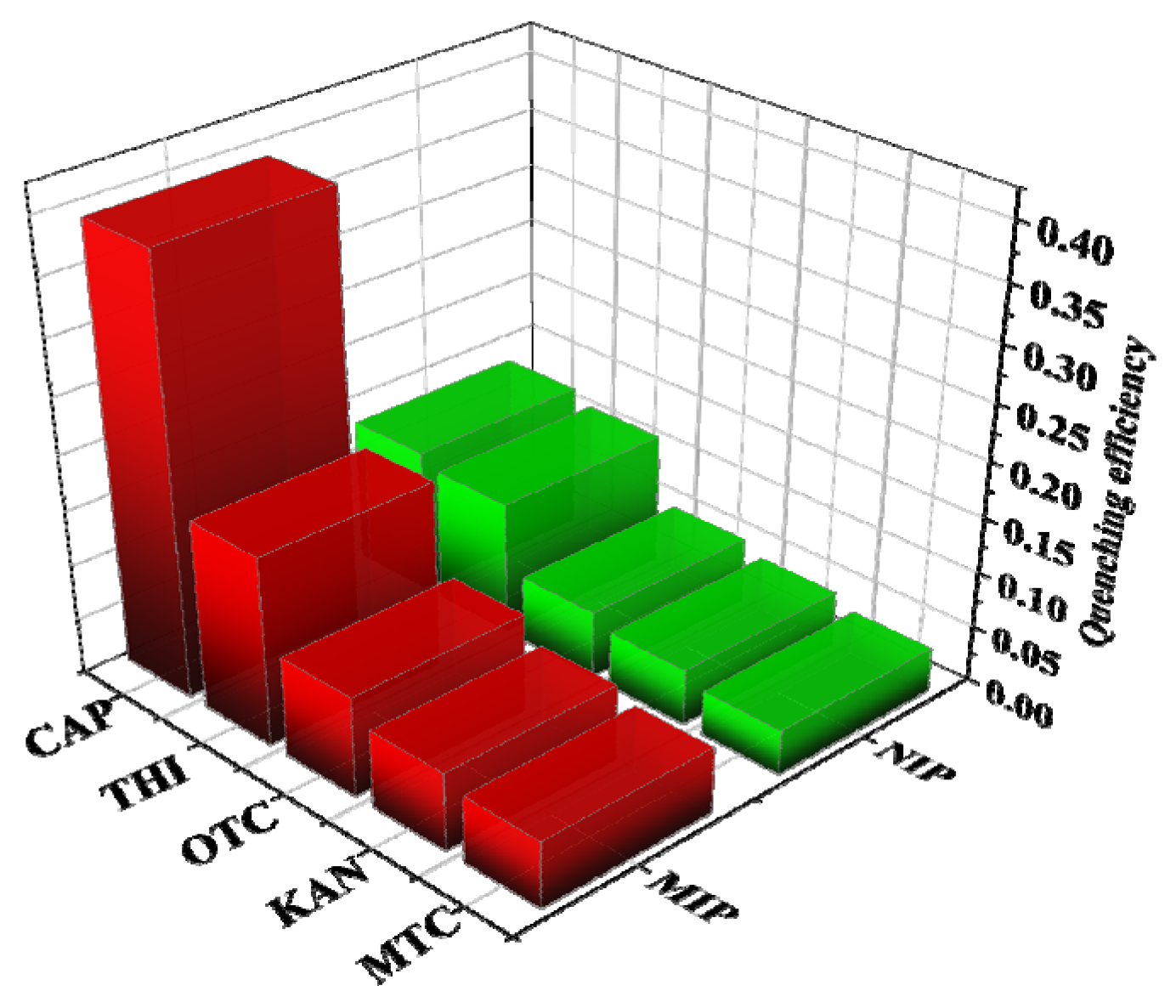
2.6. Selectivity of MIP@SiO2@QDs
2.7. Real Sample Detection
3. Experiment
3.1. Material and Reagents
3.2. Instruments
3.3. Synthesis of TGA-Stabilized CdTe QDs
3.4. Synthesis of MIP@SiO2@QDs and NIP@SiO2@QDs
3.5. Adsorption Experiment
3.6. Optimization of Elution Times Experiment
3.7. Exploration of Acid and Alkali Resistance of MIP@SiO2@QDs
3.8. Selective Experiment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Huang, J.F.; Zhang, H.J.; Feng, Y.Q. Chloramphenicol extraction from honey, milk, and eggs using polymer monolith microextraction followed by liquid chromatography-mass spectrometry determination. J. Chromatogr. B 2006, 54, 9279–9286. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Wu, A.; Zheng, S.; Li, R.; Zhang, D. Molecularly imprinted polymer microspheres for solid-phase extraction of chloramphenicol residues in foods. J. Chromatogr. B 2007, 850, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.D.; He, J.S.; Xie, A.T.; Gao, L.; Pan, J.M.; Chen, X.; Zhou, Z.P.; Wei, X.; Yan, Y.S. Novel pitaya-inspired well-defined core-shell nanospheres with ultrathin surface imprinted nanofilm from magnetic mesoporous nanosilica for highly efficient chloramphenicol removal. Chem. Eng. J. 2016, 284, 812–822. [Google Scholar] [CrossRef]
- Liang, B.C.; Hong, Y.; Kong, D.Y.; Gao, S.H.; Lee, D.J. Accelerated Reduction of Chlorinated Nitroaromatic Antibiotic Chloramphenicol by Biocathode. Environ. Sci. Technol. 2013, 47, 5353–5361. [Google Scholar] [CrossRef] [PubMed]
- Nie, M.H.; Yang, Y.; Zhang, Z.J.; Yan, C.X.; Wang, X.N.; Li, H.J.; Dong, W.B. Degradation of chloramphenicol by thermally activated persulfate in aqueous solution. Chem. Eng. J. 2014, 246, 373–382. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Hameed, B.H. Ordered mesoporous carbons originated from non-edible polyethylene glycol 400 (PEG-400) for chloramphenicol antibiotic recovery from liquid phase. Chem. Eng. J. 2015, 260, 730–739. [Google Scholar]
- Rizzo, S.; Russo, M.; Labra, M.; Campone, L.; Rastrelli, L. Determination of Chloramphenicol in Honey Using Salting-Out Assisted Liquid-Liquid Extraction Coupled with Liquid Chromatography-Tandem Mass Spectrometry and Validation According to 2002/657 European Commission Decision. Molecules 2020, 25, 3481. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.J.; Deng, J.J.; Kang, X.J. Packed-nanofiber solid phase extraction coupled with HPLC for the determination of chloramphenicol in milk. Anal. Methods 2017, 9, 6499–6506. [Google Scholar] [CrossRef]
- Wang, M.H.; Gu, J.H.; Ma, V.; Wu, Y.C.; Lin, Y.J.; Chia, Y.M.; Huang, S.T. A rapid fluorescence detecting platform: Applicable to sense carnitine and chloramphenicol in food samples. RSC Adv. 2014, 4, 64112–64118. [Google Scholar] [CrossRef]
- Liang, X.; Fang, X.; Yao, M.; Yang, Y.; Li, J.; Liu, H.; Wang, L. Direct competitive chemiluminescence immunoassays based on gold-coated magnetic particles for detection of chloramphenicol. Luminescence 2016, 31, 168–172. [Google Scholar] [CrossRef]
- Dong, B.; Li, H.; Sun, J.; Li, Y.; Wang, Z. Magnetic assisted fluorescent immunoassay for sensitive chloramphenicol detection using carbon dots@CaCO3 nanocomposites. J. Hazard. Mater. 2020, 402, 123942. [Google Scholar] [CrossRef]
- Borowiec, J.; Wang, R.; Zhu, L.H.; Zhang, J.D. Synthesis of nitrogen-doped graphene nanosheets decorated with gold nanoparticles as an improved sensor for electrochemical determination of chloramphenicol. Electrochim. Acta 2013, 99, 138–144. [Google Scholar] [CrossRef]
- Petr, J.; Veronika, U.; Zdenka, M.; Radek, Z. Advanced Sensing of Antibiotics with Magnetic Gold Nanocomposite: Electrochemical Detection of Chloramphenicol. Chem. -A Eur. J. 2016, 22, 14279–14284. [Google Scholar]
- Chang, C.C.; Wang, G.Q.; Takarada, T.; Maeda, M. Iodine-mediated etching of triangular gold nanoplates for colorimetric sensing of copper ion and aptasensing of chloramphenicol. ACS Appl. Mater. Interfaces 2017, 9, 34518–34525. [Google Scholar] [CrossRef]
- Chen, L.X.; Xu, S.F.; Li, J.H. Recent advances in molecular imprinting technology: Current status, challenges and highlighted applications. Chem. Soc. Rev. 2011, 40, 2922–2942. [Google Scholar] [CrossRef] [PubMed]
- Beyazit, S.; Bui, B.; Haupt, K.; Gonzato, C. Molecularly imprinted polymer nanomaterials and nanocomposites by controlled/living radical polymerization. Prog. Polym. Sci. 2016, 62, 1–21. [Google Scholar] [CrossRef]
- Li, D.; Bie, Z.; Wang, F.; Guo, E. Efficient synthesis of riboflavin-imprinted magnetic nanoparticles by boronate affinity-based surface imprinting for the selective recognition of riboflavin. Analyst 2018, 143, 4936–4943. [Google Scholar] [CrossRef] [PubMed]
- Bie, Z.Z.; Zhao, W.; Lv, Z.; Liu, S.; Chen, Y. Preparation of salbutamol imprinted magnetic nanoparticles boronate affinity oriented surface imprinting for the selective analysis of trace salbutamol residues. Analyst 2019, 144, 3128–3135. [Google Scholar] [CrossRef]
- Jing, F.A.; Yw, A.; Jw, A.; Cw, B.; Hs, A. Study of molecularly imprinted solid-phase extraction of diphenylguanidine and its structural analogs. Anal. Chim. Acta 2009, 639, 42–50. [Google Scholar]
- Yan, H.Y.; Row, K.H.; Yang, J.T. Water-compatible molecularly imprinted polymers for selective extraction of ciprofloxacin from human urine. Talanta 2008, 75, 227–232. [Google Scholar] [CrossRef]
- Alexander, C.; Andersson, H.S.; Andersson, L.I.; Ansell, R.J.; Whitcombe, M.J. Molecular imprinting science and technology: A survey of the literature for the years up to and including 2003. J. Mol. Recognit. 2010, 19, 106–180. [Google Scholar] [CrossRef]
- Jing, X.; Yang, L.; Zhao, W.; Wang, F.; Chen, Z.; Ma, L.; Jia, L.; Wang, X. Evaporation-assisted dispersive liquid–liquid microextraction based on the solidification of floating organic droplets for the determination of triazole fungicides in water samples by high-performance liquid chromatography. J. Chromatogr. A 2019, 1597, 46–53. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, X.; Wang, M.; Wang, Y.; Zhou, Q. Dummy molecularly imprinted microspheres prepared by Pickering emulsion polymerization for matrix solid-phase dispersion extraction of three azole fungicides from fish samples. J. Chromatogr. A 2020, 461013. [Google Scholar] [CrossRef]
- Sehit, E.; Drzazgowska, J.; Buchenau, D.; Yesildag, C.; Lensen, M.; Altintas, Z. Ultrasensitive nonenzymatic electrochemical glucose sensor based on gold nanoparticles and molecularly imprinted polymers. Biosens. Bioelectron. 2020, 165, 112432. [Google Scholar] [CrossRef]
- Mars, A.; Mejri, A.; Hamzui, A.H.; Elfil, H. Molecularly imprinted curcumin nanoparticles decorated paper for electrochemical and fluorescence dual-mode sensing of bisphenol A. Microchim. Acta 2021, 188, 94. [Google Scholar] [CrossRef]
- Tong, P.; Meng, Y.; Liang, J.; Li, J. Molecularly imprinted electrochemical luminescence sensor based on core–shell magnetic particles with ZIF-8 imprinted material. Sens. Actuators B Chem. 2020, 330, 129405. [Google Scholar] [CrossRef]
- Chen, P.; Qu, R.; Peng, W.; Wang, X.; Huang, K.; He, Y.; Zhang, X.; Meng, Y.; Liu, T.; Chen, J.; et al. Visual and dual-fluorescence homogeneous sensor for the detection of pyrophosphatase in clinical hyperthyroidism samples based on selective recognition of CdTe QDs and coordination polymerization of Ce3+. J. Mater. Chem. C. 2021, 9, 4141–4149. [Google Scholar] [CrossRef]
- Xu, Y.H.; Wang, X.X.; Zhang, W.L.; Lv, F.; Guo, S.J. Recent progress in two-dimensional inorganic quantum dots. Chem. Soc. Rev. 2018, 47, 586–625. [Google Scholar] [CrossRef] [PubMed]
- Liao, B.T.; Chang, C.J.; Wang, C.F.; Lu, C.H.; Chen, J.K. Controlled antibody orientation on Fe3O4 nanoparticles and CdTe quantum dots enhanced sensitivity of a sandwich-structured electrogenerated chemiluminescence immunosensor for the determination of human serum albumin. Sens. Actuators B Chem. 2021, 336, 129710. [Google Scholar] [CrossRef]
- Li, D.; Wang, N.; Wang, F.; Zhao, Q. Boronate affinity-based surface-imprinted quantum dots as novel fluorescent nanosensors for the rapid and efficient detection of rutin. Anal. Methods 2019, 11, 3212–3220. [Google Scholar] [CrossRef]
- Li, D.J.; Zhai, S.M.; Song, R.M.; Liu, Z.Y.; Wang, W.Z. Determination of cis-diol-containing flavonoids in real samples using boronate affinity quantum dots coated with imprinted silica based on controllable oriented surface imprinting approach - ScienceDirect. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2020, 227, 117542. [Google Scholar] [CrossRef]
- Yang, Q.; Li, J.H.; Wang, X.Y.; Xiong, H.; Chen, L.X. Ternary Emission of Blue, Green and Red Based Molecular Imprinting Fluorescence Sensor for the Multiplexed and Visual Detection of Bovine Hemoglobin. Anal. Chem. 2019, 91, 6561–6568. [Google Scholar] [CrossRef]
- Zhou, Z.; Ying, H.; Liu, Y.; Xu, W.; Yang, Y.; Luan, Y.; Lu, Y.; Liu, T.; Yu, S.; Yang, W. Synthesis of surface molecular imprinting polymer on SiO2-coated CdTe quantum dots as sensor for selective detection of sulfadimidine. Appl. Surf. Sci. 2017, 404, 188–196. [Google Scholar] [CrossRef]
- Wei, X.; Chen, H. Ratiometric fluorescence molecularly imprinted sensor based on dual-emission quantum dots hybrid for determination of tetracycline. Anal. Bioanal. Chem. 2019, 411, 5809–5816. [Google Scholar] [CrossRef]
- Wang, X.Y.; Yu, J.L.; Wu, X.Q.; Fu, J.Q.; Kang, Q.; Shen, D.Z.; Li, J.H.; Chen, L.X. A molecular imprinting-based turn-on Ratiometric fluorescence sensor for highly selective and sensitive detection of 2,4-dichlorophenoxyacetic acid (2,4-D). Biosens. Bioelectron. 2016, 81, 438–444. [Google Scholar] [CrossRef]
- Shi, T.; Fu, H.; Tan, L.; Wang, J. CdTe quantum dots coated with a molecularly imprinted polymer for fluorometric determination of norfloxacin in seawater. Microchim. Acta 2019, 186, 644. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, Y.; Jing, C. Molecularly Imprinted Silica Nanospheres Embedded CdSe Quantum Dots for Highly Selective and Sensitive Optosensing of Pyrethroids. Chem. Mater. 2010, 22, 2451–2457. [Google Scholar] [CrossRef]
- Hua, M.; Yang, S.; Ma, J.; He, W.; Kuang, L.; Hua, D.T. Highly selective and sensitive determination of uranyl ion by the probe of CdTe quantum dot with a specific size. Talanta 2018, 190, 278–283. [Google Scholar] [CrossRef]
- Yu, J.L.; Wang, X.Y.; Kang, Q.; Li, J.H.; Shen, D.Z.; Chen, L.X. One-pot synthesis of a quantum dot-based molecular imprinting nanosensor for highly selective and sensitive fluorescence detection of 4-nitrophenol in environmental waters. Environ. Sci. Nano 2017, 4, 493. [Google Scholar] [CrossRef]
- Li, D.J.; Tu, T.Y.; Yang, M.K.; Xu, C. Efficient preparation of surface imprinted magnetic nanoparticles using poly (2-anilinoethanol) as imprinting coating for the selective recognition of glycoprotein. Talanta 2018, 184, 316–324. [Google Scholar] [CrossRef]
- Shirani, M.P.; Rezaei, B.; Ensafi, A.A.; Ramezani, M. Development of an eco-friendly fluorescence nanosensor based on molecularly imprinted polymer on silica-carbon quantum dot for the rapid indoxacarb detection. Food Chem. 2020, 339, 127920. [Google Scholar] [CrossRef]
- Chen, L.G.; Liu, B. Magnetic molecularly imprinted polymer extraction of chloramphenicol from honey. Food Chem. 2013, 141, 23–28. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Esfahani, P.G.; Rezaei, B. Simultaneous detection of folic acid and methotrexate by an optical sensor based on molecularly imprinted polymers on dual-color CdTe quantum dots. Analytica Chimica Acta 2017, 996, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Zhang, F.; Zhang, H.; Wen, Y.; Saren, T. Substitution of antibody with molecularly imprinted 96-well plate in chemiluminescence enzyme immunoassay for the determination of chloramphenicol residues. Food Agric. Immunol. 2014, 408, 411–422. [Google Scholar]
- Zhou, Y.; Qiu, Z.B.; Zeng, Y.B.; Zhou, T.S.; Shi, G.Y. A novel composite of graphene quantum dots and molecularly imprinted polymer for fluorescent detection of paranitrophenol. Biosens. Bioelectron. 2014, 52, 317–323. [Google Scholar] [CrossRef] [PubMed]



| Sample | Found/μmol × L−1 | Added/μmol × L−1 | Found/μmol × L−1 | Recovery/% |
|---|---|---|---|---|
| Lake water | 0.00 | 10.00 | 10.40 ± 0.35 | 104.0 ± 3.4 |
| 0.00 | 85.00 | 85.65 ± 0.65 | 100.8 ± 0.76 | |
| 0.00 | 150.0 | 150.78 ± 0.79 | 100.5 ± 0.52 | |
| 0.00 | 300.0 | 300.75 ± 0.86 | 100.2 ± 0.28 |
| Methods | Samples | Linear Range (μmol × L−1) | LOD (μmol × L−1) | Recovery (%) | RSD (%) | Ref. |
|---|---|---|---|---|---|---|
| HPLC | Milk | 0.50–10.0 | 0.20 | 97.5–104.0 | 8.5 | [8] |
| CL | Honey | 0.50–10.0 | 0.36 | - | - | [9] |
| ELISA | Sea cucumber | 0.38–9.28 | 0.10 | 89.0–98.7 | 11.9 | [44] |
| Electrochemical analysis | Eye drops | 2.00–80.0 | 0.59 | 98.9–103.0 | 2.84 | [12] |
| Aptamer Sensor | Milk powder | 10.0–1000 | 5.00 | - | - | [14] |
| MIP-QDs | Lake water | 1.00–400 | 0.35 | 102.0–104.0 | 2.06 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Liu, Y.; Li, P.; Xing, Y.; Huang, C. Molecularly Imprinted Silica-Coated CdTe Quantum Dots for Fluorometric Determination of Trace Chloramphenicol. Molecules 2021, 26, 5965. https://doi.org/10.3390/molecules26195965
Chen X, Liu Y, Li P, Xing Y, Huang C. Molecularly Imprinted Silica-Coated CdTe Quantum Dots for Fluorometric Determination of Trace Chloramphenicol. Molecules. 2021; 26(19):5965. https://doi.org/10.3390/molecules26195965
Chicago/Turabian StyleChen, Xiaoxiao, Yang Liu, Pu Li, Yichen Xing, and Chaobiao Huang. 2021. "Molecularly Imprinted Silica-Coated CdTe Quantum Dots for Fluorometric Determination of Trace Chloramphenicol" Molecules 26, no. 19: 5965. https://doi.org/10.3390/molecules26195965





