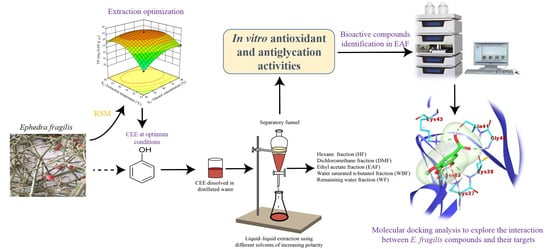
Bioactive Compounds from Ephedra fragilis: Extraction Optimization, Chemical Characterization, Antioxidant and AntiGlycation Activities
Abstract
:1. Introduction
2. Results and Discussion
2.1. Fitting the Models
2.2. Effects of Extraction Variables on TP Content
2.3. Effects of Extraction Variables on TF Content
2.4. Validation of Optimized Conditions
2.5. Extraction Yield and Phytochemical Analysis
2.6. In Vitro Antioxidant Activity
2.6.1. DPPH• Scavenging Activity
2.6.2. ABTS•+ Scavenging Activity
2.6.3. H2O2 Scavenging Activity
2.6.4. Reducing Power
2.6.5. Phosphomolybdenum Assay
2.6.6. β-Carotene–Linoleate Model System
2.7. Antiglycation Activity
2.7.1. UV-Visible Analysis
2.7.2. Inhibition of Protein Glycation in the BSA-Glu Model
2.7.3. Effects on Glycation-Induced Protein Oxidation
2.8. Identified Phenolic Compounds in the EAF
2.9. Molecular Docking Study of Identified Compounds
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Materials
3.3. Experimental Design
3.3.1. Selection of Variables
3.3.2. BBD for Extraction Optimization
3.3.3. Extraction Procedure
3.4. Fractionation of the CEE Obtained under Optimum Condition
3.5. Phytochemical Analysis
3.6. Biological Activities
3.7. RP-HPLC Analysis of EAF
3.8. Molecular Docking
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Martins, N.; Barros, L.; Santos-Buelga, C.; Silva, S.; Henriques, M.; Ferreira, I.C.F.R. Decoction, Infusion and Hydroalcoholic Extract of Cultivated Thyme: Antioxidant and Antibacterial Activities, and Phenolic Characterisation. Food Chem. 2015, 167, 131–137. [Google Scholar] [CrossRef] [Green Version]
- Deetae, P.; Parichanon, P.; Trakunleewatthana, P.; Chanseetis, C.; Lertsiri, S. Antioxidant and Anti-Glycation Properties of Thai Herbal Teas in Comparison with Conventional Teas. Food Chem. 2012, 133, 953–959. [Google Scholar] [CrossRef]
- Yao, Q.; Liang, Y.; Shao, Y.; Bian, W.; Fu, H.; Xu, J.; Sui, L.; Yao, B.; Li, M. Advanced Glycation End Product Concentrations in Follicular Fluid of Women Undergoing IVF/ICSI with a GnRH Agonist Protocol. Reprod. Biomed. Online 2018, 36, 20–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimm, S.; Ott, C.; Hörlacher, M.; Weber, D.; Höhn, A.; Grune, T. Advanced-Glycation-End-Product-Induced Formation of Immunoproteasomes: Involvement of RAGE and Jak2/STAT1. Biochem. J. 2012, 448, 127–139. [Google Scholar] [CrossRef] [Green Version]
- Meenatchi, P.; Purushothaman, A.; Maneemegalai, S. Antioxidant, Antiglycation and Insulinotrophic Properties of Coccinia Grandis (L.) in Vitro: Possible Role in Prevention of Diabetic Complications. J. Tradit. Complement. Med. 2017, 7, 54–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakagawa, T.; Yokozawa, T.; Terasawa, K.; Shu, S.; Juneja, L.R. Protective Activity of Green Tea against Free Radical- and Glucose-Mediated Protein Damage. J. Agric. Food Chem. 2002, 50, 2418–2422. [Google Scholar] [CrossRef] [PubMed]
- Xi, M.; Hai, C.; Tang, H.; Chen, M.; Fang, K.; Liang, X. Antioxidant and Antiglycation Properties of Total Saponins Extracted from Traditional Chinese Medicine Used to Treat Diabetes Mellitus. Phytother. Res. 2008, 22, 228–237. [Google Scholar] [CrossRef]
- Caveney, S.; Charlet, D.A.; Freitag, H.; Maier-Stolte, M.; Starratt, A.N. New Observations on the Secondary Chemistry of World Ephedra (Ephedraceae). Am. J. Bot. 2001, 88, 1199–1208. [Google Scholar] [CrossRef] [Green Version]
- Yamada, I.; Goto, T.; Takeuchi, S.; Ohshima, S.; Yoneyama, K.; Shibuya, T.; Kataoka, E.; Segawa, D.; Sato, W.; Dohmen, T.; et al. Mao (Ephedra sinica Stapf) Protects against d-Galactosamine and Lipopolysaccharide-Induced Hepatic Failure. Cytokine 2008, 41, 293–301. [Google Scholar] [CrossRef]
- Nam, N.-H.; Lee, C.-W.; Hong, D.-H.; Kim, H.-M.; Bae, K.-H.; Ahn, B.-Z. Antiinvasive, Antiangiogenic and Antitumour Activity of Ephedra sinica Extract. Phytother. Res. 2003, 17, 70–76. [Google Scholar] [CrossRef]
- Danciu, C.; Muntean, D.; Alexa, E.; Farcas, C.; Oprean, C.; Zupko, I.; Bor, A.; Minda, D.; Proks, M.; Buda, V.; et al. Phytochemical Characterization and Evaluation of the Antimicrobial, Antiproliferative and Pro-Apoptotic Potential of Ephedra alata Decne. Hydroalcoholic Extract against the MCF-7 Breast Cancer Cell Line. Molecules 2018, 24, 13. [Google Scholar] [CrossRef] [Green Version]
- Zuo, S.; Li, W.; Li, Q.; Zhao, H.; Tang, J.; Chen, Q.; Liu, X.; Zhang, J.H.; Chen, Y.; Feng, H. Protective Effects of Ephedra sinica Extract on Blood–Brain Barrier Integrity and Neurological Function Correlate with Complement C3 Reduction after Subarachnoid Hemorrhage in Rats. Neurosci. Lett. 2015, 609, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, M.; Azarnia, M.; Jamali, M.; Mirabolghasemi, G.; Nazarian, S.; Naghizadeh, M.M.; Rajabi, M.; Tahamtani, Y. Protective Effects of Ephedra pachyclada Extract on Mouse Models of Carbon Tetrachloride- Induced Chronic and Acute Liver Failure. Tissue Cell 2014, 46, 78–85. [Google Scholar] [CrossRef]
- Zhang, B.-M.; Wang, Z.-B.; Xin, P.; Wang, Q.-H.; Bu, H.; Kuang, H.-X. Phytochemistry and Pharmacology of Genus Ephedra. Chin. J. Nat. Med. 2018, 16, 811–828. [Google Scholar] [CrossRef]
- Chen, S.; Zeng, Z.; Hu, N.; Bai, B.; Wang, H.; Suo, Y. Simultaneous Optimization of the Ultrasound-Assisted Extraction for Phenolic Compounds Content and Antioxidant Activity of Lycium ruthenicum Murr. Fruit using Response Surface Methodology. Food Chem. 2018, 242, 1–8. [Google Scholar] [CrossRef]
- Mokrani, A.; Madani, K. Effect of Solvent, Time and Temperature on the Extraction of Phenolic Compounds and Antioxidant Capacity of Peach (Prunus persica L.) Fruit. Sep. Purif. Technol. 2016, 162, 68–76. [Google Scholar] [CrossRef]
- Box, G.E.P.; Behnken, D.W. Some New Three Level Designs for the Study of Quantitative Variables. Technometrics 1960, 2, 455–475. [Google Scholar] [CrossRef]
- Martín-García, B.; Pimentel-Moral, S.; Gómez-Caravaca, A.M.; Arráez-Román, D.; Segura-Carretero, A. Box-Behnken Experimental Design for a Green Extraction Method of Phenolic Compounds from Olive Leaves. Ind. Crops Prod. 2020, 154, 112741. [Google Scholar] [CrossRef]
- Pandey, A.; Belwal, T.; Sekar, K.C.; Bhatt, I.D.; Rawal, R.S. Optimization of Ultrasonic-Assisted Extraction (UAE) of Phenolics and Antioxidant Compounds from Rhizomes of Rheum moorcroftianum using Response Surface Methodology (RSM). Ind. Crops Prod. 2018, 119, 218–225. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Y.; Chen, G.; Yue, W.; Liang, Q.; Wu, Q. Optimisation of Ultrasound Assisted Extraction of Phenolic Compounds from Sparganii rhizoma with Response Surface Methodology. Ultrason. Sonochem. 2013, 20, 846–854. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, S.; Liao, M. Optimization of Ultrasonic Extraction of Phenolic Compounds from Euryale ferox Seed Shells using Response Surface Methodology. Ind. Crops Prod. 2013, 49, 837–843. [Google Scholar] [CrossRef]
- Favre, L.C.; dos Santos, C.; López-Fernández, M.P.; Mazzobre, M.F.; del Pilar Buera, M. Optimization of β-Cyclodextrin-Based Extraction of Antioxidant and Anti-Browning Activities from Thyme Leaves by Response Surface Methodology. Food Chem. 2018, 265, 86–95. [Google Scholar] [CrossRef]
- Naczk, M.; Shahidi, F. Extraction and Analysis of Phenolics in Food. J. Chromatogr. A 2004, 1054, 95–111. [Google Scholar] [CrossRef]
- Belwal, T.; Dhyani, P.; Bhatt, I.D.; Rawal, R.S.; Pande, V. Optimization Extraction Conditions for Improving Phenolic Content and Antioxidant Activity in Berberis Asiatica Fruits using Response Surface Methodology (RSM). Food Chem. 2016, 207, 115–124. [Google Scholar] [CrossRef]
- Zhang, D.-S.; Guo, C.-Y.; Wang, J.; Hou, Y.; Zhao, Y.-M.; Shen, L.-X. Orthogonal Test Design for Optimizing the Extraction of Total Flavonoids from Inula helenium. Pharmacogn. Mag. 2013, 9, 192. [Google Scholar] [CrossRef] [Green Version]
- Pinelo, M.; Sineiro, J.; Núñez, M.J. Mass Transfer during Continuous Solid–Liquid Extraction of Antioxidants from Grape Byproducts. J. Food Eng. 2006, 77, 57–63. [Google Scholar] [CrossRef]
- Yao, X.-H.; Zhang, D.-Y.; Zu, Y.-G.; Fu, Y.; Luo, M.; Gu, C.-B.; Li, C.-Y.; Mu, F.-S.; Efferth, T. Free Radical Scavenging Capability, Antioxidant Activity and Chemical Constituents of Pyrola incarnata Fisch. Leaves. Ind. Crops Prod. 2013, 49, 247–255. [Google Scholar] [CrossRef]
- Bhardwaj, P.; Naryal, A.; Thakur, M.S.; Aggarwal, N.K.; Saxena, S.; Chaurasia, O.P.; Kumar, R. Comparative Antioxidant, Antibacterial, and GC-MS Analysis of Methanol Extract’s Fractions and Isolation of Luteolin from Leaves of Trans-Himalayan Codonopsis clematidea. Ind. Crops Prod. 2020, 144, 112046. [Google Scholar] [CrossRef]
- Kaewseejan, N.; Siriamornpun, S. Bioactive Components and Properties of Ethanolic Extract and Its Fractions from Gynura procumbens Leaves. Ind. Crops Prod. 2015, 74, 271–278. [Google Scholar] [CrossRef]
- Koleva, I.I.; van Beek, T.A.; Linssen, J.P.H.; de Groot, A.; Evstatieva, L.N. Screening of Plant Extracts for Antioxidant Activity: A Comparative Study on Three Testing Methods. Phytochem. Anal. 2002, 13, 8–17. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, M.-F.; Tu, Z.-C.; Zhao, Y.; Wang, H.; Li, G.-J.; Wang, H.; Sha, X.-M. A-Glucosidase Inhibition, Anti-Glycation and Antioxidant Activities of Liquidambar formosana Hance Leaf, and Identification of Phytochemical Profile. S. Afr. J. Bot. 2017, 113, 239–247. [Google Scholar] [CrossRef]
- Adebiyi, O.E.; Olayemi, F.O.; Ning-Hua, T.; Guang-Zhi, Z. In Vitro Antioxidant Activity, Total Phenolic and Flavonoid Contents of Ethanol Extract of Stem and Leaf of Grewia carpinifolia. Beni-Suef Univ. J. Basic Appl. Sci. 2017, 6, 10–14. [Google Scholar] [CrossRef]
- Wu, L.; Hsu, H.-W.; Chen, Y.-C.; Chiu, C.-C.; Lin, Y.-I.; Ho, J.A. Antioxidant and Antiproliferative Activities of Red Pitaya. Food Chem. 2006, 95, 319–327. [Google Scholar] [CrossRef]
- Sies, H. Hydrogen Peroxide as a Central Redox Signaling Molecule in Physiological Oxidative Stress: Oxidative Eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef]
- Namiki, M. Antioxidants/Antimutagens in Food. Crit. Rev. Food Sci. Nutr. 1990, 29, 273–300. [Google Scholar] [CrossRef]
- Halliwell, B. Reactive Oxygen Species in Living Systems: Source, Biochemistry, and Role in Human Disease. Am. J. Med. 1991, 91, S14–S22. [Google Scholar] [CrossRef]
- Guenaou, I.; Hmimid, F.; Lahlou, F.A.; Errami, A.; Irahal, I.N.; Fahde, S.; L’Houcine, O.; Bourhim, N. Cytoprotective Effect of Ethyl Acetate Fraction from Ephedra fragilis on H2O2-Induced Oxidative Damage in Tetrahymena pyriformis. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 239, 108899. [Google Scholar] [CrossRef] [PubMed]
- Sroka, Z.; Cisowski, W. Hydrogen Peroxide Scavenging, Antioxidant and Anti-Radical Activity of Some Phenolic Acids. Food Chem. Toxicol. 2003, 41, 753–758. [Google Scholar] [CrossRef]
- Kalaivani, T.; Mathew, L. Free Radical Scavenging Activity from Leaves of Acacia nilotica (L.) Wild. Ex Delile, an Indian Medicinal Tree. Food Chem. Toxicol. 2010, 48, 298–305. [Google Scholar] [CrossRef]
- Meir, S.; Kanner, J.; Akiri, B.; Philosoph-Hadas, S. Determination and Involvement of Aqueous Reducing Compounds in Oxidative Defense Systems of Various Senescing Leaves. J. Agric. Food Chem. 1995, 43, 1813–1819. [Google Scholar] [CrossRef]
- Duh, P.-D.; Tu, Y.-Y.; Yen, G.-C. Antioxidant Activity of Water Extract of Harng Jyur (Chrysanthemum morifolium Ramat). LWT Food Sci. Technol. 1999, 32, 269–277. [Google Scholar] [CrossRef]
- Sharifi, B.; Goli, S.A.H.; Maghsoudlou, Y. Antioxidant Activity and Chemical Composition of the Methanolic Extract and Related Fractions of Dracocephalum kotschyi Leaves using Liquid Chromatography–Tandem Mass Spectrometry. Ind. Crops Prod. 2017, 104, 111–119. [Google Scholar] [CrossRef]
- Trabelsi, N.; Oueslati, S.; Falleh, H.; Waffo-Téguo, P.; Papastamoulis, Y.; Mérillon, J.-M.; Abdelly, C.; Ksouri, R. Isolation of Powerful Antioxidants from the Medicinal Halophyte Limoniastrum guyonianum. Food Chem. 2012, 135, 1419–1424. [Google Scholar] [CrossRef] [PubMed]
- Sarikurkcu, C.; Tepe, B.; Daferera, D.; Polissiou, M.; Harmandar, M. Studies on the Antioxidant Activity of the Essential Oil and Methanol Extract of Marrubium globosum Subsp. globosum (Lamiaceae) by Three Different Chemical Assays. Bioresour. Technol. 2008, 99, 4239–4246. [Google Scholar] [CrossRef]
- Singh, R.P.; Chidambara Murthy, K.N.; Jayaprakasha, G.K. Studies on the Antioxidant Activity of Pomegranate (Punica granatum) Peel and Seed Extracts using in Vitro Models. J. Agric. Food Chem. 2002, 50, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Janitha, P.K.; Wanasundara, P.D. Phenolic Antioxidants. Crit. Rev. Food Sci. Nutr. 1992, 32, 67–103. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Xia, Y.; Zeng, M.; Wang, W.; He, Y.; Liu, J. Caffeic Acid Inhibits the Formation of Advanced Glycation End Products (AGEs) and Mitigates the AGEs-Induced Oxidative Stress and Inflammation Reaction in Human Umbilical Vein Endothelial Cells (HUVECs). Chem. Biodivers. 2019, 16, e1900174. [Google Scholar] [CrossRef] [PubMed]
- Yeh, W.-J.; Hsia, S.-M.; Lee, W.-H.; Wu, C.-H. Polyphenols with Antiglycation Activity and Mechanisms of Action: A Review of Recent Findings. J. Food Drug Anal. 2017, 25, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Beal, M.F. Oxidatively Modified Proteins in Aging and Disease. Free Radic. Biol. Med. 2002, 32, 797–803. [Google Scholar] [CrossRef]
- Telci, A.; Çakatay, U.; Salman, S.; Satman, İ.; Sivas, A. Oxidative Protein Damage in Early Stage Type 1 Diabetic Patients. Diabetes Res. Clin. Pract. 2000, 50, 213–223. [Google Scholar] [CrossRef]
- Ardestani, A.; Yazdanparast, R. Inhibitory Effects of Ethyl Acetate Extract of Teucrium polium on in Vitro Protein Glycoxidation. Food Chem. Toxicol. 2007, 45, 2402–2411. [Google Scholar] [CrossRef]
- Golshahi, F.; Bahramikia, S. Protective Effects of Organic Fractions of Trachyspermum copticum against Advance Glycation End Products in Vitro. Adv. Tradit. Med. 2020, 20, 143–151. [Google Scholar] [CrossRef]
- Soumaya, B.; Yosra, E.; Rim, B.M.; Sarra, D.; Sawsen, S.; Sarra, B.; Kamel, M.; Wissem, A.-W.; Isoda, H.; Wided, M.-K. Preliminary Phytochemical Analysis, Antioxidant, Anti-Inflammatory and Anticancer Activities of Two Tunisian Ephedra Species: Ephedra alata and Ephedra fragilis. S. Afr. J. Bot. 2020, 135, 421–428. [Google Scholar] [CrossRef]
- De Groot, A.C.; Schmidt, E. Tea Tree Oil: Contact Allergy and Chemical Composition. Contact Dermat. 2016, 75, 129–143. [Google Scholar] [CrossRef] [Green Version]
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant Properties of Phenolic Compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Awasthi, S.; Saraswathi, N.T. Vanillin Restrains Non-Enzymatic Glycation and Aggregation of Albumin by Chemical Chaperone like Function. Int. J. Biol. Macromol. 2016, 87, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Cepas, V.; Collino, M.; Mayo, J.C.; Sainz, R.M. Redox Signaling and Advanced Glycation Endproducts (AGEs) in Diet-Related Diseases. Antioxidants 2020, 9, 142. [Google Scholar] [CrossRef] [Green Version]
- Ravichandran, G.; Lakshmanan, D.K.; Murugesan, S.; Elangovan, A.; Rajasekaran, N.S.; Thilagar, S. Attenuation of Protein Glycation by Functional Polyphenolics of Dragon Fruit (Hylocereus polyrhizus); An in Vitro and in Silico Evaluation. Food Res. Int. 2021, 140, 110081. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Zhang, L.-L.; Yue, X.-Y.; Liang, J.; Jiang, J.; Gao, X.-L.; Yue, P.-X. Optimization of Ultrasound-Assisted Extraction of Phenolic Compounds and Anthocyanins from Blueberry (Vaccinium ashei) Wine Pomace. Food Chem. 2016, 204, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Celli, G.B.; Ghanem, A.; Brooks, M.S.-L. Optimization of Ultrasound-Assisted Extraction of Anthocyanins from Haskap Berries (Lonicera caerulea L.) Using Response Surface Methodology. Ultrason. Sonochem. 2015, 27, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.; Kumazawa, S.; Usui, Y.; Nakamura, J.; Matsuka, M.; Zhu, F.; Nakayama, T. Antioxidant Activity and Constituents of Propolis Collected in Various Areas of China. Food Chem. 2007, 101, 1383–1392. [Google Scholar] [CrossRef]
- Slinkard, K.; Singleton, V.L. Total Phenol Analysis: Automation and Comparison with Manual Methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Dorman, H.J.D.; Hiltunen, R. Fe(III) Reductive and Free Radical-Scavenging Properties of Summer Savory (Satureja hortensis L.) Extract and Subfractions. Food Chem. 2004, 88, 193–199. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on Products of Browning Reaction. Antioxidative Activities of Products of Browning Reaction Prepared from Glucosamine. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef] [Green Version]
- Ruch, R.J.; Cheng, S.; Klaunig, J.E. Prevention of Cytotoxicity and Inhibition of Intercellular Communication by Antioxidant Catechins Isolated from Chinese Green Tea. Carcinogenesis 1989, 10, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Tepe, B.; Daferera, D.; Sokmen, A.; Sokmen, M.; Polissiou, M. Antimicrobial and Antioxidant Activities of the Essential Oil and Various Extracts of Salvia tomentosa Miller (Lamiaceae). Food Chem. 2005, 90, 333–340. [Google Scholar] [CrossRef]
- Adisakwattana, S.; Sompong, W.; Meeprom, A.; Ngamukote, S.; Yibchok-Anun, S. Cinnamic Acid and Its Derivatives Inhibit Fructose-Mediated Protein Glycation. Int. J. Mol. Sci. 2012, 13, 1778–1789. [Google Scholar] [CrossRef]
- Levine, R.L. Carbonyl Modified Proteins in Cellular Regulation, Aging, and Disease. Free Radic. Biol. Med. 2002, 32, 790–796. [Google Scholar] [CrossRef]
- Sedlak, J.; Lindsay, R.H. Estimation of Total, Protein-Bound, and Nonprotein Sulfhydryl Groups in Tissue with Ellman’s Reagent. Anal. Biochem. 1968, 25, 192–205. [Google Scholar] [CrossRef]
- Bellahcen, T.O.; AAmiri, A.; Touam, I.; Hmimid, F.; Amrani, A.E.; Cherif, A.; Cherki, M. Evaluation of Moroccan Microalgae: Spirulina platensis as a Potential Source of Natural Antioxidants. J. Complement. Integr. Med. 2020, 17, 20190036. [Google Scholar] [CrossRef] [PubMed]






| Variable | Units | Symbol | Variable Levels | ||
|---|---|---|---|---|---|
| Low (−1) | Middle (0) | Hight (+1) | |||
| Ethanol concentration | % | X1 | 40 | 60 | 80 |
| Extraction temperature | °C | X2 | 25 | 42.5 | 60 |
| Extraction time | h | X3 | 6 | 15 | 24 |
| Run | Extraction conditions | Experimental results | |||
| X1 (ethanol concentration, %) | X2 (extraction temperature, °C) | X3 (extraction time, h) | TP (mg GAE/g dw) * | TF (mg QE/g dw) * | |
| 1 | 40 (−1) | 42.5 (0) | 24 (+1) | 12.41 | 2.82 |
| 2 | 40 (−1) | 25 (−1) | 15 (0) | 11.59 | 2.75 |
| 3 | 60 (0) | 42.5 (0) | 15 (0) | 15.26 | 2.98 |
| 4 | 60 (0) | 42.5 (0) | 15 (0) | 15.39 | 2.96 |
| 5 | 60 (0) | 60 (+1) | 6 (−1) | 11.57 | 2.73 |
| 6 | 80 (+1) | 60 (+1) | 15 (0) | 13.21 | 2.78 |
| 7 | 60 (0) | 60 (+1) | 24 (+1) | 13.65 | 2.81 |
| 8 | 40 (−1) | 60 (+1) | 15 (0) | 13.67 | 2.84 |
| 9 | 60 (0) | 25 (−1) | 6 (−1) | 12.18 | 2.72 |
| 10 | 80 (+1) | 25 (−1) | 15 (0) | 13.39 | 2.86 |
| 11 | 60 (0) | 42.5 (0) | 15 (0) | 15.26 | 2.98 |
| 12 | 80 (+1) | 42.5 (0) | 6 (−1) | 12.41 | 2.81 |
| 13 | 60 (0) | 25 (−1) | 24 (+1) | 10.31 | 2.77 |
| 14 | 80 (+1) | 42.5 (0) | 24 (+1) | 13.19 | 2.79 |
| 15 | 40 (−1) | 42.5 (0) | 6 (−1) | 12.13 | 2.69 |
| Source | Total Phenolics (TP) Content (mg GAE/g dw) | Total Flavonoids (TF) Content (mg QE/g dw) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sum of Squares | DF a | Mean Square | F-Value | p-Value | Sum of Squares | DF | Mean Square | F-Value | p-Value | |
| Model | 30.2 | 9 | 3.36 | 84.96 | <0.0001 *** | 0.1172 | 9 | 0.0130 | 90.83 | <0.0001 *** |
| X1 -Ethanol | 0.72 | 1 | 0.7200 | 18.23 | 0.0079 ** | 0.0024 | 1 | 0.0024 | 17.09 | 0.0090 ** |
| X2 -Temperature | 2.68 | 1 | 2.68 | 67.84 | 0.0004 *** | 0.0004 | 1 | 0.0004 | 3.14 | 0.1366 ns |
| X3- Time | 0.2016 | 1 | 0.2016 | 5.10 | 0.0734 ns | 0.0072 | 1 | 0.0072 | 50.23 | 0.0009 *** |
| X12 | 2.77 | 1 | 2.77 | 70.01 | 0.0004 *** | 0.0196 | 1 | 0.0196 | 136.96 | <0.0001 *** |
| X22 | 8.01 | 1 | 8.01 | 202.80 | <0.0001 *** | 0.0319 | 1 | 0.0319 | 222.40 | <0.0001 *** |
| X32 | 13.37 | 1 | 13.37 | 338.50 | <0.0001 *** | 0.0558 | 1 | 0.0558 | 389.20 | <0.0001 *** |
| X1 X2 | 1.28 | 1 | 1.28 | 32.33 | 0.0023 ** | 0.0072 | 1 | 0.0072 | 50.41 | 0.0009 *** |
| X1 X3 | 0.0625 | 1 | 0.0625 | 1.58 | 0.2640 ns | 0.0056 | 1 | 0.0056 | 39.24 | 0.0015 ** |
| X2 X3 | 3.90 | 1 | 3.90 | 98.75 | 0.0002 *** | 0.0002 | 1 | 0.0002 | 1.57 | 0.2656 ns |
| Residual | 0.1975 | 5 | 0.0395 | 0.0007 | 5 | 0.0001 | ||||
| Lack of fit | 0.1862 | 3 | 0.0621 | 11.02 | 0.0843 ns | 0.0005 | 3 | 0.0002 | 1.13 | 0.5024 ns |
| Pure error | 0.0113 | 2 | 0.0056 | 0.0003 | 2 | 0.0001 | ||||
| Cor Total | 30.40 | 14 | 0.1179 | 14 | ||||||
| R2 | 0.9935 | 0.9939 | ||||||||
| Adjusted R2 | 0.9818 | 0.9829 | ||||||||
| Predicted R² | 0.9012 | 0.9338 | ||||||||
| C.V % b | 1.52 | 0.4246 | ||||||||
| Adeq Precision | 29.4772 | 28.7290 | ||||||||
| Extraction Variables | TP a (mg GAE/g of dw) | TF a (mg QE/g of dw) | ||||
|---|---|---|---|---|---|---|
| X1 (Ethanol Concentration, %) | X2 (Temperature, °C) | X3 (Time, h) | Predicted Value | Experimental Value b | Predicted Value | Experimental Value b |
| 61.93 | 44.43 | 15.84 | 15.373 | 14.98 ± 0.29 | 2.975 | 2.92 ± 0.09 |
| Fractions | Yield (%, w/w) | TP (mg GAE/g of dw) | TF (mg QE/ g of dw) |
|---|---|---|---|
| CEE | 10.6 ± 0.98 d | 14.98 ± 0.29 c | 2.92 ± 0.09 b |
| HF | 0.78 ± 0.05 a | 8.04 ± 0.17 a | 1.65 ± 0.13 a |
| DMF | 1.34 ± 0.08 a,b | 19.21 ± 0.22 d | 4.29 ± 0.18 c |
| EAF | 0.93 ± 0.03 a | 32.78 ± 0.49 f | 10.50 ± 0.11 e |
| WBF | 2.04 ± 0.11 a,b,c | 25.02 ± 1.01 e | 7.64 ± 0.21 d |
| WF | 2.73 ± 0.17 c | 10.47 ± 0.71 b | 1.86 ± 0.28 a |
| TP | TF | DPPH | ABTS | H2O2 | RP | TAC | β-carotene | AGEs | |
|---|---|---|---|---|---|---|---|---|---|
| TP | 1 | 0.986 ** | 0.963 ** | 0.921 ** | 0.926 ** | 0.975 ** | 0.978 ** | 0.850 * | 0.950 ** |
| TF | 1 | 0.949 ** | 0.891 * | 0.934 ** | 0.987 ** | 0.978 ** | 0.885 * | 0.972 ** | |
| DPPH | 1 | 0.873 * | 0.908 ** | 0.952 ** | 0.924 ** | 0.860 * | 0. 930 ** | ||
| ABTS | 1 | 0.960 ** | 0.866 * | 0.835 * | 0.892 * | 0.914 * | |||
| H2O2 | 1 | 0.929 ** | 0.864 * | 0.979 ** | 0.983 ** | ||||
| RP | 1 | 0.979 ** | 0.884 * | 0.975 ** | |||||
| TAC | 1 | 0.787 ns | 0.923 ** | ||||||
| β-carotene | 1 | 0.963 ** | |||||||
| Anti-AGEs | 1 |
| Compounds | Informations | Chemical Structure | Docking Score (kcal/mol) | |
|---|---|---|---|---|
| BSA | RAGE | |||
| Rutin | MW: 610.5 g/mol MF: C27H30O16 H-bound donor: 10 H-bound acceptor: 16 PubChem ID: CID 5280805 |  | −4.41 | −4.89 |
| Caffeic acid | MW: 180.16 g/mol MF: C9H8O4 H-bound donor: 3 H-bound acceptor: 4 PubChem ID: CID 689043 |  | −5.84 | −5.89 |
| Ferulic acid | MW: 194.18 g/mol MF: C10H10O4 H-bound donor: 2 H-bound acceptor: 4 PubChem ID: CID 445858 |  | −6.35 | −5.94 |
| Galic acid | MW: 170.12 g/mol MF: C7H6O5 H-bound donor: 4 H-bound acceptor: 5 PubChem ID: CID 811292 |  | −5.25 | −6.8 |
| Quercetin | MW: 302.23 g/mol MF: C15H10O7 H-bound donor: 5 H-bound acceptor: 7 PubChem ID: CID 5280343 |  | −7.7 | −5.58 |
| Vanillic acid | MW: 168.15 g/mol MF: C8H8O4 H-bound donor: 2 H-bound acceptor: 4 PubChem ID: CID 8468 |  | −6.05 | −6.68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guenaou, I.; Nait Irahal, I.; Errami, A.; Lahlou, F.A.; Hmimid, F.; Bourhim, N. Bioactive Compounds from Ephedra fragilis: Extraction Optimization, Chemical Characterization, Antioxidant and AntiGlycation Activities. Molecules 2021, 26, 5998. https://doi.org/10.3390/molecules26195998
Guenaou I, Nait Irahal I, Errami A, Lahlou FA, Hmimid F, Bourhim N. Bioactive Compounds from Ephedra fragilis: Extraction Optimization, Chemical Characterization, Antioxidant and AntiGlycation Activities. Molecules. 2021; 26(19):5998. https://doi.org/10.3390/molecules26195998
Chicago/Turabian StyleGuenaou, Ismail, Imane Nait Irahal, Ahmed Errami, Fatima Azzahra Lahlou, Fouzia Hmimid, and Noureddine Bourhim. 2021. "Bioactive Compounds from Ephedra fragilis: Extraction Optimization, Chemical Characterization, Antioxidant and AntiGlycation Activities" Molecules 26, no. 19: 5998. https://doi.org/10.3390/molecules26195998
APA StyleGuenaou, I., Nait Irahal, I., Errami, A., Lahlou, F. A., Hmimid, F., & Bourhim, N. (2021). Bioactive Compounds from Ephedra fragilis: Extraction Optimization, Chemical Characterization, Antioxidant and AntiGlycation Activities. Molecules, 26(19), 5998. https://doi.org/10.3390/molecules26195998