Corn Husk Phenolics Modulate Hepatic Antioxidant Response in Nile Tilapia (Oreochromis niloticus) Exposed to Hypoxia
Abstract
1. Introduction
2. Results
2.1. Phenolic Compounds Profile from Corn Husk
2.2. Growth Performance and Feed Efficiency
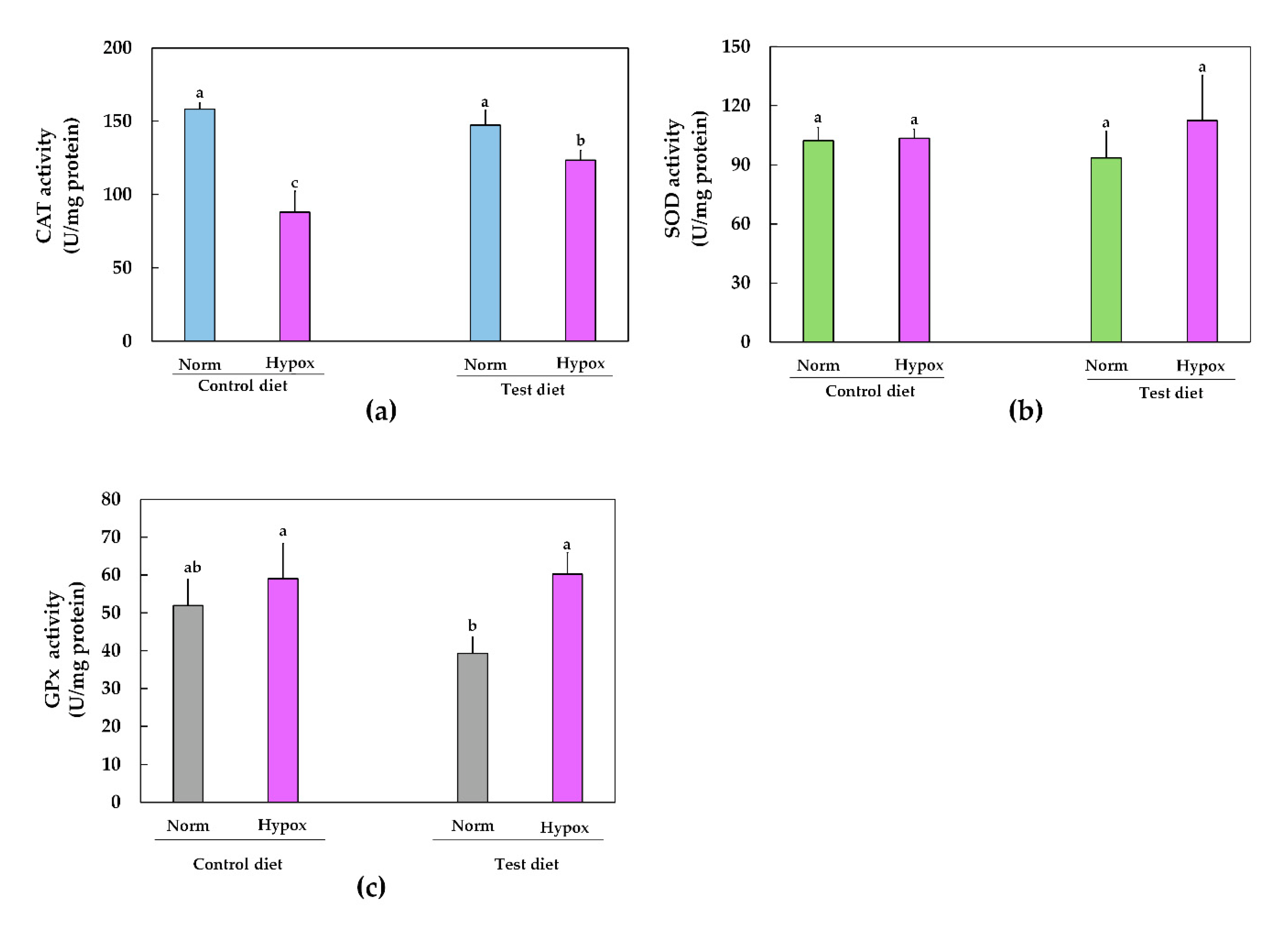
2.3. Hepatic Antioxidant-Enzyme Activities
3. Discussion
4. Material and Methods
4.1. Processing of Corn Husk
4.2. Phenolic Compound Extraction from Corn Husk
4.3. Identification and Quantification of PCs by UPLC-ESI-Q-ToF-MS/MS
4.4. Diet Preparation
4.5. Chemical Analysis of Diets
4.6. Feeding Trial (Experiment 1)
4.6.1. Growth Parameters and Feed Efficiency (Experiment 1)
4.6.2. Liver Collection in Normoxia Condition (Experiment 1)
4.7. Hypoxia-Induced Oxidative Stress and Antioxidant Enzymes (Experiment 2)
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Dawood, M.A.O.; Eweedah, N.M.; Moustafa, E.M.; Farahat, E.M. Probiotic effects of Aspergillus oryzae on the oxidative status, heat shock protein, and immune related gene expression of Nile tilapia (Oreochromis niloticus) under hypoxia challenge. Aquaculture 2020, 520, 734669. [Google Scholar] [CrossRef]
- Elbialy, Z.I.; Rizk, M.; Al-Hawary, I.I.; Salah, A.S.; Mohammed, R.A.; Assar, D.H.; Almeer, R.; Dawood, M.A.O. Yucca schidigera extract mediated the growth performance, hepato-renal function, antioxidative status and histopathological alterations in Nile tilapia (Oreochromis niloticus) exposed to hypoxia stress. Aquac. Res. 2021, 52, 1965–1976. [Google Scholar] [CrossRef]
- Mennerich, D.; Kellokumpu, S.; Kietzmann, T. Hypoxia and Reactive Oxygen Species as Modulators of Endoplasmic Reticulum and Golgi Homeostasis. Antioxid. Redox Signal. 2018, 30, 113–137. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.-L.; Qiang, J.; Tao, Y.-F.; Bao, J.-W.; Zhu, H.-J.; Li, L.-G.; Xu, P. Multi-omics analysis reveals the glycolipid metabolism response mechanism in the liver of genetically improved farmed Tilapia (GIFT, Oreochromis niloticus) under hypoxia stress. BMC Genom. 2021, 22, 105. [Google Scholar] [CrossRef] [PubMed]
- Lizárraga-Velázquez, C.E.; Hernández, C.; González-Aguilar, G.A.; Basilio-Heredia, J. Propiedades antioxidantes e inmunoestimulantes de polifenoles en peces carnívoros de cultivo. CienciaUAT 2018, 12, 127–136. [Google Scholar] [CrossRef][Green Version]
- Leyva-López, N.; Lizárraga-Velázquez, C.E.; Hernández, C.; Sánchez-Gutiérrez, E.Y. Exploitation of agro-industrial waste as potential source of bioactive compounds for aquaculture. Foods 2020, 9, 843. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Torres-Osorio, V.; Urrego, R.; Echeverri-Zuluaga, J.J.; López-Herrera, A. Estrés oxidativo y el uso de antioxidantes en la producción in vitro de embriones mamíferos. Revisión. Rev. Mex. Cienc. Pecu. 2019, 10, 433–459. [Google Scholar] [CrossRef]
- Chen, N.; Wu, M.; Tang, G.-P.; Wang, H.-J.; Huang, C.-X.; Wu, X.-J.; He, Y.; Zhang, B.; Huang, C.-H.; Liu, H.; et al. Effects of acute hypoxia and reoxygenation on physiological and immune responses and redox balance of wuchang bream (Megalobrama amblycephala Yih, 1955). Front. Physiol. 2017, 8, 375. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Jiang, X.; Kong, X.; Di, G.; Nie, G.; Li, X. Effects of hypoxia on lysozyme activity and antioxidant defences in the kidney and spleen of Carassius auratus. Aquac. Res. 2017, 48, 223–235. [Google Scholar] [CrossRef]
- Ahmed, M.K.; Islam, K.N.; Ibrahim, M.; Sultana, G.N.N.; Khan, M.S.; Akter, M.S.; Shahinuzzaman, A.; Kundu, G.K.; Hossain, A. Oxidative stress mediated antioxidant enzyme responses in tilapia (Oreochromis mossambicus) and silver carp (Hypophthalmichthys molitrix) fingerlings during hypoxic transportation and reoxygenation. Biores. Commun. 2016, 2, 264–269. [Google Scholar]
- Lizárraga-Velázquez, C.E.; Hernández, C.; González-Aguilar, G.A.; Heredia, J.B. Effect of dietary intake of phenolic compounds from mango peel extract on growth, lipid peroxidation and antioxidant enzyme activities in zebrafish (Danio rerio). Lat. Am. J. Aquat. Res. 2019, 47, 602–611. [Google Scholar] [CrossRef]
- Vicente, I.S.T.; Fleuri, L.F.; Carvalho, P.L.P.F.; Guimarães, M.G.; Naliato, R.F.; Müller, H.d.C.; Sartori, M.M.P.; Pezzato, L.E.; Barros, M.M. Orange peel fragment improves antioxidant capacity and haematological profile of Nile tilapia subjected to heat/dissolved oxygen-induced stress. Aquac. Res. 2019, 50, 80–92. [Google Scholar] [CrossRef]
- Baldissera, M.D.; Souza, C.F.; Descovi, S.N.; Petrolli, T.G.; da Silva, A.S.; Baldisserotto, B. A caffeine-supplemented diet modulates oxidative stress markers and prevents oxidative damage in the livers of Nile tilapia (Oreochromis niloticus) exposed to hypoxia. Fish Physiol. Biochem. 2019, 45, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Galeana-López, J.A.; Gómez-Gil, B.; Hernández, C.; Leyva-López, N.; Lizárraga-Velázquez, C.E.; Zenteno-Savín, T. Use of corn husk meal in the development of a functional diet for Nile tilapia (Oreochromis niloticus) fingerlings: Effect on growth performance, antioxidant status and intestinal microbiota. Waste Biomass Valorization 2021, 1–11. [Google Scholar] [CrossRef]
- Vazquez-Olivo, G.; López-Martínez, L.X.; Contreras-Angulo, L.; Heredia, J.B. Antioxidant capacity of lignin and phenolic compounds from corn stover. Waste Biomass Valorization 2019, 10, 95–102. [Google Scholar] [CrossRef]
- Tacon, A.G.J. Trends in global aquaculture and aquafeed production: 2000–2017. Rev. Fish. Sci. Aquac. 2020, 28, 43–56. [Google Scholar] [CrossRef]
- Shourbela, R.M.; El-Hawarry, W.N.; Abd El-Latif, A.M.; Abo-Kora, S.Y. Potentiality of Moringa oleifera aqueous extract as a growth modulator and antistress in acute hypoxic Nile tilapia Oreochromis niloticus. IFRO 2020, 19, 67–84. [Google Scholar]
- Santana, P.A.; Jara-Gutiérrez, C.; Mellado, M.; Forero, J.C.; Guzmán, F.; Barriga, A.; Albericio, F.; Álvarez, C.A. Effects of elderflower extract enriched with polyphenols on antioxidant defense of salmon leukocytes. Electron. J. Biotechnol. 2021, 52, 13–20. [Google Scholar] [CrossRef]
- Giri, S.S.; Jun, J.W.; Sukumaran, V.; Park, S.C. Dietary administration of banana (Musa acuminata) peel flour affects the growth, antioxidant status, cytokine responses, and disease susceptibility of rohu, Labeo rohita. J. Immunol. Res. 2016, 2016, 4086591. [Google Scholar] [CrossRef]
- Galeana-López, J.A.; Hernández, C.; Leyva-López, N.; Lizárraga-Velázquez, C.E.; Sánchez-Gutiérrez, E.Y.; Basilio Heredia, J. Corn husk extracts as an antioxidant additive in diets for Nile tilapia (Oreochromis niloticus) fingerlings: Effect on growth performance, feed intake and toxicity. Biotecnia 2020, 22, 147–154. [Google Scholar] [CrossRef]
- Maoka, T.; Tanimoto, F.; Sano, M.; Tsurukawa, K.; Tsuno, T.; Tsujiwaki, S.; Ishimaru, K.; Takii, K. Effects of dietary supplementation of ferulic acid and gamma-oryzanol on integument color and suppression of oxidative stress in cultured red sea bream, Pagrus major. J. Oleo Sci. 2008, 57, 133–137. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lizárraga-Velázquez, C.E.; Hernández, C.; González-Aguilar, G.A.; Heredia, J.B. Effect of hydrophilic and lipophilic antioxidants from mango peel (Mangifera indica L. cv. Ataulfo) on lipid peroxidation in fish oil. CyTA J. Food 2018, 16, 1095–1101. [Google Scholar] [CrossRef]
- Izli, N.; Izli, G.; Taskin, O. Impact of different drying methods on the drying kinetics, color, total phenolic content and antioxidant capacity of pineapple. CyTA J. Food 2018, 16, 213–221. [Google Scholar] [CrossRef]
- Wojdyło, A.; Lech, K.; Nowicka, P.; Hernandez, F.; Figiel, A.; Carbonell-Barrachina, A.A. Influence of different drying techniques on phenolic compounds, antioxidant capacity and colour of Ziziphus jujube Mill. fruits. Molecules 2019, 24, 2361. [Google Scholar] [CrossRef] [PubMed]
- Dongmeza, E.; Siddhuraju, P.; Francis, G.; Becker, K. Effects of dehydrated methanol extracts of moringa (Moringa oleifera Lam.) leaves and three of its fractions on growth performance and feed nutrient assimilation in Nile tilapia (Oreochromis niloticus (L.)). Aquaculture 2006, 261, 407–422. [Google Scholar] [CrossRef]
- Hassan, A.A.M.; Yacout, M.H.; Khalel, M.S.; Hafsa, S.H.A.; Ibrahim, M.A.R.; Mocuta, D.N.; Turek Rahoveanu, A.; Dediu, L. Effects of some herbal plant supplements on growth performance and the immune response in Nile tilapia (Oreochromis niloticus). Agric. Life Life Agric. Conf. Proc. 2018, 1, 134–141. [Google Scholar] [CrossRef]
- Peng, K.; Wang, G.; Wang, Y.; Chen, B.; Sun, Y.; Mo, W.; Li, G.; Huang, Y. Condensed tannins enhanced antioxidant capacity and hypoxic stress survivability but not growth performance and fatty acid profile of juvenile Japanese seabass (Lateolabrax japonicus). Anim. Feed Sci. Technol. 2020, 269, 114671. [Google Scholar] [CrossRef]
- Wheaton, W.W.; Chandel, N.S. Hypoxia. 2. Hypoxia regulates cellular metabolism. Am. J. Physiol. Cell Physiol. 2010, 300, C385–C393. [Google Scholar] [CrossRef] [PubMed]
- Bell Eric, L.; Klimova Tatyana, A.; Eisenbart, J.; Schumacker Paul, T.; Chandel Navdeep, S. Mitochondrial reactive oxygen species trigger hypoxia-inducible factor-dependent extension of the replicative life span during hypoxia. Mol. Cell. Biol. 2007, 27, 5737–5745. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmann, D.C.; Brüne, B. Mitochondrial composition and function under the control of hypoxia. Redox Biol. 2017, 12, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Qader, M.; Xu, J.; Yang, Y.; Liu, Y.; Cao, S. Natural Nrf2 activators from juices, wines, coffee, and cocoa. Beverages 2020, 6, 68. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, Z.; Lu, H.; Xu, Z.; Tong, R.; Shi, J.; Jia, G. Recent advances of natural polyphenols activators for Keap1-Nrf2 signaling pathway. Chem. Biodivers. 2019, 16, e1900400. [Google Scholar] [CrossRef]
- Jia, R.; Li, Y.; Cao, L.; Du, J.; Zheng, T.; Qian, H.; Gu, Z.; Jeney, G.; Xu, P.; Yin, G. Antioxidative, anti-inflammatory and hepatoprotective effects of resveratrol on oxidative stress-induced liver damage in tilapia (Oreochromis niloticus). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2019, 215, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Adom, K.K.; Liu, R.H. Antioxidant activity of grains. J. Agric. Food Chem. 2002, 50, 6182–6187. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 18th ed.; Association of Official Analytical Chemists: Rockville, MD, USA, 2011. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
| Phenolic Compound | Rt * [min] | m/z [M − H]− | Fragment Ions MS/MS (m/z) [M − H]− | Molecular Formula | Concentration (mg/100 g) |
|---|---|---|---|---|---|
| Chlorogenic acid | 3.73 | 353.08 | 191.05,353.08 | C16H18O9 | 10.15 |
| Ferulic acid | 5.45 | 193.058 | 134.03,178.02,193.04 | C10H10O4 | 1293.41 |
| p-coumaric acid | 5.06 | 163.047 | 117.04,163.03 | C9H8O3 | 573.87 |
| p-hydroxybenzoic acid | 3.79 | 137.032 | 137.02,122.88,137.01 | C7H6O3 | 15.38 |
| Variable | Control Diet (without CHM) | Test Diet (25 g CHM) |
|---|---|---|
| IW (g) | 5.09 ± 0.10 | 5.09 ± 0.08 |
| FW (g) | 21.40 ± 1.34 | 21.92 ± 1.34 |
| WG (%) | 320.50 ± 26.33 | 330.71 ± 24.25 |
| SGR (%/day) | 3.99 ± 0.18 | 4.05 ± 0.15 |
| FI (mg/fish/day) | 782.73 ± 64.43 | 803.79 ± 70.56 |
| FCR | 1.73 ± 0.02 | 1.72 ± 0.03 |
| HSI (%) | 1.92 ± 0.52 | 2.06 ± 0.66 |
| S (%) | 93.33 ± 6.67 | 95.56 ± 3.85 |
| Ingredient (g/kg) | Control Diet (without CHM) | Test Diet (25 g CHM) |
|---|---|---|
| Fish meal (sardine) a | 615 | 615 |
| Fish oil b | 43 | 43 |
| Cellulose c | 50 | 25 |
| Corn husk meal d | - | 25 |
| Cornstarch c | 261.4 | 261.4 |
| Alginate c | 20 | 20 |
| * Mineral premix e | 5 | 5 |
| ** Vitamin premix e | 5 | 5 |
| Vitamin C f | 0.6 | 0.6 |
| Composition (g/kg dry matter) | ||
| Dry matter | 946.45 | 945.96 |
| Crude protein | 449.99 | 449.63 |
| Crude lipid | 93.95 | 93.54 |
| Ash | 201.04 | 203.76 |
| NFE g | 255.02 | 252.79 |
| Phenolic content (g GAE/kg) | 0.00 | 0.28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galeana-López, J.A.; Lizárraga-Velázquez, C.E.; Hernández, C.; Leyva-López, N.; Heredia, J.B. Corn Husk Phenolics Modulate Hepatic Antioxidant Response in Nile Tilapia (Oreochromis niloticus) Exposed to Hypoxia. Molecules 2021, 26, 6161. https://doi.org/10.3390/molecules26206161
Galeana-López JA, Lizárraga-Velázquez CE, Hernández C, Leyva-López N, Heredia JB. Corn Husk Phenolics Modulate Hepatic Antioxidant Response in Nile Tilapia (Oreochromis niloticus) Exposed to Hypoxia. Molecules. 2021; 26(20):6161. https://doi.org/10.3390/molecules26206161
Chicago/Turabian StyleGaleana-López, José Andrés, Cynthia E. Lizárraga-Velázquez, Crisantema Hernández, Nayely Leyva-López, and J. Basilio Heredia. 2021. "Corn Husk Phenolics Modulate Hepatic Antioxidant Response in Nile Tilapia (Oreochromis niloticus) Exposed to Hypoxia" Molecules 26, no. 20: 6161. https://doi.org/10.3390/molecules26206161
APA StyleGaleana-López, J. A., Lizárraga-Velázquez, C. E., Hernández, C., Leyva-López, N., & Heredia, J. B. (2021). Corn Husk Phenolics Modulate Hepatic Antioxidant Response in Nile Tilapia (Oreochromis niloticus) Exposed to Hypoxia. Molecules, 26(20), 6161. https://doi.org/10.3390/molecules26206161