Multifunctional Small Molecules as Potential Anti-Alzheimer’s Disease Agents
Abstract
:1. Introduction
2. Results and Discussion
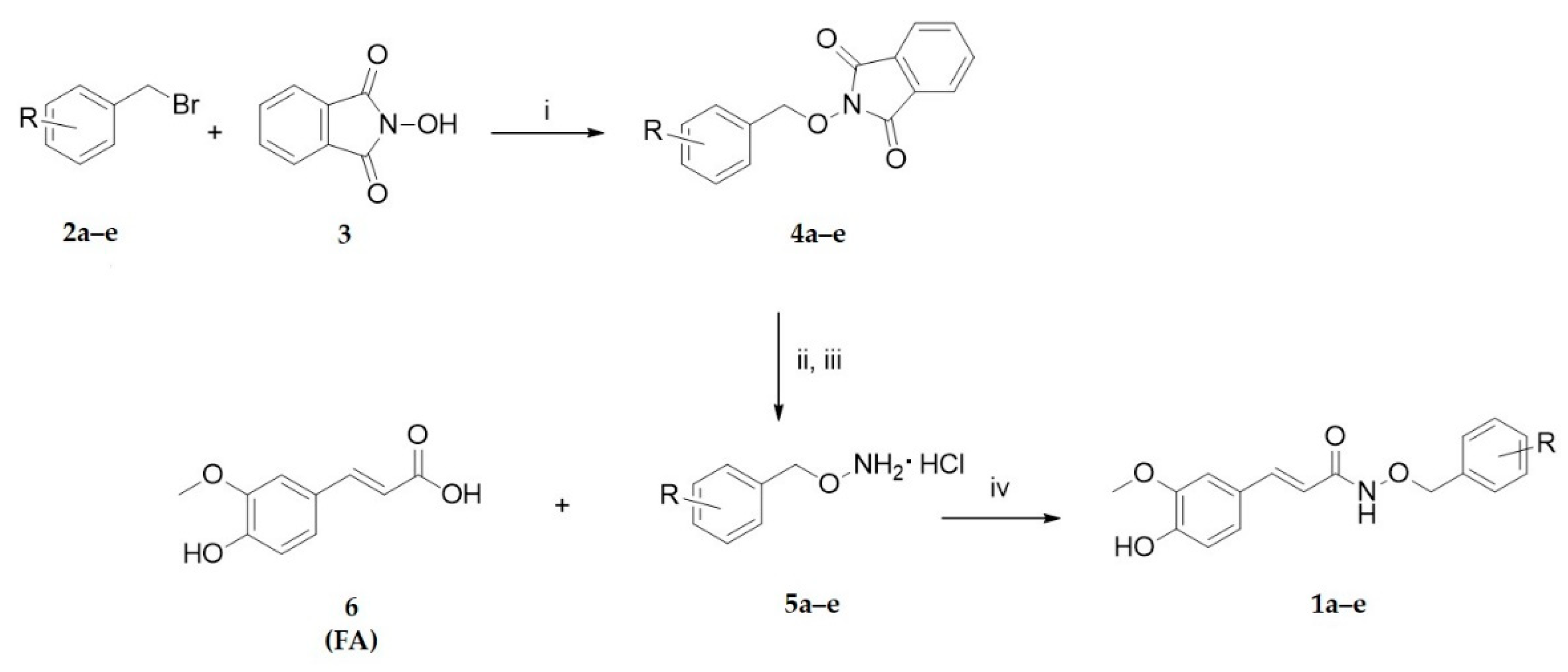
2.1. Chemistry
2.2. Physicochemical Studies
2.2.1. Antioxidant Activity
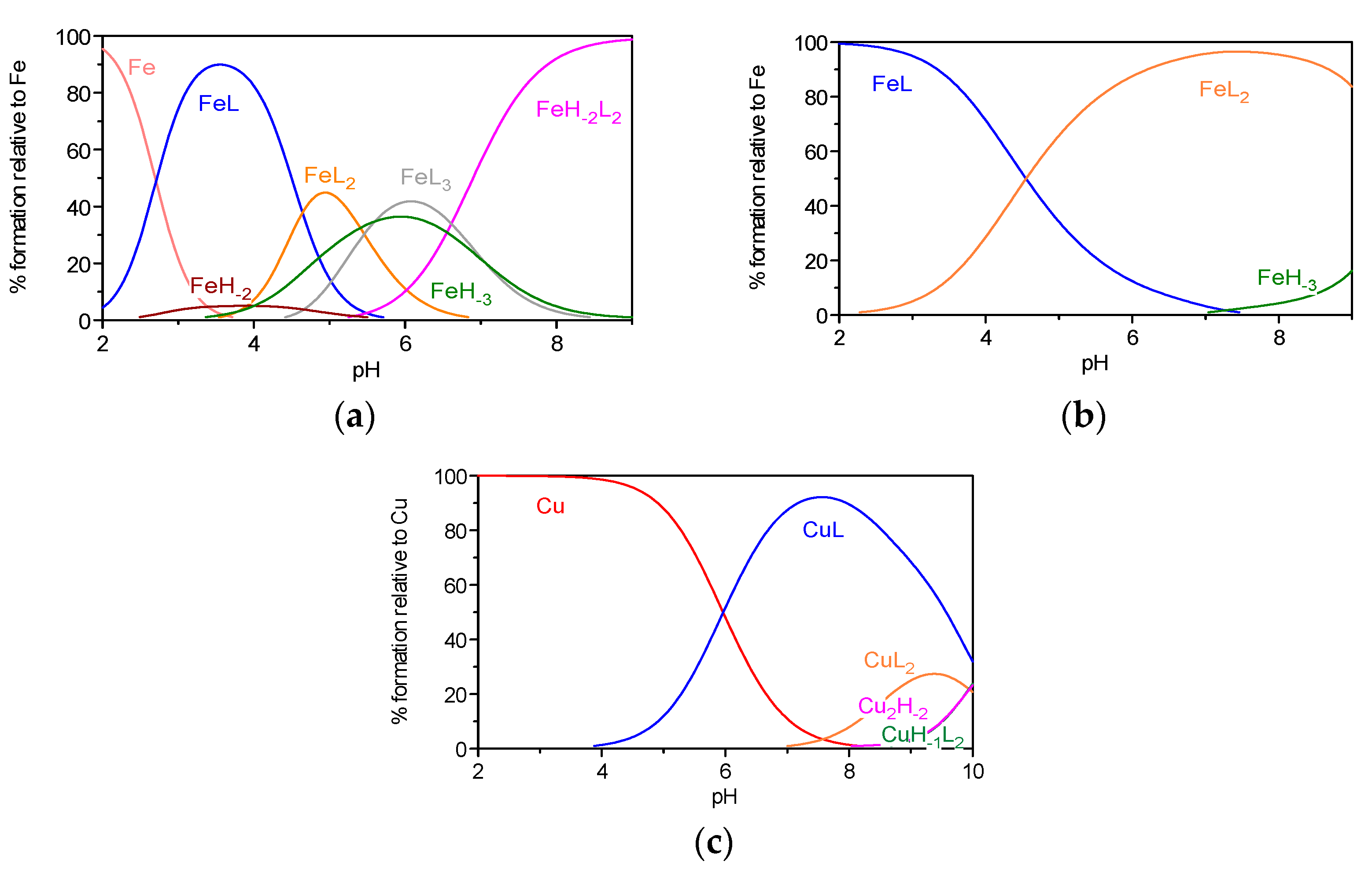
2.2.2. Metal Chelation Studies
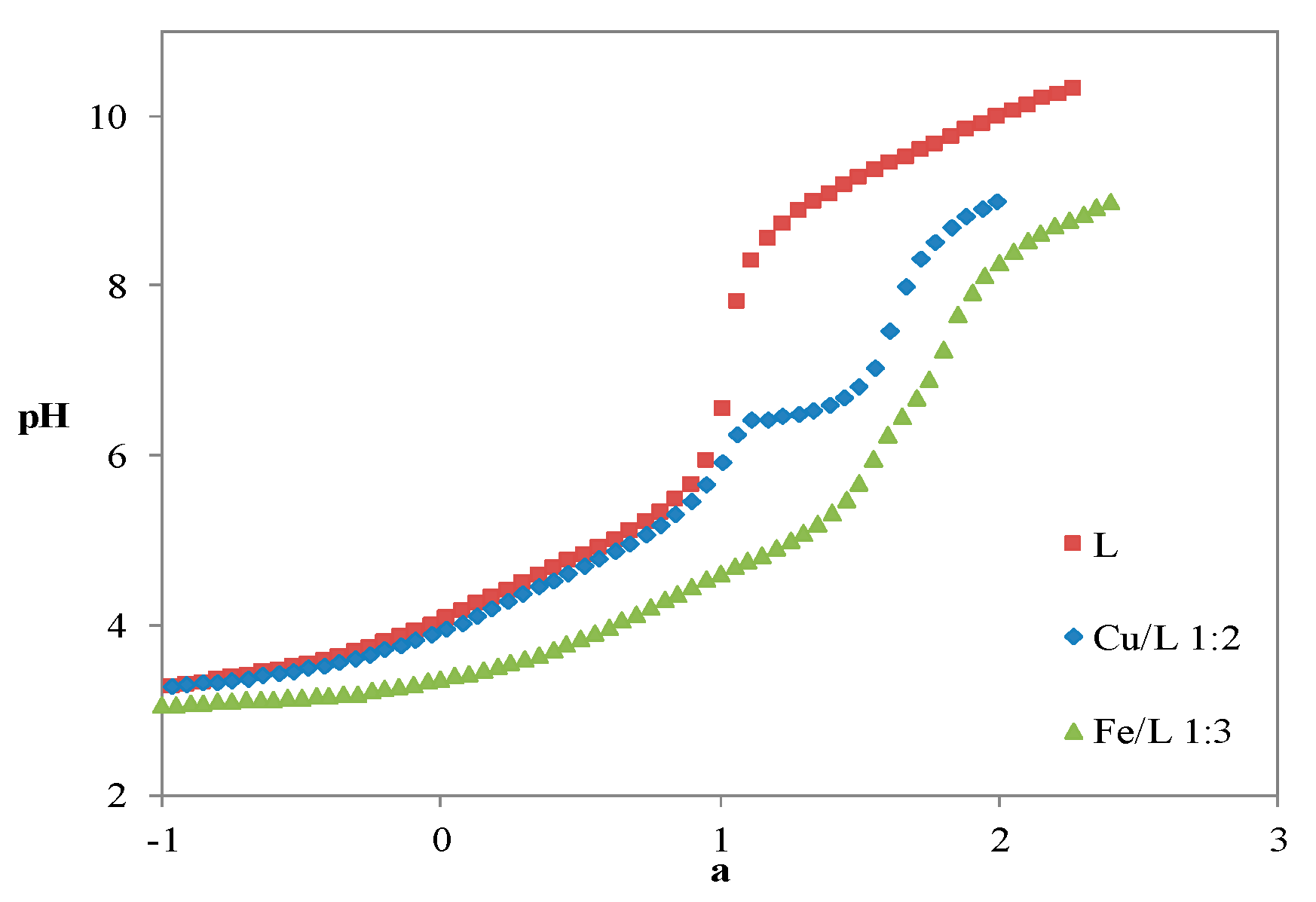
Acid-Base Properties
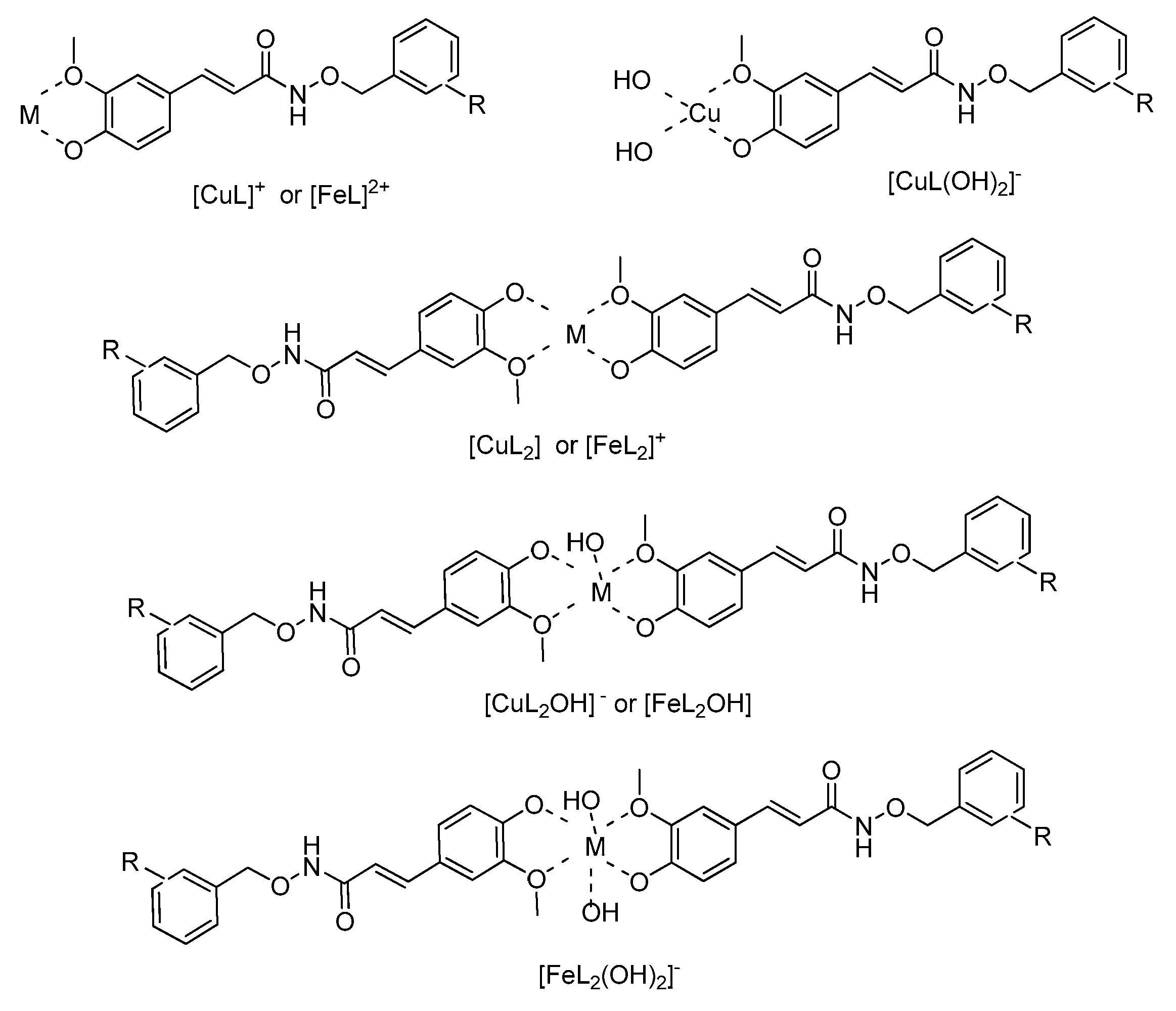
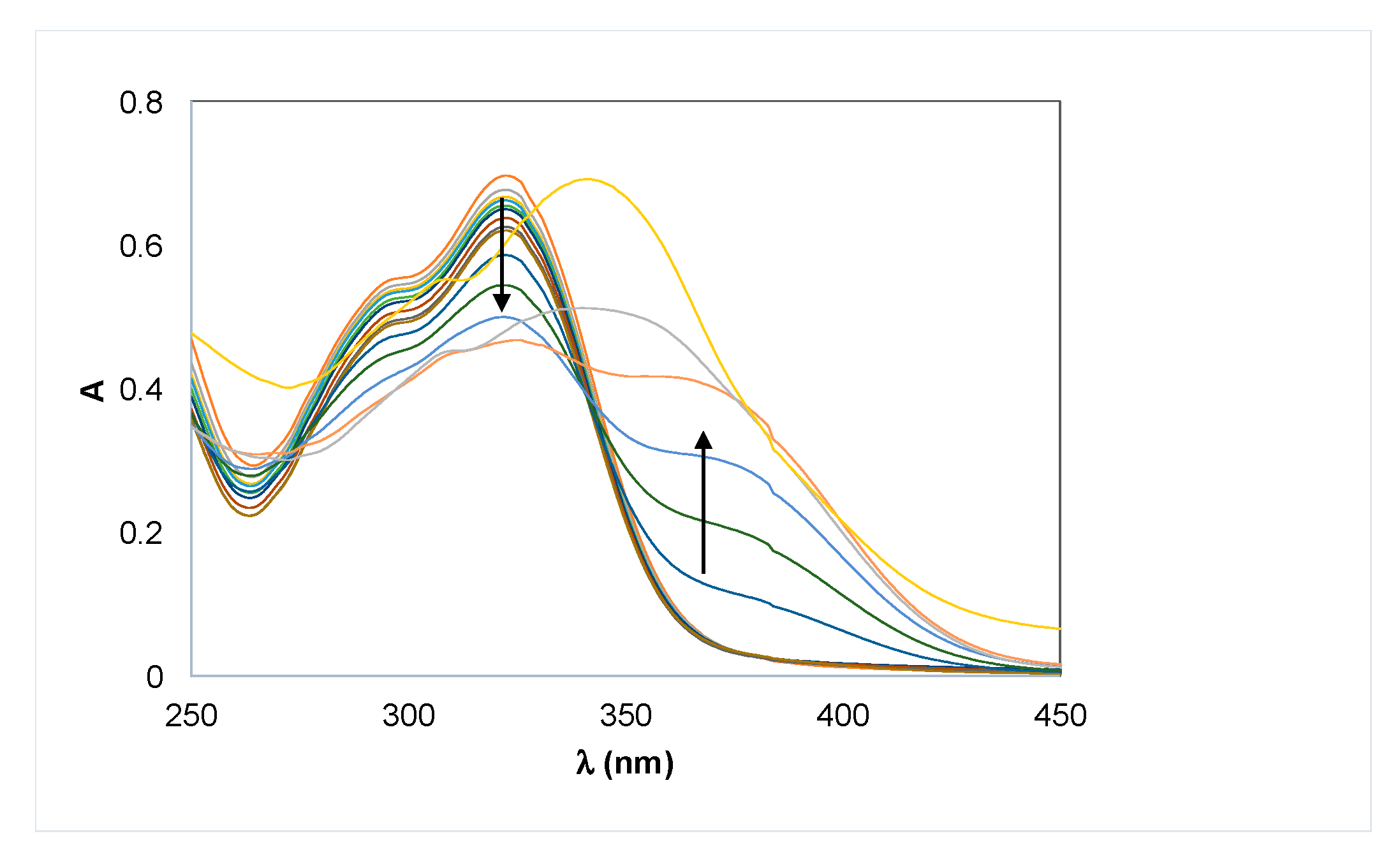
Metal Complexation
2.3. In Vitro and In Silico Studies
2.3.1. Inhibition of Aβ1–42 Aggregation
2.3.2. In Silico Pharmacokinetic Properties
3. Materials and Methods
3.1. Chemistry
3.1.1. Materials and Methods
3.1.2. General Procedure for the Synthesis of Ferulic Acid Benzyloxyamidic Derivatives (1a–e)
- (E)-3-(4-hydroxy-3-methoxyphenyl)-N-((2-methoxybenzyl)oxy)acrylamide (1a) Compound 1a was obtained as a pale yellow solid. Yield: 57%; m.p.: 58 °C. 1H-NMR (400 MHz, CD3OD-d4) δ: 7.54–7.50 (d, 1H, J = 15.8 Hz, CH=CH); 7.39–7.32 (m, 2H, Ar); 7.11 (s, 1H, Ar) 7.05–6.94 (m, 4H, Ar); 6.81–6.79 (d, 1H, J = 8.0 Hz, Ar); 6.25–6.21 (d, 1H, J = 15.8 Hz, CH=CH); 4.98 (s, 2H, OCH2); 3.88 (s, 3H, OCH3); 3.85 (s, 3H, OCH3). 13C-NMR (100 MHz, CD3OD-d4) δ: 166.9 (1C, C=O); 159.7 (1C, Ar-OCH3); 150.2 (1C, Ar-OCH3); 149.3 (1C, Ar-OH); 142.9 (1C, CH=CH); 132.2 (1C, Ar); 131.3 (1C, Ar-CH=CH); 128.1, 125.1, 123.3 (3C, Ar); 121.4 (1C, CH=CH), 116.5, 114.9, 111.8 (4C, Ar); 74.0 (OCH2); 56.4, 56.0 (2C, OCH3). m/z ESI-MS: [M + H]+ 329.96.
- (E)-3-(4-hydroxy-3-methoxyphenyl)-N-((3-methoxybenzyl)oxy)acrylamide (1b) Compound 1b was obtained as a solid. Yield: 58.3%; m.p.: 63–65 °C. 1H-NMR (400 MHz, CD3OD-d4) δ: 7.54–7.50 (d, 1H, J = 16.0 Hz, CH=CH); 7.31-7.25 (m, 1H, Ar); 7.10 (s, 1H, Ar); 7.04–6.99 (m, 3H, Ar); 6.92-6.90 (dd, 2H, J1 = 8.2 Hz, J2 = 2.0 Hz, Ar); 6.80–6.78 (d, 1H, J = 8.2 Hz, Ar); 6.25-6.21 (d, 1H, J = 16.0 Hz, CH=CH); 4.98 (s, 2H, OCH2); 3.88 (s, 3H, OCH3); 3.81 (s, 3H, OCH3). 13C-NMR (100 MHz, CD3OD-d4) δ: 166.9 (1C, C=O); 161.3, 150.2 (2C, Ar-OCH3); 149.3 (1C, Ar-OH), 143.1 (1C, Ar-CH2O); 138.5 (1C, CH=CH); 130.5 (1C, Ar-CH=CH); 128.0, 123.4 (2C, Ar); 122.4 (1C, CH=CH), 116.5, 115.3, 114.8, 111.7 (5C, Ar); 79.1 (1C, OCH2); 56.4, 55.7 (2C, OCH3). m/z ESI-MS: [M + H]+ 329.96.
- (E)-3-(4-hydroxy-3-methoxyphenyl)-N-((2-(trifluoromethyl)benzyl)oxy)acrylamide (1c) Compound 1c was obtained as a solid. Yield: 57%; m.p.: 76–77 °C. 1H-NMR (400 MHz, CD3OD-d4) δ: 7.82 (s, 1H, Ar); 7.81–7.75 (d, 1H, J = 15.2 Hz, CH=CH); 7.73–7.67 (m, 1H, Ar); 7.58–7.53 (m, 2H, Ar); 7.12 (s, 1H, J = 1.5 Hz, Ar); 7.06–7.04 (d, 1H, J = 8.0 Hz, Ar); 6.83–6.81 (d, 1H, J = 8.0 Hz, Ar); 6.28–6.25 (d, 1H, J = 15.2 Hz, CH=CH); 5.15 (s, 2H, OCH2); 3.89 (s, 3H, OCH3). 13C-NMR (100 MHz, CD3OD-d4) δ: 165.7 (1C, C=O); 148.8 (1C, Ar-OCH3); 147.9 (1C, Ar-OH); 141.8 (1C, CH=CH); 134.1, 132.0 (2C, Ar); 131.1 (1C, Ar-CH=CH); 128.5 (1C, Ar); 126.6 (1C, Ar-CF3); 125.7 (1C, Ar); 125.5 (1C, Ar); 123.0 (1C, CF3); 122.0 (1C, Ar); 115.1 (1C, CH=CH); 113.2, 110.3 (2C, Ar); 73.7 (1C, OCH2); 55.0 (1C, OCH3). m/z ESI-MS: [M + H]+ 368.08.
- (E)-N-((3-chlorobenzyl)oxy)-3-(4-hydroxy-3-methoxyphenyl)acrylamide (1d) Compound 1d was obtained as a yellow solid. Yield: 64%; m.p.: 47–48 °C. 1H-NMR (400 MHz, CD3OD-d4) δ: 7.54–7.50 (d, 1H, J = 15.0 Hz, CH=CH); 7.50 (s, 1H, Ar); 7.38–7.35 (m, 3H, Ar); 7.11–7.10 (d, 1H, J = 1.9 Hz, Ar); 7.04-7.01 (dd, 1H, J1 = 8.4 Hz, J2 = 1.9 Hz, Ar); 6.80–6.78 (d, 1H, J = 8.4 Hz, Ar); 6.25-6.21 (d, 1H, J = 15.0 Hz, CH=CH); 4.90 (s, 2H, OCH2); 3.87 (s, 3H, OCH3). 13C-NMR (100 MHz, CD3OD-d4) δ: 167.0 (1C, C=O); 150.1 (1C, Ar-OCH3); 149.2 (1C, Ar-OH) 143.1 (1C, Ar-CH2O); 139.3 (1C, CH=CH); 135.3 (1C, Ar-Cl); 130.9 (1C, Ar-CH=CH); 130.0, 129.5, 128.4, 127.8, 123.3 (5C, Ar); 116.4 (1C, CH=CH); 114.5, 111.5 (2C, Ar); 78.2 (1C, OCH2); 56.3 (1C, OCH3). m/z ESI-MS: [M + H]+ 334.04.
- (E)-N-((2,4-dichlorobenzyl)oxy)-3-(4-hydroxy-3-methoxyphenyl)acrylamide (1e) Compound 1e was obtained as a pale yellow solid. Yield: 69%; m.p.: 164.3-166.5 °C. 1H-NMR (400 MHz, CD3OD-d4) δ: 7.57 (s, 2H, Ar); 7.55–7.51 (d, 1H, J = 15.0 Hz, CH=CH); 7.39–7.37 (dd, 1H, J1 = 8.2 Hz, J2 = 1.8 Hz, Ar); 7.11–7.10 (d, 1H, J = 1.8 Hz, Ar); 7.04-7.02 (dd, 1H, J1 = 8.2 Hz, J2 = 1.8 Hz, Ar); 6.81–6.79 (d, 1H, J = 8.2 Hz, Ar); 6.24–6.20 (d, 1H, J = 15.0 Hz, CH=CH); 5.04 (s, 2H, OCH2); 3.88 (s, 3H, OCH3). 13C-NMR (100 MHz, CD3OD-d4) δ: 167.2 (1C, C=O); 150.3 (1C, Ar-OCH3); 149.3 (1C, Ar-OH) 143.3 (1C, CH=CH); 136.4 (1C, Ar-CH2O); 133.7 (2C, Ar-Cl); 130.3 (1C, Ar-CH=CH); 128.4, 128.0, 123.5 (3C, Ar); 123.4 (1C, CH=CH); 116.5, 114.6, 111.7 (3C, Ar); 75.3 (1C, OCH2); 56.4 (1C, OCH3). m/z ESI-MS: [M + H]+ 367.94.
3.2. Physicochemical and Biological Properties
3.2.1. Materials and Methods
3.2.2. Antioxidant Activity
3.2.3. Metal Chelation: Potentiometric and Spectrophotometric Studies
3.2.4. Inhibition of Self and Cu2+-Mediated Aβ1–42 Aggregation
3.2.5. In Silico ADME Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Alzheimer’s Association. 2019 Alzheimer’s Disease Facts and Figures. Alzheimer Dement. 2019, 15, 321–387. [Google Scholar] [CrossRef]
- Alzheimer’s Disease International World Alzheimer Report 2019. Available online: https://www.alz.co.uk/research/WorldAlzheimerReport2019.pdf (accessed on 26 February 2021).
- Ittner, L.M.; Götz, J. Amyloid-β and Tau—A toxic pas de deux in Alzheimer’s disease. Nat. Rev. Neurosci. 2011, 12, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Frontiers in Clinical Drug Research—Alzheimer Disorders. Available online: https://www.eurekaselect.com/170302/volume/8 (accessed on 30 March 2021).
- Canady, V.A. FDA Approves First Drug Therapy for Alzheimer’s in 18 Years. Ment. Health Wkly. 2021, 31, 3–4. [Google Scholar] [CrossRef]
- Liao, L.; Cheng, D.; Wang, J.; Duong, D.M.; Losik, T.G.; Gearing, M.; Rees, H.D.; Lah, J.J.; Levey, A.I.; Peng, J. Proteomic Characterization of Postmortem Amyloid Plaques Isolated by Laser Capture Microdissection. J. Biol. Chem. 2004, 279, 37061–37068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciccone, L.; Shi, C.; di Lorenzo, D.; Van Baelen, A.-C.; Tonali, N. The Positive Side of the Alzheimer’s Disease Amyloid Cross-Interactions: The Case of the Aβ 1-42 Peptide with Tau, TTR, CysC, and ApoA1. Molecules 2020, 25, 2439. [Google Scholar] [CrossRef]
- Ciccone, L.; Fruchart-Gaillard, C.; Mourier, G.; Savko, M.; Nencetti, S.; Orlandini, E.; Servent, D.; Stura, E.A.; Shepard, W. Copper Mediated Amyloid- β Binding to Transthyretin. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- An, S.; Fu, L. Small-Molecule PROTACs: An Emerging and Promising Approach for the Development of Targeted Therapy Drugs. EBioMedicine 2018, 36, 553–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xi, M.; Chen, Y.; Yang, H.; Xu, H.; Du, K.; Wu, C.; Xu, Y.; Deng, L.; Luo, X.; Yu, L.; et al. Small Molecule PROTACs in Targeted Therapy: An Emerging Strategy to Induce Protein Degradation. Eur. J. Med. Chem. 2019, 174, 159–180. [Google Scholar] [CrossRef]
- Tonali, N.; Nencetti, S.; Orlandini, E.; Ciccone, L. Application of PROTAC Strategy to TTR-Aβ Protein-Protein Interaction for the Development of Alzheimer’s Disease Drugs. Neural Regen. Res. 2021, 16, 1554–1555. [Google Scholar] [CrossRef]
- Raj, K.; Kaur, P.; Gupta, G.D.; Singh, S. Metals Associated Neurodegeneration in Parkinson’s Disease: Insight to Physiological, Pathological Mechanisms and Management. Neurosci. Lett. 2021, 753, 135873. [Google Scholar] [CrossRef]
- Kenche, V.B.; Barnham, K.J. Alzheimer’s Disease & Metals: Therapeutic Opportunities. Br. J. Pharmacol. 2011, 163, 211–219. [Google Scholar] [CrossRef] [Green Version]
- Ciccone, L.; Policar, C.; Stura, E.A.; Shepard, W. Human TTR Conformation Altered by Rhenium Tris-Carbonyl Derivatives. J. Struct. Biol. 2016, 195, 353–364. [Google Scholar] [CrossRef]
- Yang, G.; Liu, H.; Ma, D.-L.; Leung, C.-H. Rebalancing Metal Dyshomeostasis for Alzheimer’s Disease Therapy. J. Biol. Inorg. Chem. 2019, 24, 1159–1170. [Google Scholar] [CrossRef]
- Shcherbatykh, I.; Carpenter, D.O. The Role of Metals in the Etiology of Alzheimer’s Disease. J. Alzheimer’s Dis. JAD 2007, 11, 191–205. [Google Scholar] [CrossRef]
- Ciccone, L.; Tonali, N.; Shepard, W.; Nencetti, S.; Orlandini, E. Physiological Metals Can Induce Conformational Changes in Transthyretin Structure: Neuroprotection or Misfolding Induction? Crystals 2021, 11, 354. [Google Scholar] [CrossRef]
- Cicero, C.E.; Mostile, G.; Vasta, R.; Rapisarda, V.; Signorelli, S.S.; Ferrante, M.; Zappia, M.; Nicoletti, A. Metals and Neurodegenerative Diseases. A Systematic Review. Environ. Res. 2017, 159, 82–94. [Google Scholar] [CrossRef]
- Nam, G.; Lim, M.H. Intertwined Pathologies of Amyloid-β and Metal Ions in Alzheimer’s Disease: Metal–Amyloid-β. Chem. Lett. 2019, 48, 951–960. [Google Scholar] [CrossRef]
- Kepp, K.P.; Squitti, R. Copper Imbalance in Alzheimer’s Disease: Convergence of the Chemistry and the Clinic. Coord. Chem. Rev. 2019, 397, 168–187. [Google Scholar] [CrossRef]
- Himes, R.A.; Park, G.Y.; Siluvai, G.S.; Blackburn, N.J.; Karlin, K.D. Structural Studies of Copper(I) Complexes of Amyloid-β Peptide Fragments: Formation of Two-Coordinate Bis(Histidine) Complexes. Angew. Chem. 2008, 120, 9224–9227. [Google Scholar] [CrossRef]
- Santos, M.A.; Chand, K.; Chaves, S. Recent Progress in Multifunctional Metal Chelators as Potential Drugs for Alzheimer’s Disease. Coord. Chem. Rev. 2016, 327–328, 287–303. [Google Scholar] [CrossRef]
- Chaves, S.; Várnagy, K.; Santos, M.A. Recent Multi-Target Approaches on the Development of Anti- Alzheimer’s Agents Integrating Metal Chelation Activity. Curr. Med. Chem. 2021, 28, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.; Valentão, P.; Andrade, P.B. Polyphenols from Brown Seaweeds (Ochrophyta, Phaeophyceae): Phlorotannins in the Pursuit of Natural Alternatives to Tackle Neurodegeneration. Mar. Drugs 2020, 18, 654. [Google Scholar] [CrossRef]
- Pereira, L.; Valado, A. The Seaweed Diet in Prevention and Treatment of the Neurodegenerative Diseases. Mar. Drugs 2021, 19, 128. [Google Scholar] [CrossRef]
- Choi, B.W.; Lee, H.S.; Shin, H.-C.; Lee, B.H. Multifunctional Activity of Polyphenolic Compounds Associated with a Potential for Alzheimer’s Disease Therapy from Ecklonia Cava. Phytother. Res. 2015, 29, 549–553. [Google Scholar] [CrossRef]
- Polsinelli, I.; Nencetti, S.; Shepard, W.; Ciccone, L.; Orlandini, E.; Stura, E.A. A New Crystal Form of Human Transthyretin Obtained with a Curcumin Derived Ligand. J. Struct. Biol. 2016, 194, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Airoldi, C.; La Ferla, B.; D’Orazio, G.; Ciaramelli, C.; Palmioli, A. Flavonoids in the Treatment of Alzheimer’s and Other Neurodegenerative Diseases. Curr. Med. Chem. 2018, 25, 3228–3246. [Google Scholar] [CrossRef] [PubMed]
- Andrade, S.; Ramalho, M.J.; Loureiro, J.A.; Pereira, M.D.C. Natural Compounds for Alzheimer’s Disease Therapy: A Systematic Review of Preclinical and Clinical Studies. Int. J. Mol. Sci. 2019, 20, 2313. [Google Scholar] [CrossRef] [Green Version]
- Martins, M.; Silva, R.; Pinto, M.M.M.; Sousa, E. Marine Natural Products, Multitarget Therapy and Repurposed Agents in Alzheimer’s Disease. Pharmaceuticals 2020, 13, 242. [Google Scholar] [CrossRef]
- Ciccone, L.; Vandooren, J.; Nencetti, S.; Orlandini, E. Natural Marine and Terrestrial Compounds as Modulators of Matrix Metalloproteinases-2 (MMP-2) and MMP-9 in Alzheimer’s Disease. Pharmaceuticals 2021, 14, 86. [Google Scholar] [CrossRef]
- Sgarbossa, A.; Giacomazza, D.; Di Carlo, M. Ferulic Acid: A Hope for Alzheimer’s Disease Therapy from Plants. Nutrients 2015, 7, 5764–5782. [Google Scholar] [CrossRef]
- Singh, Y.P.; Rai, H.; Singh, G.; Singh, G.K.; Mishra, S.; Kumar, S.; Srikrishna, S.; Modi, G. A Review on Ferulic Acid and Analogs Based Scaffolds for the Management of Alzheimer’s Disease. Eur. J. Med. Chem. 2021, 215, 113278. [Google Scholar] [CrossRef]
- Balsamo, A.; Belfiore, M.; Macchia, M.; Martini, C.; Nencetti, S.; Orlandini, E.; Rossello, A. Synthesis and Aldose Reductase Inhibitory Activity of N-(Arylsulfonyl)- and N-(Aroyl)-N-(Arylmethyloxy)Glycines. Eur. J. Med. Chem. 1994, 29, 787–794. [Google Scholar] [CrossRef]
- Gentili, D.; Macchia, M.; Menchini, E.; Nencetti, S.; Orlandini, E.; Rossello, A.; Broccali, G.; Limonta, D. Synthesis and Antimicrobial Properties of Cephalosporin Derivatives Substituted on the C(7) Nitrogen with Arylmethyloxyimino or Arylmethyloxyamino Alkanoyl Groups. Il Farm. 1999, 54, 224–231. [Google Scholar] [CrossRef]
- Ciccone, L.; Nencetti, S.; Camodeca, C.; Ortore, G.; Cuffaro, D.; Socci, S.; Orlandini, E. Synthesis and Evaluation of Monoaryl Derivatives as Transthyretin Fibril Formation Inhibitors. Pharm. Chem. J. 2021. accepted. [Google Scholar]
- Borges, F.; Lima, J.L.F.C.; Pinto, I.; Reis, S.; Siquet, C. Application of a Potentiometric System with Data-Analysis Computer Programs to the Quantification of Metal-Chelating Activity of Two Natural Antioxidants: Caffeic Acid and Ferulic Acid. Helv. Chim. Acta 2003, 86, 3081–3087. [Google Scholar] [CrossRef]
- Šebestík, J.; Marques, S.M.; Falé, P.L.; Santos, S.; Arduíno, D.M.; Cardoso, S.M.; Oliveira, C.R.; Serralheiro, M.L.M.; Santos, M.A. Bifunctional Phenolic-Choline Conjugates as Anti-Oxidants and Acetylcholinesterase Inhibitors. J. Enzym. Inhib. Med. Chem. 2011, 26, 485–497. [Google Scholar] [CrossRef]
- David, J.; Barreiros, A.; David, J. Antioxidant Phenylpropanoid Esters of Triterpenes from Dioclea Lasiophylla. Pharm. Biol. 2004, 42, 36–38. [Google Scholar] [CrossRef]
- Naksuriya, O.; Okonogi, S. Comparison and Combination Effects on Antioxidant Power of Curcumin with Gallic Acid, Ascorbic Acid, and Xanthone. Drug Discov. Ther. 2015, 9, 136–141. [Google Scholar] [CrossRef] [Green Version]
- Gans, P.; Sabatini, A.; Vacca, A. Investigation of Equilibria in Solution. Determination of Equilibrium Constants with the HYPERQUAD Suite of Programs. Talanta 1996, 43, 1739–1753. [Google Scholar] [CrossRef]
- Zékány, L.; Nagypál, I.; Peintler, G. PSEQUAD, Version 5.01; Technical Software Distributors: Baltimore, MD, USA, 2001. [Google Scholar]
- Beneduci, A.; Furia, E.; Russo, N.; Marino, T. Complexation Behaviour of Caffeic, Ferulic and p-Coumaric Acids towards Aluminium Cations: A Combined Experimental and Theoretical Approach. New J. Chem. 2017, 41, 5182–5190. [Google Scholar] [CrossRef]
- Casolaro, M.; Anselmi, C.; Picciocchi, G. The Protonation Thermodynamics of Ferulic Acid/γ-Cyclodextrin Inclusion Compounds. Thermochim. Acta 2005, 425, 143–147. [Google Scholar] [CrossRef]
- Angkawijaya, A.E.; Fazary, A.E.; Hernowo, E.; Taha, M.; Ju, Y.-H. Iron(III), Chromium(III), and Copper(II) Complexes of l -Norvaline and Ferulic Acid. J. Chem. Eng. Data 2011, 56, 532–540. [Google Scholar] [CrossRef]
- Khvan, A.M.; Kristallovich, E.L.; Abduazimov, K.A. Complexation of Caffeic and Ferulic Acids by Transition-Metal Ions. Chem. Nat. Compd. 2001, 37, 72–75. [Google Scholar] [CrossRef]
- Raymond, K.N.; Carrano, C.J. Coordination Chemistry and Microbial Iron Transport. Acc. Chem. Res. 1979, 12, 183–190. [Google Scholar] [CrossRef]
- Biancalana, M.; Koide, S. Molecular Mechanism of Thioflavin-T Binding to Amyloid Fibrils. Biochim. Et Biophys. Acta (BBA) Proteins Proteom. 2010, 1804, 1405–1412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piemontese, L.; Tomás, D.; Hiremathad, A.; Capriati, V.; Candeias, E.; Cardoso, S.M.; Chaves, S.; Santos, M.A. Donepezil Structure-Based Hybrids as Potential Multifunctional Anti-Alzheimer’s Drug Candidates. J. Enzym. Inhib. Med. Chem. 2018, 33, 1212–1224. [Google Scholar] [CrossRef] [Green Version]
- Chao, X.; He, X.; Yang, Y.; Zhou, X.; Jin, M.; Liu, S.; Cheng, Z.; Liu, P.; Wang, Y.; Yu, J.; et al. Design, Synthesis and Pharmacological Evaluation of Novel Tacrine–Caffeic Acid Hybrids as Multi-Targeted Compounds against Alzheimer’s Disease. Bioorg. Med. Chem. Lett. 2012, 22, 6498–6502. [Google Scholar] [CrossRef]
- QikProp, Version 2.5; Schrödinger LLC: New York, NY, USA, 2005.
- Artursson, P.; Palm, K.; Luthman, K. Caco-2 Monolayers in Experimental and Theoretical Predictions of Drug Transport. Adv. Drug Deliv. Rev. 2012, 10, 280–289. [Google Scholar] [CrossRef]
- Rossotti, F.J.C.; Rossotti, H. Potentiometric Titrations Using Gran Plots: A Textbook Omission. J. Chem. Educ. 1965, 42, 375. [Google Scholar] [CrossRef]


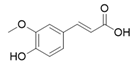
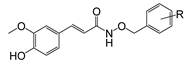 Compound | R | Radical Scavenging Activity a (EC50, μM) |
|---|---|---|
| 1a | 2-OCH3 | 34 ± 1 |
| 1b | 3-OCH3 | 40 ± 2 |
| 1c | 2-CF3 | 34 ± 1 |
| 1d | 3-Cl | 33 ± 1 |
| 1e | 2,4-Cl | 32 ± 3 |
| Ferulicacid | - | 36 ± 2 |
| Ascorbic acid | - | 25 ± 1 [40] |
| Compound | MmHhLl (mhl) | log Ki | log β | |
|---|---|---|---|---|
| (FemHhLl) a | (CumHhLl) a | |||
 FA | (011) (021) (111) (101) (1–11) (102) (1–12) (1–22) (103) pM | 9.43(2) c 4.83(4) c | - 12.15(6) c - 20.31(6) c - 7.81(8) c 26.70(8) c 17.4 | 13.30(4) c 6.52(8) c - 11.14(6) c 1.90(8) c - - 6.2 |
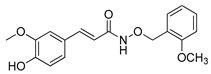 1a | (011) (101) (1–21) (102) (1–12) pM | 8.75(2) d | 16.49(3) d - 23.69(4) d - 17.6 | 6.27(7) d −13.16(7) d - 1.12(5) d 6.3 |
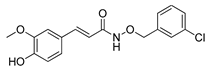 1d | (011) (101) (102) (1–12) (1–22) pM | 8.93(3) d | 15.42(7) d 25.43(5) d - 8.50(5) 18.2 | 7.49(7) d 12.14(7) d 2.20(8) d - 7.0 |
| Compound | R | Inhibition of Aβ1–42 Aggregation (%) | |
|---|---|---|---|
| Self-Aβ Aggr. | Cu-Induced Aβ Aggr. | ||
| 1a | 2-OCH3 | 42.6 | 79.6 |
| 1b | 3-OCH3 | 52.8 | 69.5 |
| 1c | 2-CF3 | 51.0 | 68.8 |
| 1d | 3-Cl | 34.1 | 76.1 |
| 1e | 2,4-Cl | 28.3 | 77.0 |
| Tacrine | - | 21.5, 22.8 [50] | - |
| Compound | MW a | clog P b | Log BB c | PCaco-2 d | Oral Absorp. e | CNS Act. f | Violations Rule of 5 g |
|---|---|---|---|---|---|---|---|
| 1a | 329.35 | −2.006 | −1.206 | 579 | 84 | -- | 0 |
| 1b | 329.35 | −2.016 | −1.196 | 579 | 84 | -- | 0 |
| 1c | 367.32 | −0.905 | −1.073 | 388 | 77 | -- | 0 |
| 1d | 333.45 | −1.073 | −0.976 | 579 | 84 | - | 0 |
| 1e | 367.9 | −0.516 | −0.752 | 732 | 85 | - | 0 |
| FA | 194.2 | 1.447 | −1.03 | 87 | 61 | -- | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bargagna, B.; Ciccone, L.; Nencetti, S.; Santos, M.A.; Chaves, S.; Camodeca, C.; Orlandini, E. Multifunctional Small Molecules as Potential Anti-Alzheimer’s Disease Agents. Molecules 2021, 26, 6015. https://doi.org/10.3390/molecules26196015
Bargagna B, Ciccone L, Nencetti S, Santos MA, Chaves S, Camodeca C, Orlandini E. Multifunctional Small Molecules as Potential Anti-Alzheimer’s Disease Agents. Molecules. 2021; 26(19):6015. https://doi.org/10.3390/molecules26196015
Chicago/Turabian StyleBargagna, Beatrice, Lidia Ciccone, Susanna Nencetti, M. Amélia Santos, Sílvia Chaves, Caterina Camodeca, and Elisabetta Orlandini. 2021. "Multifunctional Small Molecules as Potential Anti-Alzheimer’s Disease Agents" Molecules 26, no. 19: 6015. https://doi.org/10.3390/molecules26196015








