Probing Electron Excitation Characters of Carboline-Based Bis-Tridentate Ir(III) Complexes
Abstract
:1. Introduction
2. Experimental Section
2.1. General Information
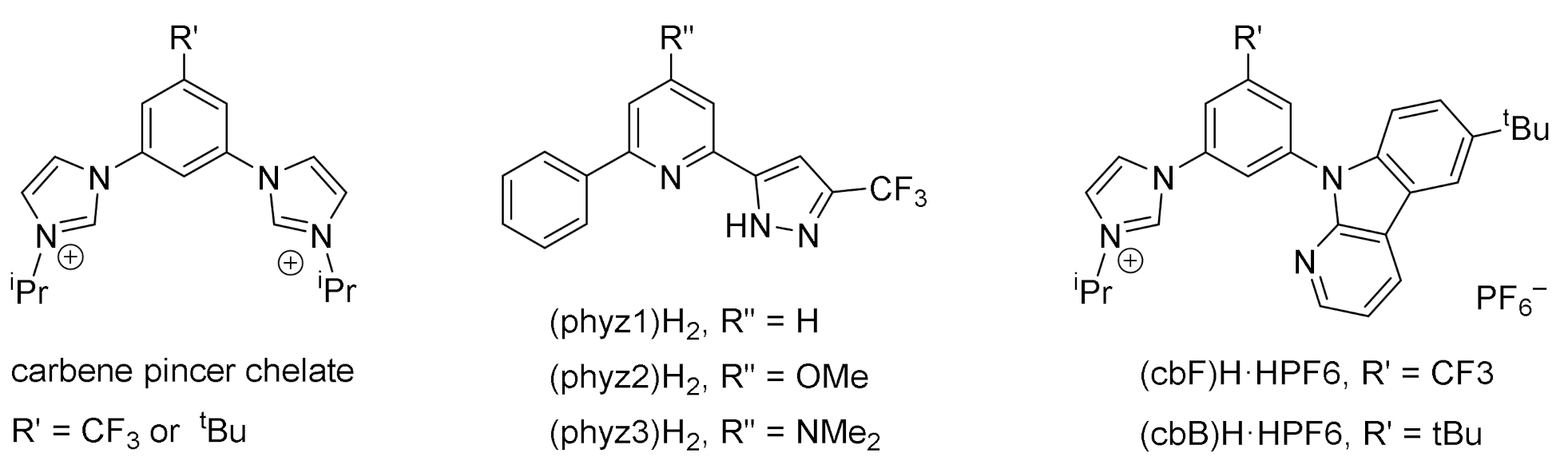
2.2. Synthesis of the Bis-Tridentate Ir(III) Metal Complexes Cb1–5
3. Results and Discussion
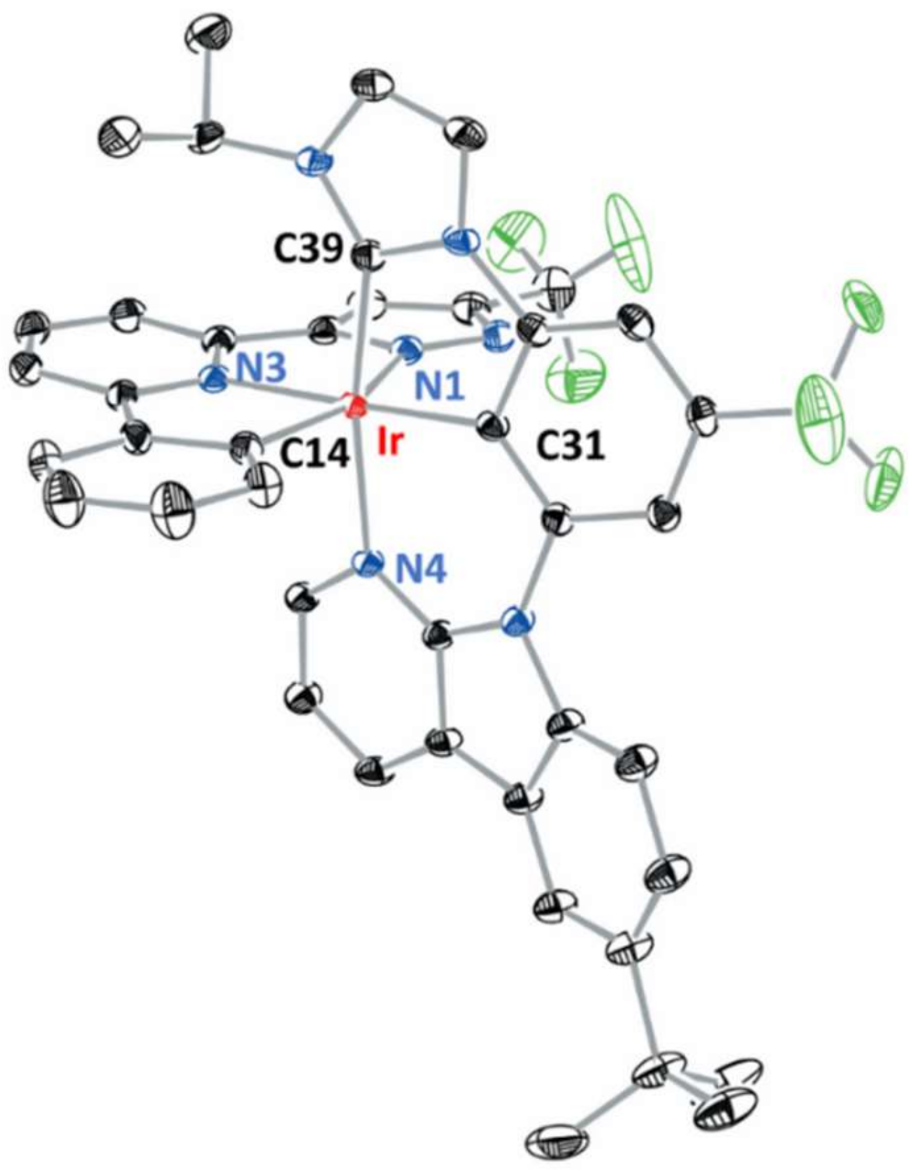
3.1. Syntheses and Characterizations
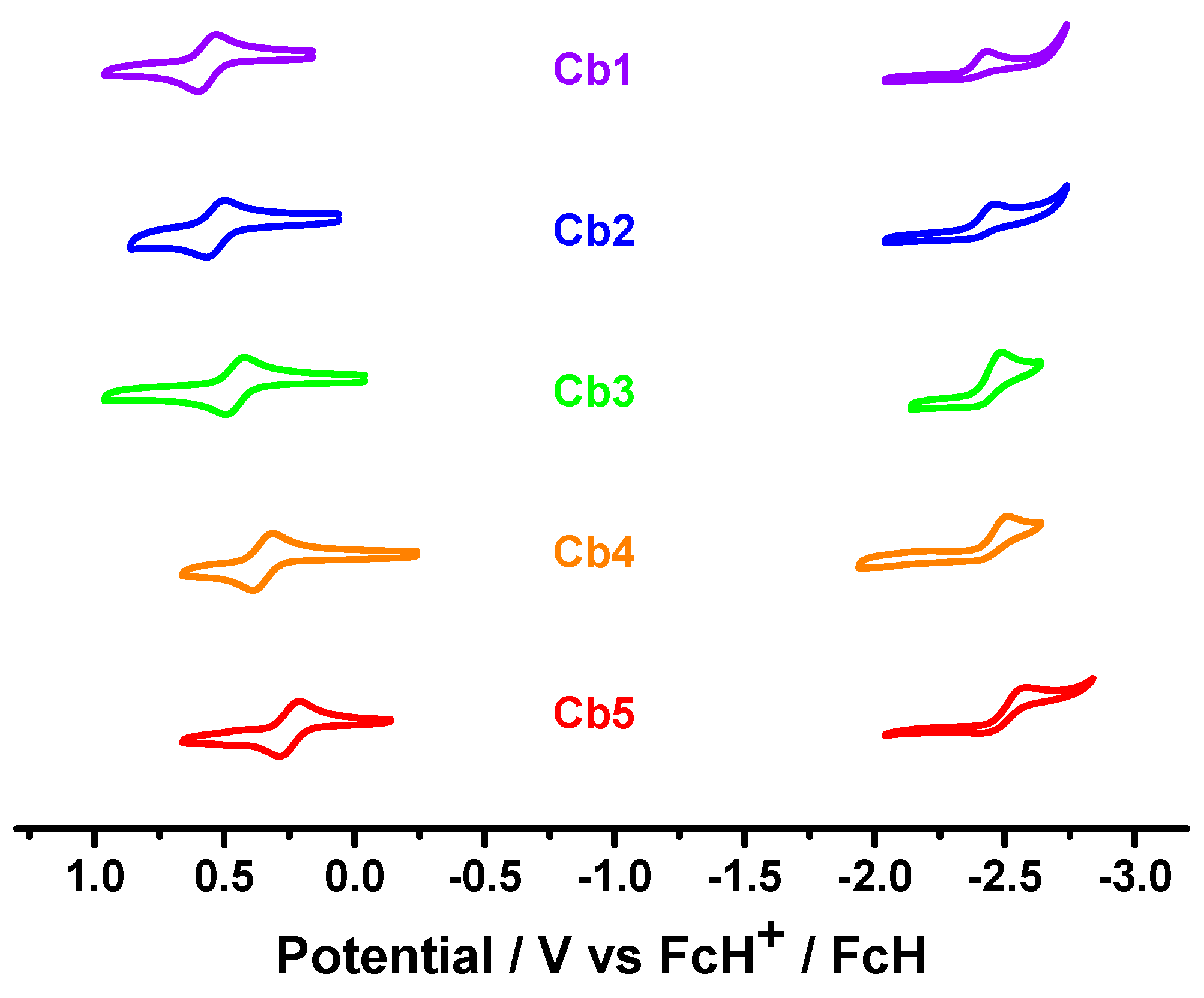
3.2. Photophysical and Electrochemical Properties
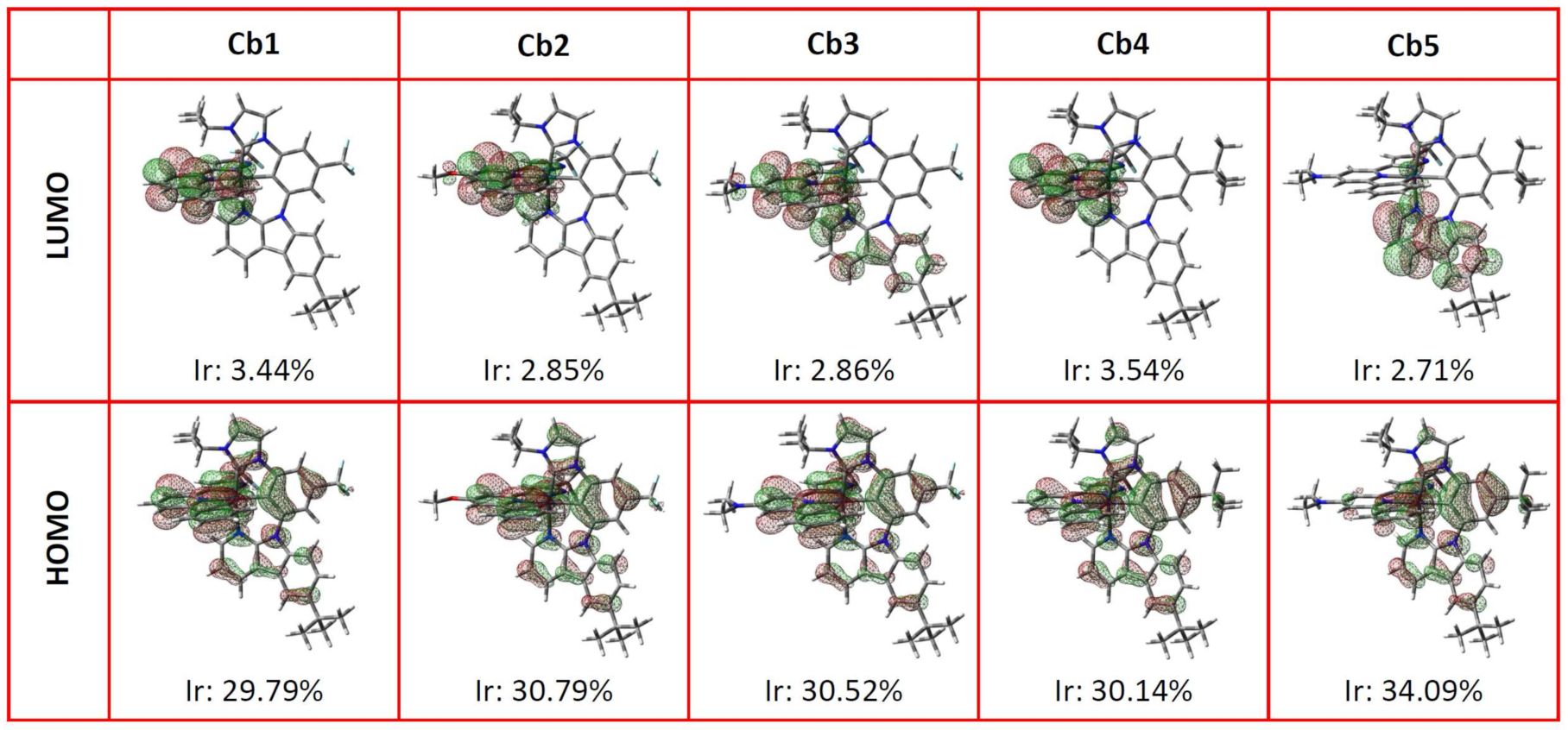
3.3. Theoretical Calculation
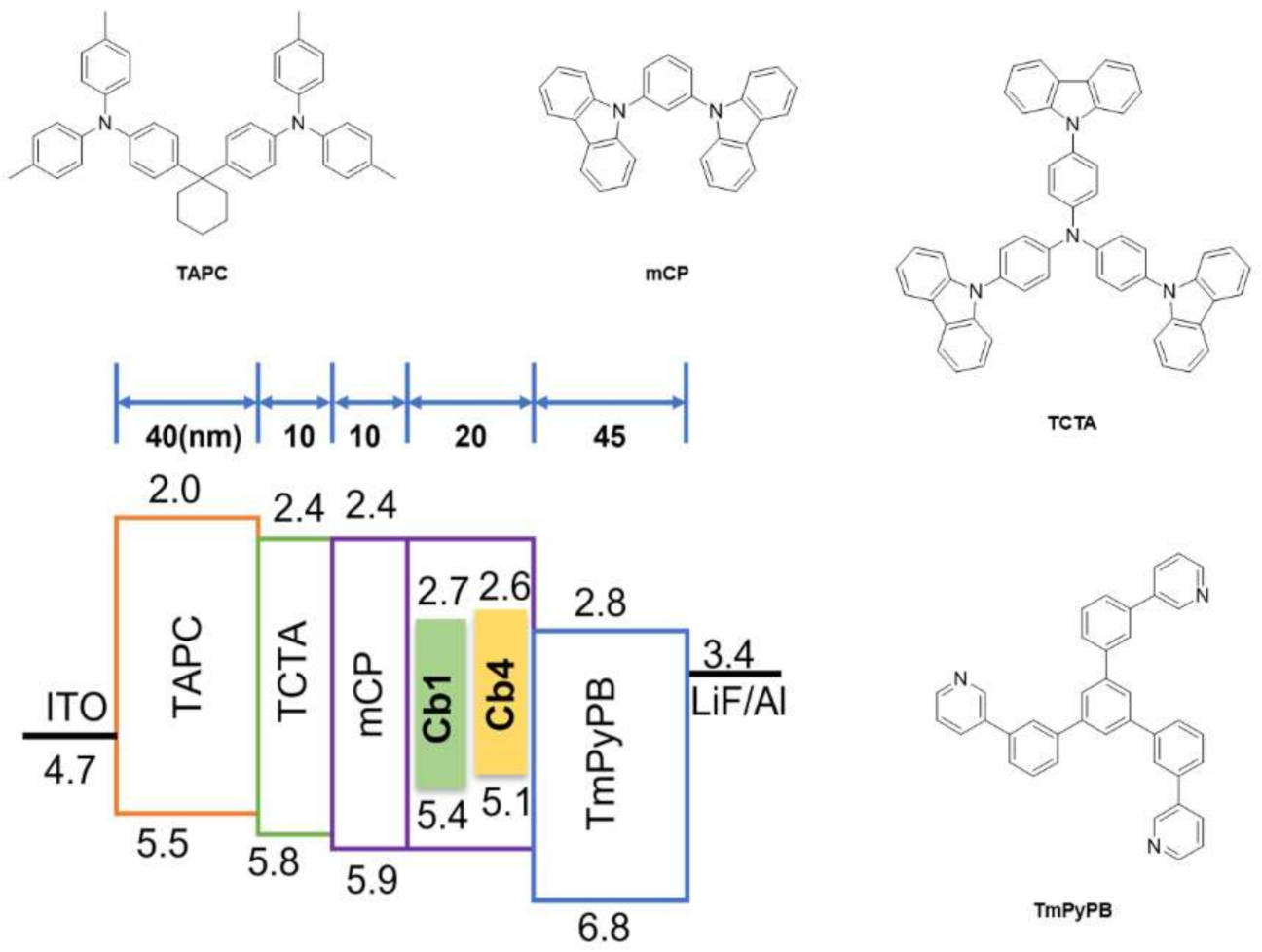
3.4. Fabrication of OLED Devices
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, D.-S.; Wu, J.; Xu, H.; Wang, Z. Emerging Light-Emitting Materials for Photonic Integration. Adv. Mater. 2021, 33, 2003733. [Google Scholar] [CrossRef] [PubMed]
- Chou, P.-T.; Chi, Y. Phosphorescent dyes for organic light-emitting diodes. Chem. Eur. J. 2007, 13, 380–395. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.; Chou, P.-T. Transition Metal Phosphors with Cyclometalating Ligands; Fundamental and Applications. Chem. Soc. Rev. 2010, 39, 638–655. [Google Scholar] [CrossRef]
- Henwood, A.F.; Zysman-Colman, E. Lessons Learned in Tuning the Optoelectronic Properties of Phosphorescent Iridium(iii) Complexes. Chem. Commun. 2017, 53, 807–826. [Google Scholar] [CrossRef] [Green Version]
- Li, T.-Y.; Wu, J.; Wu, Z.-G.; Zheng, Y.-X.; Zuo, J.-L.; Pan, Y. Rational Design of Phosphorescent Iridium(III) Complexes for Emission Color Tunability and Their Applications in OLEDs. Coord. Chem. Rev. 2018, 374, 55–92. [Google Scholar] [CrossRef]
- Li, G.; Congrave, D.G.; Zhu, D.; Su, Z.; Bryce, M.R. Recent advances in luminescent dinuclear iridium(III) complexes and their application in organic electroluminescent devices. Polyhedron 2018, 140, 146–157. [Google Scholar] [CrossRef]
- Li, G.; Zhu, D.; Wang, X.; Su, Z.; Bryce, M.R. Dinuclear metal complexes: Multifunctional properties and applications. Chem. Soc. Rev. 2020, 49, 765–838. [Google Scholar] [CrossRef] [Green Version]
- Sajoto, T.; Djurovich, P.I.; Tamayo, A.; Yousufuddin, M.; Bau, R.; Thompson, M.E.; Holmes, R.J.; Forrest, S.R. Blue and Near-UV Phosphorescence from Iridium Complexes with Cyclometalated Pyrazolyl or N-Heterocyclic Carbene Ligands. Inorg. Chem. 2005, 44, 7992–8003. [Google Scholar] [CrossRef] [PubMed]
- Daniels, R.E.; Culham, S.; Hunter, M.; Durrant, M.C.; Probert, M.R.; Clegg, W.; Williams, J.A.G.; Kozhevnikov, V.N. When two are better than one: Bright phosphorescence from non-stereogenic dinuclear iridium(iii) complexes. Dalton Trans. 2016, 45, 6949–6962. [Google Scholar] [CrossRef] [Green Version]
- Yan, Z.-P.; Luo, X.-F.; Liao, K.; Lin, Z.-X.; Wu, Z.-G.; Zhou, Y.-H.; Zheng, Y.-X. The Taiji and Eight Trigrams chemistry philosophy of chiral iridium(iii) complexes with triplex stereogenic centers. Dalton Trans. 2018, 47, 4045–4048. [Google Scholar] [CrossRef]
- Shafikov, M.Z.; Martinscroft, R.; Hodgson, C.; Hayer, A.; Auch, A.; Kozhevnikov, V.N. Non-Stereogenic Dinuclear Ir(III) Complex with a Molecular Rack Design to Afford Efficient Thermally Enhanced Red Emission. Inorg. Chem. 2021, 60, 1780–1789. [Google Scholar] [CrossRef]
- Chi, Y.; Chang, T.-K.; Ganesan, P.; Rajakannu, P. Emissive Bis-Tridentate Ir(III) Metal Complexes: Tactics, Photophysics and Applications. Coord. Chem. Rev. 2017, 346, 91–100. [Google Scholar] [CrossRef]
- Pandit, V.; Jang, J.; Babazadeh, M.; Ranasinghe, C.S.K.; Huang, D.M.; Burn, P.L.; Puttock, E.V. A solution-processed bis-tridentate iridium(iii) complex-cored dendrimer for green OLEDs. J. Mater. Chem. C 2021. [Google Scholar] [CrossRef]
- Williams, J.A.G. The coordination chemistry of dipyridylbenzene: N-deficient terpyridine or panacea for brightly luminescent metal complexes? Chem. Soc. Rev. 2009, 38, 1783–1801. [Google Scholar] [CrossRef]
- Pal, A.K.; Hanan, G.S. Design, synthesis and excited-state properties of mononuclear Ru(ii) complexes of tridentate heterocyclic ligands. Chem. Soc. Rev. 2014, 43, 6184–6197. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.D.; Lozada, I.B.; Kolodziej, C.; Burda, C.; Newman, K.M.E.; van Lierop, J.; Davis, R.L.; Herbert, D.E. Iron(ii) coordination complexes with panchromatic absorption and nanosecond charge-transfer excited state lifetimes. Nat. Chem. 2019, 11, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, A.J.; Goeta, A.E.; Foster, C.E.; Williams, J.A.G. Synthesis and Luminescence of a Charge-Neutral, Cyclometalated Iridium(III) Complex Containing N^C^N- and C^N^C-Coordinating Terdentate Ligands. Inorg. Chem. 2004, 43, 6513–6515. [Google Scholar] [CrossRef]
- Koga, Y.; Kamo, M.; Yamada, Y.; Matsumoto, T.; Matsubara, K. Synthesis, Structures, and Unique Luminescent Properties of Tridentate C^C^N Cyclometalated Complexes of Iridium. Eur. J. Inorg. Chem. 2011, 2011, 2869–2878. [Google Scholar] [CrossRef]
- Esteruelas, M.A.; Gómez-Bautista, D.; López, A.M.; Oñate, E.; Tsai, J.-Y.; Xia, C. η1-Arene Complexes as Intermediates in the Preparation of Molecular Phosphorescent Iridium(III) Complexes. Chem. Eur. J. 2017, 23, 15729–15737. [Google Scholar] [CrossRef] [Green Version]
- Boudreault, P.-L.T.; Esteruelas, M.A.; Gómez-Bautista, D.; Izquierdo, S.; López, A.M.; Oñate, E.; Raga, E.; Tsai, J.-Y. Preparation and Photophysical Properties of Bis(tridentate) Iridium(III) Emitters: Pincer Coordination of 2,6-Di(2-pyridyl)phenyl. Inorg. Chem. 2020, 59, 3838–3849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuei, C.-Y.; Liu, S.-H.; Chou, P.-T.; Lee, G.-H.; Chi, Y. Room Temperature Blue Phosphorescence; A Combined Experimental and Theoretical Study on the Bis-tridentate Ir(III) Metal Complexes. Dalton Trans. 2016, 45, 15364–15373. [Google Scholar] [CrossRef]
- Kuei, C.-Y.; Tsai, W.-L.; Tong, B.; Jiao, M.; Lee, W.-K.; Chi, Y.; Wu, C.-C.; Liu, S.-H.; Lee, G.-H.; Chou, P.-T. Bis-Tridentate Ir(III) Complexes with Nearly Unitary RGB Phosphorescence and Organic Light-Emitting Diodes with External Quantum Efficiency Exceeding 31%. Adv. Mater. 2016, 28, 2795–2800. [Google Scholar] [CrossRef]
- Kuo, H.-H.; Chen, Y.-T.; Devereux, L.R.; Wu, C.-C.; Fox, M.A.; Kuei, C.-Y.; Chi, Y.; Lee, G.-H. Bis-Tridentate Ir(III) Metal Phosphors for Efficient Deep-Blue Organic Light-Emitting Diodes. Adv. Mater. 2017, 29, 1702464. [Google Scholar] [CrossRef] [Green Version]
- Kuo, H.-H.; Zhu, Z.-L.; Lee, C.-S.; Chen, Y.-K.; Liu, S.-H.; Chou, P.-T.; Jen, A.K.-Y.; Chi, Y. Bis-tridentate Iridium(III) Phosphors with Very High Photostability and Fabrication of Blue-Emitting OLEDs. Adv. Sci. 2018, 5, 1800846. [Google Scholar] [CrossRef] [PubMed]
- Tong, B.; Ku, H.Y.; Chen, I.J.; Chi, Y.; Kao, H.-C.; Yeh, C.-C.; Chang, C.-H.; Liu, S.-H.; Lee, G.-H.; Chou, P.-T. Heteroleptic Ir(III) Phosphors with Bis-Tridentate Chelating Architecture for High Efficiency OLEDs. J. Mater. Chem. C 2015, 3, 3460–3471. [Google Scholar] [CrossRef]
- Lin, J.; Chau, N.-Y.; Liao, J.-L.; Wong, W.-Y.; Lu, C.-Y.; Sie, Z.-T.; Chang, C.-H.; Fox, M.A.; Low, P.J.; Lee, G.-H.; et al. Bis-Tridentate Iridium(III) Phosphors Bearing Functional 2-Phenyl-6-(imidazol-2-ylidene)pyridine and 2-(Pyrazol-3-yl)-6-phenylpyridine Chelates for Efficient OLEDs. Organometallics 2016, 35, 1813–1824. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.; Wang, Y.; Gnanasekaran, P.; Chiang, Y.-C.; Yang, C.-C.; Chang, C.-H.; Liu, S.-H.; Lee, G.-H.; Chou, P.-T.; Chi, Y.; et al. Unprecedented Homoleptic Bis-Tridentate Iridium(III) Phosphors: Facile, Scaled-Up Production, and Superior Chemical Stability. Adv. Funct. Mater. 2017, 27, 1702856. [Google Scholar] [CrossRef]
- Hsu, L.-Y.; Chen, D.-G.; Liu, S.-H.; Chiu, T.-Y.; Chang, C.-H.; Jen, A.K.Y.; Chou, P.-T.; Chi, Y. Roles of Ancillary Chelates and Overall Charges of Bis-tridentate Ir(III) Phosphors for OLED Applications. ACS Appl. Mater. Interfaces 2020, 12, 1084–1093. [Google Scholar] [CrossRef]
- Zheng, Y.; Batsanov, A.S.; Edkins, R.M.; Beeby, A.; Bryce, M.R. Thermally Induced Defluorination during a mer to fac Transformation of a Blue-Green Phosphorescent Cyclometalated Iridium(III) Complex. Inorg. Chem. 2012, 51, 290–297. [Google Scholar] [CrossRef]
- Zhang, D.-Y.; Zheng, Y.; Zhang, H.; Sun, J.-H.; Tan, C.-P.; He, L.; Zhang, W.; Ji, L.-N.; Mao, Z.-W. Delivery of Phosphorescent Anticancer Iridium(III) Complexes by Polydopamine Nanoparticles for Targeted Combined Photothermal-Chemotherapy and Thermal/Photoacoustic/Lifetime Imaging. Adv. Sci. 2018, 5, 1800581. [Google Scholar] [CrossRef] [Green Version]
- Motoyama, T.; Sasabe, H.; Seino, Y.; Takamatsu, J.-i.; Kido, J. An α-Carboline-containing Host Material for High-efficiency Blue and Green Phosphorescent OLEDs. Chem. Lett. 2011, 40, 306–308. [Google Scholar] [CrossRef]
- Cho, M.J.; Kim, S.J.; Yoon, S.H.; Shin, J.; Hong, T.R.; Kim, H.J.; Son, Y.H.; Kang, J.S.; Um, H.A.; Lee, T.W.; et al. New Bipolar Host Materials for Realizing Blue Phosphorescent Organic Light-Emitting Diodes with High Efficiency at 1000 cd/m2. ACS Appl. Mater. Interfaces 2014, 6, 19808–19815. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-K.; Deng, Y.-L.; Liu, X.-Y.; Yuan, X.-D.; Jiang, Z.-Q.; Liao, L.-S. A facile way to synthesize high-triplet-energy hosts for blue phosphorescent organic light-emitting diodes with high glass transition temperature and low driving voltage. Dyes and Pigm. 2015, 122, 6–12. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, M.; Cao, X.; Zhang, D.; Sun, N.; Wan, S.; Tao, Y. Carbazole/α-carboline hybrid bipolar compounds as electron acceptors in exciplex or non-exciplex mixed cohosts and exciplex-TADF emitters for high-efficiency OLEDs. J. Mater. Chem. C 2018, 6, 8784–8792. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, H.; Zang, C.; Liu, S.; Zhang, L.; Xie, W. Stable and efficient phosphorescent organic light-emitting device utilizing a δ-carboline-containing host displaying thermally activated delayed fluorescence. J. Mater. Chem. C 2020, 8, 3800–3806. [Google Scholar] [CrossRef]
- Neumann, U.; Vögtle, F. 4,4′-Donor-substituierte und 6,6′-difunktionalisierte 2,2′-Bipyridine. Chem. Ber. 1989, 122, 589–591. [Google Scholar] [CrossRef]
- Zhu, Z.-L.; Chen, W.-C.; Ni, S.-F.; Yan, J.; Wang, S.F.; Fu, L.-W.; Tsai, H.-Y.; Chi, Y.; Lee, C.-S. Constructing Deep-Blue Bis-tridentate Ir(III) Phosphors with Fluorene-Based Dianionic Chelates. J. Mater. Chem. C 2021, 9, 1318–1325. [Google Scholar] [CrossRef]
- Mairhofer, E.; Flemmich, L.; Kreutz, C.; Micura, R. Access to 3-Deazaguanosine Building Blocks for RNA Solid-Phase Synthesis Involving Hartwig–Buchwald C–N Cross-Coupling. Org. Lett. 2019, 21, 3900–3903. [Google Scholar] [CrossRef]
- Viricel, W.; Mbarek, A.; Leblond, J. Switchable Lipids: Conformational Change for Fast pH-Triggered Cytoplasmic Delivery. Angew. Chem. Int. Ed. 2015, 54, 12743–12747. [Google Scholar] [CrossRef]
- He, L.; Allwein, S.P.; Dugan, B.J.; Knouse, K.W.; Ott, G.R.; Zificsak, C.A. Synthesis of α-carboline. Org. Synth. 2016, 93, 272–293. [Google Scholar] [CrossRef] [Green Version]
- Hsu, C.-W.; Ho, S.-T.; Wu, K.-L.; Chi, Y.; Liu, S.-H.; Chou, P.-T. Ru(ii) sensitizers with a tridentate heterocyclic cyclometalate for dye-sensitized solar cells. Energy Environ. Sci. 2012, 5, 7549–7554. [Google Scholar] [CrossRef]
- Tamayo, A.B.; Alleyne, B.D.; Djurovich, P.I.; Lamansky, S.; Tsyba, I.; Ho, N.N.; Bau, R.; Thompson, M.E. Synthesis and Characterization of Facial and Meridional Tris-cyclometalated Iridium(III) Complexes. J. Am. Chem. Soc. 2003, 125, 7377–7387. [Google Scholar] [CrossRef]
- Tai, W.-S.; Gnanasekaran, P.; Chen, Y.-Y.; Hung, W.-Y.; Zhou, X.; Chou, T.-C.; Lee, G.-H.; Chou, P.-T.; You, C.; Chi, Y. Rational Tuning of Bis-Tridentate Ir(III) Phosphors to Deep-Blue with High Efficiency and Sub-microsecond Lifetime. ACS Appl. Mater. Interfaces 2021, 13, 15437–15447. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.L.; Hsu, L.Y.; Tai, W.S.; Ni, S.F.; Lee, C.S.; Chi, Y. Revealing the Role of 1,2,4-Triazolate Fragment of Blue-Emitting Bis-tridentate Ir(III) Phosphors: Photophysical Properties, Photo-stabilities, and Applications. Mater. Today Energy 2021, 20, 100636. [Google Scholar] [CrossRef]
- von Malm, N.; Steiger, J.; Schmechel, R.; von Seggern, H. Trap engineering in organic hole transport materials. J. Appl. Phys. 2001, 89, 5559–5563. [Google Scholar] [CrossRef]
- Liao, J.-L.; Chi, Y.; Sie, Z.-T.; Ku, C.-H.; Chang, C.-H.; Fox, M.A.; Low, P.J.; Tseng, M.-R.; Lee, G.-H. Ir(III)-Based Phosphors with Bipyrazolate Ancillaries; Rational Design, Photophysics, and Applications in Organic Light-Emitting Diodes. Inorg. Chem. 2015, 54, 10811–10821. [Google Scholar] [CrossRef] [Green Version]

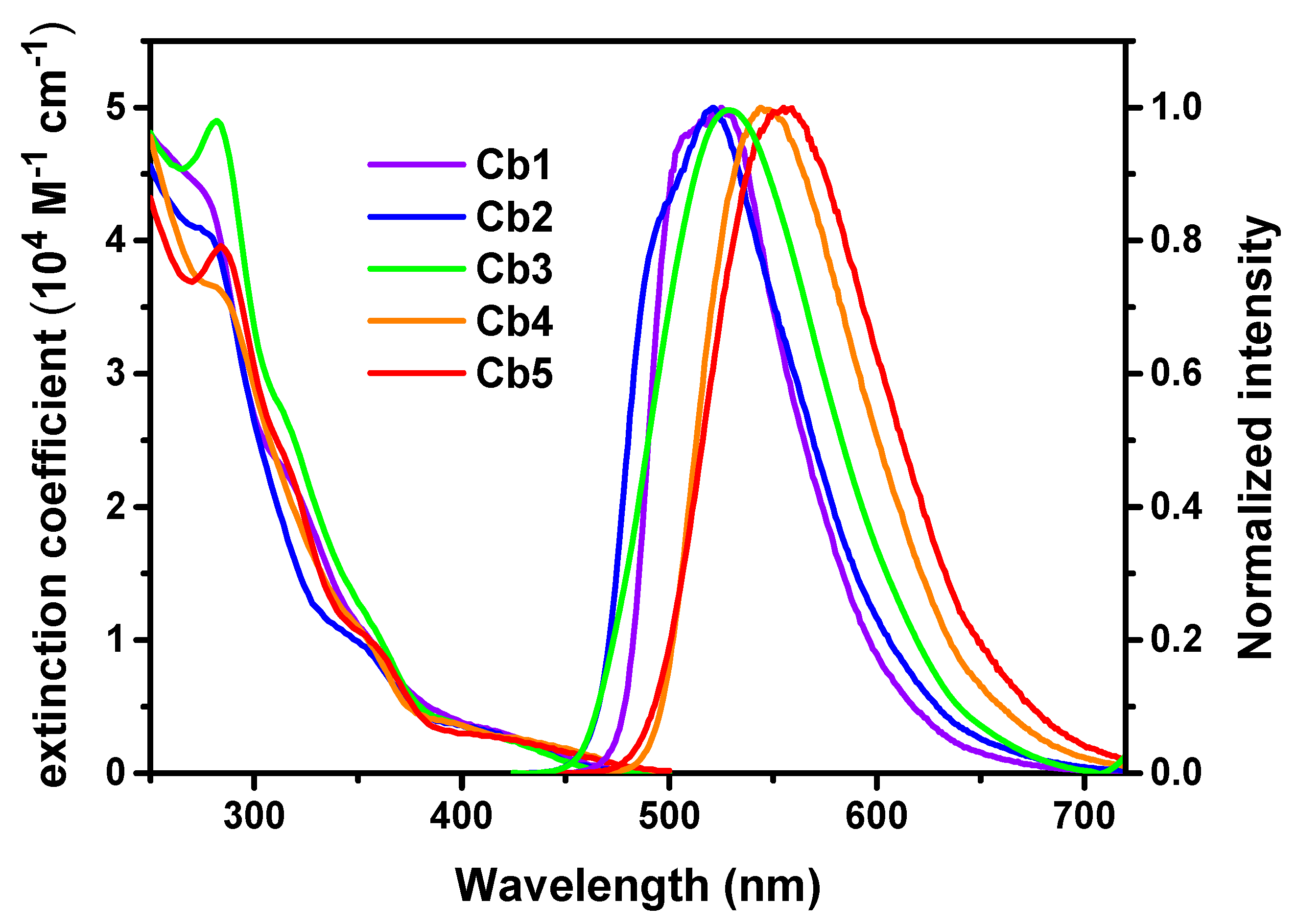

| λabs (nm); (ε × 104 M−1 cm−1) [a] | λem (nm) [b] | Φ (%) [b,c] | τobs (μs) [b] | kr (s−1) [d] | knr (s−1) [d] | |
|---|---|---|---|---|---|---|
| Cb1 | 278 (4.31), 318 (2.19), 358 (0.96), 420 (0.3) | 506(sh), 525 | 69 | 3.4 | 2.0 × 105 | 0.91 × 105 |
| Cb2 | 280 (4.02), 352 (0.96), 416 (0.3) | 495(sh), 521 | 48 | 1.5 | 3.2 × 105 | 3.4 × 105 |
| Cb3 | 282 (4.9), 316 (2.63), 358 (1.09), 418 (0.28) | 529 | 41 | 1.2 | 3.4 × 105 | 4.9 × 105 |
| Cb4 | 284 (3.62), 354 (1.02), 398 (0.37), 446 (0.2) | 544 | 58 | 2.0 | 2.9 × 105 | 2.1 × 105 |
| Cb5 | 284 (3.96), 316 (2.32), 356(0.99), 418 (0.27) | 555 | 46 | 1.4 | 3.3 × 105 | 3.8 × 105 |
| Eox½ (V) (ΔEp) [a] | Erepc (V) [b] | HOMO (eV) [c] | Energy Gap (eV) [d] | LUMO (eV) [e] | |
|---|---|---|---|---|---|
| Cb1 | 0.56 (0.07) | −2.42 | −5.36 | 2.64 | −2.72 |
| Cb2 | 0.53 (0.07) | −2.45 | −5.33 | 2.66 | −2.67 |
| Cb3 | 0.45 (0.07) | −2.48 | −5.25 | 2.65 | −2.60 |
| Cb4 | 0.35 (0.08) | −2.50 | −5.15 | 2.52 | −2.63 |
| Cb5 | 0.25 (0.07) | −2.56 | −5.05 | 2.53 | −2.52 |
| Complex | State | λ (nm) | f | Main Assignments | MLCT |
|---|---|---|---|---|---|
| Cb1 | S0 → T1 | 459.1 | 0 | HOMO → LUMO + 1 (19%) | 13.52% |
| S0 → S1 | 402.7 | 0.0105 | HOMO → LUMO + 1 (87%) | 24.82% | |
| T1 → S0 | 588.4 | 0 | LUMO → HOMO (72%) | 18.97% | |
| S1 → S0 | 488.2 | 0.0083 | LUMO → HOMO (96%) | 30.30% | |
| Cb2 | S0 → T1 | 449.2 | 0 | HOMO → LUMO (29%) | 18.62% |
| S0 → S1 | 391.2 | 0.0425 | HOMO → LUMO (94%) | 29.18% | |
| T1 → S0 | 573 | 0 | LUMO → HOMO (73%) | 20.40% | |
| S1 → S0 | 484.2 | 0.053 | LUMO → HOMO (98%) | 30.01% | |
| Cb3 | S0 → T1 | 437.4 | 0 | HOMO → LUMO (26%) | 19.13% |
| S0 → S1 | 394.8 | 0.0509 | HOMO → LUMO (94%) | 30.36% | |
| T1 → S0 | 557.4 | 0 | LUMO → HOMO (72%) | 19.92% | |
| S1 → S0 | 490 | 0.0553 | LUMO → HOMO (98%) | 31.27% | |
| Cb4 | S0 → T1 | 462.1 | 0 | HOMO → LUMO + 1 (87%) | 23.64% |
| S0 → S1 | 417.8 | 0.0074 | HOMO → LUMO + 1 (85%) | 23.09% | |
| T1 → S0 | 587.7 | 0 | LUMO → HOMO (72%) | 19.15% | |
| S1 → S0 | 511 | 0.0062 | LUMO → HOMO (96%) | 27.39% | |
| Cb5 | S0 → T1 | 449.5 | 0 | HOMO → LUMO (83%) | 25.75% |
| S0 → S1 | 413.4 | 0.0432 | HOMO → LUMO (96%) | 29.78% | |
| T1 → S0 | 598.9 | 0 | LUMO → HOMO (76%) | 23.85% | |
| S1 → S0 | 520.2 | 0.0423 | LUMO → HOMO (98%) | 28.86% |
| λEL [nm] | Von [V] [a] | Max. Luminescence [cd/m2] (@voltage [V]) | EQE [%] [b] | CE [cd/A] [b] | |
|---|---|---|---|---|---|
| Cb1 | 503(sh), 530 | 3.5 | 12420 (11.5) | 16.6, 16.5, 15.4 | 55.2, 52.9, 51.1 |
| Cb4 | 559 | 3.5 | 21480 (13) | 13.9, 13.1, 12.1 | 43.8, 43.0, 38.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, J.; Zhu, Z.-L.; Lee, C.-S.; Liu, S.-H.; Chou, P.-T.; Chi, Y. Probing Electron Excitation Characters of Carboline-Based Bis-Tridentate Ir(III) Complexes. Molecules 2021, 26, 6048. https://doi.org/10.3390/molecules26196048
Yan J, Zhu Z-L, Lee C-S, Liu S-H, Chou P-T, Chi Y. Probing Electron Excitation Characters of Carboline-Based Bis-Tridentate Ir(III) Complexes. Molecules. 2021; 26(19):6048. https://doi.org/10.3390/molecules26196048
Chicago/Turabian StyleYan, Jie, Ze-Lin Zhu, Chun-Sing Lee, Shih-Hung Liu, Pi-Tai Chou, and Yun Chi. 2021. "Probing Electron Excitation Characters of Carboline-Based Bis-Tridentate Ir(III) Complexes" Molecules 26, no. 19: 6048. https://doi.org/10.3390/molecules26196048







