Synthesis and Pharmacological Evaluation of Novel 2,3,4,5-tetrahydro[1,3]diazepino[1,2-a]benzimidazole Derivatives as Promising Anxiolytic and Analgesic Agents
Abstract
:1. Introduction
2. Results and Discussion
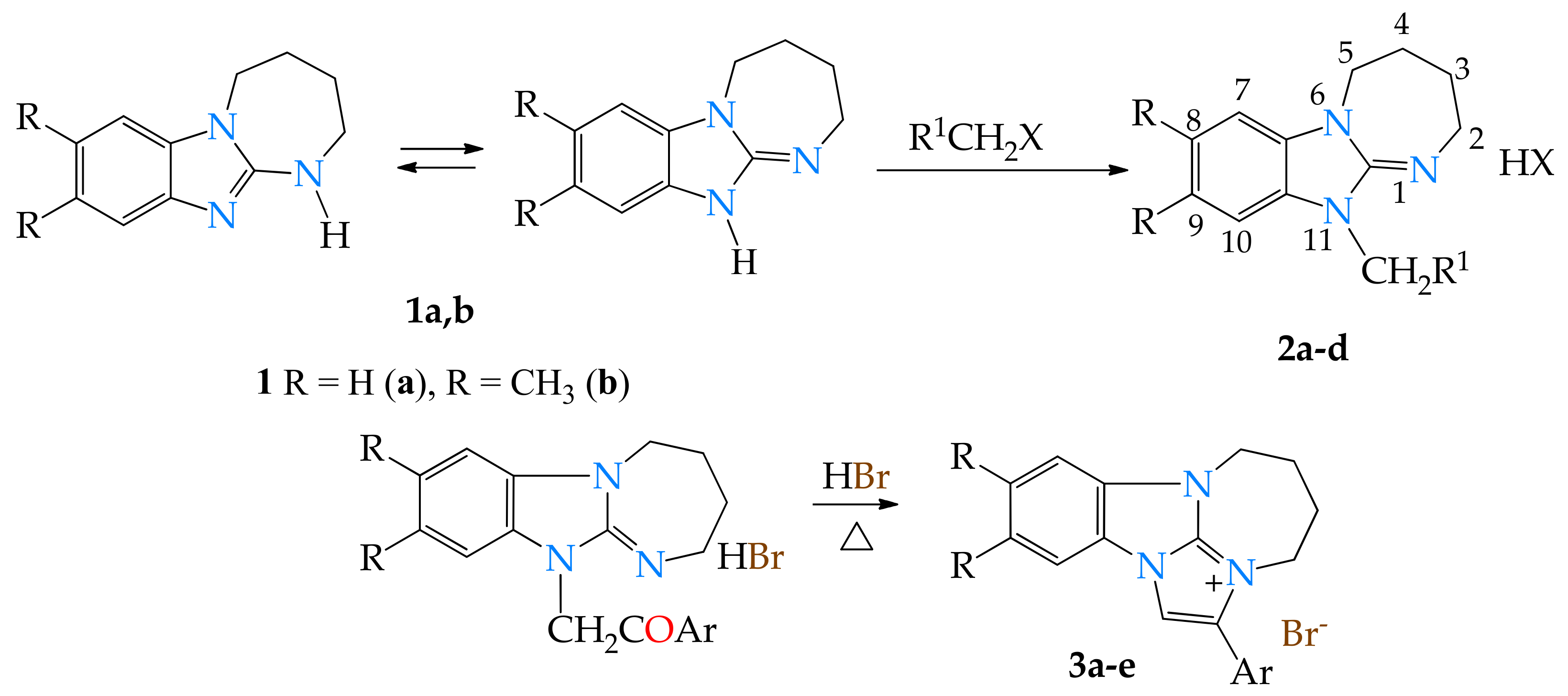
2.1. Chemistry
2.2. Pharmacology
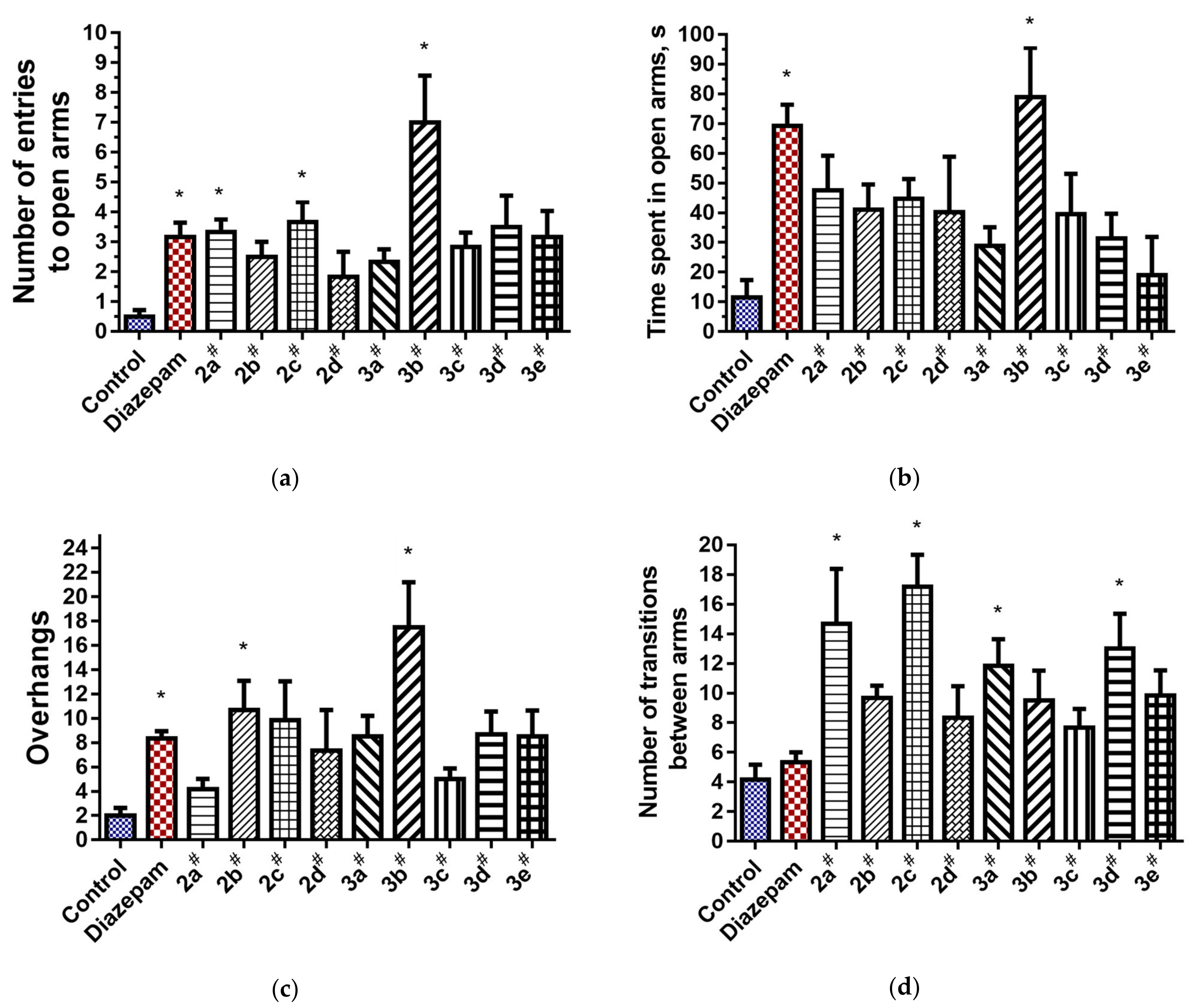
2.2.1. Elevated Plus Maze Test
2.2.2. Open Field and Spontaneous Locomotor Activity Assessment
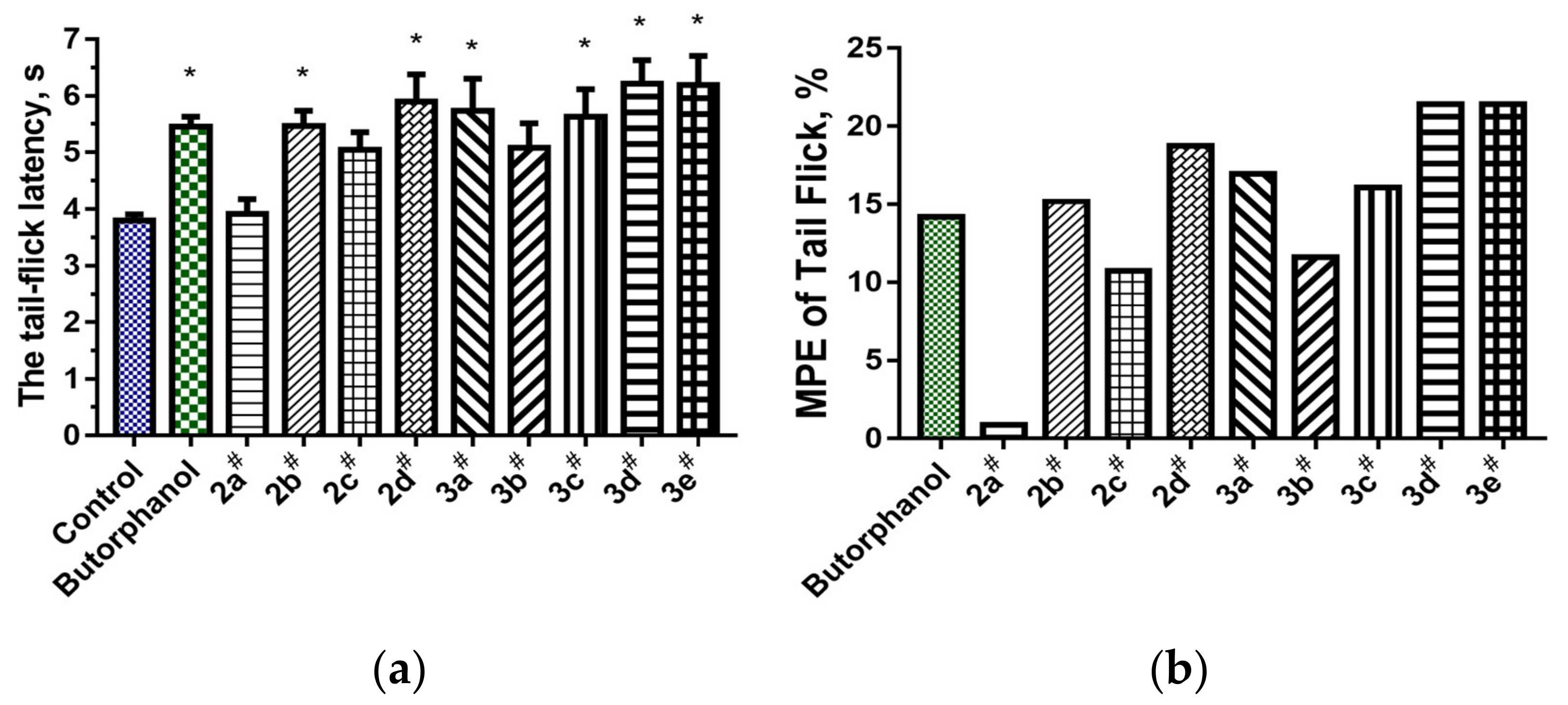
2.2.3. Tail-Flick Test
2.2.4. Hot Plate Test
2.2.5. Structure–Activity Relationships of Compounds 2 and 3
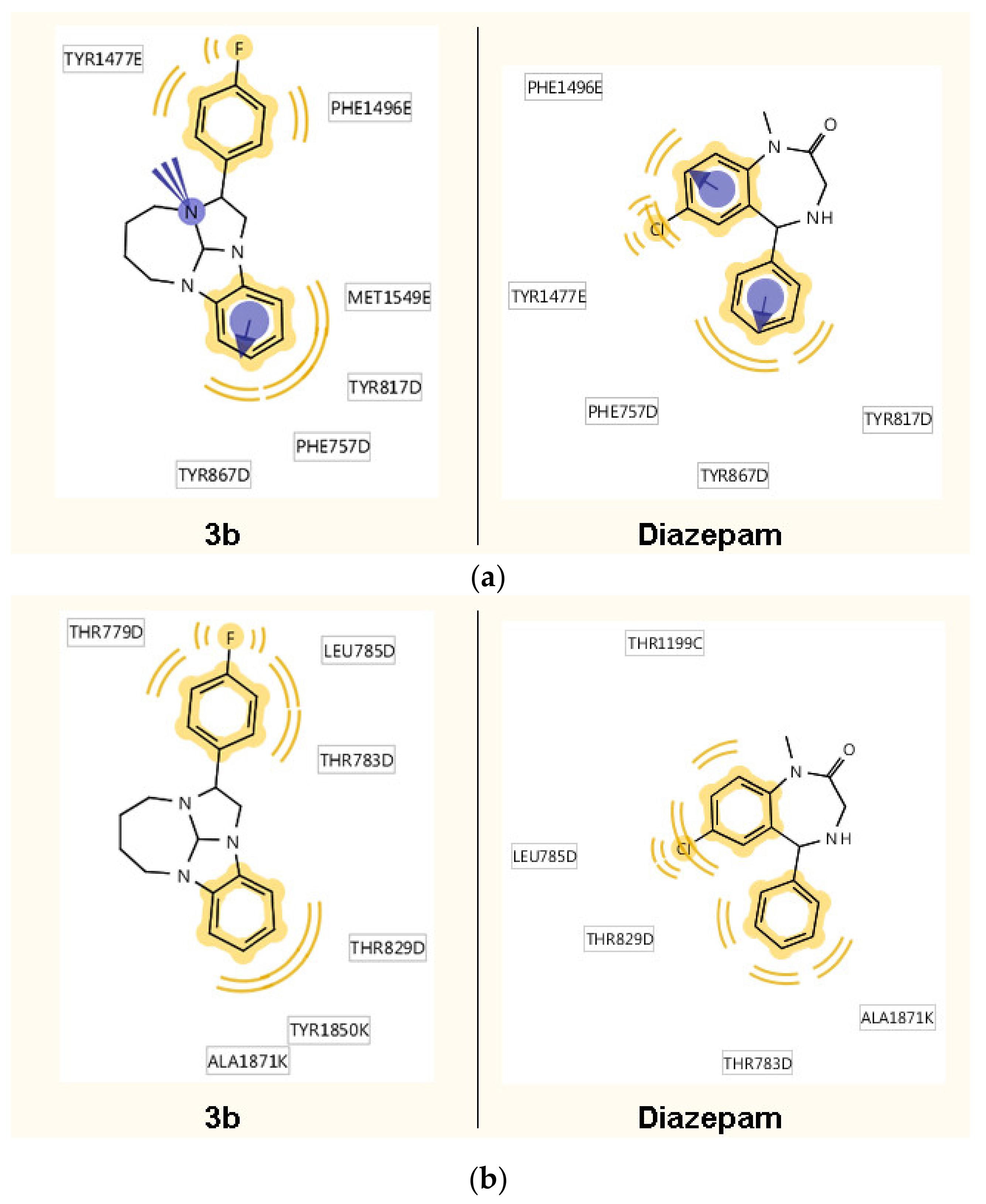
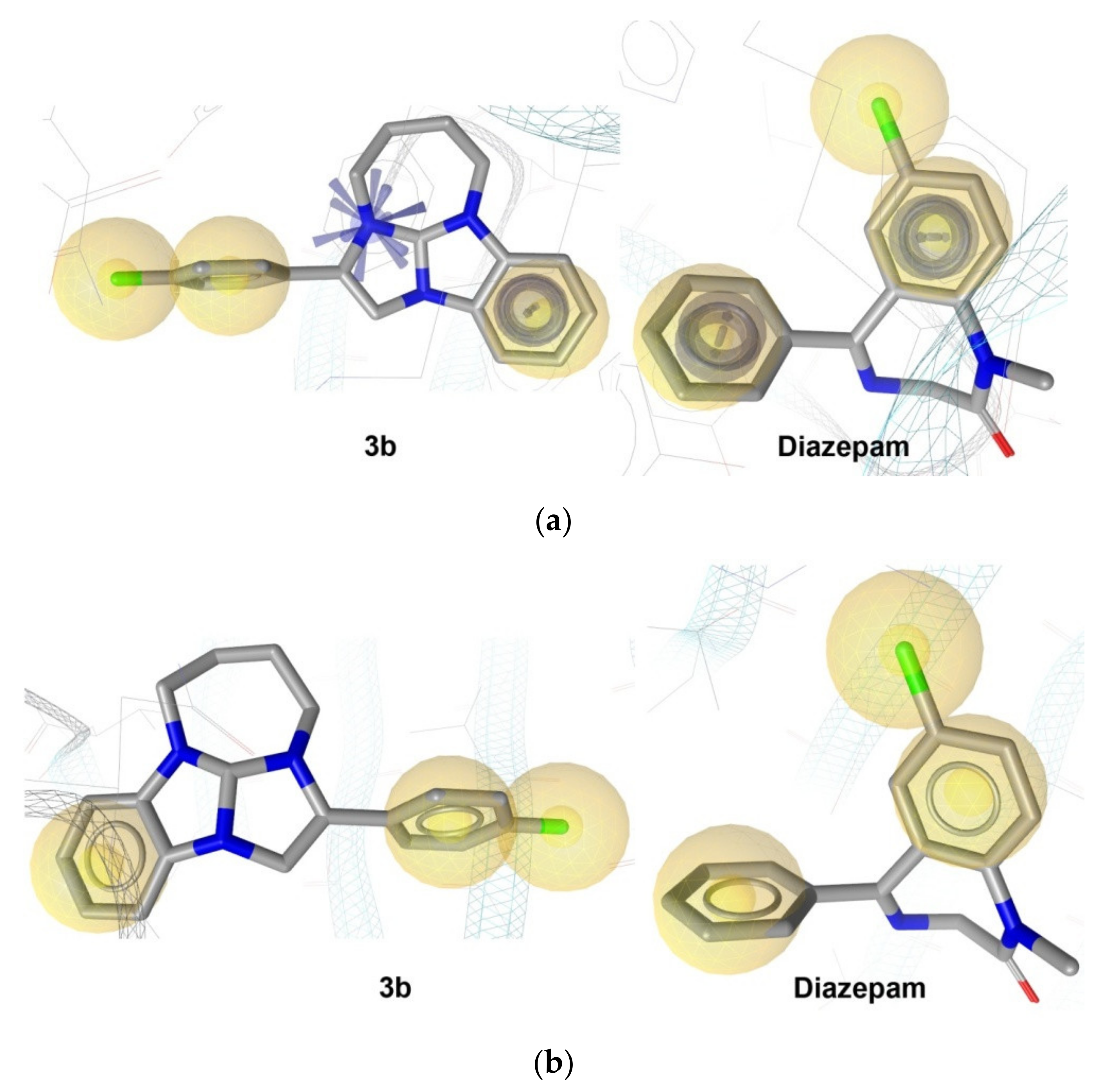
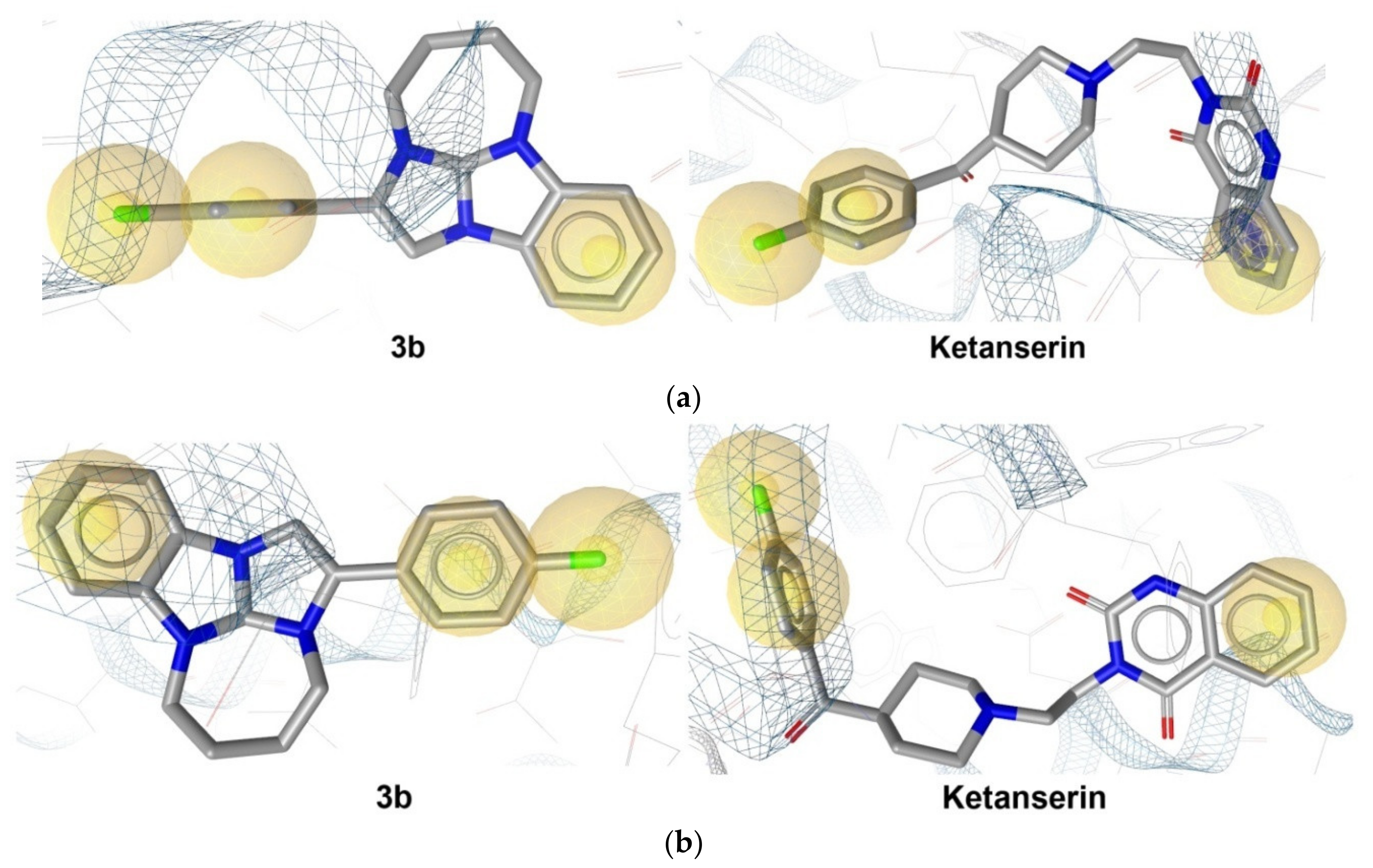
2.2.6. In Silico Analysis
3. Materials and Methods
3.1. General Procedure for the Synthesis of Compounds 2
3.1.1. 11-(4-chlorobenzyl)-2,3,4,5-tetrahydro[1,3]diazepino[1,2-a]benzimidazole Hydrochloride (2a)
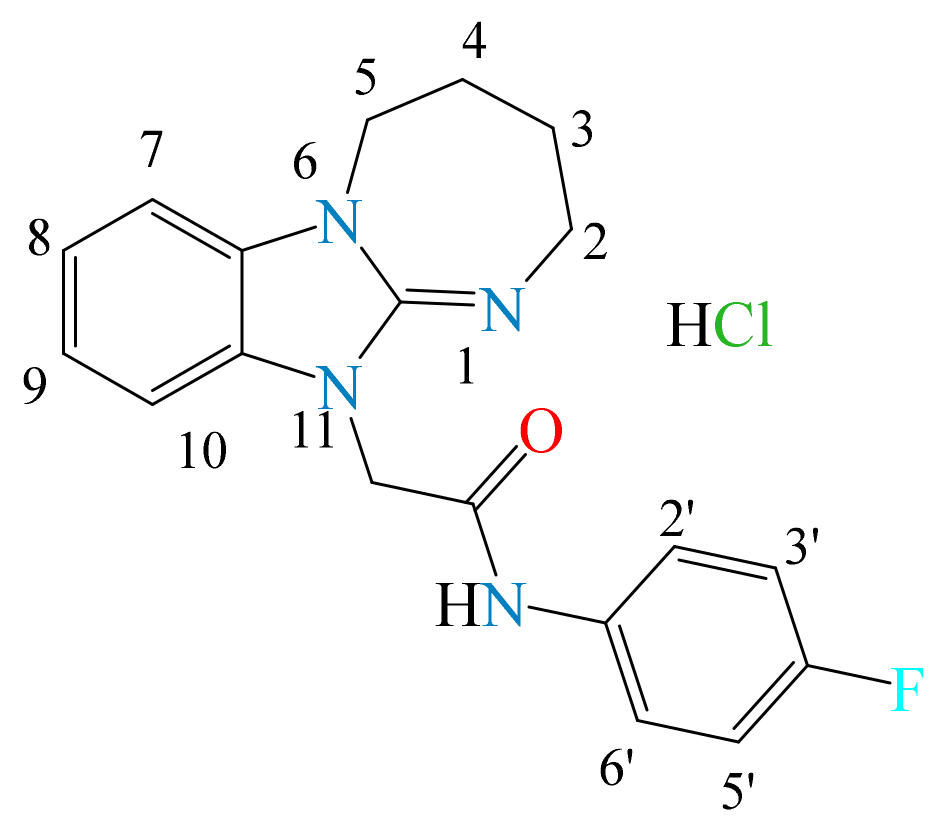
3.1.2. N-(4-fluorophenyl)-2-(2,3,4,5-tetrahydro[1,3]diazepino[1,2-a]benzimidazol-11-yl)acetamide Hydrochloride (2b)
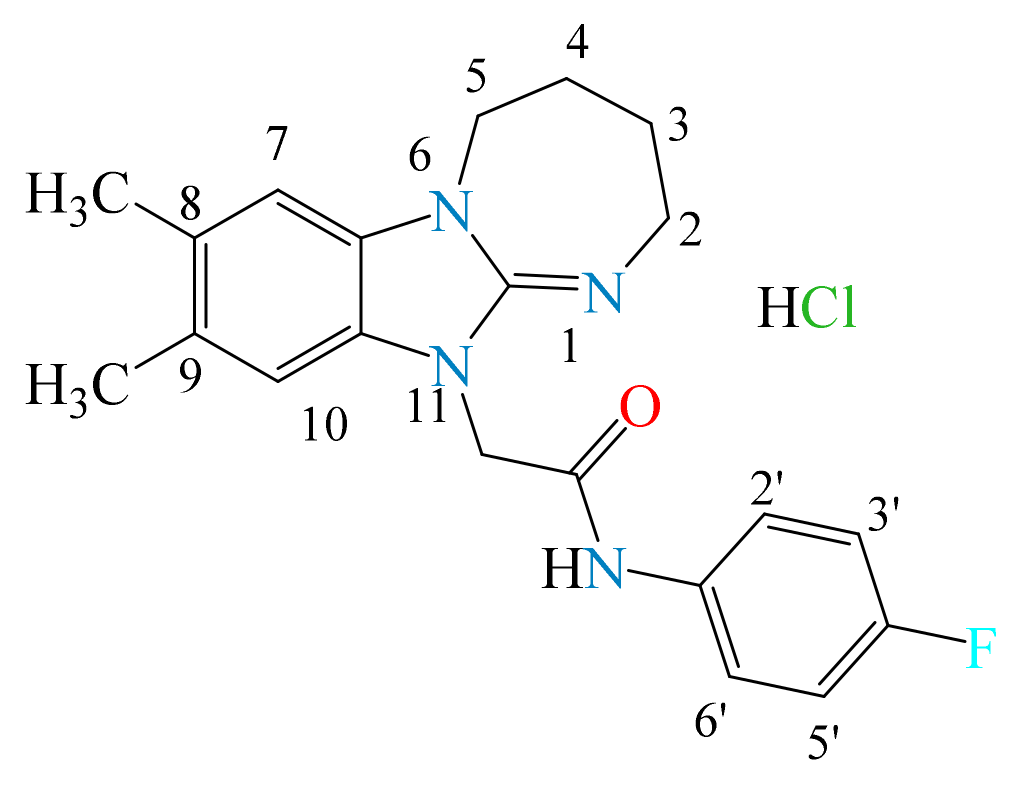
3.1.3. N-(4-fluorophenyl)-2-(8,9-dimethyl-2,3,4,5-tetrahydro[1,3]diazepino[1,2-a]benzimidazol-11-yl)acetamide Hydrochloride (2c)
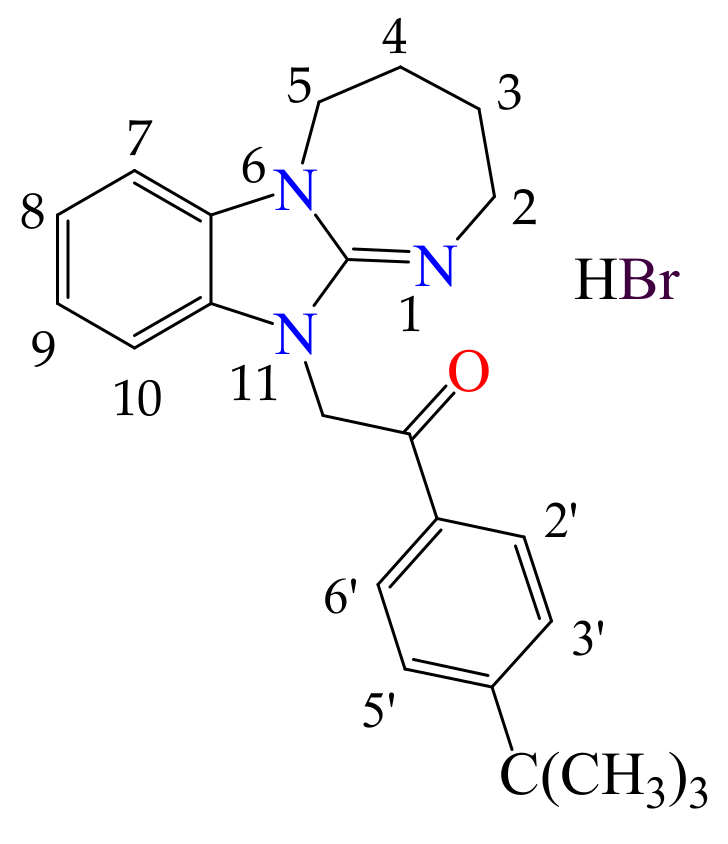
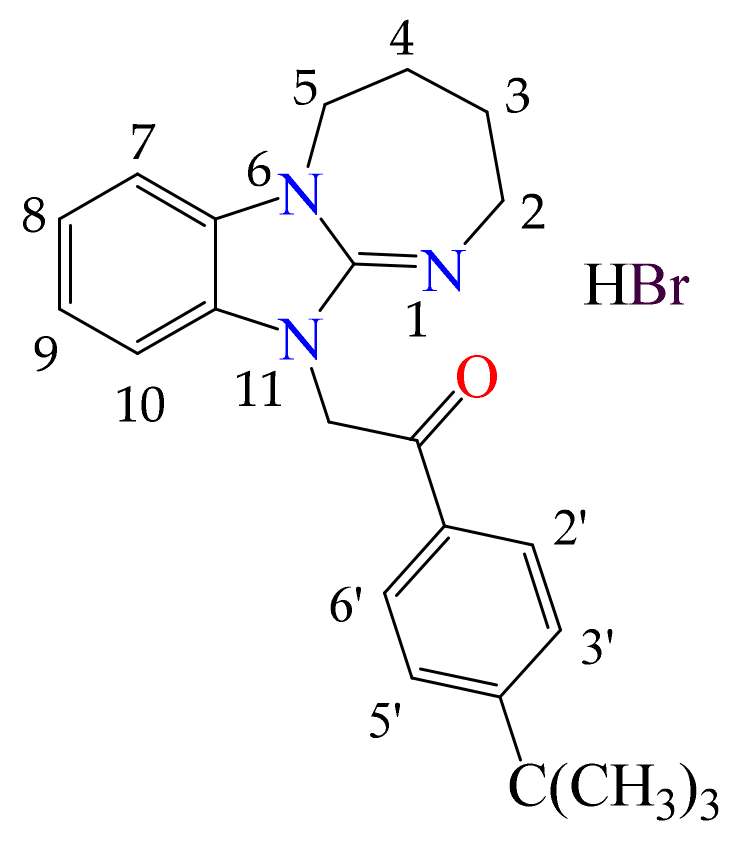
3.1.4. 11. -(4-tert-butylphenyl)-2-(2,3,4,5-tetrahydro[1,3]diazepino[1,2-a]benzimidazol-11-yl)ethanone Hydrobromide (2d)
4. Experimental Procedure
4.1. Animals
4.2. Drugs and Treatment
4.3. Anxiolytic Assay
4.4. Analgesic Assay
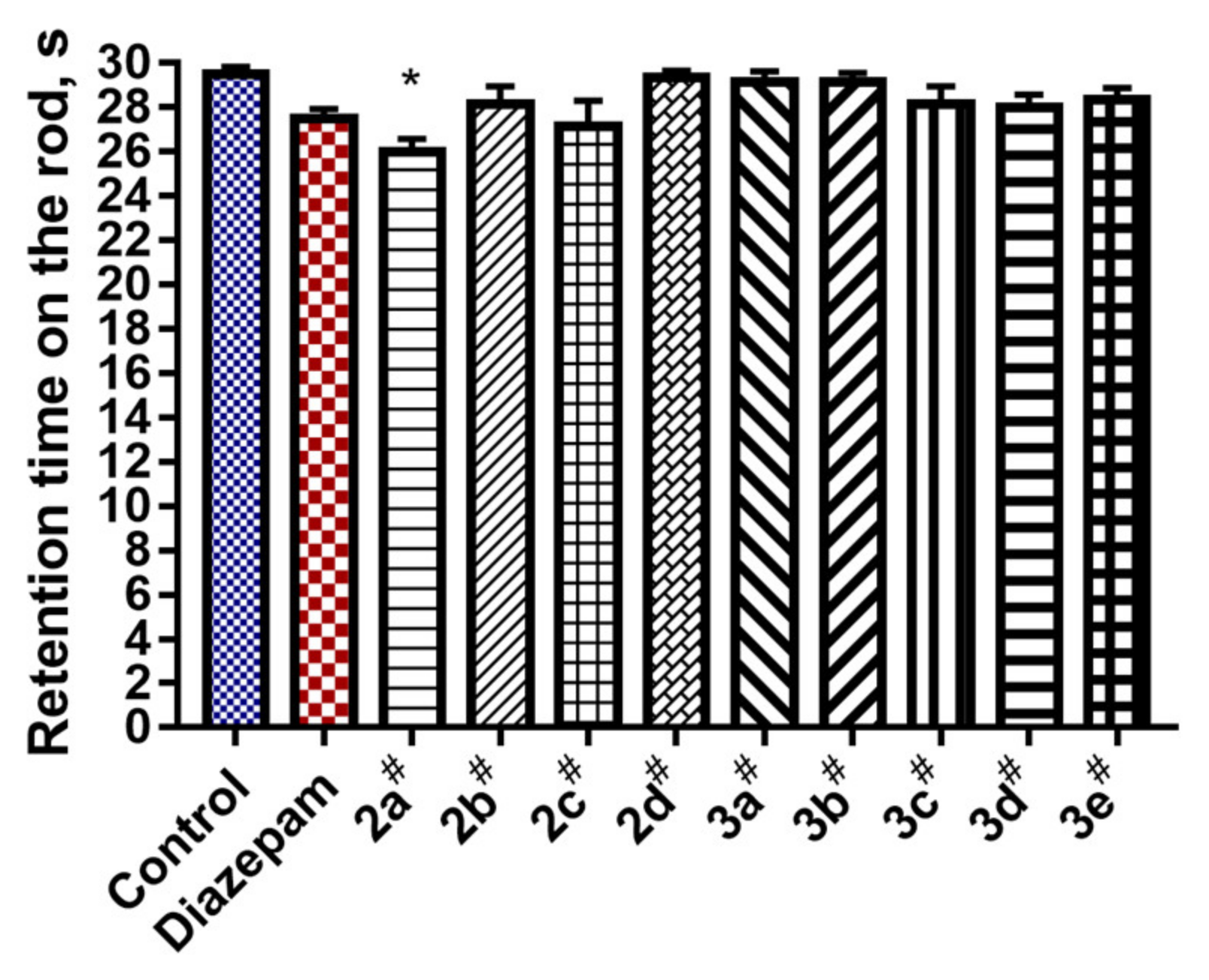
4.5. Muscle Relaxant Properties
4.6. In Silico Analysis
5. Statistical Analysis
6. Conclusions
- Derivatives of diazepino[1,2-a]benzimidazole 2 and 3 showed anxiolytic and analgesic activities of varying severity.
- The presence of the 2,3,4,5-tetrahydro[1,3]diazepino[1,2-a]benzimidazole fragment in the structure of the substances tends to manifest a moderate anxiolytic effect (2a–d) in terms of the time spent in the open arms of the EPM. According to the totality of anxiolytic parameters in the series 2a–d, chlorobenzyl derivative 2a and fluorophenylacetamide 2c were the most active. In Open Field, 2a–d had a psychostimulating effect. The presence of electronegative Cl and F atoms in the radical part of the structure of the compounds was found to be less favorable for analgesic activity than for anti-anxiety activity.
- In compounds 3, according to the parameters characterizing the anxiety state of animals (the number of entries and time spent in open arms, the number of overhangs), the substances under the code 3a–e showed a statistically significant difference from the control group; however, the effect at the level of diazepam was shown only for the fluorophenyl derivative 3b. Administration of compounds 3a–e positively influenced the search activity of mice in Open Field.
- For the analgesic action of compounds of series 2, the most effective was the substitution of (4-fluorophenyl)acetamide (2b) and 4-tert-butylphenacyl (2d), in addition to the hydrogen atoms in positions 8 and 9. Derivatives of 2 mainly affect the supraspinal regulation of pain, and 3 mainly affects the spinal regulation.
- In compounds 3, the most significant aspect for the analgesic effects was the presence in the molecule of methyl substituents at positions 8 and 9, and a 4-fluorophenyl substituent of the core structure (3e). Compounds 3a and 3c, containing hydrogen atoms instead of methyl substituents (3e) and an aryl or hydroxyl substituent in the p-position of the phenyl radical, had a somewhat less pronounced analgesic effect.
- According to the in silico study, 3b is characterized by the interaction with the GABAA receptor and by a prominent 5-HT2A antagonistic action.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bandelow, B. Current and Novel Psychopharmacological Drugs for Anxiety Disorders. Adv. Exp. Med. Biol. 2020, 1191, 347–365. [Google Scholar] [CrossRef]
- Yekkirala, A.S.; Roberson, D.P.; Bean, B.P.; Woolf, C.J. Breaking barriers to novel analgesic drug development. Nat. Rev. Drug Discov. 2017, 16, 545–564. [Google Scholar] [CrossRef] [Green Version]
- Morrison, I.; Perini, I.; Dunham, J. Facets and mechanisms of adaptive pain behavior: Predictive regulation and action. Front. Hum. Neurosci. 2013, 7, 755. [Google Scholar] [CrossRef] [Green Version]
- Cimpean, A.; David, D. The mechanisms of pain tolerance and pain-related anxiety in acute pain. Health Psychol. Open 2019, 6, 2055102919865161. [Google Scholar] [CrossRef] [PubMed]
- Rosen, J.B.; Schulkin, J. From normal fear to pathological anxiety. Psychol. Rev. 1998, 105, 325–350. [Google Scholar] [CrossRef] [PubMed]
- Gelenberg, A.J. Psychiatric and Somatic Markers of Anxiety: Identification and Pharmacologic Treatment. Prim. Care Companion J. Clin. Psychiatry 2000, 2, 49–54. [Google Scholar] [CrossRef]
- Gal, Z.; Huse, R.J.; Gonda, X.; Kumar, S.; Juhasz, G.; Bagdy, G.; Petschner, P. Anxiety and depression—The role of blood-brain barrier integrity. Neuropsychopharmacol. Hung. 2019, 21, 19–25. [Google Scholar]
- Davari, M.; Amani, B.; Amani, B.; Khanijahani, A.; Akbarzadeh, A.; Shabestan, R. Pregabalin and gabapentin in neuropathic pain management after spinal cord injury: A systematic review and meta-analysis. Korean J. Pain 2019, 33, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Mifflin, K.A.; Kerr, B.J. The transition from acute to chronic pain: Understanding how different biological systems interact. Can. J. Anaesth. 2013, 61, 112–122. [Google Scholar] [CrossRef]
- Bektas, N.; Nemutlu, D.; Arslan, R. The imidazoline receptors and ligands in pain modulation. Indian J. Pharmacol. 2015, 47, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Griebel, G.; Holmes, A. 50 years of hurdles and hope in anxiolytic drug discovery. Nat. Rev. Drug Discov. 2013, 12, 667–687. [Google Scholar] [CrossRef] [Green Version]
- Sultanova, K.T.; Yakovlev, D.S.; Maltsev, D.V.; Miroshnikov, M.V.; Morkovina, Y.V.; Anisimova, V.; Morkovnik, A.S. Anxiolytic Properties of the RU-31 Compound. Bull. Volgograd State Med. Univ. 2018, 3, 28–32. [Google Scholar] [CrossRef]
- Spasov, A.A.; Yakovlev, D.S.; Maltsev, D.V.; Zhukovskaya, O.N.; Anisimova, V.A.; Kovalev, G.I.; Zimin, I.A.; Morkovina, Y.V. The derivatives of imidazo[1,2-a]benzimidazole as 5-HT2A receptor antagonists. Russ. J. Bioorg. Chem. 2016, 42, 397–403. [Google Scholar] [CrossRef]
- Yakovlev, D.S.; Lyubashina, O.A.; Busygina, I.I.; Maltsev, D.V.; Anisimova, V.A.; Zhukovskaya, O.N.; Morkovnik, A.S.; Spasov, A.A. Anispasmodic and visceral analgesic effects of new 5-HT3 receptor antagonist emetazol in experiment. Exp. Clin. Pharmacol. 2018, 81, 8–12. [Google Scholar] [CrossRef]
- Maltsev, D.V.; Yakovlev, D.S.; Matokhin, D.G.; Samsonik, Y.V.; Spasov, A.A.; Anisimova, V.A. P.4.c.002 Anxiolytic action of a new 5-HT2A antagonist RU-476. Eur. Neuropsychopharmacol. 2013, 23, 519–520. [Google Scholar] [CrossRef]
- Spasov, A.A.; Zhukovskaya, O.N.; Maltsev, D.V.; Miroshnikov, M.V.; Skripka, M.O.; Sultanova, K.T.; Morkovnik, A.S. Anxiolytic Activity of 11H-2,3,4,5-tetrahydro[1,3]diazepino[1,2-a]benzimidazole and 2-Mercaptobenzimizadole Derivatives. Russ. J. Bioorg. Chem. 2020, 46, 107–114. [Google Scholar] [CrossRef]
- Achar, K.C.; Hosamani, K.M.; Seetharamareddy, H.R. In-vivo analgesic and anti-inflammatory activities of newly synthesized benzimidazole derivatives. Eur. J. Med. Chem. 2010, 45, 2048–2054. [Google Scholar] [CrossRef] [PubMed]
- Keri, R.S.; Hiremathad, A.; Budagumpi, S.; Nagaraja, B.M. Comprehensive Review in Current Developments of Benzimidazole-Based Medicinal Chemistry. Chem. Biol. Drug Des. 2015, 86, 19–65. [Google Scholar] [CrossRef] [PubMed]
- Cheretaev, I.V.; Korenyuk, I.I.; Nozdrachev, A.D. Neurotropic, Psychoactive, and Analgesic Properties of Benzimidazole and Its Derivatives: Physiological Mechanisms. Neurosci. Behav. Physiol. 2018, 48, 848–853. [Google Scholar] [CrossRef]
- Spasov, A.A.; Grechko, O.Y.; Shtareva, D.M.; Rashchenko, A.I.; Eliseeva, N.V.; Anisimova, V.A. Analgesic activity of the kappa opioid receptor agonist—RU-1205 in rats. J. Clin. Health Sci. 2018, 2, 13–18. [Google Scholar] [CrossRef] [Green Version]
- Kalitin, K.Y.; Spasov, A.A.; Grechko, O.Y.; Sukhov, A.G.; Vislobokov, A.I.; Anisimova, V.A.; Matukhno, A.E. Anticonvulsant and membranotropic activity of RU-1205 compound. Exp. Clin. Pharmacol. 2017, 9, 28–34. [Google Scholar] [CrossRef]
- Liu, Y.; Liao, C.; Zhou, J.; Liu, C.; Li, Q.; Jiang, Y.; Qian, H. Novel benzodiazepines derivatives as analgesic modulating for Transient receptor potential vanilloid 1. Bioorg. Med. Chem. 2018, 26, 4567–4573. [Google Scholar] [CrossRef]
- Witkin, J.M.; Cerne, R.; Davis, P.G.; Freeman, K.B.; Carmo, J.M.D.; Rowlett, J.K.; Methuku, K.R.; Okun, A.; Gleason, S.D.; Li, X.; et al. The α2,3-selective potentiator of GABAA receptors, KRM-II-81, reduces nociceptive-associated behaviors induced by formalin and spinal nerve ligation in rats. Pharmacol. Biochem. Behav. 2019, 180, 22–31. [Google Scholar] [CrossRef] [Green Version]
- Spasov, A.A.; Divaeva, L.N.; Maltsev, D.V.; Kuzmenko, T.A.; Morkovnik, A.S.; Miroshnikov, M.V.; Taran, A.S.; Zolotova, E.A. Anxiolytic potential of a new series of diazepinobenzimidazole derivatives. Bull. Volgogr. State Med. Univ. 2018, 3, 19–23. [Google Scholar] [CrossRef]
- Taran, A.S.; Maltsev, D.V.; Yakovlev, D.S.; Karavaeva, T.V.; Tkachenko, Y.O.; Divaeva, L.N.; Morkovnik, A.S.; Kuzmenko, T.A. Study of anxiolytic activity in a series of new diazepinobenzimidazole derivatives using the “ElevatedPlusMaze” installation. Volgogr. J. Med. Sci. Res. 2017, 1, 24–26, [in Russian]. [Google Scholar]
- Divaeva, L.N.; Spasov, A.A.; Maltsev, D.V.; Kuzmenko, T.A.; Morkovnik, A.S.; Yakovlev, D.S.; Taran, A.S.; Petrov, V.I.; Anisimova, V.A. 11-(4-Tert-Butylbenzyl)- and Phenacyl-Substituted 2,3,4,5-Tetrahydro[1,3]Diazepino [1,2-a]Benzimidazoles, Possessing Anxiolytic Activity. Patent of Russia 2629022, 14 October 2016. [Google Scholar]
- Divaeva, L.N.; Spasov, A.A.; Kuzmenko, T.A.; Maltsev, D.V.; Morkovnik, A.S.; Taran, A.S.; Petrov, V.I. 11-(4-Tert-Butylbenzyl) -2,3,4,5-Tetrahydro-[1,3]-Diazepino-[1,2-a]-Benzimidazole Hydrobromide and Triazabenzo [a]-Cyclopenta-[cd]-Azulenia, Possessing Anxiolytic and Anticonvulsant Activity. Patent of Russia 2662242, 25 July 2017. [Google Scholar]
- Divaeva, L.N.; Spasov, A.A.; Maltsev, D.V.; Kuzmenko, T.A.; Morkovnik, A.S.; Yakovlev, D.S.; Taran, A.S.; Petrov, V.I.; Bogoslavtseva, M.V. 1-(3,4-Dimethoxyphenyl)-2-(7,8-Dimethyl-2,3,4,5-Tetrahydro [1,3]Diazepino [1,2-a]Benzimidazol-11-yl) EthanoneHydrohalide, Possessing Analgesic and Anxiolytic Activity. Patent of Russia 2636785, 1 December 2017. [Google Scholar]
- Miroshnikov, M.V.; Divaeva, L.N.; Anisimova, V.A.; Morkovnik, A.S.; Skripka, M.O.; Zolotova, E.A.; Maltsev, D.V.; Spasov, A.A. Study of the anxiolytic activity of new diazepinobenzimidazole derivatives under the codes DAB-21 and DAB-31. Exp. Clin. Pharmacol. 2018, 81, 160–161. [Google Scholar]
- Kuzmenko, T.A.; Morkovnik, A.S.; Divaeva, L.N.; Bogoslavtseva, M.V. Peri-Cyclization of 11-aroylmethyl-2,3,4,5-tetrahydro [1,3]-diazepinobenzimidazoles into 2-aryl-3,4,5,6-tetrahydro-2a,6a,10b-triazabenzo[a]cyclopenta[cd]azulenium bromides. Russ. J. Org. Chem. 2017, 53, 1253–1257. [Google Scholar] [CrossRef]
- Morkovnik, A.S.; Spasov, A.A.; Kuzmenko, T.A.; Kucheryavenko, A.F.; Divaeva, L.N.; Koshchienko, Y.V.; Anisimova, V.A.; Kuzmina, L.G.; Rogova, N.V.; Kuznetsova, V.A.; et al. Prototropic equilibrium in 1(11)H-2,3,4,5-tetrahydro[1, 3]diazepino[1, 2-a]benzimidazole, synthesis and pharmacological properties of its N-substituted derivatives. Russ. Chem. Bull. 2015, 64, 2622–2631. [Google Scholar] [CrossRef]
- Voronina, T.A.; Seredenin, S.B.; Yarkova, M.A.; Voronin, M.V. Methodical recommendations for preclinical study of the tranquilizing (anxiolytic) action of drugs. In Guidelines for Conducting Preclinical Studies of Drugs; Part 1; Mironov, A.N., Ed.; GRIF-K: Moscow, Russia, 2012; pp. 264–275, [manual in Russian]. [Google Scholar]
- Ostrovskaya, R.U.; Raevsky, K.S.; Voronina, T.A.; Garibova, T.L.; Kovalev, G.I.; Kudrin, V.S.; Narkevich, V.B.; Klodt, P.M. Methodical recommendations for the study of neuroleptic activity of drugs. In Guidelines for Conducting Preclinical Studies of Drugs; Part 1; Mironov, A.N., Ed.; GRIF-K: Moscow, Russia, 2012; pp. 251–263, [manual in Russian]. [Google Scholar]
- Voronina, T.A.; Guzevatykh, L.S. Methodical recommendations for the study of the analgesic activity of drugs. In Guidelines for Conducting Preclinical Studies of Drugs; Mironov, A.N., Ed.; Part 1; GRIF-K: Moscow, Russia, 2012; pp. 197–218, [manual in Russian]. [Google Scholar]
- Harris, L.S.; Pierson, A.K. Some narcotic antagonists in the benzomorphan series. J. Pharmacol. Exp. Ther. 1964, 143, 141–148. [Google Scholar] [PubMed]
- Paul, A.K.; Gueven, N.; Dietis, N. Profiling the Effects of Repetitive Morphine Administration on Motor Behavior in Rats. Molecules 2021, 26, 4355. [Google Scholar] [CrossRef] [PubMed]
- MarvinSketch, ChemAxonKft. Available online: http://www.chemaxon.com/products/marvin/marvinsketch/ (accessed on 27 September 2021).
- MOPAC, Stewart Computational Chemistry. Available online: http://openmopac.net (accessed on 27 September 2021).
- Vassiliev, P.M.; Spasov, A.A.; Yanaliyeva, L.R.; Kochetkov, A.N.; Vorfolomeyeva, V.V.; Klochkov, V.G.; Appazova, D.T. Neural network modeling of multitarget RAGE inhibitory activity. Biochem. Moscow Suppl. Ser. B Biomed. Chem. 2019, 13, 256–263. [Google Scholar] [CrossRef]
- RCSB PDB, Protein Data Bank. Available online: https://www.rcsb.org/ (accessed on 27 September 2021).
- Bergmann, R.; Kongsbak, K.; Sørensen, P.L.; Sander, T.; Balle, T. A Unified Model of the GABAA Receptor Comprising Agonist and Benzodiazepine Binding Sites. PLoS ONE 2013, 8, e52323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ModBase: Database of Comparative Protein Structure Models. Available online: https://modbase.compbio.ucsf.edu/ (accessed on 27 September 2021).
- Trott, O.; Olson, A.J. AutoDockVina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comp. Chem. 2010, 31, 455–461. [Google Scholar]
- LigandScout, Inte: Ligand GmbH. Available online: http://www.inteligand.com/ligandscout/ (accessed on 27 September 2021).



 RU-31 |  RU-1276 | 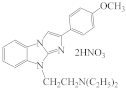 RU-476 |  AZH-57 |
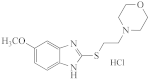 AZH-58 | 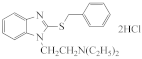 AZH-59 | 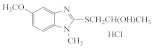 AZH-60 | 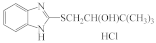 AZH-61 |
 AZH-62 |  AZH-63 |  AZH-64 |  AZH-65 |
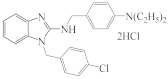 AZH-66 | 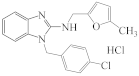 AZH-67 | 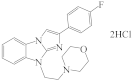 RU-1205 |  TR TR |
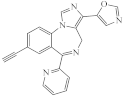 KRM-II-81 | 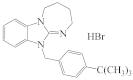 DAB-19 | 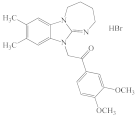 DAB-31 DAB-31 |
| Compound | R | R1 | Compound | R | Ar |
|---|---|---|---|---|---|
| 2a | H |  | 3a | H |  |
| 2b | H |  | 3b | H |  |
| 2c | CH3 | 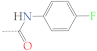 | 3c | H |  |
| 2d | H |  | 3d | CH3 |  |
| 3e | CH3 |  |
| Compound | GABAA Receptor | 5-HT2A Receptor | ||||||
|---|---|---|---|---|---|---|---|---|
| GABA Site | Benzodiazepine Site | Specific Site | Allosteric Site | |||||
| ΔE, kcal/mol | K, nM | ΔE, kcal/mol | K, nM | ΔE, kcal/mol | K, nM | ΔE, kcal/mol | K, nM | |
| 3b | −7.5 | 3410 | −10.8 | 13.4 | −9.7 | 84.9 | −11.1 | 8.1 |
| diazepam | −7.1 | 6674 | −9.8 | 71.8 | −8.5 | 637 | −9.3 | 166 |
| ketanserin | — | — | −9.9 | 60.7 | −11.1 | 8.1 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maltsev, D.V.; Spasov, A.A.; Vassiliev, P.M.; Skripka, M.O.; Miroshnikov, M.V.; Kochetkov, A.N.; Eliseeva, N.V.; Lifanova, Y.V.; Kuzmenko, T.A.; Divaeva, L.N.; et al. Synthesis and Pharmacological Evaluation of Novel 2,3,4,5-tetrahydro[1,3]diazepino[1,2-a]benzimidazole Derivatives as Promising Anxiolytic and Analgesic Agents. Molecules 2021, 26, 6049. https://doi.org/10.3390/molecules26196049
Maltsev DV, Spasov AA, Vassiliev PM, Skripka MO, Miroshnikov MV, Kochetkov AN, Eliseeva NV, Lifanova YV, Kuzmenko TA, Divaeva LN, et al. Synthesis and Pharmacological Evaluation of Novel 2,3,4,5-tetrahydro[1,3]diazepino[1,2-a]benzimidazole Derivatives as Promising Anxiolytic and Analgesic Agents. Molecules. 2021; 26(19):6049. https://doi.org/10.3390/molecules26196049
Chicago/Turabian StyleMaltsev, Dmitriy V., Alexander A. Spasov, Pavel M. Vassiliev, Maria O. Skripka, Mikhail V. Miroshnikov, Andrey N. Kochetkov, Nataliya V. Eliseeva, Yuliya V. Lifanova, Tatyana A. Kuzmenko, Lyudmila N. Divaeva, and et al. 2021. "Synthesis and Pharmacological Evaluation of Novel 2,3,4,5-tetrahydro[1,3]diazepino[1,2-a]benzimidazole Derivatives as Promising Anxiolytic and Analgesic Agents" Molecules 26, no. 19: 6049. https://doi.org/10.3390/molecules26196049
APA StyleMaltsev, D. V., Spasov, A. A., Vassiliev, P. M., Skripka, M. O., Miroshnikov, M. V., Kochetkov, A. N., Eliseeva, N. V., Lifanova, Y. V., Kuzmenko, T. A., Divaeva, L. N., & Morkovnik, A. S. (2021). Synthesis and Pharmacological Evaluation of Novel 2,3,4,5-tetrahydro[1,3]diazepino[1,2-a]benzimidazole Derivatives as Promising Anxiolytic and Analgesic Agents. Molecules, 26(19), 6049. https://doi.org/10.3390/molecules26196049







