Formation of Copper Oxide Nanotextures on Porous Calcium Carbonate Templates for Water Treatment
Abstract
:1. Introduction
2. Results and Discussion
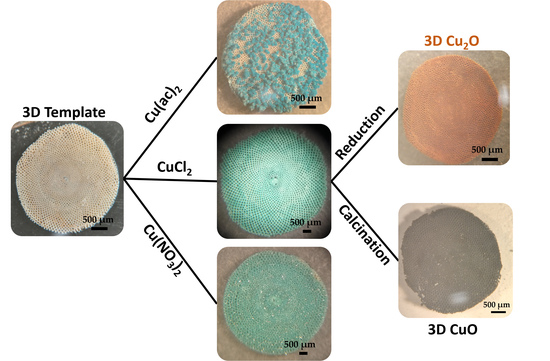
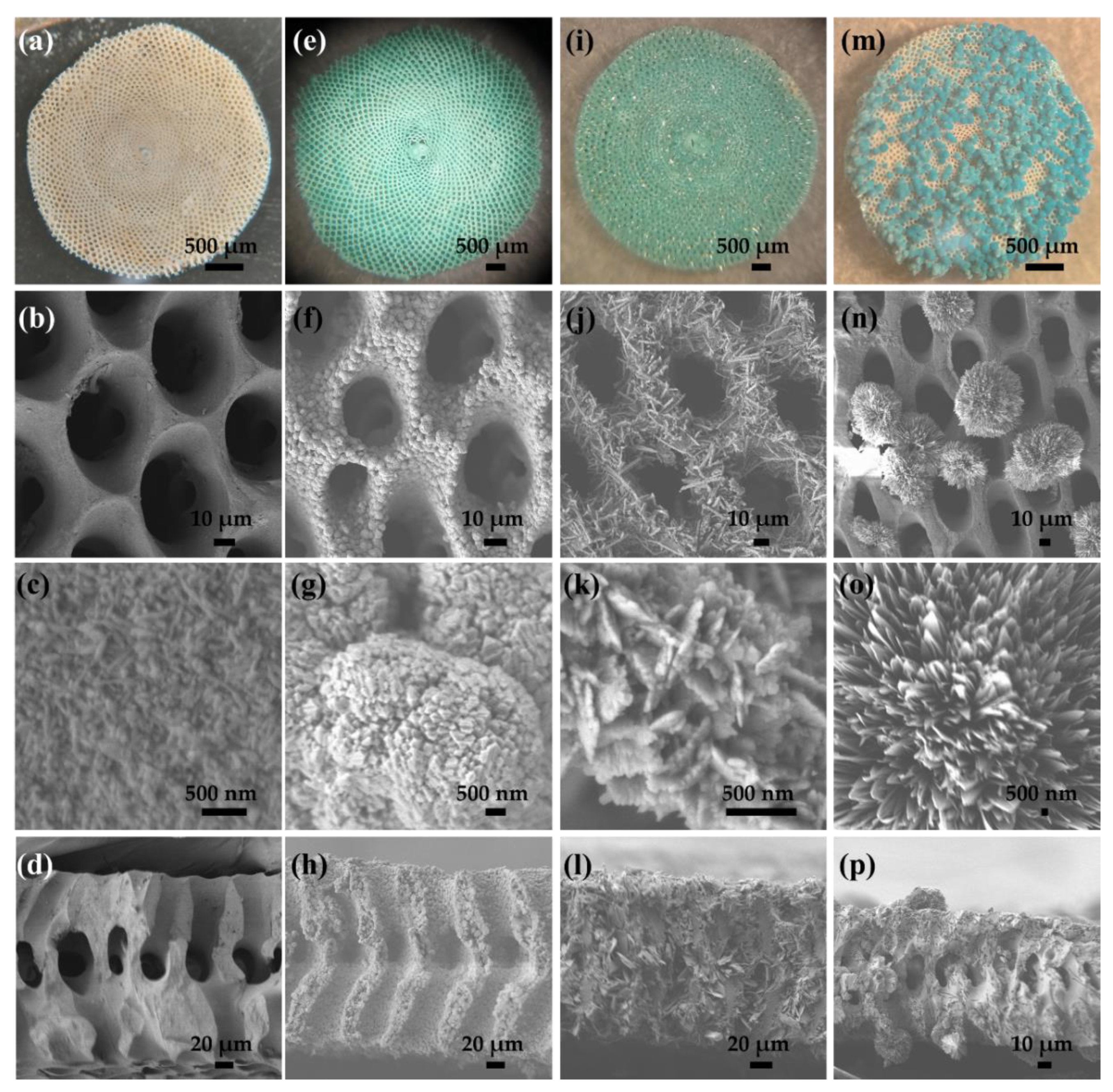
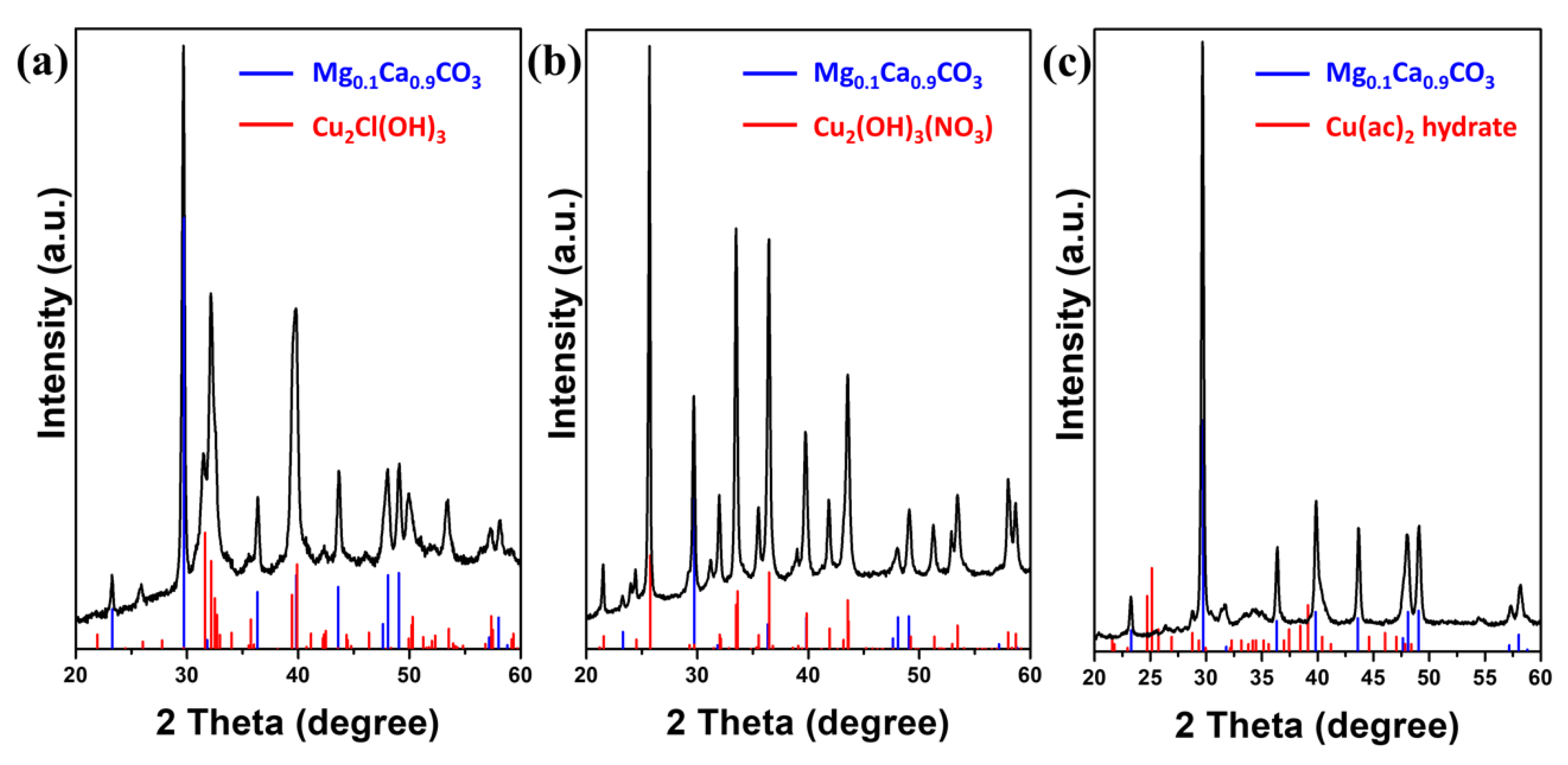
2.1. Formation of 3D Structures of Sorites@Copper-Based Hydroxide Using Various Copper Salts
2.2. Using Sorites@Cu2Cl(OH)3 as a Prototype to Further Investigate the Coating Process and Their Conversion to Sorites@CuO
2.3. Potential Application of Sorites@CuO in the Removal of Heavy Metal Ions and Degradation of an Organic Model Dye Molecule
3. Materials and Methods
3.1. Materials
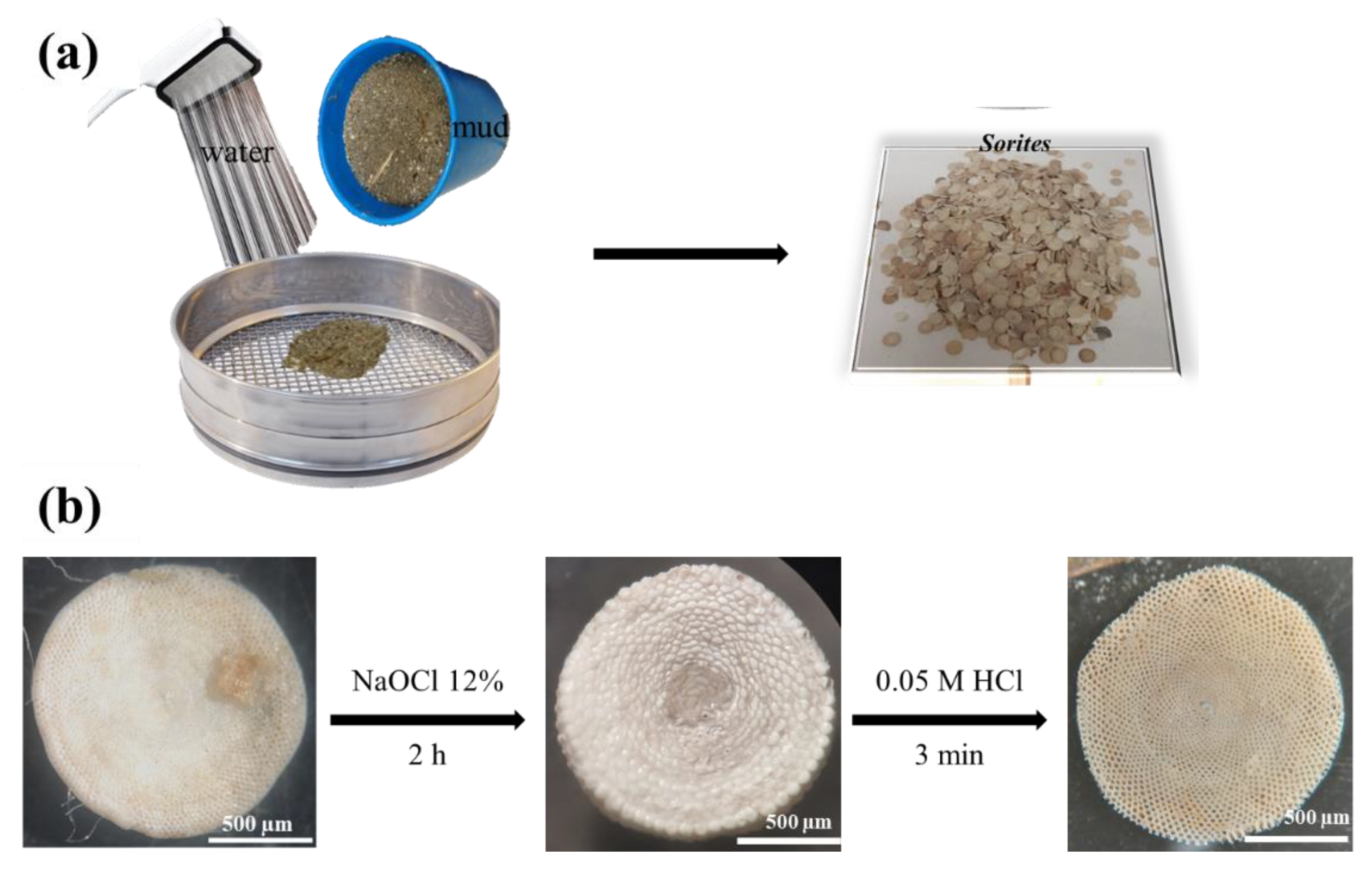
3.2. Purification and Pretreatment of Sorites Bio-Template
3.3. Formation of a Copper Oxide Porous 3D Structure
3.4. Removal of Heavy Metal Ions
3.5. Dye Degradation
3.6. Structural Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/drinking-water (accessed on 1 September 2021).
- Hashim, M.A.; Mukhopadhyay, S.; Sahu, J.N.; Sengupta, B. Remediation technologies for heavy metal contaminated groundwater. J. Environ. Manag. 2011, 92, 2355–2388. [Google Scholar] [CrossRef] [PubMed]
- Barakat, M.A. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef] [Green Version]
- Gupta, V.K.; Ali, I.; Saleh, T.A.; Nayak, A.; Agarwal, S. Chemical treatment technologies for waste-water recycling—An overview. RSC Adv. 2012, 2, 6380–6388. [Google Scholar] [CrossRef]
- Dongare, P.D.; Alabastri, A.; Pedersen, S.; Zodrow, K.R.; Hogan, N.J.; Neumann, O.; Wu, J.; Wang, T.; Deshmukh, A.; Elimelech, M.; et al. Nanophotonics-enabled solar membrane distillation for off-grid water purification. Proc. Natl. Acad. Sci. USA 2017, 114, 6936–6941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaufman, Y.; Berman, A.; Freger, V. Supported lipid bilayer membranes for water purification by reverse osmosis. Langmuir 2010, 26, 7388–7395. [Google Scholar] [CrossRef] [PubMed]
- Reed, B.E.; Vaughan, R.; Jiang, L. As(III), As(V), Hg, and Pb Removal by Fe-Oxide Impregnated Activated Carbon. J. Environ. Eng. 2000, 126, 869–873. [Google Scholar] [CrossRef]
- Sakamoto, T.; Ogawa, T.; Nada, H.; Nakatsuji, K.; Mitani, M.; Soberats, B.; Kawata, K.; Yoshio, M.; Tomioka, H.; Sasaki, T.; et al. Development of Nanostructured Water Treatment Membranes Based on Thermotropic Liquid Crystals: Molecular Design of Sub-Nanoporous Materials. Adv. Sci. 2018, 5, 1700405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farghali, A.A.; Bahgat, M.; Enaiet Allah, A.; Khedr, M.H. Adsorption of Pb(II) ions from aqueous solutions using copper oxide nanostructures. Beni-Suef Univ. J. Basic Appl. Sci. 2013, 2, 61–71. [Google Scholar] [CrossRef] [Green Version]
- Raul, P.K.; Senapati, S.; Sahoo, A.K.; Umlong, I.M.; Devi, R.R.; Thakur, A.J.; Veer, V. CuO nanorods: A potential and efficient adsorbent in water purification. RSC Adv. 2014, 4, 40580–40587. [Google Scholar] [CrossRef]
- Hua, M.; Zhang, S.; Pan, B.; Zhang, W.; Lv, L.; Zhang, Q. Heavy metal removal from water/wastewater by nanosized metal oxides: A review. J. Hazard. Mater. 2012, 211–212, 317–331. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Q.; Gao, S.; Shang, J.K. Exceptional As(III) sorption capacity by highly porous magnesium oxide nanoflakes made from hydrothermal synthesis. J. Am. Ceram. Soc. 2011, 94, 217–223. [Google Scholar] [CrossRef]
- Li, L.H.; Xiao, J.; Liu, P.; Yang, G.W. Super adsorption capability from amorphousization of metal oxide nanoparticles for dye removal. Sci. Rep. 2015, 5, 9028. [Google Scholar] [CrossRef]
- Krivec, M.; Žagar, K.; Suhadolnik, L.; Čeh, M.; Dražić, G. Highly efficient TiO2-based microreactor for photocatalytic applications. ACS Appl. Mater. Interfaces 2013, 5, 9088–9094. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Chandra, R.; Tyagi, I.; Verma, M. Removal of hexavalent chromium ions using CuO nanoparticles for water purification applications. J. Colloid Interface Sci. 2016, 478, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Bhanjana, G.; Dilbaghi, N.; Singhal, N.K.; Kim, K.H.; Kumar, S. Copper oxide nanoblades as novel adsorbent material for cadmium removal. Ceram. Int. 2017, 43, 6075–6081. [Google Scholar] [CrossRef]
- Roy Choudhury, P.; Majumdar, S.; Sahoo, G.C.; Saha, S.; Mondal, P. High pressure ultrafiltration CuO/hydroxyethyl cellulose composite ceramic membrane for separation of Cr (VI) and Pb (II) from contaminated water. Chem. Eng. J. 2018, 336, 570–578. [Google Scholar] [CrossRef]
- Choudhury, P.; Mondal, P.; Majumdar, S.; Saha, S.; Sahoo, G.C. Preparation of ceramic ultrafiltration membrane using green synthesized CuO nanoparticles for chromium (VI) removal and optimization by response surface methodology. J. Clean. Prod. 2018, 203, 511–520. [Google Scholar] [CrossRef]
- Khew, S.Y.; Tan, C.F.; Yan, H.; Lin, S.; Thian, E.S.; Zhou, R.; Hong, M. Nanosecond laser ablation for enhanced adhesion of CuO nanowires on copper substrate and its application for oil-water separation. Appl. Surf. Sci. 2019, 465, 995–1002. [Google Scholar] [CrossRef]
- Silva Junior, O.J.; Monteiro, A.F.F.; Oliveira, J.B.L.; Araújo, A.M.U.; Silva, D.G.; Kulesza, J.; Barros, B.S. Coordination polymer-derived CuO catalysts for oxidative degradation of methylene blue. Mater. Chem. Phys. 2019, 235, 121737–121744. [Google Scholar] [CrossRef]
- Yang, M.; He, J. Fine tuning of the morphology of copper oxide nanostructures and their application in ambient degradation of methylene blue. J. Colloid Interface Sci. 2011, 355, 15–22. [Google Scholar] [CrossRef]
- Devi, H.; Singh, D. Synthesis of Copper Oxide Nanoparticles by a Novel Method and its Application in the Degradation of Methyl Orange. Adv. Electron. Electr. Eng. 2014, 4, 83. [Google Scholar]
- Karim, M.N.; Singh, M.; Weerathunge, P.; Bian, P.; Zheng, R.; Dekiwadia, C.; Ahmed, T.; Walia, S.; Della Gaspera, E.; Singh, S.; et al. Visible-Light-Triggered Reactive-Oxygen-Species-Mediated Antibacterial Activity of Peroxidase-Mimic CuO Nanorods. ACS Appl. Nano Mater. 2018, 1, 1694–1704. [Google Scholar] [CrossRef]
- Yecheskel, Y.; Dror, I.; Berkowitz, B. Catalytic degradation of brominated flame retardants by copper oxide nanoparticles. Chemosphere 2013, 93, 172–177. [Google Scholar] [CrossRef]
- Černík, M.; Thekkae Padil, V.V. Green synthesis of copper oxide nanoparticles using gum karaya as a biotemplate and their antibacterial application. Int. J. Nanomed. 2013, 8, 889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wongpisutpaisan, N.; Charoonsuk, P.; Vittayakorn, N.; Pecharapa, W. Sonochemical synthesis and characterization of copper oxide nanoparticles. Energy Procedia 2011, 9, 404–409. [Google Scholar] [CrossRef] [Green Version]
- Phiwdang, K.; Suphankij, S.; Mekprasart, W.; Pecharapa, W. Synthesis of CuO nanoparticles by precipitation method using different precursors. Energy Procedia 2013, 34, 740–745. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.; Zeng, H.C. Controlled synthesis and self-assembly of single-crystalline CuO nanorods and nanoribbons. Cryst. Growth Des. 2004, 4, 397–402. [Google Scholar] [CrossRef]
- Moosavifard, S.E.; El-Kady, M.F.; Rahmanifar, M.S.; Kaner, R.B.; Mousavi, M.F. Designing 3D highly ordered nanoporous CuO electrodes for high-performance asymmetric supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 4851–4860. [Google Scholar] [CrossRef]
- Wang, C.; Yue, L.; Wang, S.; Pu, Y.; Zhang, X.; Hao, X.; Wang, W.; Chen, S. Role of Electric Field and Reactive Oxygen Species in Enhancing Antibacterial Activity: A Case Study of 3D Cu Foam Electrode with Branched CuO-ZnO NWs. J. Phys. Chem. C 2018, 122, 26454–26463. [Google Scholar] [CrossRef]
- Shen, L.; Ali, M.; Gu, Z.; Min, B.; Kim, D.; Park, C. Preparation of anodic aluminum oxide (AAO) nano-template on silicon and its application to one-dimensional copper nano-pillar array formation. Korean J. Chem. Eng. 2013, 30, 221–227. [Google Scholar] [CrossRef]
- Crick, C.R.; Parkin, I.P. CVD of copper and copper oxide thin films via the in situ reduction of copper(ii) nitrate—A route to conformal superhydrophobic coatings. J. Mater. Chem. 2011, 21, 14712–14716. [Google Scholar] [CrossRef]
- Diab, M.; Moshofsky, B.; Jen-La Plante, I.; Mokari, T. A facile one-step approach for the synthesis and assembly of copper and copper-oxide nanocrystals. J. Mater. Chem. 2011, 21, 11626. [Google Scholar] [CrossRef]
- Diab, M.; Mokari, T. Bioinspired Hierarchical Porous Structures for Engineering Advanced Functional Inorganic Materials. Adv. Mater. 2018, 30, 1706349. [Google Scholar] [CrossRef] [PubMed]
- Diab, M.; Shreteh, K.; Afik, N.; Volokh, M.; Abramovich, S.; Abdu, U.; Mokari, T. Design of Hierarchal 3D Metal Oxide Structures for Water Oxidation and Purification. Adv. Sustain. Syst. 2018, 2, 1800001. [Google Scholar] [CrossRef]
- Diab, M.; Shreteh, K.; Volokh, M.; Abramovich, S.; Abdu, U.; Mokari, T. Calcareous Foraminiferal Shells as a Template for the Formation of Hierarchal Structures of Inorganic Nanomaterials. ACS Appl. Mater. Interfaces 2019, 11, 6456–6462. [Google Scholar] [CrossRef]
- Pan, L.; Zou, J.J.; Zhang, T.; Wang, S.; Li, Z.; Wang, L.; Zhang, X. Cu2O film via hydrothermal redox approach: Morphology and photocatalytic performance. J. Phys. Chem. C 2014, 118, 16335–16343. [Google Scholar] [CrossRef]
- Švarcová, S.; Klementová, M.; Bezdička, P.; Śasocha, W.; Dušek, M.; Hradil, D. Synthesis and characterization of single crystals of the layered copper hydroxide acetate Cu2(OH)3(CH3COO) ·H2O. Cryst. Res. Technol. 2011, 46, 1051–1057. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Y.; Zhu, P.; Zhou, F.; Zeng, W.; Lu, D.D.; Sun, R.; Wong, C. Copper Salts Mediated Morphological Transformation of Cu2O from Cubes to Hierarchical Flower-like or Microspheres and Their Supercapacitors Performances. Sci. Rep. 2015, 5, 9672. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, J.M.; Gutiérrez, A.; Giraldo, O. Simple route for the synthesis of copper hydroxy salts. J. Braz. Chem. Soc. 2011, 22, 546–551. [Google Scholar] [CrossRef]
- Lide, D.R. (Ed.) CRC Handbook of Chemistry and Physics: A Ready-Reference of Chemical and Physical Data, 85th ed.; CRC Press LLC: Boca Raton, FL, USA, 2004. [Google Scholar]
- Diab, M.; Mokari, T. Role of the Counteranions on the Formation of Different Crystal Structures of Iron Oxyhydroxides via Redox Reaction. Cryst. Growth Des. 2017, 17, 527–533. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diab, M.; Shreteh, K.; Volokh, M.; Mokari, T. Formation of Copper Oxide Nanotextures on Porous Calcium Carbonate Templates for Water Treatment. Molecules 2021, 26, 6067. https://doi.org/10.3390/molecules26196067
Diab M, Shreteh K, Volokh M, Mokari T. Formation of Copper Oxide Nanotextures on Porous Calcium Carbonate Templates for Water Treatment. Molecules. 2021; 26(19):6067. https://doi.org/10.3390/molecules26196067
Chicago/Turabian StyleDiab, Mahmud, Karam Shreteh, Michael Volokh, and Taleb Mokari. 2021. "Formation of Copper Oxide Nanotextures on Porous Calcium Carbonate Templates for Water Treatment" Molecules 26, no. 19: 6067. https://doi.org/10.3390/molecules26196067
APA StyleDiab, M., Shreteh, K., Volokh, M., & Mokari, T. (2021). Formation of Copper Oxide Nanotextures on Porous Calcium Carbonate Templates for Water Treatment. Molecules, 26(19), 6067. https://doi.org/10.3390/molecules26196067