Breeding Targets to Improve Biomass Quality in Miscanthus
Abstract
1. Introduction
1.1. Miscanthus for Industrial Use: Advantages, Challenges and Applications
1.2. Improving Biomass Quality in Miscanthus and Breeding Efforts
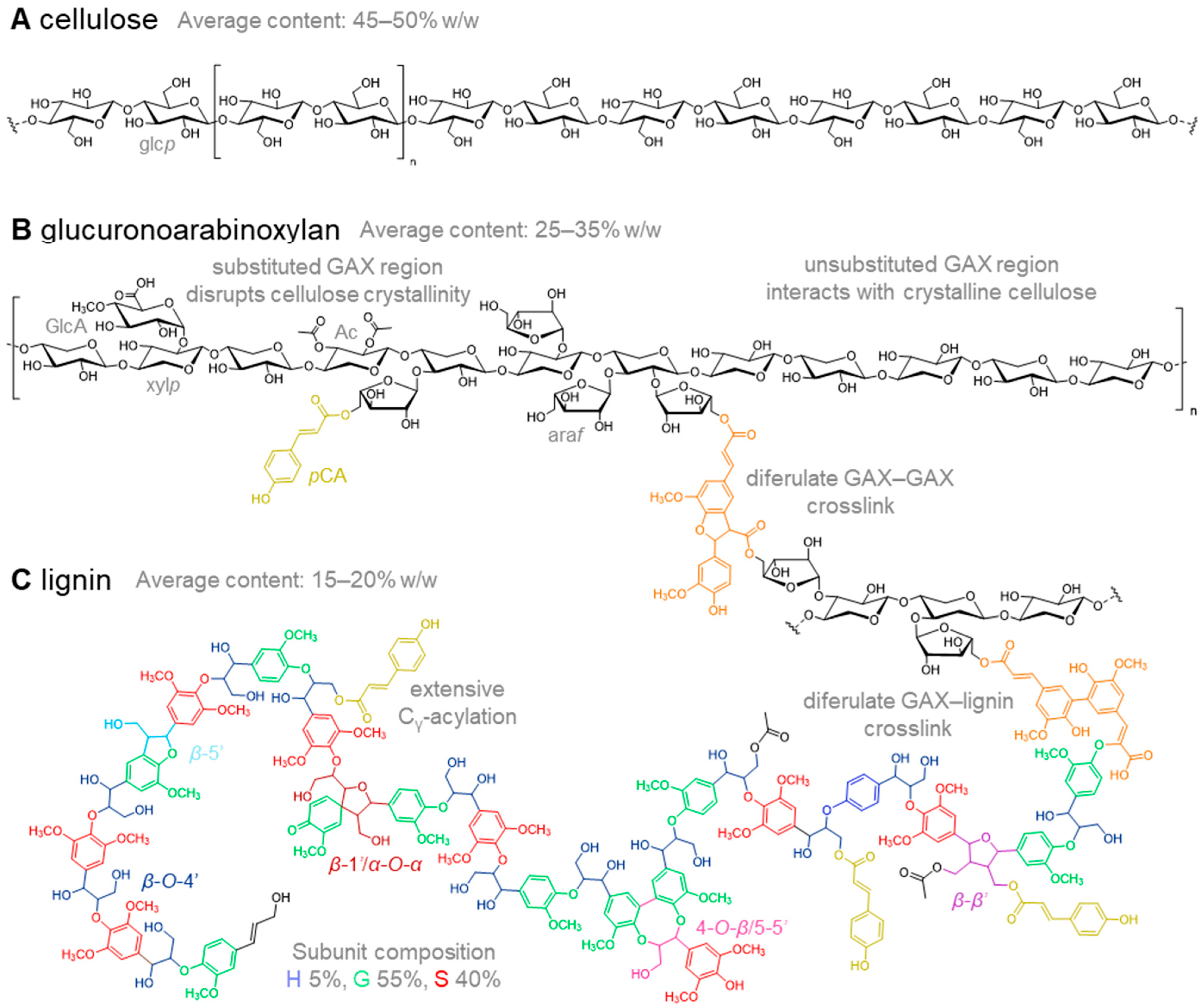
2. Cell Wall Composition in Relation to Cell Wall Digestibility
2.1. Cellulose in Miscanthus Cell Walls
2.2. Hemicellulose Composition in Cell Walls of Miscanthus
2.3. Lignin in Miscanthus Cell Walls
2.4. Content and Structure of Pectin in Miscanthus Primary Cell Walls
2.5. Crosslinking of Polymers in Miscanthus Secondary Cell Walls
3. Interdependence of Biomass Quality and Pretreatment Efficiency
3.1. Hydrothermal Pretreatment
3.2. Dilute Acid Pretreatment
3.3. Alkaline Pretreatment
3.4. Pretreatment Efficiency
4. Breeding for Improved Feedstock Quality
4.1. Breeding for More Digestible Cell Walls
4.2. Improving the Polysaccharide Composition to Enhance Cell Wall Digestibility
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lacis, A.A.; Schmidt, G.A.; Rind, D.; Ruedy, R.A. Atmospheric CO2: Principal Control Knob Governing Earth’s Temperature. Science 2010, 330, 356–359. [Google Scholar] [CrossRef]
- Huang, J.; Yu, H.; Dai, A.; Wei, Y.; Kang, L. Drylands face potential threat under 2 °C global warming target. Nat. Clim. Chang. 2017, 7, 417–422. [Google Scholar] [CrossRef]
- Rogelj, J.; Den Elzen, M.; Höhne, N.; Fransen, T.; Fekete, H.; Winkler, H.; Schaeffer, R.; Sha, F.; Riahi, K.; Meinshausen, M. Paris Agreement climate proposals need a boost to keep warming well below 2 °C. Nature 2016, 534, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Heede, R. Tracing anthropogenic carbon dioxide and methane emissions to fossil fuel and cement producers, 1854-2010. Clim. Chang. 2014, 122, 229–241. [Google Scholar] [CrossRef]
- Liu, Z.; Feng, K.; Davis, S.J.; Guan, D.; Chen, B.; Hubacek, K.; Yan, J. Understanding the energy consumption and greenhouse gas emissions and the implication for achieving climate change mitigation targets. Appl. Energy 2016, 184, 741–747. [Google Scholar] [CrossRef]
- Anderson, K.; Peters, G. The trouble with negative emissions. Science 2016, 354, 182–183. [Google Scholar] [CrossRef]
- Souza, G.M.; Ballester, M.V.R.; de Brito Cruz, C.H.; Chum, H.; Dale, B.; Dale, V.H.; Fernandes, E.C.M.; Foust, T.; Karp, A.; Lynd, L.; et al. The role of bioenergy in a climate-changing world. Environ. Dev. 2017, 23, 57–64. [Google Scholar] [CrossRef]
- Fajardy, M.; Mac Dowell, N. Can BECCS deliver sustainable and resource efficient negative emissions? Energy Environ. Sci. 2017, 10, 1389–1426. [Google Scholar] [CrossRef]
- Laibach, N.; Börner, J.; Bröring, S. Exploring the future of the bioeconomy: An expert-based scoping study examining key enabling technology fields with potential to foster the transition toward a bio-based economy. Technol. Soc. 2019, 58, 101118. [Google Scholar] [CrossRef]
- Vandermeulen, V.; der Steen, M.; Stevens, C.V.; Van Huylenbroeck, G. Industry expectations regarding the transition toward a biobased economy. Biofuels Bioprod. Biorefining 2012, 6, 453–464. [Google Scholar] [CrossRef]
- Zanchi, G.; Pena, N.; Bird, N. Is woody bioenergy carbon neutral? A comparative assessment of emissions from consumption of woody bioenergy and fossil fuel. GCB Bioenergy 2012, 4, 761–772. [Google Scholar] [CrossRef]
- Dhillon, R.S.; von Wuehlisch, G. Mitigation of global warming through renewable biomass. Biomass Bioenergy 2013, 48, 75–89. [Google Scholar] [CrossRef]
- Saba, N.; Jawaid, M.; Sultan, M.T.H.; Alothman, O.Y. Green Biocomposites for Structural Applications. In Green Biocomposites: Design and Applications; Springer: Berlin, Germany, 2017; ISBN 9783319493817. [Google Scholar]
- McKendry, P. Energy production from biomass (part 1): Overview of biomass. Bioresour. Technol. 2002, 83, 37–46. [Google Scholar] [CrossRef]
- Lal, R. Crop residues as soil amendments and feedstock for bioethanol production. Waste Manag. 2008, 28, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Scarlat, N.; Martinov, M.; Dallemand, J.F. Assessment of the availability of agricultural crop residues in the European Union: Potential and limitations for bioenergy use. Waste Manag. 2010, 30, 1889–1897. [Google Scholar] [CrossRef]
- Mitchell, R.B.; Schmer, M.R.; Anderson, W.F.; Jin, V.; Balkcom, K.S.; Kiniry, J.; Coffin, A.; White, P. Dedicated Energy Crops and Crop Residues for Bioenergy Feedstocks in the Central and Eastern USA. Bioenergy Res. 2016, 9, 384–398. [Google Scholar] [CrossRef]
- Van der Weijde, T.; Alvim Kamei, C.L.; Torres, A.F.; Vermerris, W.; Dolstra, O.; Visser, R.G.F.; Trindade, L.M. The potential of C4 grasses for cellulosic biofuel production. Front. Plant Sci. 2013, 4, 1–18. [Google Scholar] [CrossRef]
- Masters, M.D.; Black, C.K.; Kantola, I.B.; Woli, K.P.; Voigt, T.; David, M.B.; DeLucia, E.H. Soil nutrient removal by four potential bioenergy crops: Zea mays, Panicum virgatum, Miscanthus × giganteus, and prairie. Agric. Ecosyst. Environ. 2016, 216, 51–60. [Google Scholar] [CrossRef]
- Tilman, D.; Socolow, R.; Foley, J.A.; Hill, J.; Larson, E.; Lynd, L.; Pacala, S.; Reilly, J.; Searchinger, T.; Somerville, C.; et al. Beneficial biofuels-The food, energy, and environment trilemma. Science 2009, 325, 270–271. [Google Scholar] [CrossRef]
- Sims, R.E.H.; Mabee, W.; Saddler, J.N.; Taylor, M. An overview of second generation biofuel technologies. Bioresour. Technol. 2010, 101, 1570–1580. [Google Scholar] [CrossRef]
- Pancaldi, F.; Trindade, L.M. Marginal Lands to Grow Novel Bio-Based Crops: A Plant Breeding Perspective. Front. Plant Sci. 2020, 11, 227. [Google Scholar] [CrossRef] [PubMed]
- Field, C.B.; Campbell, J.E.; Lobell, D.B. Biomass energy: The scale of the potential resource. Trends Ecol. Evol. 2008, 23, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Searchinger, T.; Heimlich, R.; Houghton, R.A.; Dong, F.; Elobeid, A.; Fabiosa, J.; Tokgoz, S.; Hayes, D.; Yu, T.-H. Use of U.S. Croplands for Biofuels Increases Greenhouse Gases Through Emissions from Land-Use Change. Science 2008, 319, 1238–1240. [Google Scholar] [CrossRef] [PubMed]
- Zeri, M.; Anderson-Teixeira, K.; Hickman, G.; Masters, M.; DeLucia, E.; Bernacchi, C.J. Carbon exchange by establishing biofuel crops in Central Illinois. Agric. Ecosyst. Environ. 2011, 144, 319–329. [Google Scholar] [CrossRef]
- Qin, Z.; Zhuang, Q.; Zhu, X. Carbon and nitrogen dynamics in bioenergy ecosystems: 2. Potential greenhouse gas emissions and global warming intensity in the conterminous United States. GCB Bioenergy 2015, 7, 25–39. [Google Scholar] [CrossRef]
- Fanous, J.; Moomaw, W.R. A Critical Look at Forest Bioenergy: Exposing a high carbon “climate solution”. GDAE Clim. Policy Br. 2018, 1–8. [Google Scholar]
- Norton, M.; Baldi, A.; Buda, V.; Carli, B.; Cudlin, P.; Jones, M.B.; Korhola, A.; Michalski, R.; Novo, F.; Oszlányi, J.; et al. Serious mismatches continue between science and policy in forest bioenergy. GCB Bioenergy 2019, 1256–1263. [Google Scholar] [CrossRef]
- Zhuang, Q.; Qin, Z.; Chen, M. Biofuel, land and water: Maize, switchgrass or Miscanthus? Environ. Res. Lett. 2013, 8. [Google Scholar] [CrossRef]
- Clifton-Brown, J.; Chiang, Y.-C.; Hodkinson, T. Miscanthus: Genetic Resources and Breeding Potential to Enhance Bioenergy Production. In Genetic Improvement of Bioenergy Crops; Springer: Berlin, Germany, 2008; pp. 273–294. ISBN 978-0-387-70804-1. [Google Scholar]
- Hodkinson, T.R.; Klaas, M.; Jones, M.B.; Prickett, R.; Barth, S. Miscanthus: A case study for the utilization of natural genetic variation. Plant Genet. Resour. Characterisation Util. 2015, 13, 219–237. [Google Scholar] [CrossRef]
- Hodkinson, T.R.; Chase, M.W.; Lledó, M.D.; Salamin, N.; Renvoize, S.A. Phylogenetics of Miscanthus, Saccharum and related genera (Saccharinae, Andropogoneae, Poaceae) based on DNA sequences from ITS nuclear ribosomal DNA and plastid trnL intron and trnL-F intergenic spacers. J. Plant Res. 2002, 115, 381–392. [Google Scholar] [CrossRef]
- Clark, L.V.; Brummer, J.E.; Głowacka, K.; Hall, M.C.; Heo, K.; Peng, J.; Yamada, T.; Yoo, J.H.; Yu, C.Y.; Zhao, H.; et al. A footprint of past climate change on the diversity and population structure of Miscanthus sinensis. Ann. Bot. 2014, 114, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, I.; Clifton-Brown, J.C.; Scurlock, J.M.O.; Huisman, W. Miscanthus: European experience with a novel energy crop. Biomass Bioenergy 2000, 19, 209–227. [Google Scholar] [CrossRef]
- Beale, C.V.; Long, S.P. Can perennial C4 grasses attain high efficiencies of radiant energy conversion in cool climates? Plant. Cell Environ. 1995, 18, 641–650. [Google Scholar] [CrossRef]
- Naidu, S.L.; Moose, S.P.; AL-Shoaibi, A.K.; Raines, C.A.; Long, S.P. Cold Tolerance of C 4 photosynthesis in Miscanthus × giganteus: Adaptation in Amounts and Sequence of C 4 Photosynthetic Enzymes. Plant Physiol. 2003, 132, 1688–1697. [Google Scholar] [CrossRef]
- Hamilton, S.K.; Hussain, M.Z.; Bhardwaj, A.K.; Basso, B.; Robertson, G.P. Comparative water use by maize, perennial crops, restored prairie, and poplar trees in the US Midwest. Environ. Res. Lett. 2015, 10, 64015. [Google Scholar] [CrossRef]
- Kørup, K.; Lærke, P.E.; Baadsgaard, H.; Andersen, M.N.; Kristensen, K.; Münnich, C.; Didion, T.; Jensen, E.S.; Mårtensson, L.M.; Jørgensen, U. Biomass production and water use efficiency in perennial grasses during and after drought stress. GCB Bioenergy 2018, 10, 12–27. [Google Scholar] [CrossRef]
- Oliveira, J.A.; West, C.P.; Afif, E.; Palencia, P. Comparison of miscanthus and switchgrass cultivars for biomass yield, soil nutrients, and nutrient removal in northwest Spain. Agron. J. 2017, 109, 122–130. [Google Scholar] [CrossRef]
- Chung, J.-H.; Kim, D.-S. Miscanthus as a potential bioenergy crop in East Asia. J. Crop Sci. Biotechnol. 2012, 15, 65–77. [Google Scholar] [CrossRef]
- Clifton-Brown, J.C.; Lewandowski, I.; Andersson, B.; Basch, G.; Christian, D.G.; Kjeldsen, J.B.; Jørgensen, U.; Mortensen, J.V.; Riche, A.B.; Schwarz, K.U.; et al. Performance of 15 Miscanthus genotypes at five sites in Europe. Agron. J. 2001, 93, 1013–1019. [Google Scholar] [CrossRef]
- Jeżowski, S.; Mos, M.; Buckby, S.; Cerazy-Waliszewska, J.; Owczarzak, W.; Mocek, A.; Kaczmarek, Z.; McCalmont, J.P. Establishment, growth, and yield potential of the perennial grass Miscanthus × Giganteus on degraded coal mine soils. Front. Plant Sci. 2017, 8, 1–8. [Google Scholar] [CrossRef]
- Yost, M.A.; Randall, B.K.; Kitchen, N.R.; Heaton, E.A.; Myers, R.L. Yield potential and nitrogen requirements of miscanthus × giganteus on eroded soil. Agron. J. 2017, 109, 684–695. [Google Scholar] [CrossRef]
- Amaducci, S.; Facciotto, G.; Bergante, S.; Perego, A.; Serra, P.; Ferrarini, A.; Chimento, C. Biomass production and energy balance of herbaceous and woody crops on marginal soils in the Po Valley. GCB Bioenergy 2017, 9, 31–45. [Google Scholar] [CrossRef]
- Barbosa, B.; Boléo, S.; Sidella, S.; Costa, J.; Duarte, M.P.; Mendes, B.; Cosentino, S.L.; Fernando, A.L. Phytoremediation of Heavy Metal-Contaminated Soils Using the Perennial Energy Crops Miscanthus spp. and Arundo donax L. Bioenergy Res. 2015, 8, 1500–1511. [Google Scholar] [CrossRef]
- Pidlisnyuk, V.; Stefanovska, T.; Lewis, E.E.; Erickson, L.E.; Davis, L.C. Miscanthus as a Productive Biofuel Crop for Phytoremediation. CRC. Crit. Rev. Plant Sci. 2014, 33, 1–19. [Google Scholar] [CrossRef]
- Nakajima, T.; Yamada, T.; Anzoua, K.G.; Kokubo, R.; Noborio, K. Carbon sequestration and yield performances of Miscanthus × giganteus and Miscanthus sinensis. Carbon Manag. 2018, 9, 415–423. [Google Scholar] [CrossRef]
- Robertson, A.D.; Whitaker, J.; Morrison, R.; Davies, C.A.; Smith, P.; McNamara, N.P. A Miscanthus plantation can be carbon neutral without increasing soil carbon stocks. GCB Bioenergy 2017, 9, 645–661. [Google Scholar] [CrossRef]
- Xue, S.; Kalinina, O.; Lewandowski, I. Present and future options for Miscanthus propagation and establishment. Renew. Sustain. Energy Rev. 2015, 49, 1233–1246. [Google Scholar] [CrossRef]
- Beccari, G.; Covarelli, L.; Balmas, V.; Tosi, L. First report of Miscanthus giganteus rhizome rot caused by Fusarium avenaceum, Fusarium oxysporum and Mucor hiemalis. Australas. Plant Dis. Notes 2010, 5, 28–29. [Google Scholar] [CrossRef]
- Falter, C.; Voigt, C.A. Comparative Cellular Analysis of Pathogenic Fungi with a Disease Incidence in Brachypodium distachyon and Miscanthus x giganteus. Bioenergy Res. 2014, 7, 958–973. [Google Scholar] [CrossRef]
- Scauflaire, J.; Gourgue, M.; Foucart, G.; Renard, F.; Vandeputte, F.; Munaut, F. Fusarium miscanthi and other Fusarium species as causal agents of Miscanthus × giganteus rhizome rot. Eur. J. Plant Pathol. 2013, 137, 1–3. [Google Scholar] [CrossRef]
- Mekete, T.; Reynolds, K.; Lopez-Nicora, H.D.; Gray, M.E.; Niblack, T.L. Plant-parasitic nematodes are potential pathogens of Miscanthus × giganteus and Panicum virgatum used for biofuels. Plant Dis. 2011, 95, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Clifton-Brown, J.C.; Lewandowski, I. Overwintering problems of newly established Miscanthus plantations can be overcome by identifying genotypes with improved rhizome cold tolerance. New Phytol. 2000, 148, 287–294. [Google Scholar] [CrossRef]
- Kucharik, C.J.; VanLoocke, A.; Lenters, J.D.; Motew, M.M. Miscanthus Establishment and Overwintering in the Midwest USA: A Regional Modeling Study of Crop Residue Management on Critical Minimum Soil Temperatures. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- De Melo Peixoto, M.; Friesen, P.C.; Sage, R.F. Winter cold-tolerance thresholds in field-grown Miscanthus hybrid rhizomes. J. Exp. Bot. 2015, 66, 4415–4425. [Google Scholar] [CrossRef]
- Van der Weijde, T.; Dolstra, O.; Visser, R.G.F.; Trindade, L.M. Stability of Cell Wall Composition and Saccharification Efficiency in Miscanthus across Diverse Environments. Front. Plant Sci. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Hodgson, E.M.; Fahmi, R.; Yates, N.; Barraclough, T.; Shield, I.; Allison, G.; Bridgwater, A.V.; Donnison, I.S. Miscanthus as a feedstock for fast-pyrolysis: Does agronomic treatment affect quality? Bioresour. Technol. 2010, 101, 6185–6191. [Google Scholar] [CrossRef]
- Lewandowski, I.; Kicherer, A. Combustion quality of biomass: Practical relevance and experiments to modify the biomass quality of Miscanthus x giganteus. Eur. J. Agron. 1997, 6, 163–177. [Google Scholar] [CrossRef]
- Moll, L.; Wever, C.; Völkering, G.; Pude, R. Increase of Miscanthus cultivation with new roles in materials production—A review. Agronomy 2020, 10, 308. [Google Scholar] [CrossRef]
- Van der Weijde, T.; Kiesel, A.; Iqbal, Y.; Muylle, H.; Dolstra, O.; Visser, R.G.F.; Lewandowski, I.; Trindade, L.M. Evaluation of Miscanthus sinensis biomass quality as feedstock for conversion into different bioenergy products. GCB Bioenergy 2017, 9, 176–190. [Google Scholar] [CrossRef]
- Kiesel, A.; Wagner, M.; Lewandowski, I. Environmental performance of miscanthus, switchgrass and maize: Can C4 perennials increase the sustainability of biogas production? Sustainability 2017, 9, 5. [Google Scholar] [CrossRef]
- Świątek, K.; Gaag, S.; Klier, A.; Kruse, A.; Sauer, J.; Steinbach, D. Acid hydrolysis of lignocellulosic biomass: Sugars and furfurals formation. Catalysts 2020, 10, 437. [Google Scholar] [CrossRef]
- Cudjoe, E.; Hunsen, M.; Xue, Z.; Way, A.E.; Barrios, E.; Olson, R.A.; Hore, M.J.A.; Rowan, S.J. Miscanthus Giganteus: A commercially viable sustainable source of cellulose nanocrystals. Carbohydr. Polym. 2017, 155, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin valorization: Improving lignin processing in the biorefinery. Science 2014, 344. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, R.; Jastrzebski, R.; Clough, M.T.; Ralph, J.; Kennema, M.; Bruijnincx, P.C.A.; Weckhuysen, B.M. Paving the Way for Lignin Valorisation: Recent Advances in Bioengineering, Biorefining and Catalysis. Angew. Chem. Int. Ed. 2016, 55, 8164–8215. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.T.; Jing, C.; Wood, C.; Nagardeolekar, A.; Kohan, N.; Dongre, P.; Amidon, T.E.; Bujanovic, B.M. Toward complete utilization of miscanthus in a hot-water extraction-based biorefinery. Energies 2018, 11, 39. [Google Scholar] [CrossRef]
- Messaoudi, Y.; Smichi, N.; Bouachir, F.; Gargouri, M. Fractionation and Biotransformation of Lignocelluloses-Based Wastes for Bioethanol, Xylose and Vanillin Production. Waste Biomass Valorization 2019, 10, 357–367. [Google Scholar] [CrossRef]
- Tu, W.C.; Hallett, J.P. Recent advances in the pretreatment of lignocellulosic biomass. Curr. Opin. Green Sustain. Chem. 2019, 20, 11–17. [Google Scholar] [CrossRef]
- Limayem, A.; Ricke, S.C. Lignocellulosic biomass for bioethanol production: Current perspectives, potential issues and future prospects. Prog. Energy Combust. Sci. 2012, 38, 449–467. [Google Scholar] [CrossRef]
- Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. Lignocellulosic Biorefineries in Europe: Current State and Prospects. Trends Biotechnol. 2019, 37, 231–234. [Google Scholar] [CrossRef]
- Adney, W.S.; Rivard, C.J.; Shiang, M.; Himmel, M.E. Anaerobic digestion of lignocellulosic biomass and wastes - Cellulases and related enzymes. Appl. Biochem. Biotechnol. 1991, 30, 165–183. [Google Scholar] [CrossRef]
- Chandra, R.; Takeuchi, H.; Hasegawa, T. Methane production from lignocellulosic agricultural crop wastes: A review in context to second generation of biofuel production. Renew. Sustain. Energy Rev. 2012, 16, 1462–1476. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhao, J.; Xu, F.; Li, Y. Pretreatment of lignocellulosic biomass for enhanced biogas production. Prog. Energy Combust. Sci. 2014, 42, 35–53. [Google Scholar] [CrossRef]
- Saini, J.K.; Saini, R.; Tewari, L. Lignocellulosic agriculture wastes as biomass feedstocks for second-generation bioethanol production: Concepts and recent developments. 3 Biotech 2015, 5, 337–353. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, I.; Clifton-Brown, J.; Trindade, L.M.; Van Der Linden, G.C.; Schwarz, K.U.; Müller-Sämann, K.; Anisimov, A.; Chen, C.L.; Dolstra, O.; Donnison, I.S.; et al. Progress on optimizing miscanthus biomass production for the european bioeconomy: Results of the EU FP7 project OPTIMISC. Front. Plant Sci. 2016, 7, 1–23. [Google Scholar] [CrossRef]
- Clifton-Brown, J.; Harfouche, A.; Casler, M.D.; Dylan Jones, H.; Macalpine, W.J.; Murphy-Bokern, D.; Smart, L.B.; Adler, A.; Ashman, C.; Awty-Carroll, D.; et al. Breeding progress and preparedness for mass-scale deployment of perennial lignocellulosic biomass crops switchgrass, miscanthus, willow and poplar. GCB Bioenergy 2019, 11, 118–151. [Google Scholar] [CrossRef] [PubMed]
- Clifton-Brown, J.; Schwarz, K.U.; Awty-Carroll, D.; Iurato, A.; Meyer, H.; Greef, J.; Gwyn, J.; Mos, M.; Ashman, C.; Hayes, C.; et al. Breeding strategies to improve Miscanthus as a sustainable source of biomass for bioenergy and biorenewable products. Agronomy 2019, 9, 673. [Google Scholar] [CrossRef]
- Jiang, J.; Guan, Y.; McCormick, S.; Juvik, J.; Lubberstedt, T.; Fei, S.Z. Gametophytic self-incompatibility is operative in Miscanthus sinensis (poaceae) and is affected by pistil age. Crop Sci. 2017, 57, 1948–1956. [Google Scholar] [CrossRef]
- Sacks, E.J.; Juvik, J.A.; Lin, Q.; Stewart, J.R.; Yamada, T. The Gene Pool of Miscanthus Species and Its Improvement. In Genomics of the Saccharinae; Paterson, A.H., Ed.; Springer: New York, NY, USA, 2013; pp. 73–101. ISBN 978-1-4419-5947-8. [Google Scholar]
- Trindade, L.M.; Dolstra, O.; Van Loo, E.N.; Visser, R.G.F. Plant breeding and its role in a biobased economy. In The Biobased Economy: Biofuels, Materials and Chemicals in the Post-oil Era; Earthscan Ltd.: London, UK, 2010; pp. 67–82. [Google Scholar]
- Dong, H.; Green, S.V.; Nishiwaki, A.; Yamada, T.; Stewart, J.R.; Deuter, M.; Sacks, E.J. Winter hardiness of Miscanthus (I): Overwintering ability and yield of new Miscanthus ×giganteus genotypes in Illinois and Arkansas. GCB Bioenergy 2019, 11, 691–705. [Google Scholar] [CrossRef]
- Clark, L.V.; Dwiyanti, M.S.; Anzoua, K.G.; Brummer, J.E.; Ghimire, B.K.; Głowacka, K.; Hall, M.; Heo, K.; Jin, X.; Lipka, A.E.; et al. Biomass yield in a genetically diverse Miscanthus sinensis germplasm panel evaluated at five locations revealed individuals with exceptional potential. GCB Bioenergy 2019, 1125–1145. [Google Scholar] [CrossRef]
- Atienza, S.G.; Satovic, Z.; Petersen, K.K.; Dolstra, O.; Martin, A. Influencing combustion quality in Miscanthus sinensis Anderss.: Identification of QTLs for calcium, phosphorus and sulphur content. Plant Breed. 2003, 122, 141–145. [Google Scholar] [CrossRef]
- Gifford, J.M.; Chae, W.B.; Swaminathan, K.; Moose, S.P.; Juvik, J.A. Mapping the genome of Miscanthus sinensis for QTL associated with biomass productivity. GCB Bioenergy 2015, 7, 797–810. [Google Scholar] [CrossRef]
- Van der Weijde, T.; Kamei, C.L.A.; Severing, E.I.; Torres, A.F.; Gomez, L.D.; Dolstra, O.; Maliepaard, C.A.; McQueen-Mason, S.J.; Visser, R.G.F.; Trindade, L.M. Genetic complexity of miscanthus cell wall composition and biomass quality for biofuels. BMC Genom. 2017, 18, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Liu, S.; Clark, L.V.; Sharma, S.; Gifford, J.M.; Juvik, J.A.; Lipka, A.E.; Sacks, E.J. Genetic mapping of biomass yield in three interconnected Miscanthus populations. GCB Bioenergy 2018, 10, 165–185. [Google Scholar] [CrossRef]
- Slavov, G.T.; Nipper, R.; Robson, P.; Farrar, K.; Allison, G.G.; Bosch, M.; Clifton-Brown, J.C.; Donnison, I.S.; Jensen, E. Genome-wide association studies and prediction of 17 traits related to phenology, biomass and cell wall composition in the energy grass Miscanthus sinensis. New Phytol. 2014, 201, 1227–1239. [Google Scholar] [CrossRef] [PubMed]
- Clark, L.V.; Dwiyanti, M.S.; Anzoua, K.G.; Brummer, J.E.; Ghimire, B.K.; Głowacka, K.; Hall, M.; Heo, K.; Jin, X.; Lipka, A.E.; et al. Genome-wide association and genomic prediction for biomass yield in a genetically diverse Miscanthus sinensis germplasm panel phenotyped at five locations in Asia and North America. GCB Bioenergy 2019, 988–1007. [Google Scholar] [CrossRef]
- Xu, F.; Yu, J.; Tesso, T.; Dowell, F.; Wang, D. Qualitative and quantitative analysis of lignocellulosic biomass using infrared techniques: A mini-review. Appl. Energy 2013, 104, 801–809. [Google Scholar] [CrossRef]
- Chadwick, D.T.; McDonnell, K.P.; Brennan, L.P.; Fagan, C.C.; Everard, C.D. Evaluation of infrared techniques for the assessment of biomass and biofuel quality parameters and conversion technology processes: A review. Renew. Sustain. Energy Rev. 2014, 30, 672–681. [Google Scholar] [CrossRef]
- Vogel, J. Unique aspects of the grass cell wall. Curr. Opin. Plant Biol. 2008, 11, 301–307. [Google Scholar] [CrossRef]
- Zhong, R.; Cui, D.; Ye, Z.H. Secondary cell wall biosynthesis. New Phytol. 2019, 221, 1703–1723. [Google Scholar] [CrossRef]
- Zhong, R.; Ye, Z.H. Secondary cell walls: Biosynthesis, patterned deposition and transcriptional regulation. Plant Cell Physiol. 2015, 56, 195–214. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Fernie, A.R.; Persson, S. Transition of primary to secondary cell wall synthesis. Sci. Bull. 2016, 61, 838–846. [Google Scholar] [CrossRef]
- Moura, J.C.M.S.; Bonine, C.A.V.; de Oliveira Fernandes Viana, J.; Dornelas, M.C.; Mazzafera, P. Abiotic and biotic stresses and changes in the lignin content and composition in plants. J. Integr. Plant Biol. 2010, 52, 360–376. [Google Scholar] [CrossRef] [PubMed]
- Van der Weijde, T.; Torres, A.F.; Dolstra, O.; Dechesne, A.; Visser, R.G.F.; Trindade, L.M. Impact of Different Lignin Fractions on Saccharification Efficiency in Diverse Species of the Bioenergy Crop Miscanthus. Bioenergy Res. 2016, 9, 146–156. [Google Scholar] [CrossRef]
- Allison, G.G.; Morris, C.; Clifton-Brown, J.; Lister, S.J.; Donnison, I.S. Genotypic variation in cell wall composition in a diverse set of 244 accessions of Miscanthus. Biomass Bioenergy 2011, 35, 4740–4747. [Google Scholar] [CrossRef]
- Battaglia, M.; Fike, J.; Fike, W.; Sadeghpour, A.; Diatta, A. Miscanthus ×giganteus biomass yield and quality in the Virginia Piedmont. Grassl. Sci. 2019, 1–8. [Google Scholar] [CrossRef]
- Da Costa, R.M.F.; Lee, S.J.; Allison, G.G.; Hazen, S.P.; Winters, A.; Bosch, M. Genotype, development and tissue-derived variation of cell-wall properties in the lignocellulosic energy crop Miscanthus. Ann. Bot. 2014, 114, 1265–1277. [Google Scholar] [CrossRef]
- Qin, J.; Yang, Y.; Jiang, J.; Yi, Z.; Xiao, L.; Ai, X.; Chen, Z. Comparison of lignocellulose composition in four major species of Miscanthus. Afr. J. Biotechnol. 2012, 11, 12529–12537. [Google Scholar] [CrossRef]
- Adams, J.M.M.; Winters, A.L.; Hodgson, E.M.; Gallagher, J.A. What cell wall components are the best indicators for Miscanthus digestibility and conversion to ethanol following variable pretreatments? Biotechnol. Biofuels 2018, 11, 1–14. [Google Scholar] [CrossRef]
- De Souza, A.P.; Kamei, C.L.A.; Torres, A.F.; Pattathil, S.; Hahn, M.G.; Trindade, L.M.; Buckeridge, M.S. How cell wall complexity influences saccharification efficiency in Miscanthus sinensis. J. Exp. Bot. 2015, 66, 4351–4365. [Google Scholar] [CrossRef]
- Da Costa, R.M.F.; Pattathil, S.; Avci, U.; Lee, S.J.; Hazen, S.P.; Winters, A.; Hahn, M.G.; Bosch, M. A cell wall reference profile for Miscanthus bioenergy crops highlights compositional and structural variations associated with development and organ origin. New Phytol. 2017, 213, 1710–1725. [Google Scholar] [CrossRef]
- Somerville, C. Cellulose Synthesis in Higher Plants. Annu. Rev. Cell Dev. Biol. 2006, 22, 53–78. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Villarreal, M.; Aispuro-Hernández, E.; Vargas-Arispuro, I.; Martínez-Téllez, M.Á. Plant cell wall polymers: Function, structure and biological activity of their derivatives. Polymerization 2012, 4, 63–86. [Google Scholar]
- Lygin, A.V.; Upton, J.; Dohleman, F.G.; Juvik, J.; Zabotina, O.A.; Widholm, J.M.; Lozovaya, V.V. Composition of cell wall phenolics and polysaccharides of the potential bioenergy crop -Miscanthus. GCB Bioenergy 2011, 3, 333–345. [Google Scholar] [CrossRef]
- Le Ngoc Huyen, T.; Rémond, C.; Dheilly, R.M.; Chabbert, B. Effect of harvesting date on the composition and saccharification of Miscanthus × giganteus. Bioresour. Technol. 2010, 101, 8224–8231. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.J.; Kim, S.H.; Chung, I.M. Comparison of lignin, cellulose, and hemicellulose contents for biofuels utilization among 4 types of lignocellulosic crops. Biomass Bioenergy 2015, 83, 322–327. [Google Scholar] [CrossRef]
- Van der Weijde, T.; Huxley, L.M.; Hawkins, S.; Sembiring, E.H.; Farrar, K.; Dolstra, O.; Visser, R.G.F.; Trindade, L.M. Impact of drought stress on growth and quality of miscanthus for biofuel production. GCB Bioenergy 2017, 9, 770–782. [Google Scholar] [CrossRef]
- Da Costa, R.M.F.; Pattathil, S.; Avci, U.; Winters, A.; Hahn, M.G.; Bosch, M. Desirable plant cell wall traits for higher-quality miscanthus lignocellulosic biomass. Biotechnol. Biofuels 2019, 12, 1–18. [Google Scholar] [CrossRef]
- Hallac, B.B.; Ragauskas, A.J. Analyzing cellulose degree of polymerization and its relevancy to cellulosic ethanol. Biofuels Bioprod. Biorefining 2011, 5, 215–225. [Google Scholar] [CrossRef]
- Gismatulina, Y.A.; Budaeva, V.V.; Veprev, S.G.; Sakovich, G.V.; Shumny, V.K. Cellulose from various parts of Soranovskii Miscanthus. Russ. J. Genet. Appl. Res. 2015, 5, 60–68. [Google Scholar] [CrossRef]
- Gismatulina, Y.A.; Budaeva, V.V. Chemical composition of five Miscanthus sinensis harvests and nitric-acid cellulose therefrom. Ind. Crops Prod. 2017, 109, 227–232. [Google Scholar] [CrossRef]
- Waliszewska, H.; Zborowska, M.; Waliszewska, B.; Borysiak, S.; Antczak, A.; Czekała, W. Transformation of Miscanthus and Sorghum cellulose during methane fermentation. Cellulose 2018, 25, 1207–1216. [Google Scholar] [CrossRef]
- Sun, D.; Alam, A.; Tu, Y.; Zhou, S.; Wang, Y.; Xia, T.; Huang, J.; Li, Y.; Zahoor; Wei, X.; et al. Steam-exploded biomass saccharification is predominately affected by lignocellulose porosity and largely enhanced by Tween-80 in Miscanthus. Bioresour. Technol. 2017, 239, 74–81. [Google Scholar] [CrossRef]
- Zhang, W.; Yi, Z.; Huang, J.; Li, F.; Hao, B.; Li, M.; Hong, S.; Lv, Y.; Sun, W.; Ragauskas, A.; et al. Three lignocellulose features that distinctively affect biomass enzymatic digestibility under NaOH and H2SO4 pretreatments in Miscanthus. Bioresour. Technol. 2013, 130, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Teeri, T.T. Crystalline cellulose degradation: New insight into the function of cellobiohydrolases. Trends Biotechnol. 1997, 15, 160–167. [Google Scholar] [CrossRef]
- Ciolacu, D.; Ciolacu, F.; Popa, V.I. Amorphous cellulose - Structure and characterization. Cellul. Chem. Technol. 2011, 45, 13–21. [Google Scholar]
- Bansal, P.; Hall, M.; Realff, M.J.; Lee, J.H.; Bommarius, A.S. Multivariate statistical analysis of X-ray data from cellulose: A new method to determine degree of crystallinity and predict hydrolysis rates. Bioresour. Technol. 2010, 101, 4461–4471. [Google Scholar] [CrossRef]
- Yoshida, M.; Liu, Y.; Uchida, S.; Kawarada, K.; Ukagami, Y.; Ichinose, H.; Kaneko, S.; Fukuda, K. Effects of cellulose crystallinity, hemicellulose, and lignin on the enzymatic hydrolysis of Miscanthus sinensis to monosaccharides. Biosci. Biotechnol. Biochem. 2008, 72, 805–810. [Google Scholar] [CrossRef]
- Xu, N.; Zhang, W.; Ren, S.; Liu, F.; Zhao, C.; Liao, H.; Xu, Z.; Huang, J.; Li, Q.; Tu, Y.; et al. Hemicelluloses negatively affect lignocellulose crystallinity for high biomass digestibility under NaOH and H2SO4 pretreatments in Miscanthus. Biotechnol. Biofuels 2012, 5, 1. [Google Scholar] [CrossRef]
- Shiga, T.M.; Xiao, W.; Yang, H.; Zhang, X.; Olek, A.T.; Donohoe, B.S.; Liu, J.; Makowski, L.; Hou, T.; McCann, M.C.; et al. Enhanced rates of enzymatic saccharification and catalytic synthesis of biofuel substrates in gelatinized cellulose generated by trifluoroacetic acid. Biotechnol. Biofuels 2017, 10, 1–15. [Google Scholar] [CrossRef]
- Qin, L.; Li, W.C.; Zhu, J.Q.; Liang, J.N.; Li, B.Z.; Yuan, Y.J. Ethylenediamine pretreatment changes cellulose allomorph and lignin structure of lignocellulose at ambient pressure. Biotechnol. Biofuels 2015, 8, 1–15. [Google Scholar] [CrossRef]
- Jeoh, T.; Ishizawa, C.I.; Davis, M.F.; Himmel, M.E.; Adney, W.S.; Johnson, D.K. Cellulase digestibility of pretreated biomass is limited by cellulose accessibility. Biotechnol. Bioeng. 2007, 98, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Pauly, M.; Gille, S.; Liu, L.; Mansoori, N.; de Souza, A.; Schultink, A.; Xiong, G. Hemicellulose biosynthesis. Planta 2013, 238, 627–642. [Google Scholar] [CrossRef]
- Li, X.; Liao, H.; Fan, C.; Hu, H.; Li, Y.; Li, J.; Yi, Z.; Cai, X.; Peng, L.; Tu, Y. Distinct geographical distribution of the Miscanthus accessions with varied biomass enzymatic saccharification. PLoS ONE 2016, 11, 1–15. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schäfer, J.; Sattler, M.; Iqbal, Y.; Lewandowski, I.; Bunzel, M. Characterization of Miscanthus cell wall polymers. GCB Bioenergy 2019, 11, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, J.; Li, Y.; Xiong, K.; Wang, Y.; Li, F.; Liu, M.; Wu, Z.; Tu, Y.; Peng, L. Ammonium oxalate-extractable uronic acids positively affect biomass enzymatic digestibility by reducing lignocellulose crystallinity in Miscanthus. Bioresour. Technol. 2015, 196, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Sorek, H.; Zimmermann, H.; Wemmer, D.E.; Pauly, M. Solution-state 2D NMR spectroscopy of plant cell Walls enabled by a dimethylsulfoxide- d 6/1-Ethyl-3-methylimidazolium acetate solvent. Anal. Chem. 2013, 85, 3213–3221. [Google Scholar] [CrossRef]
- Gírio, F.M.; Fonseca, C.; Carvalheiro, F.; Duarte, L.C.; Marques, S.; Bogel-Łukasik, R. Hemicelluloses for fuel ethanol: A review. Bioresour. Technol. 2010, 101, 4775–4800. [Google Scholar] [CrossRef]
- Hatfield, R.D.; Rancour, D.M.; Marita, J.M. Grass cell walls: A story of cross-linking. Front. Plant Sci. 2017, 7. [Google Scholar] [CrossRef]
- Lapierre, C.; Voxeur, A.; Karlen, S.D.; Helm, R.F.; Ralph, J. Evaluation of Feruloylated and p-Coumaroylated Arabinosyl Units in Grass Arabinoxylans by Acidolysis in Dioxane/Methanol. J. Agric. Food Chem. 2018, 66, 5418–5424. [Google Scholar] [CrossRef]
- Klímek, P.; Wimmer, R.; Meinlschmidt, P.; Kúdela, J. Utilizing Miscanthus stalks as raw material for particleboards. Ind. Crops Prod. 2018, 111, 270–276. [Google Scholar] [CrossRef]
- Falter, C.; Zwikowics, C.; Eggert, D.; Blümke, A.; Naumann, M.; Wolff, K.; Ellinger, D.; Reimer, R.; Voigt, C.A. Glucanocellulosic ethanol: The undiscovered biofuel potential in energy crops and marine biomass. Sci. Rep. 2015, 5, 1–9. [Google Scholar] [CrossRef]
- Menardo, S.; Bauer, A.; Theuretzbacher, F.; Piringer, G.; Nilsen, P.J.; Balsari, P.; Pavliska, O.; Amon, T. Biogas Production from Steam-Exploded Miscanthus and Utilization of Biogas Energy and CO2 in Greenhouses. Bioenergy Res. 2013, 6, 620–630. [Google Scholar] [CrossRef]
- Mangold, A.; Lewandowski, I.; Möhring, J.; Clifton-Brown, J.; Krzyżak, J.; Mos, M.; Pogrzeba, M.; Kiesel, A. Harvest date and leaf:stem ratio determine methane hectare yield of miscanthus biomass. GCB Bioenergy 2019, 11, 21–33. [Google Scholar] [CrossRef]
- Li, W.; Khalid, H.; Zhu, Z.; Zhang, R.; Liu, G.; Chen, C.; Thorin, E. Methane production through anaerobic digestion: Participation and digestion characteristics of cellulose, hemicellulose and lignin. Appl. Energy 2018, 226, 1219–1228. [Google Scholar] [CrossRef]
- Qing, Q.; Yang, B.; Wyman, C.E. Xylooligomers are strong inhibitors of cellulose hydrolysis by enzymes. Bioresour. Technol. 2010, 101, 9624–9630. [Google Scholar] [CrossRef]
- Li, F.; Ren, S.; Zhang, W.; Xu, Z.; Xie, G.; Chen, Y.; Tu, Y.; Li, Q.; Zhou, S.; Li, Y.; et al. Arabinose substitution degree in xylan positively affects lignocellulose enzymatic digestibility after various NaOH/H2SO4 pretreatments in Miscanthus. Bioresour. Technol. 2013, 130, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Simmons, T.J.; Mortimer, J.C.; Bernardinelli, O.D.; Pöppler, A.C.; Brown, S.P.; DeAzevedo, E.R.; Dupree, R.; Dupree, P. Folding of xylan onto cellulose fibrils in plant cell walls revealed by solid-state NMR. Nat. Commun. 2016, 7, 1–10. [Google Scholar] [CrossRef]
- Kabel, M.A.; van den Borne, H.; Vincken, J.P.; Voragen, A.G.J.; Schols, H.A. Structural differences of xylans affect their interaction with cellulose. Carbohydr. Polym. 2007, 69, 94–105. [Google Scholar] [CrossRef]
- Köhnke, T.; Östlund, Å.; Brelid, H. Adsorption of arabinoxylan on cellulosic surfaces: Influence of degree of substitution and substitution pattern on adsorption characteristics. Biomacromolecules 2011, 12, 2633–2641. [Google Scholar] [CrossRef]
- Park, Y.B.; Lee, C.M.; Kafle, K.; Park, S.; Cosgrove, D.J.; Kim, S.H. Effects of plant cell wall matrix polysaccharides on bacterial cellulose structure studied with vibrational sum frequency generation spectroscopy and x-ray diffraction. Biomacromolecules 2014, 15, 2718–2724. [Google Scholar] [CrossRef]
- Mikkelsen, D.; Flanagan, B.M.; Wilson, S.M.; Bacic, A.; Gidley, M.J. Interactions of Arabinoxylan and (1,3)(1,4)-β-Glucan with Cellulose Networks. Biomacromolecules 2015, 16, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.S.; Silveira, R.L.; Dupree, P.; Skaf, M.S. Effects of Xylan Side-Chain Substitutions on Xylan-Cellulose Interactions and Implications for Thermal Pretreatment of Cellulosic Biomass. Biomacromolecules 2017, 18, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, U.R.; Smith, S.; Pingali, S.V.; Yang, H.; Zahran, M.; Breunig, L.; Wilson, L.A.; Kowali, M.; Kubicki, J.D.; Cosgrove, D.J.; et al. Arabinose substitution effect on xylan rigidity and self-aggregation. Cellulose 2019, 26, 2267–2278. [Google Scholar] [CrossRef]
- Vanholme, R.; Demedts, B.; Morreel, K.; Ralph, J.; Boerjan, W. Lignin biosynthesis and structure. Plant Physiol. 2010, 153, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, H.; Hatakeyama, T. Lignin Structure, Properties, and Applications. Adv. Polym. Sci. 2010, 1–63. [Google Scholar]
- Ralph, J.; Lapierre, C.; Boerjan, W. Lignin structure and its engineering. Curr. Opin. Biotechnol. 2019, 56, 240–249. [Google Scholar] [CrossRef]
- Grabber, J.H.; Ralph, J.; Lapierre, C.; Barrière, Y. Genetic and molecular basis of grass cell-wall degradability. I. Lignin-cell wall matrix interactions. Comptes Rendus Biol. 2004, 327, 455–465. [Google Scholar] [CrossRef]
- Del Río, J.C.; Lino, A.G.; Colodette, J.L.; Lima, C.F.; Gutiérrez, A.; Martínez, Á.T.; Lu, F.; Ralph, J.; Rencoret, J. Differences in the chemical structure of the lignins from sugarcane bagasse and straw. Biomass Bioenergy 2015, 81, 322–338. [Google Scholar] [CrossRef]
- Ralph, J. Hydroxycinnamates in lignification. Phytochem. Rev. 2010, 9, 65–83. [Google Scholar] [CrossRef]
- Lan, W.; Rencoret, J.; Lu, F.; Karlen, S.D.; Smith, B.G.; Harris, P.J.; del Río, J.C.; Ralph, J. Tricin-lignins: Occurrence and quantitation of tricin in relation to phylogeny. Plant J. 2016, 88, 1046–1057. [Google Scholar] [CrossRef]
- Van Erven, G.; De Visser, R.; Merkx, D.W.H.; Strolenberg, W.; De Gijsel, P.; Gruppen, H.; Kabel, M.A. Quantification of Lignin and Its Structural Features in Plant Biomass Using 13C Lignin as Internal Standard for Pyrolysis-GC-SIM-MS. Anal. Chem. 2017, 89, 10907–10916. [Google Scholar] [CrossRef] [PubMed]
- Lan, W.; Yue, F.; Rencoret, J.; del Río, J.C.; Boerjan, W.; Lu, F.; Ralph, J. Elucidating tricin-lignin structures: Assigning correlations in HSQC spectra of monocot lignins. Polymers (Basel) 2018, 10, 916. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Li, J.; Yu, L.; Chai, G.; He, G.; Hu, R.; Qi, G.; Kong, Y.; Fu, C.; Zhou, G. Cell wall polysaccharide distribution in Miscanthus lutarioriparius stem using immuno-detection. Plant Cell Rep. 2014, 33, 643–653. [Google Scholar] [CrossRef]
- Li, M.; Pu, Y.; Ragauskas, A.J. Current understanding of the correlation of lignin structure with biomass recalcitrance. Front. Chem. 2016, 4, 1–8. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, L.; Liu, D. Biomass recalcitrance. Part I: The chemical compositions and physical structures affecting the enzymatic hydrolysis of lignocellulose. Biofuels Bioprod. Biorefining 2012, 6, 465–482. [Google Scholar] [CrossRef]
- Djajadi, D.T.; Jensen, M.M.; Oliveira, M.; Jensen, A.; Thygesen, L.G.; Pinelo, M.; Glasius, M.; Jørgensen, H.; Meyer, A.S. Lignin from hydrothermally pretreated grass biomass retards enzymatic cellulose degradation by acting as a physical barrier rather than by inducing nonproductive adsorption of enzymes. Biotechnol. Biofuels 2018, 11, 1–13. [Google Scholar] [CrossRef]
- Dandikas, V.; Heuwinkel, H.; Lichti, F.; Drewes, J.E.; Koch, K. Correlation between biogas yield and chemical composition of energy crops. Bioresour. Technol. 2014, 174, 316–320. [Google Scholar] [CrossRef]
- El Hage, R.; Brosse, N.; Chrusciel, L.; Sanchez, C.; Sannigrahi, P.; Ragauskas, A. Characterization of milled wood lignin and ethanol organosolv lignin from miscanthus. Polym. Degrad. Stab. 2009, 94, 1632–1638. [Google Scholar] [CrossRef]
- Villaverde, J.J.; Li, J.; Ek, M.; Ligero, P.; De Vega, A. Native lignin structure of Miscanthus x giganteus and its changes during acetic and formic acid fractionation. J. Agric. Food Chem. 2009, 57, 6262–6270. [Google Scholar] [CrossRef]
- Bauer, S.; Sorek, H.; Mitchell, V.D.; Ibáñez, A.B.; Wemmer, D.E. Characterization of Miscanthus giganteus lignin isolated by ethanol organosolv process under reflux condition. J. Agric. Food Chem. 2012, 60, 8203–8212. [Google Scholar] [CrossRef]
- Bergs, M.; Völkering, G.; Kraska, T.; Pude, R.; Do, X.T.; Kusch, P.; Monakhova, Y.; Konow, C.; Schulze, M. Miscanthus x giganteus stem versus leaf-derived lignins differing in monolignol ratio and linkage. Int. J. Mol. Sci. 2019, 20, 1200. [Google Scholar] [CrossRef] [PubMed]
- You, T.T.; Mao, J.Z.; Yuan, T.Q.; Wen, J.L.; Xu, F. Structural elucidation of the lignins from stems and foliage of Arundo donax Linn. J. Agric. Food Chem. 2013, 61, 5361–5370. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Cao, T.; Gu, F.; Wu, W.; Jin, Y. Comparison of the Structural Characteristics of Cellulolytic Enzyme Lignin Preparations Isolated from Wheat Straw Stem and Leaf. ACS Sustain. Chem. Eng. 2017, 5, 342–349. [Google Scholar] [CrossRef]
- Ralph, J.; Brunow, G.; Harris, P.J.; Dixon, R.A.; Schatz, P.F.; Boerjan, W. Lignification: Are lignins biosynthesized via simple combinatorial chemistry or via proteinaceous control and template replication. In Recent Advances in Polyphenol Research; Wiley-Blackwell: Oxford, UK, 2009; Volume 1, pp. 36–66. [Google Scholar]
- Anderson, E.M.; Stone, M.L.; Katahira, R.; Reed, M.; Muchero, W.; Ramirez, K.J.; Beckham, G.T.; Román-Leshkov, Y. Differences in S/G ratio in natural poplar variants do not predict catalytic depolymerization monomer yields. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Li, X.; Ximenes, E.; Kim, Y.; Slininger, M.; Meilan, R.; Ladisch, M.; Chapple, C. Lignin monomer composition affects Arabidopsis cell-wall degradability after liquid hot water pretreatment. Biotechnol. Biofuels 2010, 3, 1–8. [Google Scholar] [CrossRef]
- Miyamoto, T.; Yamamura, M.; Tobimatsu, Y.; Suzuki, S.; Kojima, M.; Takabe, K.; Terajima, Y.; Mihashi, A.; Kobayashi, Y.; Umezawa, T. A comparative study of the biomass properties of Erianthus and sugarcane: Lignocellulose structure, alkaline delignification rate, and enzymatic saccharification efficiency. Biosci. Biotechnol. Biochem. 2018, 82, 1143–1152. [Google Scholar] [CrossRef]
- Halpin, C. Lignin engineering to improve saccharification and digestibility in grasses. Curr. Opin. Biotechnol. 2019, 56, 223–229. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, C.; Zha, Y.; Wan, C.; Si, S.; Liu, F.; Zhang, R.; Li, F.; Yu, B.; Yi, Z.; et al. The minor wall-networks between monolignols and interlinked-phenolics predominantly affect biomass enzymatic digestibility in Miscanthus. PLoS ONE 2014, 9. [Google Scholar] [CrossRef]
- Li, M.; Si, S.; Hao, B.; Zha, Y.; Wan, C.; Hong, S.; Kang, Y.; Jia, J.; Zhang, J.; Li, M.; et al. Mild alkali-pretreatment effectively extracts guaiacyl-rich lignin for high lignocellulose digestibility coupled with largely diminishing yeast fermentation inhibitors in Miscanthus. Bioresour. Technol. 2014, 169, 447–454. [Google Scholar] [CrossRef]
- He, Y.; Mouthier, T.M.B.; Kabel, M.A.; Dijkstra, J.; Hendriks, W.H.; Struik, P.C.; Cone, J.W. Lignin composition is more important than content for maize stem cell wall degradation. J. Sci. Food Agric. 2018, 98, 384–390. [Google Scholar] [CrossRef]
- Fu, C.; Mielenz, J.R.; Xiao, X.; Ge, Y.; Hamilton, C.Y.; Rodriguez, M.; Chen, F.; Foston, M.; Ragauskas, A.; Bouton, J.; et al. Genetic manipulation of lignin reduces recalcitrance and improves ethanol production from switchgrass. Proc. Natl. Acad. Sci. USA 2011, 108, 3803–3808. [Google Scholar] [CrossRef]
- Baxter, H.L.; Mazarei, M.; Labbe, N.; Kline, L.M.; Cheng, Q.; Windham, M.T.; Mann, D.G.J.; Fu, C.; Ziebell, A.; Sykes, R.W.; et al. Two-year field analysis of reduced recalcitrance transgenic switchgrass. Plant Biotechnol. J. 2014, 12, 914–924. [Google Scholar] [CrossRef] [PubMed]
- Piquemal, J.; Chamayou, S.; Nadaud, I.; Beckert, M.; Barrie, Y.; Lapierre, C.; Rigau, J.; Puigdomenech, P.; Jauneau, A.; Digonnet, C.; et al. Down-Regulation of Caffeic Acid. Society 2002, 130, 1675–1685. [Google Scholar] [CrossRef]
- Jung, J.H.; Vermerris, W.; Gallo, M.; Fedenko, J.R.; Erickson, J.E.; Altpeter, F. RNA interference suppression of lignin biosynthesis increases fermentable sugar yields for biofuel production from field-grown sugarcane. Plant Biotechnol. J. 2013, 11, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Rochfort, S.; Liu, Z.; Ran, Y.; Griffith, M.; Badenhorst, P.; Louie, G.V.; Bowman, M.E.; Smith, K.F.; Noel, J.P.; et al. Functional analyses of caffeic acid O-methyltransferase and Cinnamoyl-CoA-reductase genes from perennial ryegrass (Lolium perenne). Plant Cell 2010, 22, 3357–3373. [Google Scholar] [CrossRef] [PubMed]
- Mohnen, D. Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 2008, 11, 266–277. [Google Scholar] [CrossRef]
- Willats, W.G.T.; McCartney, L.; Mackie, W.; Knox, J.P. Pectin: Cell biology and prospects for functional analysis. Plant Mol. Biol. 2001, 47, 9–27. [Google Scholar] [CrossRef]
- Latarullo, M.B.G.; Tavares, E.Q.P.; Maldonado, G.P.; Leite, D.C.C.; Buckeridge, M.S. Pectins, endopolygalacturonases, and bioenergy. Front. Plant Sci. 2016, 7, 1–7. [Google Scholar] [CrossRef]
- Harholt, J.; Suttangkakul, A.; Scheller, H.V. Biosynthesis of pectin. Plant Physiol. 2010, 153, 384–395. [Google Scholar] [CrossRef]
- De Vrije, T.; De Haas, G.; Tan, G.B.; Keijsers, E.R.P.; Claassen, P.A.M. Pretreatment of Miscanthus for hydrogen production by Thermotoga elfii. Int. J. Hydrogen Energy 2002, 27, 1381–1390. [Google Scholar] [CrossRef]
- Ligero, P.; van der Kolk, J.C.; de Vega, A.; van Dam, J.E.G. Production of xylo-oligosaccharides from Miscanthus x giganteus by autohydrolysis. BioResources 2011, 6, 4417–4429. [Google Scholar]
- Cheng, S.; Yu, H.; Hu, M.; Wu, Y.; Cheng, L.; Cai, Q.; Tu, Y.; Xia, T.; Peng, L. Miscanthus accessions distinctively accumulate cadmium for largely enhanced biomass enzymatic saccharification by increasing hemicellulose and pectin and reducing cellulose CrI and DP. Bioresour. Technol. 2018, 263, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Choi, I.S.; Lee, S.; Hwang, I.M.; Chun, H.H.; Wi, S.G.; Kim, J.-C.; Shin, T.Y.; Kim, J.C.; Kim, J.S.; et al. Advanced strategy to produce insecticidal destruxins from lignocellulosic biomass Miscanthus. Biotechnol. Biofuels 2019, 12, 188. [Google Scholar] [CrossRef] [PubMed]
- Biswal, A.K.; Atmodjo, M.A.; Li, M.; Baxter, H.L.; Yoo, C.G.; Pu, Y.; Lee, Y.C.; Mazarei, M.; Black, I.M.; Zhang, J.Y.; et al. Sugar release and growth of biofuel crops are improved by downregulation of pectin biosynthesis. Nat. Biotechnol. 2018, 36, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yoo, C.G.; Pu, Y.; Biswal, A.K.; Tolbert, A.K.; Mohnen, D.; Ragauskas, A.J. Downregulation of pectin biosynthesis gene GAUT4 leads to reduced ferulate and lignin-carbohydrate cross-linking in switchgrass. Commun. Biol. 2019, 2, 1–11. [Google Scholar] [CrossRef]
- Fry, S.C. Cross-Linking of Matrix Polymers in the Growing Cell Walls of Angiosperms. Annu. Rev. Plant Physiol. 1986, 37, 165–186. [Google Scholar] [CrossRef]
- Hartley, R.D.; Morrison, W.H.; Himmelsbach, D.S.; Borneman, W.S. Cross-linking of cell wall phenolic arabinoxylans in graminaceous plants. Phytochemistry 1990, 29, 3705–3709. [Google Scholar] [CrossRef]
- Ralph, J.; Quideau, S.; Grabber, J.H.; Hatfield, R.D. Identification and synthesis of new ferulic acid dehydrodimers present in grass cell walls. J. Chem. Soc. Perkin Trans. 1 1994, 3485–3498. [Google Scholar] [CrossRef]
- De O. Buanafina, M.M. Feruloylation in Grasses: Current and Future Perspectives. Mol. Plant 2009, 2, 861–872. [Google Scholar] [CrossRef]
- Terrett, O.M.; Dupree, P. Covalent interactions between lignin and hemicelluloses in plant secondary cell walls. Curr. Opin. Biotechnol. 2019, 56, 97–104. [Google Scholar] [CrossRef]
- Grabber, J.H.; Mertens, D.R.; Kim, H.; Funk, C.; Lu, F.; Ralph, J. Cell wall fermentation kinetics are impacted more by lignin content and ferulate cross-linking than by lignin composition. J. Sci. Food Agric. 2009, 89, 122–129. [Google Scholar] [CrossRef]
- Belmokhtar, N.; Habrant, A.; Lopes Ferreira, N.; Chabbert, B. Changes in Phenolics Distribution After Chemical Pretreatment and Enzymatic Conversion of Miscanthus × giganteus Internode. Bioenergy Res. 2013, 6, 506–518. [Google Scholar] [CrossRef]
- Ji, Z.; Zhang, X.; Ling, Z.; Zhou, X.; Ramaswamy, S.; Xu, F. Visualization of Miscanthus × giganteus cell wall deconstruction subjected to dilute acid pretreatment for enhanced enzymatic digestibility. Biotechnol. Biofuels 2015, 8, 1–14. [Google Scholar] [CrossRef]
- Kim, T.H.; Im, D.; Oh, K.K. Effects of organosolv pretreatment using temperature-controlled bench-scale ball milling on enzymatic saccharification of miscanthus × giganteus. Energies 2018, 11, 2657. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, L.; Liu, D. Biomass recalcitrance. Part II: Fundamentals of different pre-treatments to increase the enzymatic digestibility of lignocellulose. Biofuels Bioprod. Biorefining 2012, 6, 561–579. [Google Scholar] [CrossRef]
- Yang, B.; Wyman, C.E. Pretreatment: The key to unlocking low-cost cellulosic ethanol. Biofuels Bioprod. Biorefining 2008, 2, 26–40. [Google Scholar] [CrossRef]
- Fu, S.F.; Chen, K.Q.; Zhu, R.; Sun, W.X.; Zou, H.; Guo, R.B. Improved anaerobic digestion performance of Miscanthus floridulus by different pretreatment methods and preliminary economic analysis. Energy Convers. Manag. 2018, 159, 121–128. [Google Scholar] [CrossRef]
- Torres, A.F.; van der Weijde, T.; Dolstra, O.; Visser, R.G.F.; Trindade, L.M. Effect of Maize Biomass Composition on the Optimization of Dilute-Acid Pretreatments and Enzymatic Saccharification. Bioenergy Res. 2013, 6, 1038–1051. [Google Scholar] [CrossRef]
- Alvira, P.; Tomás-Pejó, E.; Ballesteros, M.; Negro, M.J. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review. Bioresour. Technol. 2010, 101, 4851–4861. [Google Scholar] [CrossRef]
- Batista, G.; Souza, R.B.A.; Pratto, B.; dos Santos-Rocha, M.S.R.; Cruz, A.J.G. Effect of severity factor on the hydrothermal pretreatment of sugarcane straw. Bioresour. Technol. 2019, 275, 321–327. [Google Scholar] [CrossRef]
- Zhou, X.; Li, Q.; Zhang, Y.; Gu, Y. Effect of hydrothermal pretreatment on Miscanthus anaerobic digestion. Bioresour. Technol. 2017, 224, 721–726. [Google Scholar] [CrossRef]
- Li, H.Q.; Li, C.L.; Sang, T.; Xu, J. Pretreatment on Miscanthus lutarioriparious by liquid hot water for efficient ethanol production. Biotechnol. Biofuels 2013, 6, 1–10. [Google Scholar] [CrossRef]
- Yelle, D.J.; Kaparaju, P.; Hunt, C.G.; Hirth, K.; Kim, H.; Ralph, J.; Felby, C. Two-Dimensional NMR Evidence for Cleavage of Lignin and Xylan Substituents in Wheat Straw Through Hydrothermal Pretreatment and Enzymatic Hydrolysis. Bioenergy Res. 2013, 6, 211–221. [Google Scholar] [CrossRef]
- Djajadi, D.T.; Hansen, A.R.; Jensen, A.; Thygesen, L.G.; Pinelo, M.; Meyer, A.S.; Jørgensen, H. Surface properties correlate to the digestibility of hydrothermally pretreated lignocellulosic Poaceae biomass feedstocks. Biotechnol. Biofuels 2017, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- El Hage, R.; Chrusciel, L.; Desharnais, L.; Brosse, N. Effect of autohydrolysis of Miscanthus x giganteus on lignin structure and organosolv delignification. Bioresour. Technol. 2010, 101, 9321–9329. [Google Scholar] [CrossRef] [PubMed]
- Demartini, J.D.; Pattathil, S.; Avci, U.; Szekalski, K.; Mazumder, K.; Hahn, M.G.; Wyman, C.E. Application of monoclonal antibodies to investigate plant cell wall deconstruction for biofuels production. Energy Environ. Sci. 2011, 4, 4332–4339. [Google Scholar] [CrossRef]
- Chen, X.; Li, H.; Sun, S.; Cao, X.; Sun, R. Effect of hydrothermal pretreatment on the structural changes of alkaline ethanol lignin from wheat straw. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Laskar, D.D.; Zeng, J.; Yan, L.; Chen, S.; Yang, B. Characterization of lignin derived from water-only flowthrough pretreatment of Miscanthus. Ind. Crops Prod. 2013, 50, 391–399. [Google Scholar] [CrossRef]
- Jensen, A.; Cabrera, Y.; Hsieh, C.W.; Nielsen, J.; Ralph, J.; Felby, C. 2D NMR characterization of wheat straw residual lignin after dilute acid pretreatment with different severities. Holzforschung 2017, 71, 461–469. [Google Scholar] [CrossRef]
- Donohoe, B.S.; Decker, S.R.; Tucker, M.P.; Himmel, M.E.; Vinzant, T.B. Visualizing lignin coalescence and migration through maize cell walls following thermochemical pretreatment. Biotechnol. Bioeng. 2008, 101, 913–925. [Google Scholar] [CrossRef]
- Li, H.; Pu, Y.; Kumar, R.; Ragauskas, A.J.; Wyman, C.E. Investigation of lignin deposition on cellulose during hydrothermal pretreatment, its effect on cellulose hydrolysis, and underlying mechanisms. Biotechnol. Bioeng. 2014, 111, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Zhang, X.; Ling, Z.; Sun, R.C.; Xu, F. Tissue specific response of Miscanthus × giganteus to dilute acid pretreatment for enhancing cellulose digestibility. Carbohydr. Polym. 2016, 154, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wu, H. Significant differences in the hydrolysis behavior of amorphous and crystalline portions within microcrystalline cellulose in hot-compressed water. Ind. Eng. Chem. Res. 2010, 49, 3902–3909. [Google Scholar] [CrossRef]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.Y.; Holtzapple, M.; Ladisch, M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef]
- Rabemanolontsoa, H.; Saka, S. Various pretreatments of lignocellulosics. Bioresour. Technol. 2016, 199, 83–91. [Google Scholar] [CrossRef]
- Yoo, H.Y.; Yang, X.; Kim, D.S.; Lee, S.K.; Lotrakul, P.; Prasongsuk, S.; Punnapayak, H.; Kim, S.W. Evaluation of the overall process on bioethanol production from miscanthus hydrolysates obtained by dilute acid pretreatment. Biotechnol. Bioprocess Eng. 2016, 21, 733–742. [Google Scholar] [CrossRef]
- Si, S.; Chen, Y.; Fan, C.; Hu, H.; Li, Y.; Huang, J.; Liao, H.; Hao, B.; Li, Q.; Peng, L.; et al. Lignin extraction distinctively enhances biomass enzymatic saccharification in hemicelluloses-rich Miscanthus species under various alkali and acid pretreatments. Bioresour. Technol. 2015, 183, 248–254. [Google Scholar] [CrossRef]
- Moxley, G.; Gaspar, A.R.; Higgins, D.; Xu, H. Structural changes of corn stover lignin during acid pretreatment. J. Ind. Microbiol. Biotechnol. 2012, 39, 1289–1299. [Google Scholar] [CrossRef]
- Teramura, H.; Sasaki, K.; Oshima, T.; Aikawa, S.; Matsuda, F.; Okamoto, M.; Shirai, T.; Kawaguchi, H.; Ogino, C.; Yamasaki, M.; et al. Changes in lignin and polysaccharide components in 13 cultivars of rice straw following dilute acid pretreatment as studied by solution-state 2D 1H-13C NMR. PLoS ONE 2015, 10, 1–17. [Google Scholar] [CrossRef]
- Samuel, R.; Pu, Y.; Raman, B.; Ragauskas, A.J. Structural characterization and comparison of switchgrass ball-milled lignin before and after dilute acid pretreatment. Appl. Biochem. Biotechnol. 2010, 162, 62–74. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, Y.Y.; Kim, T.H. A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour. Technol. 2016, 199, 42–48. [Google Scholar] [CrossRef]
- Jung, W.; Savithri, D.; Sharma-Shivappa, R.; Kolar, P. Effect of Sodium Hydroxide Pretreatment on Lignin Monomeric Components of Miscanthus × giganteus and Enzymatic Hydrolysis. Waste Biomass Valorization 2019. [Google Scholar] [CrossRef]
- Liu, Z.; Padmanabhan, S.; Cheng, K.; Schwyter, P.; Pauly, M.; Bell, A.T.; Prausnitz, J.M. Aqueous-ammonia delignification of miscanthus followed by enzymatic hydrolysis to sugars. Bioresour. Technol. 2013, 135, 23–29. [Google Scholar] [CrossRef]
- Lapierre, C.; Jouin, D.; Monties, B. On the molecular origin of the alkali solubility of Gramineae lignins. Phytochemistry 1989, 28, 1401–1403. [Google Scholar] [CrossRef]
- Lapierre, C. Determining Lignin Structure by Chemical Degradations. Lignin Lignans 2010, 11–48. [Google Scholar] [CrossRef]
- Sibout, R.; Le Bris, P.; Legée, F.; Cézard, L.; Renault, H.; Lapierre, C. Structural redesigning Arabidopsis Lignins into alkali-soluble Lignins through the expression of p-coumaroyl-CoA:Monolignol transferase PMT. Plant Physiol. 2016, 170, 1358–1366. [Google Scholar] [CrossRef]
- Guo, H.; Zhao, Y.; Chen, X.; Shao, Q.; Qin, W. Pretreatment of Miscanthus with biomass-degrading bacteria for increasing delignification and enzymatic hydrolysability. Microb. Biotechnol. 2019, 12, 787–798. [Google Scholar] [CrossRef]
- Brosse, N.; Sannigrahi, P.; Ragauskas, A. Pretreatment of miscanthus x giganteus using the ethanol organosolv process for ethanol production. Ind. Eng. Chem. Res. 2009, 48, 8328–8334. [Google Scholar] [CrossRef]
- Gschwend, F.J.V.; Malaret, F.; Shinde, S.; Brandt-Talbot, A.; Hallett, J.P. Rapid pretreatment of: Miscanthus using the low-cost ionic liquid triethylammonium hydrogen sulfate at elevated temperatures. Green Chem. 2018, 20, 3486–3498. [Google Scholar] [CrossRef]
- Bhatia, R.; Winters, A.; Bryant, D.N.; Bosch, M.; Clifton-Brown, J.; Leak, D.; Gallagher, J. Pilot-scale production of xylo-oligosaccharides and fermentable sugars from Miscanthus using steam explosion pretreatment. Bioresour. Technol. 2020, 296, 122285. [Google Scholar] [CrossRef]
- Yeh, R.H.; Lin, Y.S.; Wang, T.H.; Kuan, W.C.; Lee, W.C. Bioethanol production from pretreated Miscanthus floridulus biomass by simultaneous saccharification and fermentation. Biomass Bioenergy 2016, 94, 110–116. [Google Scholar] [CrossRef]
- Vasco-Correa, J.; Ge, X.; Li, Y. Fungal pretreatment of non-sterile miscanthus for enhanced enzymatic hydrolysis. Bioresour. Technol. 2016, 203, 118–123. [Google Scholar] [CrossRef]
- Zhu, Z.; Simister, R.; Bird, S.; McQueen-Mason, S.J.; Gomez, L.D.; Macquarrie, D.J. Microwave assisted acid and alkali pretreatment of Miscanthus biomass for biorefineries. AIMS Bioeng. 2015, 2, 449–468. [Google Scholar] [CrossRef]
- Kumar, A.K.; Sharma, S. Recent updates on different methods of pretreatment of lignocellulosic feedstocks: A review. Bioresour. Bioprocess. 2017, 4. [Google Scholar] [CrossRef]
- Cunha, J.T.; Romaní, A.; Costa, C.E.; Sá-Correia, I.; Domingues, L. Molecular and physiological basis of Saccharomyces cerevisiae tolerance to adverse lignocellulose-based process conditions. Appl. Microbiol. Biotechnol. 2019, 103, 159–175. [Google Scholar] [CrossRef] [PubMed]
- Sannigrahi, P.; Kim, D.H.; Jung, S.; Ragauskas, A. Pseudo-lignin and pretreatment chemistry. Energy Environ. Sci. 2011, 4, 1306–1310. [Google Scholar] [CrossRef]
- Kärcher, M.A.; Iqbal, Y.; Lewandowski, I.; Senn, T. Comparing the performance of Miscanthus x giganteus and wheat straw biomass in sulfuric acid based pretreatment. Bioresour. Technol. 2015, 180, 360–364. [Google Scholar] [CrossRef]
- Alam, A.; Zhang, R.; Liu, P.; Huang, J.; Wang, Y.; Hu, Z.; Madadi, M.; Sun, D.; Hu, R.; Ragauskas, A.J.; et al. A finalized determinant for complete lignocellulose enzymatic saccharification potential to maximize bioethanol production in bioenergy Miscanthus. Biotechnol. Biofuels 2019, 12, 1–22. [Google Scholar] [CrossRef]
- Belmokhtar, N.; Arnoult, S.; Chabbert, B.; Charpentier, J.P.; Brancourt-Hulmel, M. Saccharification performances of miscanthus at the pilot and miniaturized assay scales: Genotype and year variabilities according to the biomass composition. Front. Plant Sci. 2017, 8, 1–13. [Google Scholar] [CrossRef]
- Łukajtis, R.; Rybarczyk, P.; Kucharska, K.; Konopacka-Łyskawa, D.; Słupek, E.; Wychodnik, K.; Kamiński, M. Optimization of saccharification conditions of lignocellulosic biomass under alkaline pre-treatment and enzymatic hydrolysis. Energies 2018, 11, 886. [Google Scholar]
- Cerazy-waliszewska, J.; Jeżowski, S.; Łysakowski, P.; Waliszewska, B.; Zborowska, M.; Sobańska, K.; Ślusarkiewicz-jarzina, A.; Białas, W. Industrial Crops & Products Potential of bioethanol production from biomass of various Miscanthus genotypes cultivated in three-year plantations in west-central Poland. Ind. Crop. Prod. 2019, 141, 111790. [Google Scholar] [CrossRef]
- Zeng, X.; Sheng, J.; Zhu, F.; Wei, T.; Zhao, L.; Hu, X.; Zheng, X.; Zhou, F.; Hu, Z.; Diao, Y.; et al. Genetic, transcriptional, and regulatory landscape of monolignol biosynthesis pathway in Miscanthus × giganteus. Biotechnol. Biofuels 2020, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Zhang, G.; Muhammad, A.; Samad, R.A.; Wang, Y.; Walton, J.D.; He, Y.; Peng, L.; Wang, L. Genetic loci simultaneously controlling lignin monomers and biomass digestibility of rice straw. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Li, X.; Weng, J.K.; Chapple, C. Improvement of biomass through lignin modification. Plant J. 2008, 54, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.H.; Seong, E.S.; Ghimire, B.K.; Heo, K.; Jin, X.; Yamada, T.; Clark, L.V.; Sacks, E.J.; Yu, C.Y. Establishment of Miscanthus sinensis with decreased lignin biosynthesis by Agrobacterium–mediated transformation using antisense COMT gene. Plant Cell. Tissue Organ Cult. 2018, 133, 359–369. [Google Scholar] [CrossRef]
- Wang, C.; He, G.; Meng, J.; Wang, S.; Kong, Y.; Jiang, J.; Hu, R.; Zhou, G. Improved lignocellulose saccharification of a Miscanthus reddish stem mutant induced by heavy-ion irradiation. GCB Bioenergy 2020, 12, 1066–1077. [Google Scholar] [CrossRef]
- Mansfield, S.D.; Kim, H.; Lu, F.; Ralph, J. Whole plant cell wall characterization using solution-state 2D NMR. Nat. Protoc. 2012, 7, 1579–1589. [Google Scholar] [CrossRef]
- Van Erven, G.; De Visser, R.; De Waard, P.; Van Berkel, W.J.H.; Kabel, M.A. Uniformly 13C Labeled Lignin Internal Standards for Quantitative Pyrolysis-GC-MS Analysis of Grass and Wood. ACS Sustain. Chem. Eng. 2019, 7, 20070–20076. [Google Scholar] [CrossRef]
- Demartini, J.D.; Pattathil, S.; Miller, J.S.; Li, H.; Hahn, M.G.; Wyman, C.E. Investigating plant cell wall components that affect biomass recalcitrance in poplar and switchgrass. Energy Environ. Sci. 2013, 6, 898–909. [Google Scholar] [CrossRef]
- Li, F.; Zhang, M.; Guo, K.; Hu, Z.; Zhang, R.; Feng, Y.; Yi, X.; Zou, W.; Wang, L.; Wu, C.; et al. High-level hemicellulosic arabinose predominately affects lignocellulose crystallinity for genetically enhancing both plant lodging resistance and biomass enzymatic digestibility in rice mutants. Plant Biotechnol. J. 2015, 13, 514–525. [Google Scholar] [CrossRef]
- Chiniquy, D.; Sharma, V.; Schultink, A.; Baidoo, E.E.; Rautengarten, C.; Cheng, K. XAX1 from glycosyltransferase family 61 mediates xylosyltransfer to rice xylan. Proc. Natl. Acad. Sci. USA 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Marriott, P.E.; Sibout, R.; Lapierre, C.; Fangel, J.U.; Willats, W.G.T.; Hofte, H.; Gómez, L.D.; McQueen-Mason, S.J. Range of cell-wall alterations enhance saccharification in Brachypodium distachyon mutants. Proc. Natl. Acad. Sci. USA 2014, 111, 14601–14606. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Heckwolf, M.; Crowe, J.D.; Williams, D.L.; Magee, T.D.; Kaeppler, S.M.; De Leon, N.; Hodge, D.B. Cell-wall properties contributing to improved deconstruction by alkaline pre-treatment and enzymatic hydrolysis in diverse maize (Zea mays L.) lines. J. Exp. Bot. 2015, 66, 4305–4315. [Google Scholar] [CrossRef] [PubMed]
- Biswal, A.K.; Hao, Z.; Pattathil, S.; Yang, X.; Winkeler, K.; Collins, C.; Mohanty, S.S.; Richardson, E.A.; Gelineo-Albersheim, I.; Hunt, K.; et al. Downregulation of GAUT12 in Populus deltoides by RNA silencing results in reduced recalcitrance, increased growth and reduced xylan and pectin in a woody biofuel feedstock. Biotechnol. Biofuels 2015, 8. [Google Scholar] [CrossRef]
| Pretreatment | Crop | Pretreatment Conditions | Extraction Hemicellulose + Lignin | Enzymatic Sacch. | Reference |
|---|---|---|---|---|---|
| Organosolv + ball milling | MxG | Liquid to solid ratio 10, Ethanol concentration 40%, 170 °C, 120 min | 62% lignin removal, 90.6% removal of xylan, mannan, galactan | 96.9% cellulose to glucose conversion (6.5% for untreated biomass) | [199] |
| Bacterial | M. sac | Laccase production on M. sac (0.5% w/v), followed by mixture of enzymes, biomass (4% w/v), buffer and laccase mediator, 37 °C, 96 h | 29.7–59.5% lignin removal, 0.24–0.61% hemicellulose removal | ~65.0–87.0% cellulose and ~40.0–78.7% pentose conversion | [232] |
| Organosolv | MxG | Pre-soaked in 500.0 mL water and 40.0 mL of 2 M sulfuric acid, treated with aqueous ethanol (water/ethanol ratio 0.8) + sulfuric acid (0.5%), 170 °C, 60 min | 70% lignin removal, 90% xylan and 95% arabinan removal | 98% cellulose to glucose conversion | [233] |
| Ionic liquid | MxG | Biomass to solvent ratio 1:5 g/g, Triethylammonium hydrogen sulfate, 180 °C, 15 min | ~82% lignin removal, ~90% hemicellulose removal, 1.6% cellulose degradation | ~75% cellulose to glucose conversion | [234] |
| Steam explosion | M. sac x M. sin | Pre-soaking + 200 °C, 15 bar, 10 min | ~4% lignin removal, 57% hemicellulose removal | ~70% cellulose to glucose conversion | [235] |
| Steam explosion | M. flo | 175 °C, 20–60 min | 42.7% lignin extraction, 70.5% hemicellulose extraction | SSF *, ethanol yield 46.4% | [236] |
| Fungal | MxG | Biomass and inoculum (MxG colonized with Ceriporiopsis subversmipora, ratio 30–50%), moisture content 60–75%, 28 °C, 28 days | 25–35% lignin degradation, 16–24% hemicellulose degradation | 35–48% glucose conversion | [237] |
| Microwave assisted chemical | MxG | 0.4 M–1.0 M NaOH, 180 °C, 20 min | 83.0–94.2% lignin removal, 46.4% hemicellulose removal | 150 nmol/mg biomass/h (10 nmol/mg biomass/h for untreated biomass) | [238] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
van der Cruijsen, K.; Al Hassan, M.; van Erven, G.; Dolstra, O.; Trindade, L.M. Breeding Targets to Improve Biomass Quality in Miscanthus. Molecules 2021, 26, 254. https://doi.org/10.3390/molecules26020254
van der Cruijsen K, Al Hassan M, van Erven G, Dolstra O, Trindade LM. Breeding Targets to Improve Biomass Quality in Miscanthus. Molecules. 2021; 26(2):254. https://doi.org/10.3390/molecules26020254
Chicago/Turabian Stylevan der Cruijsen, Kasper, Mohamad Al Hassan, Gijs van Erven, Oene Dolstra, and Luisa M. Trindade. 2021. "Breeding Targets to Improve Biomass Quality in Miscanthus" Molecules 26, no. 2: 254. https://doi.org/10.3390/molecules26020254
APA Stylevan der Cruijsen, K., Al Hassan, M., van Erven, G., Dolstra, O., & Trindade, L. M. (2021). Breeding Targets to Improve Biomass Quality in Miscanthus. Molecules, 26(2), 254. https://doi.org/10.3390/molecules26020254






