The Usefulness of Trivalent Phosphorus for the Synthesis of Dendrimers
Abstract
1. Introduction
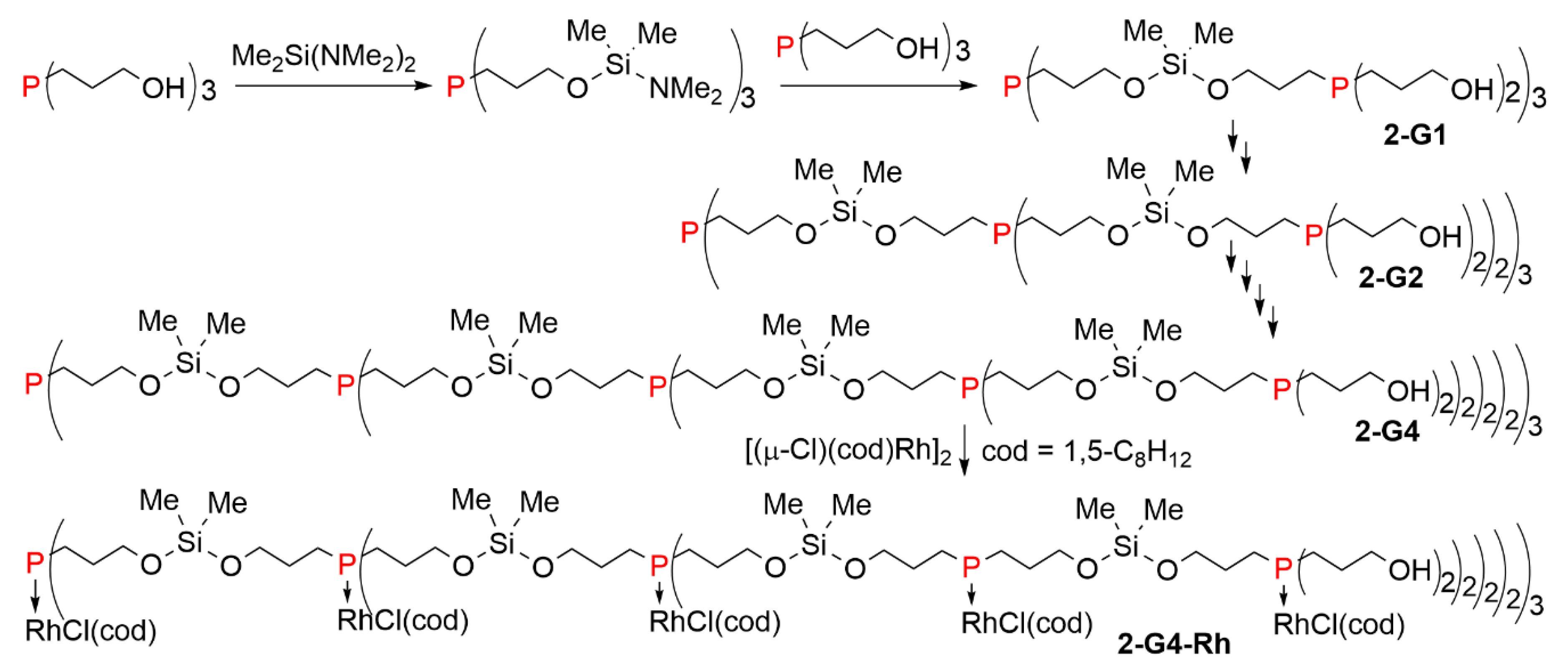
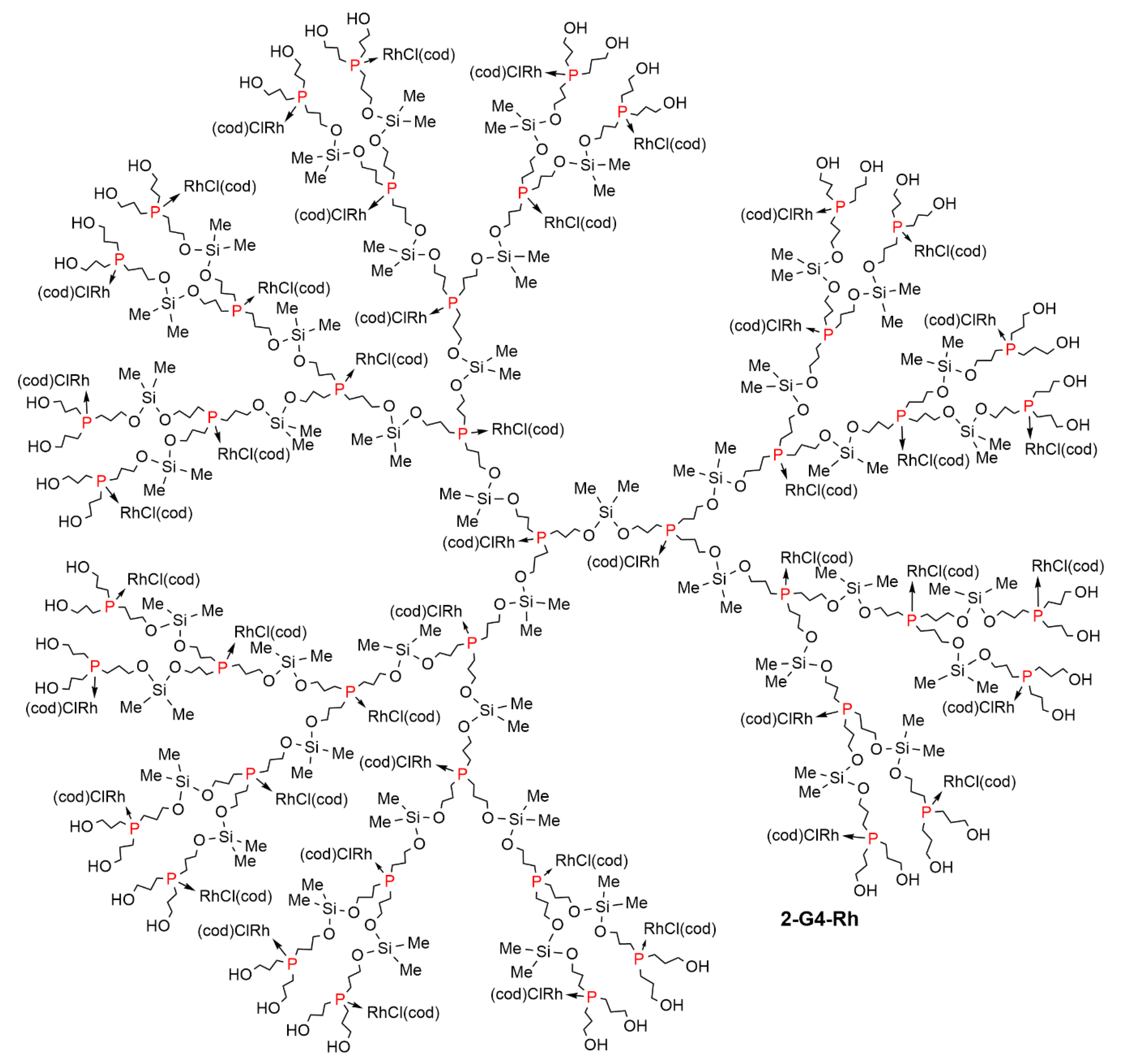
2. Dendrimers Constituted of a Trivalent Phosphorus Derivative at Each Branching Point
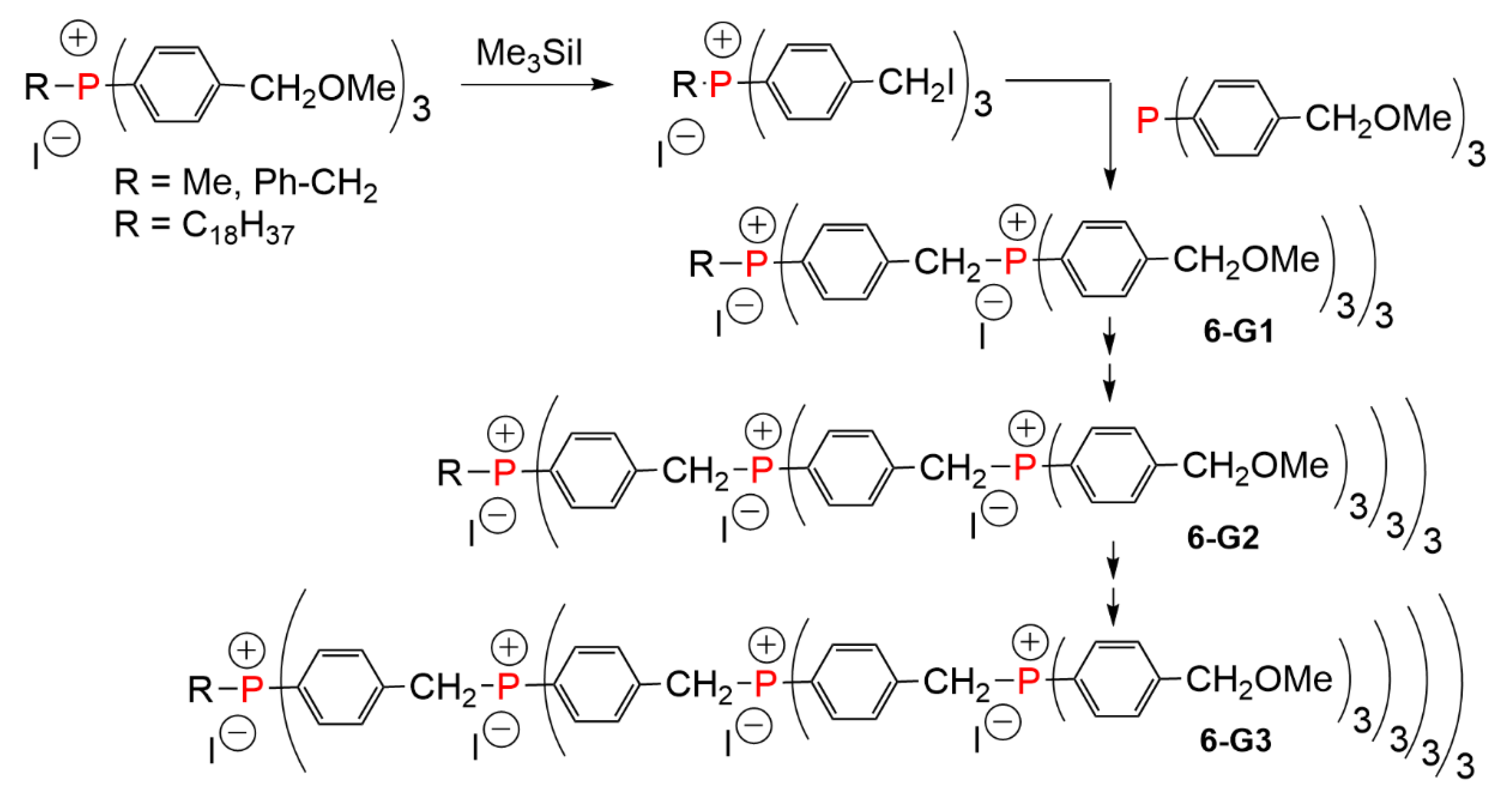
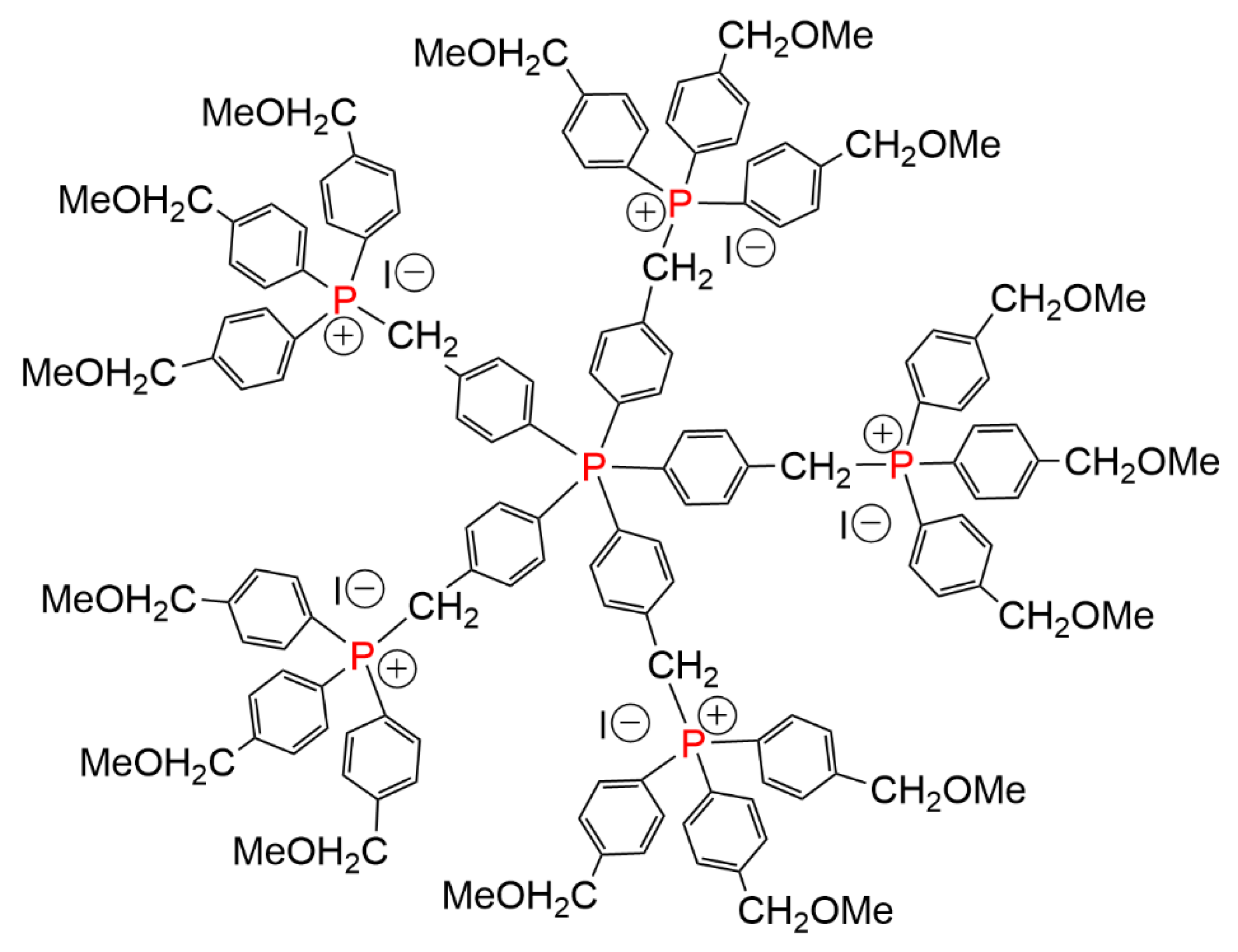
3. Dendrimers Synthesized by Alkylation of Phosphines
4. Phosphoramidite Reagents for the Synthesis of Dendrimers
5. The Staudinger Reaction
5.1. The Staudinger Reaction for the Synthesis of Regular Dendrimers
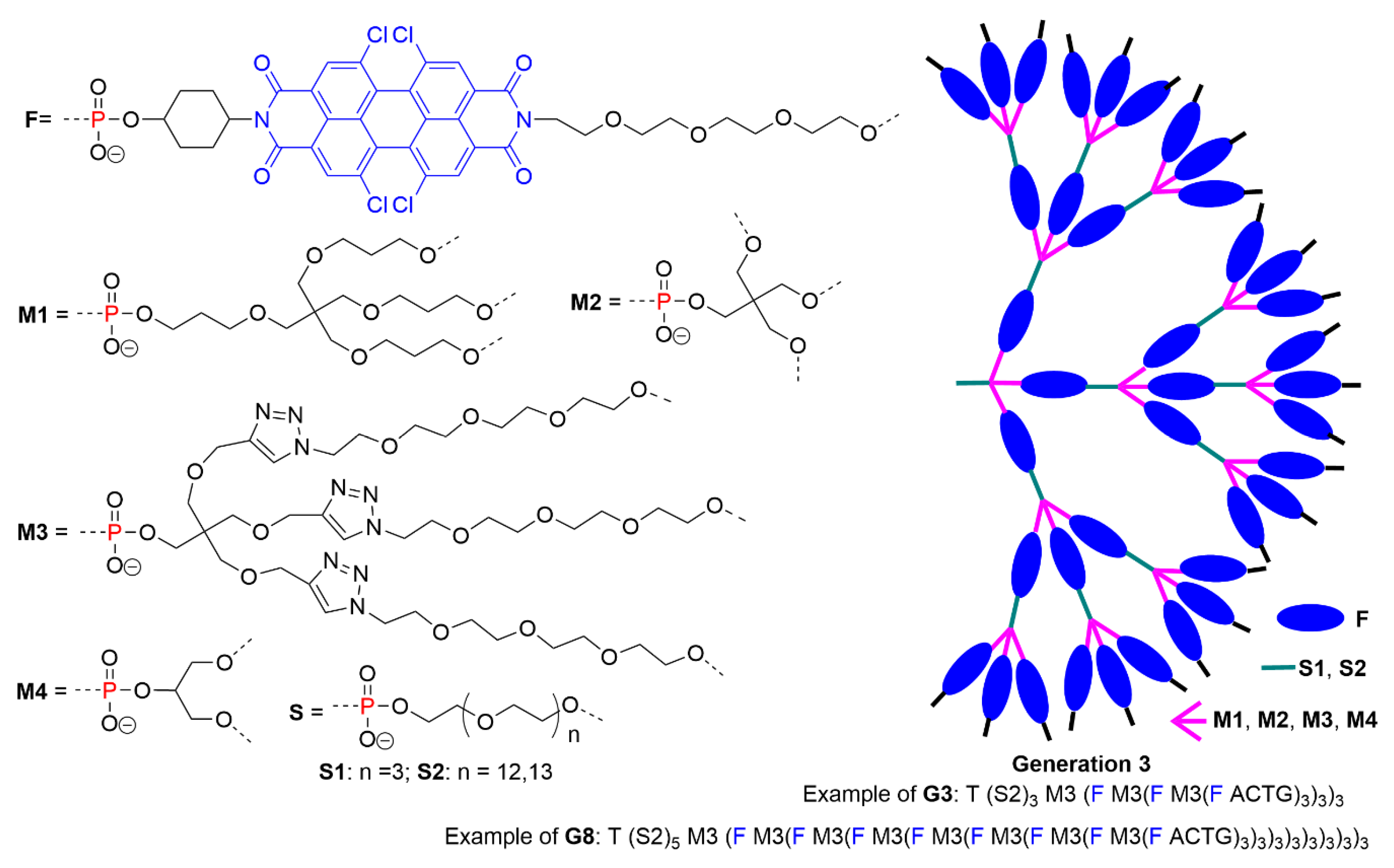
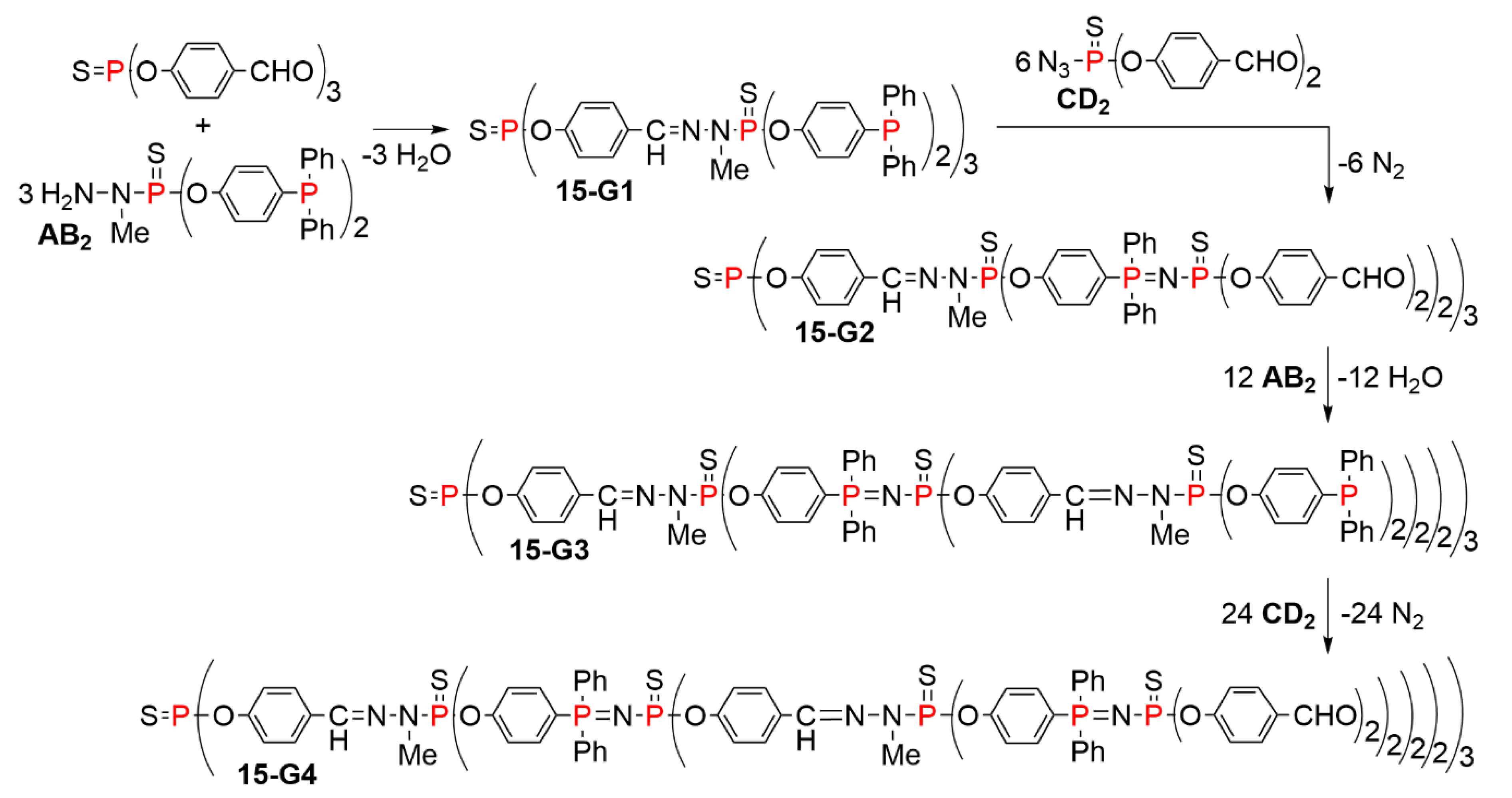
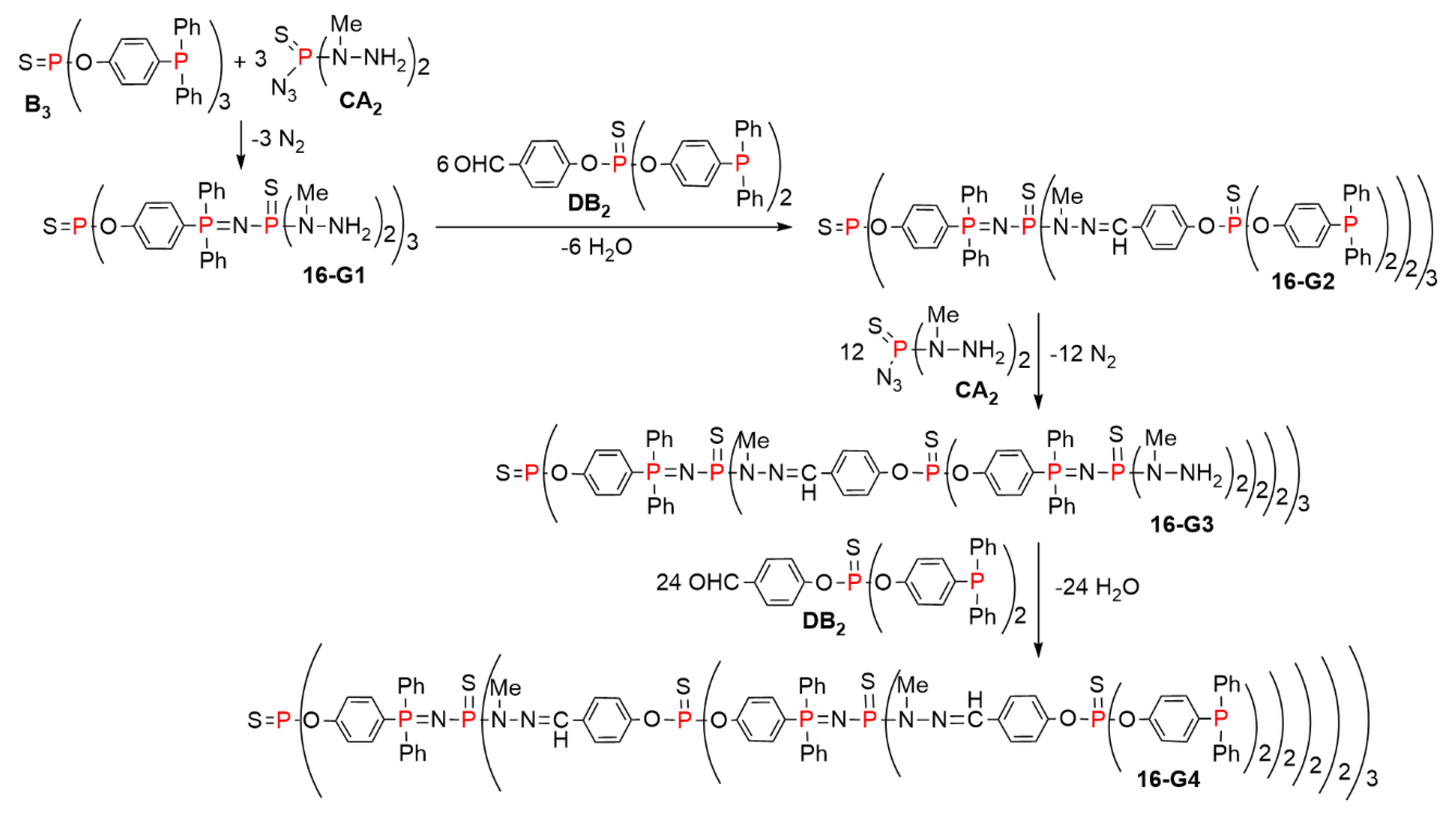
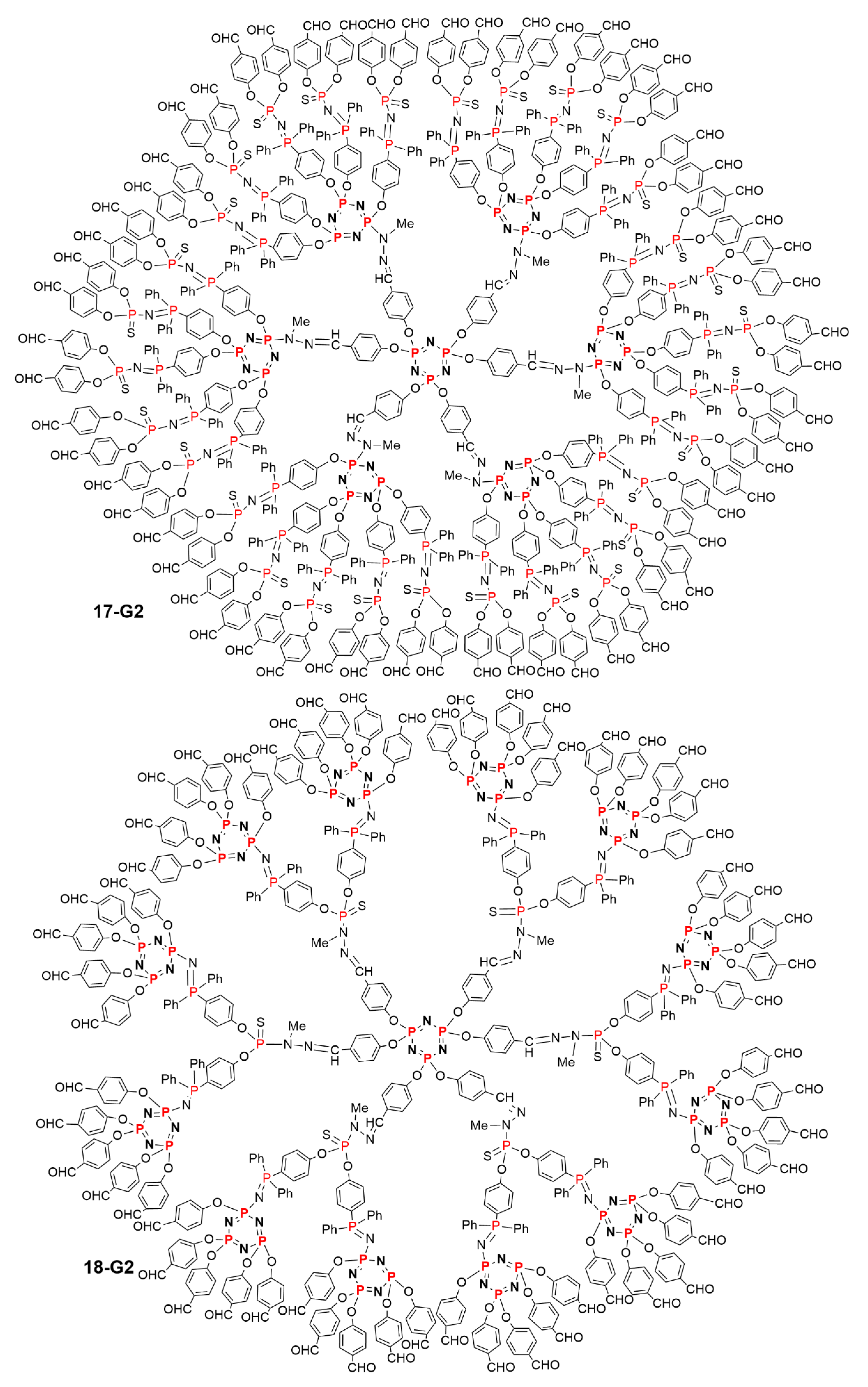
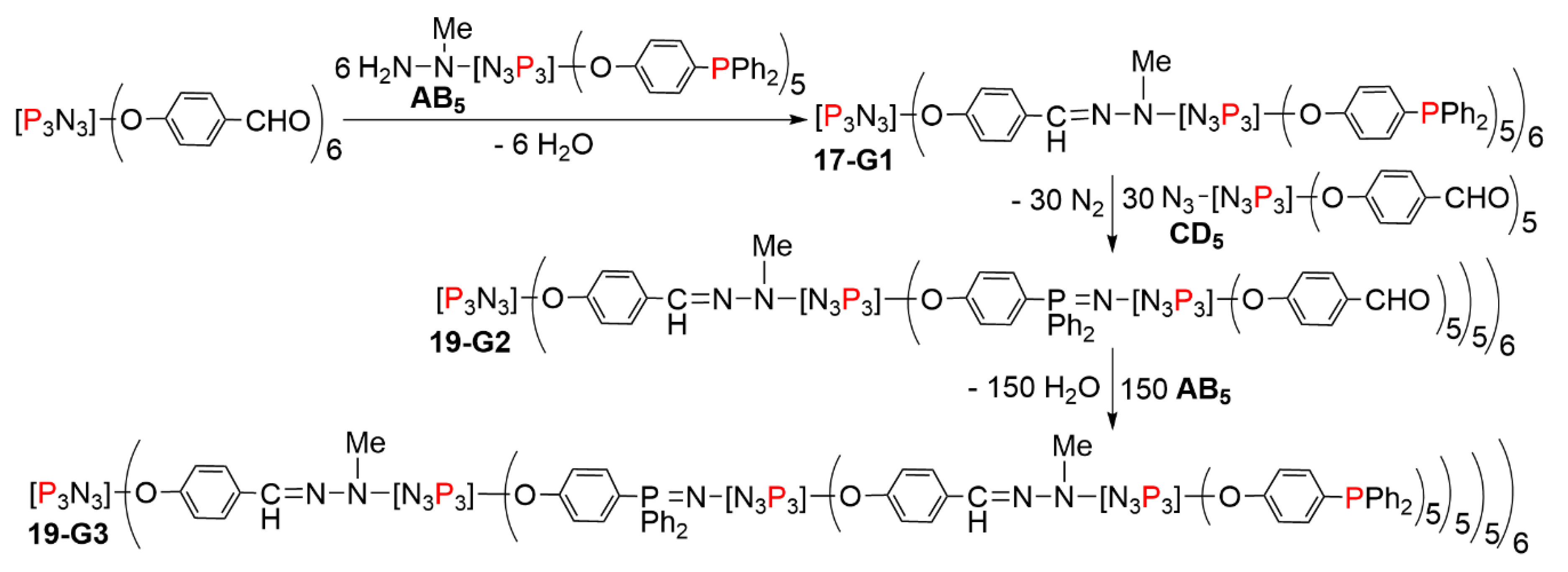
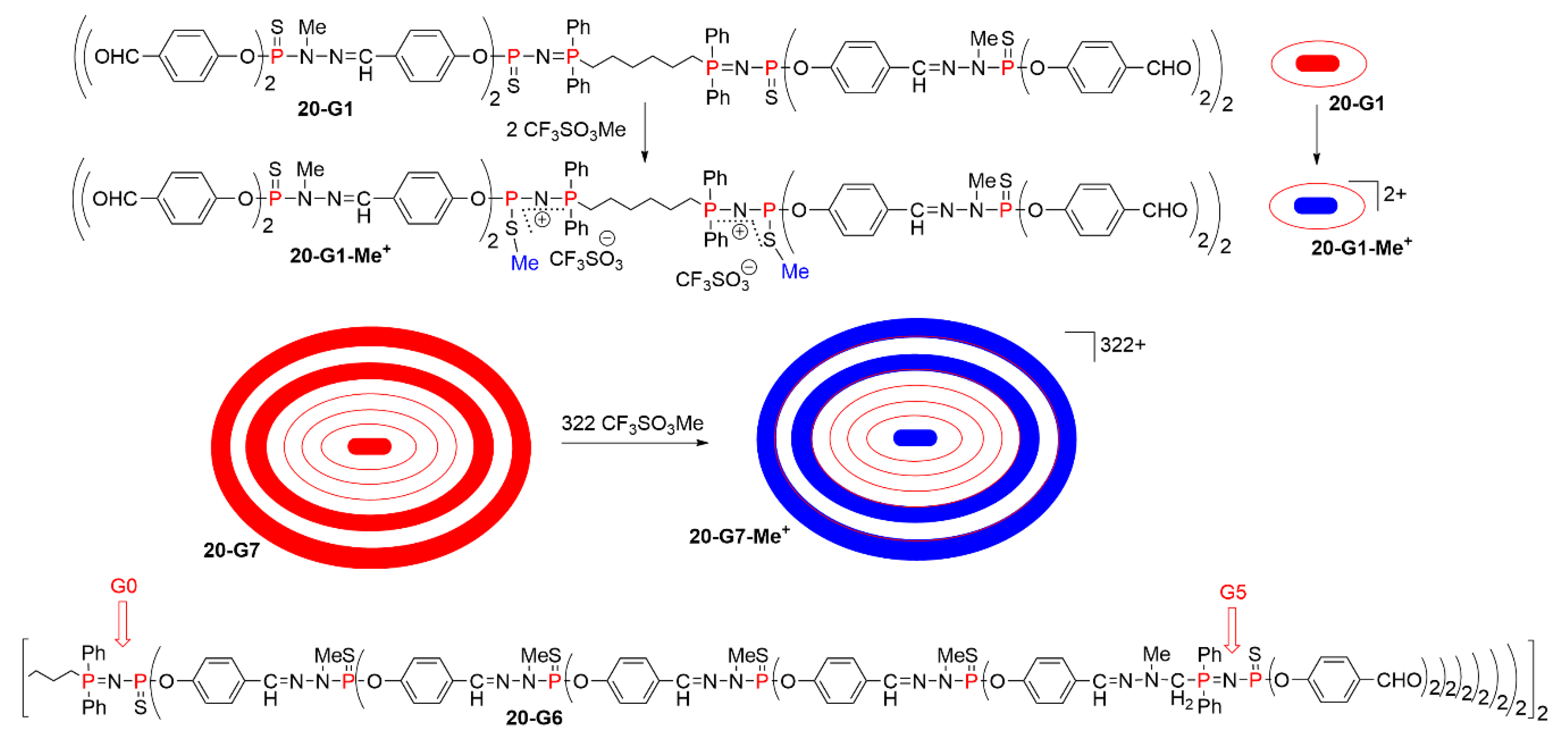
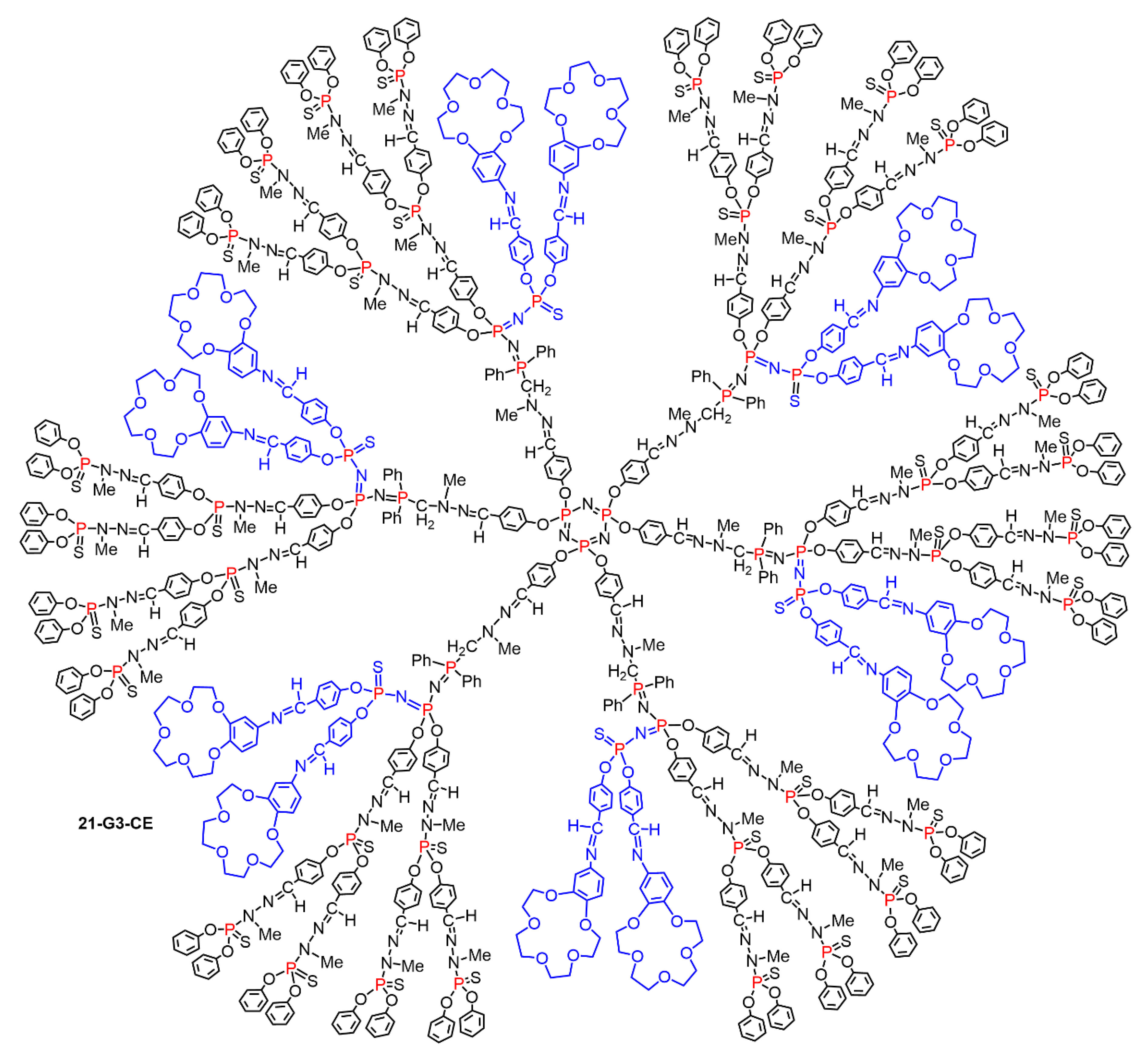
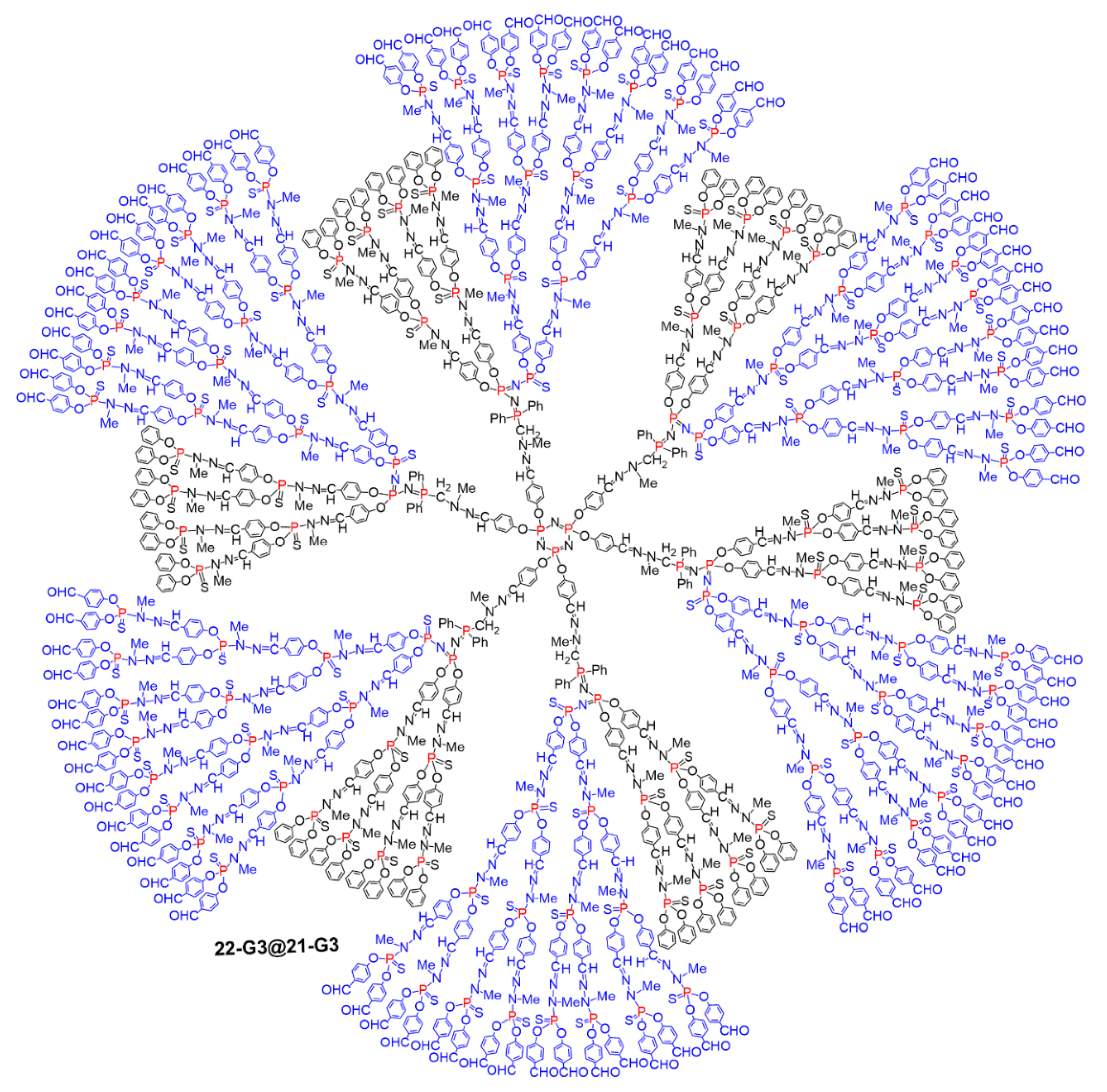
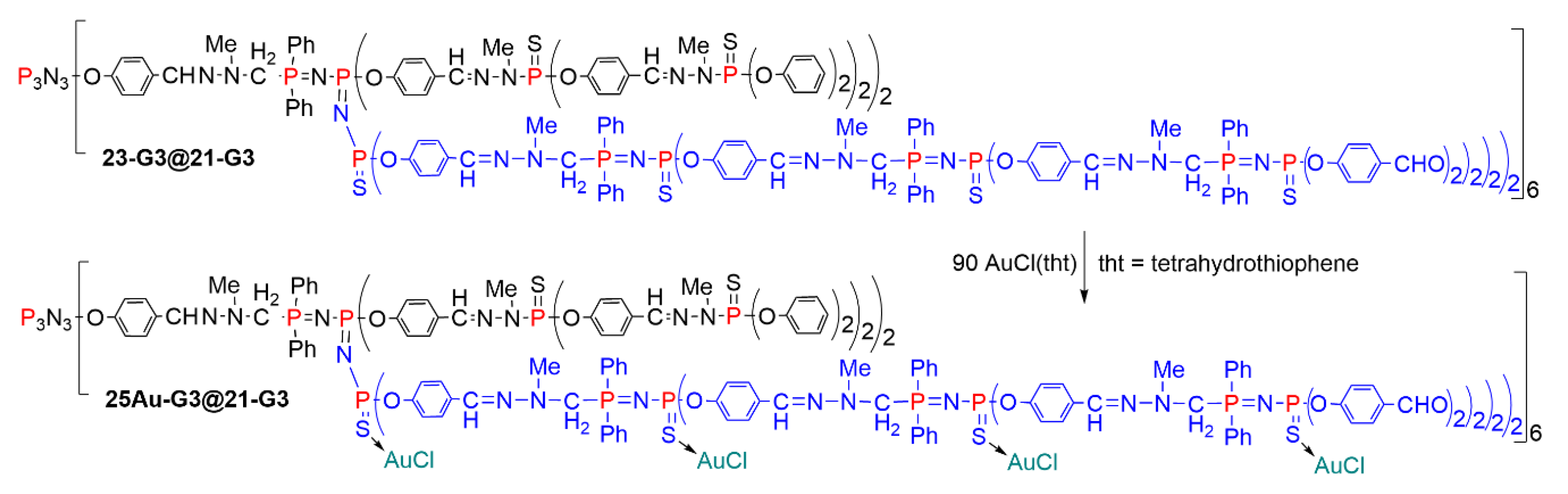
5.2. The Usefulness of P=N-P=S Linkages for the Synthesis of Sophisticated Dendritic Architectures
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Majoral, J.P.; Caminade, A.M. Dendrimers containing heteroatoms (Si, P, B, Ge, or Bi). Chem. Rev. 1999, 99, 845–880. [Google Scholar] [CrossRef]
- Caminade, A.M. Inorganic dendrimers: Recent advances for catalysis, nanomaterials, and nanomedicine. Chem. Soc. Rev. 2016, 45, 5174–5186. [Google Scholar] [CrossRef]
- Rengan, K.; Engel, R. Phosphonium cascade molecules. J. Chem. Soc. Chem. Commun. 1990, 1084–1085. [Google Scholar] [CrossRef]
- Caminade, A.M.; Laurent, R.; Turrin, C.O.; Rebout, C.; Delavaux-Nicot, B.; Ouali, A.; Zablocka, M.; Majoral, J.P. Phosphorus dendrimers as viewed by P-31 NMR spectroscopy; synthesis and characterization. Comptes Rendus Chim. 2010, 13, 1006–1027. [Google Scholar] [CrossRef]
- Majoral, J.-P.; Caminade, A.-M. Phosphorhydrazones as Useful Building Blocks for Special Architectures: Macrocycles and Dendrimers. Eur. J. Inorg. Chem. 2019, 1457–1475. [Google Scholar] [CrossRef]
- Caminade, A.-M.; Majoral, J.-P. Which Dendrimer to Attain the Desired Properties? Focus on Phosphorhydrazone Dendrimers. Molecules (Basel Switz.) 2018, 23, 622. [Google Scholar] [CrossRef] [PubMed]
- Staudinger, H.; Meyer, J. Über neue organische Phosphorverbindungen III. Phosphinmethylenderivate und Phosphinimine. Helv. Chim. Acta 1919, 2, 635–646. [Google Scholar] [CrossRef]
- Miedaner, A.; Curtis, C.J.; Barkley, R.M.; DuBois, D.L. Electrochemical Reduction of CO2 Catalyzed by Small Organophosphine Dendrimers Containing Palladium. Inorg. Chem. 1994, 33, 5482–5490. [Google Scholar] [CrossRef]
- Petrucci-Samija, M.; Guillemette, V.; Dasgupta, M.; Kakkar, A.K. A new divergent route to the synthesis of organophosphine and metallodendrimers via simple acid-base hydrolytic chemistry. J. Am. Chem. Soc. 1999, 121, 1968–1969. [Google Scholar] [CrossRef]
- Huc, V.; Balueva, A.; Sebastian, R.M.; Caminade, A.M.; Majoral, J.P. Synthesis of functionalized mono-, di-, tri-, and tetraphosphines: Attempted application to prepare hyperbranched polymers and dendrimers built with phosphines at each branching point. Synthesis 2000, 2000, 726–730. [Google Scholar] [CrossRef]
- Poniatowska, E.; Salamonczyk, G.M. Phosphite dendrimers and their organometallic derivatives. Tetrahedron Lett. 2003, 44, 4315–4317. [Google Scholar] [CrossRef]
- Rengan, K.; Engel, R. The synthesis of phosphonium cascade molecules. J. Chem. Soc. Perkin Trans. 1 1991, 1, 987–990. [Google Scholar] [CrossRef]
- Engel, R.; Rengan, K.; Chan, C.S. New cascade molecules centered about phosphorus. Heteroat. Chem. 1993, 4, 181–184. [Google Scholar] [CrossRef]
- Caruthers, M.H. Gene synthesis machines: DNA chemistry and its uses. Science 1985, 230, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Hudson, R.H.E.; Damha, M.J. Nucleic-Acid Dendrimers—Novel Biopolymer Structures. J. Am. Chem. Soc. 1993, 115, 2119–2124. [Google Scholar] [CrossRef]
- Hudson, R.H.E.; Robidoux, S.; Damha, M.J. Divergent solid-phase synthesis of nucleic acid dendrimers. Tetrahedron Lett. 1998, 39, 1299–1302. [Google Scholar] [CrossRef]
- Shchepinov, M.S.; Udalova, I.A.; Bridgman, A.J.; Southern, E.M. Oligonucleotide dendrimers: Synthesis and use as polylabelled DNA probes. Nucleic Acids Res. 1997, 25, 4447–4454. [Google Scholar] [CrossRef]
- Shchepinov, M.S.; Mir, K.U.; Elder, J.K.; Frank-Kamenetskii, M.D.; Southern, E.M. Oligonucleotide dendrimers: Stable nano-structures. Nucleic Acids Res. 1999, 27, 3035–3041. [Google Scholar] [CrossRef]
- Hussain, M.; Shchepinov, M.S.; Sohail, M.; Benter, I.F.; Hollins, A.J.; Southern, E.M.; Akhtar, S. A novel anionic dendrimer for improved cellular delivery of antisense oligonucleotides. J. Control. Release 2004, 99, 139–155. [Google Scholar] [CrossRef]
- Salamonczyk, G.M.; Kuznikowski, M.; Skowronska, A. A divergent synthesis of thiophosphate-based dendrimers. Tetrahedron Lett. 2000, 41, 1643–1645. [Google Scholar] [CrossRef]
- Salamonczyk, G.M.; Kuznikowski, M.; Poniatowska, E. Synthesis and oxygenation of selenophosphate dendrimers. Chem. Commun. 2001, 2202–2203. [Google Scholar] [CrossRef]
- Salamonczyk, G.M.; Kuznikowski, M.; Poniatowska, E. Dendrimers bearing three types of branching functions. Tetrahedron Lett. 2002, 43, 1747–1749. [Google Scholar] [CrossRef]
- Salamonczyk, G.M. Acyclovir terminated thiophosphate dendrimers. Tetrahedron Lett. 2003, 44, 7449–7453. [Google Scholar] [CrossRef]
- Salamonczyk, G.M. New water-soluble polyanionic dendrimers-phosphoric and 1,3,5-benzenetricarboxylic acid derivatives. Tetrahedron 2012, 68, 10209–10217. [Google Scholar] [CrossRef]
- Salamonczyk, G.M. Efficient synthesis of water-soluble, phosphonate-terminated polyester dendrimers. Tetrahedron Lett. 2015, 56, 7161–7164. [Google Scholar] [CrossRef]
- Domanski, D.M.; Bryszewska, M.; Salamonczyk, G. Preliminary evaluation of the behavior of fifth-generation thiophosphate dendrimer in biological systems. Biomacromolecules 2004, 5, 2007–2012. [Google Scholar] [CrossRef]
- Shaller, A.D.; Wan, W.; Zhao, B.M.; Li, A.D.Q. Chromophoric and Dendritic Phosphoramidites Enable Construction of Functional Dendrimers with Exceptional Brightness and Water Solubility. Chem. Eur. J. 2014, 20, 12165–12171. [Google Scholar] [CrossRef]
- Furer, V.L.; Vandyukov, A.E.; Majoral, J.P.; Caminade, A.M.; Gottis, S.; Laurent, R.; Kovalenko, V.I. Vibrational spectroscopic investigation of the gold complexation within the cascade structure of phosphorus-containing dendrimer. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 203, 118–126. [Google Scholar] [CrossRef]
- Furer, V.L.; Vandyukov, A.E.; Majoral, J.P.; Caminade, A.M.; Gottis, S.; Laurent, R.; Kovalenko, V.I. DFT study of structure, IR and Raman spectra of phosphorus-containing dendron with azide functional group. Vib. Spectrosc. 2014, 75, 1–10. [Google Scholar] [CrossRef]
- Larre, C.; Donnadieu, B.; Caminade, A.M.; Majoral, J.P. N-thiophosphorylated and N-phosphorylated iminophosphoranes R3P=N-P(X)R ‘(2); X = O, S as models for dendrimers: Synthesis, reactivity and crystal structures. Eur. J. Inorg. Chem. 1999, 601–611. [Google Scholar] [CrossRef]
- Galliot, C.; Prevote, D.; Caminade, A.M.; Majoral, J.P. Polyaminophosphines containing dendrimers—Syntheses and characterizations. J. Am. Chem. Soc. 1995, 117, 5470–5476. [Google Scholar] [CrossRef]
- Launay, N.; Galliot, C.; Caminade, A.M.; Majoral, J.P. Synthesis of small phosphorus dendrimers from (S)P(N(Me)-NH2)3. Bull. Soc. Chim. Fr. 1995, 132, 1149–1155. [Google Scholar]
- Merino, S.; Brauge, L.; Caminade, A.M.; Majoral, J.P.; Taton, D.; Gnanou, Y. Synthesis and characterization of linear, hyperbranched, and dendrimer-like polymers constituted of the same repeating unit. Chem.-Eur. J. 2001, 7, 3095–3105. [Google Scholar] [CrossRef]
- Balueva, A.; Merino, S.; Caminade, A.M.; Majoral, J.P. Synthesis of dendrimers with phosphine end groups at each generation. J. Organomet. Chem. 2002, 643, 112–124. [Google Scholar] [CrossRef]
- Maraval, V.; Laurent, R.; Marchand, P.; Caminade, A.M.; Majoral, J.P. Accelerated methods of synthesis of phosphorus-containing dendrimers. J. Organomet. Chem. 2005, 690, 2458–2471. [Google Scholar] [CrossRef]
- Brauge, L.; Magro, G.; Caminade, A.M.; Majoral, J.P. First divergent strategy using two AB(2) unprotected monomers for the rapid synthesis of dendrimers. J. Am. Chem. Soc. 2001, 123, 6698–6699. [Google Scholar] [CrossRef]
- Maraval, V.; Pyzowski, J.; Caminade, A.M.; Majoral, J.P. “Lego” chemistry for the straightforward synthesis of dendrimers. J. Org. Chem. 2003, 68, 6043–6046. [Google Scholar] [CrossRef]
- Caminade, A.-M.; Hameau, A.; Majoral, J.-P. The specific functionalization of cyclotriphosphazene for the synthesis of smart dendrimers. Dalton Trans. 2016, 45, 1810–1822. [Google Scholar] [CrossRef]
- Maraval, V.; Caminade, A.M.; Majoral, J.P.; Blais, J.C. Dendrimer design: How to circumvent the dilemma of a reduction of steps or an increase of function multiplicity? Angew. Chem. Int. Ed. 2003, 42, 1822–1826. [Google Scholar] [CrossRef]
- Furer, V.L.; Vandyukov, A.E.; Majoral, J.P.; Caminade, A.M.; Gottis, S.; Laurent, R.; Kovalenko, V.I. Comparative DFT study of structure, reactivity and IR spectra of phosphorus-containing dendrons with P=N-P=S linkages, vinyl and azide functional groups. J. Mol. Struct. 2015, 1091, 6–15. [Google Scholar] [CrossRef]
- Larre, C.; Caminade, A.M.; Majoral, J.P. Chemoselective polyalkylations of phosphorus-containing dendrimers. Angew. Chem. Int. Edit. Engl. 1997, 36, 596–599. [Google Scholar] [CrossRef]
- Larre, C.; Donnadieu, B.; Caminade, A.M.; Majoral, J.P. Phosphorus-containing dendrimers: Chemoselective functionalization of internal layers. J. Am. Chem. Soc. 1998, 120, 4029–4030. [Google Scholar] [CrossRef]
- Larre, C.; Bressolles, D.; Turrin, C.; Donnadieu, B.; Caminade, A.M.; Majoral, J.P. Chemistry within megamolecules: Regiospecific functionalization after construction of phosphorus dendrimers. J. Am. Chem. Soc. 1998, 120, 13070–13082. [Google Scholar] [CrossRef]
- Launay, N.; Caminade, A.M.; Lahana, R.; Majoral, J.P. A general synthetic strategy for neutral phosphorus-containing dendrimers. Angew. Chem. Int. Edit. Engl. 1994, 33, 1589–1592. [Google Scholar] [CrossRef]
- Launay, N.; Caminade, A.M.; Majoral, J.P. Synthesis and reactivity of unusual phosphorus dendrimers—A useful divergent growth approach up to the 7th generation. J. Am. Chem. Soc. 1995, 117, 3282–3283. [Google Scholar] [CrossRef]
- Slany, M.; Bardaji, M.; Casanove, M.J.; Caminade, A.M.; Majoral, J.P.; Chaudret, B. Dendrimer surface-chemistry—Facile route to polyphosphines and their gold complexes. J. Am. Chem. Soc. 1995, 117, 9764–9765. [Google Scholar] [CrossRef]
- Lartigue, M.L.; Donnadieu, B.; Galliot, C.; Caminade, A.M.; Majoral, J.P.; Fayet, J.P. Large dipole moments of phosphorus-containing dendrimers. Macromolecules 1997, 30, 7335–7337. [Google Scholar] [CrossRef]
- Galliot, C.; Larre, C.; Caminade, A.M.; Majoral, J.P. Regioselective stepwise growth of dendrimer units in the internal voids of a main dendrimer. Science 1997, 277, 1981–1984. [Google Scholar] [CrossRef]
- Maraval, V.; Laurent, R.; Merino, S.; Caminade, A.M.; Majoral, J.P. Michael-type addition of amines to the vinyl core of dendrons—Application to the synthesis of multidendritic systems. Eur. J. Org. Chem. 2000, 3555–3568. [Google Scholar] [CrossRef]
- Furer, V.L.; Vandyukov, A.E.; Majoral, J.P.; Caminade, A.M.; Gottis, S.; Laurent, R.; Kovalenko, V.I. DFT study of structure, IR and Raman spectra of dendrimer with P=N-P=S linkages and its complexation with gold. J. Mol. Struct. 2015, 1084, 103–113. [Google Scholar] [CrossRef]
- Larre, C.; Donnadieu, B.; Caminade, A.M.; Majoral, J.P. Regioselective gold complexation within the cascade structure of phosphorus-containing dendrimers. Chem. Eur. J. 1998, 4, 2031–2036. [Google Scholar] [CrossRef]
- Gottis, S.; Laurent, R.; Colliere, V.; Caminade, A.-M. Straightforward synthesis of gold nanoparticles by adding water to an engineered small dendrimer. Beilstein J. Nanotechnol. 2020, 11, 1110–1118. [Google Scholar] [CrossRef] [PubMed]



























Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caminade, A.-M.; Moineau-Chane Ching, K.I.; Delavaux-Nicot, B. The Usefulness of Trivalent Phosphorus for the Synthesis of Dendrimers. Molecules 2021, 26, 269. https://doi.org/10.3390/molecules26020269
Caminade A-M, Moineau-Chane Ching KI, Delavaux-Nicot B. The Usefulness of Trivalent Phosphorus for the Synthesis of Dendrimers. Molecules. 2021; 26(2):269. https://doi.org/10.3390/molecules26020269
Chicago/Turabian StyleCaminade, Anne-Marie, Kathleen I. Moineau-Chane Ching, and Béatrice Delavaux-Nicot. 2021. "The Usefulness of Trivalent Phosphorus for the Synthesis of Dendrimers" Molecules 26, no. 2: 269. https://doi.org/10.3390/molecules26020269
APA StyleCaminade, A.-M., Moineau-Chane Ching, K. I., & Delavaux-Nicot, B. (2021). The Usefulness of Trivalent Phosphorus for the Synthesis of Dendrimers. Molecules, 26(2), 269. https://doi.org/10.3390/molecules26020269





