Forty-One Plant Extracts Screened for Dual Antidiabetic and Antioxidant Functions: Evaluating the Types of Correlation between α-Amylase Inhibition and Free Radical Scavenging
Abstract
:1. Introduction
2. Results
2.1. Screening Plant Extracts for Their Antidiabetic and Free Radical Scavenging Activities
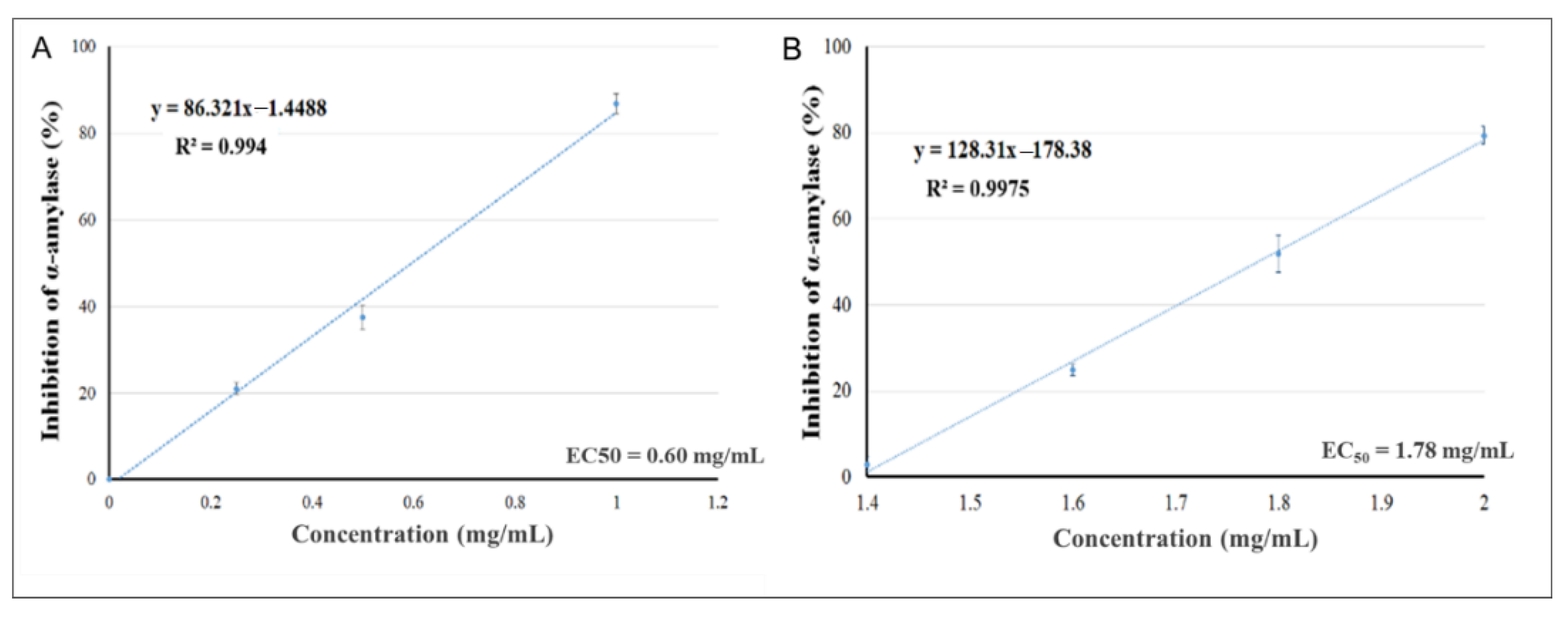
2.2. In Vitro α-Amylase Inhibition
2.3. Effects of the Plant Extracts on Free Radical Scavenging
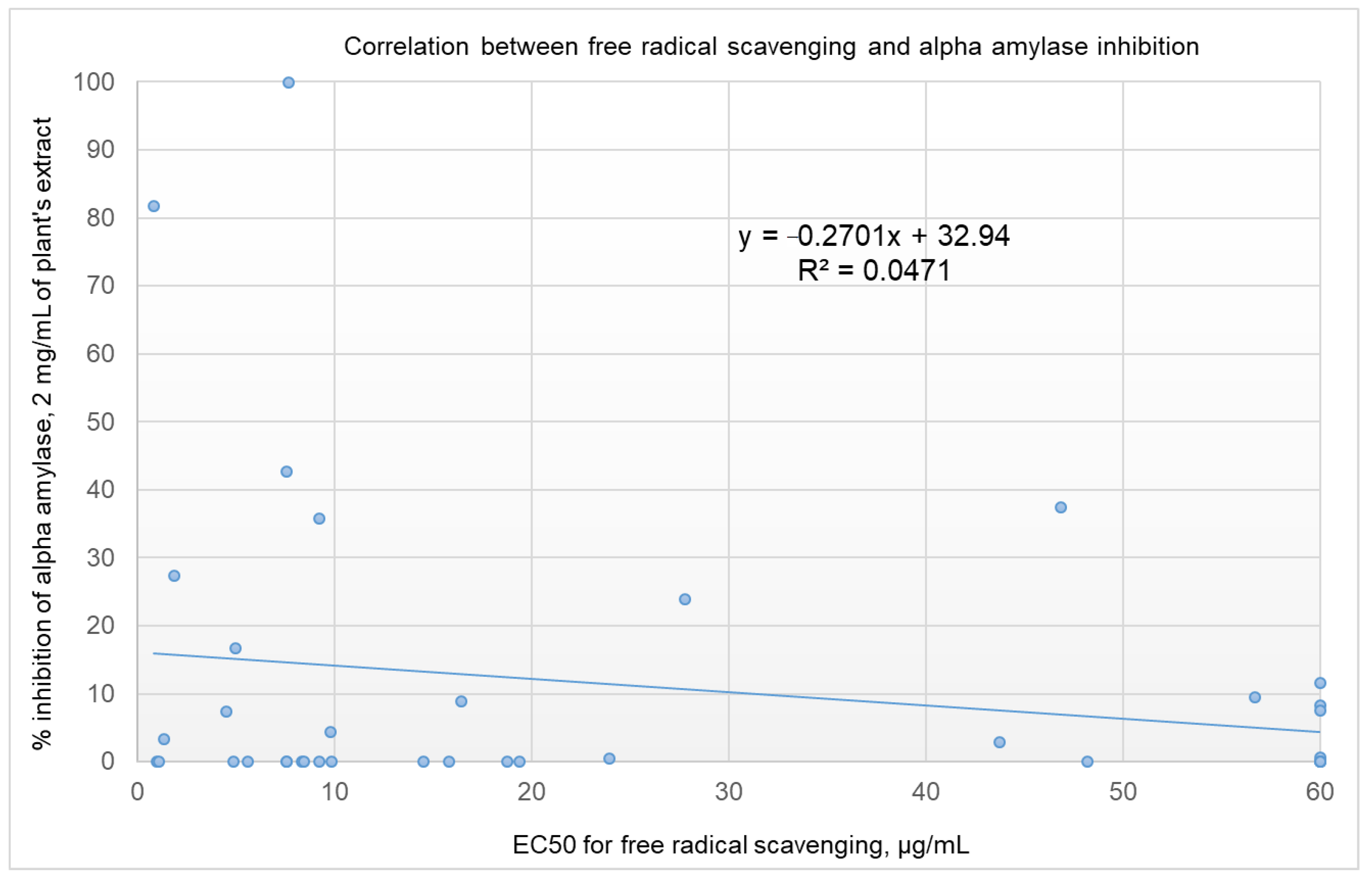
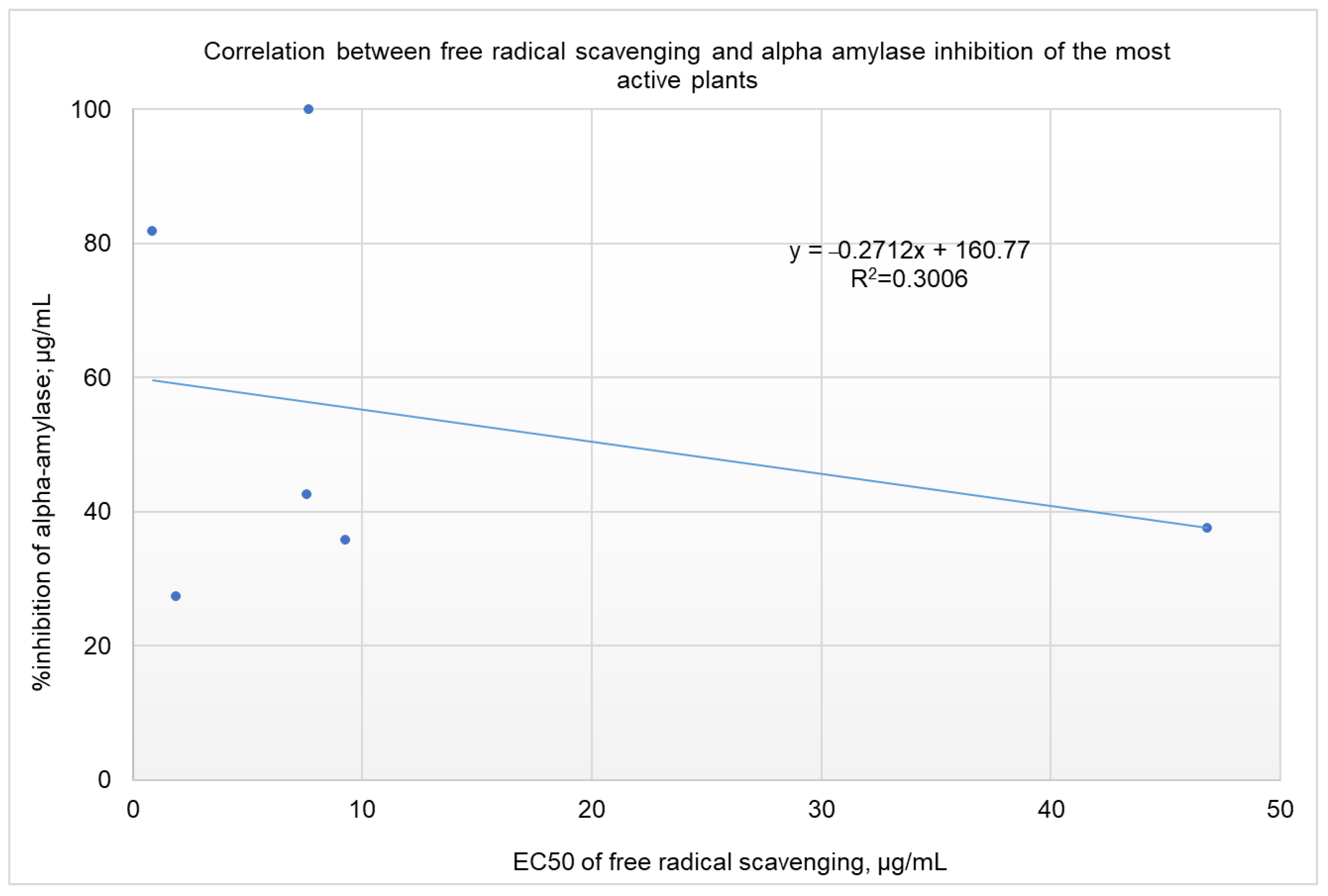
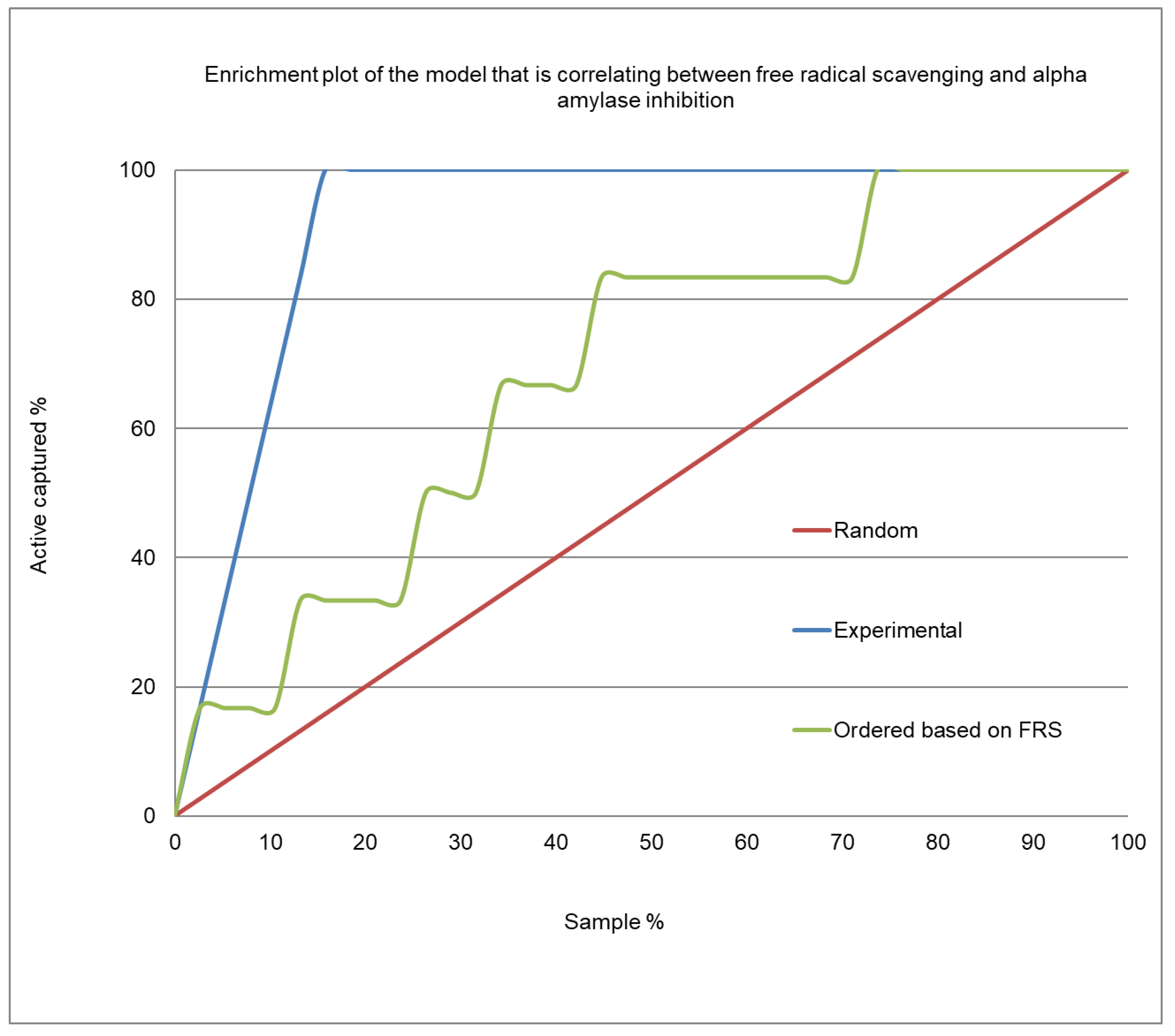
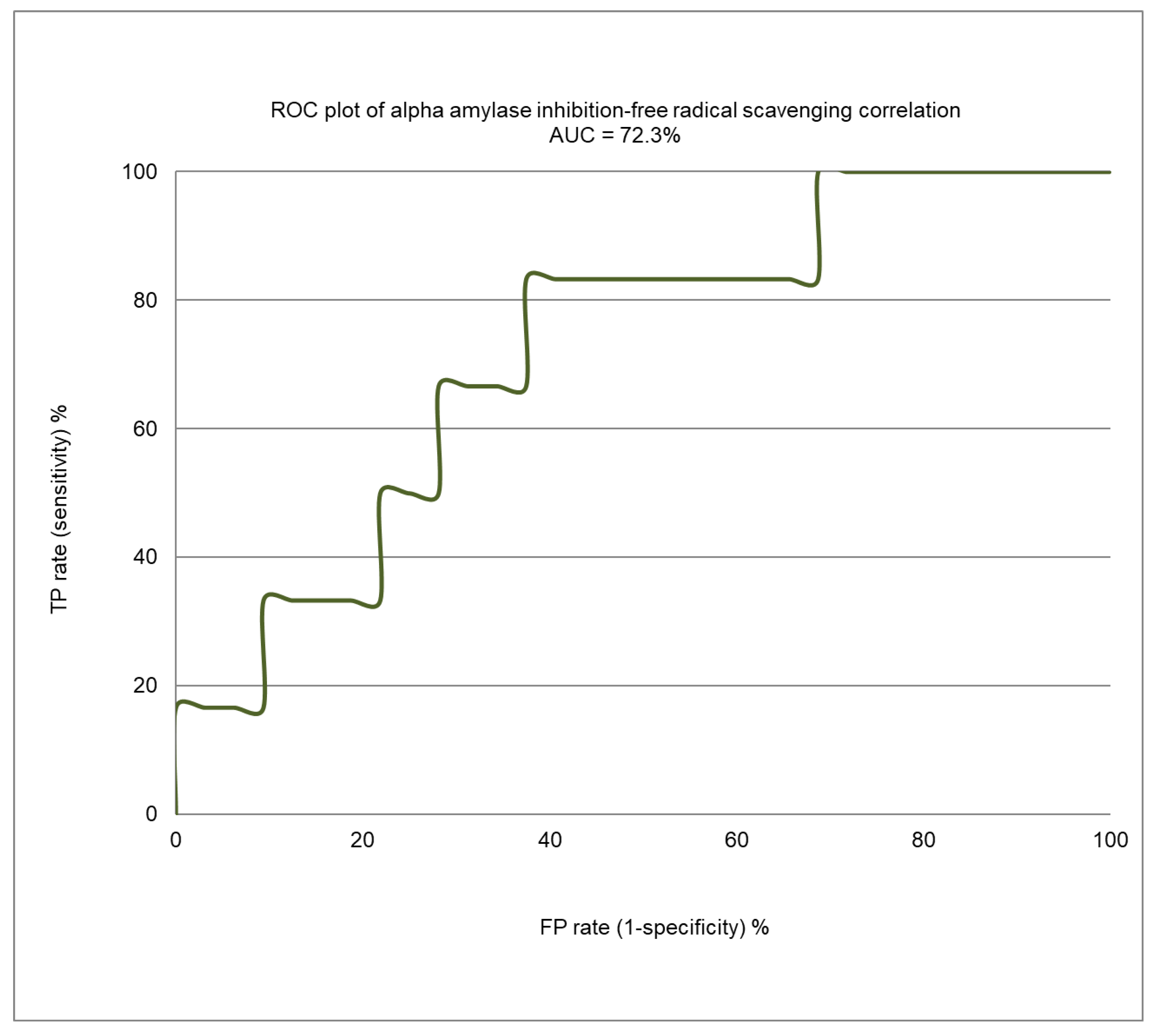
2.4. Correlation Analysis between α-Amylase Inhibition and Free Radical Scavenging Activity
2.5. Effects of the Plant Extracts on Glucose Uptake in Skeletal Muscles
2.5.1. Effects of the Plant Extracts on Cell Viability
2.5.2. Effects of Pelargonium spp. and Rhus coriaria Extracts on GLUT4 Translocation
3. Materials and Methods
3.1. Materials
3.2. Preparation of Plant Extracts
3.3. α-Amylase Activity
3.4. Free Radical Scavenging Activity (FRSA)
3.5. Cell Viability
3.6. Glucose Uptake
3.7. Model Assessments
3.8. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Miao, M.; Jiang, B.; Cui, S.W.; Zhang, T.; Jin, Z. Slowly digestible starch—A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1642–1657. [Google Scholar] [CrossRef] [PubMed]
- Warren, F.; Zhang, B.; Waltzer, G.; Gidley, M.; Dhital, S. The interplay of α-amylase and amyloglucosidase activities on the digestion of starch in in vitro enzymic systems. Carbohydr. Polym. 2015, 117, 192–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanders, L.M. Carbohydrate: Digestion, Absorption and Metabolism. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Oxford, UK, 2016; pp. 643–650. [Google Scholar]
- Lebovitz, H.E. Chapter 42-Hyperglycemia Secondary to Nondiabetic Conditions and Therapies. In Endocrinology: Adult and Pediatric, 7th ed.; Jameson, J.L., De Groot, L.J., de Kretser, D.M., Giudice, L.C., Grossman, A.B., Melmed, S., Potts, J.T., Weir, G.C., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2016; pp. 737–751.e6. [Google Scholar]
- Brockman, R.P. Roles of glucagon and insulin in the regulation of metabolism in ruminants. A review. Can. Vet. J. 1978, 19, 55–62. [Google Scholar] [PubMed]
- Alatrach, M.; Agyin, C.; Mehta, R.; Adams, J.; DeFronzo, R.A.; Abdul-Ghani, M. Glucose-Mediated Glucose Disposal at Baseline Insulin Is Impaired in IFG. J. Clin. Endocrinol. Metab. 2019, 104, 163–171. [Google Scholar] [CrossRef] [Green Version]
- Liu, A.; Liu, J.; Chen, X.; Lu, B.; Zeng, C.; Ye, H. A novel crustacean hyperglycemic hormone (CHH) from the mud crab Scylla paramamosain regulating carbohydrate metabolism. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2019, 231, 49–55. [Google Scholar] [CrossRef]
- Tabish, S.A. Is Diabetes Becoming the Biggest Epidemic of the Twenty-first Century? Int. J. Health Sci. (Qassim) 2007, 1, V–VIII. [Google Scholar]
- Samuel, V.T.; Shulman, G.I. The pathogenesis of insulin resistance: Integrating signaling pathways and substrate flux. J Clin. Investig. 2016, 126, 12–22. [Google Scholar] [CrossRef] [Green Version]
- Carnagarin, R.; Dharmarajan, A.M.; Dass, C.R. Molecular aspects of glucose homeostasis in skeletal muscle--A focus on the molecular mechanisms of insulin resistance. Mol. Cell Endocrinol. 2015, 417, 52–62. [Google Scholar] [CrossRef]
- Lund, S.; Holman, G.D.; Schmitz, O.; Pedersen, O. Glut 4 content in the plasma membrane of rat skeletal muscle: Comparative studies of the subcellular fractionation method and the exofacial photolabelling technique using ATB-BMPA. FEBS Lett. 1993, 330, 312–318. [Google Scholar] [CrossRef] [Green Version]
- Lund, S.; Holman, G.D.; Zierath, J.R.; Rincon, J.; Nolte, L.A.; Clark, A.E.; Schmitz, O.; Pedersen, O.; Wallberg-Henriksson, H. Effect of insulin on GLUT4 cell surface content and turnover rate in human skeletal muscle as measured by the exofacial bis-mannose photolabeling technique. Diabetes 1997, 46, 1965–1969. [Google Scholar] [CrossRef]
- Wilson, C.M.; Cushman, S.W. Insulin stimulation of glucose transport activity in rat skeletal muscle: Increase in cell surface GLUT4 as assessed by photolabelling. Biochem. J. 1994, 755–759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gray, S.P.; Jandeleit-Dahm, K. The pathobiology of diabetic vascular complications--cardiovascular and kidney disease. J. Mol. Med. (Berl.) 2014, 92, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Einarson, T.R.; Acs, A.; Ludwig, C.; Panton, U.H. Prevalence of cardiovascular disease in type 2 diabetes: A systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc. Diabetol. 2018, 17, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parim, B.; Sathibabu Uddandrao, V.V.; Saravanan, G. Diabetic cardiomyopathy: Molecular mechanisms, detrimental effects of conventional treatment, and beneficial effects of natural therapy. Heart Fail. Rev. 2019, 24, 279–299. [Google Scholar] [CrossRef] [PubMed]
- van Sloten, T.T.; Sedaghat, S.; Carnethon, M.R.; Launer, L.J.; Stehouwer, C.D.A. Cerebral microvascular complications of type 2 diabetes: Stroke, cognitive dysfunction, and depression. Lancet Diabetes Endocrinol. 2020, 8, 325–336. [Google Scholar] [CrossRef]
- Tian, J.; Ogawa, Y.; Shi, J.; Chen, S.; Zhang, H.; Liu, D.; Ye, X. The microstructure of starchy food modulates its digestibility. Crit. Rev. Food Sci. Nutr. 2019, 59, 3117–3128. [Google Scholar] [CrossRef]
- Ye, J.; Luo, S.; Huang, A.; Chen, J.; Liu, W.; McClements, D. Synthesis and characterization of citric acid esterified rice starch by reactive extrusion: A new method of producing resistant starch. Food Hydrocoll. 2019, 92, 135–142. [Google Scholar] [CrossRef]
- Robertson, M.D. Dietary-resistant starch and glucose metabolism. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 362–367. [Google Scholar] [CrossRef]
- Miao, M.; Jiang, H.; Jiang, B.; Li, Y.; Cui, S.W.; Zhang, T. Structure elucidation of catechins for modulation of starch digestion. LWT Food Sci.Technol. 2014, 57, 188–193. [Google Scholar] [CrossRef]
- Sun, L.; Miao, M. Dietary polyphenols modulate starch digestion and glycaemic level: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 541–555. [Google Scholar] [CrossRef]
- Miao, M.; Jiang, B.; Jiang, H.; Zhang, T.; Li, X. Interaction mechanism between green tea extract and human alpha-amylase for reducing starch digestion. Food Chem. 2015, 186, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Sales, P.M.; Souza, P.M.; Simeoni, L.A.; Silveira, D. α-Amylase inhibitors: A review of raw material and isolated compounds from plant source. J. Pharm. Pharm. Sci. 2012, 15, 141–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, Y.; Chang, S.K.C.; Zhang, Y. Comparison of alpha-amylase, alpha-glucosidase and lipase inhibitory activity of the phenolic substances in two black legumes of different genera. Food Chem. 2017, 214, 259–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, M.; Jiang, H.; Jiang, B.; Zhang, T.; Cui, S.W.; Jin, Z. Phytonutrients for controlling starch digestion: Evaluation of grape skin extract. Food Chem. 2014, 145, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Visvanathan, R.; Jayathilake, C.; Liyanage, R. A simple microplate-based method for the determination of alpha-amylase activity using the glucose assay kit (GOD method). Food Chem. 2016, 211, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Shi, J.; Tang, Z.; Sawhney, M.; Hu, H.; Shi, L.; Fonseca, V.; Dong, H. Comparison of glucose lowering effect of metformin and acarbose in type 2 diabetes mellitus: A meta-analysis. PLoS ONE 2015, 10, e0126704. [Google Scholar] [CrossRef]
- Dileep, K.V.; Nithiyanandan, K.; Remya, C. Binding of acarbose, an anti-diabetic drug to lysozyme: A combined structural and thermodynamic study. J. Biomol. Struct. Dyn. 2018, 36, 3354–3361. [Google Scholar] [CrossRef]
- Singla, R.K.; Dubey, A.K. Phytochemical profiling, GC-MS analysis and alpha-amylase inhibitory potential of ethanolic ectract of cocos nucifera Linn endocarp. Endocr. Metab. Immune Disord. Drug. Targets 2019, 19, 419–442. [Google Scholar] [CrossRef]
- Mojica, L.; de Mejia, E.G. Characterization and Comparison of Protein and Peptide Profiles and their Biological Activities of Improved Common Bean Cultivars (Phaseolus vulgaris L.) from Mexico and Brazil. Plant Foods Hum. Nutr. 2015, 70, 105–112. [Google Scholar] [CrossRef]
- Mattio, L.M.; Marengo, M.; Parravicini, C.; Eberini, I.; Dallavalle, S.; Bonomi, F.; Iametti, S.; Pinto, A. Inhibition of Pancreatic alpha-amylase by Resveratrol Derivatives: Biological Activity and Molecular Modelling Evidence for Cooperativity between Viniferin Enantiomers. Molecules 2019, 24, 3225. [Google Scholar] [CrossRef] [Green Version]
- Parizad, P.A.; Capraro, J.; Scarafoni, A.; Bonomi, F.; Blandino, M.; Marengo, M.; Giordano, D.; Carpen, A.; Iametti, S. The Bio-Functional Properties of Pigmented Cereals may Involve Synergies among Different Bioactive Species. Plant Foods Hum. Nutr. 2019, 74, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Tajaldini, M.; Samadi, F.; Khosravi, A.; Ghasemnejad, A.; Asadi, J. Protective and anticancer effects of orange peel extract and naringin in doxorubicin treated esophageal cancer stem cell xenograft tumor mouse model. Biomed. Pharmacother. 2020, 121, 109594. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.R.; Chang, C.H.; Hsu, C.F.; Tsai, M.J.; Cheng, H.; Leong, M.K.; Sung, P.J.; Chen, J.C.; Weng, C.F. Natural compounds as potential adjuvants to cancer therapy: Preclinical evidence. Br. J. Pharmacol. 2020, 177, 1409–1423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dombe, S.; Shirote, P. Nanosponges Encapsulated Phytochemicals for Targeting Cancer: A Review. Curr. Drug Targets 2020. [Google Scholar] [CrossRef] [PubMed]
- Alam, F.; Shafique, Z.; Amjad, S.T.; Bin Asad, M.H.H. Enzyme inhibitors from natural sources with antidiabetic activity: A review. Phytother. Res. 2019, 33, 41–54. [Google Scholar] [CrossRef] [Green Version]
- Zaid, H.; Raiyn, J.; Nasser, A.; Saad, B.; Rayan, A. Physicochemical Properties of Natural Based Products versus Synthetic Chemicals. Open Nutraceuticals J. 2010, 3, 194–202. [Google Scholar] [CrossRef]
- Yilmazer-Musa, M.; Griffith, A.M.; Michels, A.J.; Schneider, E.; Frei, B. Grape seed and tea extracts and catechin 3-gallates are potent inhibitors of alpha-amylase and alpha-glucosidase activity. J. Agric. Food Chem. 2012, 60, 8924–8929. [Google Scholar] [CrossRef] [Green Version]
- Farnsworth, N.R.; Akerele, O.; Bingel, A.S.; Soejarto, D.D.; Guo, Z. Medicinal plants in therapy. Bull. World Health Organ. 1985, 63, 965–981. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, S.; Montasser Kouhsari, S.; Monavar Feshani, A. Antidiabetic properties of the ethanolic extract of Rhus coriaria fruits in rats. Daru 2010, 18, 270–275. [Google Scholar]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [Green Version]
- Carocho, M.; Ferreira, I.C.F.R. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol. 2013, 51, 15–25. [Google Scholar] [CrossRef]
- Maritim, A.C.; Sanders, R.A.; Watkins, J.B. Diabetes, Oxidative stress, and antioxidants: A review. J. Biochem. Mol. Toxicol. 2003, 17, 24–38. [Google Scholar] [CrossRef]
- Oguntibeju, O.O. Type 2 diabetes mellitus, oxidative stress and inflammation: Examining the links. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 45–63. [Google Scholar]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Perrone, S.; Santacroce, A.; Longini, M.; Proietti, F.; Bazzini, F.; Buonocore, G. The Free Radical Diseases of Prematurity: From Cellular Mechanisms to Bedside. Oxid. Med. Cell Longev. 2018, 2018, 7483062. [Google Scholar] [CrossRef] [Green Version]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Yang, S.C.; Hsu, C.Y.; Chou, W.L.; Fang, J.Y.; Chuang, S.Y. Bioactive agent discovery from the natural compounds for the treatment type 2 diabetes rat model. Molecules 2020, 25, 5713. [Google Scholar] [CrossRef]
- Boojar, F.M.A.; Aghaei, R.; Mashhadi Akbar Boojar, M. Data on possible in vitro anti-diabetic effects of verticinone on β-TC6 pancreatic and C2C12 skeletal muscle cells. Data Brief 2020, 28, 104828. [Google Scholar] [CrossRef]
- Lichota, A.; Gwozdzinski, L.; Gwozdzinski, K. Therapeutic potential of natural compounds in inflammation and chronic venous insufficiency. Eur. J. Med. Chem. 2019, 176, 68–91. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Gan, R.Y.; Li, S.; Zhou, Y.; Li, A.N.; Xu, D.P.; Li, H.B. Antioxidant Phytochemicals for the Prevention and Treatment of Chronic Diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef]
- Luo, Y.; Peng, B.; Wei, W.; Tian, X.; Wu, Z. Antioxidant and anti-diabetic activities of polysaccharides from guava leaves. Molecules 2019, 24, 1343. [Google Scholar] [CrossRef] [Green Version]
- Caicai, K.; Limin, H.; Liming, Z.; Zhiqiang, Z.; Yongwu, Y. Isolation, purification and antioxidant activity of polysaccharides from the leaves of maca (Lepidium Meyenii). Int. J. Biol. Macromol. 2018, 107, 2611–2619. [Google Scholar] [CrossRef]
- Khemakhem, I.; Abdelhedi, O.; Trigui, I.; Ayadi, M.A.; Bouaziz, M. Structural, antioxidant and antibacterial activities of polysaccharides extracted from olive leaves. Int. J. Biol. Macromol. 2018, 106, 425–432. [Google Scholar] [CrossRef]
- Isakoff, S.J.; Taha, C.; Rose, E.; Marcusohn, J.; Klip, A.; Skolnik, E.Y. The inability of phosphatidylinositol 3-kinase activation to stimulate GLUT4 translocation indicates additional signaling pathways are required for insulin-stimulated glucose uptake. Proc. Natl. Acad. Sci. USA 1995, 92, 10247–10251. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.M.; Venkataranganna, M.V.; Manjunath, K.; Viswanatha, G.L.; Ashok, G. Methanolic extract of Momordica cymbalaria enhances glucose uptake in L6 myotubes in vitro by up-regulating PPAR-γ and GLUT-4. Chin. J. Nat. Med. 2014, 12, 895–900. [Google Scholar] [CrossRef] [Green Version]
- Zhao, P.; Ming, Q.; Qiu, J.; Tian, D.; Liu, J.; Shen, J.; Liu, Q.H.; Yang, X. Ethanolic extract of Folium Sennae Mediates the glucose uptake of L6 cells by GLUT4 and Ca2+. Molecules 2018, 23, 2934. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Houseknecht, K.L.; Stenbit, A.E.; Katz, E.B.; Charron, M.J. Reduced glucose uptake precedes insulin signaling defects in adipocytes from heterozygous GLUT4 knockout mice. FASEB J. 2000, 14, 1117–1125. [Google Scholar] [CrossRef]
- Stenbit, A.E.; Tsao, T.S.; Li, J.; Burcelin, R.; Geenen, D.L.; Factor, S.M.; Houseknecht, K.; Katz, E.B.; Charron, M.J. GLUT4 heterozygous knockout mice develop muscle insulin resistance and diabetes. Nat. Med. 1997, 3, 1096–1101. [Google Scholar] [CrossRef]
- Tsao, T.S.; Li, J.; Chang, K.S.; Stenbit, A.E.; Galuska, D.; Anderson, J.E.; Zierath, J.R.; McCarter, R.J.; Charron, M.J. Metabolic adaptations in skeletal muscle overexpressing GLUT4: Effects on muscle and physical activity. FASEB J. 2001, 15, 958–969. [Google Scholar] [CrossRef] [Green Version]
- Abdul-Ghani, M.A.; DeFronzo, R.A. Pathogenesis of insulin resistance in skeletal muscle. J. Biomed. Biotech. 2010, 2010, 476279. [Google Scholar] [CrossRef] [Green Version]
- Kadan, S.; Saad, B.; Sasson, Y.; Zaid, H. In vitro evaluation of anti-diabetic activity and cytotoxicity of chemically analysed Ocimum basilicum extracts. Food Chem. 2016, 196, 1066–1074. [Google Scholar] [CrossRef]
- Kacergius, T.; Abu-Lafi, S.; Kirkliauskiene, A.; Gabe, V.; Adawi, A.; Rayan, M.; Qutob, M.; Stukas, R.; Utkus, A.; Zeidan, M.; et al. Inhibitory capacity of Rhus coriaria L. extract and its major component methyl gallate on Streptococcus mutans biofilm formation by optical profilometry: Potential applications for oral health. Mol. Med. Rep. 2017, 16, 949–956. [Google Scholar] [CrossRef] [Green Version]
- Frank, A.; Abu-Lafi, S.; Adawi, A.; Schwed, J.S.; Stark, H.; Rayan, A. From medicinal plant extracts to defined chemical compounds targeting the histamine H4 receptor: Curcuma longa in the treatment of inflammation. Inflamm. Res. 2017, 66, 923–929. [Google Scholar] [CrossRef]
- Gabe, V.; Kacergius, T.; Abu-Lafi, S.; Zeidan, M.; Abu-Farich, B.; Austys, D.; Masalha, M.; Rayan, A. Suppressive Effects of Octyl Gallate on Streptococcus mutans Biofilm Formation, Acidogenicity, and Gene Expression. Molecules 2019, 24, 3170. [Google Scholar] [CrossRef] [Green Version]
- Gabe, V.; Kacergius, T.; Abu-Lafi, S.; Kalesinskas, P.; Masalha, M.; Falah, M.; Abu-Farich, B.; Melninkaitis, A.; Zeidan, M.; Rayan, A. Inhibitory Effects of Ethyl Gallate on Streptococcus mutans Biofilm Formation by Optical Profilometry and Gene Expression Analysis. Molecules 2019, 24, 529. [Google Scholar] [CrossRef] [Green Version]
- Rayan, A.; Raiyn, J.; Falah, M. Nature is the best source of anticancer drugs: Indexing natural products for their anticancer bioactivity. PLoS ONE 2017, 12, e0187925. [Google Scholar] [CrossRef]
- Rayan, M.; Abdallah, Z.; Abu-Lafi, S.; Masalha, M.; Rayan, A. Indexing natural products for their antifungal activity by filters-based approach: Disclosure of discriminative properties. Curr. Comput. Aided Drug Des. 2019, 15, 235–242. [Google Scholar] [CrossRef]
- Masalha, M.; Rayan, M.; Adawi, A.; Abdallah, Z.; Rayan, A. Capturing antibacterial natural products with in silico techniques. Mol. Med. Rep. 2018, 18, 763–770. [Google Scholar] [CrossRef] [Green Version]
- Aswad, M.; Rayan, M.; Abu-Lafi, S.; Falah, M.; Raiyn, J.; Abdallah, Z.; Rayan, A. Nature is the best source of anti-inflammatory drugs: Indexing natural products for their anti-inflammatory bioactivity. Inflamm. Res. 2018, 67, 67–75. [Google Scholar] [CrossRef]
- Zeidan, M.; Rayan, M.; Zeidan, N.; Falah, M.; Rayan, A. Indexing Natural Products for Their Potential Anti-Diabetic Activity: Filtering and Mapping Discriminative Physicochemical Properties. Molecules 2017, 22, 1563. [Google Scholar] [CrossRef] [Green Version]


| No. | Plant Name | % Inhibition of α-Amylase Activity | Free radical Scavenging EC50 (μg/mL) |
|---|---|---|---|
| 1 | Rhus coriaria | 81.75 ± 0.51 | 0.87 |
| 2 | Curcuma longa | 0 | 0.98 |
| 3 | Green tea | 0 | 1.10 |
| 4 | Camelia sinensis | 3.27 ± 2.24 | 1.38 |
| 5 | Punica granatum | 27.39 ± 1.30 | 1.89 |
| 6 | Angelica sylvestris | 7.41 ± 1.41 | 4.53 |
| 7 | Thymus vulgaris | 0 | 4.87 |
| 8 | Mentha piperita | 16.67 ± 0.89 | 4.98 |
| 9 | Humulus lupupus | 0 | 5.63 |
| 10 | Olea europaea | 42.62 ± 1.17 | 7.59 |
| 11 | Thymus capitatus | 0 | 7.59 |
| 12 | Vitis vinifera | 0 | 7.59 |
| 13 | Pelargonium spp. | 99.89 ± 0.68 | 7.69 |
| 14 | Rubus idaeus | 0 | 8.33 |
| 15 | Lippia citriodora | 0 | 8.46 |
| 16 | Corchorus olitorius | 0 | 9.22 |
| 17 | Stevia rebaudiana | 35.73 ± 1.25 | 9.26 |
| 18 | Rosmarinus officinalis | 4.37 ± 1.04 | 9.80 |
| 19 | Laurus nobilis | 0 | 9.88 |
| 20 | Cymbopogon citratus | 0 | 14.53 |
| 21 | Salvia officinalis | 0 | 15.82 |
| 22 | Ocimum basilicum | 8.90 ± 2.33 | 16.46 |
| 23 | Cynara cardunculus | 0 | 18.78 |
| 24 | Senna acutifolia | 0 | 19.40 |
| 25 | Melissa officinalis | 0.51 ± 0.93 | 23.96 |
| 26 | Avena sativa | 23.85 ± 0.72 | 27.78 |
| 27 | Cuminum cyminum | 2.86 ± 1.84 | 43.73 |
| 28 | Petroselinum crispum | 37.48 ± 0.33 | 46.82 |
| 29 | Chamomile | 0 | 48.19 |
| 30 | Ceratonia siliqua | 9.43 ± 0.87 | 56.66 |
| 31 | Centaurea | 11.63 ± 0.86 | >60 |
| 32 | Gentiana | 8.33 ± 1.14 | >60 |
| 33 | Gundelia tournefortii | 0.62 ± 1.24 | >60 |
| 34 | Malva | 7.53 ± 1.02 | >60 |
| 35 | Petroselinum | 0 | >60 |
| 36 | Portulaca oleracea | 0 | >60 |
| 37 | Vitex agnus-castus | 0 | >60 |
| 38 | Zingiber officinale | 0 | >60 |
| 39 | Foeniculurn vulgare | 0 | n.d. |
| 40 | Urtica urens/pilulifera | 0 | n.d. |
| 41 | Cinnamomum aromaticum | - * | 0.60 |
| Acarbose (1.25 mM) | 89.67 ± 4.8 | n.d. |
| Plant Name | Plant Picture | EC50 of α-Amylase Inhibition | EC50 of Free Radical Scavenging |
|---|---|---|---|
| Pelargonium spp. |  | 0.60 mg/mL | 7.69 µg/mL |
| Rhus coriaria |  | 1.78 mg/mL | 0.87 µg/mL |
| FRSA EC50 Cutoffs | All | |||
|---|---|---|---|---|
| ≤5 µg/mL | ≤10 µg/mL | ≤60 µg/mL | - | |
| Number of active plants (true positives) 1 | 2 | 5 | 6 | 6 |
| Number of inactive plants (false positives) 2 | 7 | 15 | 25 | 35 |
| Number of inactive plants (true negatives) 3 | 28 | 20 | 10 | 0 |
| Number of active plants (false negatives) 4 | 4 | 1 | 0 | 0 |
| Precision | 0.22 | 0.25 | 0.194 | 0.146 |
| Accuracy | 0.73 | 0.61 | 0.39 | 0.146 |
| Enrichment factor | 1.5 | 1.7 | 1.3 | 1.0 |
| MCC | 0.114 | 0.286 | 0.235 | 0.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bashkin, A.; Ghanim, M.; Abu-Farich, B.; Rayan, M.; Miari, R.; Srouji, S.; Rayan, A.; Falah, M. Forty-One Plant Extracts Screened for Dual Antidiabetic and Antioxidant Functions: Evaluating the Types of Correlation between α-Amylase Inhibition and Free Radical Scavenging. Molecules 2021, 26, 317. https://doi.org/10.3390/molecules26020317
Bashkin A, Ghanim M, Abu-Farich B, Rayan M, Miari R, Srouji S, Rayan A, Falah M. Forty-One Plant Extracts Screened for Dual Antidiabetic and Antioxidant Functions: Evaluating the Types of Correlation between α-Amylase Inhibition and Free Radical Scavenging. Molecules. 2021; 26(2):317. https://doi.org/10.3390/molecules26020317
Chicago/Turabian StyleBashkin, Amir, Manar Ghanim, Basheer Abu-Farich, Mahmoud Rayan, Reem Miari, Samer Srouji, Anwar Rayan, and Mizied Falah. 2021. "Forty-One Plant Extracts Screened for Dual Antidiabetic and Antioxidant Functions: Evaluating the Types of Correlation between α-Amylase Inhibition and Free Radical Scavenging" Molecules 26, no. 2: 317. https://doi.org/10.3390/molecules26020317
APA StyleBashkin, A., Ghanim, M., Abu-Farich, B., Rayan, M., Miari, R., Srouji, S., Rayan, A., & Falah, M. (2021). Forty-One Plant Extracts Screened for Dual Antidiabetic and Antioxidant Functions: Evaluating the Types of Correlation between α-Amylase Inhibition and Free Radical Scavenging. Molecules, 26(2), 317. https://doi.org/10.3390/molecules26020317







