HIV-Infected Patients: Cross Site-Specific Hydrolysis of H3 and H4 Histones and Myelin Basic Protein with Antibodies against These Three Proteins
Abstract
:1. Introduction
2. Results
2.1. Purification of Antibodies
2.2. ELISA of Anti-MBP and Anti-Histones Autoantibodies
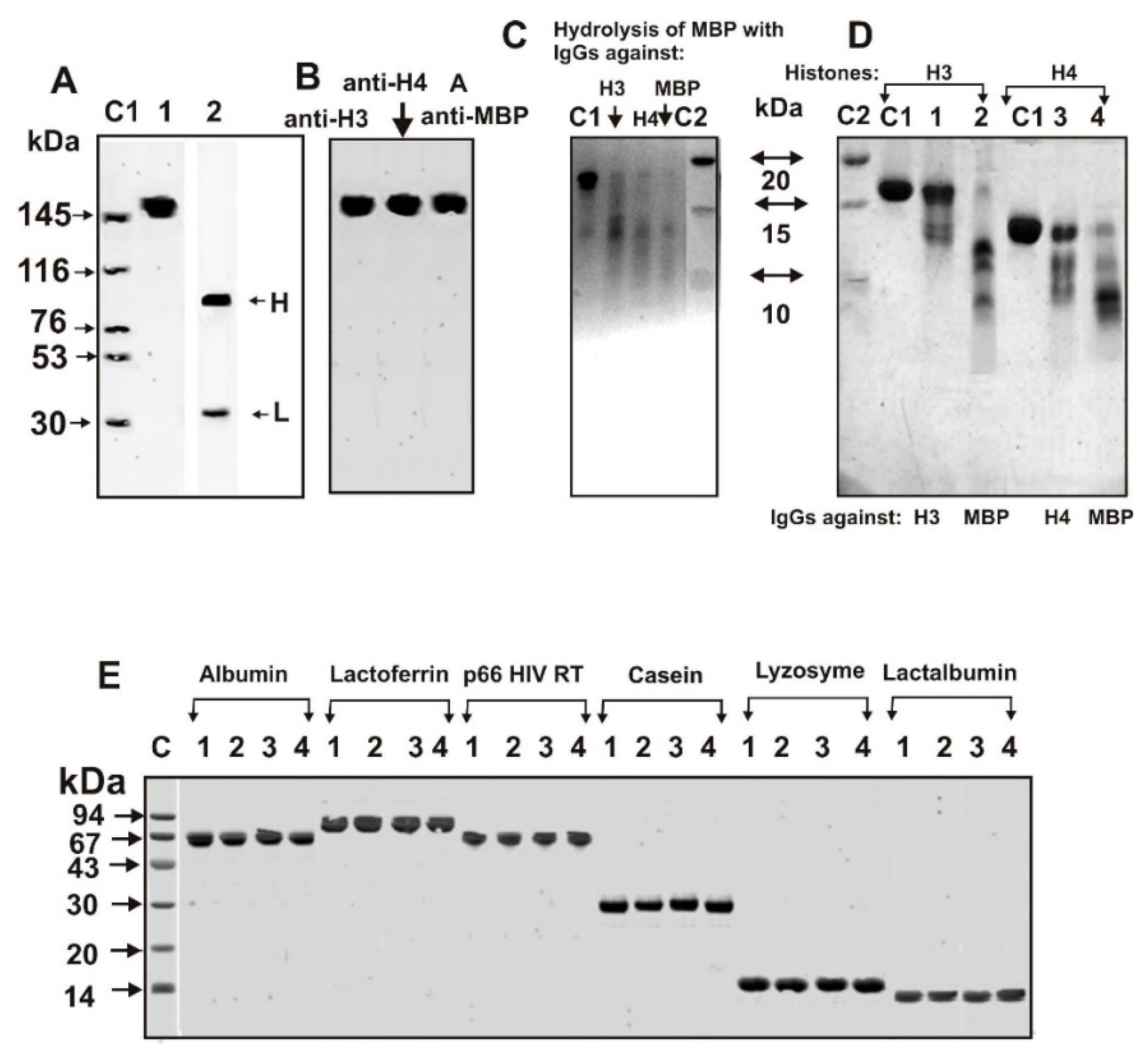
2.3. SDS-PAGE Analysis of Catalytic Cross-Reactivity
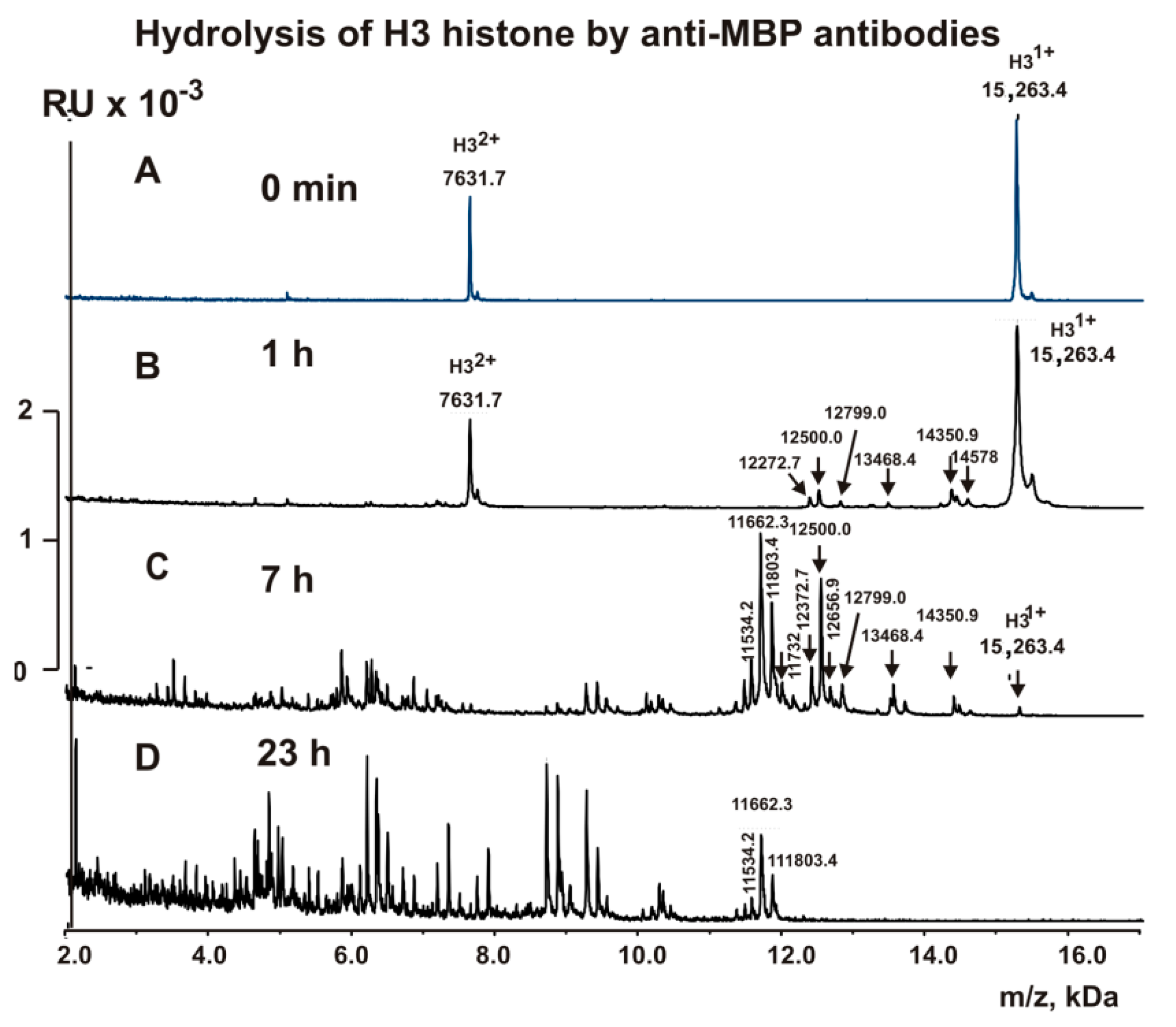
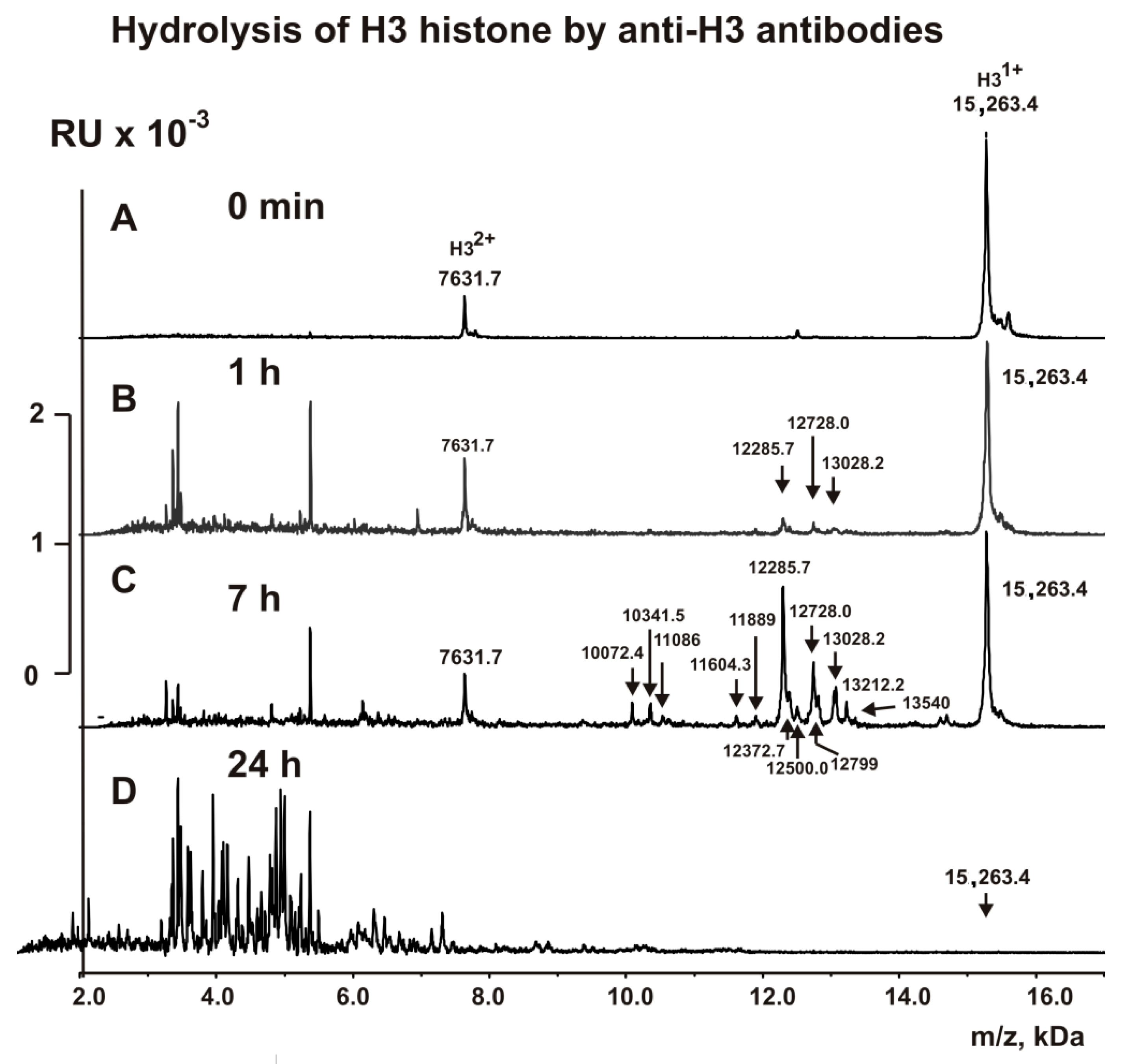
2.4. MALDI Analysis of H3 Histone Hydrolysis
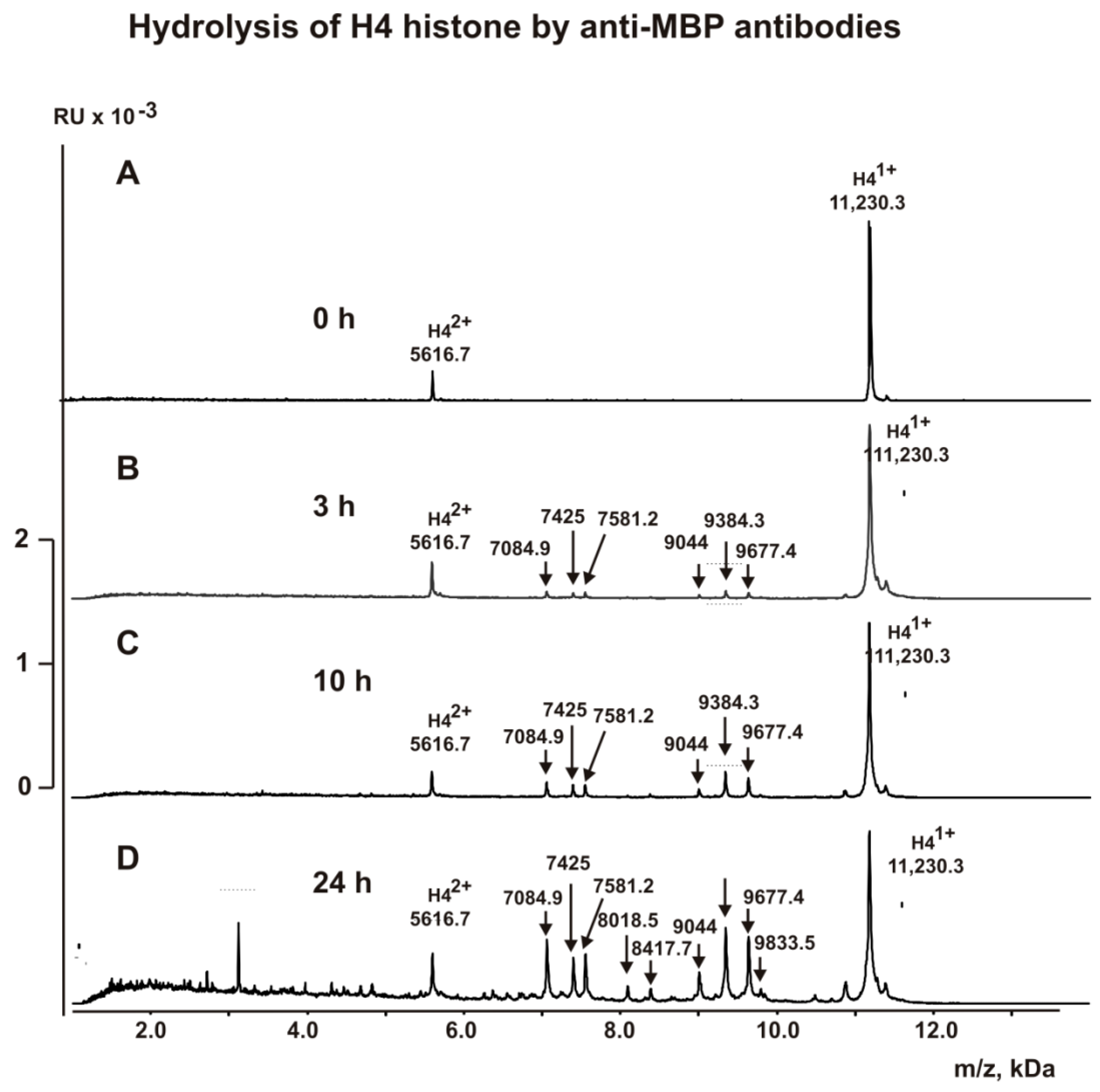
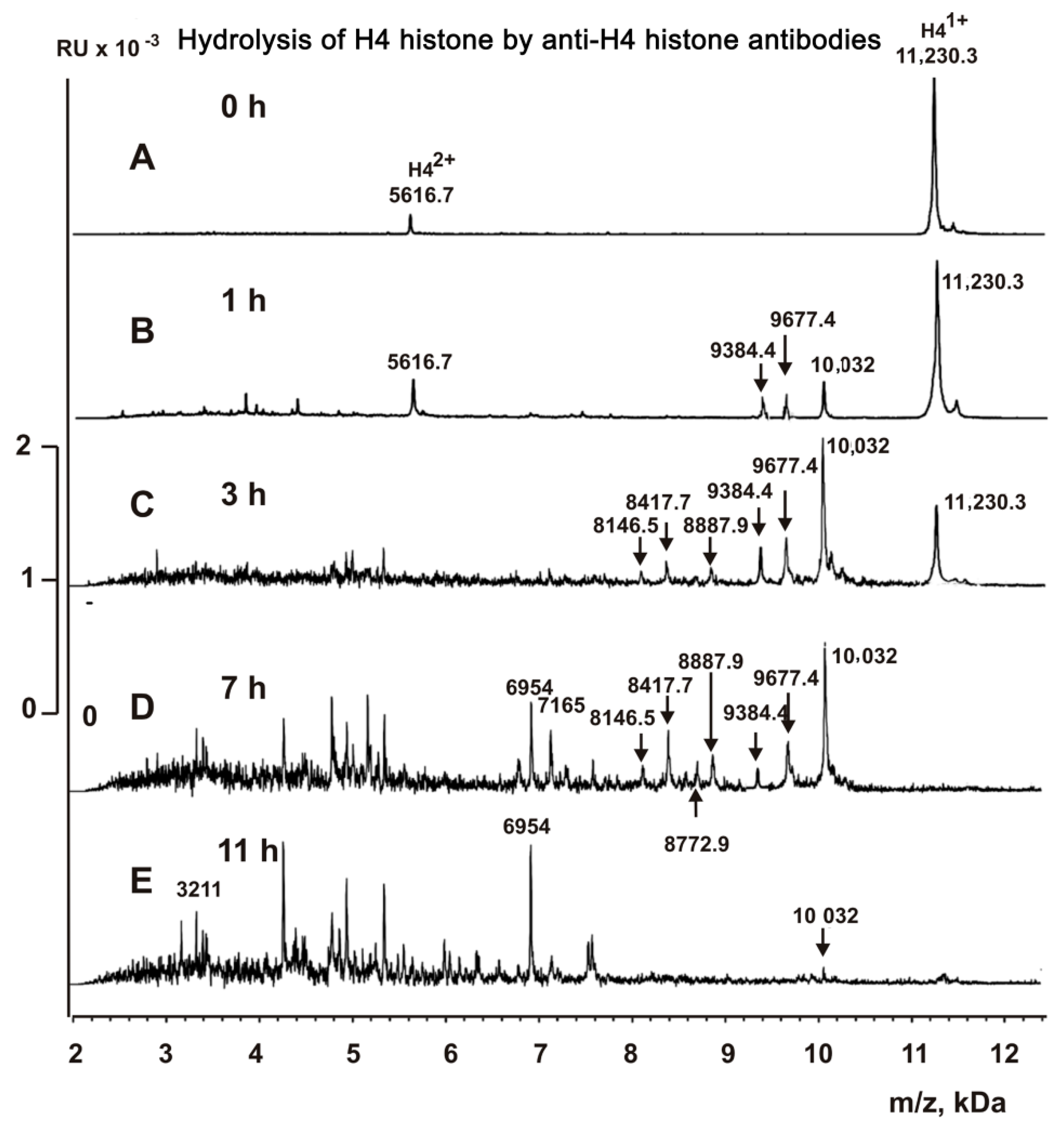
2.5. MALDI Analysis of H4 Histone Hydrolysis
2.6. Analysis of Possible Homology of H3 and H4 Histones with MBP
2.7. Affinity of IgGs for Histones
3. Discussion
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Antibody Purification
4.3. ELISA of Anti-MBP and Anti-Histones Autoantibodies
4.4. Proteolytic Activity Assay
4.5. Kinetic Analysis
4.6. MALDI-TOF Analysis of Histones Hydrolysis
4.7. Analysis of Sequence Homology
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Abbreviations
| Ab | antibody |
| Abz | abzyme |
| AI | autoimmune |
| AA | amino acid |
| AIDS | acquired immune deficiency syndrome |
| GL | generalized lymphadenopathy |
| AGDs | antigenic determinants |
| HIV-1 | human immunodeficiency virus type 1 |
| OP | oligopeptide |
| MALDI-TOF | matrix-assisted laser ionization/desorption time-of-flight mass spectrometry |
| MS | multiple sclerosis |
| MBP | myelin basic protein |
| SDS-PAGE | sodium dodecyl sulfate-polyacrylamide gel electrophoresis |
| SLE | systemic lupus erythematosus |
References
- Chen, R.; Kang, R.; Fan, X.-G.; Tang, D. Release and activity of histone in diseases. Cell Death. Dis. 2014, 5, e1370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suwa, A. Specificities and clinical significance of autoantibodies directed against histones. Nihon Rinsho Meneki Gakkai Kaishi 2005, 28, 123–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasselmayer, O.; Demirhan, I.; Chandra, A.; Bayer, M.; Müller, R.; Chandra, P. Inhibition of histone-mediated gene transfer in eucaryotic cells by anti-histone IgG. Anticancer Res. 2001, 21, 2377–2386. [Google Scholar] [PubMed]
- Founel, S.; Muller, S. Antinucleosome antibodies and T-cell response in systemic lupus erythematosus. Ann. Med. Interne. 2002, 153, 513–519. [Google Scholar]
- Fauci, A.S.; Braunwald, E.; Kasper, D.L.; Hauser, S.L.; Longo, D.L.; Jameson, L.J.; Loscalzo, J. Harrison’s Principles of Internal Medicine, 17th ed.; McGraw-Hill Professional: New York, NY, USA, 2008. [Google Scholar]
- Ternynck, P.; Falanga, B.; Unterkircher, C.; Gregoire, J.; Da Silva, L.P.; Avrameas, S. Induction of high levels of IgG autoantibodies in mice infected with Plasmodium chabaudi. Int. Immunol. 1991, 3, 29–37. [Google Scholar] [CrossRef]
- Hentati, B.; Sato, M.N.; Payelle, B.; Avrameas, S.; Ternynck, T. Beneficial effect of polyclonal immunoglobulins from malaria-infected BALB/c mice on the lupus-like syndrome of (NZB x NZW)F1 mice. Eur. J. Immunol. 1994, 24, 8–15. [Google Scholar] [CrossRef]
- Matsiota-Bernard, P.; Mahana, W.; Avrameas, S.; Nauciel, C. Specific and natural antibody production during Salmonella typhimurium infection in genetically susceptible and resistant mice. Immunology 1993, 79, 375–380. [Google Scholar]
- Barzilai, O.; Ram, M.; Shoenfeld, Y. Viral infection can induce the production of autoantibodies. Curr. Opin. Rheumatol. 2007, 19, 636–643. [Google Scholar] [CrossRef]
- Zandman-Goddard, G.; Shoenfeld, Y. HIV and autoimmunity. Autoimmun. Rev. 2002, 1, 329–337. [Google Scholar] [CrossRef]
- Baranova, S.V.; Buneva, V.N.; Nevinsky, G.A. Antibodies from the sera of HIV-infected patients efficiently hydrolyze all human histones. J. Mol. Recognit. 2016, 29, 346–362. [Google Scholar] [CrossRef]
- Baranova, S.V.; Dmitrienok, P.S.; Buneva, V.N.; Nevinsky, G.A. Autoantibodies in HIV-infected patients: Cross site-specific hydrolysis of H1 histone and myelin basic protein. Biofactors 2019, 45, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.H.; Tzioufas, A.G.; Notkins, A.L. Properties and function of polyreactive antibodies and polyreactive antigen-binding B cells. J Autoimmun. 2007, 29, 219–228. [Google Scholar] [CrossRef] [Green Version]
- Lerner, R.A.; Tramontano, A. Antibodies as enzymes. Trends Bioch. Sci. 1987, 12, 427–438. [Google Scholar] [CrossRef]
- Schultz, P.G.; Lerner, R.A. From molecular diversity to catalysis: Lessons from the immune system. Science 1995, 269, 1835–1842. [Google Scholar] [CrossRef] [PubMed]
- Keinan, E. (Ed.) Catalytic Antibodies; Wiley-VCH Verlag GmbH and Co. KgaA: Weinheim, Germany, 2005; pp. 1–586. [Google Scholar]
- Nevinsky, G.A.; Buneva, V.N. Natural catalytic antibodies–abzymes. In Catalytic Antibodies; Keinan, E., Ed.; VCH-Wiley Press: Weinheim, Germany, 2005; pp. 505–569. [Google Scholar]
- Nevinsky, G.A. Natural catalytic antibodies in norm and in autoimmune diseases. In Autoimmune Diseases: Symptoms, Diagnosis and Treatment; Brenner, K.J., Ed.; Nova Science Publishers Inc.: New York, NY, USA, 2010; pp. 1–107. [Google Scholar]
- Nevinsky, G.A. Natural catalytic antibodies in norm and in HIV-infected patients. In Understanding HIV/AIDS Management and Care—Pandemic Approaches the 21st Century; Kasenga, F.H., Ed.; InTech: Rijeka, Croatia, 2011; pp. 151–192. [Google Scholar]
- Nevinsky, G.A. Autoimmune processes in multiple sclerosis: Production of harmful catalytic antibodies associated with significant changes in the hematopoietic stem cell differentiation and proliferation. In Multiple Sclerosis; Conzalez-Quevedo, A., Ed.; InTech: Rijeka, Croatia, 2016; pp. 100–147. [Google Scholar]
- Nevinsky, G.A. Catalytic antibodies in norm and systemic lupus erythematosus. In Lupus; Khan, W.A., Ed.; InTech: Rijeka, Croatia, 2017; pp. 41–101. [Google Scholar]
- Kalaga, R.; Li, L.; O’Dell, J.R.; Paul, S. Unexpected presence of polyreactive catalytic antibodies in IgG from unimmunized donors and decreased levels in rheumatoid arthritis. J. Immunol. 1995, 155, 2695–2702. [Google Scholar]
- Paul, S.; Volle, D.J.; Beach, C.M.; Johnson, D.R.; Powell, M.J.; Massey, R.J. Catalytic hydrolysis of vasoactive intestinal peptide by human autoantibody. Science 1989, 244, 1158–1162. [Google Scholar] [CrossRef]
- Lacroix-Desmazes, S.; Moreau, A.; Bonnemain, C.; Stieltjes, N.; Pashov, A.; Sultan, Y.; Hoebeke, J.; Kazatchkine, M.D.; Kaveri, S.V. Catalytic activity of antibodies against factor VIII in patients with hemophilia A. Nat. Med. 1999, 5, 1044–1047. [Google Scholar] [CrossRef]
- Thiagarajan, P.; Dannenbring, R.; Matsuura, K.; Tramontano, A.; Gololobov, G.; Paul, S. Monoclonal antibody light chain with prothrombinase activity. Biochemistry 2000, 39, 6459–6465. [Google Scholar] [CrossRef]
- Polosukhina, D.I.; Kanyshkova, T.G.; Doronin, B.M.; Tyshkevich, O.B.; Buneva, V.N.; Boiko, A.N.; Gusev, E.I.; Favorova, O.O.; Nevinsky, G.A. Hydrolysis of myelin basic protein by polyclonal catalytic IgGs from the sera of patients with multiple sclerosis. J. Cell Mol. Med. 2004, 8, 359–368. [Google Scholar] [CrossRef]
- Polosukhina, D.I.; Kanyshkova, T.G.; Doronin, B.M.; Tyshkevich, O.B.; Buneva, V.N.; Boiko, A.N.; Gusev, E.I.; Nevinsky, G.A.; Favorova, O.O. Metal-dependent hydrolysis of myelin basic protein by IgGs from the sera of patients with multiple sclerosis. Immunol. Lett. 2006, 103, 75–81. [Google Scholar] [CrossRef]
- Polosukhina, D.I.; Buneva, V.N.; Doronin, B.M.; Tyshkevich, O.B.; Boiko, A.N.; Gusev, E.I.; Favorova, O.O.; Nevinsky, G.A. Hydrolysis of myelin basic protein by IgM and IgA antibodies from the sera of patients with multiple sclerosis. Med. Sci. Monit. 2005, 11, BR266–BR272. [Google Scholar] [PubMed]
- Savel’ev, A.N.; Eneyskaya, E.V.; Shabalin, K.A.; Filatov, M.V.; Neustroev, K.N. Antibodies with amylolytic activity. Protein Peptide Lett. 1999, 6, 179–181. [Google Scholar]
- Paul, S.; Planque, S.A.; Nishiyama, Y.; Hanson, C.V.; Massey, R.J. Nature and nurture of catalytic antibodies. Adv. Exp. Med. Biol. 2012, 750, 56–75. [Google Scholar] [PubMed]
- Planque, S.A.; Nishiyama, Y.; Hara, M.; Sonoda, S.; Murphy, S.K.; Watanabe, K.; Mitsuda, Y.; Brown, E.L.; Massey, R.J.; Primmer, S.R.; et al. Physiological IgM class catalytic antibodies selective for transthyretin amyloid. J. Biol. Chem. 2014, 289, 13243–13258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bezuglova, A.V.; Konenkova, L.P.; Doronin, B.M.; Buneva, V.N.; Nevinsky, G.A. Affinity and catalytic heterogeneity and metal-dependence of polyclonal myelin basic protein-hydrolyzing IgGs from sera of patients with systemic lupus erythematosus. J. Mol. Recognit. 2011, 24, 960–974. [Google Scholar] [CrossRef]
- Bezuglova, A.M.; Dmitrenok, P.S.; Konenkova, L.P.; Buneva, V.N.; Nevinsky, G.A. Multiple sites of the cleavage of 17- and 19-mer encephalytogenic oligopeptides corresponding to human myelin basic protein (MBP) by specific anti-MBP antibodies from patients with systemic lupus erythematosus. Peptides 2012, 37, 69–78. [Google Scholar] [CrossRef] [Green Version]
- Timofeeva, A.M.; Dmitrenok, P.S.; Konenkova, L.P.; Buneva, V.N.; Nevinsky, G.A. Multiple sites of the cleavage of 21- and 25-mer encephalytogenic oligopeptides corresponding to human myelin basic protein (MBP) by specific anti-MBP antibodies from patients with systemic systemic lupus erythematosus. PLoS ONE 2013, 8, e51600. [Google Scholar] [CrossRef]
- Parshukova, D.; Smirnova, L.P.; Ermakov, E.A.; Bokhan, N.A.; Semke, A.V.; Ivanova, S.A.; Buneva, V.N.; Nevinsky, G.A. Autoimmunity and immune system dysregulation in schizophrenia: IgGs from sera of patients hydrolyze myelin basic protein. J. Mol. Recognit. 2019, 32, e2759. [Google Scholar] [CrossRef]
- Odintsova, E.S.; Kharitonova, M.A.; Baranovskii, A.G.; Siziakina, L.P.; Buneva, V.N.; Nevinsky, G.A. DNA-hydrolyzing IgG antibodies from the blood of patients with acquired immune deficiency syndrome. Mol. Biol. 2006, 40, 857–864. [Google Scholar] [CrossRef]
- Baranova, S.V.; Dmitrienok, P.S.; Ivanisenko, N.V.; Buneva, V.N.; Nevinsky, G.A. Antibodies to H1 histone from the sera of HIV-infected patients recognize and catalyze site-specific degradation of this histone. J. Mol. Recognit. 2017, 30. [Google Scholar] [CrossRef]
- Baranova, S.V.; Dmitrienok, P.S.; Ivanisenko, N.V.; Buneva, V.N.; Nevinsky, G.A. Antibodies to H2A and H2B histones from the sera of HIV-infected patients catalyze site-specific degradation of these histones. Mol. Biosyst. 2017, 13, 1090–1101. [Google Scholar] [CrossRef] [PubMed]
- Baranova, S.V.; Dmitrenok, P.S.; Zubkova, A.D.; Ivanisenko, N.V.; Odintsova, E.S.; Buneva, V.N.; Nevinsky, G.A. Antibodies against H3 and H4 histones from the sera of HIV-infected patients catalyze site-specific degradation of these histones. J. Mol. Recognit. 2018, 31, e2703. [Google Scholar] [CrossRef] [PubMed]
- Baranova, S.V.; Buneva, V.N.; Kharitonova, M.A.; Sizyakina, L.P.; Calmels, C.; Andreola, M.L.; Parissi, V.; Nevinsky, G.A. HIV-1 integrase-hydrolyzing antibodies from sera of HIV-infected patients. Biochimie 2009, 91, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Baranova, S.V.; Buneva, V.N.; Kharitonova, M.A.; Sizyakina, L.P.; Calmels, C.; Andreola, M.L.; Parissi, V.; Nevinsky, G.A. HIV-1 integrase-hydrolyzing IgM antibodies from sera of HIV-infected patients. Int. Immunol. 2010, 22, 671–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odintsova, E.S.; Baranova, S.V.; Dmitrenok, P.S.; Rasskazov, V.A.; Calmels, C.; Parissi, V.; Andreola, M.L.; Buneva, V.N.; Zakharova, O.D.; Nevinsky, G.A. Antibodies to HIV integrase catalyze site-specific degradation of their antigen. Int. Immunol. 2011, 23, 601–612. [Google Scholar] [CrossRef] [Green Version]
- Odintsova, E.S.; Baranova, S.V.; Dmitrenok, P.S.; Calmels, C.; Parissi, V.; Nevinsky, G.A. Anti-integrase abzymes from the sera of HIV-infected patients specifically hydrolyze integrase but nonspecifically cleave short oligopeptides. J. Mol. Recognit. 2012, 25, 193–207. [Google Scholar] [CrossRef]
- Odintsova, E.S.; Kharitonova, M.A.; Baranovskii, A.G.; Siziakina, L.P.; Buneva, V.N.; Nevinsky, G.A. Proteolytic activity of IgG antibodies from blood of acquired immunodeficiency syndrome patients. Biochemistry 2006, 71, 251–261. [Google Scholar] [CrossRef]
- Libbey, J.E.; Cusick, M.F.; Fujinami, R.S. Role of pathogens in multiple sclerosis. Int. Rev. Immunol. 2014, 33, 266–283. [Google Scholar] [CrossRef] [Green Version]
- Andersen, O.; Lygner, P.E.; Bergstrom, T. Viral infections trigger multiple sclerosis relapses: A prospective seroepidemiological study. J. Neurol. 1993, 240, 417–422. [Google Scholar] [CrossRef]
- Nishi, Y. Evolution of catalytic antibody repertoire in autoimmune mice. J. Immunol. Methods 2002, 269, 213–233. [Google Scholar] [CrossRef]
- Tawfik, D.S.; Chap, R.; Green, B.S.; Sela, M.; Eshhar, Z. Unexpectedly high occurrence of catalytic antibodies in MRL/lpr and SJL mice immunized with a transition-state analog: Is there a linkage to autoimmunity? Proc. Natl. Acad. Sci. USA 2002, 92, 2145–2149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikehara, S.; Kawamura, M.; Takao, F. Organ-specific and systemic autoimmune diseases originate from defects in hematopoietic stem cells. Proc. Natl. Acad. Sci. USA 1990, 87, 8341–8344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andryushkova, A.S.; Kuznetsova, I.A.; Buneva, V.N.; Toporkova, L.B.; Sakhno, L.V.; Tikhonova, M.A.; Chernykh, E.R.; Orlovskaya, I.A.; Nevinsky, G.A. Formation of different abzymes in autoimmune-prone MRL-lpr/lpr mice is associated with changes in colony formation of haematopoetic progenitors. J. Cell Mol. Med. 2007, 11, 531–551. [Google Scholar] [CrossRef] [PubMed]
- Doronin, V.B.; Parkhomenko, T.A.; Korablev, A.; Toporkova, L.B.; Lopatnikova, J.A.; Alshevskaja, A.A.; Sennikov, S.V.; Buneva, V.N.; Budde, T.; Meuth, S.G.; et al. Changes in different parameters, lymphocyte proliferation and hematopoietic progenitor colony formation in EAE mice treated with myelin oligodendrocyte glycoprotein. J. Cell Mol. Med. 2016, 20, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Aulova, K.S.; Toporkova, L.B.; Lopatnikova, J.A.; Alshevskaya, A.A.; Sennikov, S.V.; Buneva, V.N.; Budde, T.; Meuth, S.G.; Popova, N.A.; Orlovskaya, I.A.; et al. Changes in haematopoietic progenitor colony differentiation and proliferation and the production of different abzymes in EAE mice treated with DNA. J. Cell Mol. Med. 2017, 21, 3795–3809. [Google Scholar] [CrossRef]
- Aulova, K.S.; Toporkova, L.B.; Lopatnikova, J.A.; Alshevskaya, A.A.; Sedykh, S.E.; Buneva, V.N.; Budde, T.; Meuth, S.G.; Popova, N.A.; Orlovskaya, I.A.; et al. Changes in cell differentiation and proliferation lead to production of abzymes in EAE mice treated with DNA-Histone complexes. J. Cell. Mol. Med. 2018, 22, 5816–5832. [Google Scholar] [CrossRef] [Green Version]
- Parkhomenko, T.A.; Doronin, V.B.; Castellazzi, M.; Padroni, M.; Pastore, M.; Buneva, V.N.; Granieri, E.; Nevinsky, G.A. Comparison of DNA-hydrolyzing antibodies from the cerebrospinal fluid and serum of patients with multiple sclerosis. PLoS ONE 2014, 9, e93001. [Google Scholar] [CrossRef]
- Doronin, V.B.; Parkhomenko, T.A.; Castellazzi, M.; Padroni, M.; Pastore, M.; Buneva, V.N.; Granieri, E.; Nevinsky, G.A. Comparison of antibodies hydrolyzing myelin basic protein from the cerebrospinal fluid and serum of patients with multiple sclerosis. PLoS ONE 2014, 9, e107807. [Google Scholar] [CrossRef] [Green Version]
- Doronin., V.B.; Parkhomenko, T.A.; Castellazzi, M.; Cesnik, E.; Buneva, V.N.; Granieri, E.; Nevinsky, G.A. Comparison of antibodies with amylase activity from cerebrospinal fluid and serum of patients with multiple sclerosis. PLoS ONE 2016, 11, e0154688. [Google Scholar]
- Fersht, A. Protein Structures: Kaleidoscope of Structural Properties and Functions; Freeman and Co.: San Francisco, CA, USA, 1980. [Google Scholar]
- Nevinsky, G.A. Important role of weak interactions in long DNA and RNA molecule recognition by enzymes. Mol. Biol. 1995, 29, 6–19. [Google Scholar]
- Nevinsky, G.A. Structural, thermodynamic, and kinetic basis of DNA and RNA-dependent enzymes functioning: Important role of weak nonspecific additive interactions between enzymes and long nucleic acids for their recognition and transformation. In Protein Structures: Kaleidoscope of Structural Properties and Functions; Uversky, V.N., Ed.; Research Signpost: Thiruvananthapuram, India, 2003; pp. 133–222. [Google Scholar]
- Nevinsky, G.A. Structural, thermodynamic, and kinetic basis for the activities of some nucleic acid repair enzymes. J. Mol. Recognit. 2011, 24, 656–677. [Google Scholar] [CrossRef]
- Andreev, S.L.; Buneva, V.N.; Nevinsky, G.A. How human IgGs against DNA recognize oligonucleotides and DNA. J. Mol. Recognit. 2016, 29, 596–610. [Google Scholar] [CrossRef] [PubMed]
- Belov, S.; Buneva, V.N.; Nevinsky, G.A. How human IgGs against myelin basic protein (MBP) recognize oligopeptides and MBP. J. Mol. Recognit. 2017, 30, e2637. [Google Scholar] [CrossRef] [PubMed]
- Baranova, S.V.; Dmitrienok, P.S.; Buneva, V.N.; Nevinsky, G.A. HIV-infected patients: Cross site-specific hydrolysis of H2a and H2b histones and myelin basic protein with antibodies against these three proteins. Biomolecules 2020, 10, 1501. [Google Scholar] [CrossRef] [PubMed]
- James, L.C.; Roversi, P.; Tawfik, D.S. Antibody multispecificity mediated by conformational diversity. Science 2003, 299, 1362–1367. [Google Scholar] [CrossRef] [Green Version]
- James, L.C.; Tawfik, D.S. Conformational diversity and protein evolution-a 60-year-old hypothesis revisited. Trends Biochem. Sci. 2003, 28, 361–368. [Google Scholar] [CrossRef]
- James, L.C.; Tawfik, D.S. The specificity of cross-reactivity: Promiscuous antibody binding involves specific hydrogen bonds rather than nonspecific hydrophobic stickiness. Protein Sci. 2003, 12, 2183–2193. [Google Scholar] [CrossRef] [Green Version]
- Thomas, J.O.; Wilson, C.M.; Hardin, J.A. The major core histone antigenic determinants in systemic lupus erythematosus are in the trypsin-sensitive regions. FEBS Lett. 1984, 169, 90–96. [Google Scholar] [CrossRef] [Green Version]
- Muller, S.; Soussanieh, A.; Bouley, J.P.; Reinbolt, J.; Van Regenmortel, M.H. Localization of two antigenic determinants in histone H4. Biochim. Biophys. Acta. 1983, 747, 100–106. [Google Scholar] [CrossRef]
- Muller, S.; Bonnier, D.; Thiry, M.; Van Regenmortel, M.H. Reactivity of autoantibodies in systemic lupus erythematosus with synthetic core histone peptides. Int. Arch. Allergy Appl. Immunol. 1989, 89, 288–296. [Google Scholar] [CrossRef]
- Ponomarenko, N.A.; Durova, O.M.; Vorobiev, I.I.; Belogurov, A.A.; Kurkova, I.N.; Petrenko, A.G.; Telegin, G.B.; Suchkov, S.V.; Kiselev, S.L.; Lagarkova, M.A.; et al. Autoantibodies to myelin basic protein catalyze site-specific degradation of their antigen. Proc. Natl. Acad. Sci. USA 2006, 103, 281–286. [Google Scholar] [CrossRef] [Green Version]
- O’Connor, K.C.; Bar-Or, A.; Hafler, D.A. The neuroimmunology of multiple sclerosis: Possible roles of T and B lymphocytes in immunopathogenesis. J. Clin. Immunol. 2001, 21, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Goldner, E.M.; Hsu, L.; Waraich, P.; Somers, J.M. Prevalence and incidence studies of schizophrenic disorders: A systematic review of the literature. Can. J. Psychiatry 2002, 47, 833–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corcoran, C.P. Neuropsychiatric changes in HIV/hepatitis C coinfected patients undergoing interferon therapy. J. Assoc. Nurses AIDS Care 2003, 14, 80S–86S. [Google Scholar] [CrossRef] [PubMed]
- Del Guerra, F.B.; Fonseca, J.L.; Figueiredo, V.M.; Ziff, E.B.; Konkiewitz, E.C. Human immunodeficiency virus-associated depression: Contributions of immuno-inflammatory, monoaminergic, neurodegenerative, and neurotrophic pathways. J. Neurovirol. 2013, 19, 314–327. [Google Scholar] [CrossRef] [PubMed]



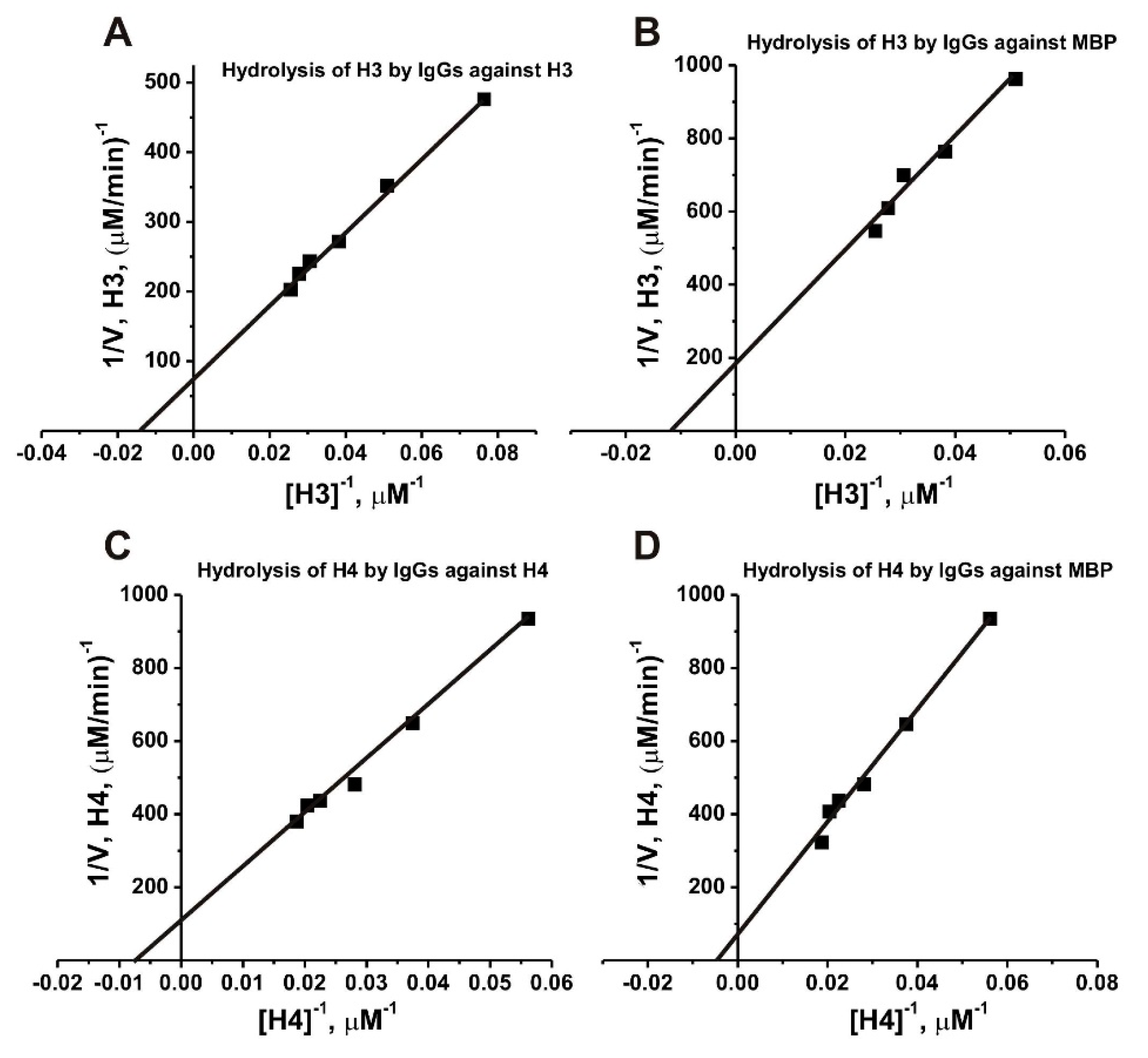
| Substrate | Abs | KM, µM | kcat, min−1 |
|---|---|---|---|
| H3 | anti-MBP IgGs | 90.0 ± 8.0 | 0.02 ± 0.002 |
| H4 | anti-MBP IgGs | 143.0 ± 10.0 | 0.037 ± 0.004 |
| H3 | anti-H3 IgGs | 76.9 ± 8.0 | 0.09 ± 0.008 |
| H4 | anti-H4 IgGs | 113.0 ± 12.0 | 0.076 ± 0.008 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baranova, S.V.; Dmitrenok, P.S.; Buneva, V.N.; Sedykh, S.E.; Nevinsky, G.A. HIV-Infected Patients: Cross Site-Specific Hydrolysis of H3 and H4 Histones and Myelin Basic Protein with Antibodies against These Three Proteins. Molecules 2021, 26, 316. https://doi.org/10.3390/molecules26020316
Baranova SV, Dmitrenok PS, Buneva VN, Sedykh SE, Nevinsky GA. HIV-Infected Patients: Cross Site-Specific Hydrolysis of H3 and H4 Histones and Myelin Basic Protein with Antibodies against These Three Proteins. Molecules. 2021; 26(2):316. https://doi.org/10.3390/molecules26020316
Chicago/Turabian StyleBaranova, Svetlana V., Pavel S. Dmitrenok, Valentina N. Buneva, Sergey E. Sedykh, and Georgy A. Nevinsky. 2021. "HIV-Infected Patients: Cross Site-Specific Hydrolysis of H3 and H4 Histones and Myelin Basic Protein with Antibodies against These Three Proteins" Molecules 26, no. 2: 316. https://doi.org/10.3390/molecules26020316








