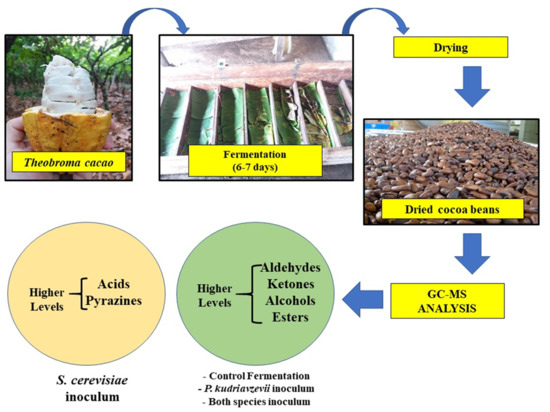
Profile of Volatile Compounds of On-Farm Fermented and Dried Cocoa Beans Inoculated with Saccharomyces cerevisiae KY794742 and Pichia kudriavzevii KY794725
Abstract
1. Introduction
2. Results and Discussion
2.1. Moisture Determination of Fermented and Dried Cocoa Beans
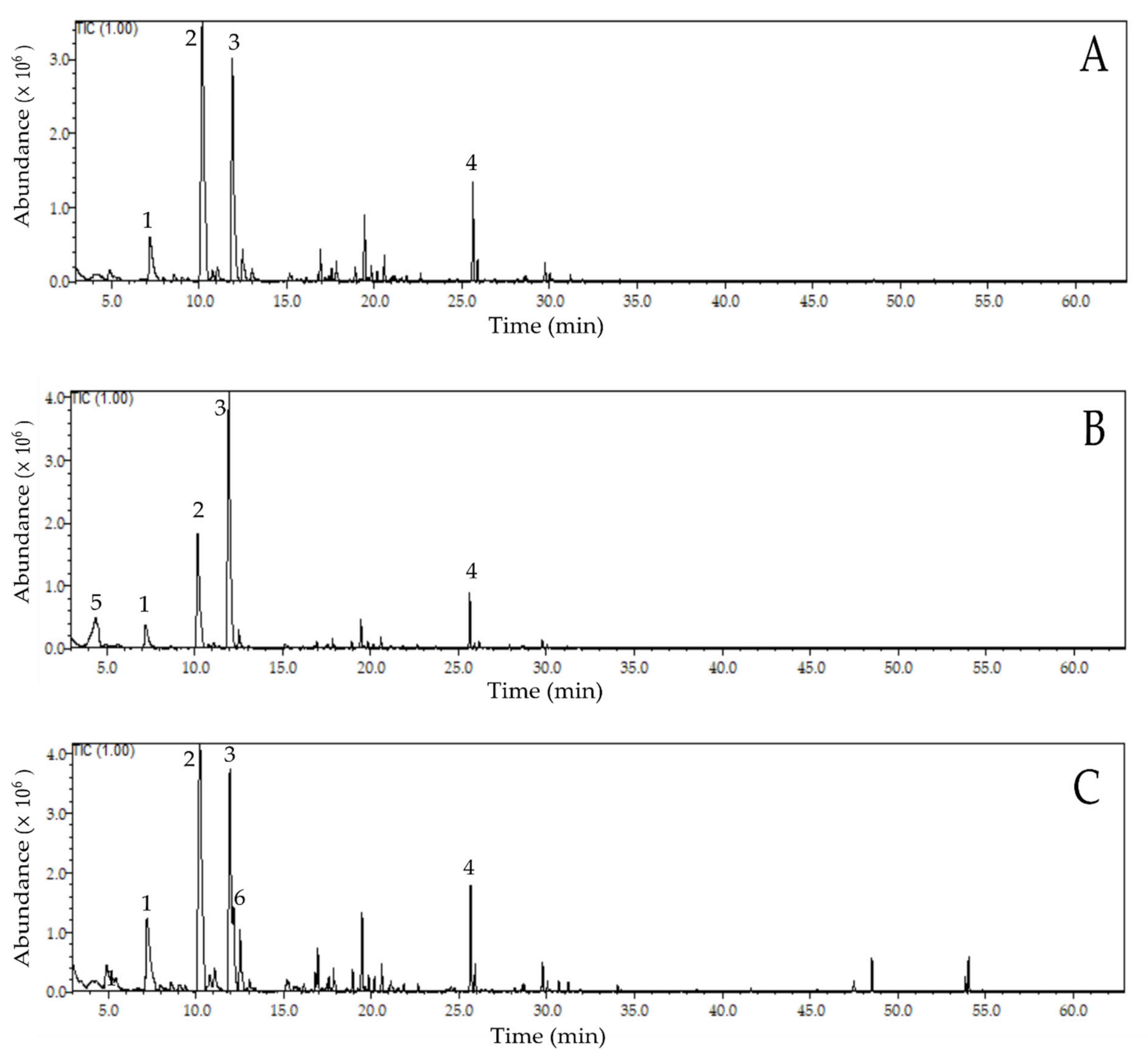
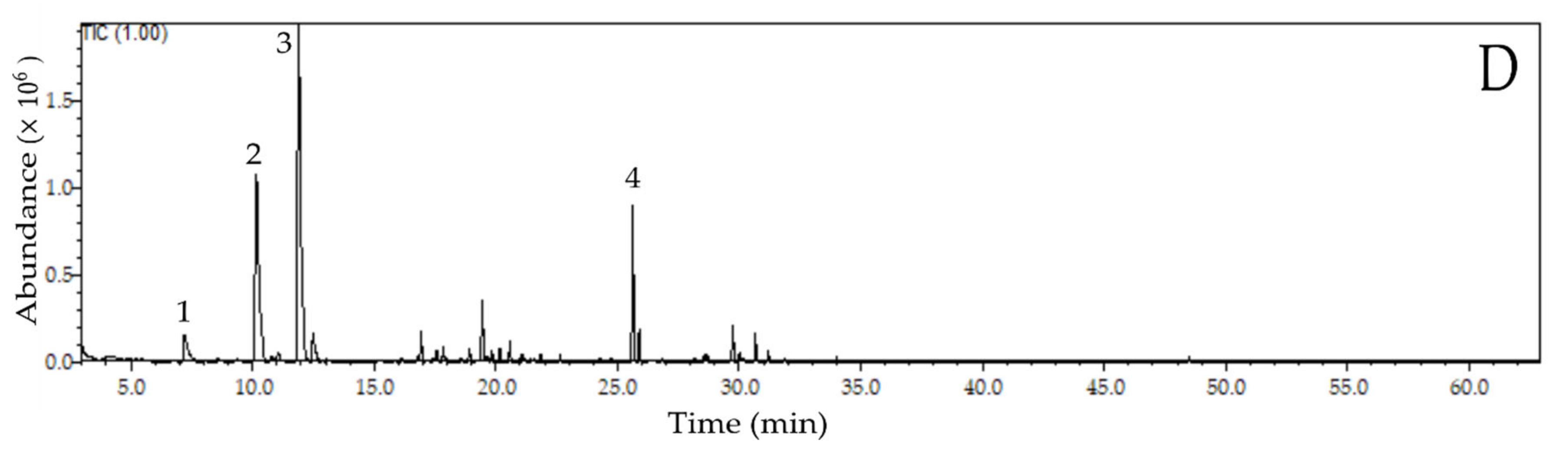
2.2. Profile of Volatile Compounds in Fermented and Dried Cocoa Beans
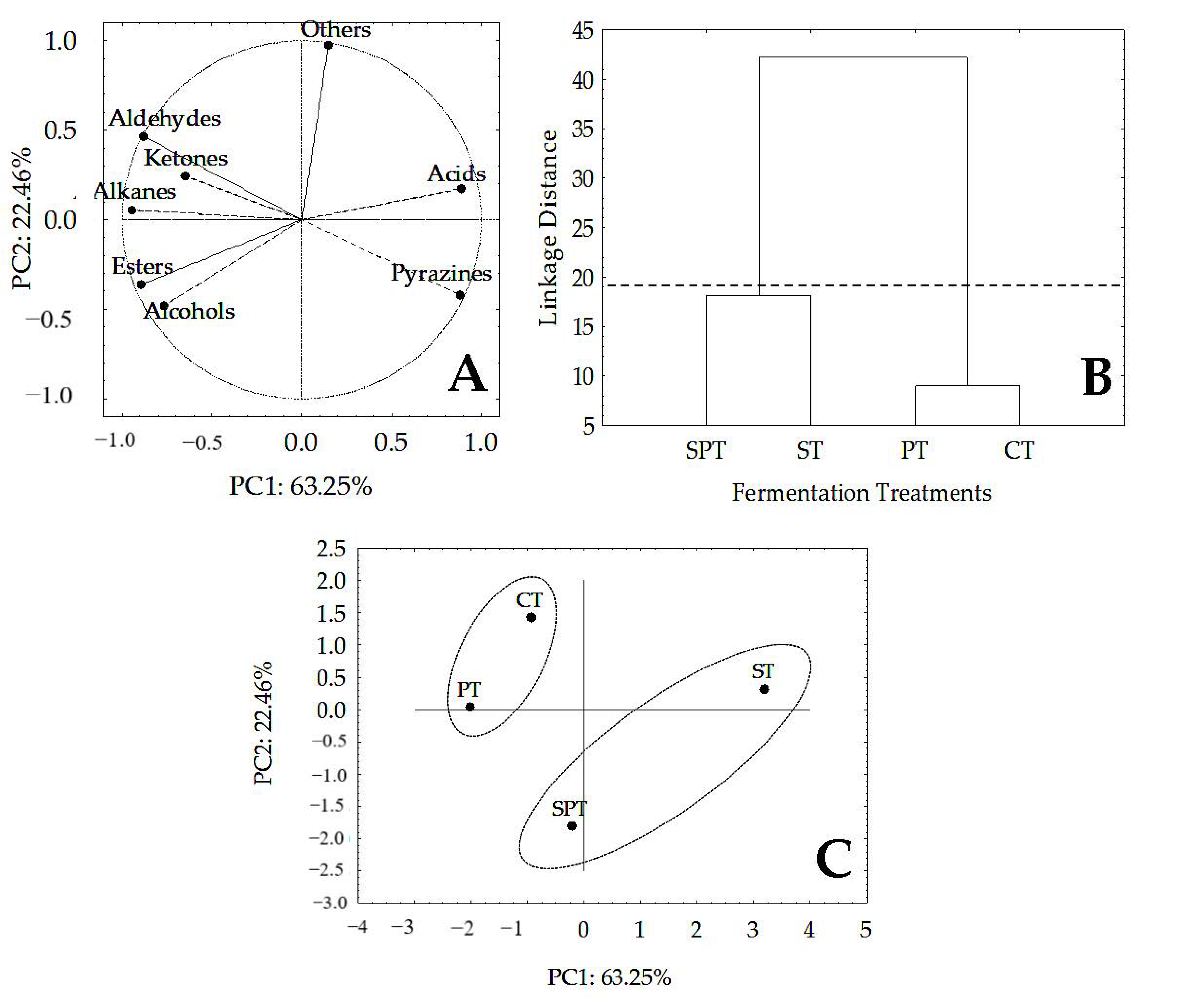
2.3. Multivariate Analysis (PCA and HCA) of Volatile Compounds in Cocoa Beans Fermented and Dried Produced with Different Yeast Inocula
3. Materials and Methods
3.1. Material
3.2. Fermentation Assay, Drying Process and Moisture Determination
3.3. Gas Chromatography-Mass Spectrometry (GC-MS) in Fermented and Dried Cocoa Beans
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ozturk, G.; Young, G.M. Food Evolution: The Impact of Society and Science on the Fermentation of Cocoa Beans. Compr. Rev. Food Sci. Food Saf. 2017, 16, 431–455. [Google Scholar] [CrossRef]
- Ramos, C.L.; Dias, D.R.; Miguel, M.G.C.P.; Schwan, R.F. Impact of different cocoa hybrids (Theobroma cacao L.) and S. cerevisiae UFLA CA11 inoculation on microbial communities and volatile compounds of cocoa fermentation. Food Res. Int. 2014, 64, 908–918. [Google Scholar] [CrossRef] [PubMed]
- Ceplac—Comissão Executiva do Plano da Lavoura Cacaueira—Pará Retoma Liderança na Produção Brasileira de Cacau, com a União de Agricultores. Available online: https://g1.globo.com/economia/agronegocios/globo-rural/noticia/2019/11/03/lideranca-na-producao-brasileira-de-cacau-volta-para-casa-no-para-com-a-uniao-de-agricultores.ghtml (accessed on 20 March 2020).
- Instituto Brasileiro de Geografia e Estatística—IBGE. Mapa das Indicações Geográficas 2019; IBGE: Brasília, Brazil, 2019. Available online: http://geoftp.ibge.gov.br/cartas_e_mapas/mapas_do_brasil/sociedade_e_economia/indicacoes_geograficas_2019_20190919.pdf (accessed on 11 July 2020).
- Schwan, R.F.; Wheals, A.E. The microbiology of cocoa fermentation and its role in chocolate quality. Crit. Rev. Food Sci. Nutr. 2004, 44, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Afoakwa, E.O.; Paterson, A.; Fowler, M.; Ryan, A. Flavor formation and character in cocoa and chocolate: A critical review. Crit. Rev. Food Sci. Nutr. 2008, 48, 840–857. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-Hernández, C.; Mota-Gutierrez, J.; Ferrocino, I.; Hernández-Estrada, Z.J.; González-Ríos, O.; Cocolin, L.; Suárez-Quiroz, M.L. The challenges and perspectives of the selection of starter cultures for fermented cocoa beans. Int. J. Food Microbiol. 2019, 301, 41–50. [Google Scholar] [CrossRef]
- Chagas Junior, G.C.A.; Ferreira, N.R.; Lopes, A.S. The microbiota diversity identified during the cocoa fermentation and the benefits of the starter cultures use: An overview. Int. J. Food Sci. Technol. 2020, 1–9. [Google Scholar] [CrossRef]
- Almeida, S.F.O.; Silva, L.R.C.; Chagas Junior, G.C.A.; Oliveira, G.; da Silva, S.H.M.; Vasconcelos, S.; Lopes, A.S. Diversity of yeasts during fermentation of cocoa from two sites in the Brazilian Amazon. Acta Amaz. 2018, 49, 64–70. [Google Scholar] [CrossRef]
- De Araújo, J.A.; Ferreira, N.R.; da Silva, S.H.M.; Oliveira, G.; Monteiro, R.C.; Alves, Y.F.M.; Lopes, A.S. Filamentous fungi diversity in the natural fermentation of Amazonian cocoa beans and the microbial enzyme activities. Ann. Microbiol. 2019. [Google Scholar] [CrossRef]
- Serra, J.L.; Moura, F.G.; Pereira, G.V.d.M.; Soccol, C.R.; Rogez, H.; Darnet, S. Determination of the microbial community in Amazonian cocoa bean fermentation by Illumina-based metagenomic sequencing. LWT Food Sci. Technol. 2019, 106, 229–239. [Google Scholar] [CrossRef]
- Batista, N.N.; Ramos, C.L.; Dias, D.R.; Pinheiro, A.C.M.; Schwan, R.F. The impact of yeast starter cultures on the microbial communities and volatile compounds in cocoa fermentation and the resulting sensory attributes of chocolate. J. Food Sci. Technol. 2016, 53, 1101–1110. [Google Scholar] [CrossRef]
- Ho, V.T.T.; Zhao, J.; Fleet, G. Yeasts are essential for cocoa bean fermentation. Int. J. Food Microbiol. 2014, 174, 72–87. [Google Scholar] [CrossRef] [PubMed]
- Moreira, I.M.V.; Vilela, L.F.; Santos, C.; Lima, N.; Schwan, R.F. Volatile compounds and protein profiles analyses of fermented cocoa beans and chocolates from different hybrids cultivated in Brazil. Food Res. Int. 2018, 109, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Visintin, S.; Ramos, L.; Batista, N.; Dolci, P.; Schwan, F.; Cocolin, L. Impact of Saccharomyces cerevisiae and Torulaspora delbrueckii starter cultures on cocoa beans fermentation. Int. J. Food Microbiol. 2017, 257, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Moreira, I.M.V.; Miguel, M.G.C.P.; Duarte, W.F.; Dias, D.R.; Schwan, R.F. Microbial succession and the dynamics of metabolites and sugars during the fermentation of three different cocoa (Theobroma cacao L.) hybrids. Food Res. Int. 2013, 54, 9–17. [Google Scholar] [CrossRef]
- Koné, M.K.; Guéhi, S.T.; Durand, N.; Ban-Koffi, L.; Berthiot, L.; Tachon, A.F.; Brou, K.; Boulanger, R.; Montet, D. Contribution of predominant yeasts to the occurrence of aroma compounds during cocoa bean fermentation. Food Res. Int. 2016, 89, 910–917. [Google Scholar] [CrossRef]
- Moreira, I.M.V.; Vilela, L.F.; Miguel, M.G.C.P.; Santos, C.; Lima, N.; Schwan, R.F. Impact of a Microbial Cocktail Used as a Starter Culture on Cocoa Fermentation and Chocolate Flavor. Molecules 2017, 22, 766. [Google Scholar] [CrossRef]
- Pereira, G.V.M.; Alvarez, J.P.; Carvalho Neto, D.P.; Soccol, V.T.; Tanobe, V.O.A.; Rogez, H.; Góes-Neto, A.; Soccol, C.R. Great intraspecies diversity of Pichia kudriavzevii in cocoa fermentation highlights the importance of yeast strain selection for flavor modulation of cocoa beans. LWT Food Sci. Technol. 2017, 84, 290–297. [Google Scholar] [CrossRef]
- Chagas Junior, G.C.A.; Ferreira, N.R.; Gloria, M.B.A.; Martins, L.H.S.; Lopes, A.S. Chemical implications and time reduction of on-farm cocoa fermentation by Saccharomyces cerevisiae and Pichia kudriavzevii. Food Chem. in press. [CrossRef]
- Sandhya, M.V.S.; Yallappa, B.S.; Varadaraj, M.C.; Puranaik, J.; Rao, L.J.; Janardhan, P.; Murthy, P.S. Inoculum of the starter consortia and interactive metabolic process in enhancing quality of cocoa bean (Theobroma cacao) fermentation. LWT Food Sci. Technol. 2016, 65, 731–738. [Google Scholar] [CrossRef]
- Ooi, T.S.; Ting, A.S.Y.; Siow, L.F. Influence of selected native yeast starter cultures on the antioxidant activities, fermentation index and total soluble solids of Malaysia cocoa beans: A simulation study. LWT Food Sci. Technol. 2020, 122, 108977. [Google Scholar] [CrossRef]
- Ferreira, A.C.R. Passo a passo na produção de cacau de qualidade. In: Indicação de procedência Sul da Bahia—Beneficiamento de Cacau de Qualidade Supeior. In Beneficiamento de Cacau de Qualidade Superior; Ferreira, A.C.R., Ed.; PCTSB: Ilhéus, Brazil, 2017; pp. 26–67. ISBN 978-85-93727-00-0. [Google Scholar]
- Zzaman, W.; Bhat, R.; Yang, T.A. Effect of superheated steam roasting on the phenolic antioxidant properties of cocoa beans. J. Food Process. Preserv. 2014, 38, 1932–1938. [Google Scholar] [CrossRef]
- Agus, B.A.P.; Mohamad, N.N.; Hussain, N. Composition of unfermented, unroasted, roasted cocoa beans and cocoa shells from Peninsular Malaysia. J. Food Meas. Charact. 2018, 12, 2581–2589. [Google Scholar] [CrossRef]
- Utrilla-Vázquez, M.; Rodríguez-Campos, J.; Avendaño-Arazate, C.H.; Gschaedler, A.; Lugo-Cervantes, E. Analysis of volatile compounds of five varieties of Maya cocoa during fermentation and drying processes by Venn diagram and PCA. Food Res. Int. 2020, 129, 108834. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Campos, J.; Escalona-Buendía, H.B.; Contreras-Ramos, S.M.; Orozco-Avila, I.; Jaramillo-Flores, E.; Lugo-Cervantes, E. Effect of fermentation time and drying temperature on volatile compounds in cocoa. Food Chem. 2012, 132, 277–288. [Google Scholar] [CrossRef]
- Barišić, V.; Kopjar, M.; Jozinović, A.; Flanjak, I.; Ačkar, Đ.; Miličević, B.; Šubarić, D.; Jokić, S.; Babić, J. The chemistry behind chocolate production. Molecules 2019, 24, 3163. [Google Scholar] [CrossRef]
- Rodriguez-Campos, J.; Escalona-Buendía, H.B.; Orozco-Avila, I.; Lugo-Cervantes, E.; Jaramillo-Flores, M.E. Dynamics of volatile and non-volatile compounds in cocoa (Theobroma cacao L.) during fermentation and drying processes using principal components analysis. Food Res. Int. 2011, 44, 250–258. [Google Scholar] [CrossRef]
- Aprotosoaie, A.C.; Luca, S.V.; Miron, A. Flavor Chemistry of Cocoa and Cocoa Products-An Overview. Compr. Rev. Food Sci. Food Saf. 2016, 15, 73–91. [Google Scholar] [CrossRef]
- Bastos, V.S.; Uekane, T.M.; Bello, N.A.; de Rezende, C.M.; Flosi Paschoalin, V.M.; Del Aguila, E.M. Dynamics of volatile compounds in TSH 565 cocoa clone fermentation and their role on chocolate flavor in Southeast Brazil. J. Food Sci. Technol. 2019, 56, 2874–2887. [Google Scholar] [CrossRef]
- Tuenter, E.; Delbaere, C.; De Winne, A.; Bijttebier, S.; Custers, D.; Foubert, K.; Van Durme, J.; Messens, K.; Dewettinck, K.; Pieters, L. Non-volatile and volatile composition of West African bulk and Ecuadorian fine-flavor cocoa liquor and chocolate. Food Res. Int. 2020, 130, 108943. [Google Scholar] [CrossRef]
- Lima, L.J.R.; Almeida, M.H.; Nout, M.J.R.; Zwietering, M.H. Theobroma cacao L., “The food of the Gods”: Quality determinants of commercial cocoa beans, with particular reference to the impact of fermentation. Crit. Rev. Food Sci. Nutr. 2011, 51, 731–761. [Google Scholar] [CrossRef]
- De Vuyst, L.; Leroy, F. Functional role of yeasts, lactic acid bacteria and acetic acid bacteria in cocoa fermentation processes. FEMS Microbiol. Rev. 2020, 44. [Google Scholar] [CrossRef] [PubMed]
- Chetschik, I.; Kneubühl, M.; Chatelain, K.; Schlüter, A.; Bernath, K.; Hühn, T. Investigations on the Aroma of Cocoa Pulp (Theobroma cacao L.) and Its Influence on the Odor of Fermented Cocoa Beans. J. Agric. Food Chem. 2018, 66, 2467–2472. [Google Scholar] [CrossRef]
- Castro-Alayo, E.M.; Idrogo-Vásquez, G.; Siche, R.; Cardenas-Toro, F.P. Formation of aromatic compounds precursors during fermentation of Criollo and Forastero cocoa. Heliyon 2019, 5. [Google Scholar] [CrossRef]
- Agyirifo, D.S.; Wamalwa, M.; Otwe, E.P.; Galyuon, I.; Runo, S.; Takrama, J.; Ngeranwa, J. Metagenomics analysis of cocoa bean fermentation microbiome identifying species diversity and putative functional capabilities. Heliyon 2019, 5, e02170. [Google Scholar] [CrossRef]
- Hamdouche, Y.; Meile, J.C.; Lebrun, M.; Guehi, T.; Boulanger, R.; Teyssier, C.; Montet, D. Impact of turning, pod storage and fermentation time on microbial ecology and volatile composition of cocoa beans. Food Res. Int. 2019, 119, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Assi-Clair, B.J.; Koné, M.K.; Kouamé, K.; Lahon, M.C.; Berthiot, L.; Durand, N.; Lebrun, M.; Julien-Ortiz, A.; Maraval, I.; Boulanger, R.; et al. Effect of aroma potential of Saccharomyces cerevisiae fermentation on the volatile profile of raw cocoa and sensory attributes of chocolate produced thereof. Eur. Food Res. Technol. 2019, 245, 1459–1471. [Google Scholar] [CrossRef]
- Santos, D.S.; Rezende, R.P.; Santos, T.F.; Marques, E.L.S.; Ferreira, A.C.R.; Silva, A.B.C.; Romano, C.C.; Santos, D.W.C.; Dias, J.C.T.; Tavares Bisneto, J.D. Fermentation in fine cocoa type Scavina: Change in standard quality as the effect of use of starters yeast in fermentation. Food Chem. 2020, 328, 7–12. [Google Scholar] [CrossRef]
- Tofalo, R.; Fusco, V.; Böhnlein, C.; Kabisch, J.; Logrieco, A.F.; Habermann, D.; Cho, G.S.; Benomar, N.; Abriouel, H.; Schmidt-Heydt, M.; et al. The life and times of yeasts in traditional food fermentations. Crit. Rev. Food Sci. Nutr. 2019, 0, 1–30. [Google Scholar] [CrossRef]
- Koné, K.M.; Assi-Clair, B.J.; Kouassi, A.D.D.; Koffi, A.Y.; Ban-Koffi, L.; Durand, N.; Lebrun, M.; Maraval, I.; Bonlanger, R.; Guehi, T.S. Pod storage time and spontaneous fermentation treatments and their impact on the generation of cocoa flavor precursor compounds. Int. J. Food Sci. Technol. 2020, 1–14. [Google Scholar] [CrossRef]
- Efraim, P.; Pezoa-García, N.H.; Jardim, D.C.P.; Nishikawa, A.; Haddad, R.; Eberlin, M.N. Influência da fermentação e secagem de amêndoas de cacau no teor de compostos fenólicos e na aceitação sensorial. Ciência Tecnol. Aliment. 2010, 30, 142–150. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy, 4th ed.; Allured Pub Corp: Carol Stream, IL, USA, 2007; ISBN 9781932633214. [Google Scholar]
- Mondello, L. Mass Spectra of Flavors and Fragrances of Natural and Synthetic Compounds; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar]
- Stein, S.; Mirokhin, D.; Tchekhovskoi, D.; Mallard, G.; Mikaia, A.; Zaikin, V.; Sparkmanm, D. NIST 2008 User Guide. In The NIST Mass Spectral Search Program for the NIST/EPA/NIH Mass Spectra Library. Standard Reference Data Program of the National Institute of Standards and Technology; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2011. [Google Scholar]
Sample Availability: Samples of the compounds fermented and dried cocoa beans are available from the authors. |
| CT | ST | PT | SPT | |
|---|---|---|---|---|
| Moisture (%) | 5.70 ± 0.00 a | 5.65 ± 0.07 ab | 5.45 ± 0.07 b | 5.55 ± 0.07 ab |
| RI | Compound | CT (%) | ST (%) | PT (%) | SPT (%) | Odour Description 2 |
|---|---|---|---|---|---|---|
| Acids | ||||||
| 841 | Isovaleric acid | - | 10.77 | - | - | Sweat, rancid (off-flavour) |
| 1173 | Octanoic acid | - | - | 0.38 | - | Sweat, fatty (off-flavour) |
| 1256 | Acetic acid | - | 2.09 | - | - | Sour, vinegar (off-flavour) |
| 1964 | Palmitic acid | - | - | 0.33 | - | |
| 2169 | Oleic acid | - | - | 1.25 | - | |
| Alcohols | ||||||
| 894 | 2-Heptanol | - | - | 0.32 | - | Fruity, citrus, herbal |
| 1099 | 2-Nonanol | - | - | 3.98 | 2.91 | Fat, green |
| 1111 | 2-Phenylethanol | 0.93 | 0.15 | 0.35 | - | Honey, rummy, floral |
| Aldehydes | ||||||
| 955 | Benzaldehyde | 6.99 | 4.68 | 10.19 | 3.30 | Roasted almonds, candy, burnt sugar |
| 1040 | Phenylacetaldehyde | 36.50 | 21.79 | 30.78 | 26.87 | Floral, honey |
| 1271 | 2-Phenylbut-2-enal | 0.45 | 0.26 | 0.56 | 0.70 | Floral, honey, powdery, cocoa |
| Dodecanal | - | - | - | 0.39 | ||
| 1490 | 5-Methyl-2-phenyl-2-hexenal | 1.01 | 0.46 | 1.22 | 1.85 | Cocoa |
| Ketones | ||||||
| 1062 | Acetophenone | 1.65 | 0.56 | 1.45 | - | Flower, almond, pungent, sweet |
| 1090 | 2-Nonanone | - | - | 5.25 | - | Fruity, sweet, waxy, green herbaceous |
| 1119 | Isophorone | 0.09 | - | - | - | |
| Esters | ||||||
| 868 | Isopenthyl acetate | 0.94 | - | 1.94 | - | |
| 1163 | Benzyl acetate | - | - | 0.34 | - | Floral, jasmine |
| 1169 | Ethyl benzoate | 0.16 | - | - | - | Camomile, flower, fruity |
| 1196 | Ethyl octanoate | 0.38 | 0.21 | - | - | Fruity, floral |
| 1255 | 2-Phenethyl acetate | 4.36 | 0.53 | 4.21 | 4.23 | Fruity, sweet, roses, honey, floral |
| 1394 | Isoamyl benzoate | 5.09 | 4.00 | 4.51 | 8.95 | Balsam, sweet |
| 1594 | Ethyl dodecanoate | 0.06 | - | 0.15 | - | Sweet, floral |
| 1994 | Ethyl hexadecanoate | 0.10 | - | 1.15 | 0.21 | |
| Pyrazines | ||||||
| 1000 | 2,3,5-Trimethylpyrazine | 0.59 | - | - | - | Roasted cocoa |
| 1084 | Tetramethylpyrazine | 24.76 | 46.77 | 19.71 | 41.20 | Roasted cocoa, chocolate |
| Alkanes | ||||||
| 1055 | 3-Methyldecane | - | - | 1.09 | - | |
| 1200 | n-Dodecane | 1.68 | 0.48 | 1.74 | 1.78 | Off-flavour |
| 1264 | 2-Methyldodecane | 0.78 | - | - | - | |
| 1280 | 4,6-Dimethyldodecane | 1.18 | - | - | - | |
| 1300 | Tridecane | 0.11 | - | 0.16 | - | Off-flavour |
| 1400 | n-Tetradecane | 0.93 | - | 0.93 | 1.51 | Off-flavour |
| 1490 | Pentadecane | 0.12 | 0.25 | 0.30 | 0.42 | Off-flavour |
| 1711 | Heptadodecane | - | - | - | 0.12 | Off-flavour |
| Others | ||||||
| 1099 | Linalool | 2.78 | 2.03 | - | - | Floral |
| 1181 | Naphthalene | 0.23 | - | 1.16 | - | |
| Total (%) | 91.87 | 95.03 | 93.45 | 94.44 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chagas Junior, G.C.A.; Ferreira, N.R.; Andrade, E.H.d.A.; Nascimento, L.D.d.; Siqueira, F.C.d.; Lopes, A.S. Profile of Volatile Compounds of On-Farm Fermented and Dried Cocoa Beans Inoculated with Saccharomyces cerevisiae KY794742 and Pichia kudriavzevii KY794725. Molecules 2021, 26, 344. https://doi.org/10.3390/molecules26020344
Chagas Junior GCA, Ferreira NR, Andrade EHdA, Nascimento LDd, Siqueira FCd, Lopes AS. Profile of Volatile Compounds of On-Farm Fermented and Dried Cocoa Beans Inoculated with Saccharomyces cerevisiae KY794742 and Pichia kudriavzevii KY794725. Molecules. 2021; 26(2):344. https://doi.org/10.3390/molecules26020344
Chicago/Turabian StyleChagas Junior, Gilson Celso Albuquerque, Nelson Rosa Ferreira, Eloisa Helena de Aguiar Andrade, Lidiane Diniz do Nascimento, Francilia Campos de Siqueira, and Alessandra Santos Lopes. 2021. "Profile of Volatile Compounds of On-Farm Fermented and Dried Cocoa Beans Inoculated with Saccharomyces cerevisiae KY794742 and Pichia kudriavzevii KY794725" Molecules 26, no. 2: 344. https://doi.org/10.3390/molecules26020344
APA StyleChagas Junior, G. C. A., Ferreira, N. R., Andrade, E. H. d. A., Nascimento, L. D. d., Siqueira, F. C. d., & Lopes, A. S. (2021). Profile of Volatile Compounds of On-Farm Fermented and Dried Cocoa Beans Inoculated with Saccharomyces cerevisiae KY794742 and Pichia kudriavzevii KY794725. Molecules, 26(2), 344. https://doi.org/10.3390/molecules26020344