Improving the Cellular Selectivity of a Membrane-Disrupting Antimicrobial Agent by Monomer Control and by Taming
Abstract
:1. Antimicrobial Agents under Siege
2. Why Consider Membrane-Disrupting Antimicrobial Agents?
3. The Issue of Toxicity
4. Discovery of a Membrane Rupture and Leakage Dichotomy
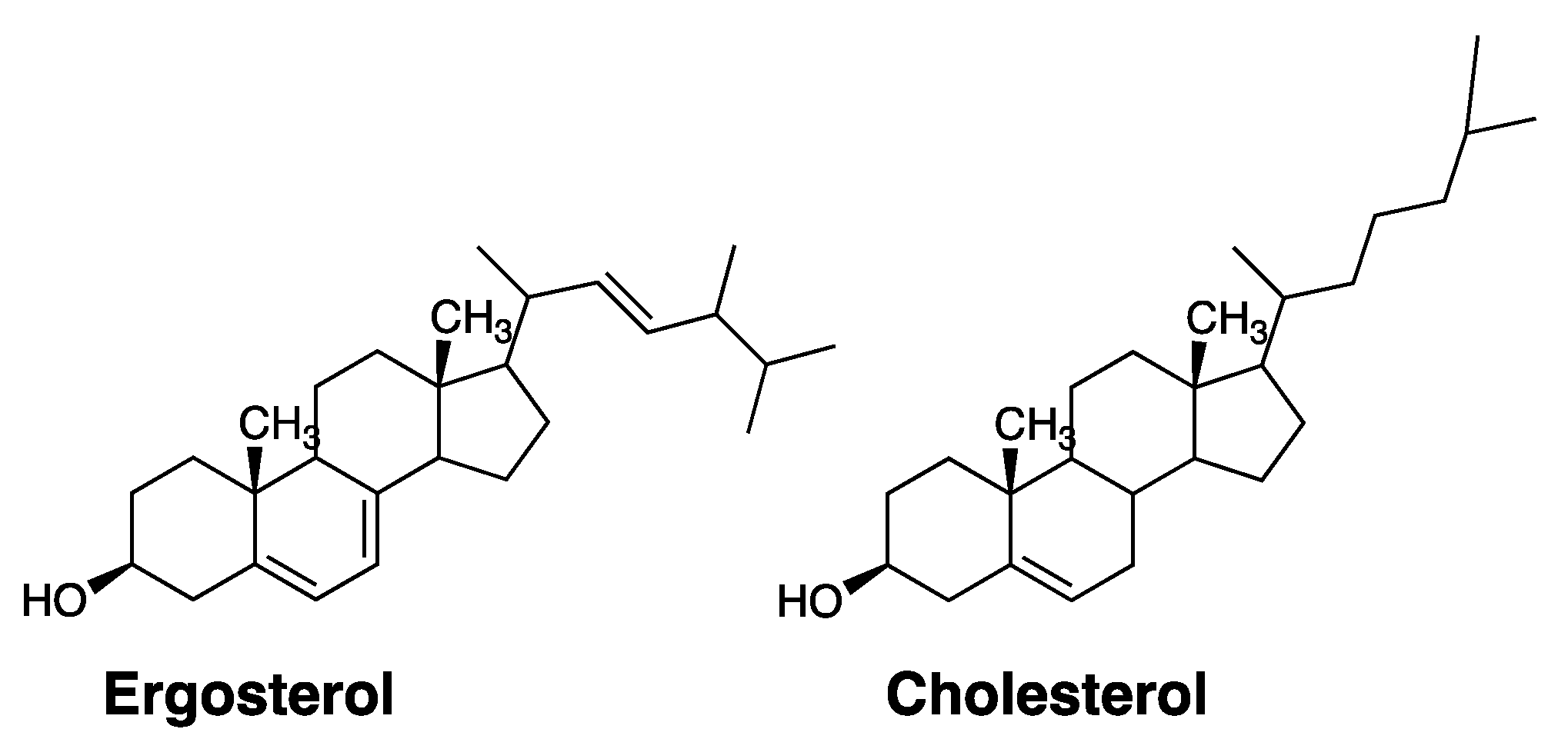
4.1. Separating Antifungal from Hemolytic Activity by Synthetic Design
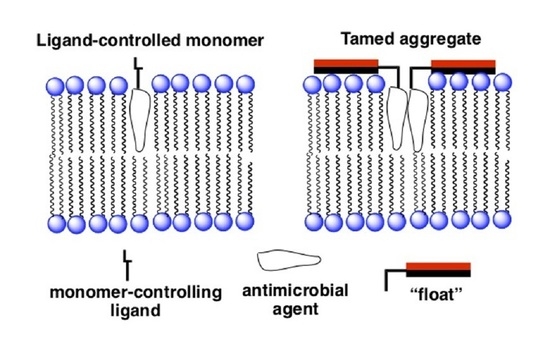
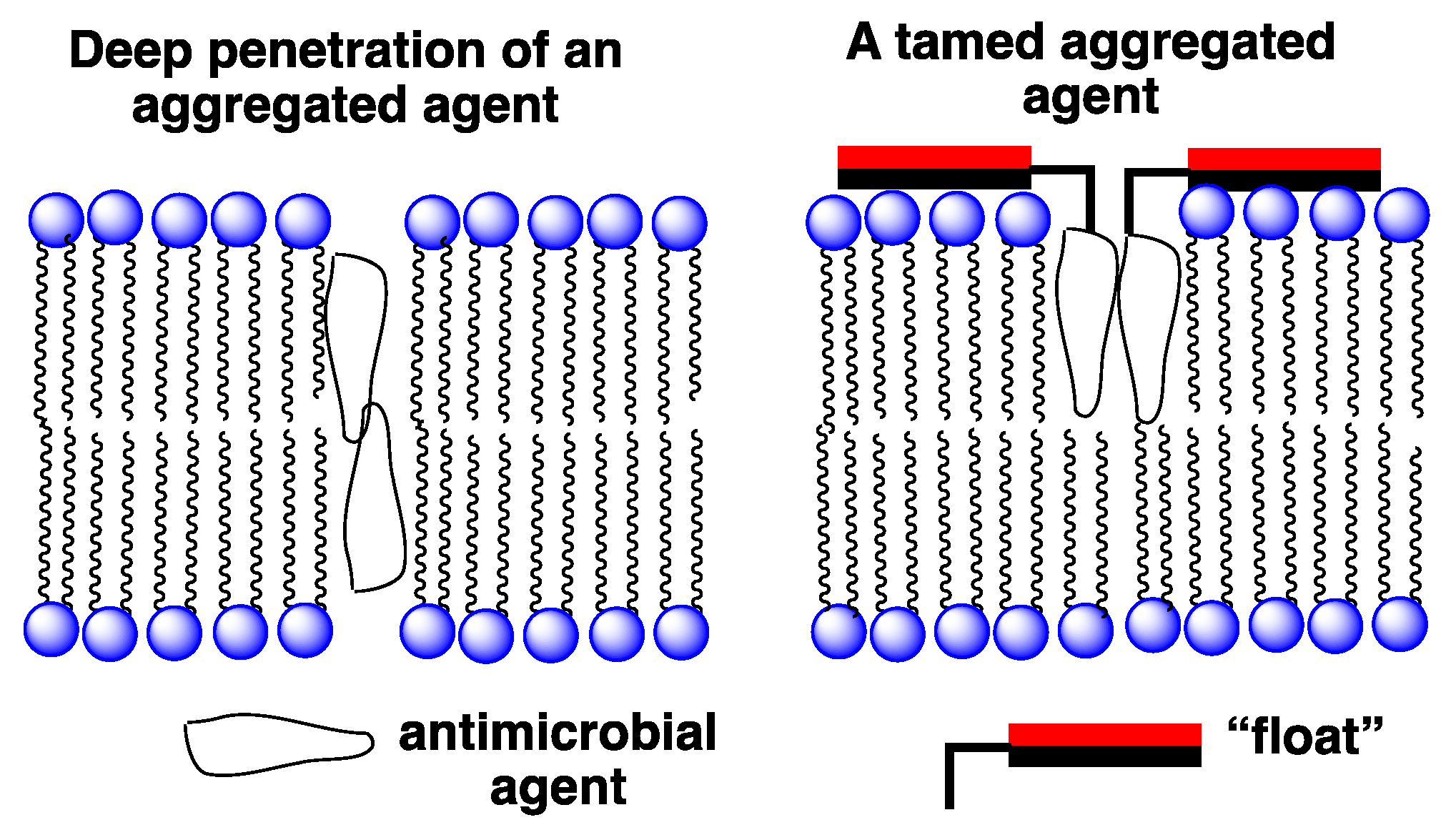
4.2. A Taming Strategy
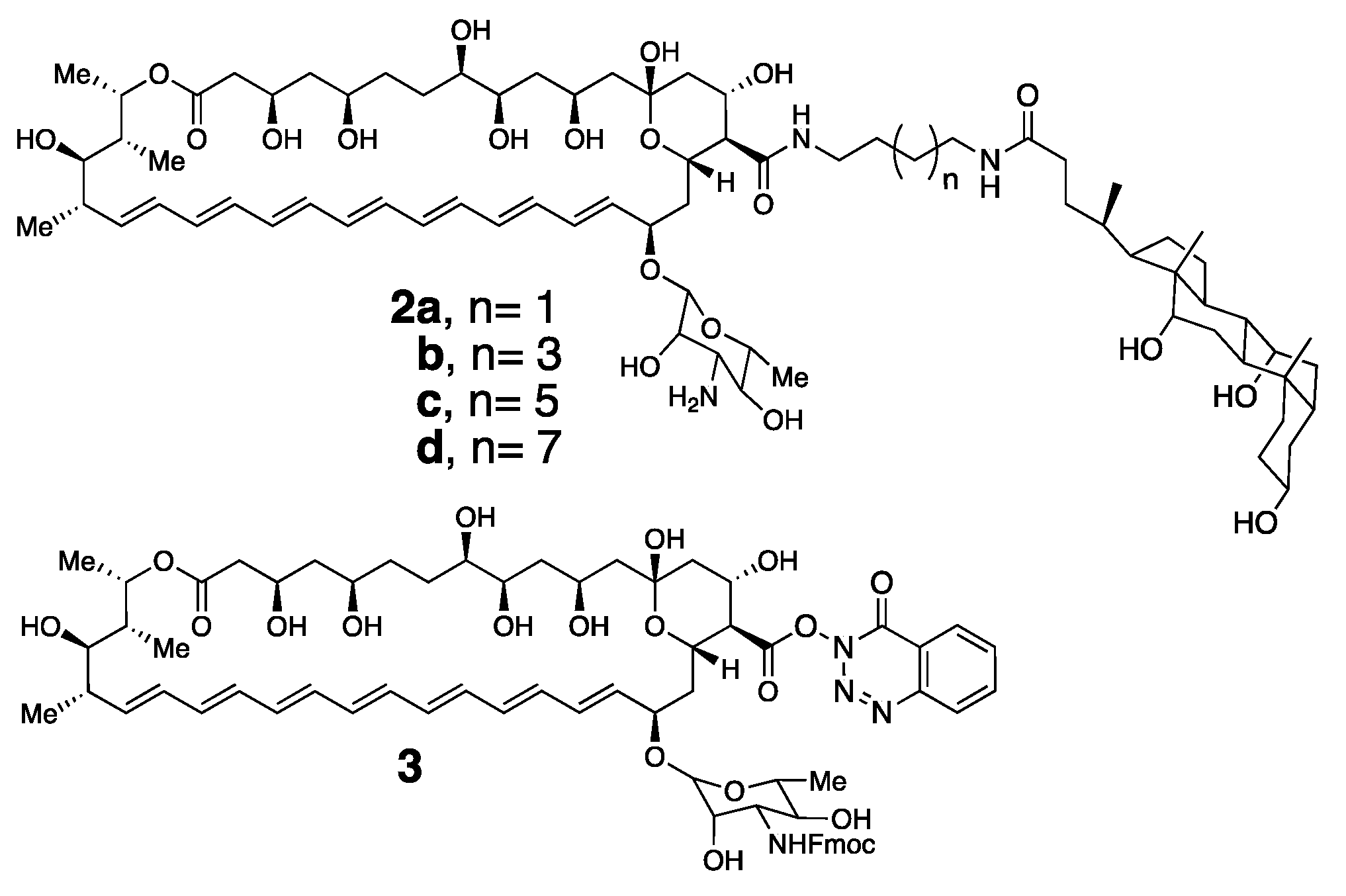
4.3. The Taming of Amphotericin B
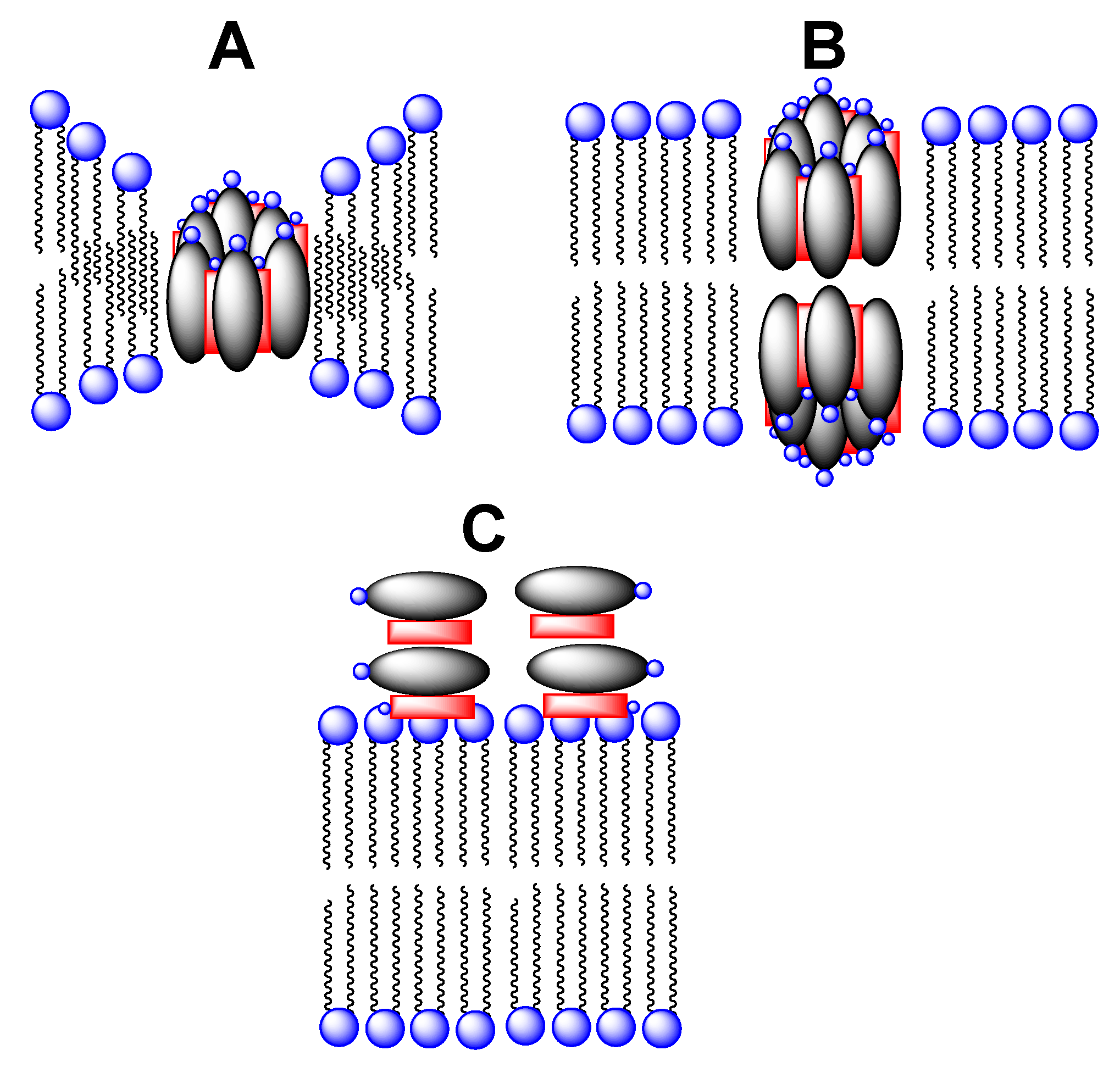
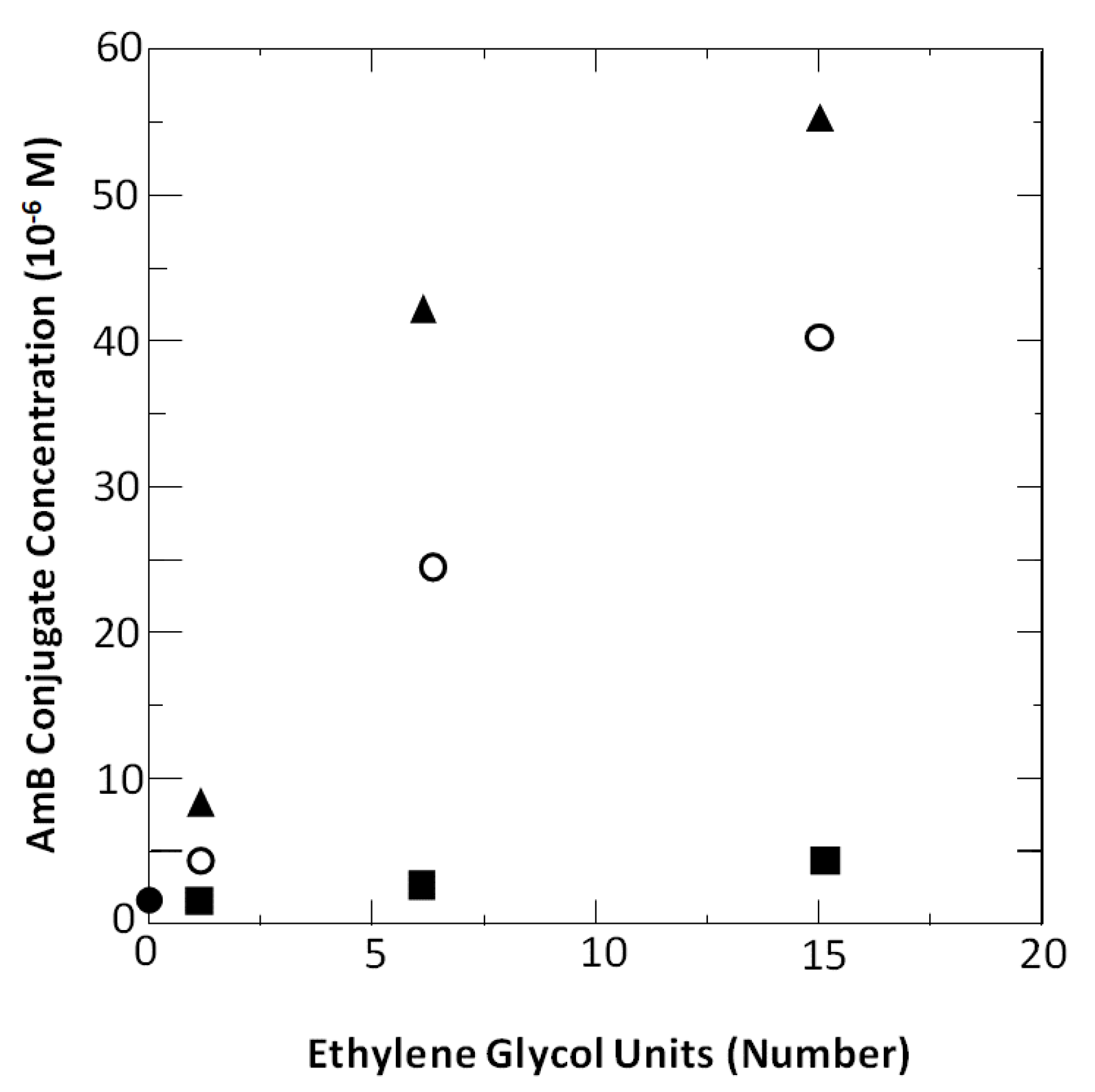
4.4. Aggregation Properties of Amphotericin B and Its Conjugates
4.5. Separation of Antifungal from Hemolytic Activity via Taming
4.6. Other Membrane-Disrupting Antimicrobial Agents Worthy of Consideration
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Antimicrobial Threats Report by the Center of Disease Control and Prevention (CDC). 2019. Available online: https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf (accessed on 20 December 2020).
- Chauhan, D.S.; Prasad, R.; Srivastava, R.; Jaggi, M.; Chauhan, S.C.; Yallapu, M.M. Comprehensive review on current interventions, diagnostics and nanotechnology perspectives against SARS-CoV-2. Bioconj. Chem. 2020, 31, 2021–2045. [Google Scholar] [CrossRef] [PubMed]
- Prajapat, M.; Sarma, P.; Shekhar, N.; Avti, P.; Sinha, S.; Kaur, H.; Kumar, S.; Bhattacharyya, A.; Kumar, H.; Bansal, S.; et al. Drug targets for coronaviruses: A systematic review. Indian J. Pharmacol. 2020, 52, 56–65. [Google Scholar] [PubMed]
- Baglivo, M.; Baronio, M.; Natalini, G.; Beccari, T.; Chiurazzi, P.; Fulcheri, E.; Petralia, P.; Michelini, S.; Fiorentini, G.; Miggiano, G.A.; et al. Natural small molecules as inhibitors of coronavirus lipid-dependent attachment to host cells: A possible strategy for reducing SARS-CoV-2 infectivity. Acta Biomed. 2020, 91, 161–164. [Google Scholar] [PubMed]
- Blaskovich, M.A.T. Antibiotics special issue: Challenges and opportunities in antibiotic discovery. ACS Infect. Dis. 2020, 6, 1286–1288. [Google Scholar] [CrossRef] [PubMed]
- Beyer, P.; Paulin, S. The antibacterial research and development pipeline needs urgent solutions. ACS Infect. Dis. 2020, 6, 1289–1291. [Google Scholar] [CrossRef]
- Kim, W.; Prosen, K.R.; Lepore, C.J.; Coukell, A. On the road to discovering urgently needed antibiotics: So close yet so far away. ACS Infect. Dis. 2020, 6, 1192–1294. [Google Scholar] [CrossRef] [PubMed]
- Shlaes, D.M. Antibacterial drugs: The last frontier. ACS Infect. Dis. 2020, 6, 1292–1294. [Google Scholar] [CrossRef]
- Alm, R.A.; Gallant, K. Innovation in antimicrobial resistance: The CARB-X perspective. ACS Infect. Dis. 2020, 6, 1317–1322. [Google Scholar] [CrossRef]
- Gray, D.A.; Wenzel, M. Multitarget approaches against multiresistant superbugs. ACS Infect. Dis. 2020, 6, 1346–1365. [Google Scholar] [CrossRef] [Green Version]
- Shlaes, D.M. The economic conundrum for antibacterial drugs. Anitimicrob. Agents Chemother. 2020, 64, e02057-19. [Google Scholar] [CrossRef]
- Steinbuch, K.B.; Fridman, M. Mechanisms of resistance to membrane-disrupting antibiotics in Gram-positive and Gram-negative bacteria. Med. Chem. Commun. 2016, 7, 86–102. [Google Scholar] [CrossRef]
- Perfect, J.R.; Dismukes, W.E.; Dromer, F.; Goldman, D.L.; Graybill, J.R.; Hamill, R.J.; Harrison, T.S.; Larsen, R.A.; Lortholary, O.; Nguyen, M.-H.; et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of america. Clin. Infect. Dis. 2010, 50, 291–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesa-Arango, A.C.; Rueda, C.; Roman, E.; Quintin, J.; Terron, M.C.; Luque, D.; Netea, M.G.; Pla, J.; Zaragoza, O. Cell wall changes in amphotericin b-resistant strains from candida tropicalis and relationship with the immune responses elicited by the host. Anitimicrob. Agents Chemother. 2016, 60, 2326–2335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, T.; Umegawa, Y.; Yamagami, M.; Suzuki, T.; Tsuchikawa, H.; Hanashima, S.; Matsumori, N.; Murata, M. The perpendicular orientation of amphotericin b methyl ester in hydrated lipid bilayers supports the barrel-stave model. Biochemistry 2019, 58, 2282–2291. [Google Scholar] [CrossRef] [PubMed]
- Van Hoogevest, P.; De Kruijff, B. Effect of amphotericin b on cholesterol-containing liposomes of egg phosphatidylcholine and didocosenoyl phosphatidylcholine: A refinement of the model for the formation of pores by amphotericin b in membranes. Biochim. Biophys. Acta 1978, 511, 397–407. [Google Scholar] [CrossRef]
- Kaminski, D.M. Recent progress in the study of the interactions of amphotericin B with cholesterol and ergosterol in lipid environments. Eur. Biophys. J. 2014, 43, 453–467. [Google Scholar] [CrossRef] [Green Version]
- Tevyashova, A.S.; Olsufyeva, E.N.; Solovieva, S.E.; Printsevskaya, S.S.; Reznikova, M.I.; Trenin, A.S.; Galatenko, O.A.; Treshalin, I.D.; Pereverzeva, E.R.; Mirchink, E.P.; et al. Structure-antifungal activity relationships of polyene antibiotics of the amphotericin b group. Antimicrob. Agents Chemother. 2013, 57, 3815–3822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volmer, A.A.; Szpilman, A.M.; Carreira, E.M. Synthesis and biological evaluation of amphotericin B derivatives. Nat. Prod. Rep. 2010, 27, 1329–1349. [Google Scholar] [CrossRef]
- Falci, D.R.; da Rosa, F.B.; Pasqualotto, A.C. Comparison of nephrotoxicity associated to different lipid formulations of amphotericin b: A real-life study. Mycoses 2015, 58, 104–112. [Google Scholar] [CrossRef]
- Davis, S.A.; Vincent, B.M.; Endo, M.M.; Whitesell, L.; Marchillo, K.; Andes, D.R.; Lindquist, S.; Burke, M.D. Nontoxic antimicrobials that evade drug resistance. Nat. Chem. Biol. 2015, 11, 481–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilcock, B.C.; Endo, M.M.; Uno, B.E.; Burke, M.D. C2’OH of Amphotericin B plays an important role in binding the primary sterol of human cells but not yeast cells. J. Am. Chem. Soc. 2013, 135, 8488–8491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, T.M.; Clay, M.C.; Cioffi, A.G.; Diaz, K.A.; Hisao, G.S.; Tuttle, M.D.; Nieuwkoop, A.J.; Comellas, G.; Maryum, N.; Wang, S.; et al. Amphotericin forms an extramembranous and fungicidal sterol sponge. Nat. Chem. Biol. 2014, 10, 400–406. [Google Scholar] [CrossRef]
- Legrand, P.; Romero, E.A.; Cohen, E.; Bolard, J. Effects of aggregation and solvent on the toxicity of amphotericin b to human erythrocytes. Anitimicrob. Agents Chemother. 1992, 36, 2518–2522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moen, M.D.; Lyseng-Williamson, K.A.; Scott, L.J. Liposomal amphotericin B: A review of its use as empirical therapy in febrile neutropenia and in the treatment of invasive fungal infections. Drugs 2009, 69, 361–392. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Regen, S.L. Control over vesicle rupture and leakage by membrane packing and by the aggregation state of an attacking surfactant. J. Am. Chem. Soc. 1993, 115, 708–713. [Google Scholar] [CrossRef]
- Yamashita, K.; Janout, V.; Bernard, E.M.; Armstrong, D.; Regen, S.L. Micelle/monomer control over the membrane-disrupting properties of an amphiphilic antibiotic. J. Am. Chem. Soc. 1995, 117, 6249–6253. [Google Scholar] [CrossRef]
- Janout, V.; Schell, W.A.; Thevenin, D.; Yu, Y.; Perfect, J.R.; Regen, S.L. Taming amphotericin b. Bioconjugate Chem. 2015, 26, 2021–2024. [Google Scholar] [CrossRef]
- Yu, Y.; Sabulski, M.J.; Schell, W.A.; Pires, M.M.; Perfect, J.R.; Regen, S.L. A simple strategy for taming membrane-disrupting antibiotics. Bioconjugate Chem. 2016, 27, 2850–2853. [Google Scholar] [CrossRef]
- Fahey, D.A.; Carey, M.C.; Donovan, J.M. Bile acid/phosphatidylcholine interactions in mixed monomolecular layers: Differences in condensation effects but not interfacial orientation between hydrophobic and hydrophilic bile acid species. Biochemistry 1995, 34, 10886–10897. [Google Scholar] [CrossRef]
- Kondo, M.; Mehiri, M.; Regen, S.L. Viewing membrane-bound molecular umbrellas by parallax analyses. J. Am. Chem. Soc. 2008, 130, 13771–13777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zharkova, M.S.; Orlov, D.S.; Golubeva, O.Y.; Chakchir, O.B.; Eliseev, I.E.; Grinchuk, T.M.; Shamova, O.V. Application of antimicrobial peptides of the innate immune system in combination with conventional antibiotics: A novel way to combat antibiotic resistance. Front. Cell. Infect. Microbiol. 2019, 9, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, J.; Sun, L.; Huang, S.; Huang, S.; Zhu, C.; Li, P.; He, J.; Mackey, V.; Coy, D.H.; He, Q. The antimicrobial peptides and their potential clinical applications. Am. J. Transl. Res. 2019, 11, 3919–3931. [Google Scholar] [PubMed]
- Mahlapuu, M.; Hakansson, J.; Ringstad, L.; Bjorn, C. Antimicrobial peptides: An emerging category of therapeutic agents. Front. Cell. Infect. Microbiol. 2016, 6, 194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordon, Y.J.; Romanowski, E.G. A review of antimicrobial peptides and their therapeutic potential as anti-infective drugs. Curr. Eye. Res. 2005, 30, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Guidotti, G.; Brambilla, L.; Rossi, D. Cell-penetrating peptides: From basic research to clinics. Trends Pharmacol. Sci. 2017, 38, 406–424. [Google Scholar] [CrossRef]
- Xie, J.; Bi, Y.; Zhang, H.; Dong, S.; Teng, L.; Lee, R.J.; Yang, Z. Cell-penetrating peptides in diagnosis and treatment of human diseases: From preclinical research to clinical application. Front. Pharmacol. 2020, 11, 697. [Google Scholar] [CrossRef]
- Henriques, S.T.; Melo, M.N.; Castanho, M.A.R.B. Cell-penetrating peptides and antimicrobial peptides: How different are they? Biochem. J. 2006, 399, 1–7. [Google Scholar] [CrossRef]
- Palm, C.; Netzereab, S.; Hallbrink, M. Quantitatively determined uptake of cell-penetrating peptides in non-mammalian cells with an evaluation of degradation and antimicrobial effects. Peptides 2006, 27, 1710–1716. [Google Scholar] [CrossRef]
- Jennings, M.C.; Minbiole, K.P.C.; Wuest, W.M. Quaternary ammonium compounds: An antimicrobial mainstay and platform for innovation to address bacterial resistance. ACS Infect. Dis. 2015, 1, 288–303. [Google Scholar] [CrossRef]
- Joondan, N.; Caumul, P.; Akerman, M.; Jhaumeer-Laulloo, S. Synthesis, micellization and interaction of novel quaternary ammonium compounds derived from L-phenylalanine with 1,2-dipalmitoyl-sn-glycero-3-phosphocholine as model membrane in relation to their antibacterial activity, and their selectivity over human red blood cells. Bioorg. Chem. 2015, 58, 117–129. [Google Scholar]
- Ongwae, G.M.; Morrison, K.R.; Allen, R.A.; Kim, S.; Im, W.; Wuest, W.M.; Pires, M.M. Broadening activity of polylmyxin by quaternary ammonium grafting. ACS Infect. Dis. 2020, 6, 1427–1435. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-C.; Boger, D.L. Maxamycins: Durable antibiotics derived by rational redesign of vancomycin. Acc. Chem. Res. 2020, 53, 2587–2599. [Google Scholar] [CrossRef] [PubMed]




| Microbe a | AmB | 2a | 2b | 2c | 2d | 4 |
|---|---|---|---|---|---|---|
| C. albicans | 0.5 | 1 | 2 | 2 | >16 | 1 |
| C. glabrata | 0.5 | 2 | 2 | >16 | >16 | 2 |
| C. neoformans | 0.3 | 1 | 1 | 1 | 1 | 1 |
| C. gatti | 0.3 | 1 | 1 | 1 | 1 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Regen, S.L. Improving the Cellular Selectivity of a Membrane-Disrupting Antimicrobial Agent by Monomer Control and by Taming. Molecules 2021, 26, 374. https://doi.org/10.3390/molecules26020374
Regen SL. Improving the Cellular Selectivity of a Membrane-Disrupting Antimicrobial Agent by Monomer Control and by Taming. Molecules. 2021; 26(2):374. https://doi.org/10.3390/molecules26020374
Chicago/Turabian StyleRegen, Steven L. 2021. "Improving the Cellular Selectivity of a Membrane-Disrupting Antimicrobial Agent by Monomer Control and by Taming" Molecules 26, no. 2: 374. https://doi.org/10.3390/molecules26020374
APA StyleRegen, S. L. (2021). Improving the Cellular Selectivity of a Membrane-Disrupting Antimicrobial Agent by Monomer Control and by Taming. Molecules, 26(2), 374. https://doi.org/10.3390/molecules26020374