Synthesis, Stability, and Antidiabetic Activity Evaluation of (?)-Epigallocatechin Gallate (EGCG) Palmitate Derived from Natural Tea Polyphenols
Abstract
:1. Introduction
2. Results and Discussion
2.1. Screen of Different Reaction Conditions
2.1.1. Effect of the Molar Ratio of Palmitoyl Chloride
2.1.2. Effect of Base
2.1.3. Effect of Solvent
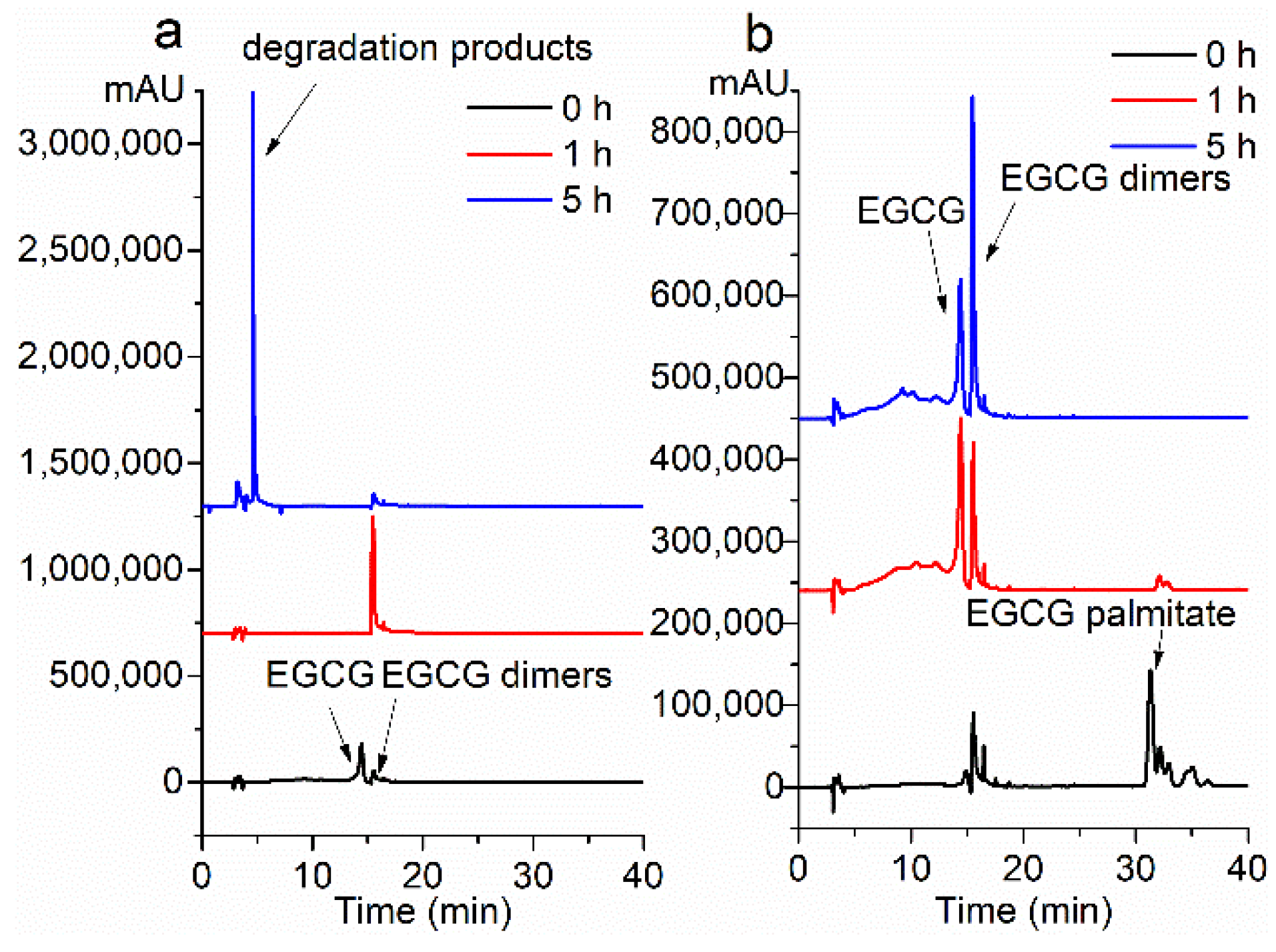
2.2. Stability under the Alkalescent Condition
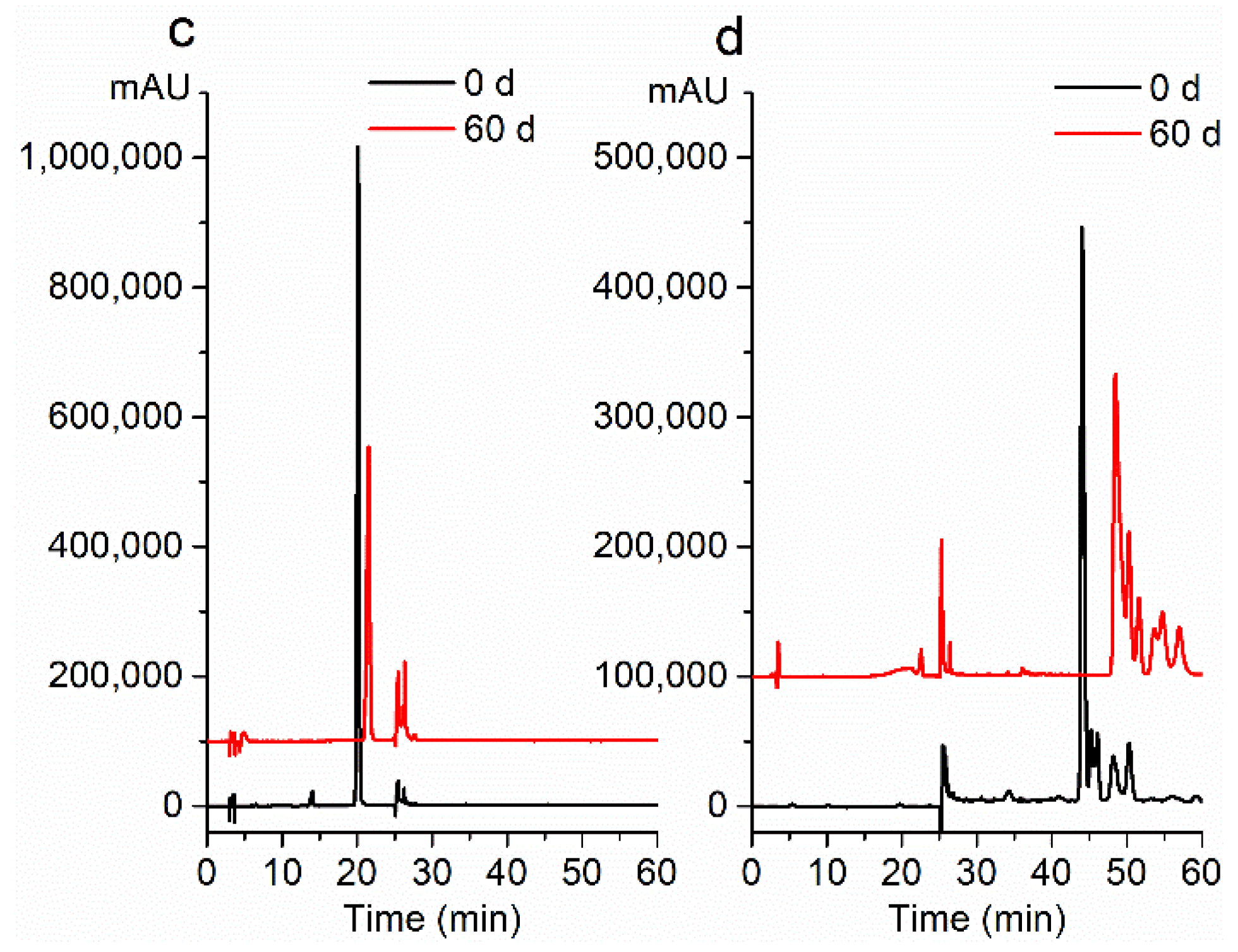
2.3. Storage Stability
2.4. Thermal Stability
2.5. Inhibition of α-Amylase
2.6. Inhibition of α-Glucosidase
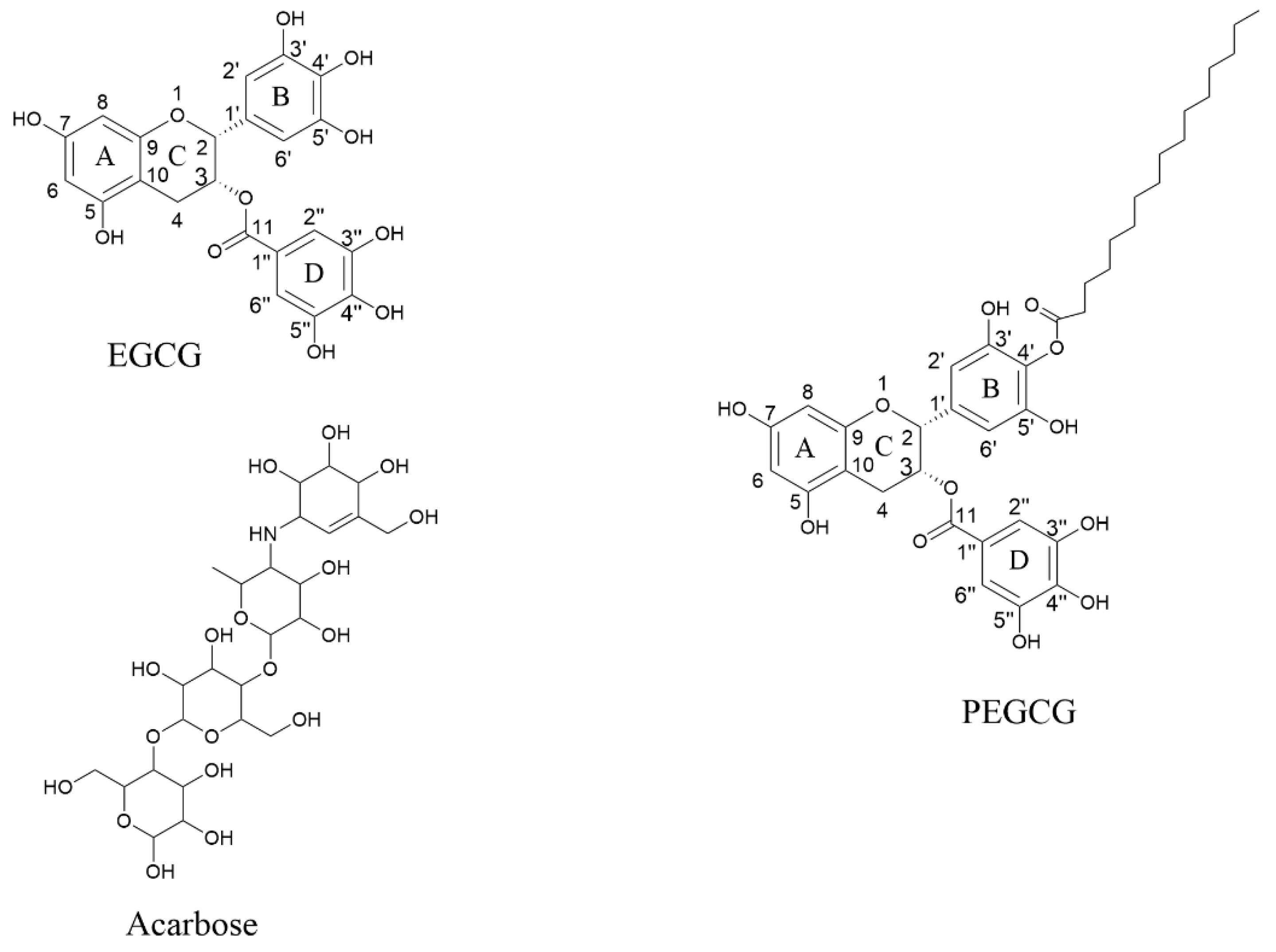
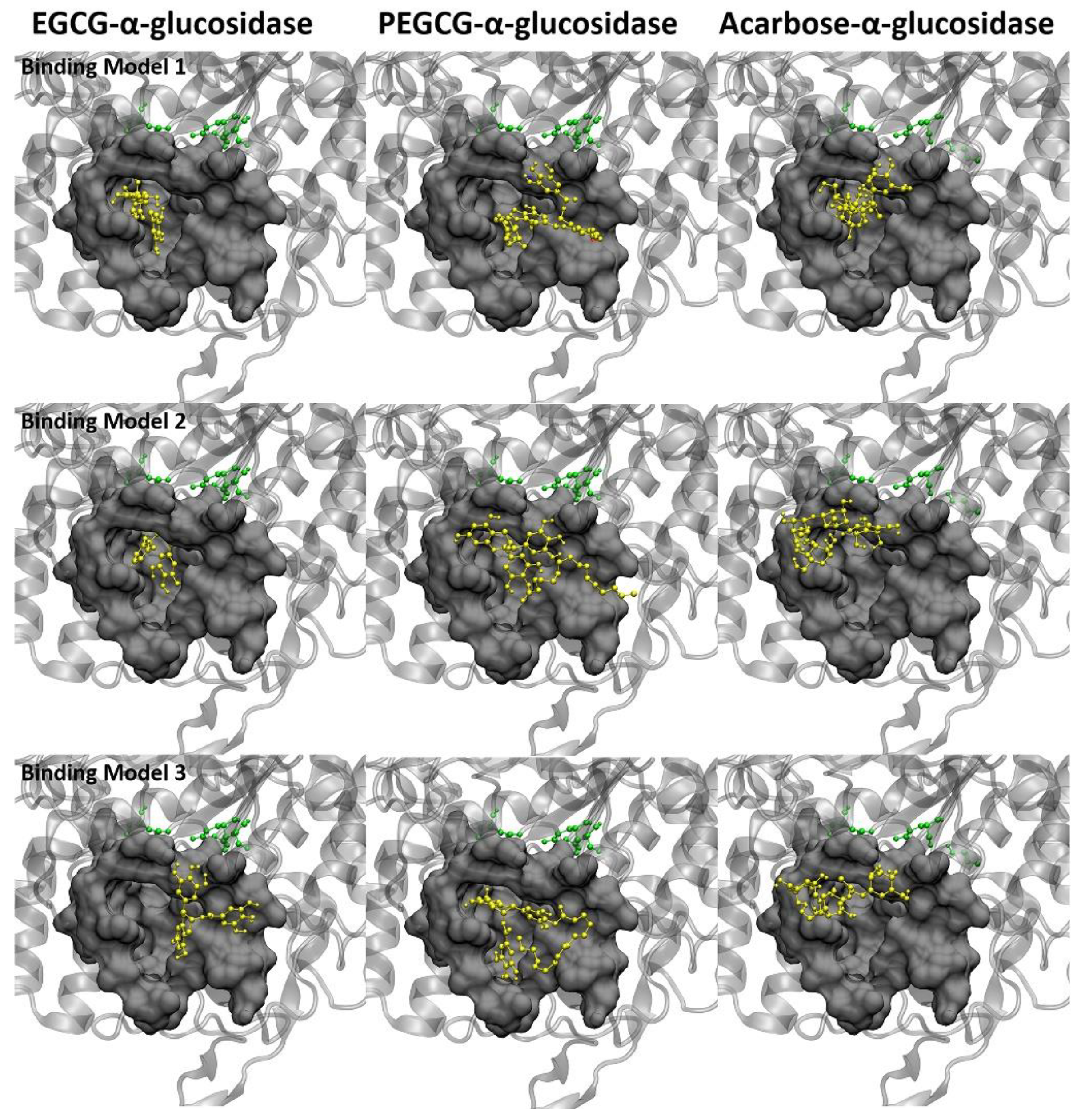
2.7. Molecular Docking of the Ligands with α-Amylase
2.8. Molecular Docking of the Ligands with α-Glucosidase
3. Experimental Section
3.1. Materials
3.2. Synthesis Procedure
3.3. HPLC-MS Analysis
3.4. Purification and Identification of the Acylation Product
3.5. HPLC Analysis of the Acylation Reaction Process
3.6. Evaluation of pH Stability in Alkalescent Condition
3.7. Evaluation of Storage Stability
3.8. Evaluation of Thermal Stability
3.9. α-Amylase Inhibition Assay
3.10. α-Glucosidase Inhibition Assay
3.11. Molecular Docking Simulation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Wang, H.; Helliwell, K.; You, X. Isocratic elution system for the determination of catechins, caffeine and gallic acid in green tea using HPLC. Food Chem. 2000, 68, 115–121. [Google Scholar] [CrossRef]
- Kurahashi, N.; Sasazuki, S.; Iwasaki, M.; Inoue, M.; Shoichiro, T.F.T.J. Green tea consumption and prostate cancer risk in Japanese men: A prospective study. Am. J. Epidemiol. 2008, 167, 71–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuriyama, S.; Shimazu, T.; Ohmori, K.; Et, A. Green tea consumption and mortality due to cardiovascular disease, cancer, and all causes in japan: The ohsaki study. JAMA 2006, 296, 1255–1265. [Google Scholar] [CrossRef] [PubMed]
- Raederstorff, D.G.; Schlachter, M.F.; Elste, V.; Weber, P. Effect of EGCG on lipid absorption and plasma lipid levels in rats. J. Nutr. Biochem. 2003, 14, 326–332. [Google Scholar] [CrossRef]
- Takechi, R.; Alfonso, H.; Hiramatsu, N.; Ishisaka, A.; Tanaka, A.; Tan, L.B.; Lee, A.H. Elevated plasma and urinary concentrations of green tea catechins associated with improved plasma lipid profile in healthy Japanese women. Nutr. Res. 2016, 36, 220–226. [Google Scholar] [CrossRef]
- Amarowicz, R.; Pegg, R.B.; Dykes, G.A.; Troszynska, A.; Shahidi, F. Antioxidant and antibacterial properties of extracts of green tea polyphenols. In Phenolic Compounds in Foods and Natural Health Products; Shahidi, F., Ho, C., Eds.; American Chemical Society: Washington, DC, USA, 2005; Volume 909, pp. 94–106. [Google Scholar]
- Wolfram, S.; Raederstorff, D.; Preller, M.; Wang, Y.; Teixeira, S.R.; Riegger, C.; Weber, P. Epigallocatechin gallate supplementation alleviates diabetes in rodents. J. Nutr. 2006, 136, 2512–2518. [Google Scholar] [CrossRef]
- Waltner-Law, M.E.; Wang, X.L.; Law, B.K.; Hall, R.K.; Nawano, M.; Granner, D.K. Epigallocatechin gallate, a constituent of green tea, represses hepatic glucose production. J. Biol. Chem. 2002, 277, 34933–34940. [Google Scholar] [CrossRef] [Green Version]
- Kamiyama, O.; Sanae, F.; Ikeda, K.; Higashi, Y.; Minami, Y.; Asano, N.; Adachi, I.; Kato, A. In vitro inhibition of α-glucosidases and glycogen phosphorylase by catechin gallates in green tea. Food Chem. 2010, 122, 1061–1066. [Google Scholar] [CrossRef]
- Kale, A.; Gawande, S.; Kotwal, S.; Netke, S.; Roomi, W.; Ivanov, V.; Niedzwiecki, A.; Rath, M. Studies on the effects of oral administration of nutrient mixture, quercetin and red onions on the bioavailability of epigallocatechin gallate from green tea extract. Phytother. Res. 2010, 24, S48–S55. [Google Scholar]
- Sang, S.; Lee, M.; Hou, Z.; Ho, C.; Yang, C.S. Stability of tea polyphenol (−)-epigallocatechin-3-gallate and formation of dimers and epimers under common experimental conditions. J. Agr. Food Chem. 2005, 53, 9478–9484. [Google Scholar] [CrossRef]
- Hong, J.; Lu, H.; Meng, X.; Ryu, J.H.; Hara, Y.; Yang, C.S. Stability, cellular uptake, biotransformation, and efflux of tea polyphenol (-)-epigallocatechin-3-gallate in HT-29 human colon adenocarcinoma cells. Cancer Res. 2002, 62, 7241–7246. [Google Scholar] [PubMed]
- Chow, H.S.; Hakim, I.A.; Vining, D.R.; Crowell, J.A.; Ranger-Moore, J.; Chew, W.M.; Celaya, C.A.; Rodney, S.R.; Hara, Y.; Alberts, D.S. Effects of dosing condition on the oral bioavailability of green tea catechins after single-dose administration of polyphenon E in healthy individuals. Clin. Cancer Res. 2005, 11, 4627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakagawa, K.; Miyazawa, T. Chemiluminescence–high-performance liquid chromatographic determination of tea catechin, (−)-epigallocatechin 3-gallate, at picomole levels in rat and human plasma. Anal. Biochem. 1997, 248, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Shahidi, F. Lipophilized epigallocatechin gallate (EGCG) derivatives as novel antioxidants. J. Agr. Food Chem. 2011, 59, 6526–6533. [Google Scholar] [CrossRef] [PubMed]
- Lam, W.H.; Kazi, A.; Kuhn, D.J.; Chow, L.M.C.; Chan, A.S.C.; Ping Dou, Q.; Chan, T.H. A potential prodrug for a green tea polyphenol proteasome inhibitor: Evaluation of the peracetate ester of (−)-epigallocatechin gallate [(−)-EGCG]. Bioorgan. Med. Chem. 2004, 12, 5587–5593. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Asadzadeh, B.; Yan, W. Solubility determination and modeling of EGCG peracetate in 12 pure solvents at temperatures from 278.15 to 318.15 K. J. Chem. Eng. Data 2019, 64, 5218–5224. [Google Scholar] [CrossRef]
- Oliveira, D.A.; Adams, S.D.; Lee, L.H.; Murray, S.R.; Hsu, S.D.; Hammond, J.R.; Dickinson, D.; Chen, P.; Chu, T. Inhibition of herpes simplex virus type 1 with the modified green tea polyphenol palmitoyl-epigallocatechin gallate. Food Chem. Toxicol. 2013, 52, 207–215. [Google Scholar] [CrossRef] [Green Version]
- Mori, S.; Miyake, S.; Kobe, T.; Nakaya, T.; Fuller, S.D.; Kato, N.; Kaihatsu, K. Enhanced anti-influenza A virus activity of (−)-epigallocatechin-3-O-gallate fatty acid monoester derivatives: Effect of alkyl chain length. Bioorg. Med. Chem. Lett. 2008, 18, 4249–4252. [Google Scholar] [CrossRef]
- Matsumura, K.; Kaihatsu, K.; Mori, S.; Cho, H.H.; Kato, N.; Hyon, S.H. Enhanced antitumor activities of (−)-epigallocatechin-3-O-gallate fatty acid monoester derivatives in vitro and in vivo. Biochem. Bioph. Res. Commun. 2008, 377, 1118–1122. [Google Scholar] [CrossRef]
- Jung, J.H.; Yun, M.; Choo, E.; Kim, S.; Jeong, M.; Jung, D.; Lee, H.; Kim, E.; Kato, N.; Kim, B.; et al. A derivative of epigallocatechin-3-gallate induces apoptosis via SHP-1-mediated suppression of BCR-ABL and STAT3 signalling in chronic myelogenous leukaemia. Br. J. Pharmacol. 2015, 172, 3565–3578. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Kaihatsu, K.; Nishino, K.; Ogawa, M.; Kato, N.; Yamaguchi, A. Antibacterial and antifungal activities of new acylated derivatives of epigallocatechin gallate. Front. Microbiol. 2012, 3, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, S.; Li, Y.; Ma, C.; Lou, Z.; Chen, S.; Dai, J.; Wang, H. Optimization of lipase-catalyzed synthesis of acetylated EGCG by response surface methodology. J. Mol. Catal. B Enzym. 2013, 97, 87–94. [Google Scholar] [CrossRef]
- Chen, P.; Tan, Y.; Sun, D.; Zheng, X. A novel long-chain acyl-derivative of epigallocatechin-3-O-gallate prepared and purified from green tea polyphenols. J. Zhejiang Univ.-Sci. A 2003, 4, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Baj, S.; Chrobok, A.; Gottwald, I. Application of solid–liquid phase transfer catalysis system for peroxyester synthesis: A kinetic study of hydroperoxides acylation in the presence of solid sodium carbonate. Appl. Catal. A Gen. 2002, 224, 89–95. [Google Scholar] [CrossRef]
- Li, T.; Liu, J.; Zhang, X.; Ji, G. Antidiabetic activity of lipophilic (−)-epigallocatechin-3-gallate derivative under its role of α-glucosidase inhibition. Biomed. Pharmacother. 2007, 61, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Tetko, I.V.; Bruneau, P. Application of ALOGPS to predict 1-octanol/water distribution coefficients, logP, and logD, of AstraZeneca in-house database. J. Pharm. Sci.-USA 2004, 93, 3103–3110. [Google Scholar] [CrossRef]
- Krupkova, O.; Ferguson, S.J.; Wuertz-Kozak, K. Stability of (−)-epigallocatechin gallate and its activity in liquid formulations and delivery systems. J. Nutr. Biochem. 2016, 37, 1–12. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, X.; Cheng, J.; Jin, C.; Zhang, Y. The reduction effect of dietary flavone C- and O-glycosides on the formation of acrylamide and its correlation and prediction with the antioxidant activity of Maillard reaction products. RSC Adv. 2014, 4, 24147–24155. [Google Scholar] [CrossRef]
- Fu, Z.; Yoo, M.J.Y.; Zhou, W.; Zhang, L.; Chen, Y.; Lu, J. Effect of (−)-epigallocatechin gallate (EGCG) extracted from green tea in reducing the formation of acrylamide during the bread baking process. Food Chem. 2018, 242, 162–168. [Google Scholar] [CrossRef]
- Etxeberria, U.; de la Garza, A.L.; Campión, J.; Martínez, J.A.; Milagro, F.I. Antidiabetic effects of natural plant extracts via inhibition of carbohydrate hydrolysis enzymes with emphasis on pancreatic alpha amylase. Expert Opin. Ther. 2012, 16, 269–297. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, S.; Shih, K.; Chou, C.; Chu, C. Evaluation of the efficacy and tolerability of miglitol in Chinese patients with type 2 diabetes mellitus inadequately controlled by diet and sulfonylureas. Acta Diabetol. 2011, 48, 71–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Hung, Y.; Qamruddin, K.; Aziz, M.F.A.; Stein, H.; Schmidt, B. International noninterventional study of acarbose treatment in patients with type 2 diabetes mellitus. Diabetes Res. Clin. Pr. 2011, 92, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Bedekar, A.; Shah, K.; Koffas, M. Chapter 2-Natural products for type II diabetes treatment. In Advances in Applied Microbiology; Academic Press: San Diego, CA, USA, 2010; Volume 71, pp. 21–73. [Google Scholar]
- Liu, B.; Yan, W. Lipophilization of EGCG and effects on antioxidant activities. Food Chem. 2019, 272, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Zhao, B.; Shen, S.; Hou, J.; Hu, J.; Xin, W. ESR study on the structure–antioxidant activity relationship of tea catechins and their epimers. Biochim. Biophys. Acta 1999, 1427, 13–23. [Google Scholar] [CrossRef]
- Salah, N.; Miller, N.J.; Paganga, G.; Tijburg, L.; Bolwell, G.P.; Riceevans, C. Polyphenolic flavanols as scavengers of aqueous phase radicals and as chain-breaking antioxidants. Arch. Biochem. Biophys. 1995, 322, 339–346. [Google Scholar] [CrossRef]
- Valcic, S.; Burr, J.A.; Timmermann, B.N.; Liebler, D.C. Antioxidant chemistry of green tea catechins. New oxidation products of (−)-epigallocatechin gallate and (−)-epigallocatechin from their reactions with peroxyl radicals. Chem. Res. Toxicol. 2000, 13, 801–810. [Google Scholar] [CrossRef]
- Nampoothiri, S.V.; Prathapan, A.; Cherian, O.L.; Raghu, K.G.; Venugopalan, V.V.; Sundaresan, A. In vitro antioxidant and inhibitory potential of Terminalia bellerica and Emblica officinalis fruits against LDL oxidation and key enzymes linked to type 2 diabetes. Food Chem. Toxicol. 2011, 49, 125–131. [Google Scholar] [CrossRef]
- Huey, R.; Morris, G.M.; Olson, A.J.; Goodsell, D.S. A semiempirical free energy force field with charge-based desolvation. J. Comput. Chem. 2007, 28, 1145–1152. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [Green Version]
- Machius, M.; Declerck, N.; Huber, R.; Wiegand, G. Activation of Bacillus licheniformis α-amylase through a disorder→order transition of the substrate-binding site mediated by a calcium–sodium–calcium metal triad. Structure 1998, 6, 281–292. [Google Scholar] [CrossRef] [Green Version]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]



| Method | Product | Catalyst | Solvent | Reaction Time | Yield | Ref. |
|---|---|---|---|---|---|---|
| Enzymatic method | Mixture of four EGCG-C16 regioisomers | Lipase PL (Alcaligenes sp.) | N,N-Dimethylformamide | 8 h | 35–39% | [19] |
| Chemical-synthesis | Mixture of four EGCG-C16 regioisomers | Triethylamine | Tetrahydrofuran | 24 h | 12–16% | [19] |
| Chemical-synthesis | EGCG-4′-O-palmitate | Pyridine | Ethyl acetate | 3 h | − | [24] |
| Chemical-synthesis | EGCG-C16 tetraester | Pyridine | Ethyl acetate | − | 57% | [15] |
| This method | EGCG-4′-O-palmitate | Sodium acetate | Acetone | 6 h | 90.6% | − |
| Reagent | Acute Toxicity Test (Mouse) a | Harm to Human Health a |
|---|---|---|
| Sodium acetate | LD50 25,956 mg/kg, non-toxic | None |
| Sodium carbonate | LD50 4090 mg/kg, mild toxicity | Irritant and corrosive |
| Sodium bicarbonate | LD50 4220 mg/kg, mild toxicity | Slight irritation to the eyes |
| Triethylamine | LD50 460 mg/kg, medium toxicity | strong irritant to the respiratory tract; after inhaling can cause pulmonary edema or even death |
| Pyridine | LD50 1580 mg/kg, mild toxicity | Strong irritant; anesthetizes the central nervous system |
| 4-dimethylaminopyridine (DMAP) | LD50 250 mg/kg, medium toxicity | Irritating to the eyes; eating poisonous |
| Base | EGCG Conversion/% | Yield/% a |
|---|---|---|
| Sodium acetate | nearly 100 | 90.6 ± 2 |
| Sodium carbonate | nearly 100 | 57.1 ± 2 |
| Sodium bicarbonate | nearly 100 | 72.4 ± 2 |
| Triethylamine | nearly 100 | 88.1 ± 2 |
| Solvent | Log P | EGCG Conversion/% a | Yield/% a |
|---|---|---|---|
| Dimethylformamide | −0.77 | 95.6 ± 2 | 45.3 ± 3 |
| Acetone | −0.29 | nearly 100 | 90.6 ± 2 |
| Butanone | 0.41 | nearly 100 | 66.2 ± 3 |
| Tetrahydrofuran | 0.35 | 92.5 ± 1 | 39.4 ± 3 |
| Ethyl acetate | 0.74 | 92.3 ± 1 | 79.3 ± 2 |
| Diethyl ether | 1.12 | nearly 100 | 47.6 ± 2 |
| Compound | IC50 (µM) for α-Amylase | IC50 (µM) for α-Glucosidase |
|---|---|---|
| EGCG | 7.44 ± 0.05 | 11.50 ± 0.02 |
| PEGCG | 1.64 ± 0.04 | 0.22 ± 0.03 |
| Acarbose | 1.10 ± 0.03 | 0.15 ± 0.02 |
| α-Amylase | EGCG | PEGCG | Acarbose |
|---|---|---|---|
| Number of contact residues | 20 | 22 | 19 |
| Number of the hydrogen bonds | 4 | 2 | 4 |
| Inhibitor | Hydrogen Bond Donor Count | Hydrogen Bond Acceptor Count |
|---|---|---|
| EGCG | 8 | 11 |
| PEGCG | 7 | 12 |
| Acarbose | 14 | 19 |
| α-Glucosidase | EGCG | PEGCG | Acarbose |
|---|---|---|---|
| Number of contact residues | 19 | 27 | 26 |
| Number of the hydrogen bonds | 3 | 4 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Kang, Z.; Yan, W. Synthesis, Stability, and Antidiabetic Activity Evaluation of (?)-Epigallocatechin Gallate (EGCG) Palmitate Derived from Natural Tea Polyphenols. Molecules 2021, 26, 393. https://doi.org/10.3390/molecules26020393
Liu B, Kang Z, Yan W. Synthesis, Stability, and Antidiabetic Activity Evaluation of (?)-Epigallocatechin Gallate (EGCG) Palmitate Derived from Natural Tea Polyphenols. Molecules. 2021; 26(2):393. https://doi.org/10.3390/molecules26020393
Chicago/Turabian StyleLiu, Bingbing, Zhengzhong Kang, and Weidong Yan. 2021. "Synthesis, Stability, and Antidiabetic Activity Evaluation of (?)-Epigallocatechin Gallate (EGCG) Palmitate Derived from Natural Tea Polyphenols" Molecules 26, no. 2: 393. https://doi.org/10.3390/molecules26020393
APA StyleLiu, B., Kang, Z., & Yan, W. (2021). Synthesis, Stability, and Antidiabetic Activity Evaluation of (?)-Epigallocatechin Gallate (EGCG) Palmitate Derived from Natural Tea Polyphenols. Molecules, 26(2), 393. https://doi.org/10.3390/molecules26020393







