Abstract
Cyclopropanated iminosugars have a locked conformation that may enhance the inhibitory activity and selectivity against different glycosidases. We show the synthesis of new cyclopropane-containing piperidines bearing five stereogenic centers from natural amino acids l-serine and l-alanine. Those prepared from the latter amino acid may mimic l-fucose, a natural-occurring monosaccharide involved in many molecular recognition events. Final compounds prepared from l-serine bear S configurations on the C5 position. The synthesis involved a stereoselective cyclopropanation reaction of an α,β-unsaturated piperidone, which was prepared through a ring-closing metathesis. The final compounds were tested as possible inhibitors of different glycosidases. The results, although, in general, with low inhibition activity, showed selectivity, depending on the compound and enzyme, and in some cases, an unexpected activity enhancement was observed.
1. Introduction
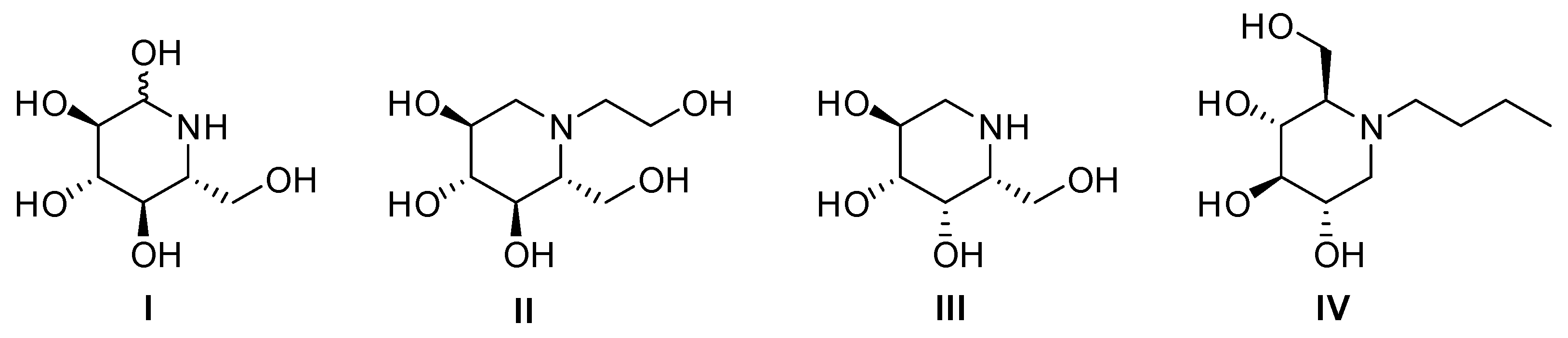
Natural or synthetic polyhydroxylated piperidines are iminosugars able to act as biomimetics of their corresponding pyranose analogs. For instance, nojirimycin, its epimers, and their deoxyanalogs have been used as lead molecules to design glycosidase and glycosyl transferase inhibitors and modulators [1,2,3]. Some iminosugars such as miglitol (Glyset®) [4], migalastat (Galafold®) [5], and miglustat (Zavesca®) [6] are commercially available, and others are actually in different clinical phases (Figure 1). The interaction with glycosidases is generally attributed to a structural similarity to diverse conformational oxocarbenium transition states formed during the hydrolysis of carbohydrates [7]. There are important variations in these transition states for different glycosidases [8,9]. Thus, there is a great interest in designing conformationally restricted inhibitors in order to achieve selective inhibition and adequate metabolic stability.
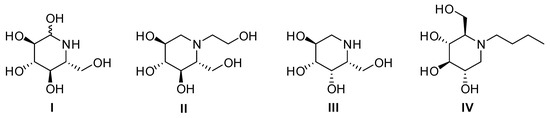
Figure 1.
Structures of nojirimycin (I), miglitol (II), migalastat (III), and miglustat (IV).
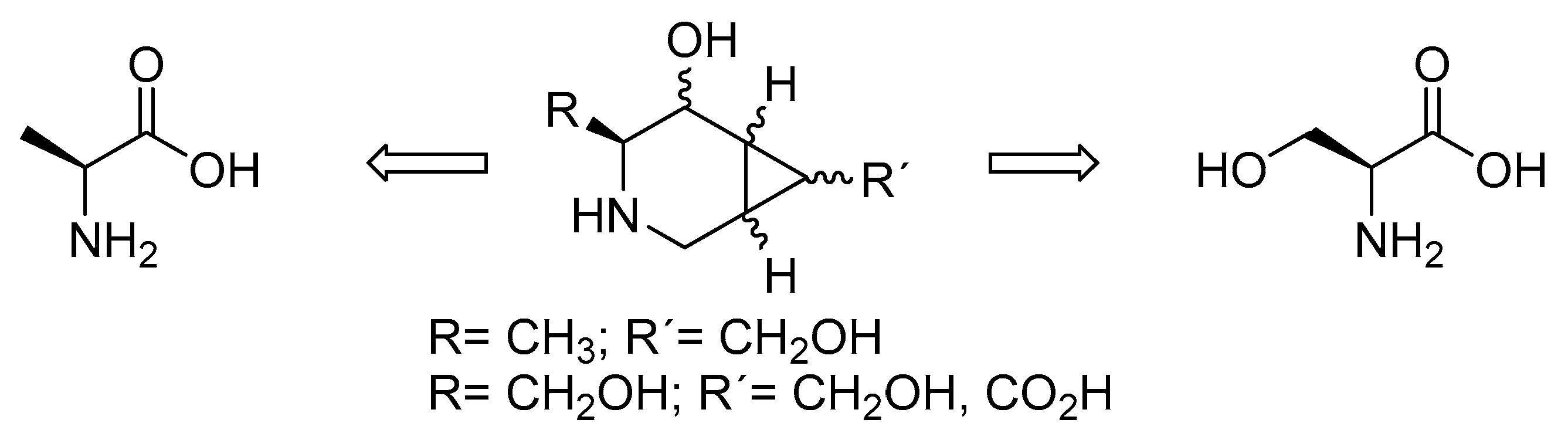
Our group engaged in the synthesis of novel piperidine iminosugars fused to a cyclopropane ring, resulting in structures with a locked conformation [10,11]. The cyclopropane renders a twist-like conformation to the piperidine ring that is found, as preferred for interactions with certain glycosidases [12,13]. We expect these compounds to be starting points for the finding of products of pharmacological interest (Figure 2). The possibility of variations in the substitution pattern of the cyclopropane allows different configurations that could direct their selectivity to different glycosidases. Although the development of synthetic routes to iminosugars has received much attention in the synthetic community [14,15], the preferred chiral pools are carbohydrates, which are transformed using reductive aminations [16,17], or other transformation strategies [18]. In our case, we developed synthetic approaches from natural amino acids, which have less precedents [19,20]. Other asymmetric or biocatalyzed approaches have been used [21]. In our previous work, starting from natural amino acid L-serine, we synthesized iminosugars bearing the R configuration on the carbon adjacent to nitrogen, which was supposed to mimic C5 in natural carbohydrates and iminosugars.
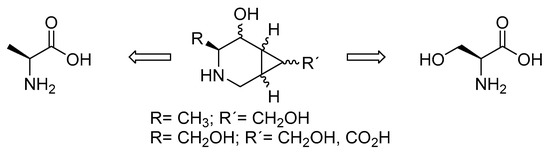
Figure 2.
Structures of the targeted compounds.
The present work is focused on new compounds prepared both from L-alanine and l-serine. In the case of L-alanine, the derived compounds could mimic 6-dehydroxylated sugars as fucose. Fucose-containing glycans, such as in blood groups and Lewis oligosaccharides and related ones, are critical for a wide range of cell events [22]. These include cell–cell adhesion, immune response, viral and bacterial infection, and tumor progression. We prepared new bicyclic iminosugars that include the cyclopropane motif fused with a piperidine that may mimic the l-fucose ring and evaluated them against fucosidase and other glycosidases. In addition, we prepared cyclopropane-containing piperidine iminosugars starting from l-serine but now with an S configuration at the carbon that mimics C5. A preliminary glycosidase inhibition evaluation is shown. The synthesis implies building final compounds with five stereogenic centers [23,24,25].
2. Results and Discussion
2.1. Chemistry
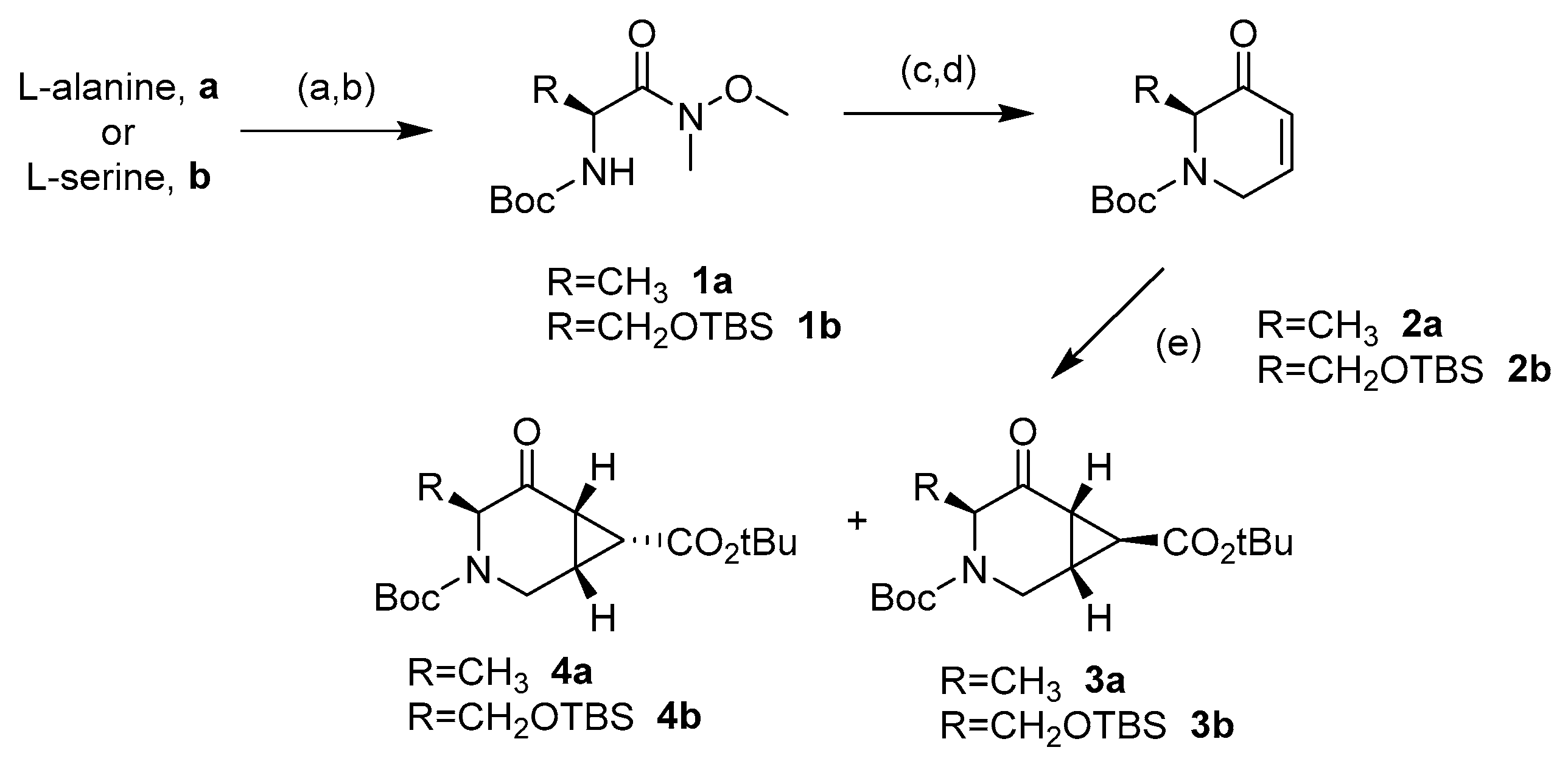
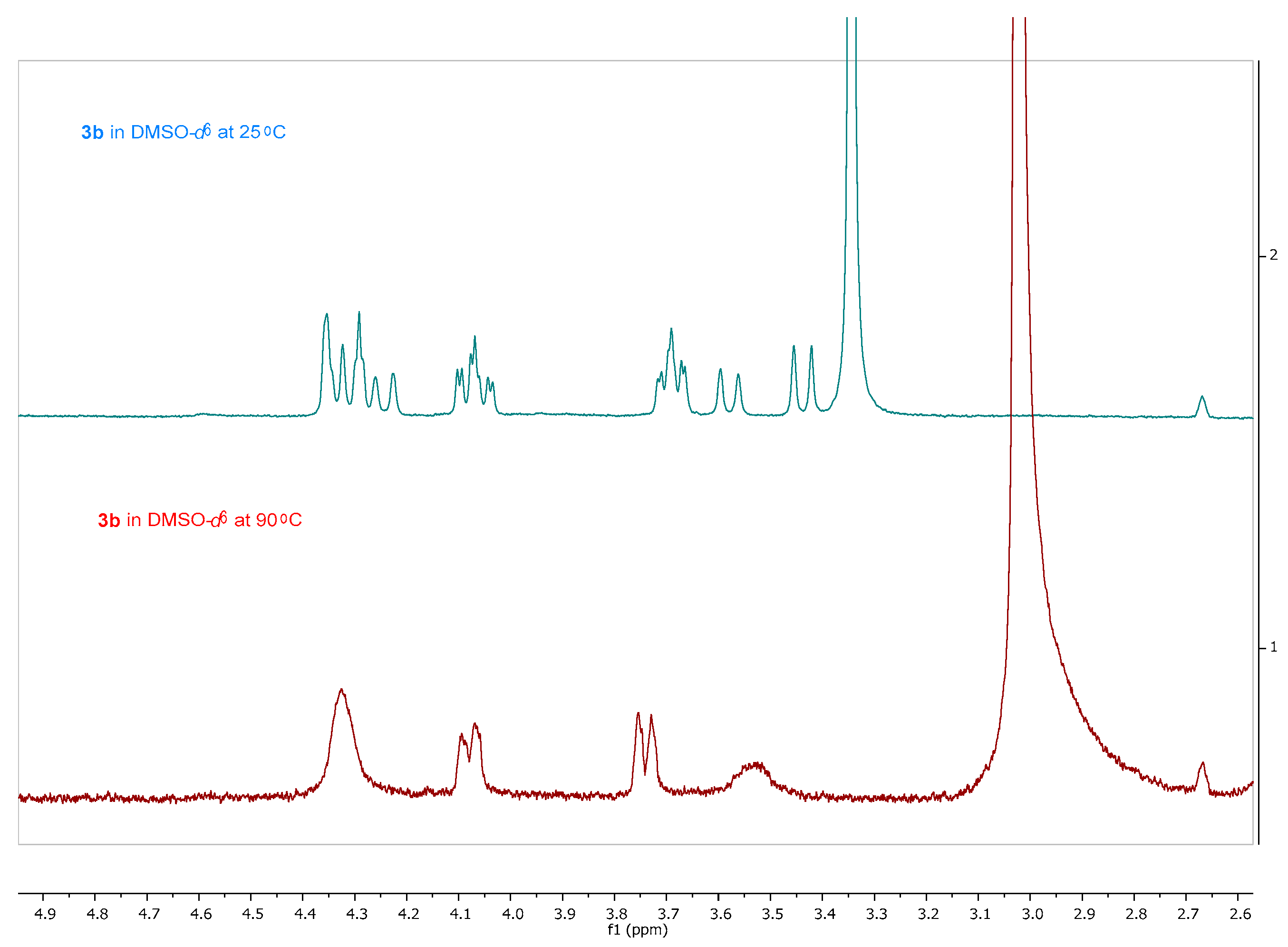
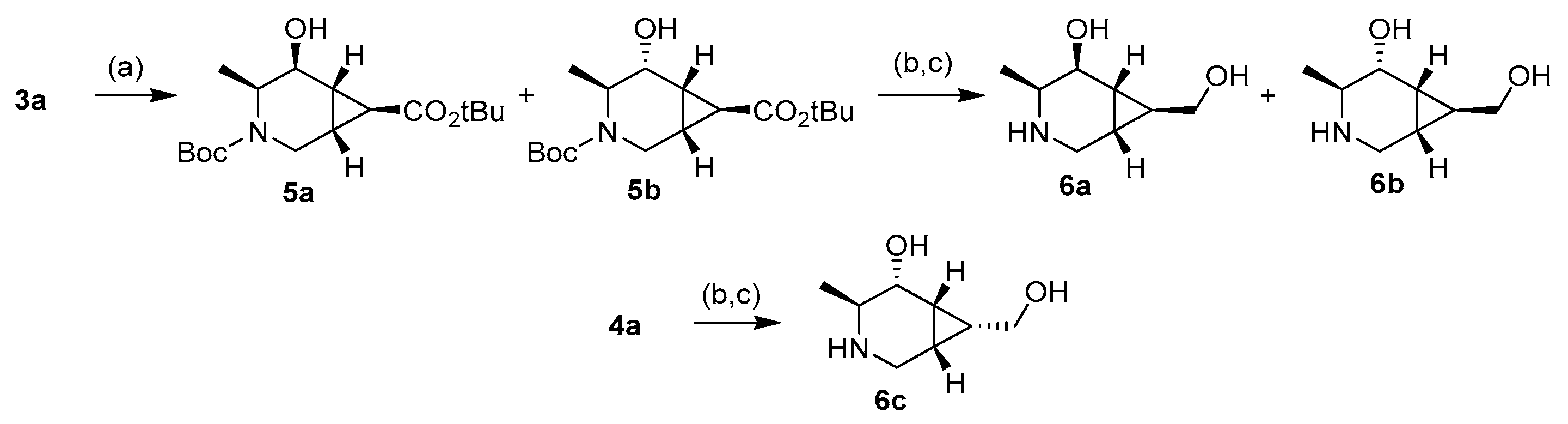
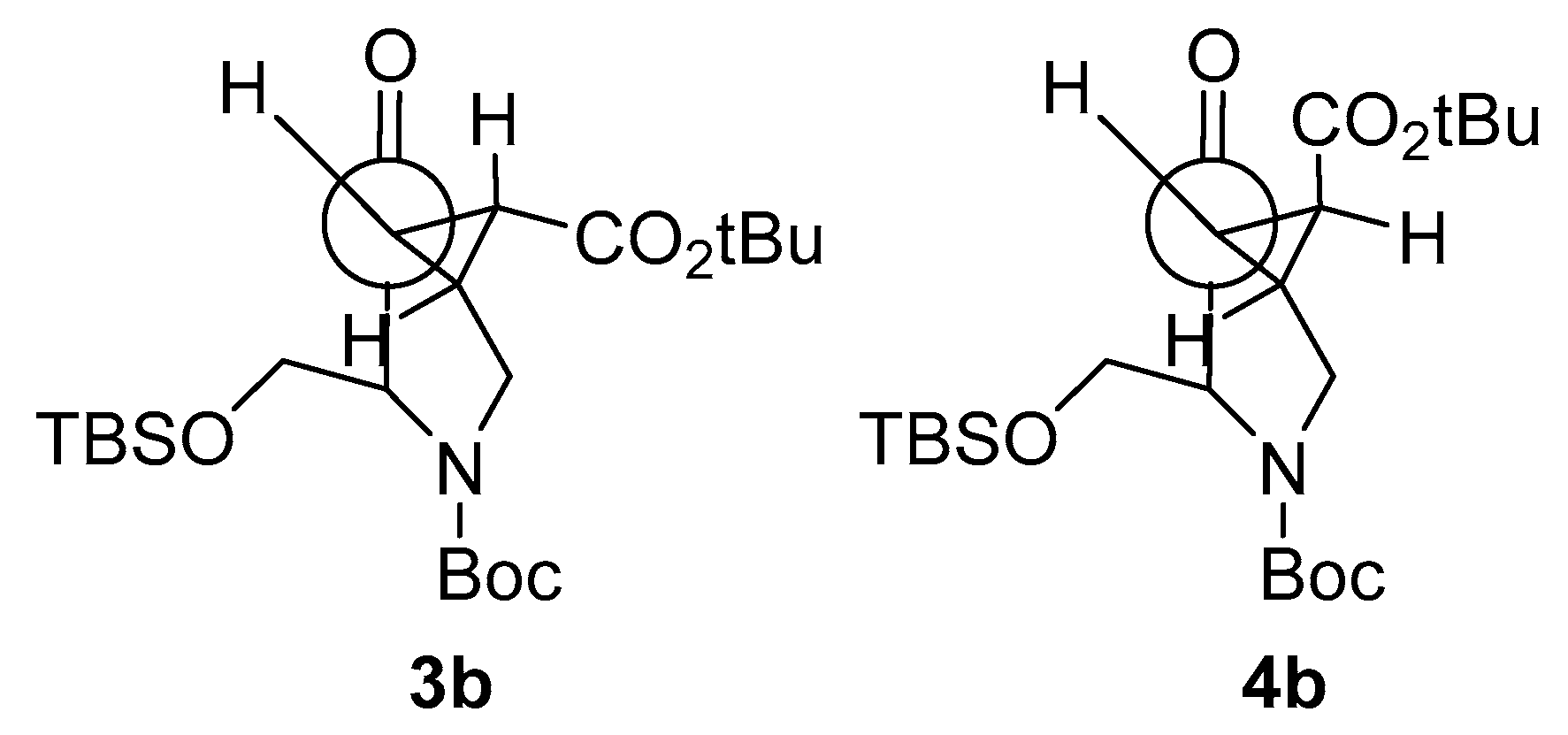
The synthesis of the novel iminosugars started from l-serine or l-alanine as the chiral pool. Both amino acids were protected using di-tert-butyl dicarbonate (Boc2O) [26]. Without further purification, these latter intermediates were submitted to coupling with N,O-dimethylhydroxylamine and, for the l-serine derivative, protection with tert-butyldimethylsilyl of the hydroxy group. Thus, intermediates 1a (78%) and 1b (76%) were obtained in good yields. Treatment with a base and allyl bromide, followed by a reaction with vinylmagnesium bromide at −30 °C, gave the precursors of the ring-closing metathesis reaction (RCM). For the RCM, a second-generation Grubbs’ catalyst (Grubbs’ Catalyst® M204, 3 mol %) was used, affording the α,β-unsaturated ketones 2a and 2b in 76% and 60% yield, respectively (Scheme 1) [27]. These compounds reacted with tert-butyl 2-(tetrahydro-1λ4-thiophen-1-ylidene)acetate to give a mixture (as seen in 1H-NMR) of cyclopropane exo:endo isomers 3a (77%) and 4a (5%) from 2a in a 15:1 ratio, while 3b (71%) and 4b (18%) from 2b in a 4:1 exo:endo ratio [28]. These isomers were isolated and separately characterized. 1H-NMR at 90 °C in DMSO-d6 determined that compounds 3b and 4b were obtained as a mixture of conformers due to the slow rotation of the Boc group in a 55:45 ratio in both cases (Figure 3).
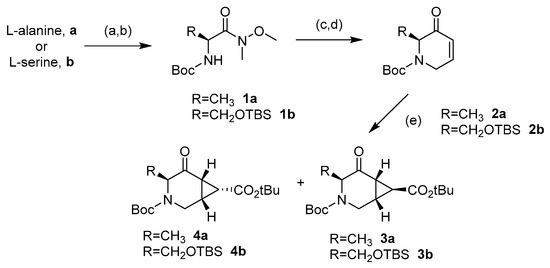
Scheme 1.
Reagents and conditions: (a) Boc2O, NaOH 1M, Dioxane, 0 °C to r.t.; then, diisopropilehtyl amine (DIPEA), N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDCI), N,O-dimethylhydroxilamine, dichloromethane (DCM), 0 °C, 78% from l-serine (1a). (b) For the product derived from l-serine: Imidazole, 4-(dimethylamino)pyridine (DMAP), tert-butyldimthylsilyl chloride (TBSCl), dimethylformamide (DMF), 0 °C to r.t., 76% from l-serine (1b). (c) NaH, allyl bromide, DMF, 0 °C to 50 °C, 78% (from 1a) and 58% (from 1b). (d) Vinylmagnesium bromide, tetrahydrofurane (THF) −30 °C; then, second-generation Grubbs’ catalyst (3 mol %), DCM, 50 °C, 76% (2a) and 60% (2b). (e) tert-Butyl (tetrahydrothiophenylidene)acetate, DCM, 0 °C to r.t., 77% (3a), 5% (4a), 71% (3b), and 18% (4b).
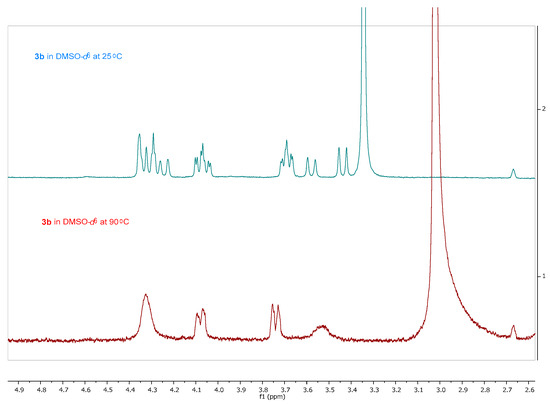
Figure 3.
1H-NMR (400 MHz, CDCl3) of 3b in DMSO-d6 at 25 °C (blue) and 90 °C (red).
The cyclopropanation reaction has two steps, ylide addition to the double bond and ring closure. It is known that the sulfur ylide attack is nonselective, and the exo:endo selectivity is determined in the second step [29,30,31]. The result depends on many different issues, such as temperature, reagent concentration, and the presence of a base. For instance, in the cyclopropanation reaction of 2a, the 15:1 exo:endo ratio was observed when carrying out the reaction at 0 °C and a low concentration of ylide (0.15 M), while a higher temperature and ylide concentration promoted higher ratios in favor of an exo isomer. In the case of the cyclopropanation of compound 2b with an ylide concentration of 2M and temperature of 20 °C, the ratio of exo/endo isomers can increase up to 20:1, as seen by NMR. Similar behavior has already been reported [32].
With the intermediates 3 and 4 in hand, the final products were prepared by a reduction of the ketone and further hydrolysis or reduction of the ester group. Thus, compound 3a was treated with NaBH4 to give isomers 5a and 5b in a 3:2 ratio (Scheme 2). These alcohols were isolated in 55% (5a) and 19% (5b) yields and separately treated with diisobutylaluminium hydride (DIBAL-H), giving the corresponding intermediates in 55% and 67% yields, respectively. A final treatment with trifluoroacetic acid (TFA) afforded compounds 6a (85%) and 6b (83%) after a final elution through a basic DOWEX resin. This two-step reduction allowed the separation of the isomers 5a and 5b. When using stronger conditions to perform the reduction in one step from 3a, a mixture of diols was obtained but could not be separated. On the other hand, isomer 4a reacted with DIBAL-H to give only a product in 32% yield, which, after hydrolysis with TFA and further elution through a basic DOWEX resin, afforded free amine 6c (81%).
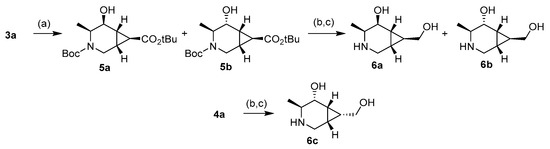
Scheme 2.
Reagents and conditions: (a) NaBH4, EtOH, 0 °C to r.t., 55% (5a) and 19% (5b). (b) DIBAL-H, DCM, 0 °C to r.t., 55% (from 5a), 67% (from 5b), and 32% (from 4a). (c) Trifluoroacetic acid (TFA), MeOH, r.t., 85% (6a), 83% (6b), and 81% (6c).
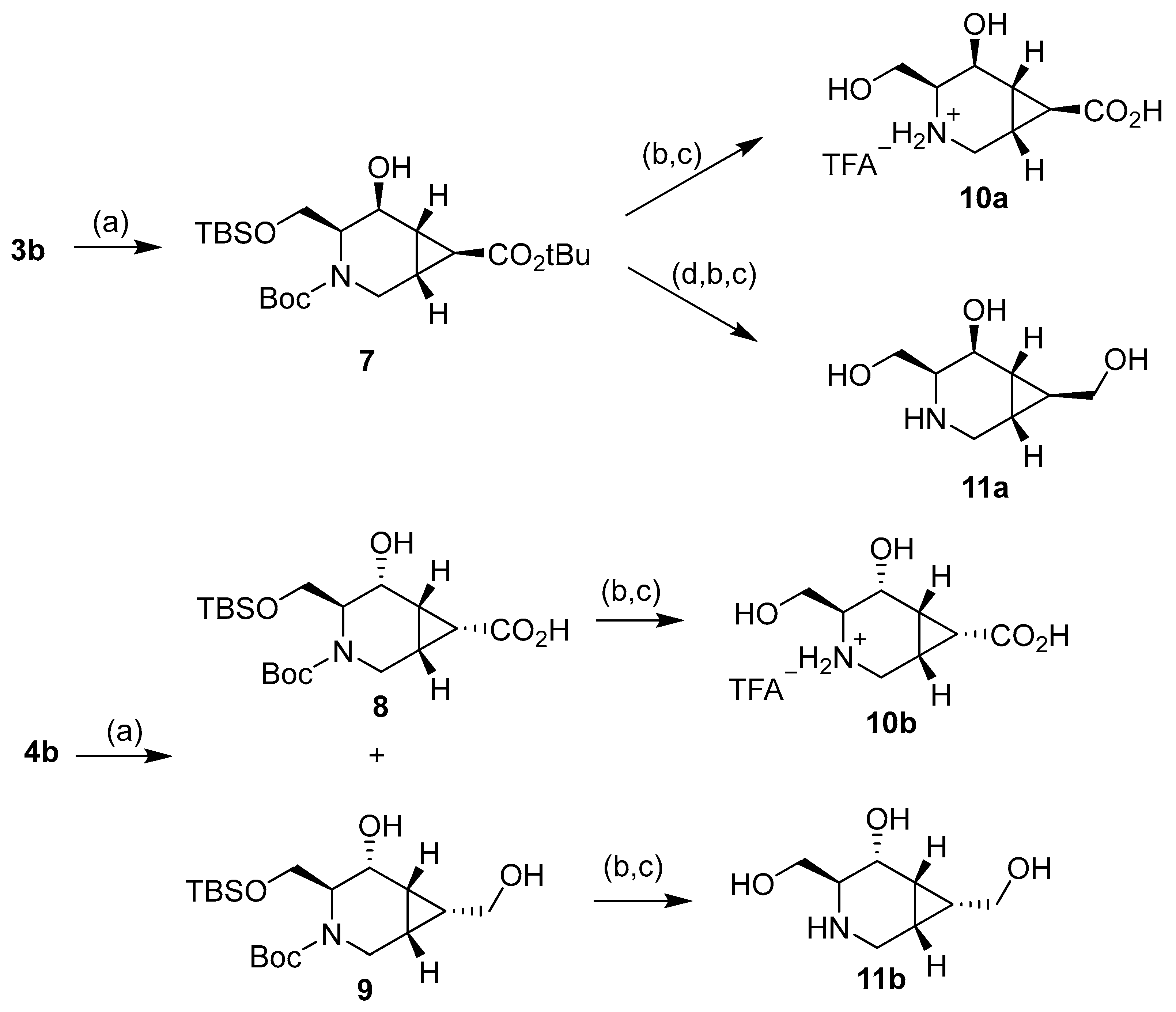
Compound 3b diastereoselectively reacted with NaBH4, resulting in 7 as the only reaction product. 4b reacted, giving a separable mixture of compounds 8 and 9. The selectivity of this reduction is governed by the configuration of the cyclopropane ring but not by the location of the bulky tert-butyldimthylsilyl (OTBS) group; as in the case of 4b, the hydride reacts by the face of the OTBS group. The Felkin-Anh model corroborates these results: on the exo isomer 3b, the tert-butyl ester group gets far from the carbonyl, but the OTBS is the one making the steric hindrance to determine the stereochemistry of the reaction. On the other hand, the endo isomer 4b has both bulky groups near the carbonyl, staying close to the ester. Moreover, CH2OTBS can rotate to get farther from carbonyl, while the tert-butyl ester cannot (Figure 4).
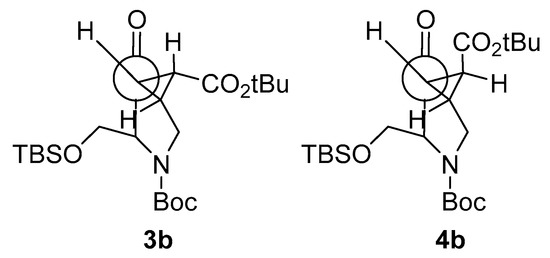
Figure 4.
The Felkin-Anh model for compounds 3b and 4b.
Deprotection of the hydroxyl group of compound 7 using tetrabutylammonium fluoride trihydrate (TBAF.3H2O) and further treatment with trifluoroacetic acid (TFA) resulted in the final product 10a as a trifluoroacetate salt (Scheme 3) [33]. On the other hand, the reduction of the tert-butyl ester in 7 with DIBAL-H gave the N-Boc and OTBS protected intermediate, which was treated as previous to give the corresponding trifluoroacetate salt. After treatment of this salt with a basic DOWEX resin, the final compound 11a was obtained. Compound 8 was deprotected to give compound 10b as a trifluoroacetate salt. Finally, compound 9 was treated similarly to afford final compound 11b.
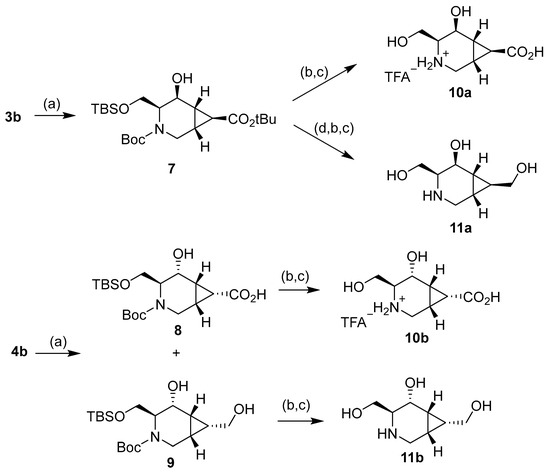
Scheme 3.
Reagents and conditions: (a) NaBH4, EtOH, 0 °C to r.t., 74% (7), 30% (8), and 50% (9). (b) TBAF.3H2O, THF, r.t., 85% (from 7 to 10a), 68% (from 7 to 11a), 88% (from 8), and 86% (from 9). (c) TFA, MeOH, r.t., 87% (10a), 91% (11a), 92% (10b), and 93% (11b). (d) DIBAL-H, DCM, 0 °C to r.t., 70%.
2.2. Modeling and NOE Experiments
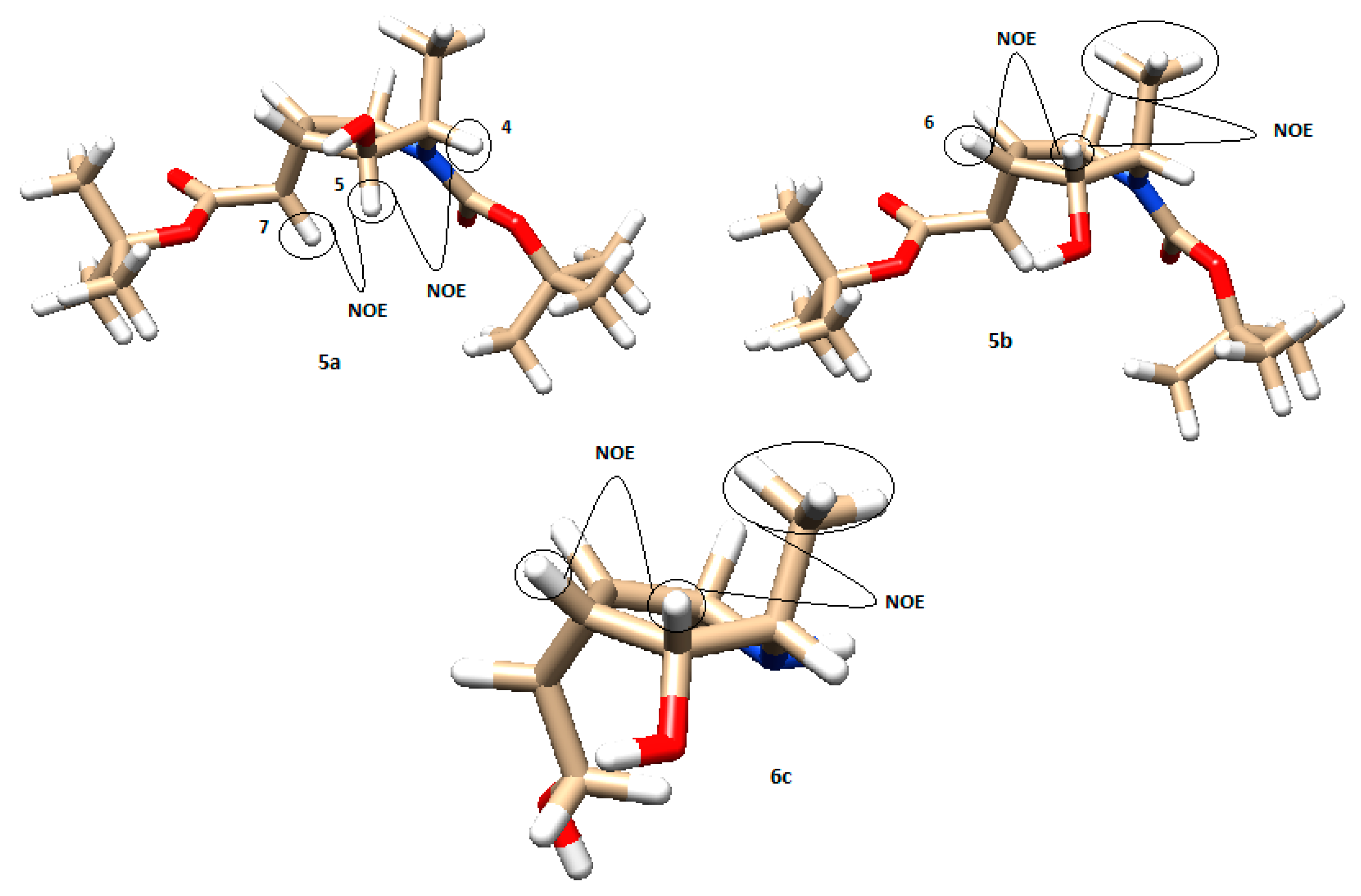
The stereochemistry of all the synthesized products was assigned by means of NOESY experiments and coupling constant calculations. All compounds were first modeled on Chimera 1.13.1, using ANTECHAMBER for the computing charges [34]. With these models, we could calculate the relevant dihedral angles and predict the expected NOE effects, which were checked with those experimentally obtained. Figure 5 shows the models and main NOE effects of compounds 5a and b and compound 6c derived from l-alanine. H5 in compound 5a shows a NOE interaction with H4 and H7. On the other hand, the other reduction isomer, 5b, gave a NOE signal between H5 and H6 and an intense effect between H5 and the methyl group. The final product 6c obtained from the endo isomer, after the cyclopropanation reaction, gave analog signals as 5b.
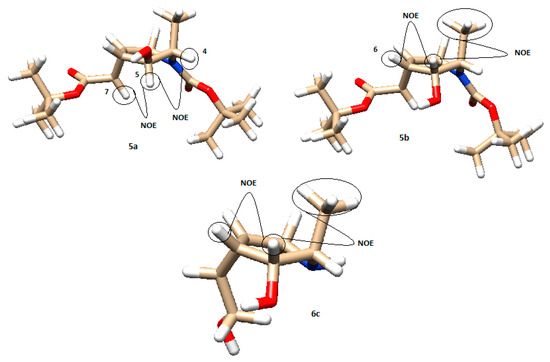
Figure 5.
Model of compounds 5a, 5b, and 6c with their main NOE interactions (brown: carbon; white: hydrogen; red: oxygen; blue: nitrogen).
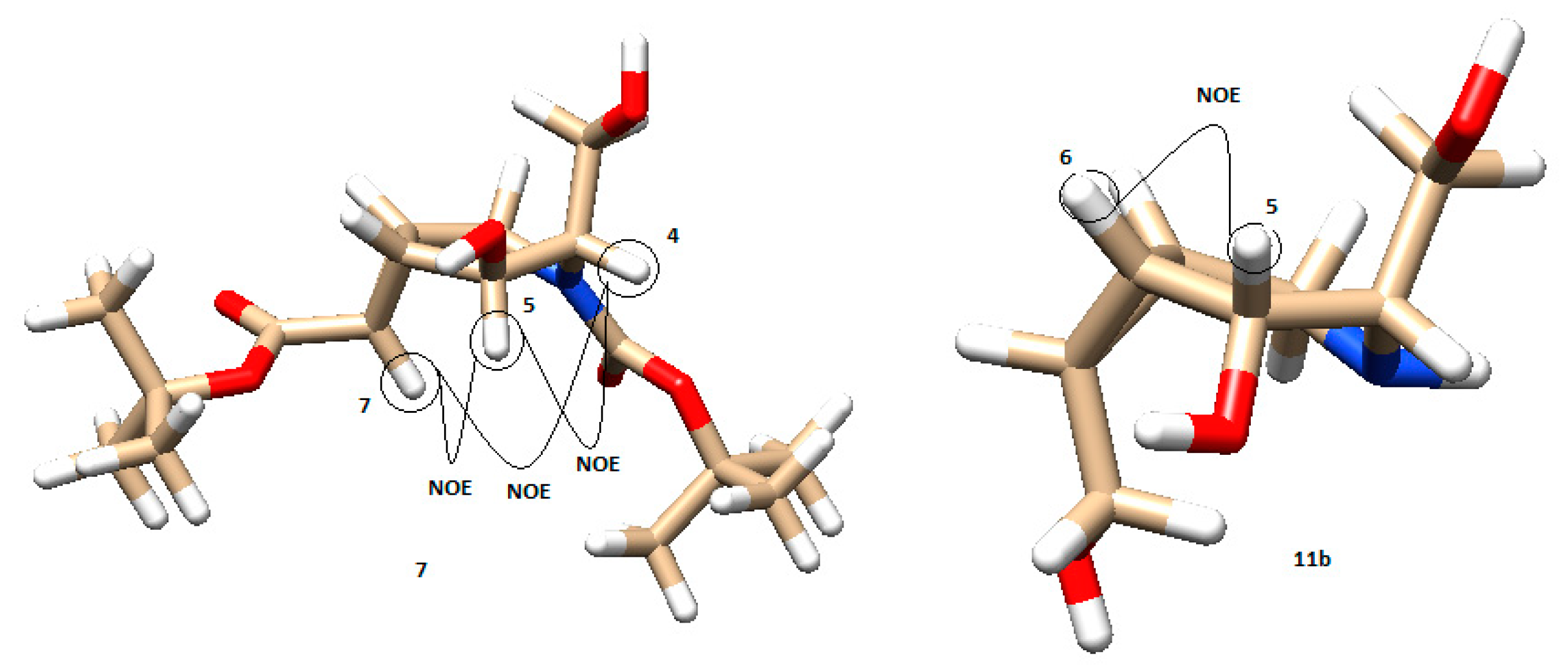
The models and NOE interactions for compounds 7 and 11b are shown in Figure 6. Compound 7 was assigned with the NOE effects observed between H7 and H5, H5 and H4, and between H7 and H4. The constant couplings measured in 1H-NMR also agreed with the modeled angles. On the other hand, compound 11b only showed one NOE signal between H5 and H6 (Figure 6). The measured coupling constant agreed with the calculated ones from models using the Carplus equation (see Supporting Information Table S1).
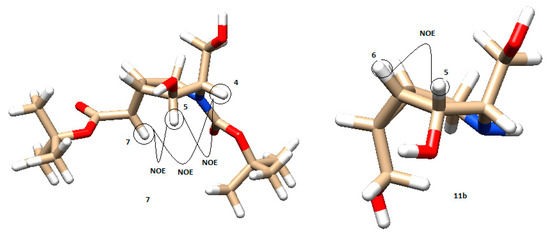
Figure 6.
Model of compounds 7 and 11b, respectively, with their main NOE interactions (brown: carbon; white: hydrogen; red: oxygen; blue: nitrogen).
2.3. Enzymatic Assays
Glycosidase activities were assessed in 80-μL reaction volumes in Eppendorf vials. Buffer composition and enzyme concentrations were adjusted depending on the enzyme: 20-mM Na2HPO4 at pH 7.3 for β-glucosidase from Almonds (3 μg/mL) and β-galactosidase from Escherichia coli (1 μg/mL), 20-mM Na2HPO4 at pH 6.8 for α-glucosidase from Bacillus stearothermophilus (1 μg/mL) and α-galactosidase from Green coffee (20 μM), 20-mM NaH2PO4 at pH 5.5 for α- and β-mannosidase from Jack beans and Helix pomatia, respectively (7 μM and 2 μM, respectively), 0.1-M NaOAc at pH 4.0 with 1 mg/mL of BSA (bovine serum albumin) for α-l-fucosidase from Homo sapiens (2 μM), and 50-mM NaOAc at pH 5.0 for neuraminidase from Vibrio cholerae (6 μM). The inhibitors were tested at 1-, 5-, and 25-mM final concentrations in the assays. Each mixture of enzyme and inhibitor was homogenized and preincubated for 10 min at 37 °C or 40 °C (α-l-fucosidase). Each reaction was initiated and brought to a final volume of 80 μL by the addition of an aliquot of the corresponding p-nitrophenyl glycoside substrate to obtain the following final concentrations in the reaction mixtures: p-nitrophenyl α- and β-d-glucopyranoside (1 mM), p-nitrophenyl α- and β-d-galactopyranoside (0.5 mM), p-nitrophenyl α- and β-d-mannopyranoside (1 mM), p-nitrophenyl α-l-fucopyranoside (1 mM), or p-nitrophenyl neuraminic acid (1 mM). After 10 min of incubation time at the same temperature, each reaction was quenched with 400 μL of 1.0-M Na2CO3, and the absorbance at 405 nm was measured. Assays were repeated twice, and the data was averaged.
The enzymatic activity was calculated by the ratio in the absorbance measured at 405 nm of the phenoxide released in the enzymatic reaction. The final compounds were screened at 1, 5, and 25 mM. The assays at 1 mM of synthesized compounds did not give clear results. Thus, the activity detected at 5 mM is shown in Table 1.

Table 1.
Residual activity of enzymes at 5 mM of the active final compounds.
None of the synthesized compounds showed activity against α-glucosidase, α-mannosidase, or neuraminidase at the concentrations used. In the case of products 11a and 6a, no activity was observed against any of the enzymes. Interestingly, products 6c and 10b inhibited only one enzyme, β-glucosidase, decreasing their activity to 43% and 39% at 5 mM, respectively. The results at 25 mM were 20% and 13% of the residual activity, respectively. Product 11b inhibited the activity of β-galactosidase to 25% at 5 mM but without an inhibition increase at 25 mM.
On the other hand, some assays showed an enhancement in the enzyme activity. Thus, compound 6b increased the activity of β-mannosidase and α-l-fucosidase up to 148% and 142% at 5 mM, respectively. This increase raised up to 240% at 25 mM. Compound 10a activated α-galactosidase and β-mannosidase up to around 155% at 5 mM.
Regarding the inhibitory results, we can conclude that these compounds are very weak inhibitors only against certain enzymes far from the inhibition values of well-known iminosugars such as deoxinojirimycin [35] or castanospermine analogs [36]. However, the activation observed in certain cases deserves some comments, as there are few precedents of this behavior [37,38], including our previous results with similar compounds [10]. This activation does not have a clear explanation. The possibility that the compounds could work as efficient transglycosidation acceptors and, thus, accelerate the nitrophenol release was checked following the enzymatic reaction in a NMR tube and recorded spectra each 5 min. However, no potential transglycosilation product was detected (see the Supporting Information). Other cases of glycosidase activity enhancements were described; thus, some glycosidases were found to activate when using multivalent iminosugars [37]. Other reports explained the activation mechanism by the introduction of a small molecule in the active site, locking the reactive form of the glycosidase [38], or through an allosteric-type interaction that changed the conformation of the enzyme into the active one. These observations need further research to explain this behavior.
3. Materials and Methods
3.1. General Information
All chemicals were obtained from Aldrich/Merck (St. Louis, MO, USA), VWR (Radnor, PA, USA), Fluorochem (Derbyshire, UK), and ABCR (Karlsruhe, Germany). TLC analyses were performed on Merck silica gel 60 F254 plates using phosphomolybdic acid or anisaldehyde and heat for detection. Silica gel NORMASIL 60 40–63 μm was used for flash chromatography. NMR spectra were recorded on a Bruker spectrometer (400 MHz or 300 MHz for 1H and 100 MHz or 75 MHz for 13C), (Billerica, MA, USA). Chemical shifts are reported in δ ppm referenced to CDCl3 (δ = 7.26 for 1H and 77.00 for 13C), CD3OD (δ = 3.31 for 1H and 49.00 for 13C), or D2O (δ = 4.79 for 1H). Bidimensional spectra (HMQC, HMBC, COSY, and NOESY) were recorded in order to carry out the assignment. Infrared spectra were done in a Perkin-Elmer spectrum 100 (Agilent, Santa Clara, CA, USA). Specific optical rotation was measured in a Polarimeter Anton Paar MCP 100 (Anton Paar, Graz, Austria). Melting points of solid compounds were determined using a Stuart Scientific Melting Point Apparatus SMP3 (Stuart, Staffordshire, UK). Microanalyses were done on a LECO CHNS-932 (LECO, St. Joseph, MI, USA). Absorbance of p-nitrophenoxyde released in the enzymatic reactions was measured at 405 nm in a Perkin-Elmer Lamba25 (PerkinElmer, Waltham, MA, USA).
3.2. Synthesis
Synthesis of 1–11
Tert-butyl (S)-(1-(methoxy(methyl)amino)-1-oxopropan-2-yl)carbamate (1a): To a solution of l-alanine (7.00 g, 78.57 mmol) in 175 mL of NaOH 1M at 0 °C was added a solution of di-tert-butyl dicarbonate (20.58 g, 94.29 mmol) in 77 mL of dioxane. The reaction was stirred 4 h at room temperature. The reaction was quenched with KHSO4 1M until pH 1 to 2. The aqueous layer was extracted with AcOEt (3 × 150 mL). The combined organic layers were dried over MgSO4, and the solvent was evaporated in vacuo. The crude was dissolved in 300 mL of DCM and cooled to 0 °C. DIPEA (13.7 mL, 78.57 mmol), EDCI (15.06 g, 78.57 mmol), and N,O-dimethylhydroxilamine hydrochloride (7.67 g, 78.57 mmol) were added. The reaction was stirred at 0 °C for 1.5 h. One hundred milliliters of a solution of HCl 1M was added to the reaction. The aqueous phase was extracted with DCM (2 × 150 mL). The combined organic layers were washed with NaHCO3 (150 mL) and brine (150 mL) and dried over MgSO4. The solvent was evaporated in vacuo. A colorless oil was obtained (14.23 g, 78% after two steps). 1H-NMR (400 MHz, CDCl3) δ 5.27 (d, J = 8.7 Hz, 1H, NH), 4.57 (brs, 1H, CHN), 3.66 (s, 3H, OCH3), 3.10 (s, 3H, NCH3), 1.33 (s, 9H, 3×CH3), 1.20 (d, J = 6.9 Hz, 3H, CH3) ppm. 13C-NMR (100 MHz, CDCl3) δ 173.6, 155.1, 79.3, 67.0, 61.5, 46.4, 28.3 (3C), 18.5 ppm. IR (NaCl): 3065, 2986, 2935, 1761, 1684 cm−1. (c 0.23 in CHCl3): −4.12. Anal. Calc. for C10H20N2O4: C, 51.7; H, 8.7; N, 12.1%. Found: C, 51.5; H, 9.0; N, 12.2%.
Tert-butyl (S)-allyl(1-(methoxy(methyl)amino)-1-oxopropan-2-yl)carbamate: To a suspension of 1a (14.23 g, 61.30 mmol) in 200 mL of DMF at 0 °C was added slowly NaH 60% w/w (4.90 g, 122.6 mmol). After 10 min, allyl bromide was added (16 mL, 183.9 mmol). The reaction was stirred at 50 °C for 2.5 h. The reaction was quenched with 200 mL of a saturated solution of NH4Cl. The aqueous phase was extracted with AcOEt (2 × 350 mL). The combined organic layers were washed with a saturated solution of NH4Cl (150 mL), a saturated solution of NaHCO3 (150 mL), and brine (150 mL) and dried over MgSO4. The solvent was evaporated in vacuo. The crude was purified in silica gel in Hex/AcOEt (9:1). A yellow oil was obtained (12.95 g, 78%). 1H-NMR (400 MHz, CDCl3) δ 5.67–5.57 (m, 1H, HC=), 5.06–4.98 (m, 1H, CHN), 4.89–4.82 (m, 2H, =CH2), 3.75–3.59 (m, 2H, CH2N), 3.53 (s, 3H, OCH3), 2.94 (s, 3H, NCH3), 1.23 (s, 9H, 3×CH3), 1.09 (d, J = 7.2 Hz, 3H, CH3) ppm. Two conformers were observed in 13C-NMR (100 MHz, CDCl3) δ 172.8, 155.1 (major), 154.4 (minor), 135.8 (major), 135.2 (minor), 115.3 (minor), 114.8 (major), 79.7 (minor), 79.4 (major), 61.2 (major), 61.0 (minor), 51.5 (minor), 49.6 (major), 46.5 (minor), 45.9 (major), 32.1 (minor), 31.9 (major), 28.0 (3C), 15.2 (minor), 15.0 (major) ppm. IR (NaCl): 3065, 2986, 2935, 1761, 1684 cm−1. (c 0.22 in DCM): −38.64. Anal. Calc. for C13H24N2O4: C, 57.3; H, 8.9; N, 10.3%. Found: C, 57.1; H, 8.7; N, 10.5%.
Tert-butyl (S)-2-methyl-3-oxo-3,6-dihydropyridine-1(2H)-carboxylate (2a): A solution of tert-butyl (S)-allyl(1-(methoxy(methyl)amino)-1-oxopropan-2-yl)carbamate (8.66 g, 31.82 mmol) in 50 mL of THF was cooled to −30 °C. A solution of vinylmagnesium bromide 0.7 M in THF (100 mL) was added slowly, keeping the temperature below −25 °C. When the addition was finished, the reaction was stirred at the same temperature for 30 min more. The reaction was poured on a mixture of 60 mL of HCl 10% and 120 mL of MeOH cooled in a bath at −15 °C. This mixture was stirred for another 15 min. The aqueous phase was extracted with AcOEt (2 × 100 mL). The combined organic layers were washed with a saturated solution of NH4Cl (100 mL), a saturated solution of NaHCO3 (100 mL), and brine (60 mL); dried over MgSO4; and evaporated in vacuo. The crude was dissolved in 150 mL of DCM and heated to reflux. When reflux began, Grubbs’ catalyst 2nd generation (812 mg, 0.96 mmol) was added. The reaction was stirred for 1.5 h. The reaction was filtered through a pad of celite. The solvent was evaporated in vacuo. The crude was purified in silica gel in Hex/AcOEt (4:1). A yellow oil was obtained as 2 conformers in a ratio 60:40 (5.11 g, 76% after two steps). 1H-NMR (400 MHz, CDCl3) δ 6.95 (brs, 1H, CH2CH=major + minor), 6.05 (dt, J = 10.3, 2.3 Hz, 1H, COCH=, major + minor), 4.72–4.54 (m, 2H, CHN+CH2N, major + minor), 3.84 (brs, 1H, CH2N major), 3.79 (brs, 1H, CH2N minor), 1.44 (s, 9H, 3×CH3, major + minor), 1.22 (d, J = 7.2 Hz, 3H, CH3, major + minor) ppm. 13C-NMR (100 MHz, CDCl3) δ 196.8, 154.1, 146.3, 125.9, 80.9, 56.5, 39.8, 28.4 (3C), 15.9 ppm. IR (NaCl): 3058, 2979, 2931, 1748, 1681 cm−1. (c 0.08 in DCM): +73.37. Anal. Calc. for C11H17NO3: C, 62.5; H, 8.1; N, 6.6%. Found: C, 62.7; H, 8.4; N, 6.4%.
Di-tert-butyl (1R,4S,6S)-4-methyl-5-oxo-3-azabicyclo[4.1.0]heptane-3,7-dicarboxylate (3a and 4a): To a solution of 2a (3.48 g, 16.46 mmol) in 17 mL of DCM at 0 °C was added a solution of tert-butyl 2-(tetrahydro-1λ4-thiophen-1-ylidene)acetate (9.98 g, 49.38 mmol) in 270 mL of DCM. The reaction was stirred 20 min at 0 °C and 20 more minutes at room temperature. Deionized water was added (150 mL), and layers were separated. The aqueous layer was extracted with DCM (2 × 100 mL). The combined organic layers were washed with brine (80 mL), dried over MgSO4, and evaporated in vacuo. The crude was purified in silica gel in Hex/AcOEt (9:1). Exo isomer (3a) was obtained as a pale brown solid (4.10 g, 77%). Endo isomer (4a) was obtained as an orange wax (280 mg, 5%). Other 2 isomers were obtained as a brown oil (10 mg, 0.2%).
Spectroscopic data for di-tert-butyl (1R,4S,6S,7S)-4-methyl-5-oxo-3-azabicyclo[4.1.0] heptane-3,7-dicarboxylate (Exo-n, 3a): 2 conformers in a ratio (64:36) were observed. 1H-NMR (400 MHz, CDCl3) δ 4.56 (q, J = 7.2 Hz, 1H, minor, CHN), 4.42 (d, J = 14.1 Hz, 1H, major, CH2N), 4.36 (q, J = 7.1 Hz, 1H, major, CHN), 4.26 (d, J = 14.1 Hz, 1H, minor, CH2N), 3.29 (d, J = 14.1 Hz, 1H, minor, CH2N), 3.20 (d, J = 14.2 Hz, 1H, major, CH2N), 2.32–2.25 (m, 4H, major + minor, CHCO + CHCO2), 2.20–2.12 (m, 2H, major + minor, CHCH2N), 1.47 (s, 9H, 3×CH3, minor), 1.45 (s, 9H, 3×CH3, major), 1.43 (s, 18H, 3×CH3, major + minor), 1.23 (d, J = 7.2 Hz, 6H, major + minor, CH3) ppm. 13C-NMR (100 MHz, CDCl3) δ 204.1 (major), 204.0 (minor), 169.4, 154.5, 82.1 (major), 82.0 (minor), 81.2, 56.9 (major), 55.9 (minor), 36.1 (minor), 34.7 (major), 32.2, 28.4 (3C), 28.1 (3C), 24.9 (major), 24.7 (minor), 24.6 (minor), 24.4 (major), 15.9 (major), 15.4 (minor) ppm. IR (KBr): 2975, 2863, 1739, 1731, 1714 cm−1. (c 0.16 in DCM): +88.22. m.p.: 98.5 °C–101.2 °C. Anal. Calc. for C17H27NO5: C, 62.8; H, 8.4; N, 4.3%. Found: C, 63.0; H, 8.2; N, 4.6%.
Spectroscopic data for di-tert-butyl (1R,4S,6S,7R)-4-methyl-5-oxo-3-azabicyclo[4.1.0] heptane-3,7-dicarboxylate (Endo-n, 4a): 2 conformers in a ratio (93:7) were observed. 1H-NMR data given of the major conformer. 1H-NMR (400 MHz, CDCl3) δ4.46–4.36 (m, 2H, CHN+CH2N), 3.38 (d, J = 13.4 Hz, 1H, CH2N), 2.26 (t, J = 9.3 Hz, 1H, CHCO2), 2.03 (dd, J = 9.6, 7.8 Hz, 1H, CHCO), 1.92-1.89 (m, 1H, CHCH2N), 1.40 (s, 9H, 3×CH3), 1.38 (s, 9H, 3×CH3), 1.22 (d, J = 7.2 Hz, 3H, CH3) ppm. 13C-NMR (100 MHz, CDCl3) δ 204.9 (major), 202.8 (minor), 168.6 (minor), 166.9 (major), 154.6, 82.3 (major), 82.2 (minor), 80.9, 57.2 (major), 56.5 (minor), 35.0 (minor), 33.9 (major), 28.7 (minor), 28.4 (3C), 28.4 (major), 28.1 (3C, minor), 28.0 (3C, major), 25.7 (major), 25.4 (minor), 21.6, 15.9 (major), 15.0 (minor) ppm. IR (NaCl): 2982, 2874, 1737, 1728, 1715 cm−1. (c 0.14 in DCM): +33.04. Anal. Calc. for C17H27NO5: C, 62.8; H, 8.4; N, 4.3%. Found: C, 63.1; H, 8.0; N, 4.2%.
Tert-butyl (S)-(3-hydroxy-1-(methoxy(methyl)amino)-1-oxopropan-2-yl)carbamate: To a solution of l-serine (10 g, 95.16 mmol) in 200 mL of NaOH 1M at 0 °C was added a solution of di-tert-butyl dicarbonate (24.74 g, 113.36 mmol) in 90 mL of dioxane. The reaction was stirred for 4 h at room temperature. The reaction was quenched with a solution of KHSO4 1M until pH 1 to 2. The aqueous phase was extracted with AcOEt (3 × 300 mL). The combined organic layers were dried over MgSO4. The solvent was evaporated in vacuo, obtaining a colorless oil. The crude was dissolved in 360 mL of DCM and cooled to 0 °C. DIPEA (16.6 mL, 95.16 mmol), EDCI (18.24 g, 95.16 mmol), and N,O-dimethylhydroxilamine hydrochloride (9.28 g, 95.16 mmol) were added. The reaction was stirred at 0 °C for 1.5 h. One hundred milliliters of a solution of HCl 1M was added. The aqueous phase was extracted with DCM (2 × 150 mL). The combined organic layers were washed with a saturated solution of NaHCO3 (100 mL) and brine (100 mL), dried over MgSO4, and evaporated in vacuo. A white solid was obtained (21.14 g, 90% after 2 steps). 1H-NMR (400 MHz, CDCl3) δ 5.65 (d, J = 8.7 Hz, 1H, NH), 4.77 (brs, 1H, CHN), 3.81–3.78 (m, 2H, CH2O), 3.76 (s, 3H, OCH3), 3.21 (s, 3H, NCH3), 1.42 (s, 9H, 3×CH3) ppm. 13C-NMR (100 MHz, CDCl3) δ 171.1, 156.0, 80.1, 67.2, 63.7, 61.7, 52.5, 28.4 (3C) ppm. IR (KBr): 3378, 2984, 2868, 1740, 1708 cm−1. (c 0.24 in CHCl3): +2.59. m.p.: 110.1–115.6 °C. Anal. Calc. for C10H20N2O5: C, 48.4; H, 8.1; N, 11.3%. Found: C, 48.7; H, 7.9; N, 11.6%.
Tert-butyl (S)-(3,8,8,9,9-pentamethyl-4-oxo-2,7-dioxa-3-aza-8-siladecan-5-yl)carbamate (1b): To a solution of tert-butyl (S)-(3-hydroxy-1-(methoxy(methyl)amino)-1-oxopropan-2-yl)carbamate (21.14 g, 85.19 mmol) in 70 mL of DMF at 0 °C was added imidazole (17.40 g, 255.58 mmol), DMAP (520 mg, 4.26 mmol), and TBSCl (15.41 g, 102.23 mmol). The reaction was stirred for 40 min at room temperature. Deionized water (500 mL) and AcOEt (300 mL) were added. The aqueous layer was extracted with AcOEt (2 × 150 mL). The combined organic layers were washed with water (3 × 300 mL) and brine (150 mL), dried over MgSO4, and evaporated in vacuo. The crude was purified in silica gel in Hex/AcOEt (9:1). A yellow oil was obtained (26.31 g, 85%). 1H-NMR (400 MHz, CDCl3) δ5.31 (d, J = 9.0 Hz, 1H, NH), 4.69–4.64 (m, 1H, CHN), 3.77 (dd, J = 10.1, 4.7 Hz, 1H, CH2O), 3.71 (dd, J = 10.0, 5.2 Hz, 1H, CH2O), 3.67 (s, 3H, OCH3), 3.13 (s, 3H, NCH3), 1.35 (s, 9H, 3×CH3), 0.78 (s, 9H, 3×CH3), −0.06 (s, 6H, 2×CH3) ppm. 13C-NMR (100 MHz, CDCl3) δ 170.7, 155.4, 79.4, 63.5, 61.4, 52.4, 32.1, 28.3 (3C), 25.8 (3C), 18.2, −5.6 (2C) ppm. IR (NaCl): 2988, 2879, 1738, 1706 cm−1. (c 0.24 in DCM): +11.08. Anal. Calc. for C16H34N2O5Si: C, 53.0; H, 9.5; N, 7.7%. Found: C, 53.1; H, 9.8; N, 7.6%.
Tert-butyl (S)-allyl(3,8,8,9,9-pentamethyl-4-oxo-2,7-dioxa-3-aza-8-siladecan-5-yl)carbamate: To a suspension of NaH 60% w/w (4.35g, 108.86 mmol) at 0 °C in 90 mL of DMF was added a solution of 1b (19.72 g, 54.53 mmol) in 100 mL of DMF. Then, allyl bromide (16 mL, 183.9 mmol) was added. The reaction was stirred for 30 min at room temperature and 2 h at 50 °C. A solution of saturated NH4Cl was added, until all salts were dissolved. The aqueous layer was extracted with AcOEt (3 × 150 mL). The combined organic layers were washed with a saturated solution of NH4Cl (150 mL) and a saturated solution of NaHCO3 (150 mL) and brine (100 mL), dried over MgSO4, and evaporated in vacuo. The crude was purified in silica gel in Hex/AcOEt (9:1). A yellow oil was obtained (12.81 g, 58%). 1H-NMR (300 MHz, CDCl3) δ 5.92–5.71 (m, 1H), 5.34–4.93 (m, 3H), 4.02–3.76 (m, 4H), 3.72 (s, 3H), 3.15 (s, 3H), 1.43 (s, 9H), 0.86 (s, 9H), 0.04 (s, 6H) ppm. 13C-NMR (75 MHz, CDCl3) δ 170.6, 155.6 (major), 155.0 (minor), 136.0 (major), 135.3 (minor), 115.4 (minor), 115.1 (major), 80.1 (minor), 79.8 (major), 61.6, 61.0 (minor), 60.7 (major), 56.9 (minor), 55.5 (major), 46.5, 32.2, 28.4 (3C), 25.8 (3C), 18.2 (minor), 18.1 (major), −5.5 (2C) ppm. IR (NaCl): 3024, 2992, 2889, 1731, 1710 cm−1. (c 0.26 in DCM): −35.63. Anal. Calc. for C19H38N2O5Si: C, 56.7; H, 9.5; N, 7.0%. Found: C, 56.3; H, 9.2; N, 7.3%.
Tert-butyl-(S)-2-(((tert-butyldimethylsilyl)oxy)methyl)-3-oxo-3,6-dihydropyridine-1(2H)-carboxylate (2b): A solution of tert-butyl(S)-allyl(3,8,8,9,9-pentamethyl-4-oxo-2,7-dioxa-3-aza-8-siladecan-5-yl)carbamate (2.18 g, 5.42 mmol) in THF (10 mL) was cooled to −30 °C. A solution of vinylmagnesium bromide 1M (12 mL) was added slowly, keeping the temperature below −25 °C. When the addition finished, the reaction was stirred for a further 30 min. The reaction was poured into a mixture of 10mL of HCl 10% and MeOH (20mL) cooled in a bath at −15 °C. This mixture was stirred for another 30 min at −15 °C. The aqueous layer was extracted with AcOEt (3 × 40 mL). The combined organic layers were washed with a saturated solution of NH4Cl (40 mL), a saturated solution of NaHCO3 (40 mL), and brine (40 mL). The organic phase was dried over MgSO4 and evaporated in vacuo. The crude mixture was dissolved in 9 mL of DCM and heated to reflux. Grubbs’ 2nd generation catalyst (179 mg, 0.21 mmol) was then added. The reaction was stirred for 1.5 h. The reaction was filtered through a pad of celite. The solvent was evaporated in vacuo, and the crude was purified in silica gel in Hex:AcOEt (9:1). An orange solid was obtained as 2 conformers in the ratio 63:37 (1.11 g, 60% after 2 steps). 1H-NMR (400 MHz, CDCl3) δ 7.01 (ddd, J = 10.4, 5.0, 2.0 Hz, 1H, =CHCH2, major), 6.90 (ddd, J = 10.4, 5.0, 2.0 Hz, 1H, =CHCH2, minor), 6.17 (d, J = 10.4 Hz, 2H, =CHCO, major + minor), 4.69 –4.60 (m, 2H, CH2N, major and CHN, minor), 4.55–4.47 (m, 2H, CH2N, minor and CHN, major), 4.00–3.92 (m, 5H, CH2N, major + minor and CH2O, major + minor), 3.82 (dd, J = 10.2, 3.1 Hz, 1H, CH2O, major), 1.49 (s, 9H, 3×CH3, minor), 1.47 (s, 9H, 3×CH3, major), 0.81 (s, 18H, 3×CH3, major + minor), −0.02 (s, 6H, CH3, major + minor), −0.04 (s, 6H, CH3, major + minor) ppm. 13C-NMR (100 MHz, CDCl3) δ 195.5, 154.4 (major), 154.3 (minor), 147.4 (major), 146.2 (minor), 127.8 (minor), 127.6 (major), 81.0 (major), 80.9 (minor), 65.9 (major), 65.7 (minor), 62.8 (major), 61.7 (minor), 44.2 (minor), 43.1 (major), 28.5 (3C), 25.9 (3C), 18.2, −5.6 (minor, 2C), −5.7 (major, 2C) ppm. IR (KBr): 3044, 2987, 2895, 1741, 1696 cm−1. (c 0.14 in DCM): +84.93. MP: 50.8 °C –55.6 °C. Anal. Calc. for C17H31NO4Si: C, 59.8; H, 9.2; N, 4.1%. Found: C, 60.0; H, 9.4; N, 3.9%.
Di-tert-butyl (1S,4S,6R)-4-(((tert-butyldimethylsilyl)oxy)methyl)-5-oxo-3-azabicyclo[4.1.0] heptane-3,7-dicarboxylate (3b and 4b): To a solution of 2b (1.11 g, 3.25 mmol) in 3 mL of DCM at 0 °C was slowly added a solution of tert-butyl 2-(tetrahydro-1λ4-thiophen-1-ylidene)acetate (1.97 g, 9.75 mmol) in 55 mL of DCM. The reaction was stirred for 20 min at 0 °C and 20 more minutes at room temperature. Deionized water was added (50 mL), and the layers were separated. The aqueous phase was extracted with DCM (2 × 50 mL). The combined organic layers were washed with brine (50 mL), dried over MgSO4, and the solvent was evaporated in vacuo. The crude was separated in silica gel in Hex:AcOEt (9:1). Exo isomer was obtained as 2 conformers in a ratio 58:42 as an orange solid (1.05 g, 71%). Endo isomer was obtained as 2 conformers in a ratio 52:48 as a brown wax (260 mg, 18%).
Spectroscopic data for di-tert-butyl (1R,4S,6S,7S)-4-(([tert-butyldimethylsilyl]oxy)methyl)-5-oxo-3-azabicyclo[4.1.0]heptane-3,7-dicarboxylate (3b): 1H-NMR (400 MHz, CDCl3) δ4.47 (dd, J = 13.5, 1.9 Hz, 1H, CH2N, major), 4.38 (t, J = 2.9 Hz, 1H, CHN, minor), 4.29 (dd, J = 13.6, 2.0 Hz, 1H, CH2N, minor), 4.21 (t, J = 3.0 Hz, 1H, CHN, major), 4.15 (dt, J = 10.0, 3.1 Hz, 2H, CH2O, major + minor), 3.79 (dd, J = 10.2, 2.7 Hz, 1H, CH2O, minor), 3.74 (d, J = 12.4 Hz, 1H, CH2N, minor), 3.71 (dd, J = 10.1, 2.7 Hz, 1H, CH2O, major), 3.63 (dd, J = 13.6, 1.8 Hz, 1H, CH2N, major), 2.34–2.31 (m, 2H, CHCO, major + minor), 2.19 (t, J = 4.4 Hz, 2H, CHCO2, major + minor), 2.18–2.09 (m, 2H, CHCH2N, major + minor), 1.42 (s, 18H, 3×CH3, major + minor), 1.415 (s, 9H, 3×CH3, minor), 1.411 (s, 9H, 3×CH3, major), 0.82 (s, 18H, 3×CH3, major + minor), −0.02 (s, 12H, 2×CH3, major + minor) ppm. 13C-NMR (100 MHz, CDCl3) δ 202.5 (major), 202.3 (minor), 169.5, 155.0 (minor), 154.7 (major), 82.0 (major), 81.9 (minor), 81.1, 66.0 (major), 65.6 (minor), 62.4 (major), 61.5 (minor), 39.4 (minor), 37.9 (major), 32.90 (minor), 32.87 (major), 28.5 (3C), 28.10 (3C, minor), 28.08 (3C, major), 25.9 (3C, minor), 25.8 (3C, major), 24.8 (major), 24.7 (minor), 24.6 (minor), 24.4 (major), 18.16, −5.5 (2C, minor), −5.7 (2C, major) ppm. IR (KBr): 2991, 2887, 1724, 1711, 1691 cm−1. (c 0.15 in DCM): +6.60. m.p.: 74.2 °C–79.0 °C. Anal. Calc. for C23H41NO6Si: C, 60.6; H, 9.1; N, 3.1%. Found: C, 60.3; H, 9.4; N, 3.3%.
Spectroscopic data for di-tert-butyl (1R,4S,6S,7R)-4-(([tert-butyldimethylsilyl]oxy)methyl)-5-oxo-3-azabicyclo[4.1.0]heptane-3,7-dicarboxylate (4b): 1H-NMR (400 MHz, CDCl3) δ 4.54 (d, J = 13.4 Hz, 1H, CH2N, major), 4.43 (d, J = 13.2 Hz, 1H, CH2N, minor), 4.24 (t, J = 2.7 Hz, 1H, CHN, minor), 4.21–4.12 (m, 3H, CHN, major and CH2O, major + minor), 3.92–3.85 (m, 2H, CH2N, minor and CH2O, minor), 3.82 (dd, J = 13.5, 3.2 Hz, 1H, CH2N, major), 3.77 (dd, J = 10.4, 2.9 Hz, 1H, CH2O, major), 2.29–2.20 (m, 2H, CHCO, major + minor), 2.09 (t, J = 8.6 Hz, 2H, CHCO2, major + minor), 1.95–1.81 (m, 2H, CHCH2N, major + minor), 1.45 (s, 9H, 3×CH3, minor), 1.40 (s, 9H, 3×CH3, major), 1.39 (s, 9H, 3×CH3, major), 1.38 (s, 9H, 3×CH3, minor), 0.84 (s, 18H, 3×CH3, major + minor), 0.00 (s, 6H, CH3, major + minor), −0.01 (s, 6H, CH3, major + minor) ppm. 13C-NMR (100 MHz, CDCl3) δ 204.0 (major), 203.4 (minor), 167.4 (major), 167.0 (minor), 154.7 (minor), 154.6 (major), 82.5 (minor), 82.1 (major), 80.9 (minor), 80.7 (major), 67.5 (major), 66.3 (minor), 62.8 (major), 62.0 (minor), 38.5 (minor), 37.5 (major), 28.9, 28.6 (3C,), 28.2 (3C, major), 28.0 (3C, minor), 27.8, 25.91 (3C, minor), 25.89 (3C major), 22.0 (major), 21.9 (minor), 18.16 (minor), 18.13 (major), −5.6 (major + minor), −5.7 (major + minor) ppm. IR (NaCl): 2995, 2894, 1721, 1716, 1699 cm−1. (c 0.09 in DCM): +34.09. Anal. Calc. for C23H41NO6Si: C, 60.6; H, 9.1; N, 3.1%. Found: C, 60.1; H, 8.9; N, 3.4%.
Di-tert-butyl (1R,4S,6S,7S)-5-hydroxy-4-methyl-3-azabicyclo[4.1.0]heptane-3,7-dicarboxylate (5a and 5b): To a solution of 3a (4.10 g, 12.61 mmol) in absolute ethanol (90 mL) at 0 °C was added NaBH4 (954 mg, 25.22 mmol). The reaction was stirred for 1 h at room temperature. Then, a saturated solution of NH4Cl was added (until salts were dissolved). The aqueous phase was extracted with AcOEt (3 × 50 mL). The combined organic layers were washed with brine (60 mL). A solid was obtained as a mixture of two isomers in a 3:2 ratio, that were separated by silica gel column chromatography using Hex:AcOEt (5:1) to Hex/AcOEt (2:1) as eluents (3.10 g, 55% (5a) and 19% (5b)).
Spectroscopic data from di-tert-butyl (1R,4S,5S,6S,7S)-5-hydroxy-4-methyl-3-azabicyclo [4.1.0]heptane-3,7-dicarboxylate (5a): White solid (2.27 g, 55%).1H-NMR (400 MHz, CDCl3) δ 4.16 (brs, 1H, CHN), 4.01 (d, J = 14.1 Hz, 1H, CH2N), 3.84 (td, J = 5.9, 5.4, 2.0 Hz, 1H, CHOH), 3.28 (dd, J = 14.1, 4.1 Hz, 1H, CH2N), 1.63–1.58 (m, 1H, CHCH2N), 1.55 (ddd, J = 9.4, 4.5, 2.0 Hz, 1H, CHCHOH), 1.434 (s, 9H, 3×CH3), 1.431 (s, 9H, 3×CH3), 1.22 (t, J = 4.6 Hz, 1H, CHCO2), 1.15 (d, J = 6.9 Hz, 3H, CH3) ppm. 13C-NMR (100 MHz, CDCl3) δ 172.6, 155.1, 80.9, 80.3, 67.5, 48.4, 36.1, 28.6 (3C), 28.3 (3C), 25.2, 25.0, 20.2, 11.4 ppm. IR (KBr): 3389, 2986, 2884, 1727, 1720 cm−1. (c 0.13 in DCM): +7.43. m.p.: 123.8 °C–127.1 °C. Anal. Calc. for C17H29NO5: C, 62.4; H, 8.9; N, 4.3%. Found: C, 62.6; H, 9.2; N, 4.1%.
Spectroscopic data from di-tert-butyl (1R,4S,5R,6S,7S)-5-hydroxy-4-methyl-3-azabicyclo [4.1.0]heptane-3,7-dicarboxylate (5b): White solid (770 mg, 19%).1H-NMR (400 MHz, CDCl3) δ 4.15–4.08 (m, 2H, CHN+CH2N), 3.99–3.97 (m, 1H, CHOH), 3.20 (d, J = 13.8 Hz, 1H, CH2N), 1.95–1.90 (m, 1H, CHCHOH), 1.67–1.63 (m, 2H, 2×CH cyclopropane), 1.46 (s, 9H, 3×CH3), 1.44 (s, 9H, 3×CH3), 1.13 (d, J = 7.2 Hz, 3H, CH3) ppm. 13C-NMR (100 MHz, CDCl3) δ 172.9, 155.9, 80.8, 80.3, 66.3, 51.3, 35.4, 28.5 (3C), 28.3 (3C), 23.6, 20.6, 19.7, 16.1 ppm. IR (KBr): 3395, 2974, 2891, 1729, 1718 cm−1. (c 0.12 in DCM): −2.61. m.p.: 96.0 °C–98.6 °C. Anal. Calc. for C17H29NO5: C, 62.4; H, 8.9; N, 4.3%. Found: C, 62.2; H, 9.1; N, 4.0%.
Tert-butyl (1S,4S,6S,7S)-5-hydroxy-7-(hydroxymethyl)-4-methyl-3-azabicyclo[4.1.0]heptane-3-carboxylate: To a solution of 5a (2.172 g, 6.62 mmol) in 55 mL of DCM cooled to 0 °C was added DIBAL-H 1.2M in toluene (22 mL). The reaction was stirred at room temperature for 1 h. The solvent was evaporated in vacuo. The crude was purified in silica gel in Hex:AcOEt (1:3). A yellow wax was obtained as two conformers in a ratio 1:1 (931 mg, 55%). 1H-NMR (400 MHz, CDCl3) δ4.05 (brs, 1H, CHN), 3.81 (dd, J = 13.8, 1.7 Hz, 1H, CH2N), 3.76–3.69 (m, 2H, CH2O+CHOH), 3.33 (dd, J = 13.8, 4.7 Hz, 1H, CH2N), 3.21 (t, J = 9.7 Hz, 1H, CH2O), 1.42 (s, 9H, 3×CH3), 1.14 (d, J = 6.7 Hz, 3H, CH3), 0.97-0.88 (m, 2H, 2×CH cyclopropane), 0.81–0.75 (m, 1H, CHCH2O) ppm. 13C-NMR (100 MHz, CDCl3) δ 155.4, 80.1, 68.6 (β), 68.5 (α), 65.5 (β), 65.4 (α), 48.7, 37.0, 28.6 (3C), 25.2, 20.23 (β), 20.19 (α), 14.7, 11.8 ppm. IR (NaCl): 3402, 2974, 2884, 1729 cm−1. (c 0.17 in DCM): +14.68. Anal. Calc. for C13H23NO4: C, 60.7; H, 9.0; N, 5.4%. Found: C, 60.3; H, 9.2; N, 5.7%.
(1S,4S,5S,6S,7S)-7-(hydroxymethyl)-4-methyl-3-azabicyclo[4.1.0]heptan-5-ol (6a): To a solution of tert-butyl (1S,4S,6S,7S)-5-hydroxy-7-(hydroxymethyl)-4-methyl-3-azabicyclo[4.1.0]heptane-3-carboxylate (931 mg, 3.62 mmol) in 0.5 mL of DCM and 1 mL of MeOH was added 30 mL of TFA. The reaction was stirred 30 min at room temperature. The solvent was evaporated in vacuo. The crude was eluted through a basic DOWEX resin. A brown wax was obtained (484 mg, 85%). 1H-NMR (300 MHz, MeOD) δ 4.09 (t, J = 1.7 Hz, 1H), 3.64 (dd, J = 13.6, 8.5 Hz, 1H), 3.48 (dd, J = 11.4, 6.0 Hz, 1H), 3.40 (dd, J = 11.4, 6.5 Hz, 1H), 3.13 (dd, J = 13.6, 2.5 Hz, 1H), 2.94 (qd, J = 6.6, 1.7 Hz, 1H), 1.34 (ddd, J = 9.1, 5.2, 1.7 Hz, 1H), 1.28 (d, J = 6.6 Hz, 3H), 1.25–1.18 (m, 1H), 1.18–1.07 (m, 1H) ppm. 13C-NMR (75 MHz, MeOD) δ 65.0, 64.8, 52.2, 44.0, 26.7, 23.9, 15.3, 11.4 ppm. IR (NaCl): 3419, 2986, 2899 cm−1. (c 0.04 in MeOH): +10.75. Anal. Calc. for C8H15NO2: C, 61.1%; H, 9.1; N, 8.9%. Found: C, 61.2; H, 9.4; N, 8.7%.
Tert-butyl (1S,4S,5R,6S,7S)-5-hydroxy-7-(hydroxymethyl)-4-methyl-3-azabicyclo[4.1.0] heptane-3-carboxylate: To a solution of 5b (770 mg, 2.34 mmol) in 55 mL of DCM cooled to 0 °C was added DIBAL-H 1.2M in toluene (22 mL). The reaction was stirred at room temperature for 1 h. The solvent was evaporated in vacuo. The crude was purified in silica gel in Hex:AcOEt (1:3). A yellow wax was obtained (400 mg, 67%). 1H-NMR (400 MHz, CDCl3) δ 4.19–3.98 (m, 3H, OH+CH2N+CHN), 3.93 (d, J = 7.2 Hz, 1H, CHOH), 3.87 (dd, J = 11.2, 4.8 Hz, 1H, CH2O), 3.13 (d, J = 13.0 Hz, 1H; CH2N), 3.00 (dd, J = 11.2, 9.1 Hz, 1H, CH2O), 2.66 (brs, 1H, OH), 1.43 (s, 9H, 3×CH3), 1.28–1.23 (m, 1H, CHCHOH), 1.17–1.12 (m, 1H, CHCH2N), 1.09 (d, J = 7.1 Hz, 3H, CH3), 0.97–0.92 (m, 1H, CHCH2OH) ppm. 13C-NMR (100 MHz, CDCl3) δ 156.1, 80.1, 66.9, 65.6, 51.2, 35.7, 28.5 (3C), 19.7, 18.6, 16.5, 15.3 ppm. IR (NaCl): 3409, 2991, 2892, 1726 cm−1. (c 0.14 in DCM): −6.84. Anal. Calc. for C13H23NO4: C, 60.7; H, 9.0; N, 5.4%. Found: C, 60.3; H, 9.2; N, 5.2%.
(1S,4S,5R,6S,7S)-7-(hydroxymethyl)-4-methyl-3-azabicyclo[4.1.0]heptan-5-ol (6b): To a solution of tert-butyl (1S,4S,5R,6S,7S)-5-hydroxy-7-(hydroxymethyl)-4-methyl-3-azabicyclo[4.1.0] heptane-3-carboxylate (400 mg, 1.55 mmol) in 1 mL of DCM and 2 mL of MeOH was added 12 mL of TFA. The reaction was stirred for 30 min at room temperature. The solvent was evaporated in vacuo. The crude was eluted through a basic DOWEX resin. A brown wax was obtained (202 mg, 83%). 1H-NMR (400 MHz, MeOD) δ 3.98 (dd, J = 9.5, 5.4 Hz, 1H, CHOH), 3.62–3.54 (m, 2H, CH2O+CH2N), 3.35-3.27 (m, 1H, CH2O), 3.03–2.99 (m, 1H, CH2N), 2.63 (dq, J = 9.5, 6.4 Hz, 1H, CHN), 1.42–1.38 (m, 2H, 2×CH cyclopropane), 1.34–1.30 (m, 4H, CH3+CHCH2OH) ppm. 13C-NMR (100 MHz, MeOD) δ 68.2, 65.0, 53.4, 43.3, 26.6, 21.9, 15.9, 15.7 ppm. IR (NaCl): 3391, 2995, 2899 cm−1. (c 0.03 in MeOH): −51.91. Anal. Calc. for C8H15NO2: C, 61.1%; H, 9.6; N, 8.9%. Found: C, 60.8; H, 9.4; N, 9.1%.
Tert-butyl (1S,4S,5R,6S,7R)-5-hydroxy-7-(hydroxymethyl)-4-methyl-3-azabicyclo[4.1.0]heptane-3-carboxylate: To a solution of 4a (280 mg, 0.86 mmol) in 10 mL of DCM at 0 °C was added DIBAL-H 1.2M in toluene (3.6 mL). The reaction was stirred 1 h at room temperature. Methanol was added (12 mL). The salts were filtered through a pad of celite and rinsed with methanol. The solvent was evaporated in vacuo and purified in silica gel in Hex:AcOEt (1:2). A yellow wax was obtained (71 mg, 32%). 1H-NMR (400 MHz, CDCl3) δ 4.24–4.18 (m, 2H, CHN+CHOH), 3.94 (d, J = 14.3 Hz, 1H, CH2N), 3.89 (dd, J = 11.6, 5.9 Hz, 1H, CH2O), 3.54 (t, J = 11.4 Hz, 1H, CH2O), 3.31 (brs, 1H, OH), 3.25 (dd, J = 14.2, 5.7 Hz, 1H, CH2N), 2.02 (brs, 1H, OH), 1.43 (s, 10H, 3×CH3+CHCHOH), 1.33–1.26 (m, 1H, CHCH2OH), 1.14–1.11 (m, 4H, CH3+CHCH2OH) ppm. 13C-NMR (100 MHz, CDCl3) δ 155.5, 80.2, 68.1, 58.8, 51.2, 34.3, 28.6 (3C), 21.0, 16.0, 14.5, 11.3 ppm. IR (NaCl): 3392, 2981, 2896, 1719 cm−1. (c 0.01 in DCM): +60.0. Anal. Calc. for C13H23NO4: C, 60.7; H, 9.0; N, 5.4%. Found: C, 60.5; H, 9.3; N, 5.1%.
(1S,4S,5R,6S,7R)-7-(hydroxymethyl)-4-methyl-3-azabicyclo[4.1.0]heptan-5-ol (6c): To a solution of tert-butyl (1S,4S,5R,6S,7R)-5-hydroxy-7-(hydroxymethyl)-4-methyl-3-azabicyclo[4.1.0]heptane-3-carboxylate (112 mg, 0.44 mmol) in 0.5 mL of DCM and 0.5 mL of MeOH was added 4 mL of TFA. The reaction was stirred for 30 min at room temperature. The solvent was evaporated in vacuo. The crude was eluted through a basic DOWEX resin. A brown wax was obtained (56 mg, 81%). 1H-NMR (400 MHz, MeOD) δ 4.08 (dd, J = 9.8, 7.2 Hz, 1H, CHOH), 3.87 (dd, J = 12.5, 7.2 Hz, 1H, CH2O), 3.80 (dd, J = 12.5, 9.2 Hz, 1H, CH2O), 3.69 (dd, J = 14.3, 9.6 Hz, 1H, CH2N), 2.95 (dd, J = 14.3, 5.1 Hz, 1H, CH2N), 2.60 (dq, J = 9.7, 6.3 Hz, 1H, CHN), 1.75 (qd, J = 9.4, 5.1 Hz, 1H, CHCH2N), 1.60 (td, J, 9.2, 7.2 Hz, 1H, CHCHOH), 1.53–1.44 (m, 1H, CHCH2OH), 1.39 (d, J = 6.3 Hz, 3H, CH3) ppm. 13C-NMR (100 MHz, MeOD) δ 69.3, 58.4, 56.0, 40.1, 24.7, 18.7, 17.2, 14.8 ppm. IR (NaCl): 3413, 2992, 2905 cm−1. (c 0.01 in MeOH): −72.95. Anal. Calc. for C8H15NO2: C, 61.1%; H, 9.6; N, 8.9%. Found: C, 61.3; H, 9.9; N, 8.7%.
Di-tert-butyl (1R,4S,5S,6S,7S)-4-(((tert-butyldimethylsilyl)oxy)methyl)-5-hydroxy-3-azabicyclo [4.1.0]heptane-3,7-dicarboxylate (7): To a solution of 3b (970 mg, 2.13 mmol) in 11 mL of absolute ethanol at 0 °C was added NaBH4 (161 mg, 4.26 mmol). The reaction was stirred for 1 h at room temperature. A saturated solution of NH4Cl was added (30 mL). The aqueous phase was extracted with AcOEt (3 × 40 mL). The combined organic layers were washed with brine (40 mL). Only one isomer was observed, which was purified in silica gel in Hex:AcOEt (4:1). A yellow wax was obtained (716 mg, 74%). 1H-NMR (400 MHz, CDCl3) δ 4.16 (brs, 1H, CHOH), 4.06–3.90 (m, 3H, CH2N+CH2O+CHN), 3.89–3.78 (m, 1H, CH2O), 3.34 (brs, 1H, CH2N), 1.78 (dt, J = 9.3, 3.8 Hz, 1H, CHCHOH), 1.71–1.65 (m, 1H, CHCH2N), 1.44 (s, 9H, 3×CH3), 1.43 (s, 9H, 3×CH3), 1.37 (t, J = 4.1 Hz, 1H, CHCO2), 0.89 (s, 9H, 3×CH3), 0.083 (s, 3H, CH3), 0.076 (s, 3H, CH3) ppm. 13C-NMR (100 MHz, CDCl3) δ 172.1, 155.2, 80.8, 80.4, 67.2, 61.9, 53.4, 37.2, 28.6 (3C), 28.2 (3C), 25.9 (3C), 25.3, 23.8, 20.2, 18.1, −5.4 (2C) ppm. IR (NaCl): 3429, 2988, 2892, 1714, 1696 cm−1. (c 0.02 in DCM): +16.49. Anal. Calc. for C23H43NO6Si: C, 60.4; H, 9.5; N, 3.1%. Found: C, 60.6; H, 9.4; N, 3.4%.
Di-tert-butyl (1R,4S,5S,6S,7S)-5-hydroxy-4-(hydroxymethyl)-3-azabicyclo [4.1.0]heptane-3,7-dicarboxylate: To a solution of 7 (300 mg, 0.66 mmol) in 3 mL of THF was added TBAF.3H2O (303 mg, 0.96 mmol). The reaction was stirred for 1 h at room temperature. The solvent was evaporated in vacuo and purified in silica gel in Hex/AcOEt (1:1). A white solid was obtained (192 mg, 85%). 1H-NMR (400 MHz, MeOD) δ 4.14 (brs, 1H, CHN), 4.10–4.01 (m, 1H, CH2N), 3.92 (dd, J = 6.1, 1.7 Hz, 1H, CHOH), 3.88-3.84 (m, 1H, CH2O), 3.73 (dd, J = 11.5, 9.7 Hz, 1H, CH2O), 3.42–3.31 (m, 1H, CH2N), 1.66–1.59 (m, 1H, CHCH2N), 1.56 (ddd, J = 9.4, 4.5, 2.1 Hz, 1H, CHCHOH), 1.46 (s, 9H, 3×CH3), 1.44 (s, 9H, 3×CH3), 1.20 (t, J = 4.5 Hz, 1H, CHCO2) ppm. 13C-NMR (100 MHz, MeOD) δ 172.7, 156.1, 80.6, 80.2, 65.7 (major), 65.5 (minor), 56.7, 54.9 (major), 53.7 (minor), 36.6 (minor), 35.4 (major), 27.2 (3C), 26.9 (3C), 25.4, 24.4, 20.5 (minor), 20.3 (major) ppm. IR (NaCl): 3435, 2991, 2884, 1716, 1692 cm−1. (c 0.02 in MeOH): +6.02. m.p.: 173.8 °C –172.5 °C. Anal. Calc. for C17H29NO6: C, 59.5; H, 8.5; N, 4.1%. Found: C, 59.2; H, 8.8; N, 4.2%.
(1R,4S,5S,6S,7S)-7-carboxy-5-hydroxy-4-(hydroxymethyl)-3-azabicyclo[4.1.0]heptan-3-ium trifluoroacetate (10a): To a solution of di-tert-butyl (1R,4S,5S,6S,7S)-5-hydroxy-4-(hydroxymethyl)-3-azabicyclo[4.1.0]heptane-3,7-dicarboxylate (192mg, 0.56mmol) in a mixture of 3 mL of DCM and 0.7 mL of MeOH was added TFA (5.6 mL). The reaction was stirred for 20 min. The solvent was evaporated in vacuo. A brown wax was obtained (140 mg, 87%). 1H-NMR (400 MHz, D2O) δ 4.47 (brs, 1H, CHOH), 3.89–3.81 (m, 2H, CH2N+CH2O), 3.78 (dd, J = 12.2, 8.5 Hz, 1H, CH2O), 3.31 (d, J = 13.5 Hz, 1H, CH2N), 3.03 (ddd, J = 8.2, 4.4, 1.5 Hz, 1H, CHN), 2.05–1.99 (m, 2H, 2×CH cyclopropane), 1.88 (t, J = 4.6 Hz, 1H, CHCO2) ppm. 13C-NMR (100 MHz, D2O) δ 175.9, 163.0 (q, J = 35.5 Hz), 116.3 (q, J = 291.7 Hz), 60.6, 59.4, 56.3, 41.6, 26.1, 24.3, 15.8 ppm. IR (NaCl): 3398, 2991, 2884, 1710, cm−1. (c 0.03 in MeOH): +4.19. Anal. Calc. for C10H14F3NO5: C, 42.1; H, 5.0; N, 4.9%. Found: C, 41.9; H, 5.4; N, 5.1%.
Tert-butyl (1S,4S,5S,6S,7S)-4-(([tert-butyldimethylsilyl]oxy)methyl)-5-hydroxy-7-(hydroxymethyl)-3-azabicyclo[4.1.0]heptane-3-carboxylate: To a solution of 7 (400 mg, 0.87 mmol) at 0 °C in 3 mL of DCM was added 2.2 mL of DIBAL-H 1.2M in toluene. The reaction was stirred 1 h at room temperature. Methanol was added (5 mL). The salts were filtered and rinsed with methanol (3 × 15 mL). The solvent was evaporated in vacuo. The crude was purified in silica gel in Hex:AcOEt (1:1). A yellow wax was obtained (270 mg, 70%). 1H-NMR (400 MHz, CDCl3) δ 4.11 (brs, 1H, CHOH), 3.95–3.71 (m, 4H, 2CH2O+CHN+CH2N), 3.56 (dd, J = 11.2, 6.2 Hz, 1H, CH2OH), 3.39–3.26 (m, 2H, CH2OH+CH2N), 1.41 (s, 9H, 3×CH3), 1.13–1.05 (m, 1H, CHCHOH), 1.06–0.99 (m, 1H, CHCH2N), 0.99–0.90 (m, 1H, CHCH2OH), 0.85 (s, 9H, 3×CH3), 0.05 (s, 3H, CH3), 0.04 (s, 3H, CH3) ppm. 13C-NMR (100 MHz, CDCl3) δ 155.5, 80.2, 67.8, 65.0, 61.7, 54.0, 37.8, 28.5 (3C), 25.8 (3C), 23.3, 20.5, 18.1, 15.1, −5.4 (2C) ppm. IR (NaCl): 3384, 2984, 2896, 1698 cm−1. (c 0.10 in CHCl3): +23.77. Anal. Calc. for C19H37NO5Si: C, 58.9; H, 9.6; N, 3.6%. Found: C, 59.1; H, 9.3; N, 3.5%.
Tert-butyl (1S,4S,5S,6S,7S)-5-hydroxy-4,7-bis(hydroxymethyl)-3-azabicyclo[4.1.0]heptane-3-carboxylate: To a solution of tert-butyl (1S,4S,5S,6S,7S)-4-(([tert-butyldimethylsilyl]oxy)methyl)-5-hydroxy-7-(hydroxymethyl)-3-azabicyclo[4.1.0]heptane-3-carboxylate (270 mg, 0.70 mmol) in 7 mL of THF was added TBAF.3H2O (243 mg, 0.77 mmol). The reaction was stirred at room temperature for 1 h. The solvent was evaporated in vacuo and purified in silica gel in AcOEt 100%. A yellow wax was obtained (185 mg, 68%). 1H-NMR (400 MHz, MeOD) δ 4.05–3.98 (m, 2H, CHN+CH2N), 3.95 (dd, J = 5.8, 2.6 Hz, 1H, CHOH), 3.91–3.81 (m, 1H, NCHCH2OH), 3.74 (dd, J = 11.5, 8.6 Hz, 1H, NCHCH2OH), 3.47–3.26 (m, 3H, 2CH2OH+CH2N), 1.45 (s, 9H, 3×CH3), 1.06–0.98 (m, 1H, CHCH2N), 1.00–0.91 (m, 1H, CHCHOH), 0.75 (tt, J = 6.8, 4.6 Hz, 1H, CHCH2OH) ppm. 13C-NMR (100 MHz, MeOD) δ 157.7, 81.3, 68.0, 65.5, 58.9, 56.5 (major), 55.5 (minor), 38.9 (minor), 37.7 (major), 28.7 (3C), 25.0, 21.6, 16.7 (minor), 16.4 (major) ppm. IR (NaCl): 3413, 2992, 2887, 1702 cm−1. (c 0.05 in MeOH): +13.02. Anal. Calc. for C13H23NO5: C, 57.1; H, 8.5; N, 5.1%. Found: C, 57.3; H, 8.3; N, 5.0%.
((1S,4S,5S,6S,7S)-4,7-bis(hydroxymethyl)-3-azabicyclo[4.1.0]heptane-5-ol (11a): To a solution of tert-butyl (1S,4S,5S,6S,7S)-5-hydroxy-4,7-bis(hydroxymethyl)-3-azabicyclo[4.1.0]heptane-3-carboxylate (185 mg, 0.68 mmol) in 3 mL of DCM and 0.7 mL of MeOH was added TFA (6.8 mL). The reaction was stirred for 20 min. The solvent was evaporated in vacuo. The crude was filtered through a basic DOWEX resin. A brown wax was obtained (107 mg, 91%). 1H-NMR (400 MHz, MeOD) δ 4.28 (brs, 1H, CHOH), 3.77–3.74 (m, 2H, NCHCH2OH), 3.70 (dd, J = 13.7, 8.3 Hz, 1H, CH2N), 3.48 (dd, J = 11.4, 6.1 Hz, 1H, CH2OH), 3.41 (dd, J = 11.4, 6.5 Hz, 1H, CH2OH), 3.12 (dd, J = 13.7, 2.4 Hz, 1H, CH2N), 2.86 (t, J = 5.9 Hz, 1H, CHN), 1.33-1.23 (m, 2H, 2×CH cyclopropane), 1.14 (p, J = 5.6 Hz, 1H, CHCH2OH) ppm. 13C-NMR (100 MHz, MeOD) δ 64.8, 62.7, 61.2, 58.5, 44.3, 26.7, 23.7, 11.9 ppm. IR (NaCl): 3429, 2988, 2892 cm−1. (c 0.02 in MeOH): +8.51. Anal. Calc. for C8H15NO3: C, 55.5; H, 8.7; N, 8.1%. Found: C, 55.9; H, 8.2; N, 8.3%.
(1R,4S,5S,6S,7R)-3-(tert-Butoxycarbonyl)-4-(([tert-butyldimethylsilyl]oxy)methyl)-5-hydroxy-3-azabicyclo[4.1.0]heptane-7-carboxylic acid (8): To a solution of 4b (260 mg, 0.57 mmol) in 3 mL of absolute ethanol was added NaBH4 (43 mg, 1.14 mmol). The reaction was stirred for 48 h. Methanol was added (5 mL). The salts were filtered and rinsed with methanol (3 × 10 mL). The solvent was evaporated in vacuo. The crude was purified in silica gel from Hex:AcOEt (4:1) to Hex:AcOEt (2:1). A yellow wax was obtained as 2 conformers in a ratio 56:44 (70 mg, 30%). 1H-NMR (400 MHz, CDCl3) δ 5.04 (brs, 2H, CHOH, major + minor), 4.18 (brs, 1H, CHN, minor), 4.13–4.04 (m, 1H, CHN, major), 3.78–3.48(m, 8H, CH2O and CH2N, major + minor), 2.56–2.47 (m, 2H, CHCHOH, major + minor), 2.22 (dd, J = 8.5, 6.2 Hz, 2H, CHCO2, major + minor), 1.82–1.70 (m, 2H, CHCH2N, major + minor), 1.44 (s, 18H, 3×CH3, major + minor), 0.89 (s, 18H, 3×CH3, major + minor), 0.07 (s, 6H, CH3, major + minor), 0.06 (s, 6H, CH3, major + minor) ppm. 13C-NMR (100 MHz, CDCl3) δ 173.7 (minor), 173.5 (major), 155.0, 80.6, 80.4, 74.1 (minor), 73.4 (major), 61.5 (major), 61.2 (minor), 52.4 (major), 51.6 (minor), 36.9 (minor), 35.9 (major), 28.4 (3C), 25.8 (3C), 23.9 (minor), 23.8 (major), 20.4 (minor), 19.8 (major), 18.1 (major), 16.3 (minor), −5.4, −5.5 ppm. IR (NaCl): 3408, 2995, 2891, 1712, 1698 cm−1. (c 0.02 in MeOH): +25.10. Anal. Calc. for C19H35NO6Si: C, 56.8; H, 8.8; N, 3.5%. Found: C, 56.4; H, 8.5; N, 3.7%.
(1R,4S,5S,6S,7R)-3-(tert-butoxycarbonyl)-5-hydroxy-4-(hydroxymethyl)-3-azabicyclo[4.1.0]heptane-7-carboxylic acid: To a solution of 8 (70 mg, 0.17 mmol) in 1 mL of THF was added TBAF.3H2O (59 mg, 0.19 mmol). The reaction was stirred for 30 min. The solvent was evaporated in vacuo. The crude was purified in silica gel in Hex:AcOEt (1:4). A yellow wax was obtained (48 mg, 88%). 1H-NMR (400 MHz, CDCl3) δ 5.03 (brs, 1H, CHOH), 4.24–4.15 (m, 1H, CHN), 3.84–3.74 (m, 2H, CH2O+CH2N), 3.73–3.63 (m, 1H, CH2O), 3.50 (d, J = 14.5 Hz, 1H, CH2N), 2.60–2.50 (m, 1H, CHCHOH), 2.23 (dd, J = 8.5, 6.2 Hz, 1H, CHCO2), 1.78 (qd, J = 8.0, 2.5 Hz, 1H, CHCH2N), 1.42 (s, 9H, 3×CH3) ppm.13C-NMR (100 MHz, CDCl3) δ 173.6, 156.1, 81.1, 73.3, 61.3, 52.6, 36.6, 28.3 (3C), 23.9, 19.9, 16.2 ppm. IR (NaCl): 3419, 2992, 2901, 1701 cm−1. (c 0.01 in MeOH): +15.41. Anal. Calc. for C13H21NO6: C, 54.4; H, 7.4; N, 4.9%. Found: C, 54.6; H, 7.1; N, 5.1%.
(1R,4S,5S,6S,7R)-7-carboxy-5-hydroxy-4-(hydroxymethyl)-3-azabicyclo[4.1.0]heptan-3-ium trifluoroacetate (10b): To a solution of (1R,4S,5S,6S,7R)-3-(tert-butoxycarbonyl)-5-hydroxy-4-(hydroxymethyl)-3-azabicyclo[4.1.0]heptane-7-carboxylic acid (48 mg, 0.17 mmol) in a mixture of 1 mL of DCM and 0.5 mL of MeOH was added TFA (1.7 mL). The reaction was stirred for 20 min. The solvent was evaporated in vacuo. A brown wax was obtained (44 mg, 92%). 1H-NMR (400 MHz, D2O) δ 5.19 (d, J = 5.5 Hz, 1H, CHOH), 4.00 (dd, J = 14.7, 8.7 Hz, 1H, CH2N), 3.93 (dd, J = 10.7, 5.4 Hz, 2H, CH2O), 3.71 (dd, J = 6.3, 4.5 Hz, 1H, CHN), 2.86–2.74 (m, 2H, CH2N+CHCHOH), 2.65 (dd, J = 8.4, 5.9 Hz, 1H, CHCO2), 2.30 (qd, J = 8.3, 6.4 Hz, 1H, CHCH2N) ppm.13C-NMR (100 MHz, D2O) δ 175.2, 163.0 (q, J = 35.5 Hz), 116.4 (q, J = 291.9 Hz), 73.6, 58.9, 54.3, 36.9, 24.7, 18.4, 14.3 ppm. IR (NaCl): 3385, 2997, 2899, 1707, cm−1. (c 0.01 in MeOH): +10.19. Anal. Calc. for C10H14F3NO5: C, 42.1; H, 5.0; N, 4.9%. Found: C, 41.8; H, 5.2; N, 5.0%.
Tert-butyl (1S,4S,5S,6S,7R)-4-(([tert-butyldimethylsilyl]oxy)methyl)-5-hydroxy-7-(hydroxymethyl)-3-azabicyclo[4.1.0]heptane-3-carboxylate (9): To a solution of 4b (260 mg, 0.57 mmol) in 3 mL of absolute ethanol was added NaBH4 (43 mg, 1.14 mmol). The reaction was stirred for 48 h. Methanol was added (5 mL). The salts were filtered and rinsed with methanol (3 × 10 mL). The solvent was evaporated in vacuo. The crude was purified in silica gel from Hex:AcOEt (4:1) to Hex:AcOEt (2:1). A yellow wax was obtained (110 mg, 50%). 1H-NMR (400 MHz, CDCl3) δ 4.35 (dd, J = 8.8, 1.4 Hz, 1H, CHOH), 4.11–4.03 (m, 1H, CHN), 3.93 (d, J = 14.2 Hz, 1H, CH2N), 3.86 (dd, J = 11.5, 5.7 Hz, 1H, CH2OH), 3.59 (dd, J = 10.1, 7.5 Hz, 1H, CH2O), 3.56–3.43 (m, 2H, CH2O+CH2OH), 3.12 (dd, J = 14.3, 5.3 Hz, 1H, CH2N), 2.87 (brs, 1H, OH), 1.67 (brs, 1H, OH), 1.43 (q, J = 8.9 Hz, 1H, CHCHOH), 1.38 (s, 9H, 3×CH3), 1.32–1.23 (m, 1H, CHCH2OH), 1.13–1.02 (m, 1H, CHCH2N), 0.82 (s, 9H, 3×CH3), 0.000 (s, 3H, CH3), −0.004 (s, 3H, CH3) ppm. 13C-NMR (100 MHz, CDCl3) δ 155.6, 80.3, 64.3, 61.6, 58.8, 57.4, 35.2, 28.6 (3C), 26.0 (3C), 21.6, 18.3, 15.3, 12.0, −5.30, −5.32 ppm. IR (NaCl): 3372, 2991, 2906, 1706 cm−1. (c 0.03 in CHCl3): +24.04. Anal. Calc. for C19H37NO5Si: C, 58.9; H, 9.6; N, 3.6%. Found: C, 59.2; H, 9.2; N, 3.4%.
Tert-butyl (1S,4S,5S,6S,7R)-5-hydroxy-4,7-bis(hydroxymethyl)-3-azabicyclo[4.1.0]heptane-3-carboxylate: To a solution of 9 (110 mg, 0.29 mmol) in 2 mL of THF was added TBAF.3H2O (101 mg, 0.32 mmol). The reaction was stirred for 30 min. The solvent was evaporated in vacuo. The crude was purified in silica gel in AcOEt. A yellow wax was obtained (68 mg, 86%). 1H-NMR (400 MHz, MeOD) δ 4.29 (d, J = 8.9 Hz, 1H, CHOH), 4.14 (t, J = 7.6 Hz, 1H, NCHCH2OH), 4.03 (d, J = 14.1 Hz, 1H, CH2N), 3.78 (dd, J = 11.7, 7.3 Hz, 1H, NCHCH2OH), 3.73–3.65 (m, 1H, CHN), 3.61 (dd, J = 11.3, 7.1 Hz, 1H, CH2OH), 3.52 (dd, J = 11.3, 8.3 Hz, 1H, CH2OH), 3.28–3.16 (m, 1H, CH2N), 1.51 (q, J = 9.1 Hz, 1H, CHCHOH), 1.46 (s, 9H, 3×CH3), 1.33–1.11 (m, 2H, 2×CH cyclopropane) ppm. 13C-NMR (100 MHz, MeOD) δ 157.6, 81.4, 64.4, 61.0, 59.6, 59.2 (major), 57.9(minor), 36.3 (minor), 35.1 (major), 28.7 (3C), 22.3, 16.2, 12.4 ppm. IR (NaCl): 3385, 2988, 2892, 1698 cm−1. (c 0.02 in MeOH): +10.68. Anal. Calc. for C13H23NO5: C, 57.1; H, 8.5; N, 5.1%. Found: C, 56.8; H, 8.3; N, 5.3%.
((1S,4S,5S,6S,7R)-4,7-bis(hydroxymethyl)-3-azabicyclo[4.1.0]heptane-5-ol (11b): To a solution of tert-butyl (1S,4S,5S,6S,7R)-5-hydroxy-4,7-bis(hydroxymethyl)-3-azabicyclo[4.1.0]heptane-3-carboxylate (68 mg, 0.25mmol) in 1.5 mL of DCM and 0.7 mL of MeOH was added TFA (2.5 mL). The reaction was stirred for 20 min. The solvent was evaporated in vacuo. The crude was filtered through a basic DOWEX resin. A brown wax was obtained (40 mg, 93%). 1H-NMR (400 MHz, MeOD) δ 4.34 (dd, J = 10.1, 7.3 Hz, 1H, CHOH), 3.94 (dd, J = 11.9, 3.0 Hz, 1H, NCHCH2OH), 3.85 (d, J = 8.3 Hz, 2H, CH2OH), 3.72 (dd, J = 12.0, 5.1 Hz, 1H, NCHCH2OH), 3.65 (dd, J = 14.3, 9.6 Hz, 1H, CH2N), 2.94 (dd, J = 14.2, 5.0 Hz, 1H, CH2N), 2.57–2.48 (m, 1H, CHN), 1.80–1.67 (m, 1H, CHCH2N), 1.60 (q, J = 8.9 Hz, 1H, CHCHOH), 1.46 (p, J = 8.4 Hz, 1H, CHCH2OH) ppm. 13C-NMR (100 MHz, MeOD) δ 63.7, 61.7, 60.4, 58.6, 40.1, 24.7, 18.9, 15.6 ppm. IR (NaCl): 3441, 2995, 2899 cm−1. (c 0.01 in MeOH): +6.38. Anal. Calc. for C8H15NO3: C, 55.5; H, 8.7; N, 8.1%. Found: C, 55.3; H, 8.9; N, 8.3%.
4. Conclusions
We described the synthesis of bicyclic piperidine-based iminosugars from natural amino acids l-alanine and l-serine. The procedure involves the preparation of enantiomerically pure α,β-unsaturated ketones in four steps and high yields from the natural amino acids. These intermediates, upon a stereoselective cyclopropanation reaction and further straightforward transformations, give the final products, which contain five stereogenic centers. The synthetic methodology used allows the obtention of different configurations at some of the asymmetric carbons, which, in this project, is interesting, because selectivity towards different enzymes could be achieved. The behavior of the products against different glycosidases showed that inhibition was generally low but selective towards one or two enzymes. The activation of the target enzymes was observed in some cases.
Supplementary Materials
The following are available online, 1D and 2D NMR spectra of all new compounds (Figures S1–S72). Figure S41: Transglycosidation monitorization by 1H-NMR). Table S1: Measured constant coupling from products derived from l-serine.
Author Contributions
Conceptualization of the work, J.P.-C. and G.D.; methodology, all authors; synthetic work, A.P.; biological assays, A.P. and F.J.C.; writing—original draft preparation, A.P. and J.P.-C.; writing—review and editing, all authors; and funding acquisition, J.P.-C. and F.J.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Spanish MINECO, grant numbers RTI2018-095588-B-I00 and RTI2018-094751-B-C22 (co-funded by the European Regional Development Fund/European Social Fund, “Investing in your future”), FUSP-CEU (PC17/17), and CIBERES, an initiative from the Spanish Institute of Health Carlos III.
Data Availability Statement
The data presented in this study are available in this article.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not available.
References
- Compain, P.; Martin, O.R. Iminosugars: From Synthesis to Therapeutical Applications; John Wiley & Sons: Chichester, UK, 2008. [Google Scholar]
- Stütz, A.E. Iminosugars as Glycosidase Inhibitors: Nojirimycin and Beyond; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 1998. [Google Scholar]
- Asano, N. Glycosidase inhibitors: Update and perspectives on practical use. Glycobiology 2003, 13, 93R–104R. [Google Scholar] [CrossRef] [PubMed]
- Joubert, P.H.; Veuter, C.P.; Joubert, H.F.; Hillebrand, I. the effect of a 1-Deoxynojirimycin derivative on post-prandial blood glucose and insulin level in healthy black and white volunteers. Eur. J. Clin. Pharmacol. 1985, 28, 705–708. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.R.; Flanagan, J.J.; Schilling, A.; Chang, H.H.; Agarwal, L.; Katz, E.; Wu, X.; Pine, C.; Wustman, B.; Desnick, R.J.; et al. The pharmacological chaperone 1-deoxygalactonojirimycin increases α-galactosidase A in Fabry patient cell lines. J. Inherit. Metab. Dis. 2009, 32, 424–440. [Google Scholar] [CrossRef] [PubMed]
- Markad, S.D.; Karanjule, N.S.; Sharma, T.; Sabharwal, S.G.; Dhavele, D.D. Synthesis and evaluation of glycosidase inhibitory activity if N-butyl-1-deoxy-D-gluco-homonojirimycin and N-butyl-1-deoxy-L-ido-homonojirimycin. Bioorg. Med. Chem. 2006, 14, 5535–5539. [Google Scholar] [CrossRef] [PubMed]
- Lillelund, V.H.; Jensen, H.H.; Liang, X.; Bols, M. Recent developments of transition-state analogue glycosidase inhibitors of non-natural product origin. Chem. Rev. 2002, 102, 515–553. [Google Scholar] [CrossRef]
- Davies, G.J.; Planas, A.; Rovira, C. Conformational analyses of the reaction coordinate of glycosidases. Acc. Chem. Res. 2012, 45, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Speciale, G.; Thompson, A.J.; Davies, G.J.; Williams, S.J. Dissecting conformational contributions to glycosidase catalysis and inhibition. Curr. Opin. Struct. Biol. 2014, 28, 1–13. [Google Scholar] [CrossRef]
- Puet, A.; Domínguez, G.; Cañada, F.J.; Pérez-Castells, J. Amino Acid-Based Synthesis and Glycosidase Inhibition of Cyclopropane-Containing Iminosugars. ACS Omega 2020, 5, 31821–31830. [Google Scholar] [CrossRef]
- López-Rodríguez, A.; Domínguez, G.; Pérez-Castells, J. Synthesis of novel iminosugars derivatives based on a 2-azabicyclo[4.1.0]heptane skeleton. Synthesis 2017, 49, 4600–4612. [Google Scholar] [CrossRef]
- Thompson, A.J.; Speciale, G.; Iglesias-Fernandez, J.; Hakki, Z.; Belz, T.; Cartmell, A.; Spears, R.J.; Chandler, E.; Temple, M.J.; Stepper, J.; et al. Evidence for a boat conformation at the transition state of GH76 α-1,6-mannanases key enzymes in bacterial and fungal mannoprotein metabolism. Angew. Chem. Int. Ed. 2015, 54, 5378–5382. [Google Scholar] [CrossRef]
- Beenakker, T.J.M.; Wander, D.P.A.; Offen, W.A.; Artola, M.; Raich, M.; Ferraz, M.J.; Li, K.-Y.; Houben, J.H.P.M.; van Rijssel, E.R.; Hansen, T.; et al. Carba-cyclophellitols are neutral retaining-glucosidase inhibitors. J. Am. Chem. Soc. 2017, 139, 6534–6537. [Google Scholar] [CrossRef] [PubMed]
- Compain, P. Glycomimetics: Design, synthesis and therapeutic applications. Molecules 2018, 23, 1658. [Google Scholar] [CrossRef]
- Stocker, B.L.; Dangerfield, E.M.; Win-Manson, A.L.; Haslett, G.W.; Timmer, M.S.M. Recent developments in the synthesis of pyrrolidine-containing iminosugars. Eur. J. Org. Chem. 2010, 9, 1615–1637. [Google Scholar] [CrossRef]
- Wang, B.; Bogh, S.A.; Navarro Poulsen, J.C.; Laursen, B.W.; Bols, M. Synthesis of isofagomine derivatives as new fluorescence pH indicators/glycosidase inhibitors. Eur. J. Org. Chem. 2020, 2020, 3989–3996. [Google Scholar] [CrossRef]
- Clemente, F.; Matassini, C.; Cardona, F. Reductive amination routes in the synthesis of piperidine iminosugars. Eur. J. Org. Chem. 2020, 2020, 4447–4462. [Google Scholar] [CrossRef]
- Moynihan, L.; Chadda, R.; McArdle, P.; Murphy, P.V. Allylic azide rearrangement in tandem with Huisgen cycloaddition for stereoselective annulations: Synthesis of C-glycosiliminosugars. Org. Lett. 2015, 17, 6226–6229. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.; Lee, J.H.; Kim, J.-W.; Kim, Y.G. Syn-Selective dihydroxylation of γ-amino-α,β-unsaturated (Z)-esters from D-serine: Setereoselctive synthesis of D-iminolyxitol. Tetrahedron Asymmetry 2007, 18, 2448–2453. [Google Scholar] [CrossRef]
- Boto, A.; Romero-Estudillo, I. One-Pot stereoselective synthesis of 1,2-amino alcohol derivatives. Org. Lett. 2011, 13, 3426–3429. [Google Scholar] [CrossRef]
- Prichard, K.L.; O’Brien, N.; Ghorbani, M.; Wood, A.; Barnes, E.; Kato, A.; Houston, T.A.; Simone, M.I. Synthetic routes to 3,4,5-trihydroxipiperidines via stereoselective and biocatalysed protocols, and strategies to N- and O-derivatisation. Eur. J. Org. Chem. 2018, 2018, 6830–6842. [Google Scholar] [CrossRef]
- Stanley, P.; Cummings, R.D. Structures common to different glycans. In Essentials of Glycobiology, 3rd ed.; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Darvill, A.G., Kinoshita, T., Packer, N.H., Prestegard, J.H., et al., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2017. [Google Scholar]
- Désiré, J.; Shipman, M. Iminoglycals in synthesis: Preparation of novel deoxymannojirimycin analogues. Synlett 2001, 2001, 1332–1334. [Google Scholar] [CrossRef]
- Taube, S.; Mallagaray, A.; Peters, T. Norovirus, glycans and attachment. Curr. Opin. Virol. 2018, 31, 33–42. [Google Scholar] [CrossRef]
- Tu, Z.; Lin, Y.-N.; Lin, C.-C. Development of fucosyltransferase and fucosidase inhibitors. Chem. Soc. Rev. 2013, 42, 4459–4475. [Google Scholar] [CrossRef]
- Decuyper, L.; Magdalenic, K.; Verstraete, M.; Jukic, M.; Sosic, I.; Sauvage, E.; Amoroso, A.M.; Verlaine, O.; Joris, B.; Gobec, S.; et al. alfa-Unsaturated 3-amino-1-carboxymethyl-beta-lactams as bacterial PBP inhibitors: Synthesis and biochemical assessment. Chem. Eur. J. 2019, 25, 16128–16140. [Google Scholar] [CrossRef]
- Dragutan, I.; Dragutan, V.; Demonceau, A. Targeted drugs by olefin metathesis: Piperidine-based iminosugars. RSC Adv. 2012, 2, 719–736. [Google Scholar] [CrossRef]
- For the synthesis of the ylide see: Henry, S.S.; Brady, M.D.; Laird, D.L.T.; Ruble, J.C.; Varie, D.L.; Monn, J.A. Improved synthesis of C4α- and C4β-Methyl analogues of 2-Aminobicyclo[3.1.0]hexane-2,6-dicarboxylate. Org. Lett. 2012, 14, 2662–2665. [Google Scholar] [CrossRef]
- Riches, S.L.; Saha, C.; Filgueira, N.F.; Grange, E.; McGarrigle, E.M.; Aggarwal, V.K. On the mechanism of ylide-mediated cyclopropanations: Evidence for a proton-transfer step and its effects on stereoselectivity. J. Am. Chem. Soc. 2010, 132, 7626–7630. [Google Scholar] [CrossRef]
- Aggarwal, V.K.; Grange, E. Asymmetric sulfonium ylide mediated cyclopropanation: Stereocontrolled synthesis of (+)-LY354740. Chem. Eur. J. 2006, 12, 568–575. [Google Scholar] [CrossRef]
- Zhang, R.; Mamai, A.; Madalengoitia, J.S. Cyclopropanation reactions of pyroglutamic acid-derived synthons with akylidene transfer reagents. J. Org. Chem. 1999, 64, 547–555. [Google Scholar] [CrossRef]
- Romo, D.; Meyers, A.I. An asymmetric route to enantiomerically pure 1,2,3-trisubstituted cyclopropanes. J. Org. Chem. 1992, 57, 6265–6270. [Google Scholar] [CrossRef]
- Andrés, J.M.; Pedrosa, R.; Pérez, A.; Pérez-Encabo, A. Diastereoselective synthesis of enantiopure γ-amino-β-hydroxy acids by Reformatsky reaction of chiral α-dibenzylamino aldehydes. Tetrahedron 2001, 57, 8521–8530. [Google Scholar] [CrossRef]
- Wang, J.; Wang, W.; Kollman, P.A.; Case, D.A. Automatic atom type and bond perception in molecular mechanical calculations. J. Mol. Graph. Model. 2006, 25, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Zamoner, L.O.B.; Aragao-Leoneti, V.; Carvalho, I. Iminosugars: Effects of stereochemistry, ring size and N-substituents on glucosidase activities. Pharmaceuticals 2019, 12, 108. [Google Scholar] [CrossRef] [PubMed]
- Gonda, J.; Siroky, M.; Martinkova, M.; Homolya, S.; Vilkova, M.; Pilatova, M.B.; Sestak, S. Synthesis and biologicak activity of diastereoisomeric octahydro-1H-indole-5,6,7-triols, analogues of castanospermine. Tetrahedron 2019, 75, 398–408. [Google Scholar] [CrossRef]
- Brissonnet, Y.; Ladevèze, S.; Tezé, D.; Fabre, E.; Deniaud, D.; Deligault, F.; Tellier, C.; Šesták, S.; Remaud-Simeon, M.; Potocki-Veronese, G.; et al. Polymeric iminosugars improve the activity of carbohydrate-processing enzymes. Bioconj. Chem. 2015, 26, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Darby, J.F.; Landström, J.; Roth, C.; He, Y.; Davies, G.J.; Hubbard, R.E. Discovery of selective small-molecule activators of a bacterial glycoside hydrolase. Angew. Chem. Int. Ed. 2014, 53, 13419–13423. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).