Oat Extract Avenanthramide-C Reverses Hippocampal Long-Term Potentiation Decline in Tg2576 Mice
Abstract
:1. Introduction
2. Results and Discussion
2.1. Avn Content in Different Oat Cultivars
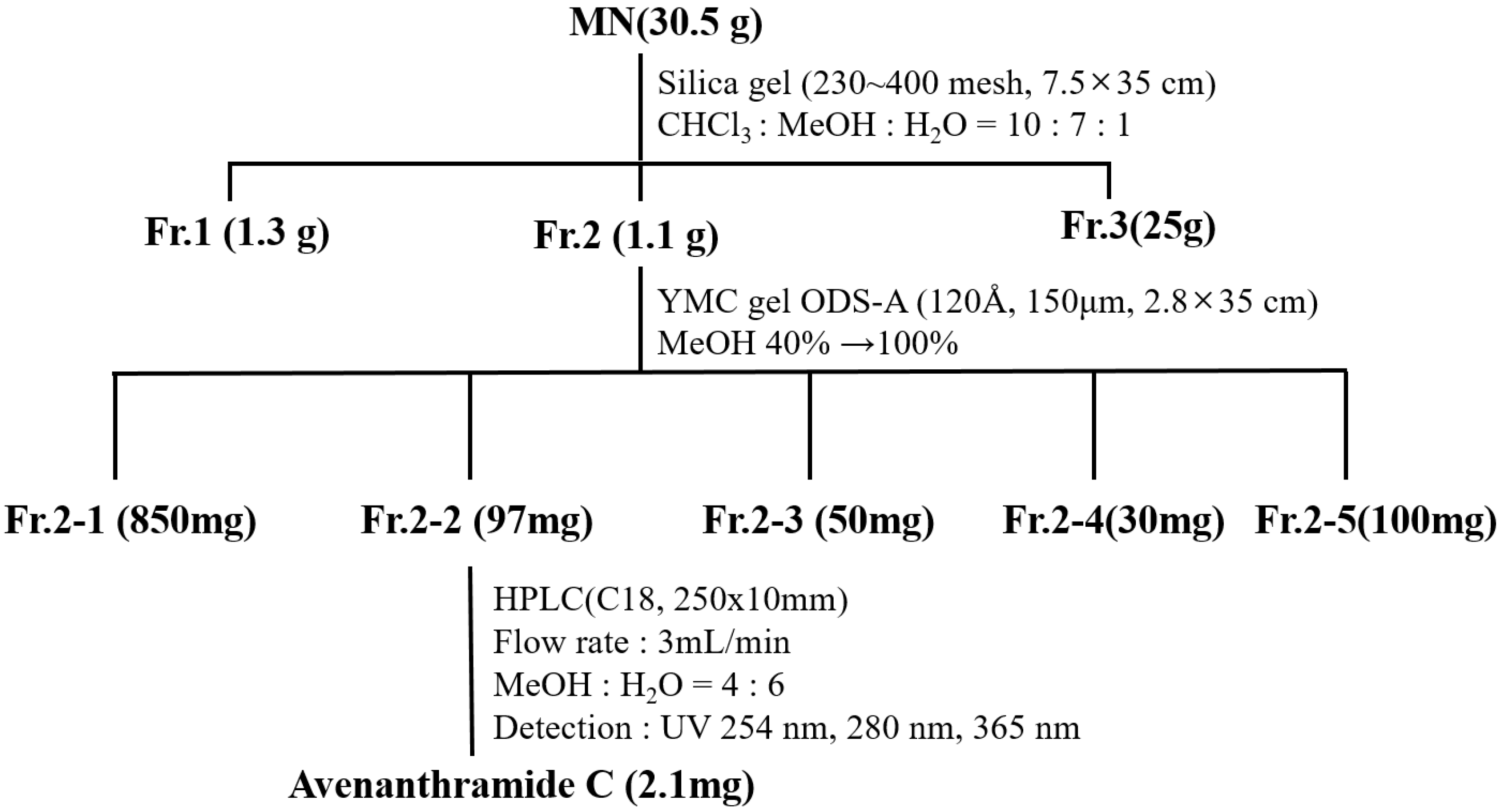
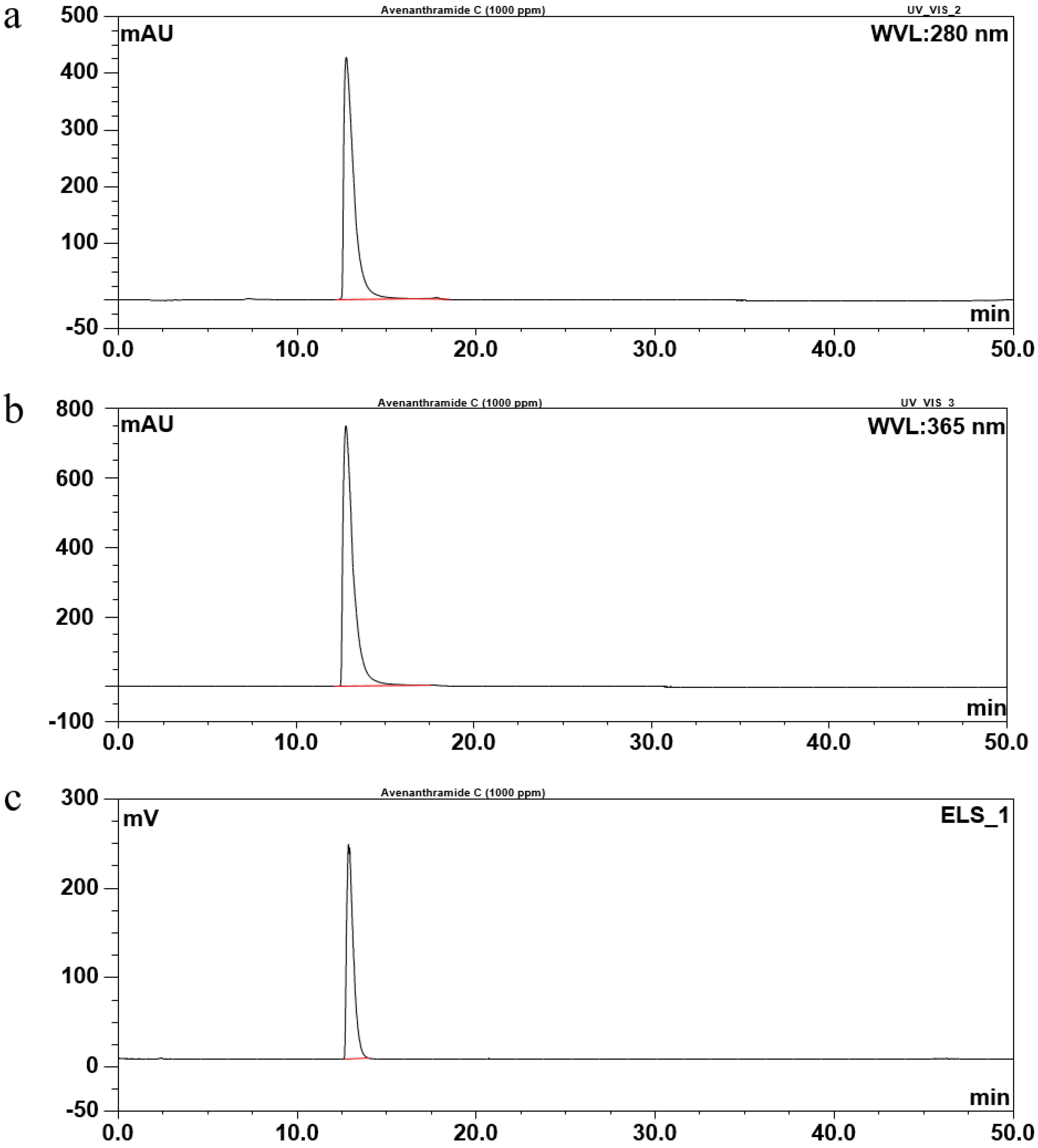
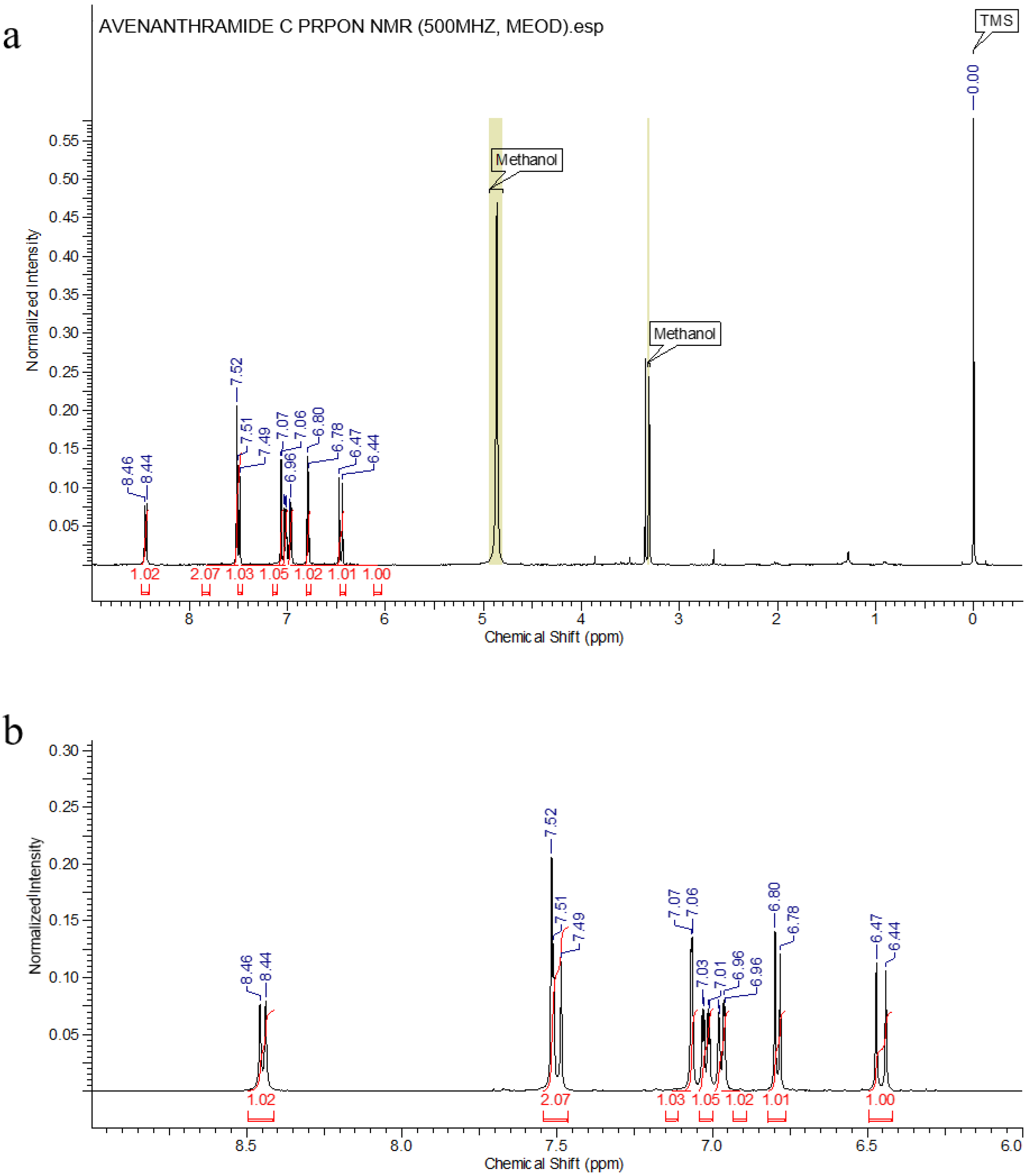
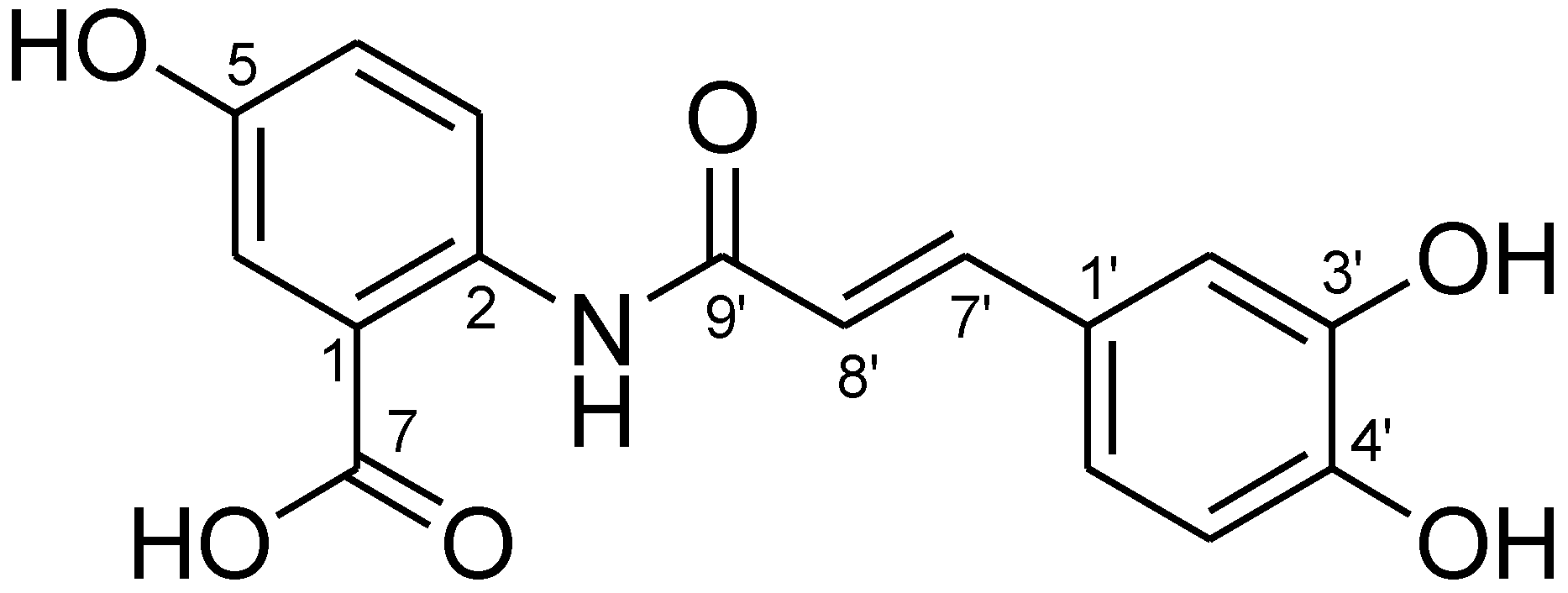
2.2. Identification of Avn-C Content in Fr. 2
2.3. Isolation and Purification of Avn-C
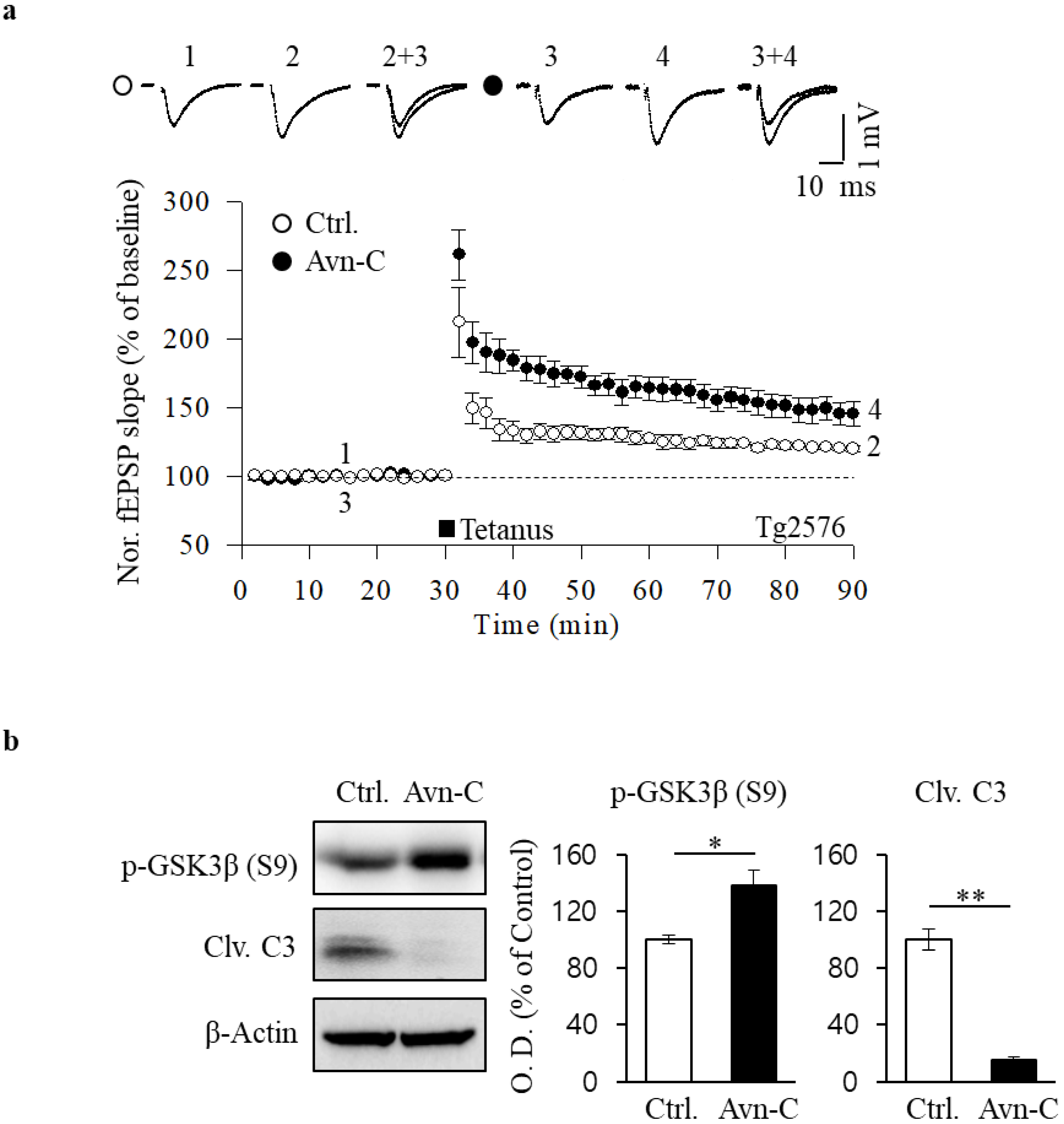
2.4. Restoration of HFS-Induced LTP by Avn-C
3. Materials and Methods
3.1. General Apparatus and Chemicals
3.2. Oat Samples
3.3. Sampling of Avns-Rich Fractions
3.3.1. Extraction
3.3.2. Purification
3.3.3. Analysis of Avns and β-Glucan Content
3.3.4. Total Phenol Quantification, DPPH, and ABTS
3.4. Animals
3.5. Hippocampal Slice Preparation
3.6. Electrophysiology
3.7. Immunoblotting
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Palop, J.J.; Mucke, L. Amyloid-beta-induced neuronal dysfunction in Alzheimer’s disease: From synapses toward neural networks. Nat. Neurosci. 2010, 13, 812–818. [Google Scholar] [CrossRef]
- Shankar, G.M.; Walsh, D.M. Alzheimer’s disease: Synaptic dysfunction and Aβ. Mol. Neurodegener. 2009, 4, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambert, M.P.; Barlow, A.K.; Chromy, B.A.; Edwards, C.; Freed, R.; Liosatos, M.; Morgan, T.E.; Rozovsky, I.; Trommer, B.; Viola, K.L.; et al. Diffusible, nonfibrillar ligands derived from Ab1–42 are potent central nervous system neurotoxins. Proc. Natl. Acad. Sci. USA 1998, 95, 6448–6453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, L.J.; Pardo, C.A.; Cork, L.C.; Price, D.L. Synaptic pathology and glial responses to neuronal injury precede the formation of senile plaques and amyloid deposits in the aging cerebral cortex. Am. J. Pathol. 1994, 145, 1358–1381. [Google Scholar] [PubMed]
- Lynch, M.A. Long-term potentiation and memory. Physiol. Rev. 2004, 84, 87–136. [Google Scholar] [CrossRef]
- Whitlock, J.R.; Heynen, A.J.; Shuler, M.G.; Bear, M.F. Learning induces long-term potentiation in the hippocampus. Science 2006, 313, 1093–1097. [Google Scholar] [CrossRef] [Green Version]
- Hwang, K.D.; Bak, M.S.; Kim, S.J.; Rhee, S.; Lee, Y.S. Restoring synaptic plasticity and memory in mouse models of Alzheimer’s disease by PKR inhibition. Mol. Brain 2017, 10, 57. [Google Scholar] [CrossRef] [Green Version]
- Prieto, G.A.; Trieu, B.H.; Dang, C.T.; Bilousova, T.; Gylys, K.H.; Berchtold, N.C.; Lynch, G.; Cotman, C.W. Pharmacological rescue of long-term potentiation in Alzheimer disease-related synapses. J. Neurosci. 2017, 37, 1197–1212. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Yoon, G.; Song, J.; Jo, J. Exendin-4 improves long-term potentiation and neuronal dendritic growth under in vivo and in vitro obesity condition. Sci. Rep. 2021, 11, 8326. [Google Scholar] [CrossRef]
- Canady, V.A. FDA Approves First Drug Therapy for Alzheimer’s in 18 Years. Ment. Health Wkly. 2021, 31, 3–4. [Google Scholar] [CrossRef]
- Ciccone, L.; Vandooren, J.; Nencetti, S.; Orlandini, E. Natural Marine and Terrestrial Compounds as Modulators of Matrix Metalloproteinases-2 (MMP-2) and MMP-9 in Alzheimer’s Disease. Pharmaceuticals 2021, 14, 86. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.P.S.; Albuquerque, H.M.T.; Cardoso, S.M.; Silva, A.M.S.; Silva, V.L.M. Dual-target compounds for Alzheimer’s disease: Natural and synthetic AChE and BACE-1 dual-inhibitors and their structure-activity relationship (SAR). Eur. J. Med. Chem. 2021, 221, 113492. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.; Silva, R.; Pinto, M.M.M.; Sousa, E. Marine Natural Products, Multitarget Therapy and Repurposed Agents in Alzheimer’s Disease. Pharmaceuticals 2020, 13, 242. [Google Scholar] [CrossRef] [PubMed]
- Peterson, D.M. Composition and nutritional characteristics of oat grains and products. Oat Sci. Technol. 1992, 33, 265–292. [Google Scholar]
- Welch, R.W. Oat, Human Nutrition and Health. In The Oat Crop, 1st ed.; Welch, R.W., Ed.; Springer: Dordrecht, The Netherlands, 1995; pp. 433–479. [Google Scholar]
- Tapola, N.; Karvonen, H.; Niskanen, L.; Mikola, M.; Sarkkinen, E. Glycemic responses of oat bran products in type 2 diabetic patients. Nutr. Metab. Cardiovasc. Dis. 2000, 15, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Zduńczyk, Z.; Flis, M.; Zieliński, H.; Wróblewska, M.; Antoszkiewicz, Z.; Juśkiewicz, J. In vitro antioxidant activities of barley, husked oat, naked oat, triticale, and buckwheat wastes and their influence on the growth and biomarkers of antioxidant status in rats. J. Agric. Food Chem. 2006, 54, 4168–4175. [Google Scholar] [CrossRef]
- Boz, H. Phenolic amides (Avenanthramides) in oats: A review. Czech J. Food Sci. 2015, 33, 399–404. [Google Scholar] [CrossRef] [Green Version]
- Emmons, C.L.; Peterson, D.M. Antioxidant activity and phenolic contents of oat groats and hulls. Cereal Chem. 1999, 76, 902–906. [Google Scholar] [CrossRef]
- Dimberg, L.H.; Theander, O.; Lingnert, H. Avenanthramides—A group of phenolic antioxidants in oats. Cereal Chem. 1993, 70, 637–641. [Google Scholar]
- Meydani, M. Potential health benefits of avenanthramides of oats. Nutr. Rev. 2009, 67, 731–735. [Google Scholar] [CrossRef]
- Verardo, V.; Serea, C.; Segal, R.; Caboni, M.F. Free and bound minor polar compounds in oats: Different extraction methods and analytical determinations. J. Cereal Sci. 2011, 54, 211–217. [Google Scholar] [CrossRef]
- Yang, J.; Ou, B.; Wise, M.L.; Chu, Y. In vitro total antioxidant capacity and anti-inflammatory activity of three common oat-derived avenanthramides. Food Chem. 2014, 160, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Wise, M.L.; Collins, F.W.; Meydani, M. Avenanthramides, polyphenols from oats, inhibit IL-1beta-induced NF-kappaB activation in endothelial cells. Free Radic. Biol. Med. 2008, 44, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Sur, R.; Nigam, A.; Grote, D.; Liebel, F.; Southall, M.D. Avenanthramides, polyphenols from oats, exhibit anti-inflammatory and anti-itch activity. Arch. Dermatol. Res. 2008, 300, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.O.; Milbury, P.E.; Collins, F.W.; Blumberg, J.B. Avenanthramides are bioavailable and have antioxidant activity in humans after acute consumption of an enriched mixture from oats. J. Nutr. 2007, 137, 1375–1382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peterson, D.M.; Hahn, M.J.; Emmons, C.L. Oat avenanthramides exhibit antioxidant activities in vitro. Food Chem. 2002, 79, 473–478. [Google Scholar] [CrossRef]
- Ramasamy, V.S.; Samidurai, M.; Park, H.J.; Wang, M.; Park, R.Y.; Yu, S.Y.; Kang, H.K.; Hong, S.; Choi, W.S.; Lee, Y.Y.; et al. Avenanthramide-C restores impaired plasticity and cognition in Alzheimer’s disease model mice. Mol. Neurobiol. 2020, 57, 315–330. [Google Scholar] [CrossRef]
- Rasane, P.; Jha, A.; Sabikhi, L.; Kumar, A.; Unnikrishnan, V.S. Nutritional advantages of oats and opportunities for its processing as value added foods—A review. J. Food Sci. Technol. 2015, 52, 662–675. [Google Scholar] [CrossRef] [Green Version]
- Emmons, C.; Peterson, D.M. Antioxidant activity and phenolic content of oat as affected by cultivar and location. Crop Sci. 2001, 41, 1676–1681. [Google Scholar] [CrossRef]
- Skoglund, M.; Peterson, D.M.; Andersson, R.; Nilsson, J.; Dimberg, L.H. Avenanthramide content and related enzyme activities in oats are affected by steeping and germination. J. Cereal Sci. 2008, 48, 294–303. [Google Scholar] [CrossRef]
- Wise, M.L. Effect of chemical systemic acquired resistance elicitors on avenanthramide biosynthesis in oat (Avena sativa). J. Agric. Food Chem. 2011, 59, 7028–7038. [Google Scholar] [CrossRef] [PubMed]
- Lesné, S.; Koh, M.T.; Kotilinek, L.; Kayed, R.; Glabe, C.G.; Yang, A.; Gallagher, M.; Ashe, K.H. A specific amyloid-β protein assembly in the brain impairs memory. Nature 2006, 440, 352–357. [Google Scholar] [CrossRef]
- Ferreira, S.T.; Lourenco, M.V.; Oliveira, M.M.; Felice, F.G.D. Soluble amyloid-β oligomers as synaptotoxins leading to cognitive impairment in Alzheimer’s disease. Front. Cell. Neurosci. 2015, 9, 191. [Google Scholar] [CrossRef] [Green Version]
- Jo, J.; Whitcomb, D.J.; Olsen, K.M.; Kerrigan, T.L.; Lo, S.C.; Bru-Mercier, G.; Dickinson, B.; Scullion, S.; Sheng, M.; Collingridge, G.; et al. Aβ (1–42) inhibition of LTP is mediated by a signaling pathway involving caspase-3, Akt1 and GSK-3β. Nat. Neurosci. 2011, 14, 545–547. [Google Scholar] [CrossRef]
- Llorens-Martín, M.; Jurado, J.; Hernández, F.; Ávila, J. GSK-3β, a pivotal kinase in Alzheimer disease. Front. Mol. Neurosci. 2014, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- D’Amelio, M.; Cavallucci, V.; Cecconi, F. Neuronal caspase-3 signaling: Not only cell death. Cell Death Differ. 2010, 17, 1104–1114. [Google Scholar] [CrossRef] [PubMed]
- Takashima, A.; Noguchi, K.; Michel, G.; Mercken, M.; Hoshi, M.; Ishiguro, K.; Imahori, K. Exposure of rat hippocampal neurons to amyloid β peptide (25–35) induces the inactivation of phosphatidyl inositol-3 kinase and the activation of tau protein kinase I/glycogen synthase kinase-3β. Neurosci. Lett. 1996, 203, 33–36. [Google Scholar] [CrossRef]
- Leroy, K.; Yilmaz, Z.; Brion, J.-P. Increased level of active GSK-3β in Alzheimer’s disease and accumulation in argyrophilic grains and in neurones at different stages of neurofibrillary degeneration. Neuropathol. Appl. Neurobiol. 2007, 33, 43–55. [Google Scholar] [CrossRef]
- Phiel, C.J.; Wilson, C.A.; Lee, V.M.-Y.; Klein, P.S. GSK-3α regulates production of Alzheimer’s disease amyloid-β peptides. Nature 2003, 423, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Petrov, D.; Ettcheto, M.; Abad, S.; Sánchez-López, E.; García, M.L.; Olloquequi, J.; Beas-Zarate, C.; Auladell, C.; Camins, A. Current Research Therapeutic Strategies for Alzheimer’s Disease Treatment. Neural Plasticity 2016, 2016, 8501693. [Google Scholar] [CrossRef] [Green Version]
- Murakami, K.; Murata, N.; Ozawa, Y.; Kinoshita, N.; Irie, K.; Shirasawa, T.; Shimizu, T. Vitamin C Restores Behavioral Deficits and Amyloid-β Oligomerization without Affecting Plaque Formation in a Mouse Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2011, 26, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Schrott, L.M.; Jackson, K.; Yi, P.; Dietz, F.; Johnson, G.S.; Basting, T.F.; Purdum, G.; Tyler, T.; Rios, J.D.; Castor, T.P.; et al. Acute Oral Bryostatin-1 Administration Improves Learning Deficits in the APP/PS1 Transgenic Mouse Model of Alzheimer’s Disease. Curr. Alzheimer Res. 2015, 12, 22–31. [Google Scholar] [CrossRef]
- Marambaud, P.; Zhao, H.; Davies, P. Resveratrol Promotes Clearance of Alzheimer’s Disease Amyloid-β Peptides. J. Biol. Chem. 2005, 280, 37377–37382. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, K.; Alonso, A.D.; Chen, S.; Chohan, M.O.; El-Akkad, E.; Gong, C.X.; Khatoon, S.; Li, B.; Liu, F.; Rahman, A.; et al. Tau pathology in Alzheimer disease and other tauopathies. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2005, 1739, 198–210. [Google Scholar] [CrossRef] [Green Version]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef] [Green Version]
- Toral-Rios, D.; Pichardo-Rojas, P.S.; Alonso-Vanegas, M.; Campos-Peña, V. GSK3 β and Tau Protein in Alzheimer’s Disease and Epilepsy. Front. Cell. Neurosci. 2020, 14, 19. [Google Scholar] [CrossRef] [PubMed]
| Type | Cultivars | Abbr. | Avn-C | Avn-A | Avn-B | Total Avn |
|---|---|---|---|---|---|---|
| Naked | Seonyang | SE | 11.7 ± 2.5 b | 13.7 ± 3.5 b | 13.4 ± 3.9 b | 38.9 ± 8.8 b |
| Daeyang | DY | 86.9 ± 24.1 a | 52.7 ± 16.7 a | 49.1 ± 24.3 a | 188.7 ± 85.1 a | |
| Choyang | CY | 7.8 ± 1.7 b | 8.6 ± 2.4 b | 10.5 ± 6.1 b | 27.0 ± 10 b | |
| Suyang | SY | 15.9 ± 0.7 b | 14.3 ± 2.7 b | 21.1 ± 9.5 b | 51.3 ± 11.6 b | |
| Jungmo2005 | JM | 22.9 ± 6.8 b | 26.4 ± 8.3 ab | 18 ± 3.1 b | 67.4 ± 18.2 b | |
| Hulled | Samhan | SH | 1.6 ± 0.2 c | 0.0 ± 0.1 c | 1.3 ± 0.1 c | 2.9 ± 0.4 c |
| Donghan | DH | 3.4 ± 1.1 c | 3.7 ± 1.0 c | 7.5 ± 1.9 bc | 14.7 ± 3.9 c | |
| Highspeed | HS | 3.3 ± 0.1 c | 2.4 ± 0.1 c | 3.6 ± 0.1 c | 9.3 ± 0.3 c | |
| Jopung | JP | 3.6 ± 0.1 c | 3.0 ± 0.0 c | 3.5 ± 0.1 c | 10.1 ± 0.2 c | |
| ** | * | ** |
| Germination Time (h) | Avn-C | Avn-A | Avn-B | Total Avns |
|---|---|---|---|---|
| 0 | 84.3 ± 3.1 b | 64.1 ± 2.3 a | 58.1 ± 2.2 a | 206.5 ± 7.6 a |
| 24 | 75.5 ± 1.7 c | 49.9 ± 0.6 a | 46.4 ± 1.2 a | 171.9 ± 3.4 a |
| 48 | 216.0 ± 1.9 a | 165.2 ± 2.2 a | 115.5 ± 1.4 a | 496.7 ± 4.0 a |
| 72 | 218.8 ± 4.0 a | 173.2 ± 2.9 a | 129.5 ± 4.2 a | 521.5 ± 11.0 a |
| Extract (1 g) | DPPH (mg TE/100 g of Sample) | ABTS (mg TE/100 g of Sample) | Polyphenol (mg GAE/g of Extract) |
|---|---|---|---|
| Germinated grain | 11.02 ± 1.23 b | 30.76 ± 2.17 b | 30.49 ± 2.39 b |
| MN | 9.05 ± 0.32 c | 25.35 ± 0.29 c | 27.40 ± 0.60 c |
| Fr. 2 | 29.77 ± 0.94 a | 37.11 ± 0.21 a | 44.01 ± 1.32 a |
| Extract | Weight (g) | Avn-C | Avn-A | Avn-B | Total Avns | Yield (%) | β-glucan % (g/100 g) |
|---|---|---|---|---|---|---|---|
| Fr. 2 | 1.1 | 31.11 | 0.01 | 0.00 | 31.12 | 0.2 | 0.52 ± 0.02 |
| Cultivar | Abbr. | Registration Year | Type | Utilization Type | Grain Yield (MT ha−1) |
|---|---|---|---|---|---|
| Seonyang | SE | 2003 | Naked | Food | 3.38 |
| Daeyang | DY | 2007 | Naked | Food | 4.18 |
| Choyang | CY | 2007 | Naked | Food | 4.67 |
| Suyang | SY | 2010 | Naked | Food | 4.35 |
| Jungmo2005 | JM | 2010 | Naked | Food | 4.35 |
| Samhan | SH | 2001 | Hulled | Forage use | 4.44 |
| Donghan | DH | 2001 | Hulled | Forage use | 4.21 |
| Highspeed | HS | 2005 | Hulled | Forage use | 5.00 |
| Jopung | JP | 2009 | Hulled | Forage use | 5.68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-Y.; Wang, M.; Son, Y.; Yang, E.-J.; Kang, M.-S.; Kim, H.-J.; Kim, H.-S.; Jo, J. Oat Extract Avenanthramide-C Reverses Hippocampal Long-Term Potentiation Decline in Tg2576 Mice. Molecules 2021, 26, 6105. https://doi.org/10.3390/molecules26206105
Lee Y-Y, Wang M, Son Y, Yang E-J, Kang M-S, Kim H-J, Kim H-S, Jo J. Oat Extract Avenanthramide-C Reverses Hippocampal Long-Term Potentiation Decline in Tg2576 Mice. Molecules. 2021; 26(20):6105. https://doi.org/10.3390/molecules26206105
Chicago/Turabian StyleLee, Yu-Young, Ming Wang, Yurim Son, Eun-Ju Yang, Moon-Seok Kang, Hyun-Joo Kim, Hyung-Seok Kim, and Jihoon Jo. 2021. "Oat Extract Avenanthramide-C Reverses Hippocampal Long-Term Potentiation Decline in Tg2576 Mice" Molecules 26, no. 20: 6105. https://doi.org/10.3390/molecules26206105
APA StyleLee, Y.-Y., Wang, M., Son, Y., Yang, E.-J., Kang, M.-S., Kim, H.-J., Kim, H.-S., & Jo, J. (2021). Oat Extract Avenanthramide-C Reverses Hippocampal Long-Term Potentiation Decline in Tg2576 Mice. Molecules, 26(20), 6105. https://doi.org/10.3390/molecules26206105





